Abstract
Streptococcus pneumoniae is a significant pathogen of young children and the elderly. Systemic infection by pneumococci is a complex process involving several bacterial and host factors. We have investigated the role of CD40L in host defense against pneumococcal infection. Treatment of mice with MR-1 antibody (anti-CD154/CD40L) markedly reduced antibody responses to the pneumococcal protein PspA, elicited by immunization of purified protein or whole bacteria. In mice immunized with whole bacteria, MR-1 treatment reduced antibody responses to capsular polysaccharides but not cell wall polysaccharides. MR-1 did not suppress antibody responses to isolated capsular polysaccharides but did reduce the production of antibody to a capsular polysaccharide-protein conjugate, indicating that when presented in the context of whole bacteria, the humoral response to capsular polysaccharides is partially T-cell dependent. Despite the reduction of the protective humoral responses to pneumococcal infection, administration of MR-1 had no effect on sepsis, lung infection, or nasal carriage in nonimmune mice inoculated with virulent pneumococci. Thus, short-term neutralization of CD40L does not compromise innate host defenses against pneumococcal invasion.
Streptococcus pneumoniae is a human pathogen that frequently causes pneumonia, otitis media, septicemia, and meningitis (47). The mucosal epithelium of the nasopharynx is the primary site of colonization, and individuals can carry up to four different serotypes asymptomatically (63). In some cases, perhaps in conjunction with a viral infection, the host is predisposed to symptomatic pneumococcal infections including sinusitis, otitis media, and pneumonia. In rare cases, sepsis develops and seeds infections at distant sites (e.g., meningitis).
Recent studies of the natural course of disease progression have suggested that pneumococcal adherence to mucosal surfaces involves cytokine-mediated upregulation of platelet-activating factor receptor (63). However, there remain considerable gaps in our understanding of the mechanism of pneumococcal invasion of host tissue. Identification of the molecules important in the disease progression has become easier with the development of mouse models of pneumococcal diseases that replicate nasopharyngeal colonization that can lead to pneumonia and sepsis (67).
Host defense against S. pneumoniae involves mainly acute-phase responses as well as antibodies to pneumococcal antigens. C-reactive protein is an acute-phase protein that binds to phosphocholine moieties within cell wall polysaccharide (C-PS) in the presence of calcium (65). C-reactive protein promotes phagocytosis of S. pneumoniae by human leukocytes (34) and protects mice against fatal pneumococcal infection (60, 71). Either short-term or chronic ablation of tumor necrosis factor and tumor necrosis factor receptor (TNF/TNFR) function renders mice more susceptible to S. pneumoniae, suggesting that cytokine-mediated inflammatory processes play an integral role in host defense against pneumococcal infection (50, 61). Classic studies demonstrated that antibodies to capsular polysaccharide (Caps-PS) are an important component of the adaptive immune response to pneumococcal infection (38). Subsequent studies, however, revealed that antibodies to C-PS (41), phosphocholine (5), or pneumococcal proteins (e.g., PspA) (7) can protect mice against pneumococcal infection. Thus, antibodies to many different pneumococcal antigens could be important in host defense.
CD40L is critical for humoral responses to T-dependent antigens, whereas antibody responses to type II T-independent antigens (e.g., TNP-Ficoll) occur independently of CD40L (22, 23, 25, 33, 51, 69). CD40L also regulates fibroblast (11, 70, 73), epithelial (72), and endothelial (17, 30, 46, 54) cell function through regulation of adhesion molecule expression and production of prostaglandins, cytokines, and chemokines. The pivotal role of CD40L in protection from viral infections (4, 42, 52, 62) and from several, but not all, intracellular pathogens or parasites is well established (10, 14, 16, 26, 29, 32, 36, 59, 74). However, the role of CD40L in protection from infection with extracellular bacteria is less well understood. A protective humoral response to Borrelia burgdorferi, the causative agent of Lyme disease, is elicited independently of CD40L (21). In a recent study, Wu et al. demonstrated that the antiphosphocholine-specific immunoglobulin G (IgG) responses elicited by immunization with a nonencapsulated, nonvirulent variant of S. pneumoniae are impaired in T-cell-deficient or CD40L(−/−) mice (68). The present study was undertaken to determine whether CD40L is essential for protection from an encapsulated strain of S. pneumoniae. The effect of anti-CD40L (MR-1) on humoral responses to pneumococcal antigens as well as susceptibility to pneumococcal infection was examined.
MATERIALS AND METHODS
Mice and bacterial strains.
Female BALB/cJ or CBA/CAHN-XID/J (CBA/N) mice were obtained from Jackson Laboratory (Bar Harbor, Maine) and used at 6 to 14 weeks of age. S. pneumoniae serotype 6B (strain BG9163) was grown in Todd-Hewitt broth enriched with 0.5% yeast extract, harvested during the log phase, and kept frozen in aliquots. This strain of S. pneumoniae causes chronic infections in immunocompetent mouse strains; these infections last several days or even weeks before the mouse either recovers or dies (5, 6). The 50% lethal dose of strain BG9163 mice is greater than 105 CFU intravenously (i.v.) for BALB/cByJ or 1,000 CFU i.v. for CBA/N mice. When inoculated intranasally (i.n.), strain BG9163 causes a carrier status, generally without disease except when it is introduced at very high inocula (5, 6).
Treatment with MR-1 and control antibodies.
Mice were injected intraperitoneally (i.p.) with (i) saline (Ringer's lactate); (ii) nonspecific polyclonal hamster IgG (Accurate Chemical, San Diego, Calif.) or Ha4/8 Armenian hamster IgG (anti-keyhole limpet hemocyanin [KLH]; Biogen, Cambridge, Mass.), 250 μg as a control antibody; or (iii) purified MR-1 (hamster monoclonal antibody specific to CD40L [Biogen]), 250 μg (49). Antibodies were injected on days −1, 1, and 2 relative to inoculation or vaccination for assessment of susceptibility to bacterial infections or on days −1, 1, and 3 relative to the primary immunization for assessment of antibody responses to pneumococcal antigens.
Immunization protocols for the study of antibody responses to S. pneumoniae antigens.
Groups of mice were immunized with various antigens as described below. The immunization protocols and procedures used for the study of antibody responses are described in detail below and outlined in Table 1.
TABLE 1.
Immunization protocols used in this studya
| Antigen | No. of animals/groupb | Time of antibody injection (day) | Immunizationc | Time of bleeding (day) |
|---|---|---|---|---|
| PspA | 8 (MR-1), 14 (HIgG), 8 (NoTx) | −1, 1, 3 | Day 0, 0.5 μg s.c. w/CFA; day 21, 0.5 μg s.c. w/PBS | 0, 33 |
| 6B-KLH | 8 (MR-1), 12 (HIgG), 8 (NoTx) | −1, 1, 3 | Day 0, 2.5 μg s.c. w/QA; day 21, 2.5 μg i.p.; day 42, 2.5 μg s.c. w/QA; day 64, 2.5 μg s.c. w/QA | 0, 53, 74 |
| 6B Polysaccharide | 8 (MR-1), 12 (HIgG), 8 (NoTx) | −1, 1, 3 | Day 0, 5 μg s.c. w/QA; day 21, 5 μg s.c. w/QA | 0, 28 |
| Bacteria | 8 (MR-1), 8 (HIgG), 10 (NoTx) | −1, 1, 3 | Days 0, 7, 14, 21, 108 bacteria i.p.; day 49, 108 bacteria i.v. | 0, 56 |
BALB/cJ mice were used for all experiments.
