Abstract
Rationale
Several Western studies have reported that participants with preserved ratio impaired spirometry (PRISm) have higher risks of airflow limitation (AFL) and death. However, evidence in East Asian populations is limited.
Objectives
To investigate the relationship between PRISm and the risks of death and incident AFL in a Japanese population.
Methods
A total of 3,032 community-dwelling Japanese participants aged ⩾40 years were seen in follow-up for a median of 5.3 years by annual spirometry examinations. Participants were classified into lung function categories at baseline as follows: normal spirometry (FEV1/FVC ⩾0.70 and FEV1 ⩾80% predicted), PRISm (⩾0.70 and <80%), AFL Global Initiative for Chronic Obstructive Lung Disease 1 (<0.70 and ⩾80%), and AFL Global Initiative for Chronic Obstructive Lung Disease 2–4 (<0.70 and <80%). Hazard ratios (HRs) and their 95% confidence intervals were computed using a Cox proportional hazards model.
Measurements and Main Results
During the follow-up period, 131 participants died, 22 of whom died of cardiovascular disease, and 218 participants developed AFL. When examining the prognosis of each baseline lung function category, participants with PRISm had higher risks of all-cause death (HR, 2.20; 95% confidence interval, 1.35–3.59) and cardiovascular death (HR, 4.07; 1.07–15.42) than those with normal spirometry after adjusting for confounders. Moreover, the multivariable-adjusted risk of incident AFL was greater in participants with PRISm than in those with normal spirometry (HR, 2.48; 1.83–3.36).
Conclusions
PRISm was associated with higher risks of all-cause and cardiovascular death and a greater risk of the development of AFL in a Japanese community.
Keywords: spirometry classification, spirometry statistics and numerical data, spirometry mortality, lung disease epidemiology
At a Glance Commentary
Scientific Knowledge on the Subject
The preserved ratio impaired spirometry (PRISm) functional pattern is a heterogeneous condition with simultaneous declines in FEV1 and FVC and is generally categorized into either restrictive or normal lung function. PRISm has been associated with a higher incidence of chronic obstructive pulmonary disease and an increased risk of mortality, especially cardiovascular disease mortality, in Western populations. However, the relationship between PRISm and the risks of mortality and incidence of chronic obstructive pulmonary disease among East Asian populations, who have lifestyles and body types different from those of Western populations, has not been fully understood.
What This Study Adds to the Field
This is the first longitudinal study examining the association of PRISm with the risk for all-cause and cause-specific mortality and lung spirometry function in a Japanese population as a subgroup of East Asians. We confirmed that Japanese participants with PRISm had increased risks of all-cause and cardiovascular death and a higher incidence of airflow limitation. These results support the recent studies showing that PRISm is an important clinical condition or a subtype of spirometry categories related to the risks of mortality and lung dysfunction in not only Westerners but also Japanese individuals.
Chronic obstructive pulmonary disease (COPD) is a progressive, life-threatening lung disease that can cause breathlessness, acute exacerbations of symptoms with worsening respiratory distress, and even death. In fact, 3.2 million people died of COPD in 2015 worldwide (1). Moreover, the number of deaths, as well as the estimated global prevalence of COPD, increased from 1990 to 2015, probably because of the aging global population and population growth (1). Therefore, COPD is a major global public health problem (1). Airflow limitation (AFL), which is a spirometric hallmark of chronic obstructive lung diseases such as COPD, asthma, and bronchiectasis, is defined by spirometry with a reduced FEV1 to FVC ratio (2, 3). When FEV1 and FVC are simultaneously decreased, the FEV1/FVC will be normal in spite of potentially low lung function. This pattern of spirometric parameters is defined as preserved ratio impaired spirometry (PRISm) (4). Patients with PRISm do not meet the criteria for COPD; these individuals are generally classified as having a Global Initiative for Chronic Obstructive Lung Disease (GOLD) unclassified or nonspecific pattern (5, 6) and are often overlooked. Nonetheless, PRISm is increasingly recognized to have a clinically important role in prognosis (7–11). Several population-based studies conducted in Western populations have shown that participants with PRISm have a higher incidence of AFL and a higher risk of mortality than those with normal lung function (7, 9–11). As far as we know, however, there have been no studies examining the association of PRISm with mortality in East Asians. Because lifestyle factors such as obesity and smoking status affect lung function, the prevalence of PRISm and its influence on mortality and lung function may differ between Asians and Westerners, who have different rates of obesity and smoking (12–19). Japanese males, for example, have a lower prevalence of obesity and a higher smoking rate than their Western counterparts (20, 21). In addition, average life expectancy and frequencies of various causes of death have been shown to differ between Japan and several Western nations (22, 23). Therefore, evaluating the relationship between PRISm and mortality and its complications in the general Japanese population as a subgroup of East Asians may be useful to provide more appropriate treatment opportunities.
The present study aimed to investigate the relationship between PRISm and the risks of all-cause and cause-specific death in a general Japanese population. We also estimated the risk of the development of AFL in participants with PRISm in a Japanese community.
Methods
Study Population and Data
The present study was approved by the Kyushu University Institutional Review Board for Clinical Research. Since 1961, a population-based prospective cohort study has been conducted longitudinally to investigate lifestyle-related diseases and their risk factors in the town of Hisayama, Japan. The age, nutrient intake, and occupational distributions of residents in the town of Hisayama are similar to those of Japan as a whole, based on data from the national census and nutrition survey (24–26). More details of this cohort study have been described elsewhere (26). As part of an annual health examination, a survey of lung function with spirometry was performed from 2012 to 2013 as a baseline survey and repeated annually until 2017. At baseline, a total of 3,396 residents aged ⩾40 years (72.6% of the whole population in the town of Hisayama in this age group) participated in a health examination and underwent a comprehensive health assessment. After 6 participants who refused to participate in the epidemiological studies and 358 participants without available spirometry data to evaluate the lung function categories were excluded, the remaining 3,032 participants (1,340 men and 1,692 women) were enrolled in the follow-up survey in the present study. For analysis of the transition of lung function categories for 5 years, 351 participants with no available spirometry data during the follow-up period were excluded. Thus, a total of 2,681 (88.4% of the baseline population) participants were included in the analysis of the transition of lung function categories. For an additional analysis of the development of AFL, 431 participants who already had AFL at the baseline survey were further excluded. Finally, a total of 2,250 participants (89.7% of the population with normal spirometry or PRISm at baseline; 917 men and 1,333 women) were included in the analysis of the development of AFL (see Figure E1 in the online supplement).
Definitions of PRISm and AFL
At the baseline survey, a prebronchodilator spirometry examination was performed following the guidelines of the Japanese Respiratory Society (27). Two to four measurements were taken using a CHESTGRAPH HI-105 electronic spirometer (Chest MI) to obtain appropriate flow–volume loops. FEV1, FVC, and FEV1/FVC were obtained from the visually finest loop having the highest sum of FEV1 and FVC as assessed by pulmonary physicians. Predicted FEV1 and FVC were calculated using reference equations for the Japanese population with the age, sex, and height that were reported by the Clinical Pulmonary Functions Committee of the Japanese Respiratory Society in 2014 (28). The lung function categories were defined according to the modified criteria of GOLD and the previous reports (2, 7) as follows: normal spirometry (FEV1/FVC ⩾0.70 and FEV1 ⩾80% predicted), PRISm (FEV1/FVC ⩾0.70 and FEV1 <80% predicted), AFL GOLD 1 (FEV1/FVC <0.70 and FEV1 ⩾80% predicted), and AFL GOLD 2–4 (FEV1/FVC <0.70 and FEV1 <80% predicted).
