Abstract
Rationale
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is associated with pulmonary endothelial dysfunction. There are limited data available on the outcomes of coronavirus disease (COVID-19) in patients with pulmonary hypertension (PH), a disease characterized by pulmonary endothelial dysfunction.
Objectives
To describe characteristics and outcomes of patients with precapillary PH and COVID-19.
Methods
We prospectively collected characteristics, management, and outcomes of adult patients with precapillary PH in the French PH network who had COVID-19 between February 1, 2020, and April 30, 2021. Clinical, functional, and hemodynamic characteristics of PH before COVID-19 were collected from the French PH registry.
Measurements and Main Results
A total of 211 patients with PH (including 123 with pulmonary arterial hypertension, 47 with chronic thromboembolic PH, and 41 with other types of PH) experienced COVID-19, and 40.3% of them were outpatients, 32.2% were hospitalized in a conventional ward, and 27.5% were in an ICU. Among hospitalized patients (n = 126), 54.0% received corticosteroids, 37.3% high-flow oxygen, and 11.1% invasive ventilation. Right ventricular and acute renal failure occurred in 30.2% and 19.8% of patients, respectively. Fifty-two patients (all hospitalized) died from COVID-19. Overall mortality was 24.6% (95% CI [confidence interval], 18.8–30.5) and in-hospital mortality 41.3% (95% CI, 32.7–49.9). Nonsurvivors were significantly older, more frequently male and suffering comorbidities (diabetes, chronic respiratory diseases, systemic hypertension, chronic cardiac diseases, and/or chronic renal failure), and had more severe PH at their most recent evaluation preceding COVID-19 diagnosis (in terms of functional class and 6-minute-walk distance; all P < 0.05). Use of pulmonary arterial hypertension therapy was similar between survivors and nonsurvivors.
Conclusions
COVID-19 in patients with precapillary PH was associated with a high in-hospital mortality. The typical risk factors for severe COVID-19 and severity of PH were associated with mortality in this population.
Keywords: COVID-19, pulmonary hypertension, pulmonary arterial hypertension, outcomes
At a Glance Commentary
Scientific Knowledge on the Subject
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has a predilection for causing pulmonary vascular injury. Patients with preexisting pulmonary vascular disease could therefore be at increased risk of adverse outcomes from coronavirus disease (COVID-19). Conversely, pulmonary arterial hypertension therapies, which improve pulmonary endothelial function, could protect from SARS-CoV-2 effects on the pulmonary endothelium. To date, the prognosis of pulmonary hypertension after SARS-CoV-2 infection has not been adequately described and there are no data that pulmonary arterial hypertension therapies mitigate COVID-19 pathophysiology or outcomes.
What This Study Adds to the Field
This was a prospective cohort study of patients with pulmonary hypertension in France with confirmed COVID-19. In-hospital mortality was high at 41% and was associated with known risk factors for COVID-19–related mortality, including underlying comorbidities, older age, male sex, as well as the severity of pulmonary hypertension before hospitalization for COVID-19. There was no suggestion that use of pulmonary arterial hypertension therapies were protective from in-hospital death. This study demonstrates that patients with pulmonary hypertension are at high risk of COVID-19 mortality, illustrating the importance of vaccination, early treatment, and other preventative measures in this population.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has effected an ongoing global pandemic of coronavirus disease (COVID-19), which has affected more than 250 million individuals leading to 5 million cumulative deaths (1).
Considerable data support an underlying SARS-CoV-2–related endothelial dysfunction with an increased risk of venous thromboembolic disease, systemic vasculitis, endothelial cell apoptosis, and inflammation in various organs, particularly in the pulmonary vasculature (2–5). The presence of endothelial dysfunction induced by SARS-CoV-2 infection has been shown to be a factor associated with the severity of COVID-19 and the occurrence of COVID-19–related acute respiratory distress syndrome (ARDS) (6).
Pulmonary vascular endothelial dysfunction is a pathophysiologic hallmark of pulmonary hypertension (PH) (7). Currently, all treatments approved for pulmonary arterial hypertension (PAH) target three pathways of endothelial dysfunction: nitric oxide, endothelin, and prostacyclin (8, 9). Thus, it is conceivable that patients with PH may be susceptible to severe forms of COVID-19. Conversely, the use of treatments targeting endothelial dysfunction could have a preventative or mitigating effect of severe COVID-19 in these patients (10–12). In addition, some patients with PH are routinely treated with anticoagulation, including those with idiopathic or heritable PAH and chronic thromboembolic pulmonary hypertension (CTEPH) (9), which could reduce the risk of acute thromboembolic events or improve the prognosis associated with COVID-19 (13).
Nevertheless, data on the effects of COVID-19 in PH are limited, with conflicting results based on small series showing unexpectedly favorable prognosis (10) or a relatively high 19–45% mortality compared with the general population (11, 14).
Thus, we launched a prospective study in the French PH network to evaluate COVID-19 and its outcomes in patients with precapillary PH, across the range of severity, etiology, and PH treatment. The aim of this study was to describe the characteristics of patients with PH who experienced COVID-19, their management, and their outcomes and to identify factors associated with poor outcomes.
Methods
Patients
From February 1, 2020, to April 30, 2021, we conducted a national multicentric prospective study of incident COVID-19 cases in the French PH network (French PH Referral Center, Hôpital Bicêtre, Université Paris Saclay, Le Kremlin-Bicêtre, and its 25 associated centers across France). Eligible patients had a history of precapillary PH and experienced COVID-19. Precapillary PH was defined by a mean pulmonary arterial pressure (mPAP) of ⩾25 mm Hg with a normal pulmonary arterial wedge pressure (PAWP) of ⩽15 mm Hg and pulmonary vascular resistance (PVR) of >3 WU (9). A COVID-19 diagnosis required a positive SARS-CoV-2 RT-PCR test or SARS-CoV-2 serology result or evidence of COVID-19 on a high-resolution computed tomography (HRCT) of the chest considered highly suggestive by clinicians after exclusion of other diagnoses.
Demographic characteristics at last evaluation for PH were reported. Comorbidities were reported, including body mass index, tobacco exposure, history of chronic respiratory diseases, systemic hypertension, diabetes, sleep disorders, chronic renal failure (defined by a creatinine clearance of <50 ml/min), and immunosuppression.
According to French epidemiological data, two COVID-19 waves were defined as “wave 1” before September 2020 (n = 53) and “wave 2” after September 1, 2020 (n = 158).
Evaluation of Pulmonary Hypertension
Parameters related to the last clinical evaluation of PH before COVID-19 diagnosis were retrospectively retrieved from the French PH registry. This registry was established in accordance with French bioethics laws (National Commission on Informatics and liberty, approval number 842063). Clinical status was assessed by the New York Heart Association Functional Class (NYHA FC). Nonencouraged 6-minute-walk test was performed according to current recommendations (2). Arterial blood gases including PaO2 were reported. Patients completed standard pulmonary function tests with spirometry, whole-body plethysmography and single-breath DlCO corrected for hemoglobin according to the European Respiratory Society/American Thoracic Society guidelines (3). Brain natriuretic peptide (BNP) or N-terminal pro–brain natriuretic peptide (NT pro-BNP) plasma concentrations were reported. The last hemodynamic evaluation by right heart catheterization recorded mPAP, PAWP, and right atrial pressure (RAP). Cardiac output (CO) was measured by the standard thermodilution technique. The cardiac index (CI) was calculated as CO divided by body surface area. PVR was calculated as (mPAP − PAWP)/CO, expressed in WU. Noninvasive parameters were collected in the year before COVID-19. Finally, approved drugs for PAH and CTEPH and use of long-term anticoagulation were collected at the time of COVID-19 diagnosis.
