Abstract
BACKGROUND:
Zambia has an estimated TB incidence of 319/100,000 population and a HIV prevalence of 11.1%. In 2020, only 49% of new people living with HIV (PLHIV) received TB preventive therapy (TPT) in Zambia. Misconceptions about the reliability of symptom screening and drug resistance among people who develop TB while on TPT are barriers to TPT scale-up. We determined the incidence and predictors of breakthrough TB during TPT among PLHIV in Zambia.
METHOD:
This was a retrospective analysis of routine TPT programme data among PLHIV collected between October 2016 and October 2019 from select primary health facilities in Zambia.
RESULTS:
Of 48,581 PLHIV enrolled on TPT, 130 (0.3%) developed breakthrough TB during TPT. Of the 130, 90 client records were accessed. The median age of the breakthrough TB cases was 35 years; 68% were males. Overall, 96% of the breakthrough TB cases had been on antiretroviral therapy (ART) for ⩽3 months; 24% were symptomatic at the beginning of TPT, 22% were asymptomatic and others had missing data. Of the 130 breakthrough TB cases, 79% developed TB in the first month after TPT initiation. The median time to TB diagnosis was 10 days (IQR 4–16).
CONCLUSION:
Breakthrough TB during TPT is rare among PHLIV on ART, and very rare after the first month of TPT initiation. It should therefore not be a barrier to TPT scale-up.
Keywords: breakthrough TB, IPT, TPT, HIV, PLHIV
Abstract
CONTEXTE :
La Zambie a une incidence de TB estimée à 319/100 000 habitants et une prévalence du VIH de 11,1%. En 2020, seulement 49% des nouvelles personnes vivant avec le VIH (PLVIH) ont reçu une thérapie préventive (TPT) contre la TB en Zambie. Les idées fausses sur la fiabilité du dépistage des symptômes et la résistance aux médicaments chez les personnes qui développent une TB alors qu’elles sont sous TPT sont des obstacles à l’extension de la TPT. Nous avons déterminé l’incidence et les facteurs prédictifs de la découverte de la TB pendant la TPT chez les PLVIH en Zambie.
MÉTHODE :
Il s’agissait d’une analyse rétrospective des données du programme TPT de routine chez les PLVIH recueillies entre octobre 2016 et octobre 2019 dans certains établissements de santé primaire en Zambie.
RÉSULTATS :
Sur 48 581 PLVIH inscrites au programme TPT, 130 (0,3%) ont développé la TB pendant le TPT. Sur ces 130, 90 dossiers de clients ont été consultés. L’âge médian des cas de TB était de 35 ans ; 68% étaient des hommes. Dans l’ensemble, 96% des cas de TB découvert étaient sous traitement antirétroviral (ART) depuis ⩽3 mois; 24% étaient symptomatiques au début du TPT, 22% étaient asymptomatiques et les autres avaient des données manquantes. Parmi les 130 cas de percée de la TB, 79% ont développé la TB au cours du premier mois après le début du TPT. Le délai médian de diagnostic de la TB était de 10 jours (IQR 4–16).
CONCLUSION :
La découverte de la TB pendant le TPT est rare chez les PLVIH sous ART, et très rare après le premier mois du début du TPT. Elle ne devrait donc pas constituer un obstacle à la généralisation des TPT.