For definitions of the groups, see the legend to Fig. 1.
s.c., subcutaneously; CFA, complete Freund's adjuvant; QA, Quil A.
(i) PspA.
BALB/cJ mice were injected subcutaneously with 0.5 μg of full-length PspA in complete Freund's adjuvant on day 0 and with 0.5 μg of PspA in phosphate-buffered saline (PBS) on day 21. PspA was isolated as described previously (7). The mice were bled on days 0 and 33. The serum samples were analyzed for anti-PspA antibodies as described below.
(ii) Polysaccharide-protein conjugate.
Caps-PS from S. pneumoniae serotype 6B conjugated to KLH (6B-KLH) was obtained from A. Verheul (Utrecht University, Utrecht, The Netherlands). Mice were immunized with 6B-KLH (2.5 μg of polysaccharide) four times. The primary, tertiary, and quaternary immunizations were given subcutaneously on days 0, 42, and 64, along with 5 μg of Quil A (partially purified Saponin from Quillaja saponaria bark, kindly provided by A. Verheul) in 200 μl of PBS. The secondary immunization was given i.p. on day 21 without adjuvant. Serum samples were obtained on days 0, 53, and 74.
(iii) Caps-PS.
Mice were immunized with 5 μg of Caps-PS from S. pneumoniae serotype 6B (from American Type Culture Collection, Rockville, Md.) on days 0 and 21. Caps-PS (187.5 μl of Caps-PS [26.8 μg/ml] in PBS) was mixed with 12.5 μl of Quil A (0.4 mg/ml) by gentle rotation overnight. Serum samples were obtained on days 0 and 28.
(iv) Pneumococci.
Mice were immunized with heat-killed (56°C for 30 min) bacterial strain BG9163 (108 CFU per dose) i.p. on days 0, 7, 14, and 21 and i.v. on day 49. Serum samples were obtained on days 0 and 56.
Mouse vaccination and inoculation.
Mice were infected i.v., intratracheally (i.t.), or i.n. with live pneumococci. The i.v. and i.n. infection was performed as previously described with log-phase bacterial cultures diluted in Ringer's lactate to the indicated number of CFU, based on optical density units (66). The i.t. inoculation was performed by a nonsurgical procedure involving a 69-mm finely drawn gel-loading plastic pipette tip (05-541-9; Fisher Scientific, Pittsburgh, Pa.). The mice were anesthetized with ketamine and xylazine and placed on their backs with their necks bent slightly back. Slight tension was applied to the tongue to expose the glottis. The gel-loading tip was inserted into the glottis, and 20 μl of bacteria in Ringer's solution was discharged into the trachea. The challenge dose was near the 50% lethal dose for untreated animals so as to maximize the increase in susceptibility due to MR-1 treatment. Numbers of CFU in inocula and animal samples are expressed as log10 values.
Assessment of bacterial infection and colonization.
The course of infection and colonization was determined by monitoring the numbers of CFU in the blood (sepsis), nasal washes, and homogenized lungs or spleens at the specified times after inoculation. Samples from blood and lungs were plated in serial dilutions on blood agar plates so that the CFU could be counted (6). Nasal wash samples were collected from mice after sacrifice. The trachea was cut at the top of the larynx, and 50 μl of Ringer's solution was injected and collected from the tip of the nose. The bacteria were plated on blood agar plates containing 4 μg of gentamicin per ml to inhibit the growth of most nonpneumococcal bacteria (15). To ensure that the bacteria observed were pneumococci, samples were plated on a second set of gentamicin-containing plates that also contained 5 μg of optochin (ethyl hydrocupreine hydrochloride [Sigma, St. Louis, Mo.]) per ml. The paucity of colonies on gentamicin-optochin plates confirmed that the bacteria observed on gentamicin plates were indeed pneumococci. Results from mice for which there were >15% as many CFU on the gentamicin-optochin plates as on the gentamicin plates were discarded. Such mice were extremely rare. In other cases, the number of CFU, if any, on the gentamicin-optochin plates were subtracted from those observed on the gentamicin plate at the same sample dilution.
Measurement of antibody levels in sera.
Serum samples were obtained from blood allowed to clot overnight at 4°C. Antibodies specific for PspA, 6B Caps-PS, or C-PS were detected by enzyme-linked immunosorbent assay (ELISA). Microtiter plates (Immulon 2; Dynatech, Chantilly, Va.) were coated with PspA (0.35 μg/ml in PBS) purified from S. pneumoniae R36A. Microtiter plates were also coated with 6B Caps-PS (American Type Culture Collection) or C-PS (Statens Seruminstitut, Copenhagen, Denmark) by adding 100 μl of PBS containing either 20 μg of 6B pneumococcal Caps-PS per ml or 10 μg of C-PS per ml to each well. The plates were blocked with 1% skim milk–PBS–0.05% Tween 20. Samples, diluted 100-fold in 1% skim milk–PBS, were placed in the first-row wells, and 4-fold serial dilutions were performed with the same diluent. For the anti-6B assay, sera were preabsorbed with C-PS by overnight incubation at 4°C of 2.5 μl of serum sample in 250 μl of the 1% skim milk buffer containing 2.5 μg of C-PS. Following a 3-h incubation at room temperature, the wells were washed three times with PBS-Tween 20 and three times with distilled water. The wells were loaded with 100 μl of anti-mouse Ig conjugated to alkaline phosphatase (Sigma). After a 2-h incubation at room temperature, the wells were washed prior to addition of substrate, p-nitrophenyl phosphate (Sigma) in diethanolamine buffer (pH 9.8). The optical densities were read at 405 nm in an ELISA reader and were converted to concentrations by comparison with the optical densities of the standard samples by a piecewise linear regression method. The standards used for ELISA were XiR278 (a monoclonal antibody to PspA [40]), Hyp6BM1 hybridoma supernatant (anti-6B pneumococcal IgM antibody), and serum 186.3 (anti-C-PS assay). Serum 186.3 was drawn from one of the mice immunized with heat-killed bacteria for this study. The antibody in this sample was almost completely absorbed by C-PS at 20 μg/ml when diluted 100-fold. Each standard was assigned a concentration of 100 units/ml. The optical density was converted into relative units by comparison with the standards used in each assay.
RESULTS
Treatment of mice with MR-1 antibody (anti-CD154/CD40L) inhibits antibody responses to a pneumococcal protein.
