Abstract
Aims
Digoxin is included in some heart failure (HF) guidelines but controversy persists about the true role for and impact of treatment with this drug, particularly in the absence of atrial fibrillation (AF). The aim of this study was to assess the association between clinical characteristics and digoxin use and between digoxin use and mortality/morbidity in a large, contemporary cohort of patients with HF with reduced ejection fraction (HFrEF) stratified by history of AF.
Methods and results
Patients with HFrEF (EF < 40%) enrolled in the Swedish HF registry between 2005 and 2018 were analysed. The independent association between digoxin use and patient characteristics was assessed by logistic regression, and between digoxin use and outcomes [composite of all-cause mortality or HF hospitalization (HFH), all-cause mortality, and HFH] by Cox regressions in a 1:1 propensity score matched population. Digoxin use was analysed at baseline and as a time-dependent variable. Of 42 456 patients with HFrEF, 16% received digoxin, 29% in the AF group and 2.8% in the non-AF group. The main independent predictors of use were advanced HF, higher heart rate, history of AF, preserved renal function, and concomitant use of beta blockers. Digoxin use was associated with lower risk of all-cause death/HFH [hazard ratio (HR): 0.95; 95% confidence interval (CI): 0.91–0.99] in AF, but with higher risk in non-AF (HR: 1.24; 95% CI: 1.09–1.43). Consistent results were observed when digoxin use was analysed as a time-dependent variable.
Conclusion
The great majority of digoxin users had a history of AF. Digoxin use was associated with lower mortality/morbidity in patients with AF, but with higher mortality/morbidity in patients without AF.
Keywords: Digoxin, Mortality, Hospitalization, Heart failure with reduced ejection fraction, Registry, SwedeHF
Introduction
Digoxin is currently recommended in patients with heart failure (HF) with reduced ejection fraction (HFrEF) and sinus rhythm who remain symptomatic despite treatment with renin–angiotensin–aldosterone system inhibitors and beta blockers (BBs) (class IIb, level of evidence B in European guidelines),1 and for rate control in those with HFrEF and atrial fibrillation (AF) (class I, level of evidence B).2
Recommendations for digoxin in patients with HFrEF and sinus rhythm are based on one randomized controlled trial (RCT), the Digitial Investigation Group (DIG) trial, in which digoxin did not affect all-cause mortality but reduced the risk for HF hospitalization (HHF) by 28%.3 However, this trial was performed more than 25 years ago, and therefore may not reflect the characteristics and contemporary management of HFrEF [i.e. patients were treated only with diuretics and angiotensin-converting enzyme inhibitors (ACE-Is)]. In the setting of HF with concomitant AF, RCTs assessing safety and efficacy linked with digoxin use are lacking, with several different analyses providing contradictory results, which might be explained by differences in methodological approaches and enrolled populations.4,5
Thus, in the large and contemporary population with HFrEF enrolled in the Swedish HF Registry (SwedeHF) we assessed (i) temporal trends in the use of digoxin; (ii) patient characteristics independently associated with digoxin use; and (iii) independent associations between digoxin use and mortality/morbidity in patients with and without a history of AF, as well as in relevant pre-specified subgroups.
Methods
Data sources
Data from the SwedeHF linked with the National Patient Registry (NPR), the Cause of Death Registry, the Dispensed Drug Registry (DDR), and Statistics Sweden were analysed. Data sources are described in the Supplementary material online.
Patients
Patients enrolled in SwedeHF between 1 December 2005 (5 months after the start of the DDR) and 14 December 2018 were considered eligible for the current analysis if they had (i) a left ventricular ejection fraction (LVEF) <40% and (ii) a follow-up length of at least 14 days (to mitigate immortal time bias). When the same patient was registered multiple times, the first registration was considered. The index date was defined as the date of hospital discharge (for inpatients) or the date of clinic visit (for outpatients). Patients who died during the hospitalization linked to first SwedeHF registration were excluded. The last date of follow-up was 31 December 2018.
Exposure, atrial fibrillation, and other variables
History of AF was defined as whether the corresponding ICD-10 code I48 was reported in the NPR during the 5 years prior to the patient's registration in SwedeHF and/or history of AF was recorded by healthcare professionals at the index visit in SwedeHF and/or AF was observed on the patient's latest electrocardiogram (ECG) prior to/at index visit in SwedeHF.
Digoxin use was defined as a dispensed prescription (from DDR) between 5 months prior to and 14 days after the index. Rates of digoxin use over time were calculated using the overall number of patients enrolled in the analysis as denominator, with the number of patients with at least one digoxin dispensation in the given year as numerator of the ratio.
A detailed definition of other variables used in this analysis is reported in the Supplementary material online, Table S1, and at https://kiheartfailure.github.io/shfdb3/.
Statistical analyses
Statistical analyses were performed in patients with HFrEF, and separately in those with and without history of AF.
Baseline characteristics
Baseline characteristics of patients receiving vs. not receiving digoxin were presented as median (25th–75th percentile) and compared by the Mann–Whitney U test if continuous, and as frequencies (percentage) and compared by the χ2 test if categorical.
Independent associations between patient characteristics and digoxin use
To identify independent predictors of digoxin use, multivariable logistic regression analyses were fitted using digoxin use as the dependent variable. Variables reported in the forest plots were included as potential predictors.
Associations between digoxin use and outcomes
Study outcomes were (i) time to all-cause mortality or first HFH (i.e. composite outcome), (ii) time to all-cause mortality, and (iii) time to first HFH. 1-Kaplan–Meier curves were fitted to present time to event for all the study outcomes. Cox regression models were performed in the overall cohort for assessing the crude association between digoxin use and outcomes, and in a 1:1 digoxin:no-digoxin propensity score (PS)-matched cohort (with the matched pairs modelled using a frailty term). Censoring occurred at end of follow-up (31 December 2018), emigration from Sweden, or, in the analyses assessing HFH, death. Outcome analysis for all-cause death/HFH was also performed in pre-specified subgroups by including an interaction term between digoxin and the respective variable.
Missing data were handled in all the multivariable models performed in this analysis by multiple imputation. The methods for multiple imputation and PS matching are reported in detail in the Supplementary material online.
Consistency analysis
We performed the following consistency analyses:
Since PS matching reduces the sample size, Cox regression models were also performed in the overall cohort adjusting for the PS as a covariate.
We included digoxin as a time-dependent variable in the Cox regression models performed in the PS-matched populations since in the main analysis digoxin use was considered as at the baseline.
A competing event analysis was performed for the outcome time to first HFH with death treated as a competing event.
A two-sided P-value of <0.05 was considered statistically significant. All analyses were performed using R version 4.0.2 (R Core Team 2019). The R code for data handling and statistical analyses are available at https://github.com/KIHeartFailure/digoxinhfref.
