To the Editor:
Alzheimer’s disease (AD) is a heterogeneous disease with multiple potential contributors to its pathophysiology, including obstructive sleep apnea (OSA) and vascular risk factors (VRFs) (e.g., hypertension [HTN]). A substantial number of patients with OSA have co-occurring VRFs, including HTN. Recently, we and others respectively showed that VRFs and OSA each act synergistically with amyloid-β (Aβ) burden to promote cognitive decline (1, 2). Therefore, it seems plausible that identifying asymptomatic individuals with co-occurring OSA and HTN who are at high risk of cognitive decline due to AD may be vital for successful prevention and/or delay of AD onset. In this study, we examined the synergistic associations of co-occurring OSA and HTN on Aβ concentrations and cognitive decline in cognitively normal older adults. Some of the results of these studies have been previously reported in the form of an abstract (3).
We recruited 98 participants between the ages of 55 and 90 from a previously described New York University cohort consisting of community-dwelling relatively healthy volunteers (4). Participants were English speaking, with minimum education of 12 years, Mini-Mental State Exam scores of higher than 27, and a Clinical Dementia Rating of 0 and had scores of 5 or less on the shorter version of the Geriatric Depression Scale. At baseline and first annual follow-up, cognitive data were available for 98 participants. At the second, third, and fourth follow-up, cognitive data were available for 79, 67, and 49 participants, respectively. For CSF-Aβ42, all 98 participants had baseline and one follow-up data with mean (SD) follow-up of 2.46 (0.64) years. Participants underwent home monitoring (clinically validated with an 89% correlation to polysomnography) for OSA during a 2-night period before baseline lumbar puncture for CSF-Aβ42. OSA was defined using the apnea–hypopnea index with 4% desaturation (AHI4% > 5 events/h), according to American Academy of Sleep Medicine guidelines. We defined HTN at baseline and follow-up as systolic blood pressure of ⩾140 mm Hg and diastolic blood pressure of ⩾90 mm Hg (5) and/or self-reported prior diagnosis of hypertension and documented use of antihypertensive medications. CSF-Aβ42 concentrations were measured using ELISA. Annual rate-of-change (Rc) in CSF-Aβ42 concentrations was calculated as RcCSF-Aβ42 = (CSF-Aβ42follow-up − CSF-Aβ42baseline) / time in years between examinations. As previously reported (6), cognitive performance data were normalized using z-scores adjusting for age, sex, race, and education. Cognitive domains included the following: episodic memory: logic 1 and 2; language: animal fluency, vegetable fluency, and Boston naming test(7); and executive function: digit symbol substitution test and trails making test A and B (8). The three domain measures were averaged to create a composite global cognitive z-score. Annual Rc in individual raw cognitive test was calculated as RcCognitiveTest = (CognitiveTestlastfollow-up − CognitiveTestbaseline) / time between examinations. The New York University Institutional Review Board approved this study.
Linear mixed-effects models with random intercept and slope were used to assess associations among OSA, HTN, and longitudinal changes in CSF-Aβ and cognition, controlling for age, sex, body mass index, years of education, APOE (Apolipoprotein E) ε4 status and their interactions with time (i.e., years from baseline for each participant). Covariates were selected a priori and included age, sex, body mass index, years of education, APOE ε4 status, clinical history of thyroid disease, diabetes, and cardiovascular disease (e.g., ischemic heart disease, heart failure, and stroke/ transient ischemic attack), and use of antihypertensive medications. However, clinical history of thyroid disease, diabetes, cardiovascular disease and use of antihypertensive medications were investigated as a potential covariate in the models but were dropped from final models because of nonsignificant results. OSA, HTN, and time were included as separate independent variables, in addition to the OSA–time and HTN–time interactions, and covariates in the interaction model. Specifically, we examined interactions of OSA with time and HTN with time in a single model (model: Delta- (CSF-Aβ or global cognition or each cognitive test measure) ≈ OSA + HTN + time + OSA × time + HTN × time + covariates × time). Next, we added an interaction term between OSA, HTN, and time to examine whether these two factors i.e., OSA and HTN increase the likelihood of prospective changes in CSF-Aβ or cognitive decline beyond their separate effects (i.e., synergistic effect model: Delta- (CSF-Aβ or global cognition or each cognitive test measure) ≈ OSA + HTN + time + OSA × time + HTN × time + covariates × time + covariates × time). In the models, time was operationalized as years from baseline for each participant. To aid comparison across measures, continuous variables (e.g., CSF-Aβ and cognitive variables) were z-transformed before model entry. The synergistic effect model examined whether OSA and HTN increased the likelihood of prospective changes in CSF-Aβ or cognitive decline beyond their separate effects.
