Abstract
Pulmonary cryptococcosis (PC) is a rare fungal lung infection that usually occurs in immunocompromised hosts. We report a rare case of PC with asymptomatic and mixed lesions of infiltrative and nodular shadows. A woman in her 50s with a 7-year history of methotrexate treatment for rheumatoid arthritis (RA) presented with abnormal chest shadows on annual examination. Chest CT revealed two tiny nodules and two infiltrative shadows in the right lower lobe of the lung. We suspected lung cancer, cryptogenic organising pneumonia or RA-interstitial lung disease. However, transbronchial lung biopsy and positivity for serum cryptococcal antigen confirmed the diagnosis of PC. We initiated treatment with fluconazole, which drastically reduced the chest shadows without any adverse events. Since PC can present with several pulmonary shadows, it should be considered as a differential diagnosis of any pulmonary lesion.
Keywords: Cryptococcosis, Cryptococcus, Infections, Pneumonia (infectious disease), Pneumonia (respiratory medicine)
Background
Pulmonary cryptococcosis (PC) is a rare lung disease caused by cryptococcal fungi, most notably Cryptococcus neoformans. It is transmitted by inhalation of C. neoformans spores, which are mainly present in pigeon droppings.1 PC is known to occur not only in immunocompromised individuals, such as those with AIDS or those receiving immunosuppressive therapy, but also in immunocompetent individuals.2 While PC in immunocompetent individuals is often asymptomatic, fever and cough are relatively common in immunocompromised individuals.2 3 Radiographic findings of PC are mostly nodular shadows, but cavity or infiltration shadows can also appear as PC findings.2 4 Because of the variety of radiographic findings that can be present, PC can sometimes be difficult to distinguish from lung cancer or tuberculosis.2 Here, we report a case of asymptomatic PC that was unexpectedly diagnosed in an immunocompromised host with mixed infiltrative and nodular shadows.
Case presentation
A woman in her 50s with a 7-year history of methotrexate (8 mg weekly) treatment for rheumatoid arthritis (RA) presented with abnormal chest shadows during an annual check-up without any symptoms. The patient had no history of smoking, allergies or contact with pigeons. Moreover, her house was also not near a shrine or park.
Investigations
Chest radiography revealed infiltrative shadows in the right lower lung. Chest CT revealed two tiny nodules and two infiltrative shadows in the right lower lobe, but no pleural effusion or lymphadenopathy (figure 1). Blood and serum tests indicated slightly elevated level of (1,3)-β-D-glucan (upper normal limit: 20 pg/mL), but no other specific findings such as elevated blood leucocyte count, C reactive protein or glycosylated haemoglobin. The results investigating for the diagnosis of the case, including immune status of the patient, fungal or Mycobacterium tests, oncology marker panel and autoimmune condition, are shown in table 1.