Zambia is a high TB-HIV burden country, with TB incidence estimated at 319/100,000 population,1 and HIV prevalence estimated at 11.1%.2 Of the 40,000 TB patients notified to the Zambia National TB Programme (NTP) in 2020, 39% were TB-HIV co-infected, and an estimated 9,100 people living with HIV (PLHIV) died from TB in the same year.1,3 To reduce the risk of TB in PLHIV and its adverse effects on HIV treatment outcomes, the WHO recommends the use of one complete course of TB preventive therapy (TPT) for PLHIV in whom TB has been ruled out.4 This is based on evidence from systematic reviews of randomised clinical trials which showed a 33% overall risk reduction for TB among PLHIV when initiated on TPT.4 This risk is further reduced when TPT is used in combination with antiretroviral therapy (ART).5–7 The protective effect of TPT has been demonstrated to last longer than 5 years among PLHIV on ART.8
Despite this, the uptake of TPT has been relatively slow in most high-burden countries, where its use is expected to have the most impact.1 For example, as of 2020 only 49% of new PLHIV received TPT in Zambia.1 One of the factors that has contributed to the slow uptake of TPT in Zambia and other settings are concerns about the reliability of symptom screening to rule out TB and the possibility of drug-resistant TB among patients who develop TB while on TPT.9–13 This is in spite of evidence that symptom screening has a high negative predictive value for TB among PLHIV (>95%),14 and that TPT is not associated with increased drug resistance.15–17 In fact, available evidence, although limited, has demonstrated that <1% of patients develop TB while on TPT (breakthrough TB).18–20 There is no literature on breakthrough TB in Zambia.
We undertook a retrospective analysis of programme data to determine the incidence and predictors of breakthrough TB among PLHIV in Zambia. The aim of this analysis was to provide local evidence on breakthrough TB among PLHIV in Zambia which would be used to inform policy and clinical decision-making.
METHODOLOGY
Study design and setting
We conducted a retrospective analysis of TPT programme data collected among PLHIV between October 2016 to October 2019 from public health facilities in the Lusaka, Western, Eastern and Southern Provinces in Zambia. The data had been collected from respectively 608, 608 and 279 facilities in 2016–2017, 2017–2018 and 2018–2019. All facilities were supported by Centers for Disease Control and Prevention (CDC)/President’s Emergency Plan for AIDS Relief (PEPFAR), and were in the process of scaling up provision of TPT to PLHIV.
During the period indicated above, TPT scale-up mainly targeted newly diagnosed HIV clients. The country was implementing test and treat for HIV,21 and eligible HIV-positive clients were started TPT at enrolment on ART. However, clients already on ART and those not yet on ART could also receive TPT. As per the national guidelines on TPT, PLHIV were screened for TB using symptom screening (any cough, weight loss in adults and adolescents/poor weight gain in children, fever and night sweats) before initiation of TPT.22 Those without TB symptoms and without any of the other contraindications to TPT (hepatitis, existing peripheral neuropathy, hypersensitivity to isoniazid, above-recommended alcohol consumption, history of convulsions and psychosis, and concomitant medication) were offered TPT, while those with any TB symptom received further evaluation for TB using Xpert® MTB/RIF (Cepheid, Sunnyvale, CA, USA) and thereafter, chest X-ray if the Xpert result was negative.23 Once TB was ruled out, the clinicians considered TPT as per their discretion.22 At the time of this analysis, 6-month isoniazid preventive therapy (IPT) was the only form of TPT available in Zambia. Vitamin B6 was given as adjuvant therapy to prevent peripheral neuropathy among those initiated on TPT. The patients were reviewed at Month 1, 3 and 6 of treatment to provide adherence support, do a symptom screen for TB and screen for adverse events. Those new on ART were also reviewed 2 weeks after ART initiation. Patients who continued to be eligible for TPT were dispensed additional doses of TPT. Patients who developed TB symptoms while on TPT received further evaluation for TB. If positive (bacteriologically confirmed) or if a clinical diagnosis was made, TPT was stopped, and they were initiated on TB treatment. We did not collect data on the development of TB after TPT completion.
Definitions
Breakthrough TB was defined as TB that develops while patients are on TPT. Patient time to TB diagnosis was defined as the time from TPT initiation to TB diagnosis.