Evaluation of Severity of COVID-19 in Hospitalized Patients
The evaluation of COVID-19 severity covered clinical symptoms ranging from asymptomatic to critical dyspnea; biochemical analysis; the degree of pulmonary parenchymal opacification on HRCT of the chest; and the management of COVID-19. The presence of more than 50% pulmonary parenchymal opacification on HRCT of the chest defined severe COVID-19 (15). Biological data at first evaluation during hospitalization including C-reactive protein (CRP), creatinine, D-dimer, NP/NT-proBNP and total lymphocyte count were collected. Occurrence of acute pulmonary embolism determined from CT pulmonary angiograms ordered at the discretion of the treating physician, acute renal failure, and right heart failure reported by physicians during hospitalization stay were collected (9).
Management of COVID-19 and Outcomes
For the hospitalized population, we assessed the proportion of patients with PH discharged alive and the proportion deceased in-hospital. In both groups, we assessed the proportion of patients needing a critical care admission. Decisions to limit escalation of care (i.e., do not intubate or resuscitate) were reported. These decisions were made at the time of hospital admission or upon deterioration among physicians, patients, and family members but were not necessarily advance care directives in place before hospitalization for COVID-19.
We recorded median length of hospital stay in days (min-max). Therapeutic management of COVID-19 was recorded, including use of corticosteroids. In cases with severe COVID-19, we described the use of high-flow oxygen, invasive mechanical ventilation, and extracorporeal membrane oxygenation (ECMO). For outpatients, vital status was ascertained from the French PH registry and by contacting the patients’ PH centers.
Statistical Analysis
We reported continuous variables as medians with interquartile ranges. Comparisons between survivors and nonsurvivors were performed using the Mann-Whitney test. Pearson’s Chi-square test was used to compare discrete variables between two groups. The 95% confidence intervals (95% CI) for the proportions were calculated using normal approximation method of the binomial confidence interval. Univariable and multivariable logistic regression models were used to assess the relationship between patient characteristics and in-hospital mortality. Only variables with a P value less than 0.1 in univariable analysis and without missing data were entered into the multivariable models. Statistical significance was defined as a P value less than 0.05.
Results
Characteristics of Patients with PH with COVID-19
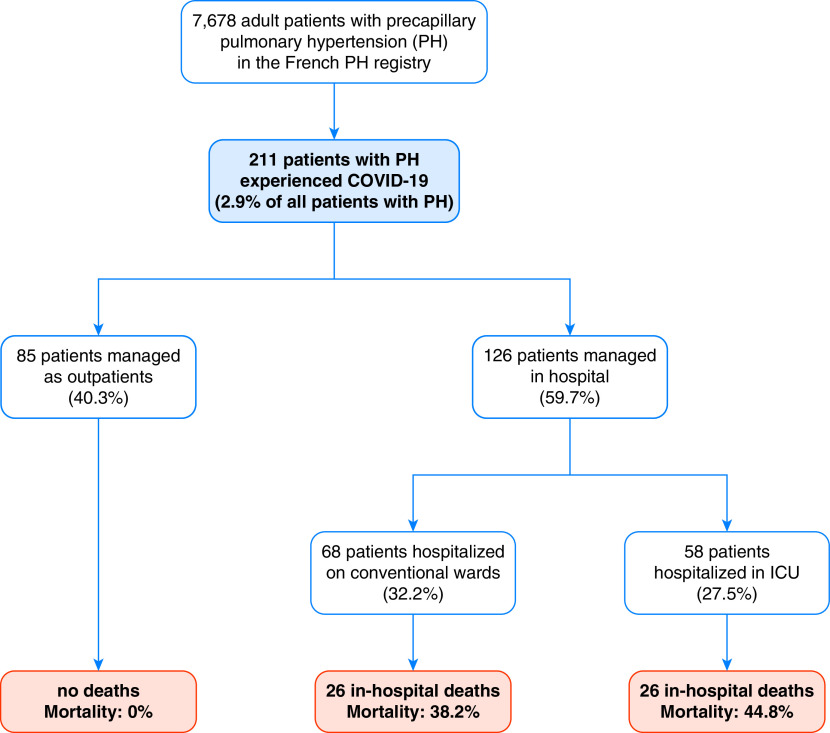
Between February 1, 2020, and April 30, 2021, we enrolled 211 patients with precapillary PH and a diagnosis of COVID-19 (Figure 1). This represents 2.7% of the 7,678 patients in the French PH registry who were alive as of February 1, 2020. Demographic and PH characteristics at the time of COVID-19 diagnosis are in Table 1. Consistent with the PH classification (16), 123 (58.3%) patients were classified as having PAH and 48 (22.7%) had CTEPH.
Figure 1.
Study flowchart. COVID-19 = coronavirus disease; PH = pulmonary hypertension.
Table 1.
Characteristics of Patients with Pulmonary Hypertension Diagnosed with COVID-19
| All Patients with PH (n = 211) | Patients Discharged Alive (n = 159) | Deceased Patients (n = 52) | P Value | |
|---|---|---|---|---|
| Outpatient | 85 (40.3%) | 85 (53.5%) | 0 | |
| Conventional wards | 68 (32.2%) | 42 (26.4%) | 26 (50.0%) | |
| Critical care | 58 (27.5%) | 32 (20.1%) | 26 (50.0%) | |
| Demographics | ||||
| Age | 63.9 (51.9–71.9) | 61.8 (47.7–70.7) | 69.4 (62.4–77.5) | <0.001 |
| Women | 115 (54.5%) | 98 (61.6%) | 17 (32.7%) | <0.001 |
| Men | 96 (45.5%) | 61 (38.4%) | 35 (67.3%) | |
| PH Diagnosis | ||||
| Group 1 | 123 (58.3%) | 100 (62.9%) | 23 (44.2%) | <0.01 |
| Group 4 | 48 (22.7%) | 38 (23.9%) | 10 (19.2%) | |
| Other groups | 40 (19.0%) | 21 (13.2%) | 19 (36.5%) | |
| Comorbidities | ||||
| Obesity (BMI > 30) | 52 (24.6%) | 37 (23.3%) | 15 (28.9%) | 0.42 |
| BMI, kg/m2 | 26 (22.5–29.4) | 26 (22.5–29.8) | 26.3 (23.1–29.4) | 0.81 |
| Chronic respiratory diseases* | 74 (35.1%) | 42 (26.4%) | 32 (61.5%) | <0.001 |
| Tobacco exposure | ||||
| None | 113 (53.6%) | 99 (62.3%) | 14 (26.9%) | <0.001 |
| Former smoker | 82 (38.9%) | 49 (30.8%) | 33 (63.5%) | |
| Active smoker | 16 (7.6%) | 11 (6.9%) | 5 (9.6%) | |
| Systemic hypertension | 80 (37.9%) | 52 (32.7%) | 28 (53.8%) | <0.01 |
| Other cardiac diseases† | 74 (35.1%) | 43 (27.0%) | 31 (59.6%) | <0.001 |
| Diabetes | 42 (19.9%) | 26 (16.4%) | 16 (30.8%) | 0.02 |
| Sleep disorders | 26 (12.3%) | 17 (10.7%) | 9 (17.3%) | 0.21 |
| Chronic renal failure (CrCl < 50 ml/min) | 58 (27.5%) | 31 (19.5%) | 27 (51.9%) | <0.001 |
