The Respiratory Microbiome: An Important and Overlooked Source of Biological and Clinical Heterogeneity
Culture-independent microbiology has transformed our understanding of the respiratory microbiome in health and disease (1). Enabled by advances in next-generation sequencing and other technologies, we now appreciate the complexity of microbial communities within the respiratory tract, together with their metabolic, immunologic, and pathophysiologic consequences. Across the spectrum of acute and chronic respiratory disease, respiratory microbiota are detectable, viable, and variable across patients (2–5); correlated with disease status and severity (1); associated with airway and alveolar inflammation (5, 6); metabolically active and immunologically consequential (7, 8); predictive of clinical outcomes (9–11); influenced by environment and geography (6, 12); and causally involved in disease pathogenesis in animal models (11, 13).
Given these insights, we believe it is prudent to consider the respiratory microbiome as an unexploited, understudied therapeutic target: a biologically potent element of respiratory homeostasis, variable across patients, that may be more readily modifiable than other sources of patient heterogeneity, such as host genomes or comorbidities. In this Perspective, we delineate the anticipated opportunities and challenges related to clinically modulating the respiratory microbiome, which refers to the communities of microbes within the human respiratory tract and their associated ecological milieu. Our scope includes both acute and chronic respiratory diseases. For a broader discussion of the state of the field, including issues related to sampling, sequencing, analysis, and interpretation, we direct readers to recent reviews and monographs (1, 14–16).
The Rationale: Why Target Respiratory Microbiota?
Although the utility of targeting respiratory microbiota in infectious lung diseases is intuitive, the notion of therapeutically modulating respiratory microbiota in noninfectious lung disease requires justification. Indeed, the past decade of research has partially blurred long-held distinctions between unambiguously infectious lung disease (e.g., bacterial pneumonia), infection-prone lung disease (e.g., bronchiectasis with periodic exacerbations), and noninfectious lung disease (e.g., idiopathic pulmonary fibrosis), with even the latter category now understood to potentially involve respiratory microbiota in its pathogenesis (10, 11, 17). Here, we review 1) evidence that respiratory microbiota play a causal role in disease pathogenesis; 2) evidence that respiratory microbiota are altered by our clinical interventions (necessary to define it as a treatable trait); and 3) evidence that respiratory microbiota play a role distinct from that of gut microbiota in lung biology and are thus worthy of their own consideration for therapeutic manipulation.
Evidence That Respiratory Microbiota Participate in Disease Pathogenesis
Although associations between airway microbiome characteristics and respiratory health are now common, demonstration of causality remains limited. From a microbial perspective, airways and alveoli that have been structurally or functionally altered by disease represent radically altered ecosystems compared with healthy states; thus, variation in the respiratory microbiome may represent a consequence of disease rather than its cause. Yet, evidence for the plausibility of causal roles—shown via animal modeling—has grown. Animal models demonstrate that the microbiome mediates lung inflammation and mortality in oxygen-induced lung injury (13) and pulmonary fibrosis (11), and commensal respiratory bacteria are protective in models of viral respiratory infections (18, 19). In cystic fibrosis, nonpathogenic oropharyngeal microbes increase the virulence of Pseudomonas aeruginosa (20). Such indirect effects of microbes on lung pathophysiology may contribute to treatment-associated benefit. For example, although inhaled tobramycin is prescribed to target chronic P. aeruginosa infection in cystic fibrosis, treatment provokes marked changes in nondominant lung microbes but minimal effect on predominant pathogens (21). In patients with chronic obstructive pulmonary disease (COPD), azithromycin treatment enriches bacterial-derived antiinflammatory metabolites, providing mechanistic plausibility to a causal role of the respiratory microbiota (7). Finally, in bronchiectasis, a key feature of the respiratory microbiome (bacterial load) identifies patients who benefit from inhaled antibiotics (22). Similarly, in asthma, baseline features of the bronchial microbiome discriminate which patients derive physiologic improvements after clarithromycin or inhaled corticosteroid intervention (23, 24). Although still indirect, this clinical evidence of heterogeneity of treatment effect (differential treatment responses based on patient variation in respiratory microbiota) lends support for a causal role for respiratory microbiota in disease pathogenesis.
Importantly, the role of airway microbiota in respiratory disease is not always detrimental. Using in vitro and in vivo models, Rothia mucilaginosa, an oral commensal frequently found in the lower airways in chronic disease, inhibits pathogen- and endotoxin-induced proinflammatory responses (25). Similarly, Prevotella dampens inflammation in P. aeruginosa infections (26). In animal models, modulation of the lung microbiome by instillation of human oropharyngeal bacteria provokes persistent T-helper cell type 17 responses, conferring persistent protection against streptococcal pneumonia (27). These animal observations, taken with now-abundant correlative human findings linking lung microbiota with poor prognosis (9–11, 17, 28) or adverse diagnoses (29–31), support the clinical and pathophysiologic significance of respiratory microbiota.
Evidence That Lung Microbiota Are Modulated by Clinical Exposures
Secondary analysis of randomized controlled trials (RCTs) clearly demonstrates that respiratory microbiota are altered by clinical exposures, including antibiotics (7, 21, 32, 33) and nonantibiotic interventions altering the lower respiratory tract environment (e.g., inhaled corticosteroids [24, 34] and CFTR modulators [35]). The critical question for the field is thus not whether we can alter respiratory microbiota but rather how are we already doing so with existing interventions and to what extent such changes inform disease and recovery. Although in murine models viable bacteria can be successfully instilled into the lower respiratory tract with persisting protective effects (27), no human study to date has determined the feasibility of respiratory probiotic therapy targeting the lower respiratory tract.
Why Target Respiratory as Opposed to Gut Microbiota?
In many respects, the gut microbiome offers a compelling alternative therapeutic target: it is more accessibly sampled and studied, it is far greater in biomass and metabolic activity, and we now have years of experience modulating it with antibiotics, probiotics, and fecal microbiota transplantation. Despite this, time-matched gut and lung specimens from animal experiments reveal that lung microbiota are consistently more tightly correlated with variation in lung immunity than are gut communities (6, 13). Experimental modulation of lung microbiota directly and persistently alters the lung immune response (8, 27), whereas the opposite interaction (the effect of immune modulation on lung microbiota) is either weaker or absent (27, 36). Thus, the supposition that lung immune tone is constantly and continuously calibrated in response to the local (pulmonary) microbial milieu is both evolutionarily plausible and empirically supported. A plausible interpretation of the literature to date is that gut and respiratory microbiota influence distinct aspects of lung immunity, and therefore neither microbial compartment should be ignored. Finally, select respiratory microbiota are known to influence host biology directly via their behavior (e.g., quorum sensing, biofilm formation, and toxin production), arguing for the importance of local microbiome–host interactions within the respiratory tract.
Efforts to Date to Modulate Lung Microbiota
Only a small number of RCTs have attempted to modulate the microbiota for therapeutic benefit in respiratory disease. These have used enteric probiotics and inhaled and systemic antibiotics. Although most have used clinical or culture-based outcomes, few have included microbiome-specific endpoints and analyses.
