Abstract
Aim
To evaluate the ability of the DrugSorb™-AntiThrombotic Removal (ATR) haemoadsorption device utilizing porous polymer bead sorbent technology to remove three commonly used antithrombotic drugs from whole blood.
Methods and results
We evaluated the removal of apixaban, rivaroxaban, and ticagrelor by the DrugSorb-ATR haemoadsorption device in a benchtop clinical scale model using bovine whole blood. Blood spiked at clinically relevant concentrations of an antithrombotic agent was continuously circulated through a 300-mL DrugSorb-ATR haemoadsorption device at a flow rate of 300 mL/min. Drug concentration was monitored over 6 h to evaluate drug removal. Results were compared with a control circuit without the haemoadsorption device. Removal rates at 30, 60, 120, and 360 minutes were: apixaban: 81.5%, 96.3%, 99.3% >99.8%; rivaroxaban: 80.7%, 95.1%, 98.9%, >99.5%; ticagrelor: 62.5%; 75%, 86.6%, >95% (all P <0.0001 vs. control). Blood pH and haematological parameters were not significantly affected by the DrugSorb-ATR haemoadsorption device when compared with the control circuit.
Conclusion
DrugSorb-ATR efficiently removes apixaban, rivaroxaban, and ticagrelor in a clinical-scale benchtop recirculation circuit with the bulk of removal occurring in the first 60 minutes. The clinical implications of these findings are currently investigated in patients undergoing on-pump cardiothoracic surgery in two US pivotal trials (ClinicalTrials.gov Identifiers: NCT04976530 and NCT05093504).
Keywords: Apixaban, Rivaroxaban, Ticagrelor, Haemoadsorption, Extracorporeal, Porous polymer beads
Background
Anticoagulant and antiplatelet therapies are widely used for the prevention and treatment of thromboembolic complications in patients with cardiovascular disease (CVD). Use of these agents is increasing due to an aging population, better diagnosis of CVD and availability of newer agents with improved benefit: risk profiles. Direct oral anticoagulants (DOACs), of which apixaban and rivaroxaban are the most widely prescribed, have largely replaced warfarin in the management of atrial fibrillation (AF) and venous thromboembolism (VTE) based on comparable or superior efficacy, improved safety, and greater ease of use.1–3 It is estimated that 2/3 of the patients on oral anticoagulants are now on a DOAC, with the lion's share being on apixaban or rivaroxaban.4 Similarly, the potent P2Y12 receptor inhibitor ticagrelor is recommended by guidelines and increasingly utilized in ACS patients who are not at high bleeding risk, based on studies like PLATO that suggest improved efficacy compared with the less potent P2Y12 receptor inhibitor clopidogrel.5–7 Approximately 40% of patients on dual antiplatelet therapy (DAPT) are on the newer generation of P2Y12 inhibitors, at least half of those being on ticagrelor.8
The trade-off between safety and efficacy with the use of antithrombotic drugs is well established and benefits must be weighed against the risk of bleeding complications.9 In addition to spontaneous bleeding events, these drugs also increase the risk of serious bleeding in patients requiring urgent surgical or invasive procedures. Accordingly, clinical practice guidelines recommend that, whenever possible, these agents should be withheld for several days prior to any surgeries with moderate to high risk of bleeding, to allow drug elimination and restoration of haemostasis.5,10,11 Specifically, for ticagrelor, recommendations are to discontinue the drug for a minimum of 3 days prior to cardiac or other high-bleeding-risk surgeries.12,13 For DOACs, such as apixaban and rivaroxaban, the recommended preoperative discontinuation period is at least 48 h, and longer in patients with impaired renal function.14 However, ∼5% of all surgeries/procedures are clinically urgent and need to be performed before the recommended washout period is completed.5,15 In such cases, bleeding complications are both frequent and severe and currently management is limited to non-specific, supportive therapies that include blood product transfusions (platelets, red blood cells, etc.) and administration of coagulation factors. Although these measures may have potential benefit in managing ongoing bleeding, their use also carries significant risks, and considerable costs.16,17 Alternate strategies are therefore urgently needed to improve the safety of patients on antithrombotic drugs undergoing urgent surgeries associated with a high perioperative bleeding risk.
Active drug removal in such settings may be beneficial, but traditional dialysis is ineffective because antithrombotic drugs are highly protein-bound.18–20 The DrugSorb™-ATR (AntiThrombotic Removal) system has a unique mechanism of action utilizing porous polymer beads that can remove drugs from blood based on their molecular weight, chemical structure, and circulating drug concentrations and can be easily incorporated into standard extracorporeal circuits (Figure 1). A similar technology, marketed as CytoSorb®, is CE mark approved in the European Union (EU) for the removal of cytokines, bilirubin, myoglobin, ticagrelor, and rivaroxaban and has been used in over 170 000 treatments to date with a favourable safety profile.21,22 The DrugSorb™-ATR system in addition to the polymer bead filled cartridge also includes disposable, specialized connector and tubing sets, disposable haemostats, roller clamps for controlling flow through the device, and a dedicated flow monitor to measure device flow rates in real time during Cardiopulmonary Bypass (CPB). Therefore, this system is specifically designed for easy integration and optimized device performance with any standard CPB setup and is currently under investigation in two FDA investigational device exemption (IDE) studies of patients undergoing on pump cardiac surgery (STAR-T: Safe and Timely Antithrombotic Removal—Ticagrelor; STAR-D: Safe and Timely Antithrombotic Removal—Direct Oral Anticoagulants; ClinicalTrials.gov Identifiers: NCT04976530 and NCT05093504 respectively).
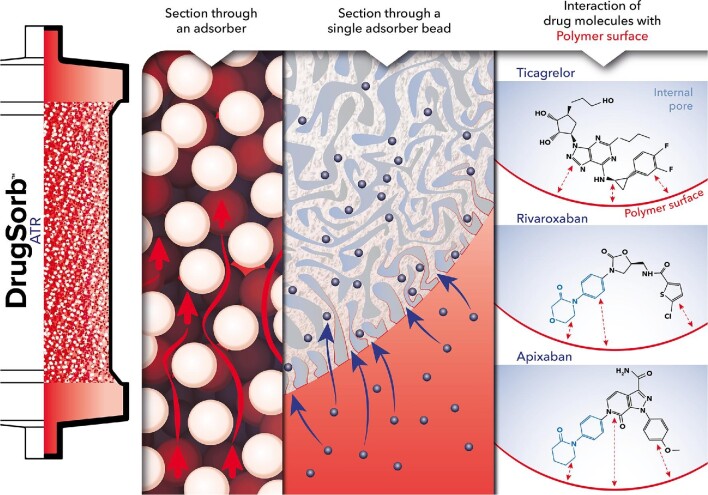
Figure 1.
