Abstract
The mechanism(s) of immune checkpoint inhibitor (ICI)-induced myasthenia gravis (MG), an immune-related adverse event (irAE) that is fatal and limits subsequent ICI use, remain unexplored. Here, through comparative genomic analysis, we identified a pathogenic p.S467C germline variant in SLC22A5 in a thymoma case with ICI-induced MG, which was found to be associated with fatty acid oxidation through its regulation on L-carnitine levels. Remarkably, ICI rechallenge with L-carnitine pretreatment led to durable response without MG-related symptoms. Thus, we provide the first clinical evidence of genetic test-directed irAE management, which integrates individualized ICI treatment into the evolving paradigm of cancer management.
Keywords: Immunotherapy, Lung Neoplasms
Insights
ICI rechallenge with L-carnitine pretreatment may be an effective treatment strategy for relieving myasthenia gravis.
Background
Thymoma is an epithelial neoplasm of the thymus. For many patients with unresectable diseases, chemotherapy might be the only treatment option, given the limited efficacy of targeted therapies and anti-angiogenics.1 With the emergence of immunotherapy, immune checkpoint inhibitor (ICI) has revolutionized the treatment landscape in a wide range of cancers.2–5 The effectiveness of ICI treatment has also been demonstrated in thymoma patients. In a phase II clinical trial of pembrolizumab for patients with thymic cancers following progression to chemotherapy, five of the seven enrolled thymoma patients had stable disease and two achieved partial response.6 However, ICI treatment is associated with a unique spectrum of side effects, referred to as immune-related adverse events (irAEs), which can largely limit the efficacy of ICIs.7 8 One emerging irAE is myasthenia gravis (MG), which is characterized by early-onset, multiple organ failures leading to permanent damages to organs, and even death.9 10 In addition, previous studies have shown that 50%–70% of patients with thymoma have one or more paraneoplastic syndromes (PNS). As MG accounts for 30%–50% of these PNS,11 thymoma patients treated with ICI may further increase the risk of MG development.
It has been widely accepted that biomarker testing is needed to enrich patients who might benefit from ICI treatment.12–15 It is equally important to identify and screen for potential biomarkers of irAEs to enable precise management and even early prevention.
Here, we present our experience in genetic testing-guided immunotherapy re-challenge in a case with carnitine deficiency and consequently dysregulated fatty acid oxidation (FAO) caused by a pathogenic SLC22A5 mutation. The addition of L-carnitine to the immunotherapy rechallenge regimen effectively relieved and prevented the reoccurrence of MG, providing the basis of durable ICI benefit in our patient.
Methods
Next generation sequencing analysis
NGS testing was performed on the patient’s tumor tissue using a targeted panel encompassing 425 cancer-relevant genes (GeneseeqPrime, Nanjing Geneseeq Technology, Nanjing, China). In addition, whole-exome sequencing (WES) was performed on blood samples from the patients and their parents. All sequencing tests were conducted with informed consents in a CAP-accredited and CLIA-certified laboratory (Nanjing Geneseeq Technology. DNA extraction, quantification, library preparation and sequencing were conducted as previously described.16 FASTQ file quality control and alignment were performed by Trimmomatic and BWA (hg19), respectively. Mutation calling was performed as previously described.17
Results
Patient characteristics and treatment overview
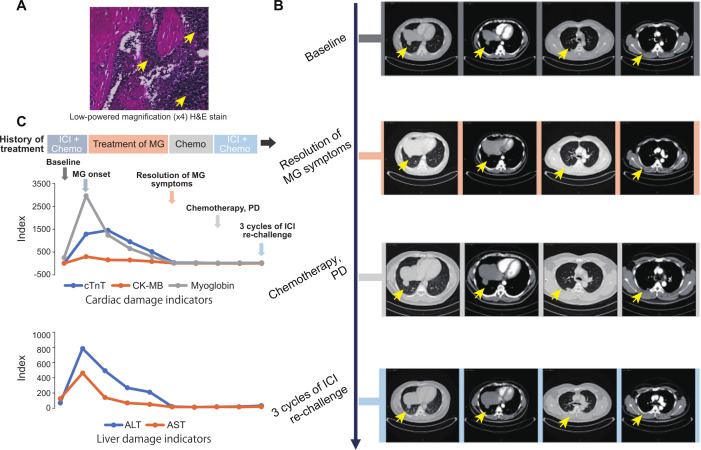
A patient was diagnosed with thymoma (figure 1A) and developed recurrence and metastasis 2 years after surgery (figure 1B). At the time of disease recurrence, the patient did not have any MG-related symptoms. The surgical specimen demonstrated positive PD-L1 expression as determined by both tumor proportion score (30%; ≥1%) and combined positive score (32; ≥1). The tumor sample was also subjected to genetic profiling using a 425-cancer gene panel (GeneseeqPrime, Nanjing, China) and no negative predictors of ICI response or hyperprogression-related biomarkers were detected.