Follow-Up Survey
For the mortality analysis, all participants were seen in follow-up for a median of 5.3 years from the date of comprehensive assessment at baseline to November 2017. When a participant died, all the medical information related to his or her death, including the cause of death, was collected from the physician records, and the death certificate was obtained. An autopsy was performed at the Department of Pathology of Kyushu University if consent for autopsy was obtained. No participants were lost to follow-up.
For analysis of the transition of lung function categories and for analysis of the development of AFL, prebronchodilator spirometry examinations were repeated at the annual health examination throughout the follow-up period until 2017 in the same way as at the baseline survey. Participants who had at least one follow-up spirometry examination were classified into the following eight categories generated on the basis of transition of lung function patterns using the data from baseline and the follow-up period as in a previous study (29):
-
1.
Consistent normal (normal spirometry at baseline and all participated follow-up surveys)
-
2.
Incident PRISm (normal spirometry at baseline and PRISm in at least one of the follow-up surveys, plus never AFL)
-
3.
Incident AFL (normal spirometry or PRISm at baseline and AFL in at least one of the follow-up surveys)
-
4.
Inconsistent PRISm (PRISm at baseline and in <50% of the follow-up surveys, plus never AFL)
-
5.
Recurrent PRISm (PRISm at baseline and in at least 50% of the follow-up surveys, plus never AFL)
-
6.
Inconsistent AFL without PRISm (AFL at baseline and in <50% of the follow-up surveys, plus never PRISm)
-
7.
Inconsistent AFL with PRISm (AFL at baseline and in <50% of the follow-up surveys, plus PRISm)
-
8.
Recurrent AFL (AFL at baseline and in at least 50% of the follow-up surveys)
Among the total of 2,681 participants entered into the analysis of the transition of lung function categories, 2,379 participants (88.7%) underwent spirometry examination more than twice during the follow-up period. The median number of spirometry examinations during the follow-up period was 4 per participant (interquartile range, 3–4).
For the analysis of the development of AFL, the onset of AFL was determined as presenting with an FEV1/FVC of <0.70 for the first time during the follow-up period. As an alternative outcome measure, the annual change rate in FEV1/FVC for each number of visits was calculated as follows: annual change rate in FEV1/FVC (%) = (FEV1/FVC at each follow-up time point [%]) − FEV1/FVC at baseline [%])/follow-up duration at each time point (years).
Clinical Evaluation and Laboratory Measurements
Participants completed a self-administered questionnaire including smoking status (never, former, and current), cumulative consumption of cigarettes, alcohol intake, physical activity, history of cancer, treatment with bronchodilators, inhaled corticosteroids (ICSs), antihypertensive agents, glucose-lowering agents (including insulin therapy), and lipid-modifying agents. The questionnaire was checked by trained interviewers. Pack-years were defined as years of smoking multiplied by the daily number of smoked cigarettes divided by 20. The medication information was collected from the drug dispensing data and checked by the pharmacists and/or physicians to confirm the contents. Bronchodilators were defined as short- or long-acting β2-agonists or muscarinic antagonists, or xanthine. ICS was defined as any ICS, such as fluticasone propionate, mometasone furoate, ciclesonide, or budesonide. Alcohol drinking was defined as current or not. A regular exercise group was defined as participants engaging in sports or other forms of exertion at least three times per week during their leisure time. Body height and weight were measured in light clothing without shoes. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Blood pressure was measured three times using an automated sphygmomanometer (BP-203 RVIIIB, Omron Healthcare) in a sitting position after rest for at least 5 minutes, and the average values were used in the analyses. Hypertension was defined as a systolic blood pressure ⩾140 mm Hg, diastolic blood pressure ⩾90 mm Hg, or current treatment with antihypertensive agents. Diabetes mellitus was defined as fasting plasma glucose levels ⩾7.0 mmol/L, 2-hour postload or casual glucose levels ⩾11.1 mmol/L, or treatment with current oral antidiabetic drugs or insulin. Serum total and high-density lipoprotein cholesterol levels were determined enzymatically. Dyslipidemia was defined as total serum cholesterol levels ⩾5.7 mmol/L, serum high-density lipoprotein cholesterol <1.03 mmol/L, or current treatment with lipid-modifying agents. Serum creatinine concentrations were measured using an enzymatic method, and the estimated glomerular filtration rate was calculated using the Japanese coefficient-modified Chronic Kidney Disease Epidemiology Collaboration equation (30). Kidney dysfunction was defined as estimated glomerular filtration rate <60 mL/min/1.73 m2. Electrocardiogram abnormalities were defined as left ventricular hypertrophy (Minnesota Code 3-1), ST depression (4-1,2, 3), or atrial fibrillation (8-3). Serum N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels were measured using an Elecsys proBNP Immunoassay (Roche Diagnostics). History of cardiovascular disease was defined as any previous events of stroke or coronary artery disease, which was adjudicated on the basis of physical examinations and a review of all available clinical information, including medical records and imaging. Cause-specific death was assessed using all available medical information, including autopsy findings, and reviewed by several physicians and then coded according to the International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD-10). Causes of death were classified into the following categories: cardiovascular disease death (ICD-10 codes I00–I99), cancer death (ICD-10 codes C00–C97), or respiratory disease death (ICD-10 codes J00–J99).
Statistical Analysis
At baseline, the means of continuous variables and the frequencies of categorical variables were compared for each of the four lung function categories using the Dunnett test and logistic regression analysis, respectively, with normal spirometry as the reference. Serum NT-proBNP and smoking pack-years were determined as the median and interquartile range from the baseline characteristics of the population. However, for the statistical analysis, NT-proBNP values were log-transformed because their distribution was skewed. Pack-years were compared for each lung function category with the Wilcoxon rank-sum test. The cumulative survival rates for all-cause death were calculated according to spirometry categories by the Kaplan-Meier method and compared using a log-rank test with Dunnett’s two-tailed comparisons of normal spirometry for each lung function category at baseline. The Cox proportional hazards model was used to estimate the hazard ratios (HRs) with 95% confidence intervals (CIs) for all-cause death, cardiovascular disease death, cancer death, and respiratory disease death. In the multivariable-adjusted analysis, the risk estimates were adjusted for potential confounding factors at baseline as follows: model 1, adjusted for 10-year age groups (multiple categorical variables), sex, current smoking, pack-years, and BMI; model 2, adjusted for the covariates included in model 1 plus hypertension, diabetes, dyslipidemia, electrocardiogram abnormalities, history of cardiovascular disease, history of cancer, current drinking, and regular exercise. As a sensitivity analysis, we used the lower limit of normal (LLN) thresholds of FEV1/FVC, in which FEV1/FVC lower than LLN was used for the definition of AFL with the Japanese reference equations (28, 31). We also performed analyses that were restricted to nonusers of bronchodilators, nonusers of ICSs, participants with NT-proBNP <300 pg/ml, or participants without kidney dysfunction. The heterogeneity by age and sex in the risk of all-cause death of participants with PRISm at baseline against those with normal spirometry at baseline was tested by adding a multiplicative interaction term to the relevant Cox model, including all the above-mentioned confounding factors. This analysis was performed by excluding 523 participants with AFL GOLD 1–4 at baseline, because we were interested only in the heterogeneity of mortality between the normal spirometry and the PRISm at baseline.
