Abstract
The ability of Vibrio vulnificus to acquire iron from the host has been shown to correlate with virulence. Many iron transport genes are regulated by iron, and in V. vulnificus, transcriptional regulation by iron depends on the fur gene. The N-terminal amino acid sequence of a 72-kDa iron-regulated outer membrane protein purified from a V. vulnificus fur mutant had 53% homology with the first 15 amino acids of the mature protein of the Vibrio cholerae vibriobactin receptor, ViuA. In this report, we describe the cloning, DNA sequence, mutagenesis, and analysis of transcriptional regulation of the structural gene for VuuA, the vulnibactin receptor of V. vulnificus. Analysis of the DNA sequence of the vuuA promoter region demonstrated a sequence identical to the upstream Fur box of V. cholerae viuA. Northern blot analysis showed that the transcript was strongly regulated by iron. The amino acid sequence of VuuA was 74% identical to the sequence of V. cholerae ViuA and was homologous to those of several TonB-dependent outer membrane receptors. An internal deletion of the V. vulnificus vuuA gene resulted in the loss of expression of the 72-kDa protein and the loss of the ability to use transferrin or vulnibactin as a source of iron. This mutant showed reduced virulence in an infant mouse model. Introduction of a plasmid containing the complete viuA coding sequence and 342 bp of upstream DNA into the mutant restored ferric vulnibactin and ferric transferrin utilization to the mutant.
Vibrio vulnificus is a halophilic marine bacterium that has been associated with primary septicemia and serious wound infections (7, 39, 40, 42). Primary septicemia is often acquired by eating raw oysters or shellfish, and wound infections are associated with exposure of wounds to seawater (30, 62). Primary septicemia is often associated with patients who have diseases predisposing them to iron overload, such as cirrhosis, hemochromatosis, and alcoholism, or who are immunocompromised (28).
Iron is an essential element for the growth of most bacteria. In the mammalian host, most intracellular iron is found as heme, ferritin, hemoglobin, and hemosiderin. The concentration of available iron in the extracellular environment is extremely low because of the binding of iron to host high-affinity iron-binding proteins, such as transferrin and lactoferrin (3). Bacteria have evolved various mechanisms for the acquisition of iron from the host, including specific uptake of iron-chelating siderophores or the use of host iron compounds directly. Production of these iron uptake systems is repressed in the presence of iron by an iron-binding repressor protein called Fur (for ferric uptake regulation) (2).
Iron appears to be particularly important in the pathogenesis of V. vulnificus infections. Stelma et al. (60) found that iron overload was a more significant risk factor for infection than impaired immune function. Virulent isolates were resistant to inactivation by serum complement, produced a phenolate (catechol) siderophore, had high titers of hemolysin, and utilized transferrin-bound iron. Avirulent isolates in that study were unable either to utilize transferrin-bound iron or to produce significant amounts of catechol siderophore. The results suggest that the phenolate (catechol) siderophore enables the virulent isolates to acquire iron from highly saturated transferrin. Morris et al. (41) also found a significant association between virulence and the utilization of transferrin as an iron source. The structure of the phenolate siderophore of V. vulnificus, named vulnibactin, has been characterized (47). We recently isolated a V. vulnificus mutant that was unable to produce catechol siderophores or to acquire iron from transferrin (34). This mutant showed reduced virulence in an infant mouse model.
A V. vulnificus fur deletion mutant overexpresses at least two normally iron-regulated outer membrane proteins having apparent molecular masses of 72 and 77 kDa (33). The gene encoding the 77-kDa iron-regulated protein (hupA) was recently cloned and found to be required for heme utilization (32). The N-terminal amino acid sequence of the 72-kDa iron-regulated protein was determined, and the gene encoding the protein was cloned. In this communication, we report the cloning, mutagenesis, DNA sequence, and characterization of the gene encoding VuuA, for vulnibactin uptake gene A, in V. vulnificus.