Figure 1.
Response to (re)immunotherapy and management of immune-related MG. (A) Low-powered magnification (×4) H&E stain of the thymic lesion. The yellow arrows indicate the thymic lesion. (B) Representative CT images of patients during the treatment (from the baseline to ICI rechallenge). The yellow arrows indicate the thymic lesion. (C), Change in levels of blood indicators of cardiac and liver damage. The arrows with different color represent the indicated stage of the treatment. ALT, alanine aminotransferase; AST, aspartate transaminase; cTnT, cardiac troponin T; CK-MB, creatine kinase-MB; ICI, immune checkpoint inhibitor; MG, myasthenia gravis.
Subsequently, the patient was treated with first-line pembrolizumab (200 mg d1) combined with chemotherapy (CAP regimen, cyclophosphamide 1000 mg d1+liposome adriamycin 40 mg d2+cisplatin 50 mg d1–2) and achieved a partial response. Seventeen days later, the patient developed right ptosis, double vision, and subsequently bilateral eyelid drooping with mild breathing and swallowing difficulties. Laboratory indicators showed high levels of CK-MB (>300 ng/mL), myoglobin (MYO >3000 ng/mL), and high-sensitive cardiac troponin T (hs-cTnT, 1087 pg/mL; figure 1C). The patient was thus diagnosed with grade III MG and was administered with a high dose of methylprednisolone (300 mg, qd). The clinical symptoms of MG gradually disappeared 2 weeks following treatment, while relevant biochemical indicators gradually reached normal levels only 3 months after (figure 1C).
Following MG relief, given the risk of MG reoccurrence with immunotherapy, we switched the patient from ICI treatment to chemotherapy. Unfortunately, after two cycles of CAP regimen and three cycles of PCb regimen (liposome paclitaxel 270 mg d1+carboplatin 600 mg d1), the patient failed to respond and experienced progressive disease (figure 1B).
Molecular mechanism of MG and ICI rechallenge
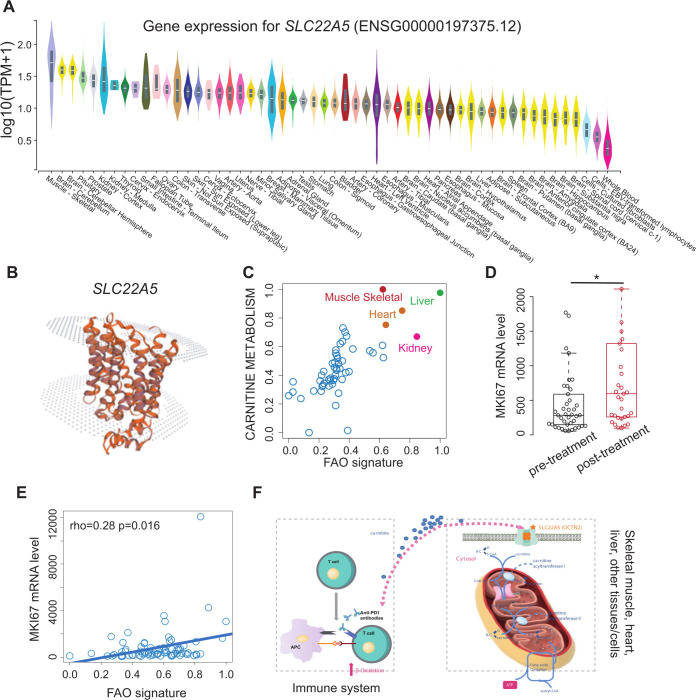
In the absence of subsequent therapeutic options, we considered the possibility of an ICI rechallenge. We sought to identify potential risk alleles of MG in our patient by performing WES on the peripheral blood samples from the patient and his parents. We found that the patient carried a maternally inherited variant in SLC22A5 (c.1400C>G; p.S467C), which encodes the high-affinity carnitine transporter, OCTN2 and has been linked to systemic primary carnitine deficiency.18 19 By performing an analysis based on Genotype-Tissue Expression (GTEx, https://gtexportal.org/home/), we found that SLC22A5 was mainly expressed in the muscle tissue (figure 2A), suggesting that mutant SLC22A5 may associate with the dysfunction of muscle tissue.20 In the wild type, as shown in the figure 2B, the structure of SLC22A5 protein acts as a pipeline for transporting molecules (eg, carnitine) from the outside into the cells. However, when we performed the MAESTRO algorithm to predict the influence of p.S467C alteration on the structure, we found the variant was a stabilizing mutation (ΔΔG pred.