Coronavirus disease (COVID-19), caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has resulted in more than 6.2 million deaths globally. Despite the expeditious development of effective vaccinations and treatments, vulnerable individuals remain susceptible to complications from COVID-19, especially patients with cardiopulmonary disease (1).
The lungs are the prime target of SARS-CoV-2 infection, and a primary cause of death is severe pneumonia resulting in respiratory failure (2). In autopsy studies of COVID-19 decedents, investigators identified pulmonary histopathologic abnormalities frequently observed in patients with acute respiratory distress syndrome (ARDS), including diffuse alveolar damage and pulmonary vascular microthrombi (3). Pulmonary vascular complications have long been a pathological hallmark of ARDS (4, 5), and patients with ARDS who develop pulmonary hypertension (PH) and right ventricular dysfunction are at increased risk of death (6, 7). Mounting evidence suggests that severe COVID-19 causes prominent pulmonary vascular dysfunction, including endotheliopathy (8, 9), pulmonary vascular microthrombi (10, 11), and increased estimated dead space fraction (11, 12).
These observations led investigators to hypothesize that PH is a risk factor for adverse outcomes from COVID-19 and, conversely, that pulmonary arterial hypertension (PAH) therapies, which ameliorate pulmonary endothelial function, could protect patients from the pulmonary vascular effects of COVID-19. However, observational data were conflicting, with some studies reporting paradoxically favorable outcomes (13, 14) and others poor outcomes (15, 16). To date, the clinical trajectory of patients with COVID-19 with precapillary PH has not been adequately described.
In this issue of the Journal, Montani and colleagues (pp. 573–583) report the largest prospective study to describe the baseline characteristics and clinical outcomes of patients with precapillary PH who received diagnoses of COVID-19 (17). In the national French PH registry, 211 of 7,678 patients (2.7%) received diagnoses of COVID-19 (Figure 1). Investigators observed a high rate of hospitalization (60%) and in-hospital mortality (41.3%), which they highlight is well above the COVID-19 mortality cited for France during the study period (∼19%). Those at highest risk of dying were male, older, had more severe baseline PH, and were more medically comorbid.
Figure 1.
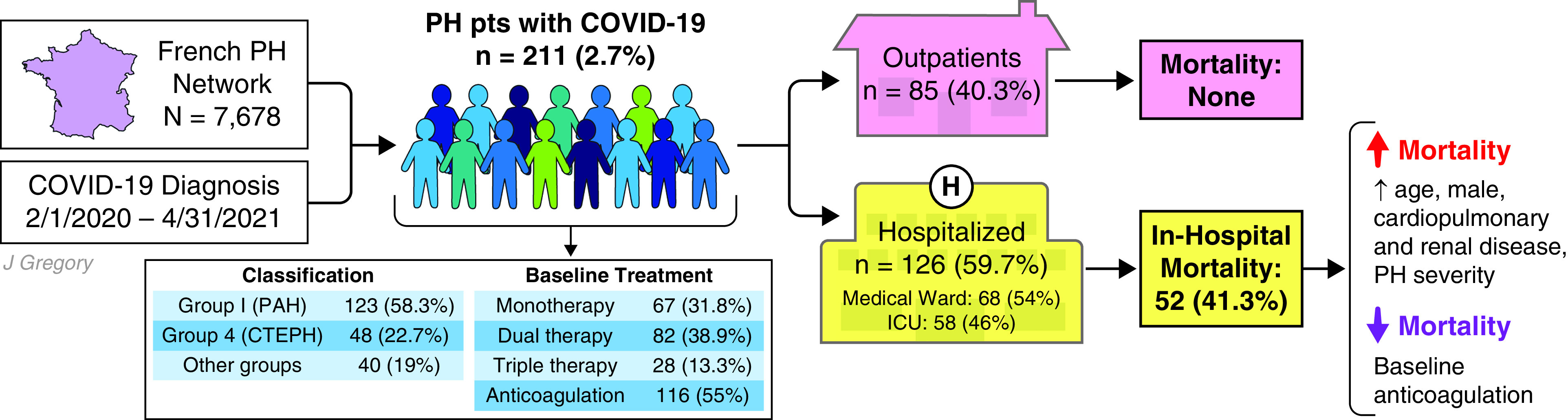
Patients with precapillary pulmonary hypertension (PH) are at increased risk of hospitalization and in-hospital mortality from coronavirus disease (COVID-19). Patients with underlying cardiopulmonary disease are at increased risk of adverse clinical outcomes from COVID-19, but observational data are conflicting regarding the risk posed to patients with PH. In this issue of the Journal, Montani and colleagues report results from the largest national prospective cohort study of patients with PH who received diagnoses of COVID-19, wherein they observed high rates of hospitalization (60%) and in-hospital mortality (41.3%). Patients at highest risk of dying were male, older, had more severe baseline PH, and were more medically comorbid. Pulmonary vasodilator therapies did not protect against in-hospital death, but patients on baseline anticoagulation were more likely to survive their hospitalization. This study establishes that patients with precapillary PH are at increased risk of adverse clinical outcomes from COVID-19, underscoring the public health importance of preventive measures in this high-risk population. CTEPH = chronic thromboembolic pulmonary hypertension; PAH = pulmonary arterial hypertension. Illustration by Jill Gregory.