Data collection and analysis
Aggregate programme data on TPT initiations and outcomes among PLHIV that had been previously collected and verified was analysed to determine the specific outcomes of individuals initiated on TPT. These outcomes included treatment completed, developed TB, died, TPT discontinued due to adverse events, loss to follow-up and not evaluated. The respective health facilities for all breakthrough TB cases were determined. The data collection team then used the paper-based TPT registers at each facility to identify the breakthrough TB cases corresponding to the period under review, and to obtain their identifying information (name, age and sex) and TPT start dates. The patient identifiers were used to access additional patient-level clinical data from either paper-based or electronic health records (Smartcare; created by Elizabeth Glaser Pediatric AIDS Foundation, Washington DC, USA; and Dimagi, Cambridge, MA, USA on behalf of the Ministry of Health, Zambia) on HIV care and the TB treatment register. From the patient files on HIV care, information on ART start date, CD4 count and results of TB symptom screening before start of TPT was collected. From the TB treatment register, we collected data on date of TB diagnosis. Where there was no documentation on a given variable in the client’s records, the data was indicated as missing.
Data collection was done remotely at some sites. Some of the client’s medical records could not be assessed, either because the sites were difficult to access and there were no resources to support data collection in the health facilities under which the patients were identified or because the TPT registers corresponding to the period when patients initiated TPT could not be found.
MS Excel (MicroSoft, Redmond, WA, USA) was used to store and clean the data and STATA statistical software v14 (StataCorp, College Station, TX, USA) was used for the analysis. A descriptive analysis was conducted to determine the incidence of breakthrough TB among PLHIV (overall and disaggregated by province), percentage of data collected by province and the characteristics of patients who developed breakthrough TB (sex, age, duration on ART, baseline CD4 count, TB symptoms and time to TB diagnosis). Age was presented both as a median with interquartile ranges (IQRs), and by percentage of individuals who were aged <15 years and ⩾15 years. Duration on ART was stratified into ⩽3 months, >3⩽6 months and >6 months, while CD4 count was stratified into <100, 100–<350, 350–<500 and ⩾500 cells/mm3 ; the proportion of individuals under each of the strata was determined. We determined the proportion of individuals who were asymptomatic and those who were symptomatic at start of ART. Among those who were symptomatic, we determined the number and type of symptoms. Finally, time to TB diagnosis was determined using both median with IQR and the number of patients developing TB every month. Kaplan–Maier failure estimates were used to determine the association between breakthrough TB and age and sex. Age was regrouped under 0–24 years, 25–40 years and >40 years to obtain more precise estimates. We tested the equality of failure rates using the log-rank test (P > 0.05). Association between breakthrough TB and CD4 count was not determined due to large numbers of missing data. Patients whose medical records could not be assessed were excluded from the analysis, as they had missing data on all the variables.
Ethics consideration
Ethics approval for the study was obtained from University of Zambia Biomedical Research and Ethics Committee, Lusaka, Zambia (Reference number (005-11-17) to analyse existing programme data. Informed consent was not obtained, as the analysis was retrospectively performed using routine data which was de-identified before analysis.
RESULTS
A total number of 48,581 PLHIV were initiated on TPT over a period of 3 years. Of these, 130 (0.3%) developed breakthrough TB during TPT (Figure 1). The TB cumulative incidence was 267 per 100,000 person-years. Western Province had the highest proportion of breakthrough TB cases (0.5%). Overall, we did not collect data on 40 (30.8%) breakthrough TB cases. In Eastern and Southern Provinces, data were collected on all breakthrough TB cases (Table 1). In Lusaka, 83% of the breakthrough TB cases had their data collected, while this number was only 30.6% in Western Province.
FIGURE 1.