Schematic representation of antithrombotic drug adsorption at the surface of porous polymer beads. The DrugSorb-ATR system incorporates a cartridge filled with biocompatible highly porous polymer beads that can actively remove hydrophobic target compounds via haemoadsorption. The extensive network of pores on the surface of each polymer bead is optimally sized for particular target molecules, and internal channels provide a vast surface area for adsorption. Beads are produced using solid state chemistry and rely on hydrophobic interactions for adsorption, rather than employing ligands, antibodies, cells, or other biologics. Pore size is such that adsorption of larger molecules (e.g. antibodies and albumin) is minimized and cells are excluded.
In this study, we determined the efficiency of the DrugSorb™-ATR system for removing apixaban, rivaroxaban, and ticagrelor from circulating blood in an in vitro recirculation circuit.
Methods
Materials and equipment
Ticagrelor [purity 99.64%, high performance liquid chromatography (HPLC)] and ticagrelor-d7 (Purity: 96%, HPLC) were procured from MuseChem, Fairfield, NJ, USA. Apixaban (purity 98%, HPLC) and rivaroxaban (purity 99.7%, HPLC) were obtained from Toronto Research Chemicals, Toronto, Ontario, Canada and AK Scientific Inc, Union City, CA, USA, respectively. All other reagents were of standard analytical grade. The test and control circuit components were procured from McMaster–Carr, Elmhurst, IL, USA and Molded Products Inc., Harlan, IA, USA. Bovine whole blood (Lampire Biological Inc., Pipersville, PA, USA) with 4.5 U/mL Na-Heparin and total protein ranging between 6.6–8.8 g/dL, was used for all runs. Maxi–Therm Pediatric Blankets 56436-I (Cincinnati Sub Zero LLC, Cincinnati, OH, USA) connected with Polystat standard 3–6 L heat/cool bath (Cole–Parmer, Vernon Hills IL, USA) were used to maintain the temperature of the blood pool at 37°C throughout the runs. All tested DrugSorb-ATR devices were gamma sterilized and used the same filling, finishing and sterilization processes as commercially available devices. All instruments used for the measurement of run parameters were calibrated using instrumentation and standards from the United States National Institute of Standards and Technology (NIST) and are compliant with ISO-10 012:2003 and ANSI/NCSL Z540-3-2006.
Experimental design
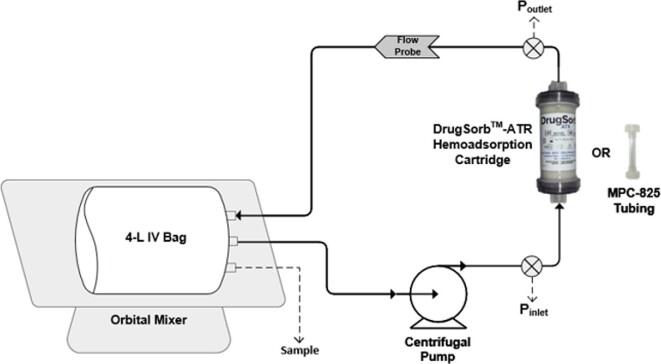
Drug removal was assessed using a full-scale benchtop recirculation model intended to mimic the intraoperative setup for haemoadsorption during cardiopulmonary bypass (Figure 2). The circuit consisted of 4 L of heparinized bovine whole blood in a blood bag placed on an orbital mixer to keep the contents well-mixed throughout the experiment. A centrifugal pump (Biomedicus 550 Centrifugal pump, Medtronic, Minneapolis, MN, USA) and pump head (BP80, International Biophysics, Austin, TX, USA) was used to pump blood at a constant flow rate of 300 mL/min. Our experimental flow rate of 300 mL/min represents the equivalent of a clinical ‘worst case removal scenario’ since flow rates through the system during CPB are typically >300 mL/min and up to 700 mL/min. The circuit contained pressure monitors (Omega Engineering, Norwalk, CT, USA) and flow monitors (Transonic Systems Inc., Ithaca, NY, USA) on both the inlet and outlet sides of the DrugSorb-ATR system. Two sets of experiments were performed with blood circulating either through the 300 mL DrugSorb-ATR system (treatment circuit) or through a 4.5′′ tubing connector (control circuit). Recirculation was continuous, for a 6-hour study period. Sampling was performed through a sampling port in the blood bag. All tubing used comprised of standard non-coated PVC material.
Figure 2.
Schematic representation of experimental recirculation circuit. The circuit includes either a 300 mL DrugSorb-ATR device (treatment) or a tubing connector (control). Drawing not to scale.
DrugSorb-ATR was flushed and primed by gravity using 2 L of saline as per manufacturer's instructions; tubing and the pump were also primed with saline in both experimental setups. Before connecting the blood bag to the circuit, an initial 5 mL blood sample was drawn for testing of total haemoglobin, pH, haematocrit, and activated clotting time (ACT) to ensure consistency in initial blood conditions between all experiments. Blood was then spiked with the drug under study to the target concentrations (apixaban: 220 ng/mL; rivaroxaban: 450 ng/mL; ticagrelor: 1000 ng/mL). Initial concentration for each drug was targeted to match clinically relevant maximum plasma concentrations as reported in the literature.23
Apixaban
Oral dosing varies according to indication, but 5 mg twice daily is most used, resulting in mean peak plasma concentrations between 125 and 220 ng/mL.23
Rivaroxaban
Standard maintenance dose is 20 mg daily (for patients with CrCl >50 mL/min), resulting in maximum plasma concentrations between 350–450 ng/mL.23
Ticagrelor
Standard loading with 180 mg, or maintenance with 90 mg twice daily yield maximum plasma concentration of ∼1000 ng/mL.24
The blood bag was allowed to mix for 20 min before obtaining baseline (t = 0 min) samples, after which the primed circuit was connected to the outlet side of the blood bag and purged of remaining saline by displacement with blood. Final step was to connect the inlet side of the blood bag to the circuit and begin recirculation.
For each drug under study, the spiked bovine blood was recirculated through either the treatment or the control circuit and the experiments were repeated five times. During the 6-hour recirculation period, blood was drawn at the following time periods to test drug levels: t = 0, 15, 30, 45, 60, 90, 120, 180, 270, and 360 min. Blood was placed into dipotassium ethylenediaminetetraacetic acid vials (BD, Franklin Lakes, NJ, USA) and processed by centrifuging at 4000 xg at 4°C for 10 min. The resulting plasma was stored at −80°C until drug concentration analysis could be performed by liquid chromatography with tandem mass spectrometry (LC-MS/MS).