Results
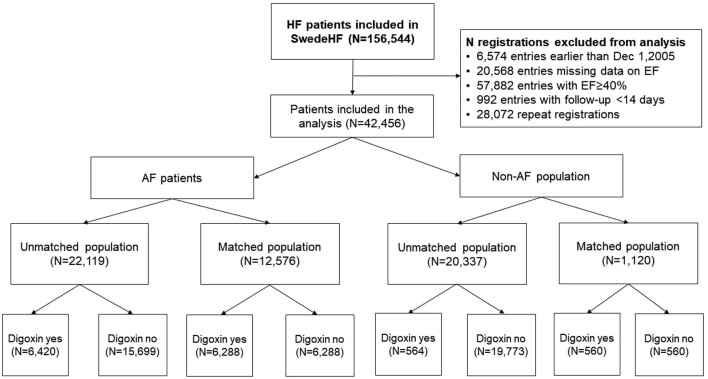
Of 42 456 patients with HFrEF, 22 119 (52.1%) had a history of AF. Overall, 6984 (16%) received digoxin, 6420 (29%) in the AF group and 564 (2.8%) in the non-AF group. Digoxin recipients had a median [inter-quartile range (IQR)] age of 74 (65–81) years, 31% were female, 54% had an LVEF <30%, and 92% had a history of AF. The CONSORT diagram of the study is reported in Figure 1. Results of all the analyses performed in the overall HFrEF population regardless of the AF status are reported in the Supplementary material online.
Figure 1.
Flow chart reporting patient selection. HF, heart failure; LVEF, left ventricular ejection fraction.
Patients with heart failure with reduced ejection fraction and a history of atrial fibrillation
Digoxin use over time
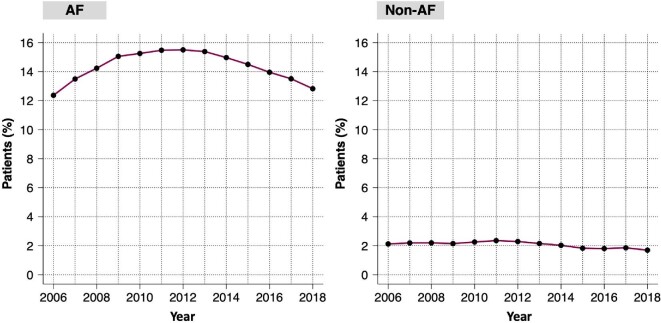
In the AF population, the use of digoxin over time showed a reverse U shape, starting with an annual use rate of 12.5% in 2006, peaking at ∼15.5% in 2011–13, and declining to 12.5% in 2018 (Figure 2).
Figure 2.
Use of digoxin over time in patients with heart failure with reduced ejection fraction and atrial fibrillation (left panel) and without atrial fibrillation (right panel). AF, atrial fibrillation; HFrEF, heart failure with reduced ejection fraction.
Digoxin users’ profile
Digoxin users were younger and had a shorter history of HF, but were more likely registered as inpatients, had lower LVEF, higher prevalence of AF at the baseline ECG, higher heart rate, and less comorbidities compared with digoxin non-users (Table 1). They were more likely to receive HF medications but less likely to have a cardiac resynchronization therapy (CRT) or implantable cardioverter defibrillator (ICD) and be followed in an HF nurse-led clinic compared with non-users.
Table 1.
Baseline characteristics of patients with heart failure with reduced ejection fraction with and without history of atrial fibrillation stratified by digoxin use in the overall population
| History of AF | No history of AF | |||||||
|---|---|---|---|---|---|---|---|---|
| Missing (%) | No digoxin | Digoxin | P | Missing (%) | No digoxin | Digoxin | P | |
| n | 15 699 | 6420 | 19 773 | 564 | ||||
| Male genderb | 0.0 | 11 806 (75.2) | 4446 (69.3) | <0.001 | 0.0 | 13 492 (68.2) | 370 (65.6) | 0.202 |
| Age (years) | 0.0 | 76.0 [69.0, 83.0] | 74.0 [66.0, 81.0] | <0.001 | 0.0 | 70.0 [61.0, 79.0] | 67.0 [59.0, 78.0] | 0.004 |
| Age ≥75 yearsb | 0.0 | 8981 (57.2) | 3140 (48.9) | <0.001 | 0.0 | 7368 (37.3) | 197 (34.9) | 0.277 |
| Outpatientsb | 0.0 | 8471 (54.0) | 3053 (47.6) | <0.001 | 0.0 | 11 617 (58.8) | 250 (44.3) | <0.001 |
| Year of inclusionb | 0.0 | <0.001 | 0.0 | <0.001 | ||||
| 2005–10 | 5099 (32.5) | 2759 (43.0) | 7174 (36.3) | 358 (63.5) | ||||
| . 2011–15 | 6135 (39.1) | 2383 (37.1) | 7255 (36.7) | 136 (24.1) | ||||
| . 2016–18 | 4465 (28.4) | 1278 (19.9) | 5344 (27.0) | 70 (12.4) | ||||
| Childrenb | 0.0 | 13 224 (84.2) | 5317 (82.8) | 0.010 | 0.0 | 15 975 (80.8) | 429 (76.1) | 0.006 |
| Living alone | 0.2 | 7122 (45.5) | 3083 (48.1) | <0.001 | 0.4 | 9063 (46.0) | 274 (48.8) | 0.198 |
| Education levelb | 1.9 | 0.040 | 2.2 | 0.062 | ||||
| Compulsory | 7002 (45.4) | 2744 (43.6) | 8100 (41.9) | 243 (44.8) | ||||
| Secondary | 5864 (38.1) | 2471 (39.2) | 8109 (41.9) | 231 (42.6) | ||||
| University | 2541 (16.5) | 1083 (17.2) | 3136 (16.2) | 68 (12.5) | ||||
| Income above mediana,b | 0.2 | 7713 (49.2) | 3296 (51.5) | 0.003 | 0.4 | 9931 (50.4) | 253 (45.1) | 0.015 |
| BMI (kg/m2) | 39.7 | 26.3 [23.4, 29.8] | 25.9 [23.1, 29.7] | 0.004 | 39.9 | 26.2 [23.3, 29.9] | 25.0 [22.2, 28.4] | <0.001 |
| BMIb ≥30 kg/m2 | 39.7 | 2314 (24.2) | 898 (23.7) | 0.491 | 39.9 | 2945 (24.7) | 59 (19.4) | 0.040 |
| HF history ≥6 mb | 2.2 | 8279 (54.0) | 3213 (50.9) | <0.001 | 2.1 | 7821 (40.4) | 368 (65.9) | <0.001 |
| NYHA classb | 27.3 | 0.162 | 25.3 | <0.001 | ||||
| I | 878 (7.8) | 323 (6.8) | 1626 (11.0) | 34 (7.9) | ||||
| II | 5047 (47.8) | 2147 (45.2) | 7436 (50.4) | 161 (37.4) | ||||
| III | 4944 (43.7) | 2097 (44.2) | 5283 (35.8) | 206 (47.8) | ||||
| IV | 457 (4.0) | 179 (3.8) | 419 (2.8) | 30 (7.0) | ||||
| SBP (mmHg) | 1.8 | 120.0 [110.0, 139.0] | 120.0 [110.0, 135.0] | <0.001 | 1.7 | 125.0 [110.0, 140.0] | 115.0 [100.0, 130.0] | <0.001 |
| DBP (mmHg) | 1.7 | 73.0 [65.0, 80.0] | 74.0 [65.0, 80.0] | 0.504 | 1.6 | 70.0 [65.0, 80.0] | 70.0 [60.0, 80.0] | <0.001 |
| MBP (mmHg) | 1.7 | 90.0 [81.7, 100.0] | 90.0 [81.3, 99.0] | 0.068 | 1.6 | 90.0 [81.7, 100.0] | 83.3 [76.7, 93.3] | <0.001 |