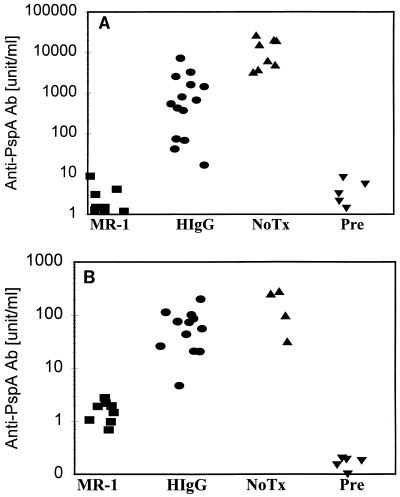
To evaluate the effect of CD40L neutralization on the responses to a protection-eliciting pneumococcal protein, we examined the effect of MR-1 treatment on immune responses to PspA. Mice were immunized with either native PspA or heat-killed strain BG9163 pneumococci. Mice receiving the control hamster immunoglobulin produced slightly less antibody to PspA (P < 0.01) than did untreated mice after immunization with PspA (Fig. 1). In contrast, the antibody response to immunization with purified PspA protein was completely suppressed by MR-1 (Fig. 1A), demonstrating that the dose of anti-CD40L antibody effectively blocked T-dependent antibody responses. Treatment of mice with MR-1 antibody also markedly reduced anti-PspA serum titers in mice immunized with whole bacteria (Fig. 1B). In two independent experiments, the antibody response to PspA was reduced between 10- and 100-fold. These data confirm that CD40L is essential for induction of protective antibody responses to pneumococcal protein antigens.
FIG. 1.
Anti-CD40L (MR-1) inhibits antibody responses to the pneumococcal protein PspA. Mice were immunized with either purified PspA or heat-killed S. pneumoniae (6B serotype, strain BG9163) as outlined in Table 1 and described in Materials and Methods. Groups labeled MR-1, HIgG, and NoTx indicate mice receiving MR-1 antibody, control hamster IgG, and no treatment, respectively. Pre indicates the antibody levels observed prior to immunization. The control hamster IgG group for panel A received either monoclonal hamster antibody Ha4/8 (seven mice), which is specific for KLH, or the nonspecific polyclonal hamster IgG (seven mice). Since the data from the two control groups were comparable, they were pooled. The Wilcoxon rank sum test for difference in medians with α = 0.05 is the statistical method used to compare groups. (A) The concentration of antibodies to PspA in serum was determined on day 33, 12 days after the second injection of purified PspA. A sample containing 7 μg of anti-PspA antibody per ml is equivalent to 1,890 U/ml. MR-1 and Pre show no statistical difference from each other, while both of the groups differ from HIgG and NoTx (P < 0.01). (B) The concentration of antibodies to pneumococcal PspA determined on day 56, 7 days after the last injection, is shown. The MR-1 group differs from both the HIgG and NoTx groups (P < 0.01).
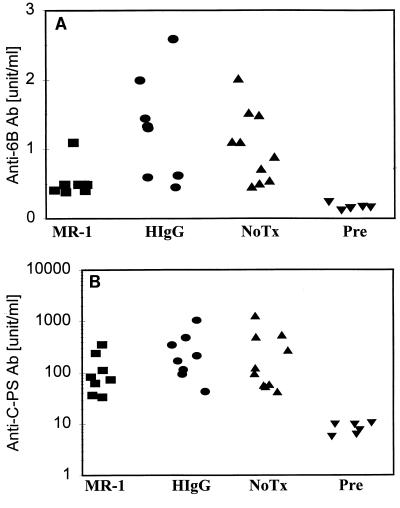
Treatment of mice with MR-1 reduces humoral responses to Caps-PS, but not C-PS, induced by immunization with heat-killed S. pneumoniae.
The humoral response to Caps-PS is an important component of the protective immune response to pneumococcal infection. To determine whether CD40L regulates antibody responses to pneumococcal polysaccharides, mice treated with MR-1 or control antibody or left untreated were immunized with heat-killed S. pneumoniae 6B (strain BG9163). In two independent experiments, MR-1 treatment reduced the anti-6B Caps-PS antibody titers. In the first experiment, anti-6B titers for the MR-1-treated group were significantly reduced relative to the HIgG control group (P = 0.012 for the HIgG group) (data not shown). In Fig. 2A the anti-6B antibody titers for the MR-1 group were also lower than those for the control antibody-treated (HIgG) or untreated (NoTx) groups (P = 0.01 for the HIgG group, and P < 0.01 for the NoTx groups). These data suggest that CD40L is a necessary component of the anti-Caps-PS antibody response elicited by infection with pneumococcal bacteria. In contrast, MR-1 treatment did not reduce anti-C-PS antibody levels (Fig. 2B), indicating that production of antibodies to C-PS elicited by immunization with whole bacteria is independent of CD40L.
FIG. 2.
MR-1 treatment reduces the antibody response to Caps-PS, but not C-PS, induced by immunization with whole bacteria. The concentrations of antibodies to 6B polysaccharide (A) or to C-PS (B) elicited in the sera of mice following immunizations with heat-inactivated S. pneumoniae (6B serotype) are shown. The blood samples were obtained on day 56. In both panels, all the experimental groups showed elevated antibody levels specific to 6B polysaccharide or C-PS compared to those of the Pre group (P < 0.01). (A) MR-1 differs from both the HIgG (P = 0.01) and NoTx (P < 0.01) groups, while the HIgG and NoTx groups are not statistically different from each other. (B) The MR-1, HIgG, and NoTX groups are not statistically different from each other. The group names are as in Fig. 1.
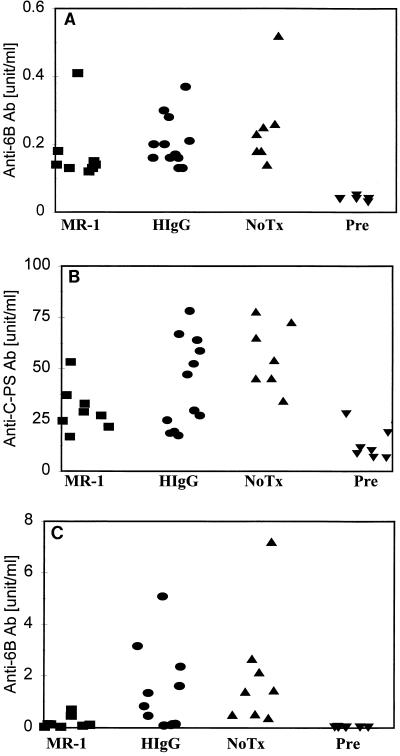
MR-1 antibody inhibits antibody responses to 6B-KLH, but not Caps-PS, in mice immunized with isolated polysaccharides.
Reduction of the anti-Caps-PS response in the experiment in Fig. 2A by MR-1 treatment was unexpected, since CD40L is not required for humoral responses to purified polysaccharide antigens (51). To confirm that the MR-1 treatment protocol selectively inhibits T-dependent antibody responses, BALB/cJ mice were immunized with unconjugated pneumococcal Caps-PS (serotype 6B) in Quil A adjuvant with or without administration of MR-1 antibody. The 6B polysaccharide is poorly immunogenic in mice, and Quil A improves the immune response to pneumococcal polysaccharide of serotypes 14 and 17F (18). In addition to antibody responses to 6B polysaccharide, those to C-PS were measured, since C-PS is present in Caps-PS preparations as a contaminant at 1 to 10% by weight (56). Antibody responses to the two polysaccharide antigens were comparable whether mice were treated with MR-1 or control hamster IgG (Fig. 3A and B). These results confirm that neutralization of CD40L does not reduce humoral responses to isolated type II T-independent antigens.
FIG. 3.