To test whether the experimental circuit had an effect on blood pH or cell counts, we performed relevant measurements in accordance with the FDA guidance document for CPB oxygenators 510(k) submissions.25 To specifically test for damage to red blood cells, we measured total haemoglobin (Hb), haematocrit (Hct), and plasma free haemoglobin (pfHb; released when red cells are damaged or lysed). Plasma pH and pfHb levels were assessed using an ABL90 FLEX PLUS blood analyzer (Radiometer America, Brea, CA, USA). Additionally, whole blood samples collected at t = 0, 90, 180, 270, and 360 min were used for direct measure of blood pH, total blood Hb, and blood glucose using an i-STAT 300 V clinical analyzer (Abbott Laboratories, Chicago, IL, USA) and red blood cell (RBC), white blood cell (WBC), and platelet counts using a HemaVet950 FS haematology analyzer (Drew Scientific, Miami Lakes, FL, USA).
Data analysis
All results are presented as a mean ± SD. Comparisons between the treatment vs. control groups at each time point were performed with Student's t-test and P values <0.05 were considered significant.
Analytical method validation
Individual bioanalytical methods for the determination of apixaban, rivaroxaban, and ticagrelor concentrations in bovine plasma were developed and validated using LC-MS/MS. The methods were specific and sensitive, having no interfering peaks in the control plasma. The methods were validated for a linear range of 1.22–1250 ng/mL for apixaban, 6.25–6250 ng/mL for rivaroxaban, and 5.0–5000 ng/mL for ticagrelor with a coefficient of determination >0.99. Criteria for acceptance of all tested parameters were met (linearity, accuracy, precision, selectivity, recovery, short- and long-term stability at various temperatures, freeze thaw stability, and stability in injection medium) as laid out in the guidance document for analytical method development from the FDA.26 Method validation parameters and other details are described as part of supplementary data file. The calibration curves for all the three validated methods are shown as supplementary material online, Figure S1.
Results
Antithrombotic drug removal
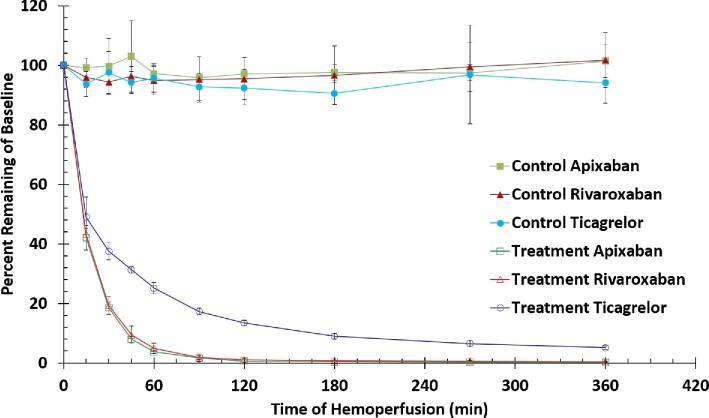
Plasma drug concentrations for all three drugs remained relatively constant in the control group (between 90–103% of baseline) with no downward trend over the entire 6-hour recirculation experiment. This indicates a relatively high stability of each tested drug under the investigational conditions. In contrast, efficient removal was observed in the treatment group for all three tested drugs with results summarized in drug concentrations over time in Table 1 and as percentage (%) drug remaining relative to baseline in Figure 3. For each drug, there is a significant difference between the control and treatment groups at all time-points except baseline.
Table 1.
Absolute plasma concentrations of apixaban, rivaroxaban, and ticagrelor during 6 h of haemoperfusion. Results represent mean ± SD, n = 5.
| Apixaban (ng/mL) | Rivaroxaban (ng/mL) | Ticagrelor (ng/mL) | ||||
|---|---|---|---|---|---|---|
| Time (min) | Control | Treatment | Control | Treatment | Control | Treatment |
| 0 | 220.4 ± 11.1 | 206.8 ± 18.0 | 449.8 ± 46.0 | 437.3 ± 47.1 | 989.6 ± 93.0 | 964.8 ± 120.8 |
| 15 | 218.3 ± 7.9 | 86.6 ± 5.2 | 430.4 ± 34.3 | 189.4 ± 22.0 | 924.6 ± 50.8 | 473.8 ± 97.7 |
| 30 | 219.7 ± 22.0 | 38.4 ± 6.6 | 423.1 ± 29.0 | 83.6 ± 6.6 | 960.8 ± 42.7 | 362.2 ± 56.4 |
| 45 | 226.5 ± 24.1 | 16.5 ± 2.8 | 432.4 ± 32.8 | 40.4 ± 8.2 | 929.8 ± 51.5 | 301.4 ± 29.8 |
| 60 | 214.3 ± 13.3 | 7.6 ± 1.3 | 425.4 ± 27.0 | 20.9 ± 5.2 | 943.8 ± 56.1 | 241.6 ± 28.8 |
| 90 | 211.2 ± 10.6 | 3.3 ± 2.3 | 426.3 ± 27.3 | 7.8 ± 2.0 | 915.6 ± 60.7 | 166.4 ± 17.2 |
| 120 | 213.9 ± 9.5 | 1.3 ± 1.0 | 427.6 ± 28.2 | 4.7 ± 1.5 | 911.8 ± 79.5 | 129.4 ± 15.6 |
| 180 | 214.9 ± 9.1 | 0.8 ± 0.1 | 431.8 ± 26.2 | 3.2 ± 0.9 | 893.4 ± 54.0 | 86 ± 11.3 |
| 270 | 214.7 ± 9.3 | 0.6 ± 0.1 | 445 ± 20.5 | 2.5 ± 0.8 | 948 ± 92.8 | 61.8 ± 6.2 |
| 360 | 223.2 ± 12.7 | 0.5 ± 0.1 | 454.2 ± 15.2 | 2.1 ± 0.4 | 926.8 ± 45.6 | 49.3 ± 4.4 |
Figure 3.
Percent removal of antithrombotic drugs. Results represent mean ± SD, n = 5. There is a statistically significance difference between treatment and control (P <0.001, student's t-test) at each time point other than t = 0 min.