MATERIALS AND METHODS
Bacterial strains and plasmids.
Characteristics of the V. vulnificus and Escherichia coli strains and plasmids used in this study are described in Table 1.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Relevant characteristicsa | Reference or source |
|---|---|---|
| V. vulnificus | ||
| CML17 | 80363 Δ(fur) | 33 |
| MO6-24 | Polyr; opaque | 63 |
| CML57 | MO6-24; Δ(vuuA) | This study |
| E. coli | ||
| DH5α | F−endA1 hsdR17 supE44 thi-1 recA1 gyrA96 relA1 Δ(argF-lacZYA)U169 (Φ80ΔlacZ M15)λ− | 23 |
| ABLE K | lac(LacZ−) [Kanr McrA− McrCB− McrA− McrF− Mrr− HsdR (rκ− mκ−)] [F′ proAB lacIqZΔ M15 Tn10 (Tetr)] | Stratagene |
| SY327λpir | Δ(lac pro) nalA recA56 araD argE(Am) λpir R6K | 38 |
| SM10λpir | thi thr leu tonA lacY supE recA::RP4-2-Tc::Mu λpir R6K Kmr | 38 |
| Plasmids | ||
| pUC19 | Cloning vector; Apr | Laboratory stock |
| pBluescript SK(−) | Phagemid derived from pUC19; Apr | Stratagene |
| pLAFR3 | Cloning vector; Tcr | 32 |
| pCML58 | V. vulnificus vuuA clone from V. vulnificus MO6-24 genomic library; 3.3-kbp chromosomal fragment in pBluescript SK(−); Apr | This study |
| pCML59 | vuuA subclone with 2.8-kb EcoRI-EcoRv insertion of V. vulnificus vuuA in pBluescript SK(−); Apr | This study |
| pCML60 | pBluescript with EcoRI-EcoRV insertion of V. vulnificus vuuA containing an internal 1.3-kb MscI-NruI deletion; Apr | This study |
| pCVD442 | Positive-selection suicide vector; pGP704 with sacB gene inserted in multiple cloning site; Apr | 18 |
| pCML61 | pCVD442 with SmaI-SalI insertion of pCML60 containing an internal 1.3-kb MscI-NruI deletion; Apr | This study |
| pCML63 | 2.8-kbp EcoRI-EcoRV vuuA fragment and pBluescript cloned into the BamHI site of pLAFR3; Tcr Apr | This study |
Apr, ampicillin resistance; Kmr, kanamycin resistance; Polyr, polymyxin B resistance; Tcr, tetracycline resistance.
Media.
Strains were routinely grown in Luria broth (LB). All strains were maintained at −70°C in LB medium containing 15% glycerol. LB solidified with agar was used for high-iron solid media. Two types of low iron media were used: LB medium with or without the addition of the iron chelator 2,2′-dipyridyl (Sigma Chemical Co., St. Louis, Mo.) to a final concentration of 0.2 mM and LB medium made iron deficient by the addition of 75 μg of ethylenediamine-di(o-hydroxyphenyl) acetic acid/ml, deferated by the method of Rogers (51). Ampicillin (100 μg/ml), kanamycin (45 μg/ml), polymyxin B (50 U/ml), tetracycline (15 μg/ml), or 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal; International Biotechnologies, Inc., New Haven, Conn.) (40 μg/ml) was added as appropriate.
Preparation and analysis of outer membrane proteins.
Enriched outer membrane proteins were prepared by previously described procedures (24) from cells grown to late logarithmic phase in LB medium with and without added 2,2′-dipyridyl. The outer membrane proteins were separated on sodium dodecyl sulfate (SDS)–10% polyacrylamide gels and were stained with Coomassie blue.
N-terminal amino acid sequence analysis.