Although oral probiotics have been trialed as therapeutic interventions in cystic fibrosis (37) and ventilator-associated pneumonia (VAP) (38), these have deliberately targeted lower intestinal microbiota rather than respiratory communities, and therefore effects on respiratory microbiota remain undetermined. No human studies to date have assessed respiratory probiotics (i.e., viable microbiota instilled or aerosolized into the lower respiratory tract). Similarly, bacteriophage therapy directed at potentially pathogenic airway bacteria may have a role based on emerging evidence in the gut (39), although experience remains anecdotal for the respiratory tract (40). It has been established that inhaled antibiotic therapies modulate the respiratory microbiota, including reducing bacterial load (22, 32).
Several studies have used systemic antibiotics to modulate respiratory microbiota. Early studies of oral cotrimoxazole in idiopathic pulmonary fibrosis (IPF) showed mixed results for clinical pulmonary endpoints (41, 42). Although one study suggested a possible mortality benefit (42), the larger the efficacy and mechanism evaluation of treating idiopathic pulmonary fibrosis with the addition of co-trimoxazole (EME-TIPAC) study found no improvement in clinical outcomes (43). Clinical efficacy of antimicrobial therapy strategy using pragmatic design in Idiopathic Pulmonary Fibrosis (CleanUP-IPF) took a pragmatic approach: subjects were randomized to cotrimoxazole or doxycycline versus usual care (44). The study terminated early because of futility, with no improvement in clinical outcomes. Unfortunately, no microbial endpoints were reported for EME-TIPAC or CleanUP-IPF. It thus remains unknown whether systemic antibiotics consistently modulate lung microbiota or whether specific subgroups (e.g., patients with IPF with high bacterial load) may respond to targeted antibiotic use.
Several studies of systemic antibiotic use have reported lung microbiome outcomes, enhancing our understanding of mechanisms underlying clinical findings (7, 45). The Bronchiectasis and Low-dose Erythromycin (BLESS) study determined that long-term use of erythromycin decreased exacerbation rates (46) and modified sputum microbiota (47) in non–cystic fibrosis bronchiectasis. Importantly, the clinical and microbiologic effects of erythromycin therapy differed depending on whether P. aeruginosa was the dominant taxon in sputum (47). Further work demonstrated that erythromycin use increases resistance gene copy number (48) and may decrease P. aeruginosa pathogenicity by inhibiting quorum sensing (49).
These insights derived from analyses of the BLESS study illustrate the value of characterizing the effects of interventions on respiratory microbiota in clinical trials, as clinical outcomes may differ depending on baseline microbiota. BLESS identified a subset of patients most likely to benefit from therapy, identified risks of long-term antibiotic use, and delineated a plausible microbiologic mechanism of action. This exemplar study should inform design of future RCTs aimed at modifying lung microbiota: future trials to modulate respiratory microbiota will be most informative if they are paired with coordinated efforts to characterize and interrogate the microbiome itself.
Practical Issues Related to Modulating the Respiratory Microbiome
If we aspire to modulate the respiratory microbiome as a therapeutic target, numerous practical challenges must be addressed and resolved (Table 1 and Figure 1). We believe that any therapeutic trials at respiratory microbiome modulation are unlikely to succeed until these barriers are overcome.
Table 1.
Opportunities and Challenges Related to Therapeutic Targeting of the Respiratory Microbiome and Key Research Questions for the Field
| Barrier | Opportunities | Challenges | Research Questions |
|---|---|---|---|