A key strength of this study is its cohort design: given the centralized nature of the PH referral network in France, the investigators assembled the largest prospective cohort study of patients with PH with comprehensive baseline phenotyping and longitudinal follow-up. Patients underwent right heart catheterization within a median of 12 months before COVID-19 diagnosis, demonstrating definitive precapillary PH. A slight majority (56.9%) were in New York Heart Association functional class I or II; elevated natriuretic peptide concentrations were present in half (49.5%) before their diagnoses; most (84%) were on at least one PAH therapy, with monotherapy, dual therapy, and triple therapy observed in 31.8%, 38.9%, and 13.3%, respectively; and just over half (55%) were on therapeutic anticoagulation.
In addition, because the data collection spanned from the onset of the pandemic (when treatment options were limited) to the spring of 2021 (when corticosteroids were more widely used), the investigators were positioned to capture national secular trends in COVID-19 management. For example, comparing the period before and after September 2020, the proportion of patients treated with corticosteroids and high-flow oxygen increased from 9.8% and 14.6% to 75.3% and 48.2% (P < 0.001), respectively, with no change in the observed rate of mechanical ventilation (12.2% vs. 10.6%; P = 0.97) or survival (46.3% vs. 38.3%; P = 0.54). Unfortunately, the impact of vaccination on this population could not be addressed during the present study period, but ongoing longitudinal follow-up will help clarify this question.
There are several observations regarding prognosis worth emphasizing. First, the investigators identified anticipated risk factors for poor clinical outcomes in addition to the sex dimorphism characteristic of prognosis in both COVID-19 and PH: men are at increased risk of in-hospital mortality, with ∼2.5 times increased odds of death (95% confidence interval, 1.12–5.40; P = 0.025). Sex-based differences in pulmonary vascular–right ventricular response to acute illness with COVID-19 warrant further study. Second, although most patients (60%) required hospitalization, only a quarter (27.5%) required ICU admission, and although natriuretic peptide elevation was observed in 54.5% of hospitalized patients, only 11% of patients developed right ventricular failure requiring inotropic support, and two received extracorporeal membrane oxygenation. Few patients (11.1%) received mechanical ventilation—a therapy generally avoided in patients with right ventricular dysfunction—but if intubated, one-third survived, and of those who received high-flow oxygen, half survived. Finally, among the 74 patients discharged alive, only 5.4% died in the subsequent year. COVID-19 is definitively morbid if patients with PH develop respiratory and/or right ventricular failure, but the prognosis is not so grim as to preclude medically appropriate, goal-concordant care.
There was no suggestion that use of PAH therapies, alone or in combination, was protective against in-hospital death. However, patients on baseline anticoagulation (55%) were more likely to survive their hospitalization (59.1% vs. 42.3%; P = 0.03), but significance was lost when adjusting for age, sex, and functional class. Prior observational studies have suggested a protective effect of baseline anticoagulation (18), but clinical trials of therapeutic anticoagulation in COVID-19 have been largely neutral (19, 20), though hospital survival may be improved if anticoagulation is initiated early in non–critically ill patients (21). Future studies are needed to determine if early administration of endothelial-targeted therapies improves clinical outcomes in individuals with COVID-19 before the onset of severe pulmonary vascular dysfunction.