Of the 98 participants, 63 (64.3%) were women and 47 (48.0%) had AHI4% > 5 events/h. The mean (SD) age was 69.6 (7.3) years, follow-up time was 2.65 (0.54) years, Epworth Sleepiness Scale was 5.0 (3.0), and total sleep time was 7.1 (0.6) hours. There were no significant differences in CSF Aβ-42 concentrations and cognitive performance between control subjects and OSA groups at baseline. The mean (SD) CSF Aβ was 681.88 (243.18) versus 657.48 (224.79) pg/ml for subjects without OSA (n = 51) versus those with OSA (n = 47; P = 0.09). Linear mixed-effects models results are summarized in Table 1. Longitudinally, OSA and HTN were each associated with faster Rc in CSF-Aβ42 (P < 0.01 for both). The interaction of OSA and HTN with time was significant (β = −3.11; 95% confidence interval [CI], −3.71 to −2.51; P < 0.01) suggesting a synergistic effect (i.e., the co-occurrence of OSA and HTN was associated with an increase in the annual Rc in CSF-Aβ42 beyond their separable effects. No significant associations were seen between OSA and global cognitive decline (P = 0.07). However, OSA was significantly associated with annual decline in executive function and language (P = 0.01 for all). HTN was associated with faster decline in global cognition, executive function, and language (P = 0.01 for all). The interaction of OSA and HTN with time was significant for global cognitive decline (β = −0.054; 95% CI, −0.063 to −0.045), as well as executive function and language cognitive domains, P < 0.01 for all, also suggesting a synergistic effect. To visualize these interactions, we compared the cognitive and CSF-Aβ42 trajectories of participants classified dichotomously according to joint OSA and HTN status (i.e., OSA+/HTN+ [n = 23], OSA+/HTN− [n = 24], OSA−/HTN− [n = 31], and OSA−/HTN+ [n = 20]) (Figure 1). At baseline, mean (SD) CSF Aβ was 612.43 (288.76) pg/ml for OSA+/HTN+, 702.53 (214.84) pg/ml for OSA+/HTN−, 711.47 (232.42) pg/ml for OSA−/HTN−, and 652.29 (225.17) for OSA−/HTN+ groups (ANOVA P ⩽ 0.05). At baseline, amyloid positivity (i.e., CSF Aβ42 ⩽ 392 pg/ml) rates did not vary across the groups with 16% positivity in OSA+/HTN+, 15% positivity in both OSA+/HTN− and OSA−/HTN+ groups, and 14% in the OSA−/HTN− group (P = 0.65). With this grouping, there was a significant association of group with annual cognitive decline/CSF-Aβ42 Rc after controlling for preselected covariates (P < 0.01). Post hoc analyses demonstrated significantly faster rate of decline in the OSA+/HTN+ group relative to OSA+/HTN− and OSA−/HTN− groups (P < 0.01 for all). There was no significant difference between the cognitive trajectories of the OSA+/HTN+ group and the OSA−/HTN+ group.
Table 1.