Treatment of mice with MR-1 inhibits antibody responses to 6B-KLH conjugate but not to 6B polysaccharide. (A and B) The concentration in serum of antibodies to 6B Caps-PS (A) or to C-PS (B) elicited in mice on day 28 following immunization with the purified Caps-PS of S. pneumoniae (6B serotype) in Quil A adjuvant are shown. The groups are labeled as in Fig. 1. (A) MR-1, HIgG, and NoTx differ from Pre (P < 0.01). The MR-1 and HIgG groups are not statistically different. (B) The anti-C-PS concentration in serum drawn from a mouse immunized with killed bacteria was assigned a concentration of 100 U/ml and used as a standard. Similar to the concentrations of anti-6B antibody shown in panel A, all the experimental groups differ statistically from the Pre group (P < 0.01), while the MR-1 and HIgG groups show no difference. (C) The concentration of antibodies to pneumococcal Caps-PS (serotype 6B) determined on day 53, 11 days after the third injection, of 6B-KLH is shown. A sample containing 2.4 μg of IgM anti-6B polysaccharide antibody per ml is equal to 100 U/ml. MR-1 differs from HIgG (P < 0.05), NoTx (P < 0.01), and Pre (P < 0.01).
On the surface of pneumococci, Caps-PS is covalently linked to peptidoglycan (57), and thus Caps-PS presented in the context of intact pneumococcus might behave more like a polysaccharide-protein conjugate vaccine. To address this possibility, the effect of MR-1 treatment on anti-6B antibody responses in mice immunized with 6B-KLH conjugate was examined. MR-1 treatment was found to inhibit antibody responses to 6B-KLH compared to treatment with hamster immunoglobulin (P < 0.05). The effect of MR-1 on antibody responses to 6B-KLH conjugate was long-lasting. Although MR-1 was administered only at the primary immunization (days −1, 1, and 3), the anti-6B polysaccharide antibody levels on day 74 were still reduced (2.8-fold less) relative to the control group (data not shown). These results demonstrate a CD40L-dependent component of antibody responses to polysaccharide-protein conjugates.
Treatment with MR-1 does not affect sepsis, lung infection, or carriage following inoculation of nonimmune mice with S. pneumoniae.
The experiments described above demonstrate that blockade of CD40L can reduce protective humoral responses to pneumococcal infection. The goal of the next set of experiments was to determine the effect of MR-1 antibody on the severity of infection following i.v. and i.t. challenge of naive mice. Mice were sacrificed at 24, 48, and 96 h after i.v. or i.t. inoculation, and the CFU in the blood and lungs were counted. No significant differences in CFU in blood were found between groups treated with MR-1, control antibody, or saline and injected i.v. with two doses of BG9163 (capsular type 6B) pneumococci (Table 2). Furthermore, treatment with MR-1 did not decrease the onset or increase the rate of mortality upon i.v. challenge with 3 × 104 CFU. Pneumococcal CFU counts in the lungs and blood following i.t. challenge with 1.7 × 107 CFU of BG9163 were slightly lower in mice treated with MR-1. These differences, however, were not statistically significant (P > 0.05) by the Wilcoxon test (Table 3). Thus, inhibition of CD40L did not exacerbate sepsis following i.v. inoculation of S. pneumoniae; if anything, it may have caused a slight protection against pulmonary infection.
TABLE 2.
Lack of effect of MR-1 treatment on sepsis in BALB/cByJ mice following i.v. inoculation of BG9163 pneumococci
| Treatmenta | Challenge dose (log10 CFU) | Mean log10 CFU/ml of blood ± SE on:
|
Days to death
|
No. alive: no. dead | |||
|---|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 4 | Mean | Median | |||
| MR-1 | 4.48 | 4.5 ± 0.9 | 3.4 ± 0.5 | 3.1 ± 0.5 | 16.7 ± 2.8 | >21 | 4:2 |
| Hamster IgG | 4.48 | 4.2 ± 0.7 | 2.7 ± 0.6 | 3.6 ± 0.7 | 19.0 ± 2.0 | >21 | 5:1 |
| Ringer's | 4.48 | 5.2 ± 0.7 | 3.2 ± 0.8 | 3.5 ± 0.9 | 14.0 ± 4.1 | 2:2 | |
| MR-1 | 6.78 | 4.9 ± 0.3 | 6.6 ± 0.4 | 7.1 ± 0.5 | 3.3 ± 0.8 | 3 | 0:6 |
| Hamster IgG | 6.78 | 5.2 ± 0.3 | 7.2 ± 0.2 | 7.5 ± 0.1 | 2.5 ± 0.3 | 2 | 0:6 |
| Ringer's | 6.78 | 5.5 ± 0.3 | 7.0 ± 0.5 | 7.5 ± 0.3 | 2.5 ± 0.5 | 2 | 0:4 |
MR-1 protocol: 250 μg of MR-1 on day −1, day +1, and day 2. No MR-1 values differed from hamster IgG, or Ringer's at P < 0.05 for any of the comparisons.
TABLE 3.
Lack of effect of MR-1 treatment on sepsis and lung infections in BALB/cByJ mice following i.t. inoculation of BG9163 pneumococci
| Treatmenta | Day of assay | n | Mean log10 CFU/lung ± SE in:
|
Mean log10 CFU/ml of blood | ||
|---|---|---|---|---|---|---|
| Right lung | Left lung | Both lungs | ||||
| MR-1 | 1 | 6 | 3.0 ± 0.1 | 2.8 ± 0.6 | 3.5 ± 0.3 | 2.7 ± 0.6 |
| Hamster IgG | 1 | 6 | 3.7 ± 0.9 | 3.8 ± 0.8 | 4.2 ± 0.7 | 3.6 ± 0.8 |
| MR-1 | 3 | 6 | 1.6 ± 0.3 | 1.4 ± 0.4 | 1.8 ± 0.4 | 2.9 ± 0.4 |
| Hamster IgG | 3 | 6 | 3.2 ± 0.9 | 2.5 ± 1.0 | 3.3 ± 0.9 | 3.3 ± 0.9 |
MR-1 protocol: mice were inoculated with BG9163 on day 0 and treated with 250 μg of MR-1 on days −1, +1, and 2. No MR-1 values differed from hamster IgG at P < 0.05 for any of the individual comparisons by the Wilcoxon rank sum test.
i.n. infections with S. pneumoniae are generally contained within the nasal tissue by host immunity (9). To address the effect of neutralizing CD40L on invasion of the lungs or blood resulting from carriage of S. pneumoniae, mice were inoculated i.n. with 4.5 × 107 CFU of BG9163 pneumococci and the numbers of CFU in the nose, lungs, and blood were determined on days 2 and 5. CBA/N mice were used for this portion of the study because nasopharyngeal carriage in this mouse strain has been well characterized (66, 67). CBA/N mice provide a more stringent test of enhanced infection resulting from MR-1 treatment due to their increased susceptibility to pneumococcal infection compared to BALB/c mice (6). Carriage of bacteria in the nasal mucosa was not affected by blockade of CD40L, since pneumococcal CFU counts in MR-1-treated mice were comparable to those in control mice (Table 4). Furthermore, MR-1 treatment had no effect on the numbers of CFU isolated from the blood and lungs. These results indicate that CD40L does not influence pneumococcal invasion of mucosal surfaces.
TABLE 4.
