Abstract
Introduction
A multidisciplinary heart team approach has been recommended by revascularisation guidelines, but how to organise and implement the heart team in a standardised way has not been validated. Inter-team and intra-team decision instability existed in the guideline-based heart team protocol, and our standardised heart team protocol based on a mixed method study may improve decision stability. The objective of this study is to evaluate the effect of the standardised heart team protocol versus the guideline-based protocol on decision-making stability in stable complex coronary artery disease (CAD).
Methods and analysis
Eighty-four eligible interventional cardiologists, cardiac surgeons or non-interventional cardiologists from 26 hospitals in China have been enrolled. They will be randomised to a standardised heart team protocol group or a guideline-based protocol group to make revascularisation decisions for 480 historic cases (from a prospective registry) with stable complex CAD. In the standardised group, we will establish 12 heart teams based on an evidence-based protocol, including specialist selection, specialist training, team composition, team training and a standardised meeting process. In the guideline-based group, we will organise 12 heart teams according to the guideline principles, including team composition and standardised meeting process. The primary outcome is the overall percent agreement in revascularisation decisions between heart teams within a group. To demonstrate the clinical implication of decision-making stability, we will further explore the association between decision stability and 1-year clinical outcomes.
Ethics and dissemination
The study was approved by the Institutional Review Board (IRB) of Fuwai Hospital (No. 2019-1303). All participants have provided informed consent and all patients included as historic cases provided written informed consent at the time of entry to the prospective registry. The results of this trial will be disseminated through manuscript publication and national/international conferences, and reported in the trial registry entry.
Trial registration number
Keywords: coronary heart disease, coronary intervention, protocols & guidelines
Strengths and limitations of this study.
The study is a randomised controlled trial testing an evidence-based standardised heart team protocol covering the whole heart team organisation process with up-to-date information provision against an approach following guideline basic recommendations.
Randomisation is used in three aspects, stratified randomisation in group allocation, randomisation in heart team membership and randomisation in case allocation, which controls the social factors that may have negative implications for true group decision-making and ensures relatively heart team exposure to case complexity.
Trial procedures will be carried out remotely, and all heart team meetings will be held via video conference using an online system, enabling full involvement and eliminating the risk of spreading COVID-19.
The cases discussed are retrospectively instead of prospectively selected, and the study does not investigate the impact of the standardised heart team protocol on true treatment decisions and clinical outcomes in routine clinical care, which is the next step to be tested.
The intervention in the standardised protocol group is an integrated approach, and the potential differential outcomes associated with its use cannot be attributed to a single point of the process.
Introduction
The heart team approach has received a class 1C/1B recommendation in European and American guidelines on myocardial revascularisation in patients with complex coronary artery disease (CAD) to optimise the treatment strategies and may lead to better outcomes.1–5 Clinical guidelines recommend that a heart team, consisting of clinical/non-interventional cardiologists, interventional cardiologists and cardiac surgeons, should take sufficient time to assess all available information on complex cases. However, there are relatively limited data on the heart team implementation in detail, such as the ideal composition, meeting frequency, the timing of decision-making and outcomes, potentially leading to suboptimal decision-making quality.
Prior efforts have noted insufficient inter-specialist consistency, intra-team reproducibility and inter-team agreement in heart team decision-making. Denvir et al found poor agreement existed between cardiac clinical specialists (kappa=0.26).6 Several studies reported that on re-discussion of the same patient data 9–12 months later, nearly 20%–24% of decisions differed from the original heart team recommendations.7 8 In our previous work, the agreement between heart teams for revascularisation decision-making was just moderate (kappa=0.58).9
Clinical guidelines and previous practice experience from different centres have summarised several critical principles in heart team implementation.10–12 Guidelines recommend the composition should be at least a cardiac surgeon, an interventional cardiologist and a non-interventional cardiologist.1 5 Sanchez et al summed up the experience of the heart team implementation from their single centre, including team composition, data collection and meeting process.11 The British Cardiovascular Society, Society for Cardiothoracic Surgery in Great Britain and Ireland and British Cardiovascular Intervention Society set out the principles for the functioning of the heart team across the UK, including composition, frequency and the type of cases discussed.12 Although these works provided essential experiences for heart team implementation, the protocols were not evidence-based and data regarding how these protocols impact decision-making stability were scarce.12
To determine the potential factors influencing heart team decision-making comprehensively and explore an evidence-based heart team protocol, we conducted a sequential explanatory mixed method study and summarised 3 themes (specialist quality, team composition and meeting process) and 10 subthemes of potential factors. In addition, nine recommendations for heart team implementation were derived based on qualitative and quantitative data, and a standardised heart team protocol was developed based on the previous experience, recommendations and guidelines, covering the whole procedure of heart team implementation.
However, the practical effect of the standardised protocol versus the guideline-based protocol on decision-making stability and clinical outcomes remains unknown, and a randomised trial for validation is warranted. Therefore, we designed this pivotal randomised trial.