Associations of Obstructive Sleep Apnea and Hypertension with Longitudinal Changes in Amyloid-β Burden and Cognition, New York University Cohort of Community-Dwelling Cognitively Normal Elderly Participants
| Outcome | Model Term | Standardized Estimate (95% CI) | P Value* |
|---|---|---|---|
| Annual rate of change of CSF-Aβ42 | OSA × time | −1.28 (−1.78 to −0.78) | <0.01 |
| Hypertension × time | −2.82 (−3.29 to −2.35) | <0.01 | |
| OSA × hypertension × time | −3.11 (−3.71 to −2.51) | <0.01 |
| Cognitive Domain Composites | |||
|---|---|---|---|
| Annual decline in global cognition | OSA × time | −0.038 (−0.091 to 0.014) | 0.07 |
| Hypertension × time | −0.047 (−0.071 to −0.024) | 0.01 | |
| OSA × hypertension × time | −0.054 (−0.063 to −0.045) | <0.001 | |
| Annual decline in executive function (DSST, trail making A and B composite) | OSA × time | −0.037 (−0.052 to −0.022) | 0.01 |
| Hypertension × time | −0.058 (−0.092 to −0.024) | <0.01 | |
| OSA × hypertension × time | −0.048 (−0.063 to −0.033) | <0.001 | |
| Annual decline in language (verbal and animal fluency and BNT composite) | OSA × time | −0.025 (−0.036 to −0.014) | 0.03 |
| Hypertension × time | −0.034 (−0.057 to −0.011) | 0.04 | |
| OSA × hypertension × time | −0.054 (−0.094 to −0.013) | <0.001 | |
| Annual decline in episodic memory (Logic-1 and 2 composite) | OSA × time | −0.026 (−0.052 to 0.019) | 0.43 |
| Hypertension × time | −0.019 (−0.038 to 0.008) | 0.34 | |
| OSA × hypertension × time | −0.037 (−0.076 to 0.021) | 0.33 | |
| Individual Cognitive Tests | |||
|---|---|---|---|
| Annual decline in cognition (verbal fluency) | OSA × time | −0.033 (−0.048 to −0.018) | 0.02 |
| Hypertension × time | −0.048 (−0.079 to −0.017) | 0.04 | |
| OSA × hypertension × time | −0.040 (−0.065 to −0.015) | <0.001 | |
| Annual decline in cognition (animal fluency) | OSA × time | −0.024 (−0.079 to 0.024) | 0.08 |
| Hypertension × time | −0.048 (−0.089 to −0.017) | 0.02 | |
| OSA × hypertension × time | −0.037 (−0.058 to −0.014) | 0.04 | |
| Annual decline in cognition (Boston naming test) | OSA × time | −0.018 (−0.069 to 0.034 ) | 0.32 |
| Hypertension × time | −0.007 (−0.028 to 0.017) | 0.41 | |
| OSA × hypertension × time | −0.008 (−0.027 to 0.009) | 0.53 | |
| Annual decline in cognition (DSST) | OSA × time | −0.040 (−0.064 to −0.016) | 0.01 |
| Hypertension × time | −0.078 (−0.098 to −0.057) | <0.01 | |
| OSA × hypertension × time | −0.058 (−0.084 to −0.033) | <0.001 | |
| Annual decline in cognition (trail making test A) | OSA × time | −0.033 (−0.065 to 0.019) | 0.34 |
| Hypertension × time | −0.052 (−0.104 to 0.022) | 0.73 | |
| OSA × hypertension × time | −0.042 (−0.084 to 0.012) | 0.54 | |
| Annual decline in cognition (trail making test B) | OSA × time | −0.038 (−0.076 to 0.014) | 0.41 |
| Hypertension × time | −0.053 (−0.107 to 0.011) | 0.74 | |
| OSA × hypertension × time | −0.044 (−0.088 to 0.015) | 0.61 | |
| Annual decline in cognition (Logic 1) | OSA × time | −0.008 (−0.017 to 0.041) | 0.53 |
| Hypertension × time | −0.013 (−0.027 to 0.041) | 0.55 | |
| OSA × hypertension × time | −0.033 (−0.087 to 0.021) | 0.43 | |
| Annual decline in cognition (Logic 2) | OSA × time | −0.018 (−0.037 to 0.029) | 0.38 |
| Hypertension × time | −0.023 (−0.046 to 0.001) | 0.24 | |
| OSA × hypertension × time | −0.040 (−0.081 to 0.024) | 0.31 | |
Definition of abbreviations: BNT = Boston naming test; CI = confidence interval; CSF Aβ-42 = cerebrospinal fluid amyloid-β42; DSST = digit symbol substitution test; OSA = obstructive sleep apnea.
Lower z-scores represent worse cognitive function. Models were adjusted for age, sex, body mass index, years of education, and APOE (Apolipoprotein E) ε4 status, which was determined by the presence of at least 1 ε4 allele. Bold text indicates instances where results reached statistical significance.
Significance level (P ⩽ 0.05).
Figure 1.