Lack of effect of MR-1 treatment on sepsis and colonization following i.n. inoculation of CBA/N mice with BG9163 pneumococci
| Groupa | Day of assay | Log10 CFU in noseb
|
Lungsb
|
Bloodb
|
|||
|---|---|---|---|---|---|---|---|
| Median | Mean ± SE | Median log10 CFU | +:− | Median log10 CFU | +:− | ||
| MR-1 | 2 | 4.75 | 4.2 ± 0.6 | <1 | 2:4 | <1 | 1:5 |
| Hamster IgG | 2 | 4.25 | 4.2 ± 0.2 | 3.25 | 4:2 | <1 | 2:5 |
| MR-1 | 5 | 3.35 | 3.7 ± 0.7 | <1 | 0:6 | <1 | 0:6 |
| Hamster IgG | 5 | 4.70 | 4.8 ± 0.8 | <1 | 0:6 | <1 | 0:6 |
MR-1 protocol: 250 μg of MR-1 on days −1, +1, and 2.
CFU values are expressed per nose, per pair of lungs, and per milliliter of blood. No MR-1 values differed from hamster IgG at P < 0.05 for individual comparisons by the Wilcoxon rank sum test. + refers to the number of mice with more than 10 CFU. − refers to the number of mice with less than 10 CFU.
DISCUSSION
Although classic studies demonstrated that circulating antibody to Caps-PS is critical for protection against invasive pneumococcal infection (38), resistance of normal mice to pneumococcal infection actually involves various components of the immune system including acute-phase responses, antigen-specific adaptive immune responses, normal phagocyte function, and inflammation (2, 8). CD40L could contribute to host defense against pneumococcal invasion of mucosal surfaces through regulation of epithelial or phagocyte cell function as well as through generation of protective humoral responses. Invasion of host tissue by S. pneumoniae involves complex interactions between the bacterial cell wall and the mucosal epithelium (63). CD40L influences epithelial-cell function through upregulation of adhesion molecule expression and cytokine production (72). CD40L is also a key component of T-dependent activation of macrophages and dendritic cells (12, 13, 58, 64) and is essential for T-dependent antibody responses (22, 23, 25, 33, 51, 69). Thus, neutralization of CD40L should diminish the T-cell-mediated adaptive immune response to pneumococcal infection.
CD40L was essential for the antibody response to pneumococcal proteins and contributed to the humoral response to Caps-PS. Neutralization of CD40L suppressed the antibody response to PspA protein (Fig. 1). To our surprise, neutralization of CD40L also reduced the protective antibody response to Caps-PS elicited by immunization with whole bacteria but not with isolated Caps-PS (Fig. 2A and 3A). The results in Fig. 2 are consistent with recent findings by Wu et al., who reported that IgG responses in mice immunized with a nonencapsulated variant of S. pneumoniae is T-cell and CD40L dependent (68). In contrast to the Caps-PS response, the antibody response to C-PS elicited by immunization with whole bacteria was unaffected by MR-1 treatment (Fig. 2B). This result is consistent with previous studies demonstrating that immunization with intact pneumococci elicits antibodies to PS antigens in a T-cell-independent manner (44). However, Caps-PS presented on the surface of extracellular bacteria may be in some ways analogous to polysaccharide-protein conjugate vaccines that elicit T-cell-dependent humoral responses (Fig. 3B) (28). Caps-PS at the surface of S. pneumoniae is covalently linked to peptidoglycan (57), and proteins expressed on the pneumococcal surface may act as carriers for attached polysaccharides (48).
Reduction of protective antibody responses by neutralization of CD40L did not compromise the host defense against S. pneumoniae in nonimmunized mice. MR-1 treatment had no effect on survival following i.t. challenge (Table 3). Since i.t. challenge of mice with capsular type 6B pneumococci generally results in sepsis rather than a focal pulmonary infection, protection from pneumococcal invasion via this route involves host defenses at the mucosal surface. These results suggest that although CD40 promotes adhesion to and cytokine production by epithelial cells at mucosal surfaces (72), blockade of CD40L does not facilitate mucosal invasion.
i.v. inoculation with S. pneumoniae leads to very rapid sepsis and death (50). Due to the speed with which acute pneumococcal infections can lead to death of mice, protection during the first several days is not dependent on a new antibody response. Therefore, failure of MR-1 treatment to affect the resistance of normal mice to pneumococcal infection indicates that CD40L is not essential for innate host defense mechanisms that include the production of acute-phase proteins, phagocyte and neutrophil activation, and complement activation via the alternative pathway.
In contrast to neutralization of CD40L, TNFR (p55) deficiency or short-term neutralization of TNFα increases the susceptibility to infection by S. pneumoniae (3, 50, 61). Both receptors regulate humoral responses to microbial antigens. A deficiency in either CD40 or p55 abrogates germinal-center formation, in which affinity maturation and differentiation of memory B cells occur (33, 39). The defect in humoral responses due to lack of TNF/TNFR signaling is more profound, since TNFR deficiency also diminishes the antibody response to TI antigens (53). However, mice succumb to pneumococcal infection long before the development of an effective primary or memory response, suggesting that TNFR (p55) regulates a component of the innate immune response to S. pneumoniae infection that is critical for host protection during the early phase of infection. To date, studies of the mechanism underlying the increased susceptibility to S. pneumoniae have failed to reveal a role for TNF in acute-phase responses and have not consistently demonstrated a role for TNF in neutrophil migration to sites of infection (50, 61). Additional studies comparing the phenotypes of TNF and CD40-CD40L with respect to host defense against pneumococcal infection are required to understand the contribution of TNF to protection from S. pneumoniae pathogenesis.
Neutralization of intercellular communication via CD40-CD40L ameliorates disease in animal models of multiple sclerosis (24, 27), graft-versus-host disease (35), systemic lupus erythematosus (31, 36, 43), and rheumatoid arthritis (19, 37, 55). Consequently, neutralization of CD40L/CD40 as well as TNF/TNFR is being considered as an immunosuppressive therapy for autoimmune diseases (20, 45). A major concern associated with these immunosuppressive therapies is the resulting increased susceptibility to microbial infection. For example, CD40L-deficient children exhibit recurrent upper respiratory tract infections (1). The results of the present study are encouraging in this respect, since short-term blockade of CD40L did not facilitate pneumococcal infection of mice. Thus, CD40L-based therapies may not increase susceptibility to infection to the same degree as neutralization of TNF. However, CD40L deficiency is associated with infection by Pneumocystiis carinii (1), indicating that continued vigilance against opportunistic infections is needed during CD40L therapy.
ACKNOWLEDGMENTS
This work was supported by Biogen Inc. and by grants from the National Institutes of Health AI-31473 (M.H.N.) and AI-65298 (D.E.B.). M.H.N. is also supported by NIAID contract NOI AI-45248.