| Barrier 1: We need to identify specific microbiologic and ecologic “targets” for therapeutic modulation |
|
|
|
| Barrier 2: An improved understanding of the efficacy and persistence of respiratory microbiome–modulating interventions is required |
|
|
|
| Barrier 3: Clinicians need to be able to access, understand, and use microbiome data for subphenotyping patients |
|
|
|
| Barrier 4: Specific concerns in special populations |
|
|
|
Figure 1.
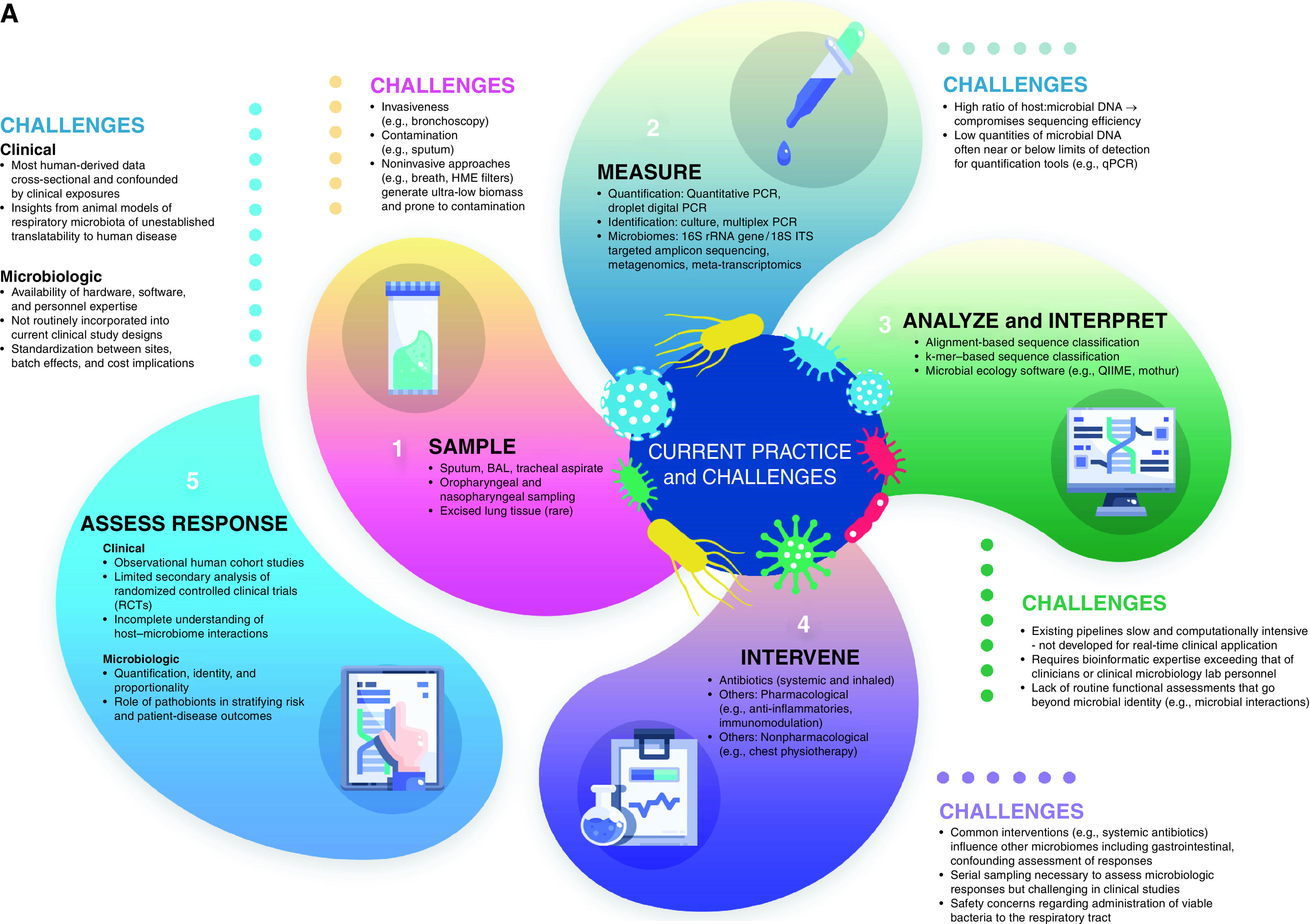
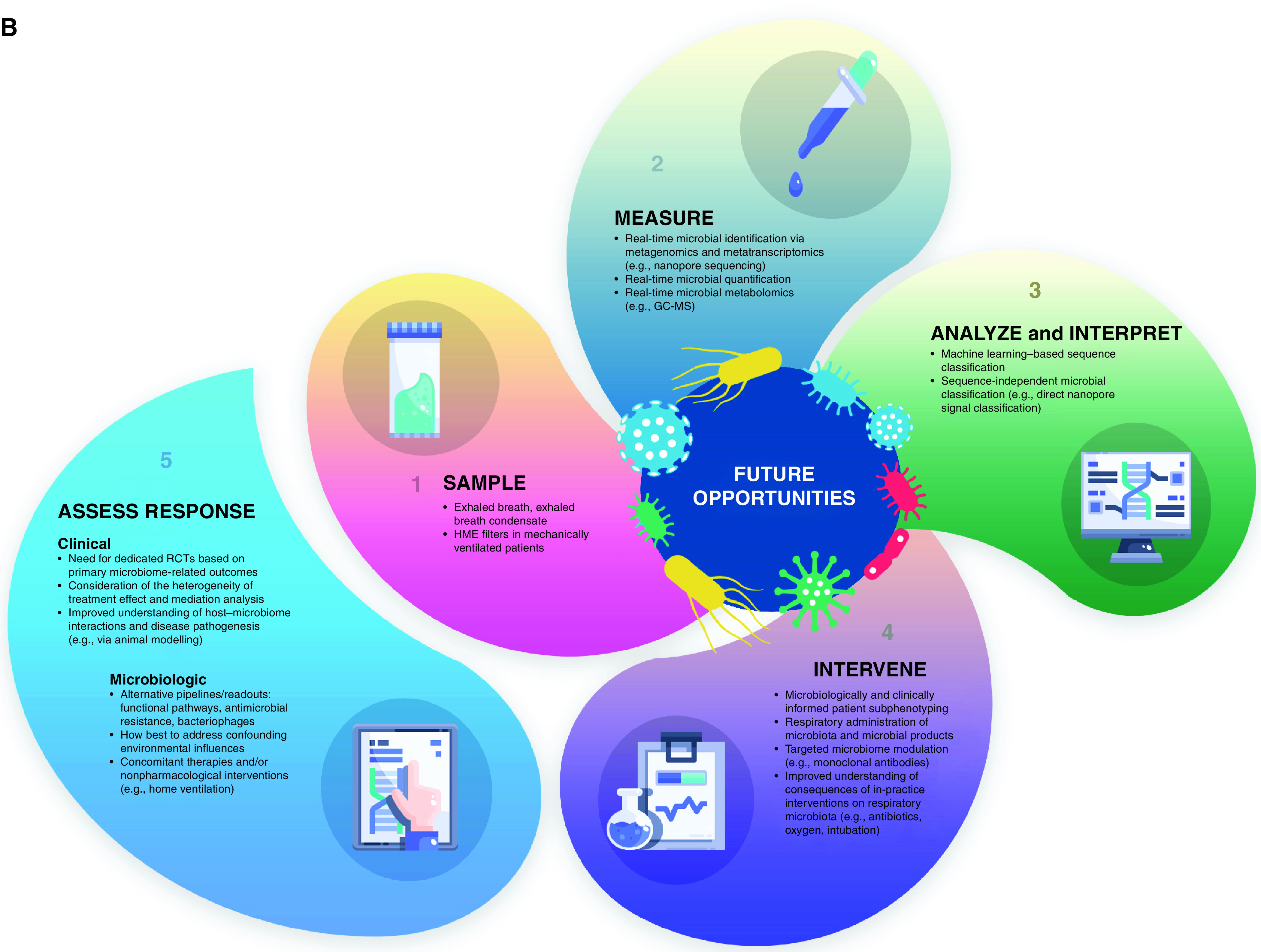
Therapeutic targeting of the respiratory microbiome: (A) current practice and challenges and (B) future opportunities. To therapeutically modulate the respiratory microbiome effectively, we must advance our means of sampling, measuring, analyzing, interpreting, and intervening on respiratory microbiota. Progress requires a rapid and accurate assessment of the microbiologic and clinical response of interventions. GC-MS = gas chromatography–mass spectrometry; HME = heat and moisture exchange; ITS = internal transcribed spacer; QIIME = quantitative insights into microbial ecology; qPCR = quantitative PCR.
Barrier 1: We Need to Identify Specific Microbiologic and Ecologic Targets for Therapeutic Modulation
In infectious lung disease, the microbiologic goal of treatment is clear: eradicate or reduce the respiratory burden of pathogens (either suspected or confirmed). In contrast, the target of intervention for respiratory microbiome therapies is less straightforward. Modulating a complex and dynamic microbial community, and anticipating second- and third-order ecological and immunological consequences, will require a far more comprehensive understanding of the respiratory microbiome and its dynamics than we currently possess.
Indeed, we do not yet even know which phenomenological scale of the microbiome we should be targeting. Should we target key species, by depletion of potential pathogens or enrichment with benign or protective commensals? Should we target collective features of communities, such as total lung bacterial burden (a prognostic feature identified in numerous diseases [9–11, 17, 28, 50])? Should we target interactions between microbes rather than focus on key species (51)? Should we target microbe–host interactions, given the intimate and bidirectional relationship between microbiota and the airway ecosystem they inhabit (5, 6, 27)? Should we target microbial function, which is uncharacterized by the DNA-based sequencing approaches that currently dominate in the field?
Current knowledge, albeit limited, supports a focus on optimizing host–microbiota interactions to enhance immune modulatory effects and/or suppress inflammation. Yet acute and chronic lung diseases arise from distinct and divergent pathophysiological processes, and most conditions contain distinct endophenotypes. A one-size-fits-all approach across and even within disease states is unlikely to be effective (52, 53). Even when disease- and endotype-associated taxa are identified, our knowledge of how (and whether) respiratory microbiota directly or indirectly interact as a community through metabolites or molecular patterns is incomplete. Studies to date have characterized microbial networks by co-occurrence analyses; this approach will ultimately require concurrent host characterization, as the host-derived respiratory microenvironment contains ecologic gradients that shape the respiratory microbiome (and are, in turn, shaped by them) (1). Preclinical animal modeling is a necessary complement to human studies to identify treatment targets (and understand the ecological consequences of our interventions, as in Barrier 2). Yet the fidelity of common respiratory disease models, both in regard to microbial and host biology, remains imperfect. Prospective human studies, with rigorous pre- and postexposure characterization of respiratory microbiota, remain the reference standard in the field.