Apixaban
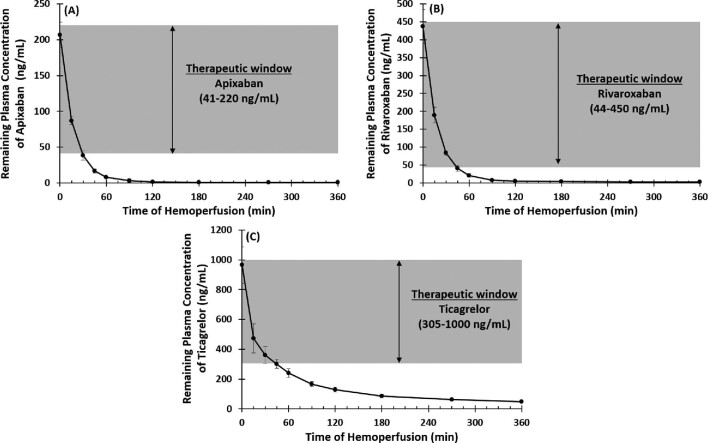
Mean plasma concentrations at baseline were similar in both the control (220.4 ± 4.9 ng/mL; n = 5) and treatment circuits (206.8 ± 18.0 ng/mL; n = 5). In the test system, DrugSorb-ATR removed 58% of the circulating apixaban within the first 15 min and >95% by 60 min with remaining plasma concentration of 7.6 ± 1.31 ng/mL which is below the expected trough plasma levels in patients receiving standard clinical dosing (5 mg apixaban twice-daily) (Figure 4A).27 After 2 h, drug levels were at or below the lower limit of quantitation (LLOQ).
Figure 4.
Therapeutic ranges and remaining plasma concentrations of (A) Apixaban, (B) Rivaroxaban and (C) Ticagrelor over 6 h of haemoperfusion. The grey shaded region represents the therapeutic window for each drug based on its clinical use. DrugSorb-ATR was able to reduce the drug concentration below the trough plasma level within 30–60 minutes for all drugs tested. Results presented as mean ± SD, n = 5.
Rivaroxaban
Mean plasma concentrations at baseline were similar in both the control (449.8 ± 45.9 ng/mL; n = 5) and treatment circuits (437.3 ± 47.1 ng/mL; n = 5). Rivaroxaban was also rapidly removed, with 57% removed within the first 15 min and >95% by 60 min with remaining plasma concentration of 20.9 ± 5.2 ng/mL which is below the mean trough plasma concentration observed in patients receiving standard clinical dosing of rivaroxaban (Figure 4B).28 Results for the first hour of removal are similar to those previously published from a scaled down recirculation system (1 L of blood) using a downsized (60 mL) sorbent device.29 In that study, however, device saturation appeared to be reached, with little additional removal observed after 1 h. In the current experiment, using a more clinically relevant blood volume (4 L) and a full-size (300 mL) DrugSorb-ATR device, we observed ongoing removal, with all time-points after 2 h yielding rivaroxaban concentrations below the LLOQ of the assay.
Ticagrelor
Mean plasma concentrations at baseline were similar in the control (989.6 ± 93.0 ng/mL; n = 5) and treatment circuits (964.8 ± 120.8 ng/mL; n = 5). Ticagrelor was also removed rapidly with 50% reduction in plasma concentration within the first 15 min and 75% by 60 min. After 2 h, ticagrelor concentration fell below expected trough plasma levels in patients receiving standard maintenance dosing (90 mg ticagrelor twice-daily) (Figure 4C).30 By the end of the 6-hour treatment period, ∼95% of circulating ticagrelor had been removed.
Blood pH, haematology, and circuit parameters
Whole blood and plasma pH
There were minimal, non-significant decreases in both whole blood and plasma pH after 6 h in both the control and treatment groups (Table 2).
Table 2.
Blood chemistry and haematology data. Unless otherwise indicated, n = 5
| Apixaban | Rivaroxaban | Ticagrelor | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Parameters | Time (h) | Control | Treatment | P-value | Control | Treatment | P-value | Control | Treatment | P-value |
| (Mean ± SD) | (Mean ± SD) | (Mean ± SD) | (Mean ± SD) | (Mean ± SD) | (Mean ± SD) | |||||
| Blood pHa | 0 | 7.32 ± 0.02 | 7.32 ± 0.00 | 0.92 | 7.28 ± 0.02 | 7.29 ± 0.02 | 0.96 | 7.36 ± 0.02 | 7.36 ± 0.02 | 1.00 |
| 6 | 7.19 ± 0.00 | 7.24 ± 0.02 | 0.07 | 7.23 ± 0.02 | 7.25 ± 0.02 | 0.23 | 7.27 ± 0.02 | 7.28 ± 0.04 | 0.51 | |
| Plasma pHa | 0 | 7.23 ± 0.10 | 7.21 ± 0.10 | 0.84 | 7.20 ± 0.09 | 7.16 ± 0.1 | 0.87 | 7.46 ± 0.07 | 7.42 ± 0.04 | 0.31 |
| 6 | 7.11 ± 0.12 | 7.20 ± 0.16 | 0.46 | 7.08 ± 0.07 | 7.06 ± 0.1 | 0.65 | 7.40 ± 0.11 | 7.42 ± 0.04 | 0.72 | |
| Total Haemoglobin (g/L)a | 0 | 90.3 ± 2.0 | 91.3 ± 2.2 | 0.87 | 106.8 ± 0.3 | 106.8 ± 0.2 | 1.00 | 100.0 ± 2.7 | 96.4 ± 1.6 | 1.50 |
| 6 | 84.0 ± 2.2 | 80.5 ± 2.7 | 0.65 | 96.8 ± 0.4 | 93.6 ± 0.2 | 0.26 | 93.4 ± 3.1 | 89.0 ± 1.6 | 1.30 | |
| Plasma free Haemoglobin | 0 | 0.5 ± 0.7 | 0.5 ± 0.7 | 0.77 | 0.5 ± 0.5 | 0.5 ± 0.2 | 0.53 | 0.4 ± 0.9 | 0.4 ± 0.4 | 0.51 |
| (g/L)a | 6 | 0.4 ± 0.4 | 0.5 ± 0.7 | 0.41 | 0.5 ± 0.5 | 0.5 ± 0.2 | 0.25 | 0.4 ± 0.7 | 0.3 ± 0.7 | 0.46 |
| Haematocrit (%)b | 0 | 31.9 ± 0.0 | 31.0 ± 0.1 | 0.72 | 31.4 ± 3.4 | 31.4 ± 3.6 | 0.79 | 29.4 ± 0.1 | 29.2 ± 0.0 | 0.94 |
| 6 | 30.0 ± 0.0 | 30.0 ± 0.0 | 0.85 | 28.4 ± 2.9 | 27.6 ± 3.8 | 0.30 | 27.4 ± 0.1 | 26.2 ± 0.0 | 0.63 | |
| Platelets (K/μL)b | 0 | 341 ± 14.4 | 328 ± 11.7 | 0.98 | 268 ± 7.1 | 261 ± 9.1 | 0.86 | 305 ± 9.9 | 301 ± 7.1 | 0.97 |
| 6 | 269 ± 13.9 | 183 ± 11.6 | 0.31 | 178 ± 14.4 | 148 ± 12.5 | 0.56 | 244 ± 12.7 | 181 ± 11.3 | 0.66 | |
| White Blood Cells (K/μL)b | 0 | 6.85 ± 1.5 | 6.88 ± 1.4 | 0.99 | 6.12 ± 0.7 | 6.06 ± 0.7 | 0.91 | 3.95 ± 0.4 | 3.99 ± 0.5 | 0.96 |
| 6 | 5.85 ± 1.4 | 5.24 ± 1.7 | 0.73 | 5.48 ± 0.7 | 4.90 ± 0.9 | 0.29 | 3.69 ± 0.4 | 3.54 ± 0.4 | 0.80 | |
a n = 4 for Apixaban group; bn = 2 for Ticagrelor group.