REFERENCES
- 1.Banatvala N, Davies J, Kanarious M, Strobel S, Levinstky R, Morgan G. Hypogammaglobulinemia associated with normal or increased IgM (the hyper IgM syndrome): a case series review. Arch Dis Child. 1994;71:150–152. doi: 10.1136/adc.71.2.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benton K A, Everson M P, Briles D E. A pneumolysin-negative mutant of Streptococcus pneumoniae causes chronic bacteremia rather than acute sepsis in mice. Infect Immun. 1995;63:448–455. doi: 10.1128/iai.63.2.448-455.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benton K A, Vancott J L, Briles D E. Role of tumor necrosis factor alpha in the host response of mice to bacteremia caused by pneumolysin-deficient Streptococcus pneumoniae. Infect Immun. 1998;66:839–842. doi: 10.1128/iai.66.2.839-842.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Borrow P, Tishon A, Lee S, Xu J, Grewal I S, Oldstone M, Flavell R A. CD40L-deficient mice show deficits in antiviral immunity and have an impaired memory CD8+ CTL response. J Exp Med. 1996;183:2129–2142. doi: 10.1084/jem.183.5.2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Briles D E, Forman C, Crain M. Mouse antibody to phosphocholine can protect mice from infection with mouse-virulent human isolates of Streptococcus pneumoniae. Infect Immun. 1992;60:1957–1962. doi: 10.1128/iai.60.5.1957-1962.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Briles D E, Forman C, Horowitz J C, Volanakis J E, Benjamin W H, Jr, McDaniel L S, Eldridge J, Brooks J. Antipneumococcal effects of C-reactive protein and monoclonal antibodies to pneumococcal cell wall and capsular antigens. Infect Immun. 1989;57:1457–1464. doi: 10.1128/iai.57.5.1457-1464.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Briles D E, King J D, Gray M A, McDaniel L S, Swiatlo E, Benton K A. PspA, a protection eliciting pneumococcal protein: immunogenicity of isolated native PspA in mice. Vaccine. 1996;14:858–867. doi: 10.1016/0264-410x(96)82948-3. [DOI] [PubMed] [Google Scholar]
- 8.Briles D E, Paton J C, Nahm M H, Swiatlo E. Immunity to Streptococcus pneumoniae. In: Cunningham M W, Fujinami R S, editors. Effects of microbes on the immune system. Philadelphia, Pa: Lippincott-Raven; 1999. pp. 263–280. [Google Scholar]
- 9.Briles D E, Tart R C, Wu H Y, Ralph B A, Russell M W, McDaniel L S. Systemic and mucosal protective immunity to pneumococcal surface protein A. Ann N Y Acad Sci. 1996;797:118–126. doi: 10.1111/j.1749-6632.1996.tb52954.x. [DOI] [PubMed] [Google Scholar]
- 10.Campos-Neto A, Ovendale P, Bement T, Kippi T A, Fanslow W C, Rossi M A, Alderson M R. CD40 ligand is not essential for the development of cell-mediated immunity and resistance to Mycobacterium tuberculosis. J Immunol. 1998;160:2037–2041. [PubMed] [Google Scholar]
- 11.Cao H J, Wang H S, Zhang Y, Lin H Y, Phipps R P, Smith T J. Activation of human orbital fibroblasts through CD40 engagement results in a dramatic induction of hyaluronan synthesis and prostaglandin endoperoxide H synthase-2 expression. Insights into potential pathogenic mechanisms of thyroid-associated ophthalmopathy. J Biol Chem. 1998;273:29615–29625. doi: 10.1074/jbc.273.45.29615. [DOI] [PubMed] [Google Scholar]
- 12.Caux C, Massacrier C, Vanbervliet B, Dubois B, Van Kooten C, Durand I, Banchereau J. Activation of human dendritic cells through CD40 crosslinking. J Exp Med. 1994;180:1263–1272. doi: 10.1084/jem.180.4.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cella M, Scheidegger D, Palmer-Lehman K, Lane P, Lanzavecchia A, Alber G. Ligation of CD40 on dendritic cells triggers production of high levels of IL-12 and enhances T cell stimulatory capacity: T-T help via APC activation. J Exp Med. 1996;184:747–752. doi: 10.1084/jem.184.2.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaussabel D, Jacobs F, de Jonge J, de Veerman M, Carlier Y, Thielemans K, Goldman M, Vray B. CD40 ligation prevents Trypanosoma cruzi infection through interleukin-12 upregulation. Infect Immun. 1999;67:1929–1934. doi: 10.1128/iai.67.4.1929-1934.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Converse G M, III, Dillon H C., Jr Epidemiological studies of Streptococcus pneumoniae in infants: methods of isolating pneumococci. J Clin Microbiol. 1977;5:293–296. doi: 10.1128/jcm.5.3.293-296.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cosyns M, Tsirkin S, Jones M, Flavell R, Kikutani H, Hayward A R. Requirement of CD40-CD40 ligand interaction for elimination of Cryptosporidium parvum from mice. Infect Immun. 1999;66:603–607. doi: 10.1128/iai.66.2.603-607.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dechanet J, Grosset C, Taupin J L, Merville P, Banchereau J, Ripoche J, Moreau F. CD40 ligand stimulates proinflammatory cytokine production by human endothelial cells. J Immunol. 1997;159:5640–5647. [PubMed] [Google Scholar]
- 18.DeVelasco E A, Dekker H A, Antal P, Jalink K P, van Strijp J A G, Verheul A F M, Verhoef J, Snippe H. Adjuvant Quil A improves protection in mice and enhances opsonic capacity of antisera induced by pneumococcal polysaccharide conjugate vaccines. Vaccine. 1994;12:1419–1422. doi: 10.1016/0264-410x(94)90151-1. [DOI] [PubMed] [Google Scholar]
- 19.Durie F H, Fava R A, Foy T M, Aruffo A, Ledbetter J, Noelle R J. Prevention of collagen-induced arthritis with an antibody to gp39, the ligand for CD40. Science. 1993;261:1328–1330. doi: 10.1126/science.7689748. [DOI] [PubMed] [Google Scholar]
- 20.Elliot M J, Maini R N, Feldman M, Long-Fox A, Charles P, Katsikis P, Brennan F M, Walker J, Bijl H, Ghrayeb J, Woody J N. Treatment of rheumatoid arthritis with chimeric monoclonal antibodies to tumor necrosis factor α. Arthritis Rheum. 1993;36:1681–1690. doi: 10.1002/art.1780361206. [DOI] [PubMed] [Google Scholar]
- 21.Fikrig E, Barthold S W, Chen M, Grewal I S, Craft J, Flavell R A. Protective antibodies in murine Lyme disease arise independently of CD40 ligand. J Immunol. 1996;157:1–3. [PubMed] [Google Scholar]