Methods and analysis
Study design
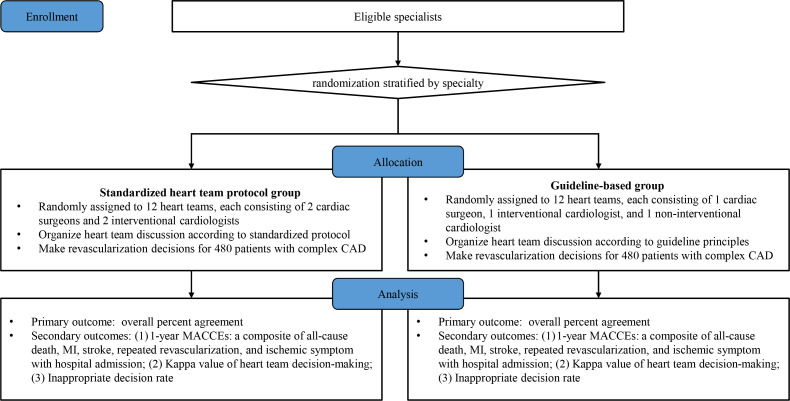
The current study is a randomised, controlled, two-arm trial involving 84 cardiac specialists from 26 hospitals in China. Eligible specialists have been randomised to a standardised implementation protocol group or a guideline-based group to establish 24 heart teams and make revascularisation decisions for 480 stable complex CAD cases retrospectively enrolled. We will evaluate the decision-making stability (figure 1). Standard Protocol Items: Recommendations for Interventional Trials,13 Consolidated Standards of Reporting Trials14 and Template for Intervention Description and Replication15 checklists are mentioned in online supplemental file 1. The study start date is 4 January 2022 and the anticipated end date is 31 January 2023.
Figure 1.
Study flow chart. Eligible specialists will be randomised to a standardised heart team protocol group or a guideline-based group and established 12 heart teams in each group to make revascularisation decisions for 480 historic cases (from a prospective registry) with stable complex CAD. CAD, coronary artery disease; MACCE, major adverse cardiovascular and cerebrovascular event; MI, myocardial infarction.
bmjopen-2022-064761supp001.pdf (551.7KB, pdf)
Objective and hypothesis
The primary objective of this study is to evaluate the effect of the standardised heart team protocol versus the guideline-based protocol on the stability of decision-making in stable complex CAD. The primary hypothesis is that heart teams organised on the standardised protocol will result in better decision-making consistency compared with those based on guideline principles. The secondary objectives of this study are to (1) evaluate the association between decision-making stability and 1-year composite of death, myocardial infarction (MI), stroke, repeated revascularisation and re-hospitalisation due to ischaemic symptoms; (2) assess the appropriateness of heart team decision-making.
Participants and recruitment
To have access to enough experienced specialists, we will enrol eligible specialists from hospitals with (1) annual volume of percutaneous coronary intervention (PCI) ≥500; (2) annual volume of coronary artery bypass grafting (CABG) ≥2001; (3) have at least two interventional cardiologists, two cardiac surgeons and one non-interventional cardiologist meeting the inclusion criteria and agreeing to participate in the study. The inclusion criteria for the heart team specialists differ from specialties and require specified operator volumes and experience (table 1). The interventional cardiologist is required to have an annual PCI volume ≥200,16 an annual left main (LM)-PCI volume ≥251 and is capable of chronic total occlusion (CTO)-PCI. The cardiac surgeon must have a total CABG volume ≥20017 and be proficient in both on-pump and off-pump CABG. We have contacted all the potential participants via emails or telephones to get their information confirmed and obtained their content from 1 December 2021 to 10 January 2022. All participating specialists have provided written informed consent for enrolment (online supplemental file 2).
Table 1.
Inclusion criteria for heart team specialists
| Disciplines | Inclusion criteria |
| Interventional cardiologist | |
| Cardiac surgeon |
|
| Non-interventional cardiologist |
|
CABG, coronary artery bypass grafting; CTO, chronic total occlusion; LM, left main; PCI, percutaneous coronary intervention.
bmjopen-2022-064761supp002.pdf (187.2KB, pdf)
Randomisation
Randomisation is stratified by specialties and conducted by a data manager using random number generation in SAS. We have randomised 36 cardiac surgeons and 36 interventional cardiologists in a 2:1 ratio to the standardised protocol group (24 surgeons and 24 interventional cardiologists) or the guideline-based group (12 surgeons and 12 interventional cardiologists). Twelve non-interventional cardiologists have been randomly selected and allocated to the guideline-based group. After the randomisation, each group of specialists will be randomly assigned to 12 heart teams and perform heart team meetings according to corresponding protocols. Research staff will be informed of the randomisation and organise the allocated specialists to establish heart teams. Participating specialists are unaware of the implementation conditions (online supplemental figure 1).
bmjopen-2022-064761supp003.pdf (1.8MB, pdf)
Case selection and preparation
Selection of cases to be discussed
Adult cases with stable CAD according to the National Cardiovascular Data Registry (NCDR) CathPCI criteria18 (stable angina, no or silent myocardial ischaemia) and angiographically confirmed 3-vessel disease or LM disease are eligible for inclusion in the study. We have randomly selected eligible cases from a prospective registry of consecutive patients who underwent coronary angiography between August 2016 and August 2017 (online supplemental figure 2).19 All cases provided written informed consent at the time of registration and agreed to use their data for subsequent approved cardiovascular-related medical research. Definitions and inclusion/exclusion criteria of cases can be seen in online supplemental methods.
Structured patient information
Patient data will be presented in a structured information form on an electronic meeting support system by non-clinical coordinators (online supplemental table 1). The structured information includes (a) demographics; (b) medical histories and clinical risk factors; (c) medical treatment histories and CVD symptoms of the index hospitalisation; (d) laboratory results; (e) non-invasive testing results (eg, ECG, echocardiogram, stress testing results); (f) diagnostic angiogram images and quantitative flow ratio (QFR)20; (g) clinical risk scores (ie, Synergy Between Percutaneous Coronary Intervention With Taxus and Cardiac Surgery (SYNTAX) score,21 SYNTAX II score,22 SYNTAX II 2020 score,23 Society of Thoracic Surgeons (STS) score,24 25 the European System for Cardiac Operative Risk Evaluation (EuroSCORE) II26 and SinoSCORE II).27 All the clinical information has been obtained from medical records according to the NCDR CathPCI data definitions.18 An independent angiographic core laboratory takes responsibility for all angiogram image screening and risk score evaluation by using a computer-based automatic calculator.