For the analysis of the development of AFL, the Cox proportional hazards model was used to estimate the HRs for PRISm against normal spirometry at baseline. In the multivariable-adjusted analysis, the risk estimates were adjusted for potential confounding factors at baseline as follows: model 1, adjusted for 10-year age groups, sex, current smoking, pack-years; model 2, adjusted for the covariates included in model 1 plus BMI, hypertension, diabetes, dyslipidemia, current drinking, regular exercise, and FEV1/FVC at baseline. For sensitivity analyses, we also performed an analysis using the LLN threshold of FEV1/FVC in which FEV1/FVC lower than LLN was used to define AFL; the onset of AFL was determined as the first time that FEV1/FVC of <0.70 was observed two or more times in spirometry examinations during the follow-up period, and a competing risk analysis was performed using the method proposed by Fine and Gray, in which all-cause death was treated as a competing event (32). We also performed analyses restricted to nonusers of bronchodilators, nonusers of ICSs, participants with NT-proBNP <300 pg/ml, or participants without kidney dysfunction. The annual change rate in FEV1/FVC over time was evaluated by using a general linear mixed model with a random slope, including the aforementioned covariates and the interaction term between the baseline spirometry categories (normal spirometry vs. PRISm) and the number of visit years during the follow-up period.
The SAS software package version 9.4 (SAS Institute) was used to perform all statistical analyses. Two-sided values of P < 0.05 were considered statistically significant in all analyses.
Results
Prevalence and Characteristics of Each Lung Function Category at Baseline for Mortality Analysis
First, to evaluate the association between each lung function category and mortality, 358 participants without available spirometry data at baseline were excluded (Figure E1). Those excluded participants at baseline had older age and more comorbidities and used a bronchodilator more frequently than the participants included in the mortality analysis (Table E1). Among the remaining 3,032 participants, the crude prevalence rates of PRISm, AFL GOLD 1, and AFL GOLD 2–4 were 9.9% (n = 301), 7.1% (n = 216), and 10.1% (n = 307), respectively. The baseline characteristics of the participants according to the lung function categories are shown in Table 1. The participants with PRISm, AFL GOLD 1, and AFL GOLD 2–4 were significantly older and had greater pack-years than those with normal spirometry. The frequencies of hypertension, antihypertensive agents, diabetes, kidney dysfunction, and history of cardiovascular disease were higher in the participants with PRISm, AFL GOLD 1, and AFL GOLD 2–4 than in those with normal spirometry. The participants with PRISm were more likely to have higher BMI, dyslipidemia, and electrocardiogram abnormalities and more likely to be using a lipid-lowering drug than those with normal spirometry.
Table 1.
Baseline Characteristics of Participants, According to Baseline Lung Function Categories
| Lung Function Categories |
||||
|---|---|---|---|---|
| Characteristics | Normal Spirometry | PRISm | AFL GOLD 1 | AFL GOLD 2–4 |
| (n = 2,208) | (n = 301) | (n = 216) | (n = 307) | |
| Age, yr | 61.2 (11.9) | 65.0 (11.6)* | 69.9 (10.1)* | 69.2 (11.9)* |
| Men, % | 41.2 | 41.5 | 53.7* | 61.6* |
| Height, cm | 158.5 (9.4) | 156.4 (9.4)* | 157.2 (8.6) | 158.4 (9.2) |
| BMI, kg/m2 | 23.2 (3.4) | 24.1 (4.0)* | 22.4 (2.9)* | 22.9 (3.6) |
| ⩾25.0 kg/m2, % | 26.4 | 39.9* | 17.6* | 22.5 |
| <18.5 kg/m2, % | 6.7 | 6.6 | 7.4 | 9.4 |
| Hypertension, % | 47.7 | 66.4* | 60.2* | 60.9* |
| Systolic blood pressure, mm Hg | 129 (19) | 134 (19)* | 131 (17) | 133 (19)* |
| Diastolic blood pressure, mm Hg | 77 (11) | 77 (12) | 76 (10) | 77 (11) |
| Antihypertensive agents, % | 32.2 | 48.5* | 47.2* | 46.3* |
| Dyslipidemia, % | 52.5 | 62.8* | 49.5 | 50.2 |
| Serum total cholesterol, mmol/L | 5.28 (0.93) | 5.23 (1.02) | 5.13 (0.93) | 5.02 (0.93)* |
| Serum HDL cholesterol, mmol/L | 1.69 (0.44) | 1.62 (0.42)* | 1.69 (0.44) | 1.59 (0.47)* |
| Lipid-modifying agents, % | 21.1 | 31.9* | 25.0 | 27.0 |
| Diabetes mellitus, % | 15.0 | 28.2* | 21.3* | 24.1* |
| Hemoglobin A1c, % | 5.7 (0.7) | 6.0 (0.9)* | 5.7 (0.5) | 5.8 (0.6) |
| Glucose-lowering agents, % | 8.1 | 17.6* | 9.7 | 10.7 |
| Kidney dysfunction, % | 13.3 | 20.9* | 27.8* | 25.4* |
| eGFR, ml/min/1.73 m2 | 73.3 (12.6) | 70.2 (14.9)* | 66.3 (12.6)* | 66.7 (14.4)* |
| ECG abnormalities, % | 10.3 | 16.3* | 13.4 | 19.9* |
| History of cardiovascular disease, % | 4.0 | 10.6* | 9.3* | 8.8* |
| History of cancer, % | 7.9 | 7.6 | 12.5 | 13.7* |
| Smoking status | ||||
| Current, % | 15.1 | 16.9 | 20.8 | 23.5* |
| Former, % | 26.0 | 25.6 | 31.5 | 42.0* |
| Never, % | 58.8 | 57.5 | 47.7* | 34.5* |
| Pack-years† | 25 (14–41) | 36 (20–46)* | 38 (23–52)* | 39 (22–54)* |
| Current drinking, % | 51.4 | 45.5 | 51.4 | 46.9 |
| Regular exercise, % | 14.6 | 15.6 | 21.3 | 13.4 |
| Bronchodilators, % | 0.8 | 1.3 | 6.5* | 14.3* |
| Inhaled corticosteroids, % | 0.5 | 1.3 | 3.7* | 11.7* |
| Serum NT-proBNP ⩾300 pg/ml, % | 3.3 | 7.6* | 6.5* | 10.1* |
| Serum NT-proBNP, pg/ml‡ | 45 (27–80) | 59 (32–121)* | 66 (40–116)* | 69 (36–138)* |
| FEV1, L | 2.40 (0.63) | 1.71 (0.45)* | 2.08 (0.45)* | 1.55 (0.45)* |
| FEV1/FVC, % | 78.0 (4.7) | 75.6 (4.4)* | 66.4 (3.1)* | 61.2 (7.8)* |
| FEV1 % predicted, % | 97.3 (10.5) | 72.9 (7.2)* | 90.4 (8.3)* | 64.7 (12.3)* |
| FVC, L | 3.08 (0.79) | 2.26 (0.60)* | 3.13 (0.68) | 2.53 (0.68)* |
| FVC % predicted, % | 99.1 (10.7) | 76.2 (8.8)* | 106.1 (9.6)* | 82.5 (12.7)* |
Definition of abbreviations: AFL = airflow limitation; BMI = body mass index; ECG = electrocardiogram; eGFR = estimated glomerular filtration rate; GOLD = Global Initiative for Chronic Obstructive Lung Disease; HDL = high-density lipoprotein; NT-proBNP = N-terminal pro-B-type natriuretic peptide; PRISm = preserved ratio impaired spirometry.
Data are presented as the mean values (standard deviation) or percentages. Numbers of participants with missing data were as follows: 2 for serum HDL cholesterol, 1 for hemoglobin A1c, 4 for ECG abnormalities, 11 for pack-years, 1 for regular exercise, 1 for bronchodilators, 1 for inhaled corticosteroids, 3 for serum NT-proBNP and serum NT-proBNP ⩾300 pg/ml.
P < 0.05 versus normal spirometry at baseline with Dunnett’s test.
The values are shown as median values (interquartile range) of pack-years calculated in only current or former smokers.
The values are shown as median values (interquartile range) of serum NT-proBNP.