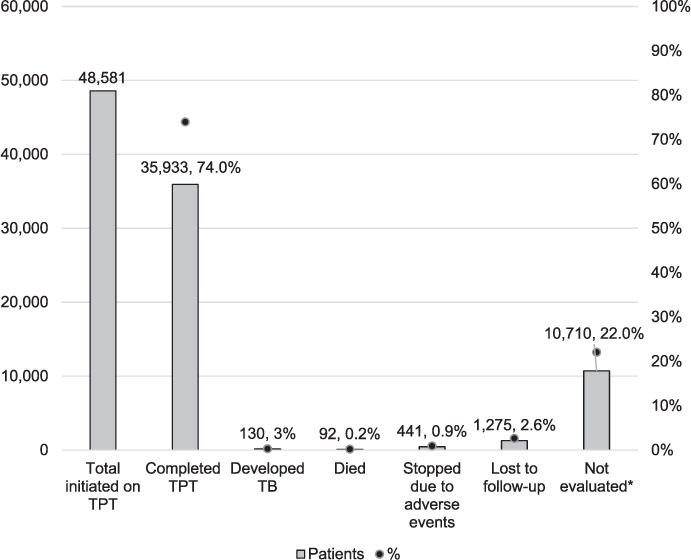
Treatment outcomes of patients initiated on TPT. *Individuals with no outcome. TPT = TB preventive therapy.
TABLE 1.
Provinces with patients with breakthrough TB
| Province | Patients initiated on TPT n | PLHIV with breakthrough TB n (%) | TB cases with data collected n (%) | Breakthrough TB cases with no data collected n (%) |
|---|---|---|---|---|
| Lusaka | 32,961 | 88 (0.3) | 73 (83.0) | 15 (17.0) |
| Western | 7,458 | 36 (0.5) | 11 (30.6) | 25 (69.4) |
| Southern | 4,071 | 5 (0.1) | 5 (100.0) | 0 (0.0) |
| Eastern | 4,091 | 1(0.02) | 1(100.0) | 0 (0.0) |
| Total | 48,581 | 130 (0.3) | 90 (69.2) | 40 (30.8) |
TPT = TB preventive therapy; PLHIV = people living with HIV.
Of the 90 patients with breakthrough TB whose medical records were accessed, the majority (n = 61, 68%) were males (Table 2). The median age of the individuals with breakthrough TB was 35 years (IQR 29.5–41). Of these individuals, 86 (96%) had been on ART for not more 3 months at diagnosis of breakthrough TB. Data on baseline CD4 count and TB symptoms before start of TPT were largely missing. The median time to TB diagnosis was 10 days (IQR 4–16) (Table 2). The majority of people with breakthrough TB (79%) were identified within the first month of being initiated on TPT, with a steep decline in the months that followed (Figure 2).
TABLE 2.
Characteristics of PLHIV initiated on TPT who developed breakthrough TB
| Variable | Sub-category | Breakthrough TB* n (%) |
|---|---|---|
| Sex | Male | 61 (68) |
| Female | 29 (32) | |
| Age, years | Median [IQR] | 35 [29.5–41] |
| <15 | 1 (1) | |
| ⩾15 | 89 (99) | |
| Duration on ART, months | ⩽3 | 86 (96) |
| >3 ⩽ 6 | 0 (0) | |
| >6 | 4 (4) | |
| Baseline CD4 count, cells/mm3 | <100 | 6 (7) |
| 100–<350 | 8 (9) | |
| 350–<500 | 0 (0) | |
| ⩾500 | 5 (5) | |
| Missing | 71(79) | |
| TB symptoms | Symptomatic before start of TPT | 22 (24) |
| 1 symptom | 6 (27) | |
| 2 symptoms | 5 (23) | |
| >2 symptoms | 11 (50) | |
| Fever | 9 (41)† | |
| Cough | 21 (95)† | |
| Weight loss | 10 (45)† | |
| Night sweats | 15 (68)† | |
| Asymptomatic before start of TPT | 20 (22) | |
| Missing data | 48 (54) | |
| Time to TB diagnosis, days | Median [IQR] | 10 [4–16] |
*Majority of people with breakthrough TB (79%) were recorded within the first month of being initiated on TPT with a steep decline in the months that followed (see Figure 2).
†Numbers with specific symptoms are not mutually exclusive.
PLHIV = people living with HIV; TPT = TB preventive therapy; IQR = interquartile range.
FIGURE 2.