Total haemoglobin, hematocrit, and plasma free haemoglobin
Minor, non-significant decreases were also noted in total Hb and Hct after 6 h in both treatment and control groups (Table 2). Importantly, levels of pfHb remained essentially unchanged in all groups, indicating no device-related haemolysis.
Other haematology
Similarly, mild decreases in white blood cell counts were noted at the end of the 6-hour study (decrease in mean values ranged from 0.26–1.64 K/μL). Decreases in platelet counts, however, were more pronounced (change in mean values ranged from 72–145 K/μL) and occurred in both groups. The drop in platelets was numerically larger in the treatment group although it remained statistically non-significant (Table 2).
Other circuit parameters
The pressure differential across the DrugSorb-ATR system remained constant at all timepoints without any evidence of clotting during any run. All circuit parameters, such as flow rate, temperature, pressure, and pump speed, were monitored during the 6 hours of haemoperfusion and remained consistent throughout (data not shown).
Discussion
There are three main observations from the current study. First, the DrugSorb-ATR system has the ability to remove apixaban, rivaroxaban, and ticagrelor from blood as shown in a clinical-scale benchtop recirculation model. Second, the bulk of the removal occurs quickly in under 60 minutes. Third, the introduction of the device in the circuit did not contribute to any significant alterations on physiologic blood parameters. The clinical implications of our observations are now being prospectively evaluated in two FDA approved investigational device exemption double-blind, randomized controlled trials.
Patients on antithrombotic drugs undergoing urgent surgical or invasive procedures are at high risk of bleeding with only supportive measures currently available to manage such complications.9 Two potentially promising strategies to address this critical unmet clinical need are: (a) antithrombotic drug reversal; and (b) antithrombotic drug removal. There are currently two FDA-approved reversal agents for DOACs (idarucizumab for dabigatran and andexanet alfa for apixaban and rivaroxaban) but none for ticagrelor (although the investigational agent bentracimab is currently in phase III trials).31,32 Both agents rapidly normalize pharmacodynamic parameters (dilute thrombin time and ecarin clotting time for idarucizumab, and anti-factor Xa activity for andexanet alfa),33,34 as measured after completion of initial infusion of these agents. Idarucizumab is approved for two reversal indications: (a) in the setting of uncontrollable, life-threatening bleeding, and (b) in patients undergoing undeferrable high bleeding-risk surgery. Andexanet alfa is only approved for use in the management of uncontrolled life-threatening bleeding. Thus, for patients requiring urgent surgery on apixaban, rivaroxaban, or ticagrelor, there is no approved option to reduce the associated serious bleeding risk. In the current analysis we report a detailed evaluation of the DrugSorb-ATR system's capacity for removal of apixaban, rivaroxaban, and ticagrelor in a clinical-scale in vitro benchtop recirculation study. Previously published studies assessing rivaroxaban and ticagrelor removal by haemoadsorption used only scaled down experimental systems.29,35 The current results are derived in a system specifically designed to mimic clinical usage scenarios with respect to blood volume, device size, and flow rate and lend mechanistic support to the emerging clinical experience showing significant reductions in bleeding complications with intraoperative haemoadsorption in patients on apixaban, rivaroxaban, and ticagrelor undergoing urgent cardiac surgery.36–38
It is also important to highlight that the bulk of the removal happens quickly with drug concentrations falling below therapeutic levels within 60 minutes. This is clinically meaningful since this interval is shorter than the median CPB duration in coronary artery bypass graft and heart valve surgeries suggesting that significant drug removal can be expected in everyday clinical practice.39 This is consistent with clinical outcomes reported in patients on ticagrelor or rivaroxaban undergoing urgent cardiac surgery where use of intraoperative haemoadsorption resulted in significantly less surgical and post-surgical bleeding evidenced by less chest tube drainage, fewer transfusions and no re-operations for bleeding control.37,38 Not surprisingly, less bleeding also resulted in shorter intensive care unit and overall hospital lengths of stay. Importantly, the authors noted that integration of the device on CPB was easy and safe without any device-related adverse events.
Haemoadsorption did not contribute any significant changes in blood and plasma pH or haematological parameters compared with the control circuit, with the exception of slightly more pronounced drops in platelet counts. Similar drops are also seen in clinical practice in patients on CPB and are likely caused by platelet adhesion to circuit surfaces which may potentially be exacerbated by the use of roller pumps or centrifugal pumps, with the latter showing better preservation of platelet counts.40 Other potential causes of this observation in vivo include haemodilution from non-blood priming solutions, platelet aggregation, activation, and removal by the reticulo–endothelial system (the latter of which would not be applicable to benchtop models).41 The literature endorses an adverse effect of CPB on red blood cells, platelets, and coagulation factors, independent of any haemodilution from the extracorporeal circuit on blood volume.42 Indeed, prolonged CPB times is considered a key factor that contributes to post-operative bleeding and increased use of blood products.41,43 The absence of any difference between the treatment and control groups for tested hematologic parameters and pH is consistent with multiple human studies (including Randomized control trials) that support a positive safety profile for this biocompatible polymer bead technology.21,44
Our study also has limitations that need to be considered when interpreting the results. First, the in vitro circuit cannot reproduce the effects of extravascular drug distribution which may be an important factor in removal rates in vivo. Second, changes in pharmacodynamic and antihaemostatic parameters were not assessed since the focus was on removal kinetics. Third, the study sample size was not powered to detect modest, but potentially significant changes, such as in circulating platelet counts. However, despite the aforementioned limitations, it is reasonable to presume that the observed in vitro drug removal capabilities are also translatable in vivo and are underlying the potentially significant clinical benefits outlined in published reports.37,38 In comparison to the efficiency of drug removal by the DrugSorb-ATR device, the contribution of intrinsic drug clearance and/or metabolism in vivo would be expected to be minimal. However, the present analysis is limited by the fact that it does not consider the possible impact of these physiologic systems when the technology is utilized in human beings. The ongoing STAR-T and STAR-D randomized clinical trials should provide a precise answer to these questions since drug levels before and after CPB will be available in both patients who received the investigational device and controls. Given the robust drug removal capabilities and the early, but promising evidence for reduction in bleeding complications reported from observational studies, this technology has the potential to improve clinical outcomes and reduce the overall cost of care for patients on antithrombotic drugs requiring urgent cardiac surgery with CPB.