Mortality by Baseline Lung Function Category
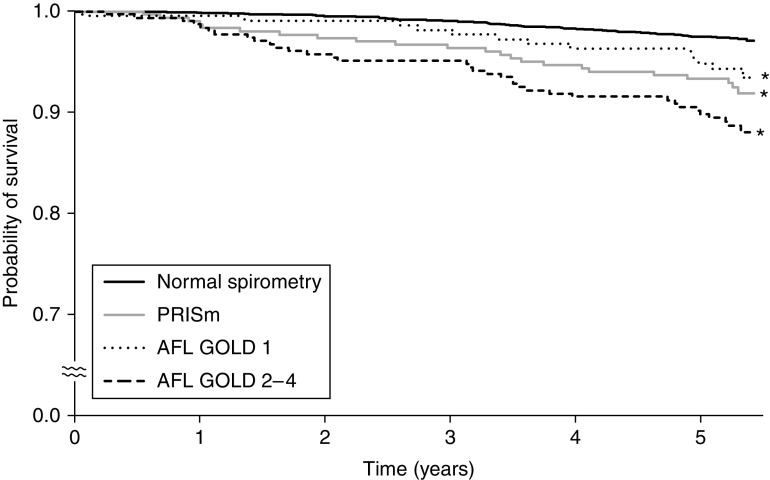
During the follow-up period, a total of 131 (4.3%) participants died. The crude cumulative survival rate was significantly lower in participants with PRISm, AFL GOLD 1, and AFL GOLD 2–4 as compared with participants with normal spirometry (Figure 1). The age- and sex-adjusted HRs for all-cause death were significantly higher in participants with PRISm and AFL GOLD 2–4 than in those with normal spirometry (both P < 0.01) (Table 2). These associations remained significant after additional adjustment for current smoking, pack-years, and BMI (model 1, HR, 2.20 [95% CI, 1.35–3.59] for PRISm; HR, 1.71 [95% CI, 1.09–2.68] for AFL GOLD 2–4) and after adjustment for other risk factors in addition to those in model 1 (model 2, HR, 2.00 [95% CI, 1.22–3.30] for PRISm; HR, 1.60 [95% CI, 1.02–2.53] for AFL GOLD 2–4).
Figure 1.
Kaplan-Meier plot of mortality by baseline lung function categories. *P < 0.05 versus normal spirometry. AFL = airflow limitation; GOLD = Global Initiative for Chronic Obstructive Lung Disease; PRISm = preserved ratio impaired spirometry.
Table 2.
Hazard Ratios and Their 95% Confidence Intervals for Mortality, According to Baseline Lung Function Categories
| Lung Function Categories | No. of Deaths/Participants | Crude Incidence Rate (per 103 Person-Years) | Hazard Ratio (95% CI) |
||
|---|---|---|---|---|---|
| Age- and Sex-adjusted | Model 1 | Model 2 | |||
| All-cause death | |||||
| Normal spirometry | 60/2,208 | 5.2 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| PRISm | 23/301 | 15.0 | 2.30 (1.42 to 3.72)* | 2.20 (1.35 to 3.59)* | 2.00 (1.22 to 3.30)* |
| AFL GOLD 1 | 13/216 | 11.7 | 1.05 (0.57 to 1.94) | 1.02 (0.55 to 1.87) | 1.01 (0.55 to 1.87) |
| AFL GOLD 2–4 | 35/307 | 22.8 | 1.88 (1.21 to 2.93)* | 1.71 (1.09 to 2.68)* | 1.60 (1.02 to 2.53)* |
| Cardiovascular death | |||||
| Normal spirometry | 5/2,208 | 0.4 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| PRISm | 4/301 | 2.6 | 4.72 (1.27 to 17.60)* | 4.07 (1.07 to 15.42)* | 3.20 (0.84 to 12.27) |
| AFL GOLD 1 | 3/216 | 2.7 | 2.93 (0.68 to 12.51) | 2.92 (0.68 to 12.50) | 2.63 (0.61 to 11.35) |
| AFL GOLD 2–4 | 10/307 | 6.5 | 6.60 (2.13 to 20.47)* | 6.02 (1.90 to 19.04)* | 4.58 (1.43 to 14.65)* |
| Cancer death | |||||
| Normal spirometry | 37/2,208 | 3.2 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| PRISm | 8/301 | 5.2 | 1.30 (0.60 to 2.80) | 1.13 (0.52 to 2.45) | 1.12 (0.51 to 2.48) |
| AFL GOLD 1 | 7/216 | 6.3 | 0.97 (0.43 to 2.20) | 0.95 (0.42 to 2.18) | 0.99 (0.43 to 2.26) |
| AFL GOLD 2–4 | 15/307 | 9.8 | 1.39 (0.74 to 2.61) | 1.27 (0.67 to 2.41) | 1.20 (0.63 to 2.31) |
| Respiratory disease death | |||||
| Normal spirometry | 5/2,208 | 0.4 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| PRISm | 2/301 | 1.3 | 2.18 (0.42 to 11.26) | 4.26 (0.74 to 24.37) | 3.39 (0.53 to 21.52) |
| AFL GOLD 1 | 0/216 | 0 | — | — | — |
| AFL GOLD 2–4 | 3/307 | 2.0 | 1.23 (0.28 to 5.48) | 1.03 (0.23 to 4.66) | 0.85 (0.17 to 4.12) |
Definition of abbreviations: AFL = airflow limitation; CI = confidence interval; GOLD = Global Initiative for Chronic Obstructive Lung Disease; PRISm = preserved ratio impaired spirometry.
Model 1 was adjusted for age, sex, current smoking, smoking pack-years, and body mass index. Model 2 was adjusted for the covariates in model 1 plus hypertension, diabetes mellitus, dyslipidemia, electrocardiogram abnormalities, history of cardiovascular diseases, history of cancer, current drinking, and regular exercise.
P < 0.05 versus normal spirometry at baseline.
Next, we performed sensitivity analyses to examine the association between each baseline lung function category and all-cause death using the criterion of LLN threshold for determining AFL (Table E2). Regardless of the criterion used, we observed significant associations of PRISm and AFL GOLD 2–4 with all-cause death. In addition, we conducted the analyses by using never smoker, past smoker, and current smoker instead of current smoker and pack-years for smoking status as adjustment factors, or by using age and age-squared instead of 10-year age groups as covariates. As a consequence, similarly significant associations were observed in these analyses (Tables E3 and E4). When we performed additional sensitivity analyses among various subgroups of participants (i.e., nonusers of bronchodilators, nonusers of ICSs, participants with serum NT-proBNP levels <300 pg/ml, and participants without kidney dysfunction), the findings were also substantially unchanged (Table E5). In the subgroup analyses of age and sex among 2,509 participants after excluding 523 participants with AFL GOLD 1–4, there was no evidence of heterogeneity in the multivariable-adjusted risk of all-cause death of participants with PRISm against those with normal spirometry: HR of 3.07 (95% CI, 0.94–10.07) for ages 40–64 years versus HR of 1.86 (95% CI, 1.05–3.30) for age ⩾65 years and HR of 2.18 (95% CI, 1.15–4.11) for men versus HR of 1.68 (95% CI, 0.68–4.18) for women (both P values for heterogeneity >0.49).
We also examined the associations of each baseline lung function category with cause-specific death. The number and frequencies of cause of death in each baseline lung function category are shown in Table E6. The participants with PRISm and AFL GOLD 2–4 had significantly greater risks of cardiovascular death than those with normal spirometry in model 1, but the increased risk of cardiovascular death in participants with PRISm did not reach the statistically significant level in model 2 (Table 2). There was no significant association of PRISm, AFL GOLD 1, or AFL GOLD 2–4 with the risk of cancer death or respiratory disease death. When we used the criterion of threshold for determining AFL, the findings were not substantially changed.