=−0.347), which may profoundly affect the transporting activities of the protein. This finding was also confirmed by other studies.1 2 Since the SLC22A5 mutation is involved in the de-regulation of the FAO pathway,20 we next asked whether ICI treatment might exacerbate the metabolic disorder in this patient. We analyzed the mRNA expression profile from the GTEx and found that both Carnitine Metabolism and the FAO pathway (MSigDB, http://www.gsea-msigdb.org/gsea/msigdb) were highly upregulated in the muscle tissues (figure 2C). Furthermore, we collected the mRNA profiles consisting of 74 cases whose T cells were extracted before and after ICI treatment (GSE141479).21 The expression of the marker of proliferation Ki-67, MKI67, was significantly higher after ICI treatment (Wilcoxon’s rank-sum test, p=0.014), suggesting ICI induced T cell proliferation (figure 2D). In addition, we found that the MKI67 levels showed a correlation with the enrichment score of FAO (via single-sample Gene set enrichment analysis, ssGSEA22 (Spearman’s correlation, r=0.32, p=0.0056) (figure 2E). As shown in the figure 2F, the findings above suggested that ICI-induced MG could be ascribed to the competition immune system and muscle/heart/liver tissues for carnitine, since both require the carnitine to sustain the FAO and acquire energy. However, the variant in SLC22A5 can reduce the efficiency of carnitine transport. Such a deficiency could be exacerbated by ICI, as the ICI could trigger the large number of activated immune cells throughout the body to hunt for carnitine, resulting in an extreme lack of carnitine for other tissues. We proposed that carnitine supplementation might restore energy production in the muscle tissues on ICI treatment. Consequently, the patient was pretreated with L-carnitine (6 mg), followed by an ICI rechallenge, to prevent MG reoccurrence. Up to now, the patient had continued to receive the combination treatment for three cycles and had a partial response without MG-related symptoms.
Figure 2.
ICI associates with fatty acid oxidation (FAO) activation. (A) Comparison of SLC22A5 mRNA level among different tissues based on genotype-tissue expression project. (B) The structure of SLC22A5 (OCTN2) visualized by SWISS-MODEL. (C) The scatter plot of carnitine metabolism (y-axis) and FAO signature (x-axis) across different tissues. The tissues with high calcium ion transport and FAO signature scores are colored in red (muscle tissue), orange (heart), green (liver), and pink (kidney). (D) Comparison of MKI67 mRNA levels between ICI pretreatment and post-treatment groups (dataset derived from GSE141479). Wilcoxon’s rank-sum test, p-value<0.05:*. (E) Correlation between FAO signature and MKI67 mRNA levels based on the expression profile derived from GSE141479. (F) Schematic diagram illustrating the competition between the immune system (left panel) and muscle/heart/liver tissues (right panel) for carnitine following ICI treatment. ICI, immune checkpoint inhibitor.