Conclusions
Haemoadsorption by the DrugSorb-ATR porous polymer bead technology can rapidly remove apixaban, rivaroxaban, and ticagrelor from whole blood in a benchtop recirculation model designed to mimic clinical use. These results have informed the design of the randomized, controlled, double blind STAR-T, and STAR-D pivotal trials, designed to validate the current results in vivo and determine whether they translate in significantly fewer bleeding events in patients on antithrombotic drugs undergoing urgent cardiac surgery with CPB.
Supplementary Material
Acknowledgment
The LC-MS/MS method validation and sample analysis for apixaban and rivaroxaban were performed at Biological Mass Spectrometry Facility of Robert Wood Johnson Medical School at Rutgers, The State University of New Jersey.
Contributor Information
Ritu Tripathi, CytoSorbents Medical Inc. 305 College Road E, Princeton, NJ-08540, USA.
Jesus Morales, CytoSorbents Medical Inc. 305 College Road E, Princeton, NJ-08540, USA.
Victoria Lee, CytoSorbents Medical Inc. 305 College Road E, Princeton, NJ-08540, USA.
C Michael Gibson, Department of Medicine at Beth Israel Deaconess Medical Center, The Baim Institute and Harvard Medical School, Boston, MA-02215, USA.
Michael J Mack, Baylor Scott & White Health, Baylor Scott & White Research Institute, Dallas, TX-75093, USA.
David J Schneider, Department of Medicine, Cardiovascular Research Institute, University of Vermont, Burlington VT-05401, USA.
James Douketis, Vascular Medicine and General Internal Medicine, St. Joseph's Healthcare Hamilton, McMaster University, ON-L9C 0E3, Canada.
Frank W Sellke, Division of Cardiothoracic Surgery, Alpert Medical School of Brown University, Providence RI-02903, USA.
Magnus E Ohman, Duke Clinical Research Institute, Duke Heart Center, Duke Program for Advanced Coronary Disease, Duke University Medical Center, Durham, NC-27701, USA.
Vinod H Thourani, Department of Cardiovascular Surgery, Marcus Valve Center, Piedmont Heart Institute, Atlanta, GA-30309, USA.
Robert F Storey, Cardiovascular Research Unit, Department of Infection, Immunity and Cardiovascular Disease, University of Sheffield, Sheffield S10 2RX, UK.
Efthymios N Deliargyris, CytoSorbents Medical Inc. 305 College Road E, Princeton, NJ-08540, USA.
Disclosures and Conflict of Interest
R.T.: Employee of CytoSorbents. J.M.: Employee of CytoSorbents. V.L.: Employee of CytoSorbents. C.M.G.: Reports research grant support from CytoSorbents and Janssen. M.J.M. : Reports no relevant disclosures for this paper. D.J.S.: Reports honorarium/Advisory Board Fees from CytoSorbents
J.D.: Reports research support from CIHR, HSFC, Boehringer Ingelheim; Consultant & Advisory Board Fees from Actelion, AGEN, AstraZeneca, Bayer, Biotie, BMS, Daiichi Sankyo, Portola, Boehringer Ingelheim, Cytori, Janssen, Leo Pharma, Pfizer, Medicines CO., Servier, Bristol Meyers Squibb; and speaker's fees from Bayer, BoehringerIngelheim, Pfizer, Leo Pharma, and Sanofi.
F.W.S.: Reports honorarium/Advisory Board Fees from CytoSorbents and has served as a consultant for Stryker, and XyloCor.
E.M.O.: Reports research support from Research Chiesi USA and Consultant fees from Abiomed, Cara Therapeutics, Cytokinetics, Milestone, Otsuka, Neurocrine, Pfizer, and Xylocor.
V.H.T.: Reports no relevant disclosures and conflict of interest for this paper.
R.F.S.: Reports institutional research grants/support from AstraZeneca, CytoSorbents, GlyCardial Diagnostics and Thromboserin; and honorarium/personal fees from Alnylam, AstraZeneca, Bayer, Bristol Myers Squibb/Pfizer, Chiesi, CSL Behring, CytoSorbents, GlyCardial Diagnostics, Hengrui, Idorsia, Intas Pharmaceuticals, Medscape, Novartis, PhaseBio, Portola, Sanofi Aventis, and Thromboserin.
E.N.D.: Employee of CytoSorbents.
Data availability
The data underlying this article are available in the article and in its online supplementary material.