For N-terminal amino acid analysis, outer membrane proteins from the fur mutant CML17 were electrophoresed by SDS-polyacrylamide gel electrophoresis (PAGE), electroblotted to polyvinylidene difluoride membranes (Bio-Rad, Richmond, Calif.), and stained with Ponceau S to localize the proteins. The 72-kDa protein was cut from the membrane, and the N-terminal amino acid sequence was determined by the Huntsman Cancer Center Peptide and DNA facility, University of Utah. The N-terminal amino acid sequence was determined by standard Edman degradation on a model 477A microsequencer (Applied Biosystems, Foster City, Calif.).
DNA manipulations and cloning.
Standard methods were followed for molecular biological techniques (54). Oligonucleotides were synthesized at the Huntsman Cancer Center Peptide and DNA facility. Oligonucleotides were radioactively labeled with T4 polynucleotide kinase, and plasmid DNA was radioactively labeled by random oligonucleotide-primed synthesis (Bethesda Research Laboratories Life Technologies, Gaithersburg, Md.).
The vuuA gene was cloned by screening a recombinant lambda ZAPII phage genomic library of V. vulnificus MO6-24 constructed as previously described (34). After infection and plating of E. coli XL1 Blue, the resulting plaques were screened with the labeled oligonucleotide by using GeneScreen Plus colony-plaque membranes (Du Pont, NEN Research Products) as described previously except under low-stringency hybridization conditions (31). Purified phage isolated from the positive plaques were excised as Bluescript plasmids (Stratagene, La Jolla, Calif.) according to the directions of the manufacturer.
Restriction enzyme-digested genomic and plasmid DNA fragments were resolved through 1.0% agarose gels, and DNA was transferred to GeneScreen Plus membranes (Du Pont, NEN Research Products) by the method of Southern (59). High-stringency hybridizations were performed at 42°C in a buffer containing 1 M NaCl, 1% SDS, and 50% formamide; the buffer used for low-stringency hybridizations contained 25% formamide instead of 50% formamide. After 6 to 24 h of hybridization, the membranes were washed according to the manufacturer's recommendations and visualized by autoradiography.
DNA sequencing.
The DNA sequence was determined by the dideoxy chain termination method of Sanger et al. (55) on double-stranded DNA plasmid templates by using a Sequenase kit from United States Biochemical Corporation, Cleveland, Ohio, and with the Prism 377 DNA sequencer from Applied Biosystems. Synthetic oligonucleotides used as primers for DNA sequencing were synthesized by the Huntsman Cancer Center DNA peptide facility.
Construction of V. vulnificus vuuA mutant.
A vuuA deletion was constructed in V. vulnificus by in vivo marker exchange by techniques described previously (9). Plasmid pCVD442 is a suicide vector containing the sacB gene, which allows positive selection with sucrose for the loss of plasmid sequences after homologous recombination into the chromosome (18). The 2.8-kb EcoRI-EcoRV fragment of pCML58 was subcloned in pBluescript and designated pCML59; a 1.3-kb MscI-NruI fragment internal to V. vulnificus vuuA was deleted by digestion and religation to yield pCML60. The 1.5-kbp fragment of pCML60 was ligated into SmaI-SalI-digested pCVD442, yielding pCML61. In vivo marker exchange was used to replace the chromosomal copy of vuuA in V. vulnificus with the internal deleted copy in pCML61 without any remaining integrated plasmid sequences, as described previously (9), to generate strain CML57. Construction of the deletion mutant was confirmed by Southern blotting.
Utilization of iron sources.
The utilization of iron sources by V. vulnificus was assayed by the procedure by Simpson and Oliver (58). Human holotransferrin (Sigma) solubilized in phosphate-buffered saline was determined to have an iron saturation of 99% by the Ferrozine assay (61) performed on a Hitachi 717 automatic analyzer (Boehringer Mannheim Corp., Indianapolis, Ind.). Hemin (Sigma) was solubilized in 10 mM NaOH, and hemoglobin was solubilized in phosphate-buffered saline. Vulnibactin, the catechol siderophore of V. vulnificus (47), was extracted from the culture supernatant of MO6-24 by the procedure of Griffiths et al. (22).