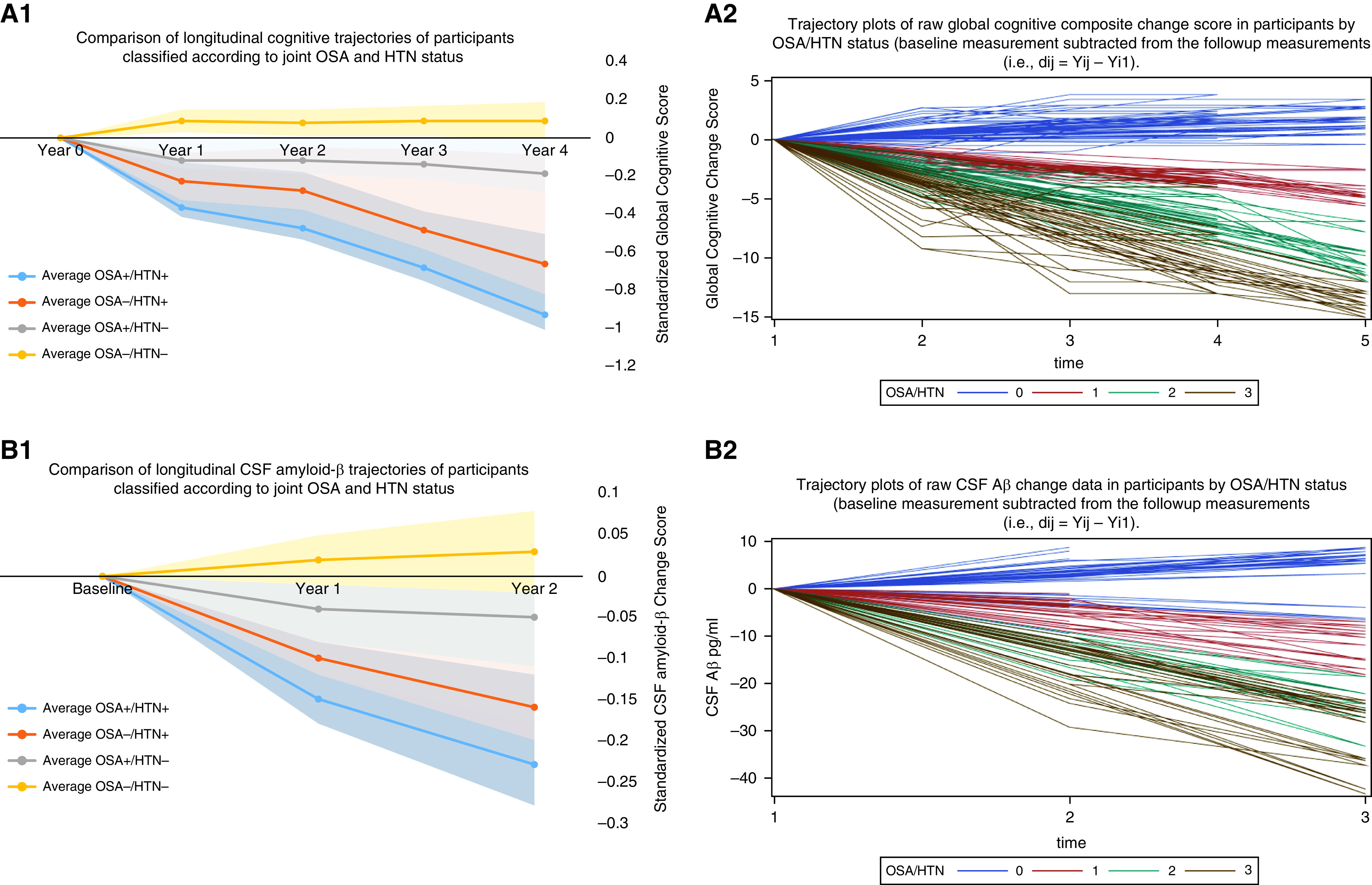
(A1) The composite global cognitive score is scaled such that 0 represents the mean score of all participants at baseline, positive scores indicate better performance, and 1 unit represents approximately 1 SD of performance. There was a significant association of group with prospective cognitive decline after controlling for age at baseline, sex, APOE4 (Apolipoprotein E4) status, and years of education (β = 0.062; 95% confidence interval [CI], 0.033 to 0.090; P = 0.004). Post hoc analyses demonstrated significantly faster rate of annual cognitive decline in the group positive for obstructive sleep apnea (OSA+)/positive for hypertension (HTN+) (n = 23) relative to OSA+/HTN− (n = 24; β = 0.13; 95% CI, 0.07 to 0.22; P = 0.04) and OSA−/HTN− (n = 31; β = 0.32; 95% CI, 0.25 to 0.41; P < 0.001) groups. There was no significant difference between the cognitive trajectories of the OSA+/HTN+ (n = 23) group and the OSA−/HTN+ (n = 20; β = 0.021; 95% CI, −0.122 to 0.079; P = 0.11). At baseline and first annual follow-up, cognitive data were available for 98 participants. At the second, third, and fourth follow-up, cognitive data were available for 79, 67, and 49 participants, respectively. Fourth follow-up data: OSA+/HTN+ (n = 13), OSA+/HTN− (n = 12), OSA−/HTN+ (n = 10), and OSA−/HTN− (n = 14). (A2) Trajectory plots of raw global cognitive composite change score in participants by OSA/HTN status over the follow-up period. Global cognitive change scores were obtained by subtracting baseline scores from original follow-up measurements. Following previously described methods, a composite measure for each cognitive domain was created. The three domain measures were then averaged to create a composite global score. Legend: Blue lines with OSA/HTN = 0 represent OSA−/HTN− (n = 31). Red lines with OSA/HTN = 1 represent OSA+/HTN− (n = 34). Green lines with OSA/HTN = 2 represent OSA−/HTN+ (n = 20). Brown lines with OSA/HTN = 3 represent OSA+/HTN+ (n = 23). At baseline and first annual follow-up, cognitive data were available for 98 participants. At the second, third, and fourth follow-up, cognitive data were available for 79, 67, and 49 participants, respectively. Fourth follow-up data: OSA+/HTN+ (n = 13), OSA+/HTN− (n = 12), OSA−/HTN+ (n = 10), and OSA−/HTN− (n = 14). Cognitive domains included the following: Episodic memory: logic 1 and 2. Language: animal fluency, vegetable fluency), and Boston naming test. Executive function: digit symbol substitution test, and trails making test A and B. The three domain measures were averaged to create a composite global cognitive score prior to z-scoring for A2 plot. Higher scores suggest better performance. (Note: Because this was not z-scored, trails making test A and B scores were excluded as lower values are indicative of better performance). At