| MBP >90 mmHgb | 1.7 | 7379 (47.8) | 2950 (46.8) | 0.170 | 1.6 | 9301 (47.8) | 174 (31.4) | <0.001 |
| Duration AF (days) | 7.1 | 536.0 [43.0, 1908.5] | 467.0 [35.0, 2004.5] | 0.064 | — | — | — | — |
| ECG (%) | 1.9 | <0.001 | 2.5 | — | — | — | ||
| Sinus rhythm | 4280 (27.8) | 734 (11.6) | 17767 (91.9) | 469 (85.7) | ||||
| AF | 8857 (57.6) | 4972 (78.7) | 0 (0.0) | 0 (0.0) | ||||
| Paced/other | 2244 (14.6) | 609 (9.6) | 1572 (8.1) | 78 (14.3) | ||||
| HR (b.p.m.) | 2.7 | 74.0 [65.0, 86.0] | 79.0 [68.0, 90.0] | <0.001 | 2.6 | 70.0 [62.0, 80.0] | 72.0 [65.0, 83.0] | <0.001 |
| HR ≥70 b.p.m.b | 2.7 | 8866 (57.9) | 4168 (67.0) | <0.001 | 2.6 | 9215 (47.8) | 304 (56.4) | <0.001 |
| Current smokerb | 22.5 | 1264 (10.4) | 631 (12.6) | 0.003 | 18.6 | 2827 (17.6) | 76 (17.1) | 0.859 |
| Diabetesb | 0.0 | 4243 (27.0) | 1642 (25.6) | 0.028 | 0.0 | 5290 (26.8) | 199 (35.3) | <0.001 |
| Hypertensionb | 0.0 | 10 134 (64.6) | 3644 (56.8) | <0.001 | 0.0 | 11 486 (58.1) | 323 (57.3) | 0.730 |
| COPDb | 0.0 | 1867 (11.9) | 811 (12.6) | 0.131 | 0.0 | 2331 (11.8) | 73 (12.9) | 0.441 |
| Strokeb | 0.0 | 2668 (17.0) | 915 (14.3) | <0.001 | 0.0 | 2080 (10.5) | 61 (10.8) | 0.878 |
| IHDb | 0.0 | 8908 (56.7) | 2862 (44.6) | <0.001 | 0.0 | 11 486 (58.1)) | 323 (57.3) | 0.730 |
| PADb | 0.0 | 1512 (9.6) | 463 (7.2) | <0.001 | 0.0 | 1616 (8.2) | 45 (8.0) | 0.930 |
| Valve diseaseb | 0.0 | 3239 (20.6) | 1204 (18.8) | 0.002 | 0.0 | 2705 (13.7) | 136 (24.1) | <0.001 |
| Cancerb | 0.0 | 2346 (14.9) | 844 (13.1) | 0.001 | 0.0 | 2311 (11.7) | 71 (12.6) | 0.555 |
| Liver diseaseb | 0.0 | 303 (1.9) | 146 (2.3) | 0.111 | 0.0 | 399 (2.0) | 25 (4.4) | <0.001 |
| ICD/CRTb | 1.2 | 1522 (9.8) | 512 (8.1) | <0.001 | 1.2 | 1507 (7.7) | 91 (16.2) | <0.001 |
| EF <30%b | 0.0 | 6926 (44.1) | 3358 (52.3) | <0.001 | 0.0 | 9897 (50.1) | 412 (73.0) | <0.001 |
| Haemoglobin (g/L) | 3.5 | 134.0 [121.0, 146.0] | 138.0 [125.0, 150.0] | <0.001 | 3.3 | 135.0 [123.0, 146.0] | 132.0 [120.0, 144.0] | 0.003 |
| Anaemiab | 3.5 | 5178 (34.3) | 1618 (25.9) | <0.001 | 3.3 | 5761 (30.1) | 194 (34.6) | 0.027 |
| GFR, (mL/min/1.73 m2) | 1.3 | 58.2 [42.4, 75.3] | 64.4 [49.1, 80.8] | <0.001 | 1.1 | 68.6 [50.6, 85.6] | 67.9 [50.1, 86.8] | 0.719 |
| GFRb | 1.3 | <0.001 | 1.1 | 0.691 | ||||
| . 30–60 | 6758 (43.6) | 2383 (37.6) | 6110 (31.3) | 181 (32.3) | ||||
| . ≥60 | 7299 (47.1) | 3683 (58.1) | 12 203 (62.4) | 348 (62.1) | ||||
| . ≤30 | 1441 (9.3) | 273 (4.3) | 1236 (6.3) | 31 (5.5) | ||||
| NT-ProBNP (pg/mL) | 49.1 | 3311.5 [1625.8, 7000.0] | 3464.5 [1815.5, 6770.8] | 0.075 | 47.4 | 2360.0 [890.0, 5835.5] | 3350.0 [1270.0, 7490.0] | <0.001 |
| NT-proBNP above medianb | 49.1 | 4618 (56.1) | 1703 (56.4) | 0.818 | 47.4 | 4525 (43.3) | 125 (49.0) | 0.082 |
| Serum potassium | 20.2 | 4.2 [3.9, 4.5] | 4.2 [3.9, 4.5] | 0.010 | 18.5 | 4.2 [3.9, 4.5] | 4.2 [4.0, 4.5] | 0.629 |
| Serum potassium groupsb | 20.2 | 0.004 | 18.5 | 0.718 | ||||
| . Normokalaemia | 11 824 (92.4) | 4524 (93.2) | 15 127 (93.4) | 347 (92.8) | ||||
| . Hypokalaemia | 525 (4.1) | 205 (4.2) | 583 (3.6) | 13 (3.5) | ||||
| . Hyperkalaemia | 452 (3.5) | 123 (2.5) | 489 (3.0) | 14 (3.7) | ||||
| Beta blockerb | 0.3 | 14 328 (91.6) | 6052 (94.5) | <0.001 | 0.3 | 17 958 (91.1) | 498 (89.1) | 0.127 |
| RASib | 1.2 | 13 820 (89.0) | 5737 (90.7) | <0.001 | 1.3 | 18 086 (92.6) | 502 (90.8) | 0.115 |
| MRAb | 0.7 | 5765 (36.9) | 2619 (41.1) | <0.001 | 0.6 | 7050 (35.9) | 299 (53.7) | <0.001 |
| Diureticsb | 0.4 | 12 603 (80.7) | 5356 (83.7) | <0.001 | 0.5 | 13 923 (70.7) | 477 (85.3) | <0.001 |
| Statinsb | 0.4 | 18 576 (52.6) | 2775 (39.9) | <0.001 | 0.4 | 11 000 (55.8) | 271 (48.5) | 0.001 |
| Nitratesb | 0.5 | 2100 (13.4) | 665 (10.4) | <0.001 | 0.4 | 2426 (12.3) | 98 (17.6) | <0.001 |
| ASAb | 0.5 | 4852 (31.0) | 1424 (22.3) | <0.001 | 0.4 | 12 761 (64.8) | 302 (54.1) | <0.001 |
| Anticoagulantb | 0.3 | 10 864 (69.4) | 5106 (79.8) | <0.001 | 0.5 | 2557 (13.0) | 167 (30.0) | <0.001 |
| FU in HF clinicb | 5.3 | 9282 (62.3) | 3558 (58.9) | <0.001 | 4.5 | 12 633 (66.9) | 282 (53.3) | <0.001 |
| FU locationb | 4.2 | 0.006 | 3.5 | 0.006 | ||||
| . Hospital | 10 877 (72.1) | 4531 (74.2) | 15 075 (79.0) | 403 (75.6) | ||||
| . Primary care | 3808 (25.3) | 1416 (23.2) | 3582 (18.8) | 108 (20.3) | ||||
| . Other | 396 (2.6) | 157 (2.6) | 3582 (18.8) | 108 (20.3) | ||||