RNA analysis.
RNAs from logarithmic-phase cultures grown under high-iron conditions (LB medium) and low-iron conditions (LB medium containing 2,2′-dipyridyl) were prepared with Trizol reagent (Bethesda Research Laboratories Life Technologies) according to the manufacturer's protocol. Northern (RNA) blot analysis was performed by using standard molecular biological techniques (54). Equivalent amounts of RNA, as calculated from the optical density at 260 nm, were loaded into all of the lanes. The internal 1.3-kbp MscI-NruI fragment of the V. vulnificus vuuA gene was used as the probe. Primer extension was performed on RNAs from cultures grown under low-iron conditions with a Promega (Madison, Wis.) primer extension kit according to the manufacturer's instructions.
DNA and protein database searches.
The National Center for Biotechnology Information services were used to consult the SwissProt, GenBank, and EMBL databases with the BLAST algorithm (1, 20). The TMpredict computer program was used to predict potential transmembrane domains (27). The program uses TMbase, a database of membrane-spanning protein segments based on SwissProt release 25, for prediction of helical-membrane-spanning domains. The secondary structure of VuuA was predicted by the computer program PredictProtein, which makes a prediction of secondary structure based on evolutionary information and neural networks (52, 53).
Virulence testing.
The 50% lethal dose (LD50) assays were performed by intragastric inoculation of 6- to 7-day-old suckling mice (CD-1; Charles River) with serial 10-fold dilutions of the bacterial suspensions of the wild type, MO6-24, and mutant CML57 grown in LB medium and suspended in 0.15 M NaHCO3 (pH 8.15). Four mice were used per dose of bacteria. The LD50 was estimated by the method of Reed and Muench (50).
Nucleotide sequence accession number.
The GenBank accession number for the sequence presented in this article is AF156494.
RESULTS
N-terminal amino acid sequence analysis of the 72-kDa iron-regulated outer membrane protein of V. vulnificus.
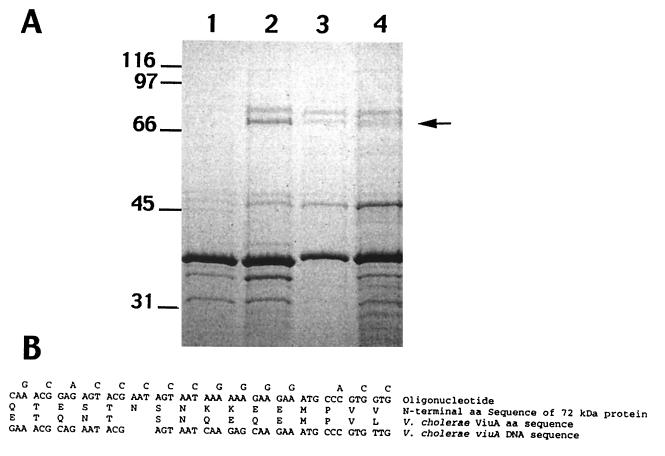
Outer membrane protein preparations of a V. vulnificus fur mutant (CML17) constitutively express at least two outer membrane proteins, 72 and 77 kDa, which are normally negatively regulated by iron in wild-type V. vulnificus (Fig. 1A). The N-terminal sequence of the 72-kDa protein isolated from the fur mutant was QTESTNSNKKEEMPVV. A BLAST search of the GenBank database found identity of this N-terminal sequence with 8 of the first 15 amino acids of the mature protein of the vibriobactin receptor of Vibrio cholerae, ViuA (Fig. 1B). This homology suggested that the 72-kDa iron-regulated protein might be the V. vulnificus vulnibactin receptor.
FIG. 1.