baseline (time 1), mean (SD) raw global cognitive score was 42.23 (8.62) for OSA+/HTN+ (n = 23), 50.51 (4.32) for OSA+/HTN− (n = 24), 51.13 (3.44) for OSA−/HTN− (n = 31), and 45.21 (5.46) for OSA−/HTN+ (n = 20; ANOVA P ⩽ 0.05). At time 2, mean (SD) raw global cognitive score was 40.18 (5.12) for OSA+/HTN+ (n = 20), 49.49 (4.22) for OSA+/HTN− (n = 19), 51.63 (3.91) for OSA−/HTN− (n = 23), and 43.51 (4.39) for OSA−/HTN+ (n = 17; ANOVA P ⩽ 0.05). At time 3, mean (SD) raw global cognitive score was 35.81 (5.21) for OSA+/HTN+ (n = 18), 48.74 (4.22) for OSA+/HTN− (n = 15), 53.68 (3.87) for OSA−/HTN− (n = 20), and 42.71 (2.81) for OSA−/HTN+ (n = 14; ANOVA P ⩽ 0.05). At time 4, mean (SD) raw global cognitive score was 32.79 (3.01) for OSA+/HTN+ (n = 13), 46.99 (3.27) for OSA+/HTN− (n = 12), 53.73 (2.21) for OSA−/HTN− (n = 14), and 40.01 (3.41) for OSA−/HTN+ (n = 10; ANOVA P ⩽ 0.05). The zero in A2 resulted from subtracting baseline score from baseline score for each individual at time 1 on the x-axis. To generate time 2 data points, baseline scores were subtracted from follow-up scores, and so on for times 3 and 4, respectively. (B1) The CSF amyloid-β (Aβ) change score is scaled such that 0 represents the mean score of all participants at baseline. For CSF Aβ, positive change scores indicate better pathology, and 1 unit represents approximately 1 SD of pathology change. There was a significant association of group with prospective CSF Aβ change after controlling for age at baseline, sex, APOE4 status, years of education (β = 1.73; 95% CI, 1.13 to 3.33; P = 0.001). Post hoc analyses demonstrated significantly faster rate of annual change in the OSA+/HTN+ (n = 23) group than OSA−/HTN+ (n = 20; β = 1.21; 95% CI, 1.02 to 2.41; P = 0.04), OSA+/HTN− (n = 24; β = 1.33; 95% CI, 1.07 to 2.59; P = 0.03), and OSA−/HTN− (n = 31; β = 1.87; 95% CI, 1.15 to 3.59; P < 0.001) groups. All 98 participants performed a lumbar puncture (LP) at baseline and follow-up, with a mean (SD) follow-up of 2.46 (0.64) years. To generate the trajectory plot, we grouped participants into those whose follow-up LP occurred within 1 to 2 years (n = 56) into Year 1 and those whose follow-up LP occurred within 2 to 3 years (n = 42) into Year 2. (Year 2 follow-up data: OSA+/HTN+ [n = 10], OSA+/HTN− [n = 10], OSA−/HTN+ [n = 9], and OSA−/HTN− [n = 13]). (B2) Trajectory plots of raw CSF Aβ change data in participants by OSA/HTN status over the follow-up period. CSF Aβ change scores were obtained by subtracting baseline scores from original follow-up measurements. Legend: Blue lines with OSA/HTN = 0 represent OSA−/HTN− (n = 31). Red lines with OSA/HTN = 1 represents OSA+/HTN− (n = 34). Green lines with OSA/HTN = 2 represents OSA−/HTN+ (n = 20). Brown lines with OSA/HTN = 3 represents OSA+/HTN+ (n = 23). All 98 participants performed an LP at baseline