There are some limitations to the work that merit consideration when interpreting these results, as noted by the authors. First, the investigators included patients without confirmed COVID-19 but with suggestive radiographic appearance (in the early period before widespread testing availability), raising concern that alternative etiologies of severe pneumonia may have been inadvertently included in the sample. However, as this represents only seven patients, it is unlikely to affect the validity of their findings. Second, 31 patients (24.2%) had medical directives limiting care either at presentation or during their hospitalization. Code status orders that limit care can influence important clinical outcomes in clinical trials, including mortality (22). Accordingly, in this cohort, patients with limitations in care have a significantly higher mortality. Finally, the overall mortality for the cohort is 25%, but by PH group, mortality is 19% and 21% for groups 1 and 4, respectively, and doubles to 46% for other groups. Although PH group was not statistically associated with mortality in univariable analysis, this inflection in mortality for other PH groups suggests that the primary driver may be the higher burden of other systemic diseases, which are independently associated with adverse outcomes in COVID-19.
In conclusion, the authors are commended for compiling the largest, most comprehensive prospective cohort of well-phenotyped patients with precapillary PH—a study they were uniquely poised to accomplish. Their work corroborates prior observational data suggesting that patients with precapillary PH are at high risk of adverse clinical outcomes from COVID-19 and, notably, underscores the public health importance of equitable access to vaccination, early treatment, and other preventive measures for this high-risk population.
Footnotes
Supported by the Harvard Catalyst (KL2TR002542), the NIH (OT2HL161847), and the Massachusetts General Hospital.
Originally Published in Press as DOI: 10.1164/rccm.202205-0884ED on May 18, 2022
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Yek C, Warner S, Wiltz JL, Sun J, Adjei S, Mancera A, et al. Risk factors for severe COVID-19 outcomes among persons aged ⩾18 years who completed a primary COVID-19 vaccination series—465 health care facilities, United States, December 2020–October 2021. MMWR Morb Mortal Wkly Rep . 2022;71:19–25. doi: 10.15585/mmwr.mm7101a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ketcham SW, Bolig TC, Molling DJ, Sjoding MW, Flanders SA, Prescott HC. Causes and circumstances of death among patients hospitalized with COVID-19: a retrospective cohort study. Ann Am Thorac Soc . 2021;18:1076–1079. doi: 10.1513/AnnalsATS.202011-1381RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Carsana L, Sonzogni A, Nasr A, Rossi RS, Pellegrinelli A, Zerbi P, et al. Pulmonary post-mortem findings in a series of COVID-19 cases from northern Italy: a two-centre descriptive study. Lancet Infect Dis . 2020;20:1135–1140. doi: 10.1016/S1473-3099(20)30434-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Greene R, Lind S, Jantsch H, Wilson R, Lynch K, Jones R, et al. Pulmonary vascular obstruction in severe ARDS: angiographic alterations after i.v. fibrinolytic therapy. AJR Am J Roentgenol . 1987;148:501–508. doi: 10.2214/ajr.148.3.501. [DOI] [PubMed] [Google Scholar]
- 5. Zapol WM, Snider MT. Pulmonary hypertension in severe acute respiratory failure. N Engl J Med . 1977;296:476–480. doi: 10.1056/NEJM197703032960903. [DOI] [PubMed] [Google Scholar]
- 6. Nuckton TJ, Alonso JA, Kallet RH, Daniel BM, Pittet JF, Eisner MD, et al. Pulmonary dead-space fraction as a risk factor for death in the acute respiratory distress syndrome. N Engl J Med . 2002;346:1281–1286. doi: 10.1056/NEJMoa012835. [DOI] [PubMed] [Google Scholar]