Case assignment
Four hundred and eighty cases will be randomised into 6 sets of 80 cases each, using a stratified randomisation procedure to ensure relatively equal heart team exposure to case complexity and a similar ratio of actual treatment strategies (CABG, PCI or medication therapy).
Intervention
Standardised heart team protocol
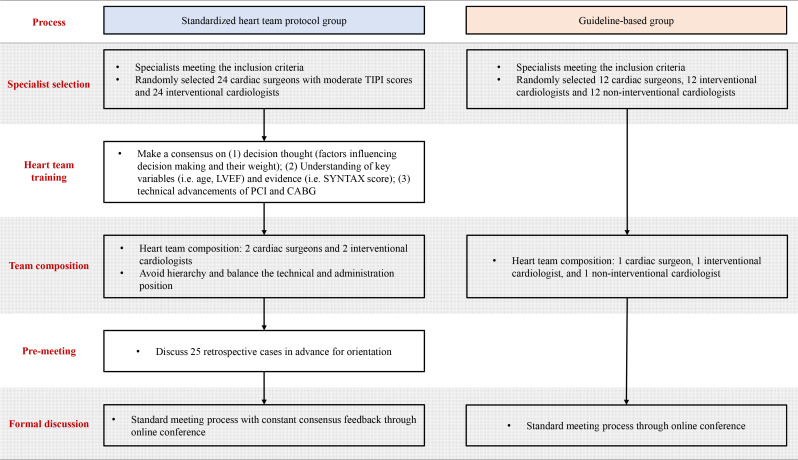
Eligible specialists randomised to this group will establish 12 heart teams and conduct heart team meetings based on the standardised heart team protocol9 (figure 2).
Figure 2.
Implementation strategies for the standardised protocol group and guideline-based group. In the standardised protocol group, the heart team will be implemented based on an evidence-based protocol including specialist selection, specialist training, team composition, team training and a standardised meeting process. In the guideline-based group, the heart team will be implemented according to the key principles mentioned in clinical guidelines, including team composition and standardised meeting process. CABG, coronary artery bypass grafting; LVEF, left ventricular ejection fraction; PCI, percutaneous coronary intervention; SYNTAX, Synergy Between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery; TIPI, Ten-Item Personality Inventory.
Specialist selection: all the cardiac surgeons are required personality tests by Ten-Item Personality Inventory in China (TIPI-C)28 and 24 surgeons with moderate scores will be randomly selected (online supplemental table 2). Twenty-four interventional cardiologists will be randomly selected without personality selection.
Specialist training: all heart team members must undergo unified training to achieve a consensus on the potential factors influencing revascularisation decisions. The training will be conducted and recorded by well-prepared coordinators. Consensus view should include clinical considerations on the essential characteristics (eg, age, left ventricular ejection fraction (LVEF) and body mass index (BMI)) and their weightage, interpretation of evidence (eg, SYNTAX trial, Evaluation of XIENCE vs Coronary Artery Bypass Surgery for Effectiveness of Left Main Revascularisation trial and the International Study of Comparative Health Effectiveness with Medical and Invasive Approaches results). Additionally, the latest technical advancements in PCI and CABG will be discussed, especially for PCI, to narrow cognitive gaps among specialists of different expertise. The consensus view document will be recorded and put onto the electronic meeting support system for reference at any time. To maintain fidelity to the consensus view, we will present each bullet point of the consensus view as a footnote under the corresponding variable.
Team composition: all specialists selected will be randomly assigned to 12 heart teams consisting of 2 cardiac surgeons and 2 interventional cardiologists. Non-interventional cardiologist or other disciplinary specialist is not required in the routine heart team unless necessary. Moreover, the technical level and administration position will be balanced in each team.
Team training: before the formal heart team meeting, a pilot discussion (25–50 retrospective cases) will be performed following the standard meeting procedure to reinforce the practice of the former consensus view for a more solid team consensus.
Standardised meeting process: heart team meetings will be conducted standardly in both groups according to the procedure widely used in the previous studies.10–12 Each heart team independently evaluates a set of cases (80 cases) through the heart team assistance system using structured online case presentations, with the members blinded to the other heart teams and the decisions of other heart teams. All specialists are required to make decisions independently among five treatment categories (PCI, CABG, PCI/CABG equipoise, medical therapy or further testing) before (round I) and after (round II) the heart team discussion. The heart team member only has access to the responses of the other heart team members after all members have submitted their independent decisions. The final treatment strategy is determined by a majority decision (online supplemental figure 3).
Guideline-based protocol
We will randomly assign eligible specialists randomised to this group to 12 heart teams based on the principles of guidelines (figure 2). Each heart team consists of one interventional cardiologist, one cardiac surgeon and one non-interventional cardiologist. This group does not require premeeting training on consensus view and pilot discussion. Formal meeting procedures follow the standardised meeting process as the other group.
All heart team meetings will be held through video conferencing, and a quiet environment will be required. For each heart team, the frequency of meetings is one or two times per week and lasts 1.5–2 hours at a time.