Figure 1.
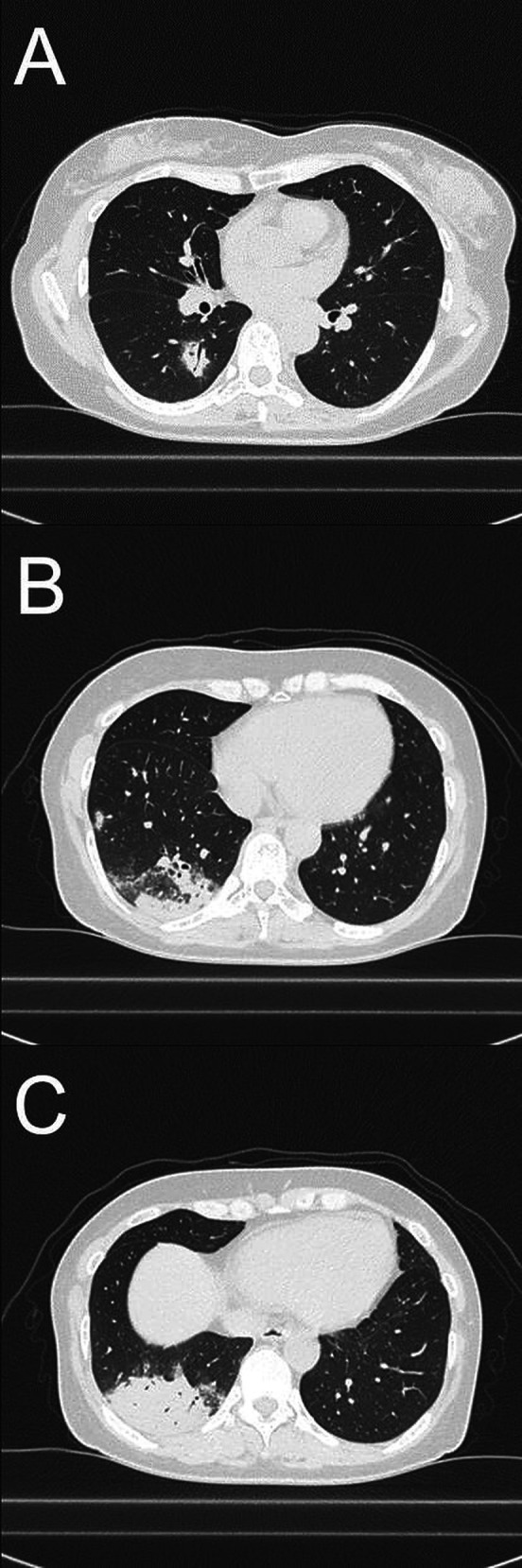
Images of chest CT. Chest CT taken at the first visit shows infiltrate shadows with an air bronchogram in the right peripheral lower lobe (A and C) and two nodular shadows in the right peripheral lower lobe (B). Pleural effusion or lymphadenopathy was not observed.
Table 1.
Results of blood and serum sample tests
| Leucocyte | 4.9 | 109/L |
| Neutrophil fraction | 68.8 | % |
| Lymphocyte fraction | 16.7 | % |
| Eosinophil fraction | 5.1 | % |
| Carcinoembryonic antigen | 1.0 | ng/mL |
| Squamous cell carcinoma antigen | 0.9 | ng/mL |
| Cytokeratin-19 fragment | 1.2 | ng/mL |
| Sialyl-Lewis x antigen | 32.7 | U/mL |
| Pro-gastrin-releasing peptide | 29.3 | pg/mL |
| Neuron-specific enolase | 12.2 | ng/mL |
| Krebs von den Lungen-6 | 237 | U/mL |
| Interferon-γ release assay | Negative | |
| Anti-HIV antibody | (−) | |
| Rheumatoid factor | <5 | IU/mL |
| Prteinase 3-antineutrophil cytoplasmic antibody (ANCA) | <1.0 | |
| Myeloperoxidase-ANCA | <1.0 | |
| Anti-aminoacyl tRNA synthetase antibody | <5.0 | |
| Anti-Sjögren syndrome (SS)-A antibody | Negative | |
| Anti-SS-B antibody | Negative | |
| Anti-nuclear antibody | 40 | titer |
| Soluble Interleukin 2 receptor | 547 | U/mL |
| (1,3)-β-D-glucan | 34.6 | pg/mL |
| Aspergillus antigen | 0.3 | |
| Candida antigen | <0.02 | U/mL |
| Capsular glucuronoxylomannan polysaccharide | Positive |
Interferon-γ release assay was performed by using T-SPOT TB.
Serum (1,3)-β-D-glucan level was elevated above the normal upper limit (20 pg/mL).
Serum capsular glucuronoxylomannan polysaccharide as a cryptococcal antigen test was positive.
Differential diagnosis
We suspected lung cancer, cryptogenic organising pneumonia (COP), RA-interstitial lung disease (ILD) and pulmonary infection. Findings of transbronchial lung biopsy for a definitive diagnosis were suggestive of cryptococcosis or other mycoses, such as mucormycosis (figure 2). While no pathogens were detected in bronchoalveolar lavage fluid cultures, the capsular glucuronoxylomannan polysaccharide (qualitative cryptococcal antigen: CrAg) was detected in serum. Based on the results of pathological assessment and CrAg, we confirmed the diagnosis of cryptococcosis. To rule out intracranial lesions, we performed contrast-enhanced head MRI and performed a lumbar puncture. Head MRI showed no space-occupying lesions, and the sample obtained by lumbar puncture showed negative in CrAg, Indian ink dyeing and fungal culture. Based on these results, the patient was diagnosed with PC.