| Immunosuppression | 35 (16.6%) | 25 (15.7%) | 10 (19.2%) | 0.56 |
| PH assessment at last evaluation | ||||
| NYHA functional class | ||||
| I/II | 120 (56.9%) | 102 (64.2%) | 18 (34.6%) | <0.001 |
| VIII/IV | 91 (43.1%) | 57 (35.8%) | 34 (65.4%) | |
| 6MWD (n = 171) | 402 (288–483) | 420 (339–508) | 291 (95–386) | <0.001 |
| Increased BNP or NT-proBNP (n = 188) | 93 (49.5%) | 61 (43.3%) | 32 (68.1%) | <0.01 |
| RAP, mm Hg (n = 202) | 7 (5–10) | 6 (4–10) | 9 (5–15) | 0.04 |
| mPAP, mm Hg (n = 207) | 39 (31–48) | 39 (30–49) | 40 (31–46) | 0.95 |
| CI, L/min/m2 (n = 204) | 2.80 (2.33–3.43) | 2.80 (2.40–3.50) | 2.80 (2.20–3.30) | 0.24 |
| PVR, WU (n = 204) | 5.2 (3.5–8.2) | 5.2 (3.3–8.3) | 6.3 (3.9–7.9) | 0.38 |
| DLCO, % predicted (n = 156) | 53.5 (35–66) | 59 (43–70) | 33 (24–48) | <0.001 |
| PaO2 on room air, mm Hg (n = 137) | 64 (58–75) | 65 (60–78) | 58 (49–68) | <0.01 |
| Treatment for PH | ||||
| Specific PH therapy | ||||
| ERA | 125 (59.2%) | 96 (60.4%) | 29 (55.8%) | 0.56 |
| PDE5i | 129 (61.2%) | 96 (60.4%) | 33 (63.5%) | 0.69 |
| Prostacyclins or derivatives | 30 (14.2%) | 26 (16.4%) | 4 (7.7%) | 0.19 |
| Riociguat | 30 (14.2%) | 26 (16.4%) | 4 (7.7%) | 0.19 |
| Calcium channel blockers‡ | 4 (1.9%) | 4 (2.5%) | 0 | — |
| None | 31 (14.7%) | 21 (13.2%) | 10 (19.2%) | 0.29 |
| Number of specific PH treatments | ||||
| Monotherapy | 66 (31.3%) | 48 (30.2%) | 18 (34.6%) | 0.40 |
| Dual therapy | 82 (38.9%) | 62 (39.0%) | 20 (38.5%) | |
| Triple Therapy | 28 (13.3%) | 24 (15.1%) | 4 (7.7%) | |
| Anticoagulation | 116 (55.0%) | 94 (59.1%) | 22 (42.3%) | 0.03 |
Definition of abbreviations: 6MWD = 6-minute-walk distance; BMI = body mass index; BNP = brain natriuretic peptide; CI = cardiac index; COVID-19 = coronavirus disease; CrCl = creatinine clearance; ERA = endothelin receptor antagonist; mPAP = mean pulmonary arterial pressure; NT-proBNP = N-terminal pro–brain natriuretic peptide; NYHA = New York Heart Association; PDE5i = phosphodiesterase type-5 inhibitor; PH = pulmonary hypertension; PVR = pulmonary vascular resistance; RAP = right atrial pressure; WU = Wood units.
Respiratory diseases other than pulmonary hypertension.
Excluding PH, right ventricular failure, and congenital heart diseases.
Calcium channel blockers prescribed for long-term calcium channel blockers responders.
The median age was 63.9 (51.9–71.9) years old, and 54.5% were female. Obesity was a comorbidity for 52 (24.6%) patients, with a median body mass index of 26 (22.5–29.4). Tobacco exposure was documented for 98 patients, including 82 (38.9%) former smokers and 16 (7.6%) active smokers. Comorbidities included chronic respiratory disease in 74 (35.1%) patients, systemic hypertension in 80 (37.9%) patients, diabetes in 42 (19.9%) patients, sleep disorders in 26 (12.3%) patients, chronic renal failure in 58 (27.5%) patients, immunosuppression in 35 (16.6%) patients (immunosuppressive medications for 33 patients including 2 patients with liver transplants and 2 with renal transplants; and 2 patients with active cancer) and cardiac diseases (other than PH, right ventricular failure, or congenital heart disease) in 74 (35.1%) patients.
At the last PH assessment (median 10.8 mo) before the diagnosis of COVID-19, 120 (56.9%) patients were in NYHA FC I or II, and 91 (43.1%) were class III or IV. The median 6-minute-walk distance (6MWD), available for 171 patients, was 402 (288–483) m. An increased BNP or NT-proBNP was present in 49.5% (93/188) of patients. The median percent predicted DlCO (n = 156) was 53.5% (35–66). Hypoxemia was common with a median PaO2 (n = 137) of 64 (58–75) mm Hg on room air. The most recent RHCs performed with a median (interquartile range) delay of 12 (5–26) months before COVID-19 showed a median RAP of 7 (5–10) mm Hg, mPAP of 39 (31–48) mm Hg, CI of 2.8 (2.33–3.43) L/min/m2, and PVR of 5.2 (3.5–8.3) mm Hg.
Specific PH therapy included an endothelin receptor antagonist for 125 (59.2%) patients, phosphodiesterase type 5 inhibitor for 129 (61.2%) patients, prostacyclins and derivatives for 30 (14.2%) patients, and riociguat for 30 (14.2%) patients. At the diagnosis of COVID-19, 177 patients were taking at least one PH therapy, including 67 (31.8%) on monotherapy, 82 (38.9%) on dual therapy, and 28 (13.3%) on triple therapy. Finally, 116 (55%) patients were on full-dose anticoagulation (81 vitamin K antagonists, 34 direct oral anticoagulants, and one low-molecular-weight heparin).
Characteristics of COVID-19 and Its Management
Among the 211 patients with PH with a diagnosis of COVID-19, 68 (32.2%) patients were hospitalized in conventional wards, 58 (27.5%) patients were in critical care units, and 85 (40.3%) were outpatients (Table 1). The median length of stay for hospitalized patients was 9 (5–15) days (Table 2).
Table 2.