Outcomes
The primary outcome is the overall percent agreement (OPA),29 defined as the proportion of patients who received coincident decision recommendations from paired heart teams. The secondary outcomes include:
One-year major adverse cardiovascular and cerebrovascular events (MACCEs): a composite of all-cause death, MI, stroke, repeated revascularisation and re-hospitalisation due to ischaemic symptoms.
Kappa value of heart team decision-making: Fleiss’s (>2 raters) and Cohen’s (2 raters) kappa coefficients to evaluate inter-team, intra-team, inter-specialist, intra-specialist and inter-round agreement for treatment decisions. To evaluate the reproducibility, all assigned cases will be re-discussed with the same clinical data but not in the same order 1 month after the completion of the initial discussion, with the heart team blinded to the outcome of the initial meeting.
Inappropriate decision rate: the final heart team recommendations will be adjudicated for appropriateness using the American College of Cardiology/American Association for Thoracic Surgery/American Heart Association 2017 Appropriate Use Criteria (AUC) and the Chinese AUC for coronary revascularisation for each case.30 31 Two investigators who do not participate in data collection will take responsibility for reviewing the team decisions and adjudicating the decision appropriateness independently. Any disputes will be settled via review by a third investigator, with a decision by consensus.
Data management and monitoring
Our IRB-approved protocol specifies plans for data entry, coding, security and data storage on a secure server. For retrospective data, all data will be double-checked or assessed by two independent coordinators. For prospective data on heart team meetings, the online meeting supporting system included several mechanisms to protect data integrity and promote data quality (eg, warning of missing values and preventing duplicate team participation). The data manager will maintain detailed data management procedures. Coordinators will report to and discuss with the principal investigator about the study progress, including participant recruitment, data collection and analysis and heart team meeting conductions. Any protocol modifications will be discussed with and approved by the IRB. Any significant changes in methods will be reported to the project’s programme officer and updated on the registration site https://ClinicalTrials.gov. This study does not need a data monitoring committee because all the cases discussed are retrospectively selected. Their revascularisation strategies would not be influenced by heart team recommendations and will be no risk for cases. As for participating specialists, heart team discussion will not interfere with their routine clinical work. The principal investigator and approved study team members will have access to the final trial datasets.
Statistical analysis
The pairwise comparison between the heart team decisions in each case provides data on the agreement (online supplemental table 3). The inter-team, intra-team, inter-specialist, intra-specialist and inter-round agreements will be assessed using OPA and Cohen’s κ coefficient, whenever applicable. Mean decision time will also be calculated. Cox proportional hazards models will be used to analyse whether the treatment decision adhering to the heart team recommendations is associated with better outcomes. Categorical variables will be expressed as frequency and percentage. Continuous variables will be expressed as mean±SD, or median and IQR. Categorical variables will be analysed with the likelihood ratio χ2 test or Fisher’s exact test if >25% of the cells have an expected frequency smaller than 5. Continuous variables will be computed with the two-sample t-test when data follow a normal distribution and will be compared with the Wilcoxon rank sum test for non-normal distribution; 95% CIs will be computed for all measurements. All the analyses will be performed at a significance level of two-sided 0.05. All tests will be performed using SAS software, V.9.4 (SAS Institute, Cary, North Carolina, USA).
Sample size
Number of assessments necessary to evaluate decision-making agreement
The primary end point of this study is to compare the OPA between the standardised protocol group and the guideline-based group. In our previous study, heart teams were established based on guidelines, and it was estimated that the OPA was 66.3% (unpublished data), serving as the reference rate of the controlled group in this study. We assumed that inter-team agreement is similar to or no better than intra-team reproducibility rate. According to relevant literature,7 8 it is estimated that the OPA of the standardised protocol group is 76% (the minimum estimate of previous literature). Under this circumstance, the standardised protocol group has a minor effect on improving decision consistency compared with the guideline-based group. Using a 5% level of two-side significance and a confidence level of 90%, it was estimated that a total number of 454 pairwise comparisons for each group would be necessary to meet the study acceptance criterion. For the convenience of case assignment, we adjusted the sample size to 480 cases.
Number of heart teams needed
Considering the feasibility of implementation and a good representation of both samples and heart teams, it was decided that 24 heart teams are needed with 12 in each arm. Teams in each group will be divided into 6 pairs randomly, and each pair of heart teams will evaluate the same randomly assigned 80 cases independently to provide inter-team agreement data, generating 480 pairwise comparisons in each group.
Number of heart team specialists
The heart team in the standardised group consists of two interventional cardiologists and two cardiac surgeons and that in the guideline-based group consists of one interventional cardiologist, one cardiac surgeon and one non-interventional cardiologist. With 12 heart teams in each group, a minimum of 36 cardiac surgeons, 36 interventional cardiologists and 12 non-interventional cardiologists are needed in the final study in total.
Subgroup analysis
The primary and secondary outcomes will be analysed in prespecified subgroups, including specialties and professional status. The analysis will also be conducted according to different cases stratified by age, LVEF, BMI, degree of the stenosis, calcified lesion, stenosis severity, tandem and bending/tortuous lesion, LM, SYNTAX stratification, SYNTAX Ⅱ recommendations and SinoSCORE stratification. The comparisons in these analyses may be not powered for hypothesis testing but are descriptive in nature.
Current status
Thirty-six cardiac surgeons, 36 interventional cardiologists and 12 non-interventional cardiologists from 26 eligible hospitals agreed to participate in this study and have provided informed consent. Four hundred and eighty cases with stable complex CAD have been randomly selected for discussion. Specialist and patient baseline data are shown in tables 2 and 3.