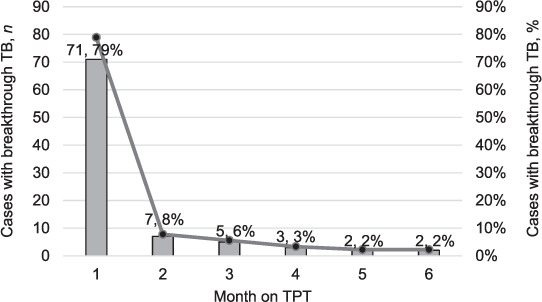
Time to TB incidence. The Kaplan–Meier failure estimates show that there was no association between sex and age with time to TB diagnosis (Figure 3A and 3B). Fifty percent of individuals aged 0–24 years, 25–40 years and >40 years developed TB by Day 25, 7 and 14 after TPT start, respectively. TPT = TB preventive therapy.
Kaplan–Meier failure estimates show that there was no association between sex and age with time to TB diagnosis; 50% of individuals aged 0–24 years, 25–40 years and >40 years developed TB by Day 25, 7 and 14 after TPT start, respectively (Figure 3A and 3B).
FIGURE 3.
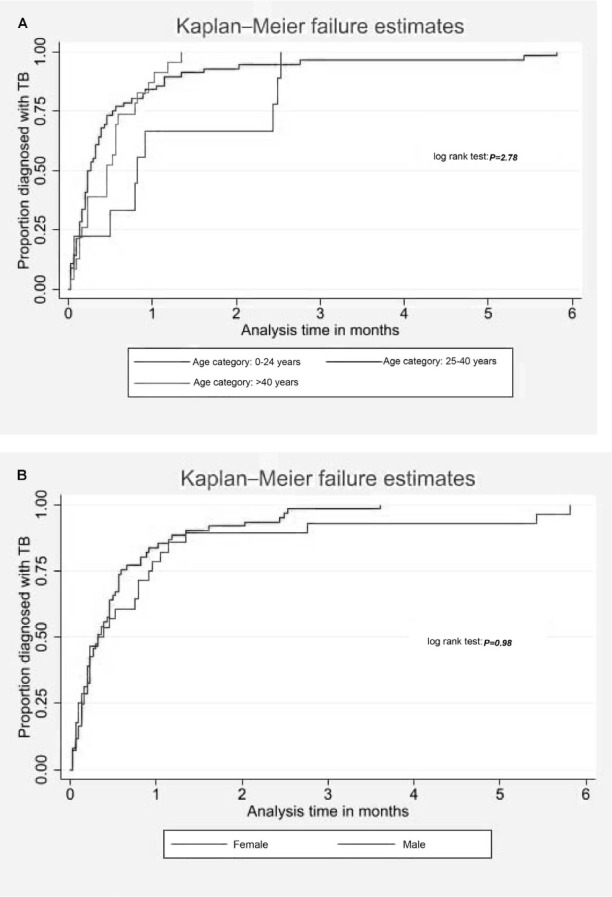
Time to TB diagnosis by patient characteristics. A) Time to TB diagnosis by age category; B) time to TB diagnosis by sex.
DISCUSSION
Breakthrough TB among PLHIV on ART occurred in 0.3% of patients during the 6 months of TPT. Of the 90 breakthrough TB cases that we reviewed, 79% developed breakthrough TB within 1 month of TPT initiation. Most results on symptom screening before start of TPT were missing; however, 24% of the breakthrough TB cases were symptomatic for TB before start of TPT. There was no association between age and sex and time to breakthrough TB.