AF, atrial fibrillation; ASA, acetylsalicylic acid; BMI, body mass index; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; DBP, diastolic blood pressure; ECG, electrocardiogram; EF, ejection fraction; FU, follow-up; GFR, glomerular filtration rate; HF, heart failure; HR, heart rate; ICD/CRT, intracardiac defibrillator/cardiac resynchronization therapy; IHD, ischaemic heart disease; MBP, mean blood pressure; MRA, mineralocorticoid receptor antagonists; NT-proBNP, N-terminal pro-brain natriuretic peptide; NYHA, New York Heart Association; RASi, renin–angiotensin system inhibitor (angiotensin-converting enzyme inhibitor, angiotensin receptor blocker, or angiotensin receptor/neprilysin inhibitor); PAD, peripheral artery disease; SBP, systolic blood pressure; and SMD, standardized mean difference.
aIncome above median for each year.
bIncluded in the multiple imputation model (although not necessarily imputed if there are no missing data) and logistic/Cox models.
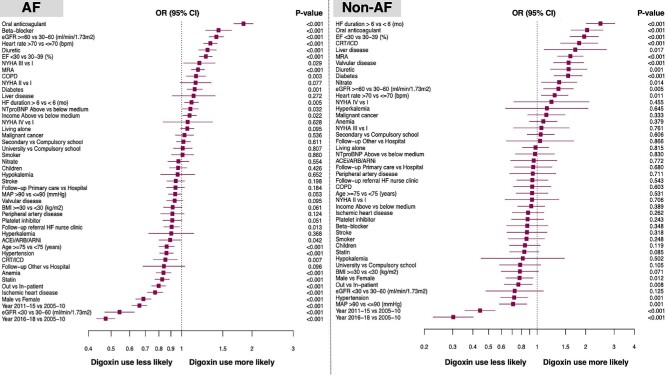
Key independent predictors of digoxin use were female sex, younger age, characteristics linked with more advanced HF, heart rate >70 b.p.m., no history of hypertension or ischaemic heart disease, history of chronic obstructive pulmonary disease, better renal function, no use of renin–angiotensin system inhibitor (RASi)/angiotensin receptor-neprilysin inhibitor (ARNI), but higher use of diuretics, BBs, and mineralocorticoid receptor antagonists (MRAs), and lack of referral to an HF nurse-led clinic (Figure 3).
Figure 3.
Independent predictors of digoxin use in patients with (left panel) and without atrial fibrillation (right panel). The forest plots report the odds ratios and 95% confidence intervals derived from multivariable logistic regression analyses using digoxin use as the dependent variable. Abbreviations as in Table 1.
Associations between digoxin use and outcomes (Table 2 and Figure 4)
Table 2.
Outcomes of patients with heart failure with reduced ejection fraction treated with vs. without digoxin
| Patients with AF | Patients without AF | |||||||
|---|---|---|---|---|---|---|---|---|
| Model | Overall Population | Matched population | Overall population | Matched population | ||||
| Digoxin no | Digoxin yes | Digoxin no | Digoxin yes | Digoxin no | Digoxin yes | Digoxin no | Digoxin yes | |
| All-cause death/first heart failure hospitalization | ||||||||
| Incidence [n of events, sum py, rate/1000py (95% CI)] | 10 162, 36 835, 276 (271–281) | 4209, 18 673, 225 (219–232) | 4156, 16 901, 246 (238–253) | 4117, 18 056, 228 (221–235) | 11 261, 59 188, 190 (187–194) | 456, 1484, 307 (280–337) | 418, 1708, 245 (222–269) | 452, 1483, 305 (277–334) |
| Crude HR (95% CI), P-value | Ref. | 0.88 (0.85–0.91), <0.001 | Ref. | 1.63(1.49–1.79) <0.001 | ||||
| Adj. (PS) HR (95% CI), P-value | Ref. | 0.98 (0.94–1.01), 0.225 | Ref. | 0.95 (0.91–0.99), 0.011 | Ref. | 1.16 (1.05–1.28), 0.003 | Ref. | 1.24 (1.09–1.43), 0.002 |
| Consistency (digoxin time-dependent) | Ref. | 0.82 (0.76–0.86), <0.001 | Ref. | 1.25 (1.08–1.45), 0.003 | ||||
| All-cause death | ||||||||
| Incidence [n of events, sum py, rate/1000py (95% CI)] | 7948, 52 676, 151 (148–154) | 3372, 25 858, 130 (126-135) | 3171, 24 835, 128 (123–132) | 3298, 24989, 132 (128-137) | 7889, 81 881, 96 (94–98) | 349, 2644, 132 (119–147) | 324, 2618, 124 (111–138) | 346, 2620, 132 (119–147) |
| Crude HR (95% CI), P-value | Ref. | 0.88 (0.84–0.91), <0.001 | Ref. | 1.39 (1.25–1.54), <0.001 | ||||
| Adj. (PS) HR (95% CI), P-value | Ref. | 1.06 (1.02–1.11), 0.007 | Ref. | 1.03 (0.99–1.09), 0.173 | Ref. | 1.08 (0.96–1.21), 0.182 | Ref. | 1.07 (0.92–1.25), 0.382 |
| Consistency (digoxin time dependent) | Ref. | 0.80 (0.76–0.84), <0.001 | Ref. | 1.04 (0.89–1.22), 0.604 | ||||
| First heart failure hospitalization | ||||||||
| Incidence [n of events, sum py, rate/1000py (95% CI)] | 6785, 36 835, 184 (180–189) | 2828, 18 673, 151 (146–157) | 2872, 16 901, 170 (164–176) | 2768, 18 056, 153 (148–159) | 7520, 59 188, 127 (124–130) | 344, 1484, 232 (208–258) | 291, 1708, 170 (151–191) | 340, 1483, 229 (206–255) |
| Crude HR (95% CI), P-value | Ref. | 0.91 (0.87–0.95), <0.001 | Ref. | 1.85 (1.66–2.07), <0.001 | ||||
| Adj. (PS) HR (95% CI), P-value | Ref. | 0.96 (0.92–1.01), 0.089 | Ref. | 0.93 (0.88–0.98), 0.004 | Ref. | 1.24 (1.11–1.39), <0.001 | Ref. | 1.34 (1.14–1.57), <0.001 |
| Consistency (digoxin time dependent) | Ref. | 0.81 (0.77–0.85), <0.001 | Ref. | 1.42 (1.19–1.69), <0.001 | ||||
| Consistency (death as competing event) | Ref. | 0.93 (0.89–0.98), 0.009 | Ref. | 1.28 (1.09–1.49), 0.002 | ||||
AF, atrial fibrillation; CI, confidence interval; HR, hazard ratio; PS, propensity score; py, patient-years; and ref, reference.