Although narrow and niche-targeted therapy is plausible, a role may exist for broad therapies targeting entire communities. For instance, reducing overall bacterial burden, altering community diversity, or recalibrating community composition may be beneficial. A prime target for further study is lung bacterial burden, which has been robustly shown to predict adverse clinical outcomes even in uninfected patients (9–11, 17, 28) and may respond to inhaled antibiotic use (22). Importantly, however, trials have yet to demonstrate clearly that lung bacterial burden is modified by systemic antibiotics (7, 21, 32), and it remains possible that lung bacterial burden is essentially fixed for a given respiratory ecosystem (i.e., antibiotics likely shift community composition without decreasing overall bacterial load).
Timing in the course of disease progression is another critical consideration. Once significant architectural destruction has occurred, physiological homeostasis may be lost, and most host–microbiota interactions may become detrimental rather than inert or beneficial. In this context, microbiota interventions may have differential effects in early versus advanced disease, further illustrating that a one-size-fits-all strategy is unlikely to be effective.
We cannot overcome this barrier unless future clinical trials characterize the respiratory microbiome of participants at baseline and after intervention. To understand the microbiome’s role as a mediator of efficacy, it is crucial to analyze communities, host–microbiota interactions, and microbe–microbe interactions through microbiological, physiological, and immunological phenotyping. The gut microbiome literature suggests that baseline heterogeneity in host–microbiome interactions can predict responses to host-based therapeutic interventions (54). Simple reliance on clinical endpoints (without characterizing microbiota) will significantly limit our understanding of the interventions’ effects, with a costly lost opportunity to improve outcomes for our patients (Box 1).
Box 1. The Ideal Respiratory Microbiome Study
Present day
-
•
Patients are screened and enrolled based on clinical characteristics (diagnosis, severity, etc.)
-
•
Interventions are chosen based on anticipated clinical benefit
-
•Respiratory and lower gut specimens are collected (for post-study sequencing and analysis):
-
•before intervention (baseline microbiota)
-
•after intervention, before primary clinical outcome (postintervention microbiota)
-
•
-
•
Systemic and respiratory indices of the host response are measured concurrently with microbiome sampling
-
•
Primary outcomes are clinically relevant endpoints (e.g., disease progression, exacerbations, mortality, or liberation from mechanical ventilation)
-
•Prespecified secondary analyses include:
-
•Heterogeneity of treatment effect: Which features of baseline microbiota predict favorable clinical response to intervention?
-
•Mediation analysis: Which changes in the respiratory microbiome (assessed using postintervention microbiota) predict favorable clinical response to intervention?
-
•Exploratory, integrative analysis of dynamic changes in host–microbiome interactions (using respiratory and systemic biomarkers) to identify potential therapeutic targets and biomarkers of clinical response
-
•Time-matched comparison of respiratory microbiota, gut microbiota, and host response
-
•
Future
-
•
Patients are screened and enrolled based on both clinical characteristics and microbiologically defined subphenotypes (e.g., high lung bacterial burden, Proteobacteria-enriched airway taxa)
-
•
Interventions are chosen based on both the anticipated microbiological effect and clinical benefit
-
•
Respiratory and lower gut specimens are collected, sequenced, analyzed, and acted on in real time (not post hoc)
-
•
Postintervention respiratory samples are characterized in real time for microbiologic response; microbiologic nonresponders are algorithmically assigned to prespecified secondary treatment strategies (response-guided trial design)
The decision of how to sample the respiratory microbiome has important implications with regard to this barrier and our efforts to overcome it. The human airways and alveolar surface area represent a vast and anatomically heterogenous ecosystem, highly variable in microbial density and community composition. Investigators must balance the potential advantages of relatively invasive approaches (e.g., the minimal influence of pharyngeal microbiota on bronchoscopically acquired specimens) with practical considerations (i.e., serial sampling is far more feasible with noninvasive specimens such as sputum), all in the context of which anatomic site is most relevant to the disease under study (upper respiratory tract, lower airways, or alveoli). Even if a microbiologic and ecologic target is identified, it will be of little value to clinicians if it cannot be readily identified using accessible specimens. Furthermore, the mucosal contiguity of the upper and lower respiratory tract necessitates that pharyngeal microbiota not be overlooked as an important contributor to lower respiratory tract pathology. Readers are directed to recent reviews and monographs for more extensive discussions of sampling considerations in respiratory microbiome studies (14, 55, 56).
Barrier 2: An Improved Understanding of the Efficacy and Persistence of Respiratory Microbiome–Modulating Interventions Is Required
Once suitable targets are identified (Barrier 1), a critical knowledge gap remains: we do not know how to optimally modulate respiratory microbiomes, using either available interventions (e.g., antibiotics) or with experimental modalities (e.g., inhaled probiotics).
Antibiotics exert effects on respiratory bacterial communities (6, 7, 21, 47) and measurable alteration to respiratory microbiota that may explain the differing clinical efficacies of antibiotics in practice (22, 57). Despite this, our understanding of these ecologic effects is coarse and nonspecific. At best, we can predict only broad trends in community diversity and phylum-level composition. We are unable to target interventions with the required granularity (i.e., genus- or species-specific interventions).
By analogy, modulating respiratory microbiota with antibiotics is currently as untargeted as modulating the host immune response with high-dose corticosteroids. If we seek to modulate respiratory microbiota with a precision comparable to that with which we currently modulate host responses, we will need microbiological therapies that are as comparably “narrow” as monoclonal antibodies.
In addition, routine nonantibiotic interventions influence the respiratory microbiome, either directly (e.g., inhaled oxygen [13]) or indirectly through host effects (e.g., corticosteroids [58]).
By corollary, other clinical interventions likely modulate respiratory microbiota, including endotracheal intubation, chest physiotherapy, and oral decontamination, and each may be mediating clinical influence through effects on the microbiome. An improved understanding of such “inadvertent” microbiome-modulating interventions is required.
A major unanswered question is whether respiratory communities can be therapeutically modulated by direct administration of respiratory probiotics (live beneficial microbes). In animal models, altered lung microbiota are evident after the instillation of viable microbial communities (27). Although such change is transient (hours to days), the protective immunologic effects are prolonged (weeks) (27). This “ecologic transience” may be advantageous for clinical translation, given legitimate concerns about the safety of instilling bacteria into the lower respiratory tract and the potential for adverse short-term effects. The administration of nonviable alternatives (bacterial products or prebiotics) may represent a more tractable initial approach.
Several potential lessons may also be learned from the past decade of attempting to therapeutically modulate the lower gut microbiome. First, microbiome “replacement” is highly variable in its efficacy across disease states. Fecal microbiota transplantation (FMT), although highly effective for recurrent Clostridium difficile infection, has proven disappointing for most other indications. Second, in situ microbial communities in the body are complex and tend to be site-adapted; modulating them is thus rarely as simple as substituting “good” microbes for “bad” ones. As examples, lower gut communities in FMT recipients diverge from those of the donor communities and retain recipient-specific taxa for months (59). Similarly, oral probiotics have negligible effects on lower gut bacterial community composition (60). Finally, the risks of treating patients with viable microbiota are far from zero; recent examples include transmission of virulent pathogens via FMT (61) and sterile site infections caused by probiotic strains in mechanically ventilated patients (38).