Transition of Lung Function Categories
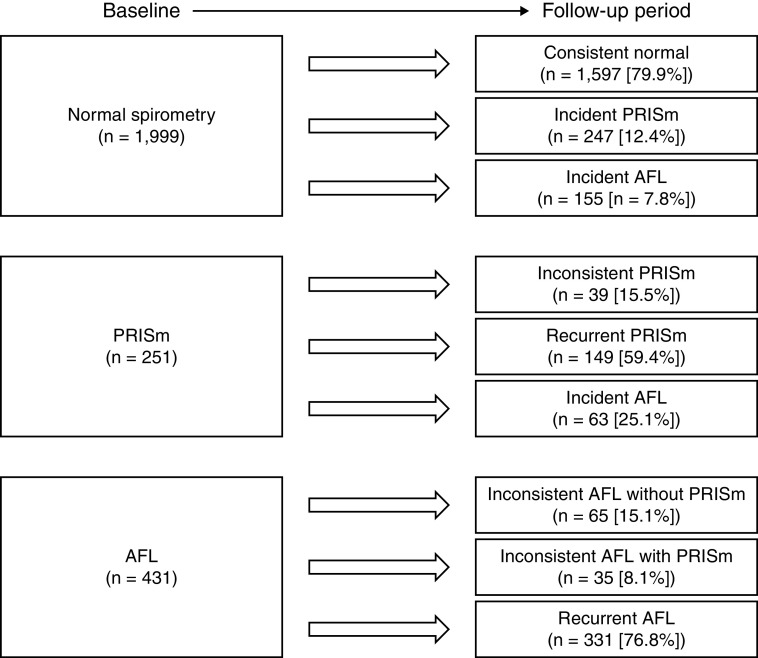
To evaluate the transition of lung function categories, 351 participants who did not attend spirometry examinations during the follow-up period were excluded (Figure E1). These excluded participants had older age, more comorbidities, and a greater frequency of bronchodilator users than the included participants in the analysis of transition spirometry categories (Table E7). Data on the remaining 2,681 participants were used to assess the transition of lung function categories. Among 1,999 participants with normal spirometry at baseline, 247 (12.4%) participants developed PRISm, and 155 (7.8%) developed AFL. Of the 251 participants with PRISm at baseline, 39 (15.5%) had an inconsistent PRISm pattern, 149 (59.4%) had a recurrent PRISm pattern, and 63 (25.1%) developed AFL (Figure 2). With regard to the development of AFL, 77 (49.7%) of 155 participants from the normal spirometry at baseline group and 38 (60.3%) of 63 participants from the PRISm at baseline group continued to exhibit AFL in at least 50% of the follow-up surveys.
Figure 2.
Transition of lung function categories from baseline to the follow-up period. AFL = airflow limitation; PRISm = preserved ratio impaired spirometry.
Cumulative Incidence of AFL
Next, we estimated the risk of the development of AFL in participants with PRISm at baseline against participants with normal spirometry at baseline among 2,250 participants without AFL at baseline who underwent spirometry at least once during the follow-up period. Baseline characteristics of these participants are shown in Table E8. Among them, 218 participants developed AFL over 5 years: 7.8% (n = 155) of participants with normal spirometry and 25.1% (n = 63) of participants with PRISm developed AFL. The age- and sex-adjusted HRs for the development of AFL increased significantly in the participants with PRISm compared with those with normal spirometry (HR, 3.33 [95% CI, 2.48–4.47]; P < 0.001) (Table 3). This association remained significant after adjustment for age, sex, current smoking, and pack-years (model 1, HR, 3.15 [95% CI, 2.34–4.24]) and for other risk factors in addition to those in model 1 (model 2, HR, 2.48 [95% CI, 1.83–3.36]). We also performed sensitivity analyses using the LLN threshold of FEV1/FVC as the criterion for AFL, using AFL onset defined as the first time that FEV1/FVC of <0.70 was observed two or more times on spirometry examinations during the follow-up period and using the competing risk analysis with the method proposed by Fine and Gray in addition to sensitivity analyses among several subgroups of participants, namely nonusers of bronchodilators, nonusers of ICSs, participants with serum NT-proBNP <300 pg/ml, and participants without kidney dysfunction (Table E9). Finally, we evaluated the annual rate of decline in FEV1/FVC across the entire follow-up period between the participants with normal spirometry and those with PRISm at baseline. Participants with PRISm had a significantly greater annual decline rate than those with normal spirometry after adjustment for the potential risk factors (−0.09 [95% CI, −0.16 to −0.03] for normal spirometry; −0.45 [95% CI, −0.64 to −0.26] for PRISm; P < 0.001).
Table 3.
Hazard Ratios and Their 95% Confidence Intervals for Development of Airflow Limitation with Global Initiative for Chronic Obstructive Lung Disease Criteria in Participants with Preserved Ratio Impaired Spirometry against Those with Normal Spirometry at Baseline
| Lung Function Categories | No. of Events/Participants | Hazard Ratio (95%CI) |
||
|---|---|---|---|---|
| Age- and Sex-adjusted | Model 1 | Model 2 | ||
| Normal spirometry | 155/1,999 | 1.00 (Reference) | 1.00 (Reference) | 1.00 (Reference) |
| PRISm | 63/251 | 3.33 (2.48 to 4.47)* | 3.15 (2.34 to 4.24)* | 2.48 (1.83 to 3.36)* |
Definition of abbreviations: CI = confidence interval; PRISm = preserved ratio impaired spirometry.
Model 1 was adjusted for age, sex, current smoking, and pack-years. Model 2 was adjusted for the covariates in model 1 plus body mass index, hypertension, diabetes mellitus, dyslipidemia, current drinking, regular exercise, and FEV1 as a percentage of FVC at baseline.
P < 0.05 versus normal spirometry at baseline.
Combined Influence of PRISm and Smoking Status on the Risk of All-Cause Death and the Development of AFL
Last, we examined the combined influence of smoking and PRISm on the risk of all-cause death and the development of AFL (Figure 3). We divided participants without AFL at baseline into four groups according to their smoking status (current smoker vs. never or former smoker) and baseline lung function category (PRISm vs. normal spirometry). Compared with the reference group of never or former smokers with normal spirometry, the participants who had both PRISm and a current smoking habit showed a significant increase in the risk of all-cause death (HR, 4.76 [95% CI, 1.77–12.78]). The combination of PRISm and current smoking tended to synergistically increase all-cause death mortality (P for interaction = 0.05) (Figure 3A). In the analyses for the development of AFL, we found that the risk of AFL was significantly increased in participants with PRISm, a current smoking habit, or both, although there was no evidence of significant interaction (P for interaction = 0.34) (Figure 3B). We also performed a sensitivity analysis by defining the reference group as never smokers with normal spirometry (Figure E2). The results showed that participants with PRISm had a significantly increased risk of all-cause death and the development of AFL, not only in the smoker group but also in the nonsmoker group.
Figure 3.