Management of Hospitalized Patients
| Hospitalized Patients with PH (n = 126) | Patients with PH Discharged Alive (n = 74) | Deceased Patients with PH (n = 52) | P Value | |
|---|---|---|---|---|
| PH network | 68 (54.0%) | 42 (56.8%) | 26 (50.0%) | 0.45 |
| Outside PH network | 58 (46.0%) | 32 (43.2%) | 26 (50.0%) | |
| PCR positive | 119 (94.4%) | 70 (94.6%) | 49 (94.2%) | 0.76 |
| Nosocomial COVID-19 | 24 (19.0%) | 13 (17.6%) | 11 (21.2%) | 0.61 |
| Extent of CT opacities (n = 89) | 0.06 | |||
| <25% | 35 (39.3%) | 25 (45.5%) | 10 (29.4%) | |
| 25–50% | 34 (38.2%) | 22 (40%) | 12 (35.3%) | |
| >50% | 20 (22.5%) | 8 (14.5%) | 12 (35.3%) | |
| Acute pulmonary embolism | 5 (4.0%) | 4 (5.4%) | 1 (1.9%) | 0.32 |
| Right heart failure | 38 (30.2%) | 15 (20.3%) | 23 (44.2%) | <0.01 |
| Acute renal failure | 25 (19.8%) | 8 (10.8%) | 17 (32.7%) | <0.01 |
| C-reactive protein, mg/l (n = 98) | 59 [31-95] | 52 (30–94) | 63 [34–117] | 0.32 |
| Lymphocytes (n = 76) | 690 [480–1,240] | 715 [500–1,255] | 585 [420–1,218] | 0.27 |
| D-dimer (n = 53) | 698 [324–1,692] | 630 [305–1,678] | 780 [430–2,045] | 0.73 |
| BNP > 300 or NT-proBNP > 1,400 ng/L (n = 77) | 42 (54.5%) | 20 (47.6%) | 22 (62.9%) | 0.18 |
| In-hospital management | ||||
| Critical care | 58 (46.0%) | 32 (43.2%) | 26 (50.0%) | 0.45 |
| Limitation of care | 31 (24.2%) | 6 (8.0%) | 25 (47.2%) | <0.001 |
| Length of stay, d | 9 (5–15) | 11 (7–18) | 5 (2–14) | <0.001 |
| Corticosteroids | 68 (54.0%) | 40 (53.3%) | 28 (52.8%) | 0.98 |
| High-flow oxygen | 47 (37.3%) | 24 (32.4%) | 23 (44.2%) | 0.18 |
| Invasive mechanical ventilation | 14 (11.1%) | 5 (6.8%) | 9 (17.3%) | 0.12 |
| ECMO | 2 (1.6%) | 1 (1.3%) | 1 (1.9%) | — |
Definition of abbreviations: BNP = brain natriuretic peptide; COVID-19 = coronavirus disease; CT = computed tomography; ECMO = extracorporeal membrane oxygenation; NT-proBNP = N-terminal pro–brain natriuretic peptide; PH = pulmonary hypertension.
In hospitalized patients (n = 126), 68 (54%) patients were at hospitals affiliated with the PH network and 58 (46%) were hospitalized outside of the PH network. COVID-19 was diagnosed by a positive SARS-CoV-2 RT-PCR test result in 119 (94.4%) hospitalized patients with PH with 24 (19%) nosocomial COVID-19 cases. The seven cases diagnosed by HRCT were all diagnosed during the first 3 months of the pandemic (March–May 2020) before widespread availability of RT-PCR testing in French hospitals. A decision to limit escalation of care (i.e., do not intubate or resuscitate) was documented for 31 (24.2%) patients (Table 2).
HRCT of the chest was performed in 89 patients and graded by the severity of parenchymal opacities, ranging from <25% to between 25% and 50%, to >50% of the total chest area in 35 (39.3%), 34 (38.2%), and 20 (22.5%) patients, respectively. The median D-dimer (n = 53) was 698 (324–1692) μg/L, and an acute pulmonary embolism was detected in five hospitalized patients. An increase in BNP or NT-proBNP was observed in 42 of 77 (54.5%) patients. Moreover, on routine blood tests, the median C-reactive protein concentration was 59 (31–95) mg/L, and the median lymphocyte count was 690 (480–1240) cells/L. Finally, acute renal failure was observed in 25 (19.8%) hospitalized patients (Table 2).
COVID-19 hospital management was performed according to the clinical judgment and discretion of physicians: 68 (54%) patients were treated by corticosteroids, 47 (37.3%) required high-flow oxygen, 14 (11.1%) patients needed invasive mechanical ventilation, and 2 (1.3%) patients needed ECMO. The proportion of patients treated with corticosteroids and high-flow nasal oxygen increased from 9.8% and 14.6% before September 2020 (wave 1, n = 41) to 75.3% and 48.2%, respectively, for patients admitted after September 1, 2020 (wave 2, n = 85) (all P < 0.001). The proportion of patients who received mechanical ventilation was similar between the two waves (12.2% vs. 10.6%; P = 0.97). For the two patients on ECMO, the first one was on the transplantation list for PH before getting COVID-19, had an emergency lung transplant 22 days after COVID-19 diagnosis (negative PCR result at that time), and is still alive. The second ECMO patient had recently been diagnosed with surgically accessible CTEPH. She progressively deteriorated and died after 17 days on ECMO before surgery. Finally, 14 (11%) patients had right heart failure requiring initiation of vasopressors and/or inotropes.
Outcomes and Mortality
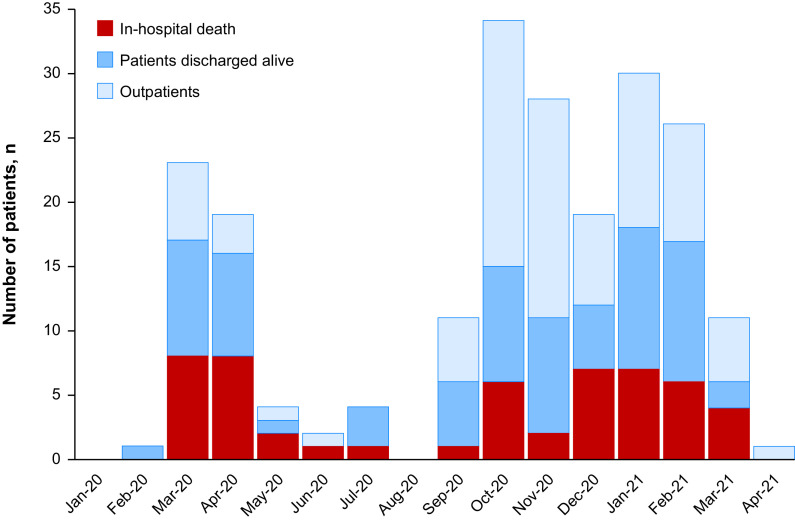
Fifty-two (24.6%; 95% CI, 18.8–30.5%) patients died, which represents 41.3% (95% CI, 32.7–49.9%) of hospitalized patients (Figure 2). Overall mortality was 23% (95% CI, 14.8–31.3%) in PH group 1, 21.3% (95% CI, 8.6–33.0%) in group 4, and 46.3% (95% CI, 31.1–61.6%) in other groups (P = 0.01). All outpatients survived. Among hospitalized patients (n = 126), mortality was 38.2% (95% CI, 26.7–49.8%) in patients managed in hospitals affiliated with the PH network and 43.1% (95% CI, 32.0–57.6%) in patients hospitalized outside of the PH network (P = 0.45) (Table 1). Despite changes in corticosteroids and high-flow nasal oxygen use between the two COVID-19 waves, mortality in hospitalized patients was similar (46.3% and 38.8%, respectively; P = 0.54). Among the 74 patients discharged alive, 4 (5.4%) patients died at 1.8, 5.3, 8.8, and 9.1 months after their diagnosis of COVID-19. The causes of death for these four patients were acute right heart failure for two patients, biventricular heart failure for one patient, and invasive aspergillosis complicated by septic shock after lung transplant in the context of PH for one patient.
Figure 2.
Distribution of occurrence of coronavirus disease (COVID-19) in patients with precapillary pulmonary hypertension. Bars represent the number of patients diagnosed with COVID-19 by month. Red bar: in-hospital deaths; dark blue bar: patients discharged alive; light blue bar: outpatients.
Risk Factors for In-Hospital Mortality
Deceased patients with PH were older and more often male (Table 1). The median age of deceased patients with PH was 69.4 (62.4–77.5) years compared with 61.8 (47.7–70.7) years for those who survived (P < 0.001). Comorbidities were also more frequent among deceased patients compared with those who survived. Deceased patients with PH had more chronic respiratory disease (61.5% vs. 26.4%; P < 0.001), systemic hypertension (53.8% vs. 32.7%; P < 0.01), diabetes (30.8% vs. 16.4%; P = 0.02), and chronic renal failure (51.9% vs. 19.5%; P < 0.001).