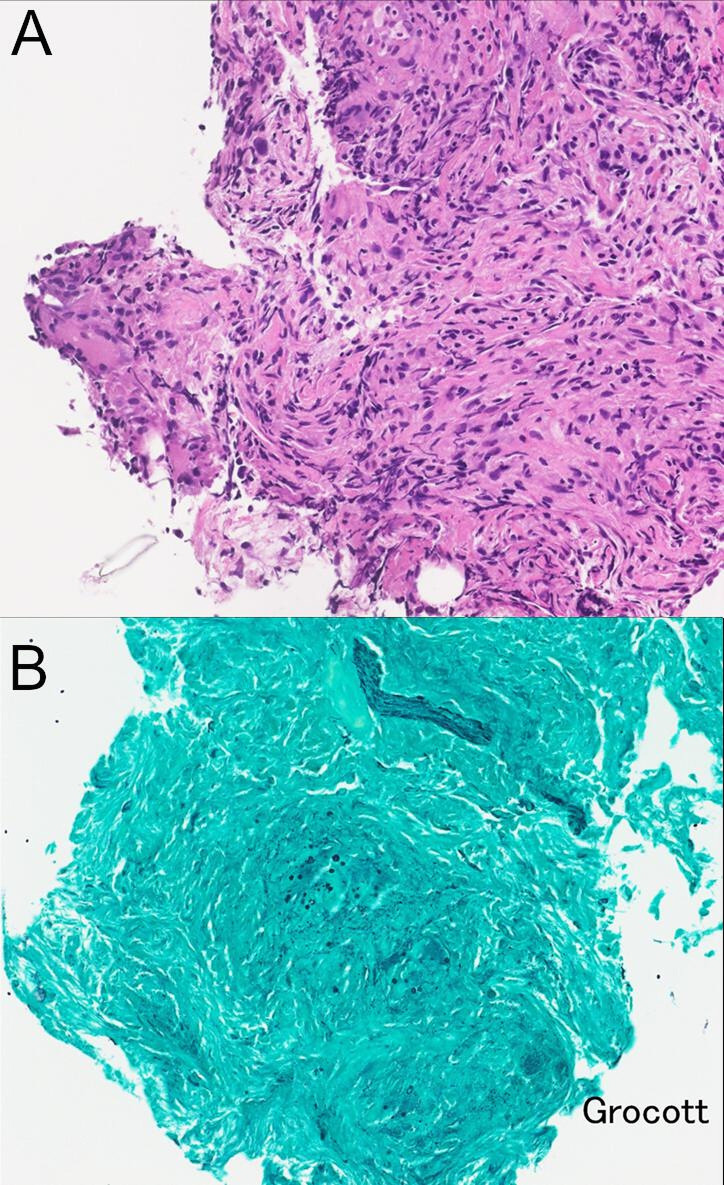
Figure 2.
Histopathology of transbronchial lung biopsy specimens. Granuloma formation with multinucleated giant cells and phagocytosing yeast-like fungi in multinucleated giant cells were observed. ((A) H&E stain, ×200; (B) Grocott stain, ×200).
Treatment
Lung lesions are often widespread in immunocompromised individuals; therefore, we administered intravenous fosfluconazole 800 mg daily for 2 days, followed by oral fluconazole 400 mg daily. The patient was discharged without any obvious adverse events.
Outcome and follow-up
Three months after the initiation of antifungal treatment, chest CT showed that the two nodules had disappeared, and that infiltrate shadows had become scarred circularly and faded (figure 3). Owing to the clinical improvement of PC and lack of adverse events with antifungal therapy, we intend to treat the patient for 6 months in accordance with the guidelines.5
Figure 3.