Discussion
According to the National Comprehensive Cancer Network (NCCN) guidelines, ICI treatment should be permanently discontinued for patients who had developed grade II or higher MG.23 As a consequence, a subset of patients, including the patient in our study, might miss the opportunity to benefit from immunotherapy rechallenge following MG relief. Following the failure of standard chemotherapy in our patient, the lack of subsequent treatment options prompted us to reconsider the possibility of the ICI challenge. We further conducted a more comprehensive evaluation of the patient’s genetic profile by WES. Remarkably, we identified a pathological germline variant in SLC22A5 (c.1400C>G), which is associated with carnitine deficiency and disruption of the FAO pathway, and consequently energy metabolism in the muscle tissues. Subsequent analysis revealed that the FAO pathway was upregulated in the T cells after ICI treatment, which may further compete for carnitine and diminish its overall level in the muscle tissues. Compared with the traditional symptomatic therapies for MG management, administration of L-carnitine, which is targeted directly against the underlying cause of MG development, may represent a more compliable and safe option for patients. In addition, our findings suggest that special focus should be placed on alterations in the FAO and other energy metabolism-related pathways in patients with immune-related MG, who might benefit from L-carnitine treatment. More importantly, this is the first clinical report, to the best of our knowledge, which integrates precision medicine into the routine management of treatment-related adverse events. While further investigations are needed to fully understand the biology and pathogenic mechanisms of irAEs, this approach might have profound implications for the future management of the broad spectrum of irAEs.
Advances in the field of genetic testing, particularly NGS technologies, have also facilitated the development of cancer immunotherapies.13 14 Stratification of responders to immunotherapy through predictive biomarkers is an effective way to overcome the low response rate to ICI treatment. The current design of clinical testing panels is mainly focused on ICI efficacy-related biomarkers. As we deepen our understanding of the mechanisms underlying irAEs, further consideration should be given to the future design of clinical testing panels.
This study is limited by the exploratory nature of the single-case report. Nevertheless, our study demonstrates that genetic testing might enable the precise identification of irAE-related mechanisms and early diagnostic assessments, and serve to guide personalized clinical decisions for subsequent treatment strategies.
jitc-2022-005970supp001.pdf (126.1KB, pdf)
Footnotes
WG, LW, SJ and JL contributed equally.
Contributors: RG, QW, and LC designed the project; WG and LW performed bioinformatics analysis and interpret the data; YWS, JCY, and LS performed bioinformatics analysis for WES; WG, SJ and JL performed the experiments and sample collection; XL and JX provided assistance for public data analysis; QG, CS, and WW provided intellectual contribution and designed early study; WG, WW, and ZW provided critical intellectual contributions throughout the project; WG and LW wrote the manuscript.
Funding: This work was supported by grants from the National Natural Science Foundation of China (81972188, 81572893, 81972358, 82141121, 82272669), the Medical Important Talents of Jiangsu Province (ZDRCA2016024), and Jiangsu Provincial Key Research and Development Program (BE2017733, BE2018713). Natural Science Foundation of Jiangsu Province (BK20211380).
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
All tumor specimens were collected after written informed consent was obtained from the patients and in accordance with the institutional review board-approved protocols of the First Affiliated Hospital of Nanjing Medical University (2021-QT-13). Participants gave informed consent to participate in the study before taking part.
References
- 1. Berghmans T, Durieux V, Holbrechts S, et al. Systemic treatments for thymoma and thymic carcinoma: a systematic review. Lung Cancer 2018;126:25–31. 10.1016/j.lungcan.2018.10.018 [DOI] [PubMed] [Google Scholar]
- 2. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC | NEJM [Internet]. Available: https://www.nejm.org/doi/full/10.1056/NEJMoa1716948 [Accessed 20 Dec 2021].