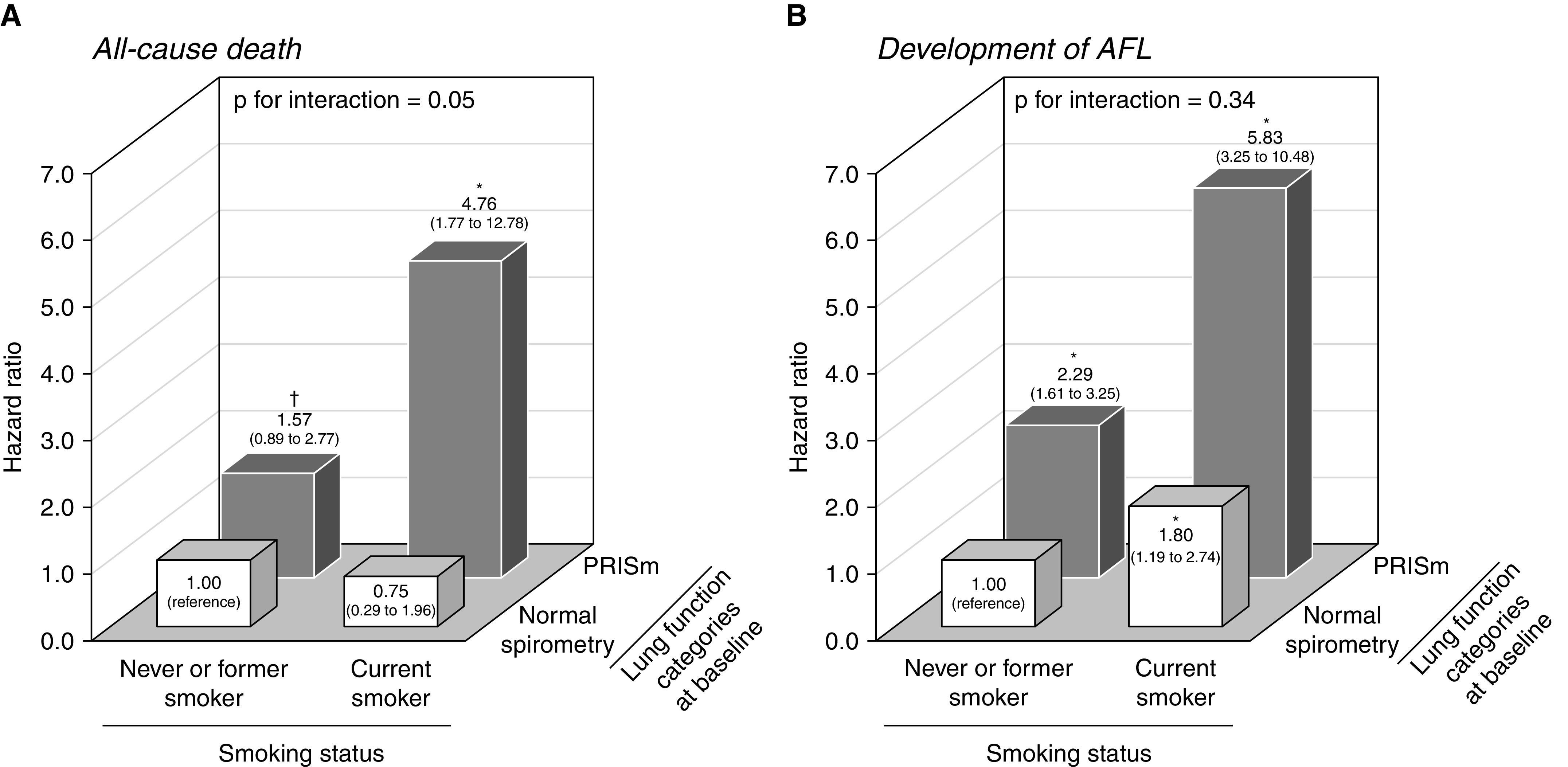
The combined influence of PRISm at baseline and current smoking on (A) the risk of all-cause death and (B) the development of airflow limitation. The hazard ratio of all-cause death (A) was estimated in 2,509 participants without airflow limitation at baseline and adjusted for age, sex, smoking pack-years, body mass index, hypertension, diabetes mellitus, dyslipidemia, electrocardiogram abnormalities, history of cardiovascular disease, history of cancer, alcohol intake, and regular exercise. The hazard ratio of the development of airflow limitation (B) was estimated in 2,250 participants with normal spirometry or PRISm at baseline and adjusted for age, sex, smoking pack-years, body mass index, hypertension, diabetes mellitus, dyslipidemia, current drinking, regular exercise, and FEV1 as a percentage of FVC at baseline. *P < 0.05 and †P < 0.20 versus the reference group (never or former smokers and participants with normal spirometry at baseline). AFL = airflow limitation; PRISm = preserved ratio impaired spirometry.
Discussion
The present study demonstrated that each of PRISm and AFL GOLD 2–4 was significantly associated with an increased risk of all-cause and cardiovascular death in a general Japanese population. The risk of all-cause death was doubled and the risk of cardiovascular death was tripled in participants with PRISm compared with those with normal spirometry. In addition, participants with PRISm had an approximately 2.5 times higher risk of developing AFL and a greater annual decline rate in FEV1/FVC than those with normal spirometry. The magnitudes of the association of PRISm with increased risks of all-cause death and the development of AFL tended to be stronger in participants with a current smoking habit than in those who were never or former smokers. To the best of our knowledge, this is the first study addressing the risks of mortality in participants with PRISm in an East Asian population.
In the present study, the prevalence of PRISm was approximately 10%, and the 5-year incidence rate of AFL in participants with PRISm was approximately 25%, and these rates were consistent with the findings of previous studies in Western populations (7, 9–11). In addition, two population-based studies (7, 11) and one hospital-based study of former and current smokers (9), which were conducted in Western populations, reported that the participants with PRISm had a two times greater risk of all-cause death and a three times higher risk of cardiovascular death than those with normal lung function. In the previous population-based study in a Western population, the risk for all-cause death and cardiovascular death in participants with PRISm was shown to be almost comparable to that of participants with AFL GOLD 2–4 (7). These findings are similar to ours. These results of the present study highlight the clinical importance of identifying participants with PRISm in Japanese populations as well as Western populations. On the one hand, Wan and colleagues also reported a significant association between PRISm and respiratory mortality (11). On the other hand, in the present study, the crude incidence rate of respiratory death on PRISm or AFL showed an upward trend, but the HRs for respiratory death in participants with PRISm or AFL did not reach a statistically significant level. This may be due to the low mortality rate from respiratory disease and the short follow-up period in the present study as compared with the previous study, which resulted in insufficient statistical power. Further investigation in other populations and settings will be needed to evaluate the association between PRISm and respiratory death.
The exact mechanism underlying the association between PRISm and death has not been fully elucidated, but fluid shifts or cardiomegaly in heart failure or kidney dysfunction or their accumulations may affect lung function via a reduction of FVC (33, 34). In the present study, however, the proportion of participants with high (⩾300 pg/ml) serum NT-proBNP levels or kidney dysfunction was not extremely high among participants with PRISm at baseline (Table 1), and the excess mortality risks in participants with PRISm at baseline were still observed after excluding the participants with serum NT-proBNP ⩾300 pg/ml or kidney dysfunction (Table E5). As other possible mechanisms, age-related changes and pulmonary diseases such as interstitial pneumonia, which are likely to reduce FVC, may contribute to the excess risk of death in participants with PRISm (4, 9). In addition, there is a possibility that PRISm is an indicator of the accumulation of cardiovascular risk factors. In support of this idea, several studies have reported an association between PRISm and obesity or metabolic syndrome, which are acknowledged to be associated with increased risks of cardiovascular disease and death (4, 7, 9, 35). Abdominal obesity might contribute to a reduction in FVC through the mechanical effects of adipose tissue on the diaphragm and the reduction of chest wall compliance, which may lead to a predisposition to PRISm (36, 37). Further studies are needed to elucidate the mechanism by which PRISm leads to worse prognosis.