SDS-PAGE of outer membrane proteins. Lane 1, wild-type V. vulnificus grown in high-iron medium; lane 2, wild-type V. vulnificus grown in low-iron medium; lane 3, CML17 grown in high-iron medium; lane 4 CML17 grown in low-iron medium. The arrow indicates the position of the 72-kDa protein which was sequenced by Edman degradation. (B) Homology of N-terminal amino acid sequence with that of V. cholerae ViuA and synthesis of an oligonucleotide probe. The top single-letter-code sequence of amino acids (aa) is the N-terminal, 16-amino-acid sequence from the 72-kDa purified outer membrane protein from V. vulnificus CML57. The lower single-letter-code sequence of amino acids corresponds to the first 15 amino acids of V. cholerae mature ViuA protein. The top nucleotide sequence is the degenerate sequence of the oligonucleotide used to probe the V. vulnificus genomic library. The bottom nucleotide sequence is the sequence of the nucleotides encoding this portion of the V. cholerae ViuA amino acid sequence.
Cloning of the gene encoding the 72-kDa protein of V. vulnificus.
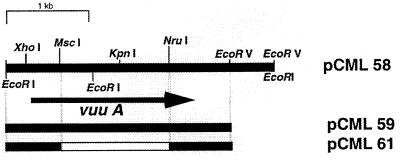
We synthesized an oligonucleotide to use as a probe to screen a V. vulnificus library. The sequence of the oligonucleotide was based on the N-terminal sequence of the 72-kDa protein, the frequency of codon usage for V. vulnificus, and the sequence of the V. cholerae viuA gene (Fig. 1B). We probed plaques from a V. vulnificus MO6-24 lambda ZAPII library with the oligonucleotide, end labeled with 32P under low-stringency conditions (25% formamide; 42°C). Several plaques hybridized strongly with the oligonucleotide probe. Purified phage isolated from the positive plaques were excised as Bluescript plasmids. One phagemid was successfully introduced to an ABLE K E. coli strain which reduces the copy number of plasmids by approximately 10-fold from the usual copy number. The vuuA gene of V. vulnificus was localized by restriction mapping, using hybridization with the oligonucleotide and subsequent DNA sequencing. The clone was designated pCML58. Subclones and a subclone containing an internal deletion of vuuA are illustrated in Fig. 2.
FIG. 2.
Restriction map of vuuA and flanking DNA. An approximately 3.3-kb fragment was cloned into lambda ZAPII and excised as a Bluescript plasmid to form pCML58. Plasmids pCML59, pCML61, and pCML63 are subclones. Plasmid pCML61 contains the EcoRI-EcoRV insert from pCML59 with an internal deletion of vuuA DNA from the MscI site to the NruI site as indicated by the open bar. In pCML59, the restriction fragment is cloned in pBluescript, and in pCML63, the same restriction fragment is cloned in pLAFR3.
Nucleotide sequence analysis and predicted protein.
The nucleotide sequence of vuuA and its promoter region was determined. The upstream genetic region, a partial amino acid sequence of the N terminus, and a portion of the C terminus are presented in Fig. 3. A 2,061-bp open reading frame begins 81 bp downstream of an XhoI restriction site. A putative Shine-Dalgarno sequence is located just upstream from the initiating methionine. An inverted repeat that may serve as a transcriptional terminator was found just beyond the termination codon. Glutamine-38 is the terminal amino acid, as confirmed by N-terminal sequence analysis, and is marked as the signal peptidase cleavage site. The precursor form of VuuA contains a leader sequence of 37 amino acids, is 687 amino acids in length, and has a predicted molecular weight of 75,740. The mature protein has a predicted molecular weight of 71,909, which is in agreement with the observed mobility on SDS-PAGE gels. The predicted pI of the mature protein is 4.85, compared to the predicted pI of 4.59 for ViuA. The predicted pIs of the N- and C-terminal regions in VuuA and ViuA are also similar. The average hydrophobicity of the mature protein is −0.43, indicating that the protein is hydrophilic in nature.
FIG. 3.