- 7. Ryan D, Frohlich S, McLoughlin P. Pulmonary vascular dysfunction in ARDS. Ann Intensive Care . 2014;4:28. doi: 10.1186/s13613-014-0028-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ackermann M, Verleden SE, Kuehnel M, Haverich A, Welte T, Laenger F, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in COVID-19. N Engl J Med . 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Potus F, Mai V, Lebret M, Malenfant S, Breton-Gagnon E, Lajoie AC, et al. Novel insights on the pulmonary vascular consequences of COVID-19. Am J Physiol Lung Cell Mol Physiol . 2020;319:L277–L288. doi: 10.1152/ajplung.00195.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hariri LP, North CM, Shih AR, Israel RA, Maley JH, Villalba JA, et al. Lung histopathology in coronavirus disease 2019 as compared with severe acute respiratory syndrome and H1N1 influenza: a systematic review. Chest . 2021;159:73–84. doi: 10.1016/j.chest.2020.09.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Alba GA, Samokhin AO, Wang R-S, et al. Pulmonary endothelial NEDD9 and the prothrombotic pathophenotype of acute respiratory distress syndrome due to SARS-CoV-2 infection. Pulm Circ . 2022;159:e12071. doi: 10.1002/pul2.12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Grasselli G, Tonetti T, Protti A, Langer T, Girardis M, et al. Pathophysiology of COVID-19-associated acute respiratory distress syndrome: a multicentre prospective observational study. Lancet Respir Med . 2020;8:1201–1208. doi: 10.1016/S2213-2600(20)30370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nuche J, Pérez-Olivares C, Segura de la Cal T, Jiménez López-Guarch C, Arribas Ynsaurriaga F, Escribano Subías P. Clinical course of COVID-19 in pulmonary arterial hypertension patients. Rev Esp Cardiol (Engl Ed) . 2020;73:775–778. doi: 10.1016/j.rec.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Belge C, Quarck R, Godinas L, Montani D, Escribano Subias P, Vachiéry JL, et al. COVID-19 in pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension: a reference centre survey. ERJ Open Res . 2020;6 doi: 10.1183/23120541.00520-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sulica R, Cefali F, Motschwiller C, Fenton R, Barroso A, Sterman D. COVID-19 in pulmonary artery hypertension (PAH) patients: observations from a large PAH center in New York City. Diagnostics (Basel) . 2021;11:128. doi: 10.3390/diagnostics11010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lee JD, Burger CD, Delossantos GB, Grinnan D, Ralph DD, Rayner SG, et al. A survey-based estimate of COVID-19 incidence and outcomes among patients with pulmonary arterial hypertension or chronic thromboembolic pulmonary hypertension and impact on the process of care. Ann Am Thorac Soc . 2020;17:1576–1582. doi: 10.1513/AnnalsATS.202005-521OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Montani D, Certain M-C, Weatherald J, Jaïs X, Bulifon S, Noel-Savina E, et al. French PH Network PULMOTENSION Investigators COVID-19 in patients with pulmonary hypertension: a national prospective cohort study. Am J Respir Crit Care Med . 2022;206:573–583. doi: 10.1164/rccm.202112-2761OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chocron R, Galand V, Cellier J, Gendron N, Pommier T, Bory O, et al. Critical COVID‐19 France Investigators Anticoagulation before hospitalization is a potential protective factor for COVID-19: insight from a French multicenter cohort study. J Am Heart Assoc . 2021;10:e018624. doi: 10.1161/JAHA.120.018624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lopes RD, de Barros E Silva PGM, Furtado RHM, Macedo AVS, Bronhara B, Damiani LP, et al. ACTION Coalition COVID-19 Brazil IV Investigators Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer concentration (ACTION): an open-label, multicentre, randomised, controlled trial. Lancet . 2021;397:2253–2263. doi: 10.1016/S0140-6736(21)01203-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Goligher EC, Bradbury CA, McVerry BJ, Lawler PR, Berger JS, Gong MN, et al. REMAP-CAP Investigators ACTIV-4a Investigators; ATTACC Investigators. Therapeutic anticoagulation with heparin in critically ill patients with COVID-19. N Engl J Med . 2021;385:777–789. doi: 10.1056/NEJMoa2103417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lawler PR, Goligher EC, Berger JS, Neal MD, McVerry BJ, Nicolau JC, et al. ATTACC Investigators ACTIV-4a Investigators; REMAP-CAP Investigators. Therapeutic anticoagulation with heparin in noncritically ill patients with COVID-19. N Engl J Med . 2021;385:790–802. doi: 10.1056/NEJMoa2105911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Turnbull AE, Ruhl AP, Lau BM, Mendez-Tellez PA, Shanholtz CB, Needham DM. Timing of limitations in life support in acute lung injury patients: a multisite study. Crit Care Med . 2014;42:296–302. doi: 10.1097/CCM.0b013e3182a272db. [DOI] [PMC free article] [PubMed] [Google Scholar]