Barrier 3: Clinicians Need to Be Able to Access, Understand, and Use Microbiome Data for Subphenotyping Patients
To date, study of the respiratory microbiome has exclusively been performed by specialists, including physician-scientists in collaboration with basic and data scientists. Although awareness of microbiome research has rapidly expanded, clinical uptake and clinician education will be necessary to realize its full potential, including the prevention of inappropriate application. This is analogous to the implementation of clinical genetics, with microbiology currently at the stage of identifying infections with a single causative organism (analogous to simple monogenic disorders). A mature clinical understanding of the respiratory microbiome will more closely resemble our current approach to polygenic traits or complex gene–environment interactions.
The inputs and readouts needed to equip clinicians with the most informative microbiome parameters remain undetermined. The complexity of these parameters dictates the level of clinical understanding required of care teams, and the clinical settings in which they may be embedded. For example, a respiratory microbiome panel to identify specific disease- and health-associated microbes performed by a hospital microbiology laboratory could be implemented with little training. But this would arguably be a poor fit for assessing the microbiome in complex disease. To extend the analogy above, a clinician does not need advanced training in medical genetics to interpret the results of a mutation test for cystic fibrosis. Yet an entire clinical discipline—medical genetics—exists to help clinicians understand and act on more complex genetic phenomena. Whether high-dimensional microbiome data can be curated and visualized sufficiently for general use remains undetermined.
Readouts of microbial function (rather than identity) could potentially prove more biologically and clinically relevant. However, the added complexity of linking microbial metabolism and lung disease phenotypes would certainly require specialist training within clinical centers of microbiome expertise. Existing studies in asthma, COPD, and lung cancer demonstrate the utility of nonmicrobial biomarkers (e.g., blood eosinophils, cancer gene mutation panels) in informing therapeutic choices, and clinicians are becoming increasingly empowered to use molecular data to inform management. Airway-derived biomarkers have proven more difficult (fractional exhaled nitric oxide in asthma being an exception), and this remains a corollary hurdle to developing and implementing lung microbiome biomarkers.
Barrier 4: Specific Concerns in Special Populations
Once feasible, respiratory microbiome interventions will either 1) aim to prevent acute or chronic disease; or 2) modulate the severity, exacerbations, or clinical course of established disease. Both scenarios present challenges. The former requires careful weighing of risk versus benefit. It is far easier to harm healthy patients than help them, especially using potent interventions like viable bacteria and antibiotics. The latter scenario encompasses difficult circumstances, with fragile patients, compromised lung structure and function, and increased potential for side effects and adverse interactions. Intervention in early-stage chronic disease may be perceived as overly aggressive, and most patients will not progress regardless of treatment. Yet, if one waits until clinical deterioration and failure of standard therapies, a pathophysiological point of no return may have been crossed, beyond which the benefits of ecological intervention are limited.
Many microbiome-related therapies will likely have limited side effects and short durations of action and thus will be administered at low dose over longer periods. However, their biologic potency and potential for harm should not be dismissed. In a prominent recent study of enteric probiotics to prevent VAP (38), patients randomized to probiotics (enteral Lactobacillus rhamnosus) derived no benefit with regard to VAP, yet they had a significantly increased number of sterile-site Lactobacillus infections (confirmed via sequencing to be the probiotic strain). This important finding should serve as a cautionary warning: if we believe in the therapeutic potency of microbiome interventions, we should not dismiss their comparably potent capacity to cause harm.
Among infants and young children, respiratory morbidity and lung function are strong predisposing factors for respiratory diseases throughout life, with early-life respiratory microbiota predicting subsequent risk for airway disease, respiratory infections, and allergy (62, 63). Early-life morbidity may thus be a reasonable indication for clinical trials of low-dose microbiome therapies, because the microbiome is still developing and adaptable, with modulation likelier to have persistent effects. Early-life intervention will, however, require specific knowledge and definition of what healthy respiratory microbiota are in an anatomically site-specific manner.
For patients with severe airway disease, microbiome-based treatments (i.e., viable bacteria) may actually be milder in potency than existing widely used therapies (e.g., recurrent broad-spectrum antibiotics) and may plausibly be tailored to patients’ needs via precision medicine approaches (64–66). As a first step, microbiome modulation may be trialed to prevent infection-related exacerbations through colonization resistance (64). More aspirationally, modulation may dampen airway inflammation to prevent lung function decline (64, 67, 68). Those with chronic airway disease remain prone to infection, and the theoretical risk of exacerbating airway inflammation via the introduction of novel microbial motifs must be considered.
Finally, although lung microbiota are prognostically significant in critically ill patients (9), the benefit of modulating respiratory microbiota in mechanically ventilated patients is unestablished, and theoretical risks are considerable. Most have impaired mucociliary clearance and blunted host defenses, and rates of VAP remain high. The strongest microbiome signal predictive of adverse clinical outcomes in this population is total bacterial load, independent of specific taxonomy. Thus, any attempt to introduce bacteria into the respiratory tract must have strong preclinical data and compelling rationale. The near-ubiquity of broad antibiotic use among critically ill patients should not be overlooked; for instance, in the recent (negative) study of enteric probiotics to prevent VAP, more than 80% of patients received antibiotics with antimicrobial activity against the administered species (40), likely dooming the intervention to failure.
Conclusions
Despite its considerable potential, therapeutic targeting of the respiratory microbiome must overcome numerous barriers—both conceptual and practical—that stand between our current understanding and our ability to effectively modulate the respiratory microbiome to prevent or treat respiratory disease (Table 1 and Figure 1). Addressing key practical issues raised in this perspective would represent a solid start, but sustained education, research, and investment alongside improved study designs will be required to realize its true potential.
Footnotes
Supported by Singapore Ministry of Health’s National Medical Research Council under its Clinician-Scientist Individual Research Grant MOH-000141 (S.H.C.) and Clinician Scientist Award MOH-000710 (S.H.C.); CSO/NRS Scottish Senior Clinical Fellowship awards SCAF/16/03 (D.B.) and SCAF/17/03 (J.D.C.); National Institutes of Health grants R01AI129958 (Y.J.H.), R00HL139996 (D.N.O’D.), R56HL155055 (D.N.O’D.), UL1TR002494 (A.A.P.), R37CA244775 (L.N.S.), R01HL144599 (R.P.D.), and T32HL007749 (R.P.D.); Nesbitt Grant G012573 (Y.J.H.); Action for Pulmonary Fibrosis Mike Bray Fellowship (P.L.M.); VAORD grants 1IK2CX001095 (A.A.P.) and 1I01CX002130 (A.A.P.); National Health and Medical Research Council Senior Research Fellowship GNT1155179 (G.B.R.); and Matthew Flinders Professorial Fellowship (G.B.R.).
Author Contributions: All authors meet International Committee of Medical Journal Editors criteria for authorship. S.H.C., D.B., J.D.C., M.J.C., P.M.H., Y.J.H., P.L.M., D.N.O’D., A.A.P., G.B.R., L.N.S., and R.P.D. all contributed to the conception and writing of this manuscript.