and follow-up, with a mean (SD) follow-up of 2.46 (0.64) years. To generate the trajectory plot, we grouped participants into those whose follow-up LP occurred within 1 to 2 years (n = 56) into Year 1 and those whose follow-up LP occurred within 2 to 3 years’ follow-up (n = 42) into Year 2. (Year 2 follow-up data: OSA+/HTN+ [n = 10], OSA+/HTN− [n = 10], OSA−/HTN+ [n = 9], and OSA−/HTN− [n = 13]). The raw CSF data are shown here: At baseline, mean (SD) CSF Aβ pg/ml was 612.43 (288.76) for OSA+/HTN+ (n = 23), 702.53 (214.84) for OSA+/HTN− (n = 24), 711.47 (232.42) for OSA−/HTN− (n = 31), and 652.29 (225.17) for OSA−/HTN+ (n = 20; ANOVA P ⩽ 0.05). At time 2 (Year 1), mean (SD) CSF Aβ pg/ml was 601.67 (258.23) for OSA+/HTN+ (n = 23), 698.43 (208.74) for OSA+/HTN− (n = 24), 713.74 (251.82) for OSA−/HTN− (n = 31), and 639.47 (211.21) for OSA−/HTN+ (n = 20; ANOVA P ⩽ 0.05). At time 3 (Year 2), mean (SD) CSF Aβ pg/ml was 594.43 (247.52) for OSA+/HTN+ (n = 10), 691.77 (197.44) for OSA+/HTN− (n = 10), 716.83 (272.52) for OSA−/HTN− (n = 13), and 619.22 (215.73) for OSA−/HTN+ (n = 9; ANOVA P ⩽ 0.05). The zero in B2 resulted from subtracting baseline score from baseline score for each individual at time 1 on the x-axis. To generate time 2 data points, baseline scores were subtracted from follow-up scores, and so on for times 3 and 4, respectively. CSF = cerebrospinal fluid.
The synergistic observation between OSA and HTN is novel, and in line with studies, showing either additive (9, 10) or synergistic (1, 2) effects of co-occurring risk factors on cognitive decline or AD. These findings underscore the importance of both OSA and HTN to CSF Aβ42 concentrations and cognitive decline in clinically normal older adults. In addition, strata-specific estimates confirmed the robust interaction between OSA and HTN such that OSA+/HTN+ individuals showed the steepest decline in cognition on follow-up, although we were not able to demonstrate that they were significantly different from HTN alone, most likely owing to the high collinearity shown by both variables. Notably, neither OSA nor HTN was associated with episodic memory; however, sleep-dependent memory tasks, in which encoding and recall are separated by a period of sleep, may be better suited to identify impacts of OSA and HTN (11).
Limitations of our study include our relatively small sample size, relatively young (mean, 69.6 yr) and well-educated (mean, 16.7 yr) participants, which may limit the generalizability of our findings. Overall, our findings highlight the importance of examining synergistic effects of lifestyle and health-related variables in the prevention of AD. Future studies examining the confluence of these exposures with other AD biomarkers as the outcome may help unravel potential causal mechanisms linking these synergistic exposures to AD progression.