- 3. Efficacy and Safety of Nivolumab Plus Ipilimumab in Patients With Advanced Hepatocellular Carcinoma Previously Treated With Sorafenib: The CheckMate 040 Randomized Clinical Trial | Gastroenterology | JAMA Oncology | JAMA Network [Internet]. Available: https://jamanetwork.com/journals/jamaoncology/fullarticle/2771012 [Accessed 20 Dec 2021]. [DOI] [PMC free article] [PubMed]
- 4. Marabelle A, Le DT, Ascierto PA, et al. Efficacy of pembrolizumab in patients with Noncolorectal high microsatellite Instability/Mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol 2020;38:1–10. 10.1200/JCO.19.02105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med 2016;375:1823–33. 10.1056/NEJMoa1606774 [DOI] [PubMed] [Google Scholar]
- 6. Cho J, Kim HS, Ku BM, et al. Pembrolizumab for patients with refractory or relapsed thymic epithelial tumor: an open-label phase II trial. J Clin Oncol 2019;37:2162–70. 10.1200/JCO.2017.77.3184 [DOI] [PubMed] [Google Scholar]
- 7. Pauken KE, Dougan M, Rose NR, et al. Adverse events following cancer immunotherapy: obstacles and opportunities. Trends Immunol 2019;40:511–23. 10.1016/j.it.2019.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Abdel-Wahab N, Alshawa A, Suarez-Almazor ME. Adverse Events in Cancer Immunotherapy [Internet]. In: Naing A, Hajjar J, eds. Immunotherapy. Cham: Springer International Publishing, 2017: 155–74. [DOI] [PubMed] [Google Scholar]
- 9. Makarious D, Horwood K, Coward JIG. Myasthenia gravis: an emerging toxicity of immune checkpoint inhibitors. Eur J Cancer 2017;82:128–36. 10.1016/j.ejca.2017.05.041 [DOI] [PubMed] [Google Scholar]
- 10. Safa H, Johnson DH, Trinh VA, et al. Immune checkpoint inhibitor related myasthenia gravis: single center experience and systematic review of the literature. J Immunother Cancer 2019;7:319. 10.1186/s40425-019-0774-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kumar R. Myasthenia gravis and thymic neoplasms: a brief review. World J Clin Cases 2015;3:980–3. 10.12998/wjcc.v3.i12.980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Patel SP, Kurzrock R. Pd-L1 expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther 2015;14:847–56. 10.1158/1535-7163.MCT-14-0983 [DOI] [PubMed] [Google Scholar]
- 13. Walk EE, Yohe SL, Beckman A, et al. The cancer immunotherapy biomarker testing landscape. Arch Pathol Lab Med 2020;144:706–24. 10.5858/arpa.2018-0584-CP [DOI] [PubMed] [Google Scholar]
- 14. Kato S, Goodman A, Walavalkar V, et al. Hyperprogressors after immunotherapy: analysis of genomic alterations associated with accelerated growth rate. Clin Cancer Res 2017;23:4242–50. 10.1158/1078-0432.CCR-16-3133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chan TA, Yarchoan M, Jaffee E, et al. Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. Ann Oncol 2019;30:44–56. 10.1093/annonc/mdy495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jin Y, Bao H, Le X, et al. Distinct co-acquired alterations and genomic evolution during TKI treatment in non-small-cell lung cancer patients with or without acquired T790M mutation. Oncogene 2020;39:1846–59. 10.1038/s41388-019-1104-z [DOI] [PubMed] [Google Scholar]
- 17. Fang W, Ma Y, Yin JC, et al. Comprehensive Genomic Profiling Identifies Novel Genetic Predictors of Response to Anti-PD-(L)1 Therapies in Non-Small Cell Lung Cancer. Clin Cancer Res 2019;25:5015–26. 10.1158/1078-0432.CCR-19-0585 [DOI] [PubMed] [Google Scholar]
- 18. Rose EC, di San Filippo CA, Ndukwe Erlingsson UC, et al. Genotype-Phenotype correlation in primary carnitine deficiency. Hum Mutat 2012;33:118–23. 10.1002/humu.21607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Magoulas PL, El-Hattab AW. Systemic primary carnitine deficiency: an overview of clinical manifestations, diagnosis, and management. Orphanet J Rare Dis 2012;7:68. 10.1186/1750-1172-7-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Longo N, Frigeni M, Pasquali M. Carnitine transport and fatty acid oxidation. Biochim Biophys Acta 2016;1863:2422–35. 10.1016/j.bbamcr.2016.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hatae R, Chamoto K, Kim YH, et al. Combination of host immune metabolic biomarkers for the PD-1 blockade cancer immunotherapy. JCI Insight 2020;5:133501. 10.1172/jci.insight.133501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Systematic RNA interference reveals that oncogenic KRAS-driven cancers require TBK1 | Nature [Internet]. Available: https://www.nature.com/articles/nature08460 [Accessed 21 Dec 2021]. [DOI] [PMC free article] [PubMed]
- 23. NCCN Version 1 . Management of Immunotherapy-Related Toxicities [Internet]. NCCN, 2021. Available: https://www.nccn.org/guidelines/guidelines-detail [Accessed 17 Jan 2022].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2022-005970supp001.pdf (126.1KB, pdf)