References
- 1. McCarty D, Robinson A. Factor Xa inhibitors: a novel therapeutic class for the treatment of non-valvular atrial fibrillation. Ther Adv Cardiovasc. Dis. 2016;10:37–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Eikelboom J, Merli G. Bleeding with direct oral anticoagulants vs. warfarin: clinical experience. Am J Emerg Med 2016;34:3–8. [DOI] [PubMed] [Google Scholar]
- 3. Lippi G, Mattiuzzi C, Adcock D, Favaloro EJ. Oral anticoagulants around the world: an updated state-of-the art analysis. Ann Blood 49–49, 2018;3. doi:10.21037/aob.2018.12.04 [Google Scholar]
- 4. Troy A, Anderson TS. National trends in use of and spending on oral anticoagulants among US medicare beneficiaries from 2011 to 2019. JAMA Health Forum 2021;2:e211693–e211693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H, Mahaffey KW, Scirica BM, Skene A, Steg PG, Storey RF, Harrington RA, Investigators P, Freij A, Thorsen M. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 2009;361:1045–1057. [DOI] [PubMed] [Google Scholar]
- 6. Collet JP, Thiele H, Barbato E, Barthelemy O, Bauersachs J, Bhatt DL, Dendale P, Dorobantu M, Edvardsen T, Folliguet T, Gale CP, Gilard M, Jobs A, Juni P, Lambrinou E, Lewis BS, Mehilli J, Meliga E, Merkely B, Mueller C, Roffi M, Rutten FH, Sibbing D, Siontis GCM, Group ESCSD . 2020 ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2021;42:1289–1367. [DOI] [PubMed] [Google Scholar]
- 7. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, Caforio ALP, Crea F, Goudevenos JA, Halvorsen S, Hindricks G, Kastrati A, Lenzen MJ, Prescott E, Roffi M, Valgimigli M, Varenhorst C, Vranckx P, Widimsky P, Group ESCSD . 2017 ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the european society of cardiology (ESC). Eur Heart J 2018;39:119–177. [DOI] [PubMed] [Google Scholar]
- 8. Dayoub EJ, Seigerman M, Tuteja S, Kobayashi T, Kolansky DM, Giri J, Groeneveld PW. Trends in platelet adenosine diphosphate P2Y12 receptor inhibitor use and adherence among antiplatelet-naive patients after percutaneous coronary intervention, 2008–2016. JAMA Intern Med 2018, 2018;178:943–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Verheugt FW, Clemmensen P, Mehran R, Agewall S, Pocock SJ, Goldstein S, Torp-Pedersen C, Simoons ML, Borer JS, Khder YM, Burton P, Deliargyris E, McMurray JJ, Berkowitz SD, Stough WG, Zannad F. Antithrombotic outcome trials in acute coronary syndromes: seeking the optimal balance between safety and efficacy. Eur Heart J 2013;34:1621–1629. [DOI] [PubMed] [Google Scholar]
- 10. Sunkara T, Ofori E, Zarubin V, Caughey ME, Gaduputi V, Reddy M. Perioperative management of direct oral anticoagulants (DOACs): a systemic review. Health Serv. Insights 2016;9s1:25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sousa-Uva M, Storey R, Huber K, Falk V, Leite-Moreira AF, Amour J, Al-Attar N, Ascione R, Taggart D, Collet JP, ESCWGoC Surgery, ESCWGo Thrombosis. Expert position paper on the management of antiplatelet therapy in patients undergoing coronary artery bypass graft surgery. Eur Heart J 2014;35:1510–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lawton JS, Tamis-Holland JE, Bangalore S, Bates ER, Beckie TM, Bischoff JM, Bittl JA, Cohen MG, DiMaio JM, Don CW, Fremes SE, Gaudino MF, Goldberger ZD, Grant MC, Jaswal JB, Kurlansky PA, Mehran R, Metkus TS Jr., Nnacheta LC, Rao SV, Sellke FW, Sharma G, Yong CM, Zwischenberger BA. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the american college of cardiology/ circulation, american heart association joint committee on clinical practice guidelines. Circulation 2022;145:e18–e114. [DOI] [PubMed] [Google Scholar]
- 13. Pagano D, Milojevic M, Meesters MI, Benedetto U, Bolliger D, von Heymann C, Jeppsson A, Koster A, Osnabrugge RL, Ranucci M, Ravn HB, Vonk ABA, Wahba A, Boer C. 2017 EACTS/EACTA guidelines on patient blood management for adult cardiac surgery. Eur J Cardiothorac Surg 2018, 2018;53:79–111. [DOI] [PubMed] [Google Scholar]
- 14. Doherty JU, Gluckman TJ, Hucker WJ, Januzzi JL Jr., Ortel TL, Saxonhouse SJ, Spinler SA. 2017 ACC expert consensus decision pathway for periprocedural management of anticoagulation in patients with non-valvular atrial fibrillation: a report of the american college of cardiology clinical expert consensus document task force. J Am Coll Cardiol 2017;69:871–898. [DOI] [PubMed] [Google Scholar]
- 15. Douketis JD, Healey JS, Brueckmann M, Fraessdorf M, Spyropoulos AC, Wallentin L, Oldgren J, Reilly P, Ezekowitz MD, Connolly SJ, Yusuf S, Eikelboom JW. Urgent surgery or procedures in patients taking dabigatran or warfarin: analysis of perioperative outcomes from the RE-LY trial. Thromb Res 2016;139:77–81. [DOI] [PubMed] [Google Scholar]
- 16. Godier A, Taylor G, Gaussem P. Inefficacy of platelet transfusion to reverse ticagrelor. N Engl J Med 2015;372:196–197. [DOI] [PubMed] [Google Scholar]
- 17. Jacobs JW, Booth GS. Blood shortages and changes to massive transfusion protocols: survey of hospital practices during the COVID-19 pandemic. Transfus Apher Sci 61, 2022:103297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Budovich A, Zargarova O, Nogid A. Role of apixaban (eliquis) in the treatment and prevention of thromboembolic disease. P T 2013;38:206–231. [PMC free article] [PubMed] [Google Scholar]
- 19. Sillen H, Cook M, Davis P. Determination of unbound ticagrelor and its active metabolite (AR-C124910XX) in human plasma by equilibrium dialysis and LC-MS/MS. J Chromatogr B 2011;879:2315–2322. [DOI] [PubMed] [Google Scholar]
- 20. Bratsos S. Pharmacokinetic properties of rivaroxaban in healthy human subjects. Cureus 2019;11:e5484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Poli EC, Alberio L, Bauer-Doerries A, Marcucci C, Roumy A, Kirsch M, De Stefano E, Liaudet L, Schneider AG. Cytokine clearance with cytosorb(R) during cardiac surgery: a pilot randomized controlled trial. Crit Care 2019;23:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Friesecke S, Trager K, Schittek GA, Molnar Z, Bach F, Kogelmann K, Bogdanski R, Weyland A, Nierhaus A, Nestler F, Olboeter D, Tomescu D, Jacob D, Haake H, Grigoryev E, Nitsch M, Baumann A, Quintel M, Schott M, Kielstein JT, Meier-Hellmann A, Born F, Schumacher U, Singer M, Kellum J, Brunkhorst FM. International registry on the use of the cytosorb (R) adsorber in ICU patients: study protocol and preliminary results. Med Klin Intensivmed Notfmed 2019;114:699–707. [DOI] [PubMed] [Google Scholar]