Acknowledgments
Acknowledgment
The authors thank the staff of the NYU Alzheimer’s Disease Research Center, Center for Brain Health, Healthy Brain Aging and Sleep Center, and all the study participants.
Footnotes
Supported by the National Institute of Aging (K23AG068534, L30-AG064670, CIRAD P30AG059303 Pilot, and P30AG066512 NYU ADRC Developmental Project [O.M.B.]; R01AG12101, R01AG022374, R01AG13616, and RF1AG057570 [M.J.d.L.]; R21AG049348, R21AG055002, and R01AG056031 [R.S.O.]; R21AG059179 and R01AG056682 [A.W.V.]; R01AG056531 [R.S.O. and G.J.-L.]; and K07AG05268503 [G.J.-L.]) and the NHLBI (R25HL105444 [G.J.-L.], R01HL118624 [R.S.O.], and K24HL109156 [I.A.]) at the NIH; the Alzheimer’s Association (AARGD-21-8488397 [O.M.B.]); and the American Academy of Sleep Medicine Foundation (BS-231-20 [O.M.B.]). The funders had no role in the conception or preparation of this manuscript.
Originally Published in Press as DOI: 10.1164/rccm.202201-0107LE on May 12, 2022
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1. Bubu OM, Umasabor-Bubu OQ, Turner AD, Parekh A, Mullins AE, Kam K, et al. Alzheimer’s Disease Neuroimaging Initiative Self-reported obstructive sleep apnea, amyloid and tau burden, and Alzheimer’s disease time-dependent progression. Alzheimers Dement . 2020 doi: 10.1002/alz.12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rabin JS, Schultz AP, Hedden T, Viswanathan A, Marshall GA, Kilpatrick E, et al. Interactive associations of vascular risk and β-amyloid burden with cognitive decline in clinically normal elderly individuals: findings from the Harvard Aging Brain Study. JAMA Neurol . 2018;75:1124–1131. doi: 10.1001/jamaneurol.2018.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bubu OM, Umasabor-Bubu OQ, Andrade A, Chung A, Parekh A, Kam K, et al. Interactive associations of obstructive sleep apnea and β-amyloid burden among clinically normal and mild cognitive impairment elderly individuals: an examination of conversion risk [abstract] Sleep . 2019;42:A123. [Google Scholar]
- 4. Sharma RA, Varga AW, Bubu OM, Pirraglia E, Kam K, Parekh A, et al. Obstructive sleep apnea severity affects amyloid burden in cognitively normal elderly. A longitudinal study. Am J Respir Crit Care Med . 2018;197:933–943. doi: 10.1164/rccm.201704-0704OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, et al. National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC 7 report. JAMA . 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 6. De Santi S, Pirraglia E, Barr W, Babb J, Williams S, Rogers K, et al. Robust and conventional neuropsychological norms: diagnosis and prediction of age-related cognitive decline. Neuropsychology . 2008;22:469–484. doi: 10.1037/0894-4105.22.4.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaplan EGH, Weintraub S. Boston Naming Test. Philadelphia: Lea and Febiger; 1983. [Google Scholar]
- 8. Dugbartey AT, Townes BD, Mahurin RK. Equivalence of the color trails test and trail making test in nonnative English-speakers. Arch Clin Neuropsychol . 2000;15:425–431. [PubMed] [Google Scholar]
- 9. Kim HJ, Yang JJ, Kwon H, Kim C, Lee JM, Chun P, et al. Relative impact of amyloid-β, lacunes, and downstream imaging markers on cognitive trajectories. Brain . 2016;139:2516–2527. doi: 10.1093/brain/aww148. [DOI] [PubMed] [Google Scholar]
- 10. Vemuri P, Lesnick TG, Przybelski SA, Knopman DS, Preboske GM, Kantarci K, et al. Vascular and amyloid pathologies are independent predictors of cognitive decline in normal elderly. Brain . 2015;138:761–771. doi: 10.1093/brain/awu393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Varga AW, Kam K. The importance of sleep-dependent memory testing in positive airway pressure treatment of obstructive sleep apnea. Am J Respir Crit Care Med . 2021;203:1064–1065. doi: 10.1164/rccm.202101-0078ED. [DOI] [PMC free article] [PubMed] [Google Scholar]