Our study adds to and extends the scarce existing literature on breakthrough TB among PLHIV. It concords with findings from the study conducted in Ethiopia, which was also a retrospective study that used existing records of patients in care and found that the incidence of breakthrough TB during TPT was low and that there was no association between breakthrough TB with age and sex.18 While the incidence of breakthrough TB reported in Ethiopia was 0.5%, which was slightly higher than what was reported by our study, this could be accounted for by the fact that not all patients on TPT in the Ethiopian study were initiated on ART. Our incidence of breakthrough TB is also comparable to that reported among PLHIV on ART in Karnataka, India (0.2%),19 and that reported among the Thibela miners, of unknown HIV status, in South Africa (0.4%).20
In our study, the majority of the breakthrough TB cases occurred within the first month of TPT initiation, with a steep decline thereafter. Within this group of patients were some missed diagnoses of TB during the initial evaluation for TPT. This is suggested by our data, which showed that 24% of the PLHIV were symptomatic for TB before start of TPT, some of whom were diagnosed with TB within 4 days of TPT initiation. This implies that some patients were initiated on TPT while being evaluated for TB, even though the national guidelines on TPT require clinicians to rule out TB among symptomatic individuals before start of TPT. Furthermore, as 54% of breakthrough TB cases had missing data on TB symptoms before start of TPT, it is not known whether they were screened for TB and documentation not done or if no screening was done. Initiation of TPT among patients being evaluated for TB raises concerns about adherence to TPT guidelines,22 while having a large amount of missing data on TB screening before start of TPT raises concerns about possible poor adherence to the TB screening and diagnostic algorithm, a concern that has also been reported in other settings.24–27
Some of the breakthrough TB cases were possibly due to TB-associated immune reconstitution inflammatory syndrome (TB-IRIS). This is supported by the fact that 22% of the individuals were asymptomatic at baseline but later developed symptoms, which prompted evaluation. Furthermore, over 96% of the breakthrough TB cases occurred within 3 months of starting ART, the time period during which TB IRIS is typically unmasked.28–30 Although data on CD4 count was largely missing, 7% of patients had CD4 count <100 cells/mm3 , a key risk factor for IRIS.31,32 This category of individuals have advanced HIV disease and could have benefitted from the use of TB lipoarabinomannan (LAM), which is currently being rolled out in the country, before start of TPT.33 The sensitivity of LAM increases with decreasing CD4 count, as opposed to other types of TB diagnostics, whose sensitivities are reduced with decreasing CD4 count.34
The strength of our study is that it involved a large number of PLHIV from various sites within Zambia. However, it has some important limitations. First, it had large amounts of missing data. Failing to obtain medical information on 31% of breakthrough TB cases may have introduced bias during the analysis of TB predictors. Additionally, the large amount of missing data on symptom screening and CD4 count limits the generalisability of the findings related to these variables in the study population. Also, the findings might not be generalisable to Western Province, where 69% of the data on breakthrough TB cases were not collected. Another limitation was that we did not collect data on the development of TB after TPT completion, so TB incidence is specific to the period during TPT. Also, as we did not monitor adherence to TPT, there is a possibility that some of these patients with breakthrough TB were not on TPT. Finally, our findings cannot be generalised to other parts of the country, as the study sites were not systematically selected to be representative of the national picture. Opportunities for future research would include evaluation of patients with breakthrough TB to determine if they are at higher risk for isoniazid-resistant TB compared to other forms of TB.
CONCLUSION
Breakthrough TB during TPT is rare among PHLIV on ART. It should hence not be a barrier to TPT scale-up, as it can be detected early with the use of the national TB programme guidelines on TB screening during TPT. There is need to strengthen adherence to the national guidelines on symptom screening among all PLHIV before start of TPT while increasing access to tools that can detect asymptomatic TB in PLHIV who have advanced HIV disease.
ACKNOWLEDGEMENTS
The authors would like to thank the health workers and community health workers who were involved in the documentation of the various registers from which data were collected.
Availability of data and materials: These will be made available from the senior author (Dr M Muyoyeta, email: monde. muyoyeta@cidrz.org) upon reasonable request.
Footnotes
Conflicts of interest: none declared.