aPropensity scores (PSs) for digoxin use were separately calculated in each imputed data set by a logistic regression model including all the variables highlighted in Table 1 as covariates, and then averaged across the 10 imputed data sets, for patients with and without AF. Adjusted Cox regression models were performed partly in the PS-matched cohort and partly in the overall cohort including the PS as a covariate.
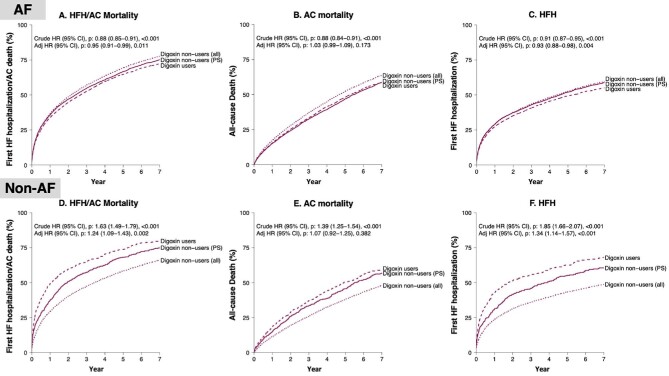
Figure 4.
Outcome analysis.
In the unmatched cohort, event rates for the all-cause death/HFH, all-cause death, and HFH were significantly lower among digoxin users vs. non-users. In the PS-matched cohort (i.e. adjusted analyses), digoxin use remained associated with a statistically significant lower risk of all-cause death/HFH [hazard ratio (HR): 0.95; 95% confidence interval (CI): 0.91–0.99] and of HFH (HR: 0.93; 95% CI: 0.88–0.98), but not of all-cause death (HR: 1.03; 95% CI: 0.99–1.09).
Consistency analyses (Table 2)
In the analyses performed adjusting rather than matching for PS, digoxin use was not associated with the risk of all-cause death/HFH and of HFH but it was associated with higher risk of all-cause death.
In the PS-matched cohort analyses, digoxin use as a time-dependent variable was independently associated with lower risk of all-cause death/HFH, all-cause mortality, and HFH.
The risk of HFH was also significantly lower with the use of digoxin when death was handled as a competing event.
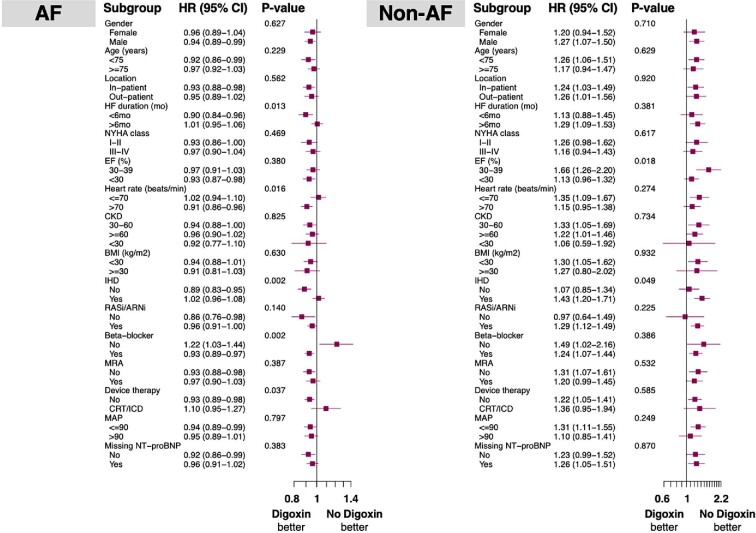
Subgroup analysis (Figure 5)
Figure 5.
Pre-specified subgroup analyses for all-cause mortality and/or first heart failure hospitalization in patients with heart failure with reduced ejection fraction with (left panel) and without (right panel) atrial fibrillation. Abbreviations as in Table 1.
The association between digoxin use and risk of all-cause death/HFH in the PS-matched analyses was consistent in most clinically relevant subgroups, but with some exceptions. In particular, digoxin use was associated with a significantly lower risk of outcome in those (i) without vs. with ischaemic heart disease; (ii) with HF history <6 months vs. ≥6 months; (iii) with heart rate >70 b.p.m. vs. ≤70 b.p.m.; (iv) receiving vs. not receiving BBs; and (v) without vs. with CRT/ICD.
Patients with heart failure with reduced ejection fraction without a history of atrial fibrillation
Digoxin use over time
In the non-AF population, the rates of digoxin use were consistent over time, ranging between 1.5% and 2.5% throughout the study period (Figure 2).
Digoxin users’ profile
Digoxin users were younger but had a longer history of HF, were more likely registered as inpatients, and had lower LVEF, lower systolic blood pressure (BP), and higher heart rate, but higher comorbidity burden compared with digoxin non-users (Table 1). They were more likely to receive diuretics and MRAs and to have a CRT/ICD, but less likely to be followed up in an HF nurse-led clinic or in hospital care compared with non-users.
Key independent predictors of digoxin use were female sex, variables linked with more severe HF, heart rate >70 b.p.m., no history of hypertension, better renal function, and use of diuretics, MRAs, and CRT/ICD (Figure 3).
Associations between digoxin use and outcomes (Table 2 and Figure 4)
In the unmatched cohort, event rates for the all-cause death/HFH, all-cause death, and HFH were significantly higher among digoxin users vs. non-users. In the PS-matched cohort, digoxin use remained associated with a statistically significant higher risk of all-cause death/HFH (HR: 1.24; 95% CI: 1.09–1.43) and of HFH (HR: 1.34; 95% CI: 1.14–1.57), but not of all-cause death (HR: 1.07; 95% CI: 0.92–1.25).