Table 2.
Specialist baseline characteristics
| Characteristics | Overall (n=84) | Cardiac surgeon (n=36) | Interventional cardiologist (n=36) |
Non-interventional cardiologist (n=12) |
| Male | 71 (84.5) | 35 (97.2) | 34 (94.4) | 2 (16.7) |
| Status | ||||
| Chief specialist | 46 (54.8) | 21 (58.3) | 19 (52.8) | 6 (50.0) |
| Associate specialist | 34 (40.5) | 15 (41.7) | 13 (36.1) | 6 (50.0) |
| Attending specialist | 4 (4.8) | 0 (0.0) | 4 (11.1) | 0 (0.0) |
| Personality (TIPI)* | 5.20 (4.80–5.70) | 5.20 (4.90–5.50) | 5.20 (4.60–5.80) | 5.45 (4.80–5.60) |
| Extraversion | 4.50 (4.00–5.00) | 4.50 (4.00–5.50) | 4.50 (4.00–5.00) | 4.50 (4.00–5.00) |
| Agreeableness | 5.50 (4.50–6.00) | 5.00 (4.50–5.50) | 5.75 (4.50–6.50) | 5.75 (5.00–6.00) |
| Conscientiousness | 5.50 (5.00–6.50) | 6.00 (5.00–6.50) | 5.50 (5.00–6.50) | 5.75 (5.00–6.00) |
| Emotional stability | 5.00 (5.00–6.00) | 5.00 (5.00–5.50) | 5.00 (4.50–6.00) | 6.00 (5.00–6.00) |
| Openness to experiences | 5.00 (4.50–5.50) | 5.00 (4.50–5.50) | 5.00 (5.00–5.50) | 4.75 (4.50–5.50) |
Data presented as n (%) and median (IQR).
*Personality was evaluated by the TIPI scale in Chinese.28
TIPI, Ten-Item Personality Inventory.
Table 3.
Demographic and clinical characteristics of retrospective patients
| Characteristics | Patients for discussion (n=480) |
| Demographics | |
| Age, years | 62.0 (55.0–67.5) |
| Male (%) | 363 (75.6) |
| Risk factors | |
| Hypertension | 334 (69.6) |
| Hyperlipidaemia | 429 (89.4) |
| Diabetes | 185 (38.5) |
| Cerebrovascular disease | 102 (21.3) |
| COPD | 7 (1.5) |
| Chronic renal disease | 14 (2.9) |
| Smoker | 226 (47.1) |
| Body mass index, kg/m2 | 25.6 (23.7–27.5) |
| Ccr <60 mL/min/1.73 m2 | 7 (1.5) |
| Cardiovascular characteristics | |
| Previous MI | 49 (10.2) |
| Previous heart failure | 10 (2.1) |
| Peripheral vascular disease | 46 (9.6) |
| Ejection fraction, % | 63.0 (59.0–65.0) |
| Ejection fraction ≤40% | 23 (4.8) |
| CAD symptoms | |
| Silent ischaemia (after medical therapy) | 90 (18.8) |
| Non-ischaemia symptom | 20 (4.2) |
| Stable angina | 370 (77.1) |
| CCS I–II | 325 (87.8) |
| CCS III–IV | 45 (12.2) |
| Number of anti-anginal medications | |
| 0 | 118 (24.6) |
| 1 | 154 (32.1) |
| 2 | 149 (31.0) |
| 3 | 59 (12.3) |
| Extent of coronary disease | |
| 3-vessel disease | 451 (94.0) |
| Left main disease | 129 (26.9) |
| Risk classification | |
| SYNTAX score | 22.5 (16.5–29.5) |
| SYNTAX score tertiles | |
| Low risk (0–22) | 237 (49.4) |
| Intermediate risk (23–32) | 157 (32.7) |
| High risk (≥33) | 86 (17.9) |
| SYNTAX score II recommendation | |
| PCI | 11 (2.3) |
| CABG | 153 (31.9) |
| Equipoise | 316 (65.8) |
| SYNTAX score II 2020 10-year mortality (%) | |
| CABG | 14.8 (9.1–24.7) |
| PCI | 19.4 (11.6–32.2) |
| Euroscore II mortality (%) | 0.80 (0.58–1.06) |
| SinoSCORE II mortality (%) | 0.82 (0.47–1.18) |
| STS score (incidence of postoperative events) | |
| Mortality (%) | 0.49 (0.36–0.70) |
| Mortality or major complications (%) | 5.30 (4.43–6.56) |
| Reoperation (%) | 1.72 (1.46–2.07) |
| Renal failure (%) | 0.43 (0.32–0.61) |
| Stroke (%) | 0.96 (0.73–1.36) |
| Prolonged ventilation (%) | 3.20 (2.62–3.98) |
| DSWI (%) | 0.10 (0.08–0.14) |
| Prolonged hospitalisation (%) | 1.79 (1.33–2.53) |
| Treatment strategy in real world | |
| PCI | 287 (59.8) |
| CABG | 116 (24.2) |
| Medical therapy | 77 (16.0) |
Data presented as median (IQR) and n (%).
CABG, coronary artery bypass graft; CAD, coronary artery disease; Ccr, creatinine clearance rate; CCS, Canadian Cardiovascular Society; COPD, chronic obstructive pulmonary disease; DSWI, deep sternal wound infection; MI, myocardial infarction; PCI, percutaneous coronary intervention; STS, Society of Thoracic Surgeons; SYNTAX, Synergy Between Percutaneous Coronary Intervention with Taxus and Cardiac Surgery.