References
- 1.World Health Organisation Global tuberculosis report, 2021. Geneva, Switzerland: WHO; 2021. [Google Scholar]
- 2.Zambia Statistics Agency, Ministry of Health of Zambia, University Teaching Hospital–Virology Laboratory, Department of Population Studies Demography Health Survey, HIV fact sheet. Lusaka, Zambia: University of Zambia; 2018. [Google Scholar]
- 3.World Health Organisation Global tuberculosis report, 2020. Geneva, Switzerland: WHO; 2020. [Google Scholar]
- 4.World Health Organisation Latent tuberculosis infection: updated and consolidated guidelines for programmatic management. Geneva, Switzerland: WHO; 2018. [PubMed] [Google Scholar]
- 5.Golub JE, et al. The impact of antiretroviral therapy and isoniazid preventive therapy on tuberculosis incidence in HIV-infected patients in Rio de Janeiro, Brazil. AIDS. 2007;21(11):1441–1448. doi: 10.1097/QAD.0b013e328216f441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Golub JE, et al. Isoniazid preventive therapy, HAART and tuberculosis risk in HIV-infected adults in South Africa: a prospective cohort. AIDS. 2009;23(5):631–636. doi: 10.1097/QAD.0b013e328327964f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yirdaw KD, et al. Beneficial effect of isoniazid preventive therapy and antiretroviral therapy on the incidence of tuberculosis in people living with HIV in Ethiopia. PLoS One. 2014;9(8):e104557. doi: 10.1371/journal.pone.0104557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Badje A, et al. Effect of isoniazid preventive therapy on risk of death in west African, HIV-infected adults with high CD4 cell counts: long-term follow-up of the Temprano ANRS 12136 trial. Lancet Global Health. 2017;5(11):e1080–e1089. doi: 10.1016/S2214-109X(17)30372-8. [DOI] [PubMed] [Google Scholar]
- 9.Kagujje M, et al. Implementation of isoniazid preventive therapy in people living with HIV in Zambia: challenges and lessons. BMC Public Health. 2019;19(1):1–4. doi: 10.1186/s12889-019-7652-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akolo C, et al. Debunking the myths perpetuating low implementation of isoniazid preventive therapy amongst human immunodeficiency virus-infected persons. World J Virol. 2015;4(2):105. doi: 10.5501/wjv.v4.i2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Getahun H, et al. Implementation of isoniazid preventive therapy for people living with HIV worldwide: barriers and solutions. AIDS. 2010;24:S57–S65. doi: 10.1097/01.aids.0000391023.03037.1f. [DOI] [PubMed] [Google Scholar]
- 12.Pathmanathan I, et al. TB preventive therapy for people living with HIV: key considerations for scale-up in resource-limited settings. Int J Tuberc Lung Dis. 2018;22(6):596–605. doi: 10.5588/ijtld.17.0758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalema N, et al. Gaps in TB preventive therapy for persons initiating antiretroviral therapy in Uganda: an explanatory sequential cascade analysis. Int J Tuberc Lung Dis. 2021;25(5):388–394. doi: 10.5588/ijtld.20.0956. [DOI] [PubMed] [Google Scholar]
- 14.Getahun H, et al. Development of a standardized screening rule for tuberculosis in people living with HIV in resource-constrained settings: individual participant data meta-analysis of observational studies. PLoS Med. 2011;8(1):e1000391. doi: 10.1371/journal.pmed.1000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Balcells ME, et al. Isoniazid preventive therapy and risk for resistant tuberculosis. Emerg Infect Dis. 2006;12(5):744. doi: 10.3201/eid1205.050681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Van Halsema CL et al. Tuberculosis outcomes and drug susceptibility in individuals exposed to isoniazid preventive therapy in a high HIV prevalence setting. AIDS. 2010;24(7):1051–1055. doi: 10.1097/QAD.0b013e32833849df. [DOI] [PubMed] [Google Scholar]