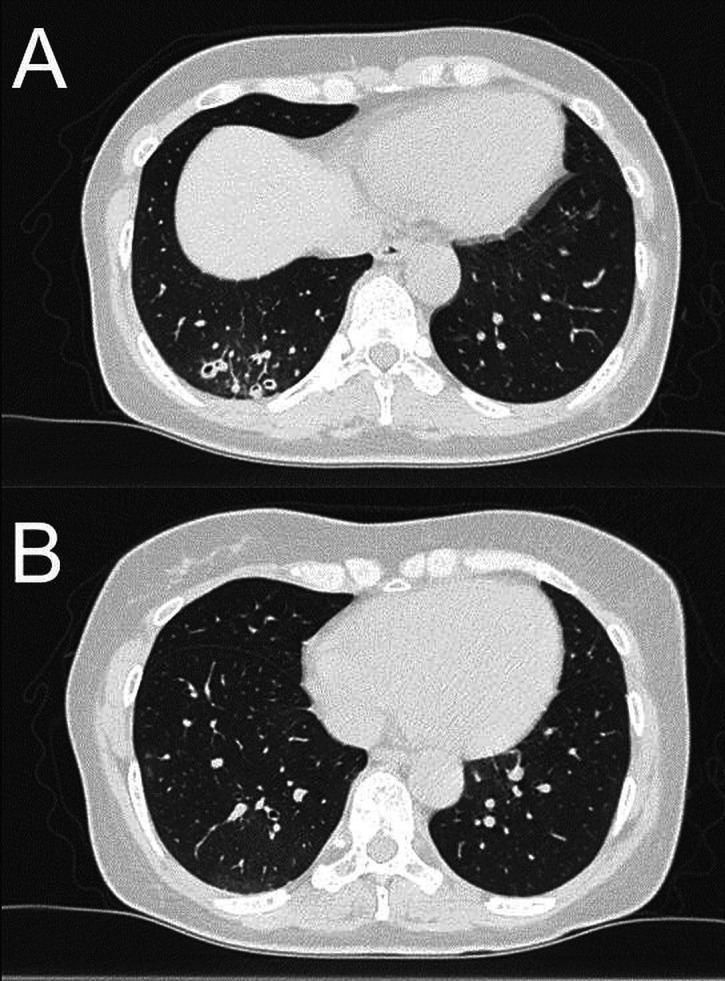
Changes in findings on CT images. Infiltrate shadows were scarred circularly and faded (A), and two nodules disappeared after the administration of antifungal therapy for 3 months (B).
Discussion
We report a case of asymptomatic PC that presented with mixed infiltrative and nodular shadows in an immunocompromised patient. We can learn three important points.
First, based on imaging findings alone, it is difficult to identify PC as a differential diagnosis. The typical chest imaging findings of PC are most often nodular shadows but may also present as cavitary or infiltrative shadows.2 4 In the present case, nodular and infiltrating shadows were mixed, but it was sometimes difficult to distinguish nodular shadows from lung cancer and an infiltrating form of ILD, such as pneumonia or COP. The patient had RA as an underlying disease; therefore, RA-ILD was easily identified. Furthermore, as there was no fever or cough, the likelihood of a respiratory tract infection was not assumed to be high on the list of differential diagnoses.
Second, in this case, PC was diagnosed because fungi were found in the lung tissue and CrAg was positive. Both tissue and culture specimens were obtained using bronchoscopy, which is a less invasive, generalised, and useful test for obtaining both tissue and culture specimens. In a previous report, many cases required video-assisted thoracic surgery (VATS) to reach the diagnosis of PC.2 3 When VATS is performed for diagnosis, tissue culture should also be performed to identify the causative organism in case of an infection. Minimally invasive bronchoscopy is the preferred diagnostic method if possible.
Third, there are some specific considerations for immunocompromised hosts. The patient had been receiving methotrexate (8 mg weekly) for 7 years for RA and was immunocompromised.6 This is because methotrexate inhibits directly or indirectly the function of nearly every cell type involved in inflammation, including neutrophils, monocytes, T cells, B cells, etc.7 On the other hand, we determined that the patient did not develop severe immune deficiency due to the lack of decrease in neutrophil or lymphocyte count in peripheral blood induced by the toxic effect of methotrexate. Opportunistic infections often have a clinical course, with few symptoms or atypical imaging findings. It is difficult to determine a specific disease based on imaging findings alone, and we should strive to confirm the diagnosis more rigorously and sometimes more invasively in immunocompromised patients than in immunocompetent patients.