Smoking has been recognized to cause bronchial and systemic chronic inflammation, which may exacerbate impaired lung function, promote target organ damage, and increase mortality risk (38–41). Notably, the present study found that a smoking habit steeply increased the risk of all-cause death and the development of AFL in participants with PRISm. This finding suggests that smoking cessation is important to reduce the risk of death and the development of AFL in participants with PRISm. On the other hand, the frequency of ever smokers among participants with PRISm in the present study was relatively lower than that in a previous Western study (7). It is unclear to what extent differences in the frequency of ever smokers affected the results of the study, but our finding of an excess risk of all-cause mortality and development of AFL among PRISm participants may be somewhat conservative. The influence of smoking on mortality in participants with PRISm should be validated in other populations.
Strengths and Limitations
The strengths of our study include the complete follow-up of participants for the mortality analysis, the accurate diagnosis of cause of death based on the available clinical information and autopsy findings, and the high number of follow-up spirometry examinations in a relatively short period of time. Several limitations should also be noted. First, our findings were based on the measurement of spirometry without a bronchodilator for the assessment of lung function, likely leading to the misclassification of asthma. However, in a sensitivity analysis for nonusers of ICSs or bronchodilators, the results for risk of mortality and development of AFL were not appreciably different from those in the main analyses. Second, approximately 10% of the study population was excluded from the mortality analysis and the analysis for the transition of lung function categories because of the absence of spirometry data at baseline and during the follow-up period, respectively. The participants excluded from the mortality analysis were older and had higher serum NT-proBNP (Table E1), and the participants excluded from the analysis for the transition of lung function categories had higher frequencies of bronchodilator users, PRISm, and AFL GOLD 2–4 at baseline (Table E7) than those included in the respective analyses. These selection biases may have affected the magnitude of the association between PRISm and the risks of death and the development of AFL to some extent, but we believe that they would not have changed our findings substantially. Third, the number of cause-specific deaths, especially respiratory disease deaths, and the length of the follow-up period were insufficient, so there is a possibility that our evaluation of the association between lung function categories and cause-specific death was underpowered.
Fourth, unmeasured risk factors such as obstructive sleep apnea or pulmonary hypertension may have influenced the results. The diffusing capacity of the lung for carbon monoxide and total lung volume were also unmeasured, and lung imaging modalities such as chest computed tomography were not included, so we could not accurately assess the effect of interstitial lung diseases on PRISm. Finally, the present study was performed in a single Japanese population, and thus the generalizability of the findings to other Japanese ethnic populations or other Asian populations with lifestyles different from those of the studied population is limited. Therefore, our findings should be validated in other populations.
Conclusion
In conclusion, the present study demonstrated that participants with PRISm, as well as participants with AFL GOLD 2–4, had significantly higher risk of all-cause death and cardiovascular death than those with normal spirometry. PRISm was also significantly associated with higher risk of developing AFL. These excess risks of death and the development of AFL were especially prominent in participants with a current smoking habit. These results suggest the importance of recognizing PRISm, a previously undiagnosed or neglected lung function category, in the clinical setting, and they also reaffirm the importance of smoking cessation. Further epidemiological studies will be required to elucidate the prognosis of individuals with a PRISm functional pattern in terms of both survival and lung function.
Acknowledgments
Acknowledgment
The authors thank the residents of the town of Hisayama for their participation in the survey and the staff of the Division of Health of Hisayama for their cooperation with this study. The statistical analyses were carried out using the computer resource offered under the category of General Projects by the Research Institute for Information Technology, Kyushu University.
Footnotes
Supported in part by the Ministry of Education, Culture, Sports, Science and Technology (Japan Society for the Promotion of Science KAKENHI grants JP21H03200, JP19K07890, JP20K10503, JP20K11020, JP21K07522, JP21K11725, JP21K10448, and JP18K17925); by Health and Labour Sciences Research Grants of the Ministry of Health, Labour and Welfare (grant JPMH20FA1002); and by the Japan Agency for Medical Research and Development (grant JP21dk0207053).
Author Contributions: Study concept and design: Y.W., S.F., K.K., H.I., K.M. Acquisition of data: Y.W., S.S., S.F., T.H., K.K., M.S., J.H., K.M., T.N. Analysis and interpretation of data: Y.W., S.S., T.N. Drafting of the manuscript: Y.W., S.S., T.N. Critical revision of the manuscript for important intellectual content: All authors. Statistical analysis: Y.W., S.S., T.N. Guarantors: K.M., T.K., T.N. Approval of final manuscript: All authors.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202110-2302OC on May 12, 2022
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. GBD 2015 Chronic Respiratory Disease Collaborators. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med . 2017;5:691–706. doi: 10.1016/S2213-2600(17)30293-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Am J Respir Crit Care Med . 2017;195:557–582. doi: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]
- 3. Qaseem A, Wilt TJ, Weinberger SE, Hanania NA, Criner G, van der Molen T, et al. American College of Physicians; American College of Chest Physicians; American Thoracic Society; European Respiratory Society Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med . 2011;155:179–191. doi: 10.7326/0003-4819-155-3-201108020-00008. [DOI] [PubMed] [Google Scholar]
- 4. Wan ES, Castaldi PJ, Cho MH, Hokanson JE, Regan EA, Make BJ, et al. COPDGene Investigators Epidemiology, genetics, and subtyping of preserved ratio impaired spirometry (PRISm) in COPDGene. Respir Res . 2014;15:89. doi: 10.1186/s12931-014-0089-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wan ES, Hokanson JE, Murphy JR, Regan EA, Make BJ, Lynch DA, et al. COPDGene Investigators Clinical and radiographic predictors of GOLD-unclassified smokers in the COPDGene study. Am J Respir Crit Care Med . 2011;184:57–63. doi: 10.1164/rccm.201101-0021OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Iyer VN, Schroeder DR, Parker KO, Hyatt RE, Scanlon PD. The nonspecific pulmonary function test: longitudinal follow-up and outcomes. Chest . 2011;139:878–886. doi: 10.1378/chest.10-0804. [DOI] [PubMed] [Google Scholar]
- 7. Wijnant SRA, De Roos E, Kavousi M, Stricker BH, Terzikhan N, Lahousse L, et al. Trajectory and mortality of preserved ratio impaired spirometry: the Rotterdam study. Eur Respir J . 2020;55:1901217. doi: 10.1183/13993003.01217-2019. [DOI] [PubMed] [Google Scholar]