Regarding the PH assessment at last evaluation before COVID-19 diagnosis, the deceased patients were more frequently in NYHA FC III/IV and had lower 6MWD and DlCO. Moreover, they more frequently had an increase in BNP or NT-proBNP and higher RAP. There was no difference in the management of specific PH therapy between deceased and alive patients. However, deceased patients were less frequently receiving anticoagulation therapy (42.3% vs. 59.1%; P = 0.03).
In univariable logistic regression, increasing age, male sex, comorbidities, NYHA FC III/IV, and lower 6MWD at last evaluation were associated with higher in-hospital mortality, whereas use of anticoagulation was associated with lower in-hospital mortality (Table 3). Multivariable analyses were limited to a maximum of five independent variables per model given 52 mortality events in the study population. Only variables that were significant in univariable analysis and had complete data were considered. One multivariable model was performed to assess the independent associations between significant demographic factors, NYHA FC III/IV, and anticoagulation treatment with in-hospital mortality (model A), and the other (model B) assessed individual comorbidities with in-hospital mortality. Because there was no association between PH group and mortality, PH group was not included in the multivariable model. In model A, the odds ratio of in-hospital death was 1.04 (95% CI, 1.00–1.07) for increasing age and was 2.46 (95% CI, 1.12–5.40) for male sex. NYHA FC III/IV was not independently associated with in-hospital mortality, and there was a trend toward lower odds of death for patients on anticoagulation (odds ratio, 0.47; 95% CI, 0.21–1.02). In model B, the presence of chronic respiratory disease and chronic renal failure were independently associated with in-hospital death, whereas other cardiac diseases and systemic hypertension were not. The addition of right atrial pressure and elevated BNP or NT-proBNP did not change the associations between comorbid conditions and mortality (see Table E1 in the online supplement).
Table 3.
Association between Prehospitalization Baseline Factors and In-Hospital Mortality (n = 126)
| Variables | n | Univariable |
Multivariable Model A |
Multivariable Model B |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | P Value | Odds Ratio | 95% CI | P Value | Odds Ratio | 95% CI | P Value | ||
| Age (per year) | 126 | 1.04 | 1.01–1.07 | 0.012 | 1.04 | 1.00–1.07 | 0.044 | — | — | — |
| Male sex | — | 2.42 | 1.16–5.07 | 0.019 | 2.46 | 1.12–5.40 | 0.025 | — | — | — |
| PH diagnosis | 126 | — | — | — | — | — | — | — | — | — |
| Group 1 | — | — | — | — | — | — | — | — | — | — |
| Group 4 | — | 1.02 | 0.40–2.60 | 0.962 | — | — | — | — | — | — |
| Other | — | 1.94 | 0.85–4.46 | 0.117 | — | — | — | — | — | — |
| Chronic respiratory diseases | 126 | 2.95 | 1.42–6.16 | 0.004 | — | — | — | 3.44 | 1.53–7.70 | 0.003 |
| Systemic hypertension | 126 | 2.03 | 0.99–4.18 | 0.05 | — | — | — | 1.54 | 0.68–3.50 | 0.30 |
| Other cardiac diseases | 126 | 2.53 | 1.21–5.28 | 0.014 | — | — | — | 2.21 | 0.98–4.98 | 0.055 |
| Diabetes | 126 | 1.90 | 0.83–4.36 | 0.13 | — | — | — | — | — | — |
| Chronic renal failure | 126 | 3.62 | 1.68–7.8 | 0.001 | — | — | — | 2.65 | 1.14–6.17 | 0.024 |
| NYHA FC III/IV (vs. I/II) | 126 | 2.35 | 1.12–4.88 | 0.022 | 1.86 | 0.84–4.10 | 0.12 | — | — | — |
| 6-min-walk distance > 440 m | 100 | 0.24 | 0.08–0.70 | 0.009 | — | — | — | — | — | — |
| Increased BNP or NT-proBNP | 113 | 1.89 | 0.87–4.13 | 0.11 | — | — | — | — | — | — |
| Right atrial pressure (mm Hg) | 120 | 1.05 | 0.99–1.11 | 0.14 | — | — | — | — | — | — |
| Anticoagulation | 126 | 0.47 | 0.23–0.97 | 0.042 | 0.47 | 0.21–1.02 | 0.056 | — | — | — |
Definition of abbreviations: BNP = brain natriuretic peptide; CI = confidence interval; NT-proBNP = N-terminal pro–brain natriuretic peptide; NYHA = New York Heart Association; PH = pulmonary hypertension.
Discussion
This is the first prospective study to describe the clinical features and outcomes of patients with precapillary PH diagnosed with COVID-19. Among 211 patients with PH diagnosed with COVID-19, we observed a high rate of hospitalization (60%) and a high rate of in-hospital mortality (41.3%). Those who died were older, more likely to be male, and had a greater number of comorbidities and more severe PH than those who survived a hospitalization for COVID-19.
The COVID-19 pandemic has had a significant impact on overall and mental health of all patients (17). Patients with PH also reported a high degree of anxiety and fears over the impact of lockdowns on their care (18, 19). An estimated 2.9% of patients in the French PH registry were diagnosed with COVID-19, which is high considering the strict public health measures in France. To date, data on the outcomes of patients with PH who contract COVID-19 are scarce. Several case series described patients with PAH who had favorable outcomes with COVID-19 (20, 21). Because SARS-CoV-2 has detrimental effects on endothelial function, it may be hypothesized the PAH medications, which improve endothelial function, could be protective (10–12). However, our data do not support this hypothesis. In the present study, we observed a high in-hospital mortality rate of 41.3% among patients with precapillary PH, including a 23% in-hospital mortality among those with group 1 PAH. The in-hospital mortality of 41% for patients with PH is more than double the overall estimated in-hospital mortality of 18.8% due to COVID-19 in France (22, 23). In addition to the deleterious effects of COVID-19 on the pulmonary vasculature (24), there is a strong relationship between right ventricular (RV) dysfunction and death in all comers who are hospitalized with COVID-19 (25). In a systematic review and meta-analysis of 29 studies, the presence of RV dysfunction was associated with a more than threefold increased odds of all-cause mortality (25). Markers of RV dysfunction are well-established predictors of mortality in patients with precapillary PH (26–30). On the basis of elevated BNP or NT-proBNP concentrations, RV dysfunction was present in approximately half of patients with PH in our study and was more frequent among those who died in-hospital than those who survived to discharge. Although the association between elevated BNP or NT-proBNP concentration at last evaluation with in-hospital outcomes was not significant in the univariable analysis, this may have been owing to missing data for 10% of patients or the interval between last evaluations and COVID-19 admission, which would not be reflective of RV function at the time of COVID-19 infection. Of the patients who developed clinical RV failure in hospital, 84% had an elevated BNP or NT-proBNP at their last evaluation. Thus, the high prevalence of preexisting RV dysfunction in patients with PH is likely an important factor mediating COVID-19 outcomes.
Patients who died in our cohort were also predominantly male. Male sex was independently associated with in-hospital mortality after adjustment for age, NYHA FC, and use of anticoagulation. Although the sex ratio is equal among confirmed COVID-19 cases, male sex is a known risk factor for mortality in COVID-19 (31, 32) and is also an independent risk factor for mortality in PAH (33). Male sex and RV dilation were both independent predictors of in-hospital death or need for ICU admission in a multicenter study of more than 2,800 hospitalized patients with COVID-19 in France (34). Hormonal factors and sex differences in RV function and adaptation in acute illness are interesting potential explanations for sex disparities in outcomes in COVID-19 that warrant further study. Of note, PH group was not statistically associated with prognosis in the univariate analysis. Conversely, comorbidities, including chronic respiratory disease, systemic hypertension, cardiac disease, and chronic renal failure, were associated with prognosis in univariate and multivariate analysis. These results confirm that the main driven prognostic factor were the comorbidities rather than PH group, although we acknowledge that the presence and severity of comorbidities may have influenced the original classification of PH.