- 23. Gulilat M, Tang A, Gryn SE, Leong-Sit P, Skanes AC, Alfonsi JE, Dresser GK, Henderson SL, Rose RV, Lizotte DJ, Teft WA, Schwarz UI, Tirona RG, Kim RB. Interpatient variation in rivaroxaban and apixaban plasma concentrations in routine care. Can J Cardiol 2017;33:1036–1043. [DOI] [PubMed] [Google Scholar]
- 24. Mazzeffi MA, Lee K, Taylor B, Tanaka KA. Perioperative management and monitoring of antiplatelet agents: a focused review on aspirin and P2Y12 inhibitors. Korean J Anesthesiol 2017;70:379–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. US Food and Drug Administration . Cardiopulmonary bypass oxygenators 510(k) submissions - Final Guidance for industry and FDA staff. https://www.fda.gov/medical-devices/guidance-documents-medical-devices-and-radiation-emitting-products/cardiopulmonary-bypass-oxygenators-510k-submissions-final-guidance-industry-and-fda-staff [Google Scholar]
- 26. US Food and Drug Administration . Bioanalytical method validation guidance for industry. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/bioanalytical-method-validation-guidance-industry [Google Scholar]
- 27. Frost C, Nepal S, Wang J, Schuster A, Byon W, Boyd RA, Yu Z, Shenker A, Barrett YC, Mosqueda-Garcia R, LaCreta F. Safety, pharmacokinetics and pharmacodynamics of multiple oral doses of apixaban, a factor xa inhibitor, in healthy subjects. Br J Clin Pharmacol 2013;76:776–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mueck W, Stampfuss J, Kubitza D, Becka M. Clinical pharmacokinetic and pharmacodynamic profile of rivaroxaban. Clin Pharmacokinet 2014;53:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Koertge A, Wasserkort R, Wild T, Mitzner S. Extracorporeal haemoperfusion as a potential therapeutic option for critical accumulation of rivaroxaban. Blood Purif. 2018;45:126–128. [DOI] [PubMed] [Google Scholar]
- 30. Husted SE, Storey RF, Bliden K, Tantry US, Høimark L, Butler K, Wei C, Teng R, Gurbel PA. Pharmacokinetics and pharmacodynamics of ticagrelor in patients with stable coronary artery disease: results from the ONSET-OFFSET and RESPOND studies. Clin Pharmacokinet 2012;51:397–409. [DOI] [PubMed] [Google Scholar]
- 31. Jaspers T, Shudofsky K, Huisman MV, Meijer K, Khorsand N. A meta-analysis of andexanet alfa and prothrombin complex concentrate in the treatment of factor Xa inhibitor-related major bleeding. Res Pract Thromb Haemos 2021;5:e12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kustos SA, Fasinu PS. Direct-acting oral anticoagulants and their reversal agents-an update. Medicines 2019;6:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pollack CV Jr., Reilly PA, van Ryn J, Eikelboom JW, Glund S, Bernstein RA, Dubiel R, Huisman MV, Hylek EM, Kam CW, Kamphuisen PW, Kreuzer J, Levy JH, Royle G, Sellke FW, Stangier J, Steiner T, Verhamme P, Wang B, Young L, Weitz JI. Idarucizumab for dabigatran reversal - Full cohort analysis. N Engl J Med 2017;377:431–441. [DOI] [PubMed] [Google Scholar]
- 34. Connolly SJ, Crowther M, Eikelboom JW, Gibson CM, Curnutte JT, Lawrence JH, Yue P, Bronson MD, Lu G, Conley PB, Verhamme P, Schmidt J, Middeldorp S, Cohen AT, Beyer-Westendorf J, Albaladejo P, Lopez-Sendon J, Demchuk AM, Pallin DJ, Concha M, Goodman S, Leeds J, Souza S, Siegal DM, Zotova E, Meeks B, Ahmad S, Nakamya J, Milling TJ Jr., Investigators A-. Full study report of andexanet alfa for bleeding associated with factor xa inhibitors. N Engl J Med 2019;380:1326–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Angheloiu GO, Gugiu GB, Ruse C, Pandey R, Dasari RR, Whatling C. Ticagrelor removal from human blood. JACC: Basic to Translational Science 2017;2:135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mendes V, Colombier S, Verdy F, Bechtold X, Schlaepfer P, Scala E, Schneider A, Kirsch M. Cytosorb (R) haemoadsorption of apixaban during emergent cardio-pulmonary bypass: a case report. Perfusion 2021;36:873–875. [DOI] [PubMed] [Google Scholar]
- 37. Hassan K, Bruning T, Caspary M, Wohlmuth P, Pioch H, Schmoeckel M, Geidel S. Haemoadsorption of rivaroxaban and ticagrelor during acute type a aortic dissection operations. Ann Thorac Cardiovasc Surg 2022. doi:10.5761/atcs.oa.21-00154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hassan K, Kannmacher J, Wohlmuth P, Budde U, Schmoeckel M, Geidel S. Cytosorb adsorption during emergency cardiac operations in patients at high risk of bleeding. Ann Thorac Surg 2019;108:45–51. [DOI] [PubMed] [Google Scholar]
- 39. Salis S, Mazzanti VV, Merli G, Salvi L, Tedesco CC, Veglia F, Sisillo E. Cardiopulmonary bypass duration is an independent predictor of morbidity and mortality after cardiac surgery. J Cardiothorac Vasc Anesth 2008;22:814–822. [DOI] [PubMed] [Google Scholar]
- 40. Klein M, Dauben HP, Schulte HD, Gams E. Centrifugal pumping during routine open heart surgery improves clinical outcome. Artif Organs 1998;22:326–336. [DOI] [PubMed] [Google Scholar]
- 41. Tettey M, Aniteye E, Sereboe L, Edwin F, Kotei D, Tamatey M, Entsuamensah K, Amuzu V, Frimpong-Boateng K. Predictors of post-operative bleeding and blood transfusion in cardiac surgery. Ghana Med J 2009;43:71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Scott BH, Seifert FC, Glass PSA, Grimson R. Blood use in patients undergoing coronary artery bypass surgery: impact of cardiopulmonary bypass pump, haematocrit, gender, age, and body weight. Anesth & Analg 2003;97:958–963. [DOI] [PubMed] [Google Scholar]
- 43. Miana LA ea. Risk factors of post-operative bleeding after adult cardiac surgery. Brasileira de Cirurgia Cardiovascular 2004;19:280–286. [Google Scholar]
- 44. Garau I, Marz A, Sehner S, Reuter DA, Reichenspurner H, Zollner C, Kubitz JC. Haemadsorption during cardiopulmonary bypass reduces interleukin 8 and tumor necrosis factor alpha serum levels in cardiac surgery: a randomized controlled trial. Minerva Anestesiol 2019;85:715–723. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.