Patient and public involvement
None.
Ethics and dissemination
Ethics
The study was reviewed and approved by the Ethics Review Committees of Fuwai Hospital (2019–1303) on 2 August 2021; subsequent amendments have been approved. All participants have provided informed consent and all patients included as historic cases provided written informed consent at the time of entry to the prospective registry.
Safety
All the eligible cases were retrospectively selected and underwent coronary angiography between August 2016 and August 2017. Heart team decisions do not affect patients’ actual treatments. There will be no adverse event or serious adverse event relating to this study.
Dissemination
The results of this trial will be reported to the participating specialists, disseminated through manuscript publication and national/international conferences, and reported in the trial registry entry.
Discussion
The optimisation of heart team implementation including team composition, operation, distribution of responsibilities and other issues still lacks verification by evidence-based trials. The present study is the first trial focusing on the heart team implementation quality assessment and improvement by evaluating the effect of the standardised heart team protocol compared with the guideline-based protocol on decision-making stability for stable complex CAD.
Stability is a potential metric of decision-making quality. As the expertise of individual specialists is specific to their professional training and experience, cardiologists and surgeons prefer PCI or CABG, respectively.10 Prior data showed that 18.1% of the overall decision-making for patients with stable angina was classified as inappropriate based on a single disciplinary decision, especially among patients undergoing PCI.32 The heart team, a medium of communication to integrate the input of numerous specialists, can help to minimise fragmented communication between specialists and eliminate specialist bias in the decision-making process. It was reported that heart team recommendations differed from those of the original treating interventional cardiologist in approximately one-third of cases.33 Sanchez et al convened 301 heart team meetings for complex CAD from 2012 to 2015 and reported the concordance of the heart team to appropriate use criteria was up to a 99.3% appropriate primary indication for coronary revascularisation.34 Therefore, qualified heart teams perform more evidence-based and neutral in revascularisation decision-making. The success of the heart team approach is apparent in a growing number of optimal revascularisation decisions made according to professional guidelines.
Notably, a dedicated and structured heart team has a potential benefit for patient survival. Sardari Nia et al reported patients treated for mitral valve disease based on a dedicated heart team decision have significantly higher survival than a general heart team, which illustrated the establishment of a dedicated heart team consisting of experienced specialists with adequate procedure volume benefits patient survival.35 In addition, appropriate revascularisation is associated with improved 1-year outcomes in patients with appropriate indications and has no benefit in those with uncertain or inappropriate indications.19 Thus, we assume that revascularisation recommendations of dedicated heart teams organised by the standardised heart team protocol would be more stable and appropriate compared with those of general heart teams based on guideline principles, which leads to better clinical outcomes.
Making the heart team approach well-structured and efficient contributes to a better quality of cardiovascular care. The current study is essential to answer the following questions: (1) Is it feasible to establish and organise heart team meetings with the guidance of the standardised heart team protocol? (2) Will the standardised heart team protocol improve the decision-making stability in patients with stable complex CAD compared with the fundamental principles of heart team organising in guidelines? Moreover, it will enhance educational opportunities for all team members involved and provide experience in the practice of heart team meetings in prospective clinical scenarios.
Several novel designs underlie the strength of this study. First, we use a randomised controlled design to demonstrate the structure and effect of an evidence-based standardised heart team protocol on decision-making stability against the controlled approach based on guideline principles, which fills the gap with no randomised data currently available in optimal heart team implementation.12 33 Second, the study applies randomisation three times. Eligible specialists are first randomly selected and assigned to different arms by stratification randomisation. Then we establish heart teams with randomised membership to reduce social factors that may have negative implications on individual decision-making.36 Cases are also randomised into 6 sets of 80 cases each to ensure relatively equal heart team exposure to case complexity. Third, all heart team training and meetings are held via video conference using an online decision-making support system, which makes it possible to involve specialists from multiple hospitals, reduce the negative influence of a few influential individuals on face-to-face decision-making and eliminate the risk of viral spreading in COVID-19.37 Fourth, we provide the most up-to-date risk scores (such as SYNTAX Ⅱ 2020 score,23 SinoSCORE Ⅱ27 and QFR,20 a novel angiography-derived physiological assessment approach, in structured information for the specialists to adjudicate the optimal treatment strategy.
The study has several limitations. First, cases discussed are retrospectively selected rather than prospectively enrolled. All cases have already been treated from August 2016 to August 2017 in the original hospitalisation, thus it is unable to reveal the causal link between heart team meetings and real-world decision-making and outcomes in routine clinical practice. Prospective design is needed for the next step. Second, the intervention in the standardised protocol group is an integrated approach and the potential differential outcomes associated with its use cannot be attributed to a single point of the process. Additional quantitative and qualitative analysis is needed to find out which steps work on the decision-making stability. Third, heart team decisions will be made independently of patient preferences, while in real-world clinical practice, patient preference is an important factor for the final treatment decision. Patient involvement in shared decision-making should be considered in future trials.
Supplementary Material
Acknowledgments
The authors thank Drs Shuo Yuan, Zhiwei Zeng, Xiaoting Su, Runchen Sun, Juntong Zeng, Xiaohong Huang, and Lihua Xie from Fuwai Hospital, National Centre for Cardiovascular Diseases, Chinese Academy of Medical Sciences and Peking Union Medical College for their contribution in the collection of standardised case information and assessment of clinical scores.
Footnotes
HM and SL contributed equally.