Nearly half of hospitalized patients were admitted to an intensive care setting; however, only 11% of patients required invasive mechanical ventilation. This is similar to the overall rates of invasive ventilation for hospitalized patients reported in other studies from the early months of the pandemic (32, 35–37). A considerable proportion of patients (24.2%) who were hospitalized and nearly half of those who died had documented limitations to care escalation (e.g., do-not-resuscitate or do-not-intubate orders). Because of the risks associated with intubation and mechanical ventilation in patients with PH, especially those with RV dysfunction, this is typically avoided, especially for older patients with PH and those with severe PH. Certain jurisdictions have recommended PH risk stratification in critical care resource triage during COVID-19 surges (38). In this study, it mainly consists of limitation of intubation, invasive ventilation, and/or cardiac resuscitation in patients with severe underlying diseases and severe COVID-19 with limited possibility of improvement. As no recommendations have been proposed regarding limitation of care in patients with PH patients, current practice is a case-by-case decision based on the severity of the underlying diseases (PH and comorbidities) and the severity of COVID-19 and its possibility of recovery. The better understanding of risk factors, prognostic factors, and less invasive management (such as high-flow nasal oxygen) of COVID-19 may have progressively changed the practice of clinicians. This pandemic highlights the importance of including discussions in future guidelines on the advance care directives limiting care for patients with PH. Nevertheless, approximately one-third of patients who were intubated (35.7%), half of those on high-flow oxygen (51.1%), and one of two who received ECMO survived. Thus, in the absence of severe resource constraints during a surge, critical care and advanced life support measures should not be categorically withheld for patients with PH who have a reasonable chance of recovery or who may be candidates for lung transplantation.
Approximately 54% of patients in our study received corticosteroids, the majority of whom were admitted after September 1, 2020, as data on the efficacy of dexamethasone for COVID-19 were not published until July 2020 (39). There was no difference in corticosteroid use between those who died in hospital and those who survived in our study. This is not surprising, as the absolute risk reduction in death with dexamethasone is relatively small (2.8%), so it is unlikely we would detect a small but important difference in this study. Furthermore, 30% of in-hospital deaths occurred in patients with mild parenchymal abnormalities on CT, suggesting that mortality was related to PH in these cases, rather than the severity of COVID-19.
Severe COVID-19 infection is associated with pulmonary vascular endothelialitis, thrombosis, and coagulopathy (40, 41). In unselected patients undergoing systematic CT pulmonary angiogram imaging at the time of hospitalization for COVID-19, the prevalence of acute pulmonary embolism was of 14.2% (95% CI, 7.5–20.8) (42, 43). A recent meta-analysis reported a high risk of venous thromboembolic event during COVID-19 of 14.7%, which is even higher among critically ill patients (23.2%) (44). Although acute pulmonary embolism was rare in our cohort (4% of hospitalized patients), patients did not undergo systematic CT pulmonary angiogram. Baseline use of anticoagulation was also high (55%), as it is indicated for patients with CTEPH and often considered for patients with idiopathic, heritable, or anorexigen-induced PAH (9). Anticoagulation use may influence the decision to order a CT pulmonary angiogram in deteriorating patients, so it is possible that acute pulmonary embolism was underdiagnosed in the anticoagulated group. Nevertheless, anticoagulation use was higher among those who survived to discharge than those who died in hospital with a signal that anticoagulation may be associated with better in hospital outcomes. Some, but not all, studies have suggested a benefit of therapeutic anticoagulation in preventing adverse outcomes from COVID-19, particularly in noncritically ill patients (45–47). Given the effects of COVID-19 on the pulmonary vasculature and poor RV reserve of patients with PH, it is conceivable that anticoagulation protected a considerable number of patients from superimposed acute pulmonary embolism and prevented additional adverse outcomes. It would be logical to consider a CT pulmonary angiogram for all patients with PH hospitalized for COVID-19, especially those who are not anticoagulated, considering the high prevalence of pulmonary embolism in severe COVID-19.
This study was conducted before the availability of COVID-19 vaccines. Vaccination is important at the population level to prevent severe COVID-19 outcomes but is evidently critical for the PH population who are at higher risk of severe illness and death due to COVID-19. Our results underscore the essential role of preventative measures such as masking, physical distancing, and vaccination for patients with PH and those around them. We anticipate that higher vaccination rates in France will have a positive impact on COVID-19–related outcomes among patients with PH going forward. Other novel therapeutics, such as monoclonal antibodies, also present an opportunity for early treatment for these high-risk patients, even if access is limited.
Strengths of this study include the prospective and multicenter design and systematic follow-up for in-hospital and outpatient outcomes. We acknowledge several limitations, such as the relatively low proportion of patients who received corticosteroids in this cohort, which is now widespread for hypoxemic hospitalized patients to reduce mortality. First, we cannot exclude that mild or asymptomatic cases of COVID-19 were underdiagnosed in our cohort of patients with PH. However, large-scale testing was deployed in France during this period, and educational communications to patients with rare diseases (including PH) were widely disseminated. In our cohort, 2.7% of patients with PH were diagnosed with COVD-19 during the first year of the pandemic. During the same period, 5.61 million people were diagnosed with COVID-19 in France, which represents 8.3% of the entire population. As patients at high risk, such as those with PH, followed stricter protective and isolating measures than general population, we can hypothesize that we still captured a fairly complete collection of COVID-19 cases. Second, a significant proportion of patients had decisions made to limit escalation to invasive critical care (e.g., intubation or cardiopulmonary resuscitation), which was decided at the time of diagnosis of the acute episode of COVID-19. These decisions were made based on the severity of COVID-19 and prognosis of the underlying disease in discussion with patients and families. Of note, this may influence the very poor prognosis of COVID-19 in patients with PH, but this study reports the landscape of COVID-19 in PH that clinicians are facing in real life. Third, for this study, we reported the most critical service (conventional wards or critical care) in which the patient was managed during this hospitalization. Management was at the discretion of physicians, as no guidelines were available for the management of COVID-19 in patients with PH. Indeed, the survey was not tailored to catch the detailed care journey of the patient, and thus, it is not possible to evaluate the impact of an early intervention in ICU on outcomes.
In conclusion, patients with chronic precapillary PH who contract COVID-19 have a high rate of hospitalization and high rate of in-hospital mortality. Risk factors for adverse outcomes in patients with PH diagnosed with COVID-19 include older age, male sex, comorbidities, and more severe PH. Anticoagulation was the only background treatment associated with lower mortality among hospitalized patients.