- 22.Foy T M, Laman J D, Ledbetter J A, Aruffo A, Claassen E, Noelle R J. gp39-CD40 interactions are essential for germinal center formation and the development of B cell memory. J Exp Med. 1994;180:157–163. doi: 10.1084/jem.180.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Foy T M, Shepherd D M, Durie F H, Aruffo A, Ledbetter J A, Noelle R J. In vivo CD40-gp39 interactions are essential for thymus-dependent humoral immunity. II. Prolonged suppression of the humoral immune response by an antibody to the ligand for CD40, gp39. J Exp Med. 1993;178:1567–1575. doi: 10.1084/jem.178.5.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerritse K, Laman J D, Noelle R J, Aruffo A, Ledbetter J A, Boersma W J A, Claassen E. CD40-CD40 ligand interactions in experimental allergic encephalomyelitis and multiple sclerosis. Proc Natl Acad Sci USA. 1996;93:2499–2504. doi: 10.1073/pnas.93.6.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gray D, Dullforce P, Jainandunsing S. Memory B cell development but not germinal center formation is impaired by in vivo blockade of CD40-CD40 ligand interaction. J Exp Med. 1994;180:141–155. doi: 10.1084/jem.180.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grewal I S, Borrow P, Pan L, Pamer E M, Oldstone M B, Flavell R A. The CD40-CD154 system in anti-infective host defense. Curr Opin Immunol. 1997;9:491–497. doi: 10.1016/s0952-7915(97)80100-8. [DOI] [PubMed] [Google Scholar]
- 27.Grewal I S, Foellmer H G, Grewal K D, Xu J, Hardardottir F, Baron J L, Janeway C A, Jr, Flavell R A. Requirement for CD40 ligand in costimulation induction, T cell activation, and experimental allergic encephalomyelitis. Science. 1996;273:1864–1867. doi: 10.1126/science.273.5283.1864. [DOI] [PubMed] [Google Scholar]
- 28.Guttormsen H-K, Wetzler L M, Finberg R W, Kasper D L. Immunologic memory induced by a glycoconjugate vaccine in a murine adoptic transfer model. Infect Immun. 1998;66:2026–2032. doi: 10.1128/iai.66.5.2026-2032.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayashi T, Rao S P, Meylan P R, Kornbuth R S, Catanzaro A. Role of CD40 ligand in Mycobacterium avium infection. Infect Immun. 1999;67:3558–3565. doi: 10.1128/iai.67.7.3558-3565.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henn V, Slupsky J R, Grafe M, Anagnostopoulos I, Forster R, Muller-Berghaus G, Kroczek R A. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 1998;391:591–594. doi: 10.1038/35393. [DOI] [PubMed] [Google Scholar]
- 31.Kalled S L, Cutler A H, Datta S K, Thomas D W. Anti-CD40 ligand antibody treatment of SNF1 mice with established nephritis: preservation of kidney function. J Immunol. 1998;160:2158–2165. [PubMed] [Google Scholar]
- 32.Kamanaka M, Yu P, Yasui T, Yoshida K, Kawabe T, Horii T, Kishimoto T, Kikutani H. Protective role of CD40 in Leishmania major infection at two distinct phases of cell-mediated immunity. Immunity. 1996;4:275–281. doi: 10.1016/s1074-7613(00)80435-5. [DOI] [PubMed] [Google Scholar]
- 33.Kawabe T, Naka T, Yoshida K, Tanaka T, Fujiwara H, Suematsu S, Yoshida N, Kishimoto T, Kikutani H. The immune response in CD40-deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity. 1994;1:167–178. doi: 10.1016/1074-7613(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 34.Kindmark C O. Stimulating effect of C-reactive protein on phagocytosis of various species of pathogenic bacteria. Clin Exp Immunol. 1971;8:941. [PMC free article] [PubMed] [Google Scholar]
- 35.Larsen C P, Peterson T M. The CD40 pathway in allograft rejection, acceptance, and tolerance. Curr Opin Immunol. 1997;9:641–647. doi: 10.1016/s0952-7915(97)80043-x. [DOI] [PubMed] [Google Scholar]
- 36.Ma J, Xu J, Madaio M P, Peng Q, Zhang J, Grewal I S, Flavell R A, Craft J. Autoimmune lpr/lpr mice deficient in CD40 ligand: spontaneous Ig class switching with dichotomy of autoantibody responses. J Immunol. 1996;157:417–426. [PubMed] [Google Scholar]
- 37.MacDonald K P, Nishioka Y, Lipsky P E, Thomas R. Functional CD40 ligand is expressed by T cells in rheumatoid arthritis. J Clin Investig. 1997;100:2404–2414. doi: 10.1172/JCI119781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.MacLeod C M, Hodges R G, Heidelberger M, Bernhard W G. Prevention of pneumococcal pneumonia by immunization with specific capsular polysaccharides. J Exp Med. 1945;82:445–465. [PMC free article] [PubMed] [Google Scholar]
- 39.Matsumoto M, Mariathasan S, Nahm M H, Baranyay F, Peschon J J, Chaplin D D. Role of lymphotoxin and type I TNF receptor in the formation of germinal centers. Science. 1996;271:1289–1291. doi: 10.1126/science.271.5253.1289. [DOI] [PubMed] [Google Scholar]
- 40.McDaniel L S, Ralph B A, McDaniel D O, Briles D E. Localization of protection-eliciting epitopes on PspA of Streptococcus pneumoniae between amino acid residues 192 and 260. Microb Pathog. 1994;17:323–337. doi: 10.1006/mpat.1994.1078. [DOI] [PubMed] [Google Scholar]
- 41.McDaniel L S, Waltman II W D, Gray B, Briles D E. A protective monoclonal antibody that reacts with a novel antigen of pneumococcal teichoic acid. Microb Pathog. 1987;3:249–260. doi: 10.1016/0882-4010(87)90058-1. [DOI] [PubMed] [Google Scholar]
- 42.McFadden G. Getting to know you: viruses meet CD40 ligand. Nat Med. 1995;1:408–409. doi: 10.1038/nm0595-408. [DOI] [PubMed] [Google Scholar]
- 43.Mohan C, Shi Y, Laman J D, Datta S K. Interaction between CD40 and its ligand gp39 in the development of murine lupus nephritis. J Immunol. 1995;154:1470–1480. [PubMed] [Google Scholar]
- 44.Mond J J, Lees A, Snapper C M. T cell-independent antigens type 2. Annu Rev Immunol. 1995;13:655–692. doi: 10.1146/annurev.iy.13.040195.003255. [DOI] [PubMed] [Google Scholar]
- 45.Moreland L W, Baumgartner S W, Schiff M H, Tindall E A, Fleischmann R M, Weaver A L, Ettlinger R E, Cohen S, Koopman W J, Mohler K, Widmer M B, Blosch C M. Treatment of rheumatoid arthritis with a recombinant human tumor necrosis factor receptor (p75)-Fc fusion protein. N Engl J Med. 1997;337:195–197. doi: 10.1056/NEJM199707173370301. [DOI] [PubMed] [Google Scholar]
- 46.Moses A V, Williams S E, Strussenberg J G, Heneveld M L, Ruhl R A, Bakke A C, Bagby G C, Nelson J A. HIV-1 induction of CD40 on endothelial cells promotes the outgrowth of AIDS-associated B-cell lymphomas. Nat Med. 1997;3:1242–1249. doi: 10.1038/nm1197-1242. [DOI] [PubMed] [Google Scholar]
- 47.Musher D M. Infections caused by Streptococcus pneumoniae: clinical spectrum, pathogenesis, immunity, and treatment. Clin Infect Dis. 1992;14:801–809. doi: 10.1093/clinids/14.4.801. [DOI] [PubMed] [Google Scholar]
- 48.Nahm M H, Apicella M A, Briles D E. Immunity to extracellular bacteria. In: Paul W E, editor. Fundamental immunology. Philadelphia, Pa: Lippincott-Raven; 1999. pp. 1373–1386. [Google Scholar]