Finally, we initiated two loading doses of intravenous fosfluconazole 800 mg daily in the present case. The recent guidelines for the treatment of PC in immunocompromised hosts with mild to moderate symptoms recommend oral fluconazole 400 mg daily for 6–12 months.5 Fluconazole takes approximately 10 days for serum concentration to stabilise due to its long elimination half-time. Two loading doses of intravenous fosfluconazole can shorten the time to achieve stable plasma concentration from 10 to 3 days.8 Rapid achievement of target steady plasma concentration seemed to be beneficial for the patient because the patient had multiple pulmonary lesions, which could be severe.
The report had one limitation. The serum sample at the first visit indicated a higher level of (1,3)-β-D-glucan than the upper limit of the normal. In cryptococcosis, serum (1,3)-β-D-glucan remains at normal levels, despite mycosis. Other serological tests for other mycoses were negative in this case; therefore, we thought that the elevation of (1,3)-β-D-glucan might be false-positive or meaningless data in actual clinical case.
We report a case of asymptomatic PC presenting with mixed infiltrative and nodular shadows in an immunocompromised patient. When an immunocompromised patient presents with abnormal chest shadows, respiratory infections, including PC, should be among the identified diagnoses, even if imaging findings are atypical.
Learning points.
Pulmonary cryptococcosis (PC) can present with a variety of radiographic findings and is sometimes difficult to distinguish from lung cancer, tuberculosis or interstitial lung diseases.
Bronchoscopy is a less invasive, generalised, and useful procedure for obtaining both tissue and culture specimens.
When an immunocompromised patient presents with abnormal chest shadows, respiratory infections, including PC, should be among the differential diagnoses, even if the imaging findings are atypical.
Acknowledgments
We would like to thank Editage (https://www.editage.jp) for English editing.
Footnotes
Contributors: TI diagnosed and treated the patient, and wrote the draft. OK planned this case report and revised the draft. KM pathologically assessed the tissue samples, helped to make the diagnosis and gave advice for the draft. KF planned this case report and revised the draft.
Funding: This study was funded by the National Hospital Organization’s fiduciary funds.
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Patient consent for publication
Obtained.
References
- 1.Sirag B, Khidir E-S, Dumyati M, et al. Cryptococcus neoformans and Other Opportunistic Cryptococcus Species in Pigeon Dropping in Saudi Arabia: Identification and Characterization by DNA Sequencing. Front Microbiol 2021;12:1–7. 10.3389/fmicb.2021.726203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kishi K, Homma S, Kurosaki A, et al. Clinical features and high-resolution CT findings of pulmonary cryptococcosis in non-AIDS patients. Respir Med 2006;100:807–12. 10.1016/j.rmed.2005.09.017 [DOI] [PubMed] [Google Scholar]
- 3.Hosoda C, Ishiguro T, Uozumi R, et al. Characteristics of pulmonary cryptococcosis in patients with rheumatoid arthritis. BMJ Open Respir Res 2021;8:e000805. 10.1136/bmjresp-2020-000805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zinck SE, Leung AN, Frost M, et al. Pulmonary cryptococcosis: CT and pathologic findings. J Comput Assist Tomogr 2002;26:330–4. 10.1097/00004728-200205000-00002 [DOI] [PubMed] [Google Scholar]
- 5.Sirag B, Khidir ES, Dumyati M. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the infectious diseases Society of America. Clin Infect Dis 2010;50:1–7. 10.1086/649858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doran MF, Crowson CS, Pond GR, et al. Frequency of infection in patients with rheumatoid arthritis compared with controls: a population-based study. Arthritis Rheum 2002;46:2287–93. 10.1002/art.10524 [DOI] [PubMed] [Google Scholar]
- 7.Cronstein BN, Aune TM. Methotrexate and its mechanisms of action in inflammatory arthritis. Nat Rev Rheumatol 2020;16:145–54. 10.1038/s41584-020-0373-9 [DOI] [PubMed] [Google Scholar]
- 8.Sobue S, Tan K, Layton G, et al. Pharmacokinetics of fosfluconazole and fluconazole following multiple intravenous administration of fosfluconazole in healthy male volunteers. Br J Clin Pharmacol 2004;58:20–5. 10.1111/j.1365-2125.2004.02107.x [DOI] [PMC free article] [PubMed] [Google Scholar]