Originally Published in Press as DOI: 10.1164/rccm.202112-2704PP on May 12, 2022
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Dickson RP, Erb-Downward JR, Martinez FJ, Huffnagle GB. The microbiome and the respiratory tract. Annu Rev Physiol . 2016;78:481–504. doi: 10.1146/annurev-physiol-021115-105238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hilty M, Burke C, Pedro H, Cardenas P, Bush A, Bossley C, et al. Disordered microbial communities in asthmatic airways. PLoS One . 2010;5:e8578. doi: 10.1371/journal.pone.0008578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dickson RP, Erb-Downward JR, Freeman CM, McCloskey L, Falkowski NR, Huffnagle GB, et al. Bacterial topography of the healthy human lower respiratory tract. MBio . 2017;8:e02287-16. doi: 10.1128/mBio.02287-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dickson RP, Erb-Downward JR, Freeman CM, McCloskey L, Beck JM, Huffnagle GB, et al. Spatial variation in the healthy human lung microbiome and the adapted island model of lung biogeography. Ann Am Thorac Soc . 2015;12:821–830. doi: 10.1513/AnnalsATS.201501-029OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Segal LN, Clemente JC, Tsay JC, Koralov SB, Keller BC, Wu BG, et al. Enrichment of the lung microbiome with oral taxa is associated with lung inflammation of a Th17 phenotype. Nat Microbiol . 2016;1:16031. doi: 10.1038/nmicrobiol.2016.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dickson RP, Erb-Downward JR, Falkowski NR, Hunter EM, Ashley SL, Huffnagle GB. The lung microbiota of healthy mice are highly variable, cluster by environment, and reflect variation in baseline lung innate immunity. Am J Respir Crit Care Med . 2018;198:497–508. doi: 10.1164/rccm.201711-2180OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Segal LN, Clemente JC, Wu BG, Wikoff WR, Gao Z, Li Y, et al. Randomised, double-blind, placebo-controlled trial with azithromycin selects for anti-inflammatory microbial metabolites in the emphysematous lung. Thorax . 2017;72:13–22. doi: 10.1136/thoraxjnl-2016-208599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sulaiman I, Wu BG, Li Y, Tsay JC, Sauthoff M, Scott AS, et al. Functional lower airways genomic profiling of the microbiome to capture active microbial metabolism. Eur Respir J . 2021;58:2003434. doi: 10.1183/13993003.03434-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dickson RP, Schultz MJ, van der Poll T, Schouten LR, Falkowski NR, Luth JE, et al. Biomarker Analysis in Septic ICU Patients (BASIC) Consortium Lung microbiota predict clinical outcomes in critically ill patients. Am J Respir Crit Care Med . 2020;201:555–563. doi: 10.1164/rccm.201907-1487OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Molyneaux PL, Cox MJ, Willis-Owen SA, Mallia P, Russell KE, Russell AM, et al. The role of bacteria in the pathogenesis and progression of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med . 2014;190:906–913. doi: 10.1164/rccm.201403-0541OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. O’Dwyer DN, Ashley SL, Gurczynski SJ, Xia M, Wilke C, Falkowski NR, et al. Lung microbiota contribute to pulmonary inflammation and disease progression in pulmonary fibrosis. Am J Respir Crit Care Med . 2019;199:1127–1138. doi: 10.1164/rccm.201809-1650OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wu BG, Kapoor B, Cummings KJ, Stanton ML, Nett RJ, Kreiss K, et al. Evidence for environmental-human microbiota transfer at a manufacturing facility with novel work-related respiratory disease. Am J Respir Crit Care Med . 2020;202:1678–1688. doi: 10.1164/rccm.202001-0197OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ashley SL, Sjoding MW, Popova AP, Cui TX, Hoostal MJ, Schmidt TM, et al. Lung and gut microbiota are altered by hyperoxia and contribute to oxygen-induced lung injury in mice. Sci Transl Med . 2020;12:eaau9959. doi: 10.1126/scitranslmed.aau9959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Carney SM, Clemente JC, Cox MJ, Dickson RP, Huang YJ, Kitsios GD, et al. Methods in lung microbiome research. Am J Respir Cell Mol Biol . 2020;62:283–299. doi: 10.1165/rcmb.2019-0273TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cox MJ, Ege MJ, von Mutius E. The lung microbiome. European Respiratory Society; 2019. [Google Scholar]
- 16.Huang YJ, Garantziotis S. The microbiome in respiratory disease; principles, tools and applications. Humana Press; 2022. [Google Scholar]
- 17. Invernizzi R, Barnett J, Rawal B, Nair A, Ghai P, Kingston S, et al. Bacterial burden in the lower airways predicts disease progression in idiopathic pulmonary fibrosis and is independent of radiological disease extent. Eur Respir J . 2020;55:1901519. doi: 10.1183/13993003.01519-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kanmani P, Clua P, Vizoso-Pinto MG, Rodriguez C, Alvarez S, Melnikov V, et al. Respiratory commensal bacteria Corynebacterium pseudodiphtheriticum improves resistance of infant mice to respiratory syncytial virus and Streptococcus pneumoniae superinfection. Front Microbiol . 2017;8:1613. doi: 10.3389/fmicb.2017.01613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ortiz Moyano R, Raya Tonetti F, Tomokiyo M, Kanmani P, Vizoso-Pinto MG, Kim H, et al. The ability of respiratory commensal bacteria to beneficially modulate the lung innate immune response is a strain dependent characteristic. Microorganisms . 2020;8:727. doi: 10.3390/microorganisms8050727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Duan K, Dammel C, Stein J, Rabin H, Surette MG. Modulation of Pseudomonas aeruginosa gene expression by host microflora through interspecies communication. Mol Microbiol . 2003;50:1477–1491. doi: 10.1046/j.1365-2958.2003.03803.x. [DOI] [PubMed] [Google Scholar]
- 21. Nelson MT, Wolter DJ, Eng A, Weiss EJ, Vo AT, Brittnacher MJ, et al. Maintenance tobramycin primarily affects untargeted bacteria in the CF sputum microbiome. Thorax . 2020;75:780–790. doi: 10.1136/thoraxjnl-2019-214187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sibila O, Laserna E, Shoemark A, Keir HR, Finch S, Rodrigo-Troyano A, et al. Airway bacterial load and inhaled antibiotic response in bronchiectasis. Am J Respir Crit Care Med . 2019;200:33–41. doi: 10.1164/rccm.201809-1651OC. [DOI] [PubMed] [Google Scholar]