- 49.Noelle R J, Roy M, Shepherd D M, Stamenkovic I, Ledbetter J A, Aruffo A A. A 39-kDa protein on activated helper T cells binds CD40 and transduces the signal for cognate activation of B cells. Proc Natl Acad Sci USA. 1992;89:6550–6554. doi: 10.1073/pnas.89.14.6550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.O'Brien D, Briles D E, Szalai A, Tu A, Sanz I, Nahm M H. Tumor necrosis factor alpha receptor I is important for survival from Streptococcus pneumoniae infections. Infect Immun. 1999;67:595–601. doi: 10.1128/iai.67.2.595-601.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Renshaw B R, Fanslow W, Armitage R J, Campbell K A, Liggitt D, Wright B, Davison B L, Maliszewski C R. Humoral immune responses in CD40 ligand-deficient mice. J Exp Med. 1994;180:1889–1900. doi: 10.1084/jem.180.5.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ruby J, Bluethmann H, Aguet M, Ramshaw I A. CD40 ligand has potent antiviral activity. Nat Med. 1995;1:437–441. doi: 10.1038/nm0595-437. [DOI] [PubMed] [Google Scholar]
- 53.Ryffel B, DiPadova F, Schreier M H, Le Hir M, Eugster H P, Quesniaux V F. Lack of type 2 T cell-independent B cell responses and defect in isotype switching in TNF-lymphotoxin alpha-deficient mice. J Immunol. 1997;158:2126–2133. [PubMed] [Google Scholar]
- 54.Schonbeck U, Mach F, Bonnefoy J-Y, Loppnow H, Flad H D, Libby P. Ligation of CD40 activates interleukin 1beta-converting enzyme (caspase-1) activity in vascular smooth muscle and endothelial cells and promotes the elaboration of active interleukin 1beta. J Biol Chem. 1997;272:19569–19574. doi: 10.1074/jbc.272.31.19569. [DOI] [PubMed] [Google Scholar]
- 55.Sekine C, Yagita H, Miyasaka N, Okumura K. Expression and function of CD40 in rheumatoid arthritis synovium. J Rheumatol. 1998;25:1048–1053. [PubMed] [Google Scholar]
- 56.Sorensen U B S, Henrichsen J. C-Polysaccharide in a pneumococcal vaccine. Acta Pathol Microbiol Immunol Scand Sect C. 1984;92:351–356. doi: 10.1111/j.1699-0463.1984.tb00099.x. [DOI] [PubMed] [Google Scholar]
- 57.Sorensen U B S, Henrichsen J, Chen H-C, Szu S C. Covalent linkage between the capsular polysaccharide and the cell wall peptidoglycan of Streptococcus pneumoniae revealed by immunochemical methods. Microb Pathog. 1990;8:325–334. doi: 10.1016/0882-4010(90)90091-4. [DOI] [PubMed] [Google Scholar]
- 58.Stout R D, Suttles J, Xu J, Grewal I S, Flavell R A. Impaired T cell-mediated macrophage activation in CD40 ligand-deficient mice. J Immunol. 1996;156:8–11. [PubMed] [Google Scholar]
- 59.Subauste C S, Wessendarp M, Sorensen R U, Leiva L E. CD40-CD40 ligand interaction is central to cell-mediated immunity against Toxoplasma gondii: patients with hyper IgM syndrome have a defective type I response that can be restored by soluble CD40 ligand trimer. J Immunol. 1999;162:6690–6700. [PubMed] [Google Scholar]
- 60.Szalai A J, Briles D E, Volanakis J E. The role of complement in C-reactive-protein-mediated protection of mice from Streptococcus pneumoniae. Infect Immun. 1996;64:4850–4853. doi: 10.1128/iai.64.11.4850-4853.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Takashima K, Tateda K, Matsumoto T, Iizawa Y, Nakao M, Yamaguchi K. Role of tumor necrosis factor alpha in pathogenesis of pneumococcal pneumonia in mice. Infect Immun. 1997;65:257–260. doi: 10.1128/iai.65.1.257-260.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thomsen A R, Christenson A N J P, Andreasen S O, Marker O. CD40 ligand is pivotal to efficient control of virus replication in mice infected with lymphocytic choriomeningitis virus. J Immunol. 1998;161:4583–4590. [PubMed] [Google Scholar]
- 63.Tuomanen E I, Austrian R, Masure H R. Pathogenesis of pneumococcal infection. N Engl J Med. 1995;332:1280–1284. doi: 10.1056/NEJM199505113321907. [DOI] [PubMed] [Google Scholar]
- 64.Vankooten C, Banchereau J. Functions of CD40 on B cells dendritic cells and other cells. Curr Opin Immunol. 1997;9:330–337. doi: 10.1016/s0952-7915(97)80078-7. [DOI] [PubMed] [Google Scholar]
- 65.Volanakis J E, Kaplan M H. Specificity of C-reactive protein for choline phosphate residues of pneumococcal C-polysaccharide. Proc Soc Exp Biol Med. 1971;136:612. doi: 10.3181/00379727-136-35323. [DOI] [PubMed] [Google Scholar]
- 66.Wu H Y, Nahm M H, Guo Y, Russell M W, Briles D E. Intranasal immunization of mice with PspA (pneumococcal surface protein A) can prevent intranasal carriage, pulmonary infection, and sepsis with Streptococcus pneumoniae. J Infect Dis. 1997;175:839–846. doi: 10.1086/513980. [DOI] [PubMed] [Google Scholar]
- 67.Wu H Y, Virolainen A, Mathews B, King J D, Russell M W, Briles D E. Establishment of a Streptococcus pneumoniae nasopharyngeal colonization model in adult mice. Microb Pathog. 1997;23:127–137. doi: 10.1006/mpat.1997.0142. [DOI] [PubMed] [Google Scholar]
- 68.Wu Z Q, Vos Q, Shen Y, Lees A, Wilson S R, Briles D E, Gause W C, Mond J J, Snapper C M. In vivo polysaccharide-specific IgG isotype responses to intact Streptococcus pneumoniae are T cell dependent and require CD40- and B7-ligand interactions. J Immunol. 1999;163:659–667. [PubMed] [Google Scholar]
- 69.Xu J, Foy T M, Laman J D, Elliott E A, Dunn J J, Waldschmidt T J, Elsemore J, Noelle R J, Flavell R A. Mice deficient for the CD40 ligand. Immunity. 1994;1:423–431. doi: 10.1016/1074-7613(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 70.Yellin M J, Winikoff S, Fortune S M, Baum D, Crow M K, Ledermann F, Chess L. Ligation of CD40 on fibroblasts induces CD54 (ICAM-1) and CD106 (VCAM-1) up-regulation and IL-6 production and proliferation. J Leukoc Biol. 1995;58:209–216. doi: 10.1002/jlb.58.2.209. [DOI] [PubMed] [Google Scholar]
- 71.Yother J, Volanakis J E, Briles D E. Human C-reactive protein is protective against fatal Streptococcus pneumoniae infection in mice. J Immunol. 1982;128:2374–2376. [PubMed] [Google Scholar]
- 72.Young L S, Eliopoulos A G, Gallagher N J, Dawson C W. CD40 and epithelial cells: across the great divide. Immunol Today. 1998;19:502–506. doi: 10.1016/s0167-5699(98)01340-1. [DOI] [PubMed] [Google Scholar]
- 73.Zhang Y, Cao H J, Graf B, Meekins H, Smith T J, Phipps R P. CD40 engagement up-regulates cyclooxygenase-2 expression and prostaglandin E2 production in human lung fibroblasts. J Immunol. 1998;160:1053–1057. [PubMed] [Google Scholar]
- 74.Zhou P, Seder R A. CD40 ligand is not essential for induction of type 1 cytokine responses after primary or secondary infection with Histoplasma capsulatum. J Exp Med. 1998;187:1315–1324. doi: 10.1084/jem.187.8.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]