Acknowledgments
French PH Network PULMOTENSION Investigators: Ahmad Kais, Université Lyon-1, Hospices Civils de Lyon, Centre de Référence des Maladies Pulmonaires Rares, Centre de Compétences de l'Hypertension Pulmonaire, Hôpital Louis Pradel, Lyon, France; Artaud-Macari Elise, Département de pneumologie, Hôpital universitaire, Rouen, France; Chabanne Céline, CHU de Rennes, Service de Cardiologie et Maladies Vasculaires, INSERM U1099, Rennes, France; Chaouat Ari, Université de Lorraine, CHU Nancy, Pôle des Spécialités Médicales, Département de Pneumologie, Vandoeuvre-lès-Nancy, France; Dauphin Claire, Service de cardiologie, Hôpital Gabriel Montpied, CHU, Clermont-Ferrand, France; Gagnadoux Frédéric, Université d'Angers, Angers University Hospital, Department of Respiratory and Sleep Medicine, INSERM UMR1063, Angers, France; Gaudoin Anne, Pneumology Department, Besançon University Hospital, Besançon, France; Hascoet Sébastien, Service de cardiopathie congénitale de l'enfant et de l'adulte, Hôpital Marie-Lannelongue, Le Plessis-Robinson, France; Horeau-Langlard Delphine, Service de pneumologie, Hôpital Laënnec, Centre Hospitalier Universitaire de Nantes, Nantes, France; Inamo Jocelyn, Département de Cardiologie, Centre Hospitalier Universitaire de Martinique, France; Lamia Bouchra, Service de pneumologie, Normandie Université, UNIROUEN, EA 3830, CHU de Rouen et Groupe Hospitalier du Havre, France; Magro Pascal, Service de Pneumologie, Centre Hospitalier Universitaire de Tours, Tours, France; Meurice Jean-Claude, Université de Poitiers, CHU de Poitiers, Service de pneumologie, INSERM CIC 1402, Poitiers, France; Poubeau Patrice, Service de Médecine Interne, Centre Hospitalier Universitaire de la Réunion, Saint-Pierre de la Réunion, France. Prevot Grégoire, Service de Pneumologie, Hôpital Larrey, Toulouse, France; Rosario Roger, Hôpital Privé St. Joseph, Marseille, France; and Servettaz, Amelie, Centre Hospitalier Universitaire de Reims, Service de Médecine Interne, Maladies Infectieuses et Immunologie Clinique, Hôpital Robert Debré, Reims, France.
Footnotes
A complete list of French PH Network PULMOTENSION Investigators may be found before the beginning of the References.
Author Contributions: D.M., M.-C.C., J.W., X.J., L.S., M.H., and O.S designed the initial concept. D.M., M.-C.C., X.J., S.B., E.N.-S., A.N., S.R., J.T., H.B., M.R., P.d.G., P.M., L.B., N.F., A.G., E.-M.J., A. Beurnier, A. Boucly, N.E., M.J., J.P., S.K., M.P., A.R., S.S., A.S., M.R.-G., V.C., and L.S. participated in acquisition and interpretation of data. D.M. and J.W. performed the statistical analysis. D.M., J.W., M.-C.C., X.J., L.S., M.H., and O.S. wrote the manuscript. All authors reviewed the manuscript.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202112-2761OC on May 12, 2022
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
Collaborators: Ahmad Kais, Artaud-Macari Elise, Chabanne Céline, Chaouat Ari, Dauphin surnameClaire, Gagnadoux Frédéric, Gaudoin Anne, Hascoet Sébastien, Horeau-Langlard Delphine, Inamo Jocelyn, Lamia Bouchra, Magro Pascal, Meurice Jean-Claude, Poubeau Patrice, Prevot Grégoire, Rosario Roger, and Servettaz
References
- 1.World Health Data Platform. https://www.who.int/data
- 2. Huertas A, Montani D, Savale L, Pichon J, Tu L, Parent F, et al. Endothelial cell dysfunction: a major player in SARS-CoV-2 infection (COVID-19)? Eur Respir J . 2020;56:2001634. doi: 10.1183/13993003.01634-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet . 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nicosia RF, Ligresti G, Caporarello N, Akilesh S, Ribatti D. COVID-19 vasculopathy: mounting evidence for an indirect mechanism of endothelial injury. Am J Pathol . 2021;191:1374–1384. doi: 10.1016/j.ajpath.2021.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, et al. Global COVID-19 Thrombosis Collaborative Group, Endorsed by the ISTH, NATF, ESVM, and the IUA, Supported by the ESC Working Group on Pulmonary Circulation and Right Ventricular Function COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up: JACC state-of-the-art review. J Am Coll Cardiol . 2020;75:2950–2973. doi: 10.1016/j.jacc.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sims JT, Krishnan V, Chang C-Y, Engle SM, Casalini G, Rodgers GH, et al. Characterization of the cytokine storm reflects hyperinflammatory endothelial dysfunction in COVID-19. J Allergy Clin Immunol . 2021;147:107–111. doi: 10.1016/j.jaci.2020.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Humbert M, Guignabert C, Bonnet S, Dorfmüller P, Klinger JR, Nicolls MR, et al. Pathology and pathobiology of pulmonary hypertension: state of the art and research perspectives. Eur Respir J . 2019;53:1801887. doi: 10.1183/13993003.01887-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sitbon O, Gomberg-Maitland M, Granton J, Lewis MI, Mathai SC, Rainisio M, et al. Clinical trial design and new therapies for pulmonary arterial hypertension. Eur Respir J . 2019;53:1801908. doi: 10.1183/13993003.01908-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Galiè N, Humbert M, Vachiery J-L, Gibbs S, Lang I, Torbicki A, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT) Eur Respir J . 2015;46:903–975. doi: 10.1183/13993003.01032-2015. [DOI] [PubMed] [Google Scholar]
- 10. Nuche J, Pérez-Olivares C, Segura de la Cal T, Jiménez López-Guarch C, Arribas Ynsaurriaga F, Escribano Subías P. Clinical course of COVID-19 in pulmonary arterial hypertension patients. Rev Esp Cardiol (Engl Ed) . 2020;73:775–778. doi: 10.1016/j.rec.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Belge C, Quarck R, Godinas L, Montani D, Escribano Subias P, Vachiéry JL, et al. COVID-19 in pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension: a reference centre survey. ERJ Open Res . 2020;6:00520-2020. doi: 10.1183/23120541.00520-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Franco V, Bradley EA, Badagliacca R, Sabanayagam A, Rajpal S, Lastinger LT, et al. Pulmonary vasodilators: beyond the bounds of pulmonary arterial hypertension therapy in COVID-19. Pulm Circ . 2020;10:2045894020970369. doi: 10.1177/2045894020970369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nadkarni GN, Lala A, Bagiella E, Chang HL, Moreno PR, Pujadas E, et al. Anticoagulation, bleeding, mortality, and pathology in hospitalized patients with COVID-19. J Am Coll Cardiol . 2020;76:1815–1826. doi: 10.1016/j.jacc.2020.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sulica R, Cefali F, Motschwiller C, Fenton R, Barroso A, Sterman D. COVID-19 in pulmonary artery hypertension (PAH) patients: observations from a large PAH center in New York City. Diagnostics (Basel) . 2021;11:128. doi: 10.3390/diagnostics11010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Remy-Jardin M, Ryerson CJ, Schiebler ML, Leung ANC, Wild JM, Hoeper MM, et al. Imaging of pulmonary hypertension in adults: a position paper from the Fleischner Society. Eur Respir J . 2021;57:2004455. doi: 10.1183/13993003.04455-2020. [DOI] [PubMed] [Google Scholar]