Consistency analyses (Table 2)
In the analyses performed adjusting rather than matching for PS, digoxin use was associated with higher risk of all-cause death/HFH and of HFH but it was not associated with risk of all-cause death.
When digoxin was analysed as a time-dependent variable in the PS-matched cohort, its use was independently associated with higher risk of all-cause death/HFH and HFH, but not death. The risk of HFH was also significantly higher with the use of digoxin when death was handled as a competing event.
Subgroup analysis (Figure 5)
The association between digoxin use and risk of all-cause death/HFH in the PS-matched analyses was consistent in most clinically relevant subgroups, but with some exceptions. Namely, digoxin use was associated with significantly higher risk of outcome in those (i) with vs. without ischaemic heart disease and (ii) with LVEF 30–39% vs. <30%.
Discussion
In this large and comprehensive analysis in patients with HFrEF, (i) overall use of digoxin was modest (16%); (ii) digoxin use was dramatically higher in AF (29%) vs. non-AF (2.8%), and had a reverse U shape over time among patients with AF, while remaining stable over time in non-AF patients; (iii) important independent predictors of digoxin use were, in addition to AF, younger age, female sex, more advanced HF, higher heart rate, and preserved kidney function; (iv) in patients with AF, digoxin was associated with lower risk of all-cause death/HFH; and (v) in patients without AF, digoxin was associated with higher risk of all-cause death/HFH and HFH.
Digoxin use over time
Our study showed that digoxin use in patients with HFrEF increased in the early 2000s but decreased thereafter. This trend was mainly attributable to changes in digoxin use over time in the subgroup of patients with a history of AF in whom treatment with digoxin was predominant (29% in AF vs. 2.8% in non-AF). Use of digoxin in patients with HFrEF without a history of AF was constantly low (between 1.5% and 2.5%) throughout the study period.
Regarding digoxin use in other populations, a 50% decrease in prescriptions was noted in the USA in 2007–14,6,7 whereas in Europe use was ∼30% in inpatients and 20% in outpatients in the European Society of Cardiology (ESC)-HF Pilot study (2009–10).8 In the ESC HF Long-Term (ESC-HF-LT) Registry (2011–13), which included HF patients regardless of LVEF, it was 25.9% in inpatients and 23% in outpatients, decreasing at 1 year of follow-up.9 Interestingly, despite the fact that presence or absence of AF in HFrEF influences guidelines´ recommendations for digoxin use,1,2 the decreasing trend has been noted in patients with HFrEF both with and without AF in the USA (from 28.9% to 8.0% and from 44.3% to 16.7% between 2005 and 14, respectively).10 Conversely, the rate of digoxin use among patients with HFrEF and AF in Europe seems to be ∼40%, although use among patients with HFrEF without AF is similar to that in the USA.11
Associations between patient characteristics and digoxin use
In our analysis, AF but also younger age, heart rate >70 b.p.m., use of BBs (only among AF patients), and preserved renal function were independently associated with digoxin use. Digoxin is indicated as a second-line treatment for rate control of AF in patients with HFrEF,2 thus explaining its higher use among patients with AF, concomitant use of a BB, and higher heart rate. On the other hand, higher use of digoxin with younger age and preserved renal function may be explained by the renal excretion of digoxin, altered drug response, and increased adverse reactions amongst the elderly and those with renal impairment.12 More severe HF was independently associated with a higher chance of receiving digoxin in both patients with and without AF, which is consistent with the current guidelines´ recommendations for its use in patients with HFrEF with continued symptoms despite use of other recommended HF treatments.1 This finding is also consistent with characteristics of the DIG study population prior to randomization.13 Furthermore, the lack of effect of digoxin on BP can explain its higher use among patients with lower BP, lower use of RASi, and no history of hypertension.
Associations between digoxin use and outcomes
In our analysis, the use of digoxin was associated with an almost 5% lower risk of all-cause death or HFH in patients with HFrEF and AF, which further decreased to 18% when digoxin use was handled as a time-dependent variable. Conversely, the use of digoxin was associated with a 24% higher risk of all-cause death or HFH in patients with HFrEF without AF, which increased to 25% when digoxin use was handled as a time-dependent variable. Importantly, in our study the majority of AF patients had AF at their baseline ECG, indicating, though not proving, that the majority of patients in the AF group suffered from chronic/persistent rather than paroxysmal AF. These findings are novel. Previous observational, post hoc, and meta-analyses have reported neutral or unfavourable associations between digoxin use and hard clinical endpoints in patients with AF and/or HFrEF.4,14–22 Methodological issues (residual confounding, use of PS) might explain the reported differences in results. Performing PS matching may be an issue in small cohorts where matching can be incomplete due to the lack of a closer potential comparator, but this is less likely in our large patient population. Additionally, we performed a consistency analysis using digoxin as a time-dependent as opposed to a single-point variable, which corroborated our results. Single-point handling of digoxin use may be viewed as a limitation of previous analyses.16,18,19
We observed some inconsistencies between our main and consistency analyses, which might be due to small fluctuations in HRs reflecting differences in sample size or adjustments for confounders in the PS-matched vs. overall analyses, leading to the observed differences in statistical significance for the same associations.
We demonstrate that digoxin use was associated with a significantly higher risk of all-cause death/HFH in patients with HFrEF and AF not receiving BB, which could be explained by use of digoxin and lack of BB therapy identifying patients at highest risk of HF events, i.e. refractory symptoms, low BP, and high risk of sudden cardiac death, or, conversely, could highlight a synergistic effect of digoxin with BBs in patients with HFrEF and AF for rate control.1,2 On the other hand, this could also underpin the arrhythmogenic side effects that digoxin-mediated inhibition of the Na+–K+ ATPase pump in cardiomyocytes and consequent increase in intracellular calcium concentration exert, especially in the absence of a BB that protects from these life-threatening arrhythmias.23,24 The use of digoxin in patients with HF and AF is further supported by the recent Rate Control Therapy Evaluation in Permanent Atrial Fibrillation (RATE-AF) RCT, which showed improved functional status, natriuretic peptide levels, and fewer adverse events with digoxin compared with bisoprolol.