- 23. Huang YJ, Nelson CE, Brodie EL, Desantis TZ, Baek MS, Liu J, et al. Airway microbiota and bronchial hyperresponsiveness in patients with suboptimally controlled asthma. J Allergy Clin Immunol . 2011;127:372–381.e1–3. doi: 10.1016/j.jaci.2010.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Durack J, Lynch SV, Nariya S, Bhakta NR, Beigelman A, Castro M, et al. National Heart, Lung and Blood Institute’s “AsthmaNet” Features of the bronchial bacterial microbiome associated with atopy, asthma, and responsiveness to inhaled corticosteroid treatment. J Allergy Clin Immunol . 2017;140:63–75. doi: 10.1016/j.jaci.2016.08.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rigauts C, Aizawa J, Taylor SL, Rogers GB, Govaerts M, Cos P, et al. Rothia mucilaginosa is an anti-inflammatory bacterium in the respiratory tract of patients with chronic lung disease. Eur Respir J . 2022;59:2101293. doi: 10.1183/13993003.01293-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bertelsen A, Elborn JS, Schock BC. Microbial interaction: Prevotella spp. reduce P. aeruginosa induced inflammation in cystic fibrosis bronchial epithelial cells. J Cyst Fibros . 2021;20:682–691. doi: 10.1016/j.jcf.2021.04.012. [DOI] [PubMed] [Google Scholar]
- 27. Wu BG, Sulaiman I, Tsay JJ, Perez L, Franca B, Li Y, et al. Episodic aspiration with oral commensals induces a MyD88-dependent, pulmonary T-helper cell type 17 response that mitigates susceptibility to Streptococcus pneumoniae. Am J Respir Crit Care Med . 2021;203:1099–1111. doi: 10.1164/rccm.202005-1596OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Combs MP, Wheeler DS, Luth JE, Falkowski NR, Walker NM, Erb-Downward JR, et al. Lung microbiota predict chronic rejection in healthy lung transplant recipients: a prospective cohort study. Lancet Respir Med . 2021;9:601–612. doi: 10.1016/S2213-2600(20)30405-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Greathouse KL, White JR, Vargas AJ, Bliskovsky VV, Beck JA, von Muhlinen N, et al. Interaction between the microbiome and TP53 in human lung cancer. Genome Biol . 2018;19:123. doi: 10.1186/s13059-018-1501-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Tsay JJ, Wu BG, Badri MH, Clemente JC, Shen N, Meyn P, et al. Airway microbiota is associated with upregulation of the PI3K pathway in lung cancer. Am J Respir Crit Care Med . 2018;198:1188–1198. doi: 10.1164/rccm.201710-2118OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Tsay JJ, Wu BG, Sulaiman I, Gershner K, Schluger R, Li Y, et al. Lower airway dysbiosis affects lung cancer progression. Cancer Discov . 2021;11:293–307. doi: 10.1158/2159-8290.CD-20-0263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Heirali AA, Workentine ML, Acosta N, Poonja A, Storey DG, Somayaji R, et al. The effects of inhaled aztreonam on the cystic fibrosis lung microbiome. Microbiome . 2017;5:51. doi: 10.1186/s40168-017-0265-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Taylor SL, Leong LEX, Mobegi FM, Choo JM, Wesselingh S, Yang IA, et al. Long-term azithromycin reduces Haemophilus influenzae and increases antibiotic resistance in severe asthma. Am J Respir Crit Care Med . 2019;200:309–317. doi: 10.1164/rccm.201809-1739OC. [DOI] [PubMed] [Google Scholar]
- 34. Contoli M, Pauletti A, Rossi MR, Spanevello A, Casolari P, Marcellini A, et al. Long-term effects of inhaled corticosteroids on sputum bacterial and viral loads in COPD. Eur Respir J . 2017;50:1700451. doi: 10.1183/13993003.00451-2017. [DOI] [PubMed] [Google Scholar]
- 35. Hisert KB, Heltshe SL, Pope C, Jorth P, Wu X, Edwards RM, et al. Restoring cystic fibrosis transmembrane conductance regulator function reduces airway bacteria and inflammation in people with cystic fibrosis and chronic lung infections. Am J Respir Crit Care Med . 2017;195:1617–1628. doi: 10.1164/rccm.201609-1954OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pantaleón García J, Hinkle KJ, Falkowski NR, Evans SE, Dickson RP. Selective modulation of the pulmonary innate immune response does not change lung microbiota in healthy mice. Am J Respir Crit Care Med . 2021;204:734–736. doi: 10.1164/rccm.202104-0836LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Anderson JL, Miles C, Tierney AC. Effect of probiotics on respiratory, gastrointestinal and nutritional outcomes in patients with cystic fibrosis: a systematic review. J Cyst Fibros . 2017;16:186–197. doi: 10.1016/j.jcf.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 38. Johnstone J, Meade M, Lauzier F, Marshall J, Duan E, Dionne J, et al. Prevention of Severe Pneumonia and Endotracheal Colonization Trial (PROSPECT) Investigators and the Canadian Critical Care Trials Group Effect of probiotics on incident ventilator-associated pneumonia in critically ill patients: a randomized clinical trial. JAMA . 2021;326:1024–1033. doi: 10.1001/jama.2021.13355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Duan Y, Llorente C, Lang S, Brandl K, Chu H, Jiang L, et al. Bacteriophage targeting of gut bacterium attenuates alcoholic liver disease. Nature . 2019;575:505–511. doi: 10.1038/s41586-019-1742-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Law N, Logan C, Yung G, Furr CL, Lehman SM, Morales S, et al. Successful adjunctive use of bacteriophage therapy for treatment of multidrug-resistant Pseudomonas aeruginosa infection in a cystic fibrosis patient. Infection . 2019;47:665–668. doi: 10.1007/s15010-019-01319-0. [DOI] [PubMed] [Google Scholar]
- 41. Varney VA, Parnell HM, Salisbury DT, Ratnatheepan S, Tayar RB. A double blind randomised placebo controlled pilot study of oral co-trimoxazole in advanced fibrotic lung disease. Pulm Pharmacol Ther . 2008;21:178–187. doi: 10.1016/j.pupt.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 42. Shulgina L, Cahn AP, Chilvers ER, Parfrey H, Clark AB, Wilson EC, et al. Treating idiopathic pulmonary fibrosis with the addition of co-trimoxazole: a randomised controlled trial. Thorax . 2013;68:155–162. doi: 10.1136/thoraxjnl-2012-202403. [DOI] [PubMed] [Google Scholar]
- 43. Wilson AM, Clark AB, Cahn T, Chilvers ER, Fraser W, Hammond M, et al. EME-TIPAC team Effect of co-trimoxazole (trimethoprim-sulfamethoxazole) vs placebo on death, lung transplant, or hospital admission in patients with moderate and severe idiopathic pulmonary fibrosis: the EME-TIPAC randomized clinical trial. JAMA . 2020;324:2282–2291. doi: 10.1001/jama.2020.22960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Martinez FJ, Yow E, Flaherty KR, Snyder LD, Durheim MT, Wisniewski SR, et al. CleanUP-IPF Investigators of the Pulmonary Trials Cooperative Effect of antimicrobial therapy on respiratory hospitalization or death in adults with idiopathic pulmonary fibrosis: the CleanUP-IPF randomized clinical trial. JAMA . 2021;325:1841–1851. doi: 10.1001/jama.2021.4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Brill SE, Law M, El-Emir E, Allinson JP, James P, Maddox V, et al. Effects of different antibiotic classes on airway bacteria in stable COPD using culture and molecular techniques: a randomised controlled trial. Thorax . 2015;70:930–938. doi: 10.1136/thoraxjnl-2015-207194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Serisier DJ, Martin ML, McGuckin MA, Lourie R, Chen AC, Brain B, et al. Effect of long-term, low-dose erythromycin on pulmonary exacerbations among patients with non-cystic fibrosis bronchiectasis: the BLESS randomized controlled trial. JAMA . 2013;309:1260–1267. doi: 10.1001/jama.2013.2290. [DOI] [PubMed] [Google Scholar]