Contributors: ZZ contributed to study conception, funding obtaining, administration and technical material support. HM, SL, XL, BX and ZZ contributed to the study design. HM and SL drafted the manuscript. XL and YW contributed to statistical consultation. HM, SL and BX contributed to data collection and interpretation. All authors revised the manuscript for important intellectual content and approved the final version.
Funding: The work was supported by grants from the Capital’s Funds for Health Improvement and Research (CFH; No. 2022-1-4031). The authors report no financial conflicts of interest.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the CFH. Funding sources had no role in the study design and will not have any role in the execution, analysis, interpretation or presentation of results.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Neumann F-J, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J 2019;40:87–165. 10.1093/eurheartj/ehy394 [DOI] [PubMed] [Google Scholar]
- 2.Yamasaki M, Abe K, Horikoshi R, et al. Enhanced outcomes for coronary artery disease obtained by a multidisciplinary heart team approach. Gen Thorac Cardiovasc Surg 2019;67:841–8. 10.1007/s11748-019-01108-4 [DOI] [PubMed] [Google Scholar]
- 3.Witberg G, Segev A, Barac YD, et al. Heart team/guidelines discordance is associated with increased mortality: data from a national survey of revascularization in patients with complex coronary artery disease. Circ Cardiovasc Interv 2021;14:e009686. 10.1161/CIRCINTERVENTIONS.120.009686 [DOI] [PubMed] [Google Scholar]
- 4.Patterson T, McConkey HZR, Ahmed-Jushuf F, et al. Long-term outcomes following heart team revascularization recommendations in complex coronary artery disease. J Am Heart Assoc 2019;8:e011279. 10.1161/JAHA.118.011279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Writing Committee Members, Lawton JS, Tamis-Holland JE, et al. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: a report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice guidelines. J Am Coll Cardiol 2022;79:e21–129. 10.1016/j.jacc.2021.09.006 [DOI] [PubMed] [Google Scholar]
- 6.Denvir MA, Pell JP, Lee AJ, et al. Variations in clinical decision-making between cardiologists and cardiac surgeons; a case for management by multidisciplinary teams? J Cardiothorac Surg 2006;1:2. 10.1186/1749-8090-1-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pavlidis AN, Perera D, Karamasis GV, et al. Implementation and consistency of heart team decision-making in complex coronary revascularisation. Int J Cardiol 2016;206:37–41. 10.1016/j.ijcard.2016.01.041 [DOI] [PubMed] [Google Scholar]
- 8.Long J, Luckraz H, Thekkudan J, et al. Heart team discussion in managing patients with coronary artery disease: outcome and reproducibility. Interact Cardiovasc Thorac Surg 2012;14:594–8. 10.1093/icvts/ivr157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma H, Lin S, Li X, et al. Optimal heart team protocol to improve revascularization decisions in patients with complex coronary artery disease: a sequential mixed method study. Eur Heart J Qual Care Clin Outcomes 2022;8:739–49. 10.1093/ehjqcco/qcab074 [DOI] [PubMed] [Google Scholar]
- 10.Head SJ, Kaul S, Mack MJ, et al. The rationale for heart team decision-making for patients with stable, complex coronary artery disease. Eur Heart J 2013;34:2510–8. 10.1093/eurheartj/eht059 [DOI] [PubMed] [Google Scholar]
- 11.Sanchez CE, Badhwar V, Dota A, et al. Practical implementation of the coronary revascularization heart team. Circ Cardiovasc Qual Outcomes 2013;6:598–603. 10.1161/CIRCOUTCOMES.113.000269 [DOI] [PubMed] [Google Scholar]
- 12.Luckraz H, Norell M, Buch M, et al. Structure and functioning of a multidisciplinary 'Heart Team' for patients with coronary artery disease: rationale and recommendations from a joint BCS/BCIS/SCTS working group. Eur J Cardiothorac Surg 2015;48:524–9. 10.1093/ejcts/ezv083 [DOI] [PubMed] [Google Scholar]
- 13.Chan A-W, Tetzlaff JM, Gøtzsche PC, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ 2013;346:e7586. 10.1136/bmj.e7586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moher D, Hopewell S, Schulz KF, et al. Consort 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c869. 10.1136/bmj.c869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoffmann TC, Glasziou PP, Boutron I, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;348:g1687. 10.1136/bmj.g1687 [DOI] [PubMed] [Google Scholar]
- 16.Dou K, Zhang D, Pan H, et al. Active SB-P versus conventional approach to the protection of high-risk side branches: the CIT-RESOLVE trial. JACC Cardiovasc Interv 2020;13:1112–22. 10.1016/j.jcin.2020.01.233 [DOI] [PubMed] [Google Scholar]
- 17.Hillis LD, Smith PK, Anderson JL, et al. 2011 ACCF/AHA guideline for coronary artery bypass graft surgery: a report of the American College of cardiology foundation/American heart association task force on practice guidelines. Circulation 2011;124:e652–735. 10.1161/CIR.0b013e31823c074e [DOI] [PubMed] [Google Scholar]
- 18.Registry C. NCDR CathPCI Registry v4.4 Coder’s Data Dictionary.