- 17.Swaminathan S, et al. Efficacy of a six-month versus a 36-month regimen for prevention of tuberculosis in HIV-infected persons in India: a randomized clinical trial. PLoS One. 2012;7(12):e47400. doi: 10.1371/journal.pone.0047400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yirdaw KD, et al. Breakthrough tuberculosis disease among people with HIV–should we be worried? A retrospective longitudinal study. PLoS One. 2019;14(2):e0211688. doi: 10.1371/journal.pone.0211688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reddy MM, et al. To start or to complete? Challenges in implementing tuberculosis preventive therapy among people living with HIV: a mixed-methods study from Karnataka, India. Global Health Action. 2020;13(1):1704540. doi: 10.1080/16549716.2019.1704540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hermans SM, et al. The timing of tuberculosis after isoniazid preventive therapy among gold miners in South Africa: a prospective cohort study. BMC Med. 2016;14(1):1–11. doi: 10.1186/s12916-016-0589-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ministry of Health Zambia Zambia Consolidated guidelines on treatment and prevention of HIV infection. Lusaka, Zambia: MoH; 2016. [Google Scholar]
- 22.Ministry of Health Zambia Managing tuberculosis in the HIV setting in Zambia. Lusaka, Zambia: MoH; 2014. [Google Scholar]
- 23.National TB and Leprosy Programme Zambia TB manual. Lusaka, Zambia: NTLP; 2017. [Google Scholar]
- 24.McCarthy K, et al. What happens after a negative test for tuberculosis? Evaluating adherence to TB diagnostic algorithms in South African Primary Health Clinics. J Acquir Immune Defic Syndr. 2016;71:e119–e126. doi: 10.1097/QAI.0000000000000907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Claassens M, et al. Tuberculosis cases missed in primary health care facilities: should we redefine case finding? Int J Tuberc Lung Dis. 2013;17(5):608–614. doi: 10.5588/ijtld.12.0506. [DOI] [PubMed] [Google Scholar]
- 26.Subbaraman R, et al. The tuberculosis cascade of care in India’s public sector: a systematic review and meta-analysis. PLoS Med. 2016;13(10):e1002149. doi: 10.1371/journal.pmed.1002149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kweza P, et al. Estimating the magnitude of pulmonary tuberculosis patients missed by primary health care clinics in South Africa. Int J Tuberc Lung Dis. 2018;22(3):264–272. doi: 10.5588/ijtld.17.0491. [DOI] [PubMed] [Google Scholar]
- 28.Meintjes G, et al. Tuberculosis-associated immune reconstitution inflammatory syndrome: case definitions for use in resource-limited settings. Lancet Infect Dis. 2008;8(8):516–523. doi: 10.1016/S1473-3099(08)70184-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Azis L, Jones-López EC, Ellner JJ. Chapter 26. HIV-associated tuberculosis. In: Volberding PA, et al., editors. Sande’s HIV/AIDS Medicine. London, UK: W. B. Saunders; 2012. pp. 325–347. [Google Scholar]
- 30.Quinn CM, et al. Tuberculosis IRIS: pathogenesis, presentation, and management across the spectrum of disease. Life. 2020;10(11):262. doi: 10.3390/life10110262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cevaal PM, Bekker L-G, Hermans S. TB-IRIS pathogenesis and new strategies for intervention: Insights from related inflammatory disorders. Tuberculosis. 2019;118:101863. doi: 10.1016/j.tube.2019.101863. [DOI] [PubMed] [Google Scholar]
- 32.Naidoo K, et al. The immune reconstitution inflammatory syndrome after antiretroviral therapy initiation in patients with tuberculosis: findings from the SAPiT trial. Ann Intern Med. 2012;157(5):313–324. doi: 10.7326/0003-4819-157-5-201209040-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ministry of Health Guidelines for management of advanced HIV disease in Zambia. Lusaka, Zambia: MoH; 2021. [Google Scholar]
- 34.World Health Organisation Geneva, Switzerland: WHO; 2021. WHO consolidated guidelines on tuberculosis. Module 3: diagnosis—rapid diagnosis for TB detection. [Google Scholar]