- 16. Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J . 2019;53:1801913. doi: 10.1183/13993003.01913-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. COVID-19 Mental Disorders Collaborators. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet . 2021;398:1700–1712. doi: 10.1016/S0140-6736(21)02143-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wieteska-Miłek M, Szmit S, Florczyk M, Kuśmierczyk-Droszcz B, Ryczek R, Dzienisiewicz M, et al. Fear of COVID-19, anxiety and depression in patients with pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension during the pandemic. J Clin Med . 2021;10:4195. doi: 10.3390/jcm10184195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Godinas L, Iyer K, Meszaros G, Quarck R, Escribano-Subias P, Vonk Noordegraaf A, et al. PH CARE COVID survey: an international patient survey on the care for pulmonary hypertension patients during the early phase of the COVID-19 pandemic. Orphanet J Rare Dis . 2021;16:196. doi: 10.1186/s13023-021-01752-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Scuri P, Iacovoni A, Abete R, Cereda A, Grosu A, Senni M. An unexpected recovery of patients with pulmonary arterial hypertension and SARS-CoV-2 pneumonia: a case series. Pulm Circ . 2020;10:2045894020956581. doi: 10.1177/2045894020956581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mandler D, Lichtblau M, Ulrich S. The course of COVID-19 in a 55-year-old patient diagnosed with severe idiopathic pulmonary arterial hypertension. Pulm Circ . 2020;10:2045894020936659. doi: 10.1177/2045894020936659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ouattara E, Bruandet A, Borde A, Lenne X, Binder-Foucard F, Le-Bourhis-Zaimi M, et al. Risk factors of mortality among patients hospitalised with COVID-19 in a critical care or hospital care unit: analysis of the French national medicoadministrative database. BMJ Open Respir Res . 2021;8:e001002. doi: 10.1136/bmjresp-2021-001002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Morin L, Savale L, Pham T, Colle R, Figueiredo S, Harrois A, et al. Writing Committee for the COMEBAC Study Group Four-month clinical status of a cohort of patients after hospitalization for COVID-19. JAMA . 2021;325:1525–1534. doi: 10.1001/jama.2021.3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Potus F, Mai V, Lebret M, Malenfant S, Breton-Gagnon E, Lajoie AC, et al. Novel insights on the pulmonary vascular consequences of COVID-19. Am J Physiol Lung Cell Mol Physiol . 2020;319:L277–L288. doi: 10.1152/ajplung.00195.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Corica B, Marra AM, Basili S, Cangemi R, Cittadini A, Proietti M, et al. Prevalence of right ventricular dysfunction and impact on all-cause death in hospitalized patients with COVID-19: a systematic review and meta-analysis. Sci Rep . 2021;11:17774. doi: 10.1038/s41598-021-96955-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Weatherald J, Boucly A, Chemla D, Savale L, Peng M, Jevnikar M, et al. Prognostic value of follow-up hemodynamic variables after initial management in pulmonary arterial hypertension. Circulation . 2018;137:693–704. doi: 10.1161/CIRCULATIONAHA.117.029254. [DOI] [PubMed] [Google Scholar]
- 27. Prins KW, Rose L, Archer SL, Pritzker M, Weir EK, Kazmirczak F, et al. Disproportionate right ventricular dysfunction and poor survival in group 3 pulmonary hypertension. Am J Respir Crit Care Med . 2018;197:1496–1499. doi: 10.1164/rccm.201712-2405LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hu S, Tan J-S, Liu S, Guo T-T, Song W, Peng F-H, et al. Development and validation of a nomogram for predicting the long-term survival in patients with chronic thromboembolic pulmonary hypertension. Am J Cardiol . 2021;163:109–116. doi: 10.1016/j.amjcard.2021.09.045. [DOI] [PubMed] [Google Scholar]
- 29. Delcroix M, Staehler G, Gall H, Grünig E, Held M, Halank M, et al. Risk assessment in medically treated chronic thromboembolic pulmonary hypertension patients. Eur Respir J . 2018;52:1800248. doi: 10.1183/13993003.00248-2018. [DOI] [PubMed] [Google Scholar]
- 30. Weatherald J, Boucly A, Launay D, Cottin V, Prévot G, Bourlier D, et al. Haemodynamics and serial risk assessment in systemic sclerosis associated pulmonary arterial hypertension. Eur Respir J . 2018;52:1800678. doi: 10.1183/13993003.00678-2018. [DOI] [PubMed] [Google Scholar]
- 31. Peckham H, de Gruijter NM, Raine C, Radziszewska A, Ciurtin C, Wedderburn LR, et al. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat Commun . 2020;11:6317. doi: 10.1038/s41467-020-19741-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW, et al. the Northwell COVID-19 Research Consortium Presenting Characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA . 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cheron C, McBride SA, Antigny F, Girerd B, Chouchana M, Chaumais MC, et al. Sex and gender in pulmonary arterial hypertension. Eur Respir Rev . 2021;30:200330. doi: 10.1183/16000617.0330-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Soulat-Dufour L, Fauvel C, Weizman O, et al. Prognostic value of right ventricular dilatation in patients with COVID-19: a multicentre study. Eur Heart J Cardiovasc Imaging . 2022;23:569–577. doi: 10.1093/ehjci/jeab067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wang D, Hu B, Hu C, Zhu F, Liu X, Zhang J, et al. clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA . 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Argenziano MG, Bruce SL, Slater CL, Tiao JR, Baldwin MR, Barr RG, et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: retrospective case series. BMJ . 2020;369:m1996. doi: 10.1136/bmj.m1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet . 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gupta S, Batt J, Bourbeau J, Chapman KR, Gershon A, Granton J, et al. Triaging access to critical care resources in patients with chronic respiratory diseases in the event of a major COVID-19 surge: key highlights from the Canadian Thoracic Society (CTS) position statement. Chest . 2020;158:2270–2274. doi: 10.1016/j.chest.2020.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. RECOVERY Collaborative Group Dexamethasone in hospitalized patients with Covid-19. N Engl J Med . 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med . 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Iba T, Levy JH, Levi M, Thachil J. Coagulopathy in COVID-19. J Thromb Haemost . 2020;18:2103–2109. doi: 10.1111/jth.14975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jevnikar M, Sanchez O, Chocron R, Andronikof M, Raphael M, Meyrignac O, et al. Prevalence of pulmonary embolism in patients with COVID-19 at the time of hospital admission. Eur Respir J . 2021;58:2100116. doi: 10.1183/13993003.00116-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Suh YJ, Hong H, Ohana M, Bompard F, Revel MP, Valle C, et al. Pulmonary embolism and deep vein thrombosis in COVID-19: a systematic review and meta-analysis. Radiology . 2021;298:E70–E80. doi: 10.1148/radiol.2020203557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tan BK, Mainbourg S, Friggeri A, Bertoletti L, Douplat M, Dargaud Y, et al. Arterial and venous thromboembolism in COVID-19: a study-level meta-analysis. Thorax . 2021;76:970–979. doi: 10.1136/thoraxjnl-2020-215383. [DOI] [PubMed] [Google Scholar]
- 45.Lopes RD, de Barros E Silva PGM, Furtado RHM, Macedo AVS, Bronhara B, Damiani LP, et al. ACTION Coalition COVID-19 Brazil IV Investigators Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer concentration (ACTION): an open-label, multicentre, randomised, controlled trial. Lancet. 2021;397:2253–2263. doi: 10.1016/S0140-6736(21)01203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lawler PR, Goligher EC, Berger JS, Neal MD, McVerry BJ, Nicolau JC, et al. ATTACC Investigators ACTIV-4a Investigators; REMAP-CAP Investigators. Therapeutic anticoagulation with heparin in noncritically ill patients with Covid-19. N Engl J Med . 2021;385:790–802. doi: 10.1056/NEJMoa2105911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Goligher EC, Bradbury CA, McVerry BJ, Lawler PR, Berger JS, Gong MN, et al. REMAP-CAP Investigators ACTIV-4a Investigators; ATTACC Investigators. Therapeutic anticoagulation with heparin in critically ill patients with Covid-19. N Engl J Med. 2021;385:777–789. doi: 10.1056/NEJMoa2103417. [DOI] [PMC free article] [PubMed] [Google Scholar]