- 8. Park HJ, Byun MK, Rhee CK, Kim K, Kim HJ, Yoo KH. Significant predictors of medically diagnosed chronic obstructive pulmonary disease in patients with preserved ratio impaired spirometry: a 3-year cohort study. Respir Res . 2018;19:185. doi: 10.1186/s12931-018-0896-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wan ES, Fortis S, Regan EA, Hokanson J, Han MK, Casaburi R, et al. COPDGene Investigators Longitudinal phenotypes and mortality in preserved ratio impaired spirometry in the COPDGene Study. Am J Respir Crit Care Med . 2018;198:1397–1405. doi: 10.1164/rccm.201804-0663OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wan ES, Hokanson JE, Regan EA, Young KA, Make BJ, DeMeo DL, et al. Significant spirometric transitions and preserved ratio impaired spirometry among ever smokers. Chest . 2022;161:651–661. doi: 10.1016/j.chest.2021.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wan ES, Balte P, Schwartz JE, Bhatt SP, Cassano PA, Couper D, et al. Association between preserved ratio impaired spirometry and clinical outcomes in US adults. JAMA . 2021;326:2287–2298. doi: 10.1001/jama.2021.20939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Quanjer PH, Stanojevic S, Cole TJ, Baur X, Hall GL, Culver BH, et al. ERS Global Lung Function Initiative Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J . 2012;40:1324–1343. doi: 10.1183/09031936.00080312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zammit C, Liddicoat H, Moonsie I, Makker H. Obesity and respiratory diseases. Int J Gen Med . 2010;3:335–343. doi: 10.2147/IJGM.S11926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Korotzer B, Ong S, Hansen JE. Ethnic differences in pulmonary function in healthy nonsmoking Asian-Americans and European-Americans. Am J Respir Crit Care Med . 2000;161:1101–1108. doi: 10.1164/ajrccm.161.4.9902063. [DOI] [PubMed] [Google Scholar]
- 15. Ishikawa C, Barbieri MA, Bettiol H, Bazo G, Ferraro AA, Vianna EO. Comparison of body composition parameters in the study of the association between body composition and pulmonary function. BMC Pulm Med . 2021;21:178. doi: 10.1186/s12890-021-01543-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Oelsner EC, Balte PP, Bhatt SP, Cassano PA, Couper D, Folsom AR, et al. Lung function decline in former smokers and low-intensity current smokers: a secondary data analysis of the NHLBI Pooled Cohorts Study. Lancet Respir Med . 2020;8:34–44. doi: 10.1016/S2213-2600(19)30276-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chen Y, Horne SL, Dosman JA. Body weight and weight gain related to pulmonary function decline in adults: a six year follow up study. Thorax . 1993;48:375–380. doi: 10.1136/thx.48.4.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sun C, Kovacs P, Guiu-Jurado E. Genetics of obesity in East Asians. Front Genet . 2020;11:575049. doi: 10.3389/fgene.2020.575049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of obesity among adults and youth: United States, 2011-2014. NCHS Data Brief . 2015;219:1–8. [PubMed] [Google Scholar]
- 20. Yatsuya H, Li Y, Hilawe EH, Ota A, Wang C, Chiang C, et al. Global trend in overweight and obesity and its association with cardiovascular disease incidence. Circ J . 2014;78:2807–2818. doi: 10.1253/circj.cj-14-0850. [DOI] [PubMed] [Google Scholar]
- 21. GBD 2019 Tobacco Collaborators. Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and attributable disease burden in 204 countries and territories, 1990-2019: a systematic analysis from the Global Burden of Disease Study 2019. Lancet . 2021;397:2337–2360. doi: 10.1016/S0140-6736(21)01169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tsugane S. Why has Japan become the world’s most long-lived country: insights from a food and nutrition perspective. Eur J Clin Nutr . 2021;75:921–928. doi: 10.1038/s41430-020-0677-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ministry of Health, Labour and Welfare. https://www.mhlw.go.jp/english/database/db-hw/dl/81-1a2en.pdf
- 24. Katsuki S, Hirota Y, Akazome T, Takeya S, Omae T, Takano S. Epidemiological studies on cerebrovascular diseases in Hisayama, Kyushu island, Japan. Part I. particular reference to cardiovascular status. Jpn Heart J . 1964;5:12–36. doi: 10.1536/ihj.5.12. [DOI] [PubMed] [Google Scholar]
- 25. Ohmura T, Ueda K, Kiyohara Y, Kato I, Iwamoto H, Nakayama K, et al. Prevalence of type 2 (non-insulin-dependent) diabetes mellitus and impaired glucose tolerance in the Japanese general population: the Hisayama Study. Diabetologia . 1993;36:1198–1203. doi: 10.1007/BF00401066. [DOI] [PubMed] [Google Scholar]
- 26. Ninomiya T. Japanese legacy cohort studies: the Hisayama Study. J Epidemiol . 2018;28:444–451. doi: 10.2188/jea.JE20180150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Japanese Respiratory Society. Guideline of respiratory function tests--spirometry, flow-volume curve, diffusion capacity of the lung [in Japanese] Nihon Kokyuki Gakkai Zasshi . 2004;(Suppl):1–56. [PubMed] [Google Scholar]
- 28. Kubota M, Kobayashi H, Quanjer PH, Omori H, Tatsumi K, Kanazawa M, Clinical Pulmonary Functions Committee of the Japanese Respiratory Society Reference values for spirometry, including vital capacity, in Japanese adults calculated with the LMS method and compared with previous values. Respir Investig . 2014;52:242–250. doi: 10.1016/j.resinv.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 29. Guerra S, Sherrill DL, Venker C, Ceccato CM, Halonen M, Martinez FD. Morbidity and mortality associated with the restrictive spirometric pattern: a longitudinal study. Thorax . 2010;65:499–504. doi: 10.1136/thx.2009.126052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Horio M, Imai E, Yasuda Y, Watanabe T, Matsuo S. Modification of the CKD epidemiology collaboration (CKD-EPI) equation for Japanese: accuracy and use for population estimates. Am J Kidney Dis . 2010;56:32–38. doi: 10.1053/j.ajkd.2010.02.344. [DOI] [PubMed] [Google Scholar]
- 31. Pellegrino R, Viegi G, Brusasco V, Crapo RO, Burgos F, Casaburi R, et al. Interpretative strategies for lung function tests. Eur Respir J . 2005;26:948–968. doi: 10.1183/09031936.05.00035205. [DOI] [PubMed] [Google Scholar]
- 32. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc . 1999;94:496–509. [Google Scholar]
- 33. Olson TP, Beck KC, Johnson BD. Pulmonary function changes associated with cardiomegaly in chronic heart failure. J Card Fail . 2007;13:100–107. doi: 10.1016/j.cardfail.2006.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Navaneethan SD, Mandayam S, Arrigain S, Rahman M, Winkelmayer WC, Schold JD. Obstructive and restrictive lung function measures and CKD: National Health and Nutrition Examination Survey (NHANES) 2007-2012. Am J Kidney Dis . 2016;68:414–421. doi: 10.1053/j.ajkd.2016.03.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Powell-Wiley TM, Poirier P, Burke LE, Després JP, Gordon-Larsen P, Lavie CJ, et al. American Heart Association Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Epidemiology and Prevention; and Stroke Council Obesity and cardiovascular disease: a scientific statement from the American Heart Association. Circulation . 2021;143:e984–e1010. doi: 10.1161/CIR.0000000000000973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jones RL, Nzekwu MM. The effects of body mass index on lung volumes. Chest . 2006;130:827–833. doi: 10.1378/chest.130.3.827. [DOI] [PubMed] [Google Scholar]
- 37. Leone N, Courbon D, Thomas F, Bean K, Jégo B, Leynaert B, et al. Lung function impairment and metabolic syndrome: the critical role of abdominal obesity. Am J Respir Crit Care Med . 2009;179:509–516. doi: 10.1164/rccm.200807-1195OC. [DOI] [PubMed] [Google Scholar]
- 38. Agustí A, Edwards LD, Rennard SI, MacNee W, Tal-Singer R, Miller BE, et al. Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) Investigators Persistent systemic inflammation is associated with poor clinical outcomes in COPD: a novel phenotype. PLoS One . 2012;7:e37483. doi: 10.1371/journal.pone.0037483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Man SF, Connett JE, Anthonisen NR, Wise RA, Tashkin DP, Sin DD. C-reactive protein and mortality in mild to moderate chronic obstructive pulmonary disease. Thorax . 2006;61:849–853. doi: 10.1136/thx.2006.059808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Churg A, Cosio M, Wright JL. Mechanisms of cigarette smoke-induced COPD: insights from animal models. Am J Physiol Lung Cell Mol Physiol . 2008;294:L612–L631. doi: 10.1152/ajplung.00390.2007. [DOI] [PubMed] [Google Scholar]
- 41. Çolak Y, Afzal S, Lange P, Nordestgaard BG. Smoking, systemic inflammation, and airflow limitation: a mendelian randomization analysis of 98085 individuals from the general population. Nicotine Tob Res . 2019;21:1036–1044. doi: 10.1093/ntr/nty077. [DOI] [PubMed] [Google Scholar]