- 47. Rogers GB, Bruce KD, Martin ML, Burr LD, Serisier DJ. The effect of long-term macrolide treatment on respiratory microbiota composition in non-cystic fibrosis bronchiectasis: an analysis from the randomised, double-blind, placebo-controlled BLESS trial. Lancet Respir Med . 2014;2:988–996. doi: 10.1016/S2213-2600(14)70213-9. [DOI] [PubMed] [Google Scholar]
- 48. Choo JM, Abell GCJ, Thomson R, Morgan L, Waterer G, Gordon DL, et al. Impact of long-term erythromycin therapy on the oropharyngeal microbiome and resistance gene reservoir in non-cystic fibrosis bronchiectasis. MSphere . 2018;3:e00103-18. doi: 10.1128/mSphere.00103-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Burr LD, Rogers GB, Chen AC, Hamilton BR, Pool GF, Taylor SL, et al. Macrolide treatment inhibits Pseudomonas aeruginosa quorum sensing in non-cystic fibrosis bronchiectasis: an analysis from the Bronchiectasis and Low-Dose Erythromycin Study trial. Ann Am Thorac Soc . 2016;13:1697–1703. doi: 10.1513/AnnalsATS.201601-044OC. [DOI] [PubMed] [Google Scholar]
- 50. Taylor SL, Leong LEX, Ivey KL, Wesselingh S, Grimwood K, Wainwright CE, et al. Total bacterial load, inflammation, and structural lung disease in paediatric cystic fibrosis. J Cyst Fibros . 2020;19:923–930. doi: 10.1016/j.jcf.2020.03.008. [DOI] [PubMed] [Google Scholar]
- 51. Mac Aogáin M, Narayana JK, Tiew PY, Ali NABM, Yong VFL, Jaggi TK, et al. Integrative microbiomics in bronchiectasis exacerbations. Nat Med . 2021;27:688–699. doi: 10.1038/s41591-021-01289-7. [DOI] [PubMed] [Google Scholar]
- 52. Moore WC, Meyers DA, Wenzel SE, Teague WG, Li H, Li X, et al. National Heart, Lung, and Blood Institute’s Severe Asthma Research Program Identification of asthma phenotypes using cluster analysis in the Severe Asthma Research Program. Am J Respir Crit Care Med . 2010;181:315–323. doi: 10.1164/rccm.200906-0896OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wang Z, Locantore N, Haldar K, Ramsheh MY, Beech AS, Ma W, et al. Inflammatory endotype-associated airway microbiome in chronic obstructive pulmonary disease clinical stability and exacerbations: a multicohort longitudinal analysis. Am J Respir Crit Care Med . 2021;203:1488–1502. doi: 10.1164/rccm.202009-3448OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Davar D, Dzutsev AK, McCulloch JA, Rodrigues RR, Chauvin JM, Morrison RM, et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science . 2021;371:595–602. doi: 10.1126/science.abf3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dickson RP, Cox MJ. In: The lung microbiome. Cox MJ, Ege MJ, von Mutius E, editors. European Respiratory Society; 2019. Sampling the lung microbiome; pp. 1–17. [Google Scholar]
- 56.Dickson RP. In: The microbiome in respiratory disease. Huang YJ, Garantziotis S, editors. Humana Press; 2022. Approaches to sampling the respiratory microbiome. [Google Scholar]
- 57. Thorsen J, Stokholm J, Rasmussen MA, Mortensen MS, Brejnrod AD, Hjelmsø M, et al. The airway microbiota modulates effect of azithromycin treatment for episodes of recurrent asthma-like symptoms in preschool children: a randomized clinical trial. Am J Respir Crit Care Med . 2021;204:149–158. doi: 10.1164/rccm.202008-3226OC. [DOI] [PubMed] [Google Scholar]
- 58. Hartmann JE, Albrich WC, Dmitrijeva M, Kahlert CR. The effects of corticosteroids on the respiratory microbiome: a systematic review. Front Med (Lausanne) . 2021;8:588584. doi: 10.3389/fmed.2021.588584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Li SS, Zhu A, Benes V, Costea PI, Hercog R, Hildebrand F, et al. Durable coexistence of donor and recipient strains after fecal microbiota transplantation. Science . 2016;352:586–589. doi: 10.1126/science.aad8852. [DOI] [PubMed] [Google Scholar]
- 60. Kristensen NB, Bryrup T, Allin KH, Nielsen T, Hansen TH, Pedersen O. Alterations in fecal microbiota composition by probiotic supplementation in healthy adults: a systematic review of randomized controlled trials. Genome Med . 2016;8:52. doi: 10.1186/s13073-016-0300-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zellmer C, Sater MRA, Huntley MH, Osman M, Olesen SW, Ramakrishna B. Shiga toxin-producing Escherichia coli transmission via fecal microbiota transplant. Clin Infect Dis . 2021;72:e876–e880. doi: 10.1093/cid/ciaa1486. [DOI] [PubMed] [Google Scholar]
- 62. Bosch AATM, de Steenhuijsen Piters WAA, van Houten MA, Chu MLJN, Biesbroek G, Kool J, et al. Maturation of the infant respiratory microbiota, environmental drivers, and health consequences. a prospective cohort study. Am J Respir Crit Care Med . 2017;196:1582–1590. doi: 10.1164/rccm.201703-0554OC. [DOI] [PubMed] [Google Scholar]
- 63. Teo SM, Mok D, Pham K, Kusel M, Serralha M, Troy N, et al. The infant nasopharyngeal microbiome impacts severity of lower respiratory infection and risk of asthma development. Cell Host Microbe . 2015;17:704–715. doi: 10.1016/j.chom.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Budden KF, Shukla SD, Rehman SF, Bowerman KL, Keely S, Hugenholtz P, et al. Functional effects of the microbiota in chronic respiratory disease. Lancet Respir Med . 2019;7:907–920. doi: 10.1016/S2213-2600(18)30510-1. [DOI] [PubMed] [Google Scholar]
- 65. Chotirmall SH, Gellatly SL, Budden KF, Mac Aogain M, Shukla SD, Wood DL, et al. Microbiomes in respiratory health and disease: an Asia-Pacific perspective. Respirology . 2017;22:240–250. doi: 10.1111/resp.12971. [DOI] [PubMed] [Google Scholar]
- 66. Gosens R, Hiemstra PS, Adcock IM, Bracke KR, Dickson RP, Hansbro PM, et al. Host-microbe cross-talk in the lung microenvironment: implications for understanding and treating chronic lung disease. Eur Respir J . 2020;56:1902320. doi: 10.1183/13993003.02320-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bowerman KL, Rehman SF, Vaughan A, Lachner N, Budden KF, Kim RY, et al. Disease-associated gut microbiome and metabolome changes in patients with chronic obstructive pulmonary disease. Nat Commun . 2020;11:5886. doi: 10.1038/s41467-020-19701-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Budden KF, Gellatly SL, Wood DL, Cooper MA, Morrison M, Hugenholtz P, et al. Emerging pathogenic links between microbiota and the gut-lung axis. Nat Rev Microbiol . 2017;15:55–63. doi: 10.1038/nrmicro.2016.142. [DOI] [PubMed] [Google Scholar]