- 19.Lin S, Zhang H, Rao C-F, et al. Assessing the association of appropriateness of coronary revascularization and 1-year clinical outcomes for patients with stable coronary artery disease in China. Chin Med J 2020;133:1–8. 10.1097/CM9.0000000000000592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu B, Tu S, Song L, et al. Angiographic quantitative flow ratio-guided coronary intervention (favor III China): a multicentre, randomised, sham-controlled trial. Lancet 2021;398:2149–59. 10.1016/S0140-6736(21)02248-0 [DOI] [PubMed] [Google Scholar]
- 21.Sianos G, Morel M-A, Kappetein AP, et al. The SYNTAX score: an angiographic tool grading the complexity of coronary artery disease. EuroIntervention 2005;1:219–27. [PubMed] [Google Scholar]
- 22.Farooq V, van Klaveren D, Steyerberg EW, et al. Anatomical and clinical characteristics to guide decision making between coronary artery bypass surgery and percutaneous coronary intervention for individual patients: development and validation of SYNTAX score II. Lancet 2013;381:639–50. 10.1016/S0140-6736(13)60108-7 [DOI] [PubMed] [Google Scholar]
- 23.Takahashi K, Serruys PW, Fuster V, et al. Redevelopment and validation of the SYNTAX score II to individualise decision making between percutaneous and surgical revascularisation in patients with complex coronary artery disease: secondary analysis of the multicentre randomised controlled SYNTAXES trial with external cohort validation. Lancet 2020;396:1399–412. 10.1016/S0140-6736(20)32114-0 [DOI] [PubMed] [Google Scholar]
- 24.Shahian DM, Jacobs JP, Badhwar V, et al. The Society of Thoracic surgeons 2018 adult cardiac surgery risk models: Part 1-background, design considerations, and model development. Ann Thorac Surg 2018;105:1411–8. 10.1016/j.athoracsur.2018.03.002 [DOI] [PubMed] [Google Scholar]
- 25.O'Brien SM, Feng L, He X, et al. The Society of thoracic surgeons 2018 adult cardiac surgery risk models: Part 2-statistical methods and results. Ann Thorac Surg 2018;105:1419–28. 10.1016/j.athoracsur.2018.03.003 [DOI] [PubMed] [Google Scholar]
- 26.Nashef SAM, Roques F, Sharples LD, et al. EuroSCORE II. Eur J Cardiothorac Surg 2012;41:discussion 44-5:734–45. 10.1093/ejcts/ezs043 [DOI] [PubMed] [Google Scholar]
- 27.Hu Z, Chen S, Du J, et al. An in-hospital mortality risk model for patients undergoing coronary artery bypass grafting in China. Ann Thorac Surg 2020;109:1234–42. 10.1016/j.athoracsur.2019.08.020 [DOI] [PubMed] [Google Scholar]
- 28.JD L. Psychometric properties of ten-Item personality inventory in China (in Chinese). China Journal of Health Psychology 2013;21:1688–92. 10.13342/j.cnki.cjhp.2013.11.008 [DOI] [Google Scholar]
- 29.Cooper WA, Russell PA, Cherian M, et al. Intra- and interobserver reproducibility assessment of PD-L1 biomarker in non-small cell lung cancer. Clin Cancer Res 2017;23:4569–77. 10.1158/1078-0432.CCR-17-0151 [DOI] [PubMed] [Google Scholar]
- 30.Patel MR, Calhoon JH, Dehmer GJ, et al. ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS 2017 appropriate use criteria for coronary revascularization in patients with stable ischemic heart disease: a report of the American College of Cardiology appropriate use criteria task force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society of Thoracic Surgeons. J Am Coll Cardiol 2017;69:2212–41. 10.1016/j.jacc.2017.02.001 [DOI] [PubMed] [Google Scholar]
- 31.Group CAUCW . National center of cardiovascular diseases. Chinese appropriate use criteria for coronary revascularization (in Chinese). Chin Circ J 2016;31:313–7. 10.3969/j.issn.1000-3614.2016.04.001 [DOI] [Google Scholar]
- 32.Lin S, Yu C, Rao C. Appropriateness of coronary revascularization in patients with stable coronary artery disease: a multicenter clinical trial. Chinese Circulation Journal 2019;34:859–65. 10.3969/j.issn.1000-3614.2019.09.004 [DOI] [Google Scholar]
- 33.Tsang MB, Schwalm JD, Gandhi S, et al. Comparison of heart team vs interventional cardiologist recommendations for the treatment of patients with multivessel coronary artery disease. JAMA Netw Open 2020;3:e2012749. 10.1001/jamanetworkopen.2020.12749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanchez CE, Dota A, Badhwar V, et al. Revascularization heart team recommendations as an adjunct to appropriate use criteria for coronary revascularization in patients with complex coronary artery disease. Catheter Cardiovasc Interv 2016;88:E103–12. 10.1002/ccd.26276 [DOI] [PubMed] [Google Scholar]
- 35.Sardari Nia P, Olsthoorn JR, Heuts S, et al. Effect of a dedicated mitral heart team compared to a general heart team on survival: a retrospective, comparative, non-randomized interventional cohort study based on prospectively registered data. Eur J Cardiothorac Surg 2021;60:263–73. 10.1093/ejcts/ezab065 [DOI] [PubMed] [Google Scholar]
- 36.Abdulrahman M, Alsabbagh A, Kuntze T, et al. Impact of hierarchy on multidisciplinary Heart-Team recommendations in patients with isolated multivessel coronary artery disease. J Clin Med 2019;8:1490. 10.3390/jcm8091490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wahadat AR, Sadeghi AH, Tanis W. Heart team meetings during COVID-19. Eur Heart J 2020;41:1872–4. 10.1093/eurheartj/ehaa412 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-064761supp001.pdf (551.7KB, pdf)
bmjopen-2022-064761supp002.pdf (187.2KB, pdf)
bmjopen-2022-064761supp003.pdf (1.8MB, pdf)