Our findings in HFrEF without AF highlighting an association between digoxin use and higher risk of all-cause death or HFH are in disagreement with what is shown in a randomized setting, i.e. the DIG trial.3 This might be at least partially explained by the significant differences between the contemporary HFrEF care and the common practice 25 years ago when the DIG trial was performed. As mentioned earlier, patients in DIG were treated with diuretics and ACE-I, but, importantly, not with BBs, MRAs, or HF devices.3 This may have major implications in patients’ mode of death and the relative effects of digoxin in these different settings, as indicated by the gradual decrease in cardiovascular mortality and HF hospitalization rates over time.25
However, we must consider that, given the difference in patient profiles between those without AF receiving vs. not receiving digoxin, our finding may merely reflect residual confounding owing to more severe disease among digoxin users. In our cohort, 90% of patients in the non-AF group were receiving a BB and nearly 50% had a heart rate ≥70 b.p.m. In this scenario and given that the BB cannot be further uptitrated, ivabradine should be the next indicated therapeutic step to achieve optimal rate control.1,26
Two RCTs are currently ongoing to test the efficacy of digoxin on top of optimal HFrEF therapy (NCT03783429).27
Limitations
A major limitation of this and other studies on digoxin is the observational design, prone to unmeasured confounding. This may be particularly problematic with digoxin, which is likely used in patients with more severe HF, especially among patients with normal sinus rhythm in whom it is used as a positive inotropic rather than as a rate controlling agent. Importantly, in our cohort the number of patients without AF receiving digoxin was relatively low [n = 564 (2.8%)]. Unfortunately, doses of digoxin and patterns of use were not readily available. Furthermore, we cannot exclude a confounding role of serum digoxin levels, which have been shown to be associated with increased mortality but were not available in our analysis.28 One additional limitation is the lack of extensive data on type of AF, though some assumptions may be made based on relevant history and baseline ECG. Finally, SwedeHF is a nationwide registry but coverage is not complete and therefore a selection bias may still be possible.
Conclusions
In patients with HFrEF, the overall use of digoxin was modest and decreased over time but was considerably higher in patients with vs. without AF. Digoxin use was associated with a lower risk of death/HFH in patients with HFrEF and AF, which supports current guideline recommendations, but was associated with higher risk of adverse events in patients with HFrEF without AF, which contrasts with the randomized DIG trial. Given the observational design of the current study, which does not allow to investigate efficacy, our findings warrant confirmation in contemporary RCTs. Nevertheless, our analysis adds important insights to the current use of digoxin in clinical practice and its association with outcomes according to the current indications, with major implications in terms of implementation of digoxin use whether or not the upcoming RCTs might show digoxin being effective.
Supplementary Material
Contributor Information
Chris J Kapelios, Cardiology Department, Royal Brompton Hospital, Royal Brompton and Harefield NHS Foundation Trust, London, UK.
Lars H Lund, Division of Cardiology, Department of Medicine, Karolinska Institutet, Stockholm, Sweden; Heart and Vascular Theme, Karolinska University Hospital, Stockholm, Sweden.
Lina Benson, Division of Cardiology, Department of Medicine, Karolinska Institutet, Stockholm, Sweden.
Ulf Dahlström, Department of Cardiology and Department of Health, Medicine and Caring Sciences, Linköping University, Linkoping, Sweden.
Giuseppe M C Rosano, Cardiovascular Clinical Academic Group, St George's Hospitals NHS Trust, University of London, Cranmer Terrace, London, UK; IRCCS San Raffaele, Pisana, Roma, Italy.
Paul J Hauptman, Graduate School of Medicine, University of Tennessee, Knoxville, TN, USA.
Gianluigi Savarese, Division of Cardiology, Department of Medicine, Karolinska Institutet, Stockholm, Sweden; Heart and Vascular Theme, Karolinska University Hospital, Stockholm, Sweden.
Funding
None declared.
Conflict of interest: G.S. reports grants and personal fees from Vifor, grants and non-financial support from Boehringer Ingelheim, personal fees from Societa´ Prodotti Antibiotici, grants and personal fees from AstraZeneca, personal fees from Roche, personal fees from Servier, grants from Novartis, personal fees from GENESIS, personal fees from Cytokinetics, personal fees from Medtronic, grants from Boston Scientific, grants from Pharmacosmos, and grants from Merck, outside the submitted work. L.H.L. reports research grants from AstraZeneca, Novartis, Boehringer Ingelheim, Boston Scientific, Vifor Pharma, Relypsa, Pharmacosmos, and consulting/speaker honoraria from AstraZeneca, Novartis, Bayer, Vifor Pharma, Sanofi, Lexicon, Myokardia, Orion Pharma, Merck/MSD, Respicardia, and Medscape. U.D. reports research grants from AstraZeneca, Vifor Pharma, Boehringer Ingelheim, Pfizer, Boston Scientific, and Roche Diagnostics and consulting honoraria from Amgen. P.J.H. reports consulting for Corvia, LivaNova, Pfizer, and BTG Pharma. U.D. reports grants outside the present work from Boehringer Ingelheim, AstraZeneca, Pfizer, Vifor, Boston Scientific, and Roche Diagnostics, consultancies from Amgen and Novartis, and speaker fees from AstraZeneca. C.J.K., G.R., and LB have no relationships to disclose.
References
- 1. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo-Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A, ESC Scientific Document Group . 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599–3726. [DOI] [PubMed] [Google Scholar]
- 2. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, Fauchier L, Filippatos G, Kalman JM, La Meir M, Lane DA, Lebeau JP, Lettino M, Lip GYH, Pinto FJ, Thomas GN, Valgimigli M, Van Gelder IC, Van Putte BP, Watkins CL, ESC Scientific Document Group . 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J 2021;42:373–498. [DOI] [PubMed] [Google Scholar]
- 3. Digitalis Investigation Group . The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med 1997;336:525–533. [DOI] [PubMed] [Google Scholar]
- 4. Vamos M, Erath JW, Benz AP, Lopes RD, Hohnloser SH. Meta-analysis of effects of digoxin on survival in patients with atrial fibrillation or heart failure: an update. Am J Cardiol 2019;123:69–74. [DOI] [PubMed] [Google Scholar]
- 5. Savarese G, Lund LH.. Digoxin: beneficial or harmful? Eur Heart J Cardiovasc Pharmacother 2017;3:127–128. [DOI] [PubMed] [Google Scholar]
- 6. Angraal S, Nuti SV, Masoudi FA, Freeman JV, Murugiah K, Shah ND, Desai NR, Ranasinghe I, Wang Y, Krumholz HM.. Digoxin use and associated adverse events among older adults. Am J Med. 2019;132:1191–1198. [DOI] [PubMed] [Google Scholar]
- 7. Weisse AB. A fond farewell to the foxglove? The decline in the use of digitalis. J Card Fail 2010;16:45–48. [DOI] [PubMed] [Google Scholar]
- 8. Maggioni AP, Dahlström U, Filippatos G, Chioncel O, Leiro MC, Drozdz J, Fruhwald F, Gullestad L, Logeart D, Metra M, Parissis J, Persson H, Ponikowski P, Rauchhaus M, Voors A, Nielsen OW, Zannad F, Tavazzi L, Heart Failure Association of ESC (HFA) . EURObservational Research Programme: the Heart Failure Pilot Survey (ESC-HF Pilot). Eur J Heart Fail 2010;12:1076–1084. [DOI] [PubMed] [Google Scholar]
- 9. Crespo-Leiro MG, Anker SD, Maggioni AP, Coats AJ, Filippatos G, Ruschitzka F, Ferrari R, Piepoli MF, Delgado Jimenez JF, Metra M, Fonseca C, Hradec J, Amir O, Logeart D, Dahlström U, Merkely B, Drozdz J, Goncalvesova E, Hassanein M, Chioncel O, Lainscak M, Seferovic PM, Tousoulis D, Kavoliuniene A, Fruhwald F, Fazlibegovic E, Temizhan A, Gatzov P, Erglis A, Laroche C, Mebazaa A, Heart Failure Association (HFA) of the European Society of Cardiology (ESC) . European Society of Cardiology Heart Failure Long-Term Registry (ESC-HF-LT): 1-year follow-up outcomes and differences across regions. Eur J Heart Fail 2016;18:613–625. [DOI] [PubMed] [Google Scholar]
- 10. Patel N, Ju C, Macon C, Thadani U, Schulte PJ, Hernandez AF, Bhatt DL, Butler J, Yancy CW, Fonarow GC. Temporal trends of digoxin use in patients hospitalized with heart failure. JACC Heart Fail 2016;4:348–356. [DOI] [PubMed] [Google Scholar]
- 11. Veenis JF, Brunner-La Rocca H-P, Linssen GCM, Smeele FJJ, Wouters NTAE, Westendorp PHM, Rademaker PC, Hemels MEW, Rienstra M, Hoes AW, Brugts JJ, CHECK-HF investigators . Atrial fibrillation in chronic heart failure patients with reduced ejection fraction: the CHECK-HF registry. Int J Cardiol 2020;308:60–66. [DOI] [PubMed] [Google Scholar]
- 12. Hauptman PJ, Blume SW, Lewis EF, Ward S. Digoxin toxicity and use of digoxin immune fab. JACC Heart Fail 2016;4:357–364. [DOI] [PubMed] [Google Scholar]
- 13. Aguirre Dávila L, Weber K, Bavendiek U, Bauersachs J, Wittes J, Yusuf S, Koch A. Digoxin–mortality: randomized vs. observational comparison in the DIG trial. Eur Heart J 2019;40:3336–3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Freeman JV, Yang J, Sung SH, Hlatky MA, Go AS. Effectiveness and safety of digoxin among contemporary adults with incident systolic heart failure. Circ Cardiovasc Qual Outcomes 2013;6:525–533. [DOI] [PubMed] [Google Scholar]
- 15. Whitbeck MG, Charnigo RJ, Khairy P, Ziada K, Bailey AL, Zegarra MM, Shah J, Morales G, Macaulay T, Sorrell VL, Campbell CL, Gurley J, Anaya P, Nasr H, Bai R, Di Biase L, Booth DC, Jondeau G, Natale A, Roy D, Smyth S, Moliterno DJ, Elayi CS.. Increased mortality among patients taking digoxin–analysis from the AFFIRM study. Eur Heart J 2013;34:1481–1488. [DOI] [PubMed] [Google Scholar]
- 16. Butler J, Anand IS, Kuskowski MA, Rector T, Carson P, Cohn JN.. Digoxin use and heart failure outcomes: results from the Valsartan Heart Failure Trial (Val-HeFT). Congest Heart Fail 2010;16:191–195. [DOI] [PubMed] [Google Scholar]
- 17. Turakhia MP, Santangeli P, Winkelmayer WC, Xu X, Ullal AJ, Than CT, Schmitt S, Holmes TH, Frayne SM, Phibbs CS, Yang F, Hoang DD, Ho PM, Heidenreich PA.. Increased mortality associated with digoxin in contemporary patients with atrial fibrillation. J Am Coll Cardiol 2014;64:660–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Al-khateeb M, Qureshi WT, Odeh R, Ahmed AM, Sakr S, Elshawi R, Bdeir MB, Al-Mallah MH. The impact of digoxin on mortality in patients with chronic systolic heart failure: a propensity-matched cohort study. Int J Cardiol 2017;228:214–218. [DOI] [PubMed] [Google Scholar]
- 19. Gheorghiade M, Fonarow GC, van Veldhuisen DJ, Cleland JGF, Butler J, Epstein AE, Patel K, Aban IB, Aronow WS, Anker SD, Ahmed A. Lack of evidence of increased mortality among patients with atrial fibrillation taking digoxin: findings from post hoc propensity-matched analysis of the AFFIRM trial. Eur Heart J 2013;34:1489–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bavishi C, Khan AR, Ather S.. Digoxin in patients with atrial fibrillation and heart failure: a meta-analysis. Int J Cardiol 2015;188:99–101. [DOI] [PubMed] [Google Scholar]
- 21. Ziff OJ, Lane DA, Samra M, Griffith M, Kirchhof P, Lip GYH, Steeds RP, Townend J, Kotecha D.. Safety and efficacy of digoxin: systematic review and meta-analysis of observational and controlled trial data. BMJ 2015;351:h4451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Singh S, Moore H, Karasik PE, Lam PH, Wopperer S, Arundel C, Tummala L, Anker MS, Faselis C, Deedwania P, Morgan CJ, Zeng Q, Allman RM, Fonarow GC, Ahmed A. Digoxin initiation and outcomes in patients with heart failure (HFrEF and HFpEF) and atrial fibrillation. Am J Med 2020;133:1460–1470. [DOI] [PubMed] [Google Scholar]
- 23. Wagner S, Maier LS, Bers DM.. Role of sodium and calcium dysregulation in tachyarrhythmias in sudden cardiac death. Circ Res 2015;116:1956–1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ziff OJ, Kotecha D.. Digoxin: the good and the bad. Trends Cardiovasc Med 2016;26:585–595. [DOI] [PubMed] [Google Scholar]
- 25. McMurray J, Packer M, Desai A, Gong J, Greenlaw N, Lefkowitz M, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, PARADIGM-HF Investigators and Committees . A putative placebo analysis of the effects of LCZ696 on clinical outcomes in heart failure. Eur Heart J 2015;36:434–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Swedberg K, Komajda M, Böhm M, Borer JS, Ford I, Dubost-Brama A, Lerebours G, Tavazzi L, SHIFT Investigators . Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study. Lancet 2010;376:875–885. [DOI] [PubMed] [Google Scholar]
- 27. Bavendiek U, Berliner D, Dávila LA, Schwab J, Maier L, Philipp SA, Rieth A, Westenfeld R, Piorkowski C, Weber K, Hänselmann A, Oldhafer M, Schallhorn S, von der Leyen H, Schröder C, Veltmann C, Störk S, Böhm M, Koch A, Bauersachs J, DIGIT-HF Investigators and Committees . Rationale and design of the DIGIT-HF trial (DIGitoxin to Improve ouTcomes in patients with advanced chronic Heart Failure): a randomized, double-blind, placebo-controlled study. Eur J Heart Fail 2019;21:676–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Adams KF, Ghali JK, Herbert Patterson J, Stough WG, Butler J, Bauman JL, Ventura HO, Sabbah H, Mackowiak JI, van Veldhuisen DJ. A perspective on re-evaluating digoxin's role in the current management of patients with chronic systolic heart failure: targeting serum concentration to reduce hospitalization and improve safety profile. Eur J Heart Fail 2014;16:483–493. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.