Partial nucleotide sequence of V. vulnificus vuuA, its promoter region starting at the upstream EcoRI site and ending at bp 513 within vuuA, and the carboxy terminus end of vuuA ending at bp 2465. The locations of certain restriction sites are indicated. The deduced amino acid sequence of VuuA is shown below the vuuA sequence. The approximate start sites of transcription are indicated by asterisks above the sequence. The −35 regions, the −10 regions, and the Shine-Dalgarno sequence (SD) are underlined and labeled. The potential Fur box is labeled by a line above the sequence. A vertical arrow marks the signal peptidase cleavage site confirmed by N-terminal sequence of the protein. A 27-bp interrupted dyad symmetric sequence overlapping the −35 box and the Fur box is indicated by opposing horizontal arrows above the sequence. The termination codon of VuuA (…) is shown. An inverted repeat that may serve as a transcriptional terminator is indicated by opposing horizontal arrows below the sequence.
With the computer program TMpredict (27), two significant transmembrane segments were predicted for VuuA. The first transmembrane segment is a 20-amino-acid hydrophobic domain spanning residues 19 to 38, which is consistent with the finding of an initial 37-amino-acid signal sequence. A second transmembrane region was also predicted spanning residues 469 to 486. The same analysis was performed on ViuA. An 18-amino-acid transmembrane region was predicted, from residues 19 to 36, similar to what was previously reported for ViuA. A second transmembrane region, like that predicted for VuuA by TM predict, however, was not predicted for ViuA.
Recently, the structure of ferric hydroxamate uptake protein component A (FhuA) has been resolved by X-ray crystallography (19, 35). Since VuuA shows 22% homology and 56% similarity with FhuA, the predicted secondary structure of VuuA was compared with the secondary structure of FhuA as revealed by X-ray crystallography (see Fig. 6A). The overall structure of the FhuA protein consists of two domains, a hollow β barrel domain (residues 194 to 756 of the unprocessed protein) consisting of 22 β strands and a plug domain (residues 34 to 193) which is located within the barrel. The secondary structure of VuuA was analyzed with the program PredictProtein and revealed approximately 19 β strands, suggesting that the secondary structure of VuuA may be similar to that of FhuA and may also consist of a hollow β barrel domain with a plug domain (see Fig. 6A for a comparison of secondary structure of the plug region of FhuA with the corresponding region of VuuA). In this region VuuA shows 21% homology and 51% similarity with FhuA. Starting with amino acid residue 88 of VuuA, there is also a fairly high degree of similarity in the secondary structure.
FIG. 6.
(A) Sequence alignment of N termini and plug domains of V. vulnificus VuuA and E. coli FhuA (EMBL accession code M12486). The numbers in parentheses indicate the position in the unprocessed protein of the first amino acid listed. Conserved amino acids between two proteins are indicated by colons, and substitutions of functionally similar amino acids are marked by periods. The assignments of helices (H) and β strands (B) for FhuA are based on the X-ray structure of the FhuA protein (35) and are noted below the residues. The secondary structure of VuuA was predicted by the PHD network method described by Rost and Sauder (52, 53) and is noted above the residues. The TonB boxes are indicated by boldface letters. Regions that were described by Lundrigan and Kadner (37) as highly homologous among TonB-dependent receptors are underlined. (B) Homology between VuuA and the TonB boxes of TonB-dependent receptors. The TonB box regions from four TonB-dependent outer membrane proteins were compared with that of the V. vulnificus VuuA protein. The proteins are E. coli BtuB, V. cholerae IrgA, V. cholerae ViuA, V. cholerae HutA, V. vulnificus HupA, and Y. enterocolitica HemR. The numbers in parentheses indicate the position in the unprocessed protein of the first amino acid shown. The most highly conserved residues in the TonB box as described by Nau and Konisky (43) are shown in boldface letters.
Primer extension analysis to localize the start site of V. vulnificus vuuA transcription.
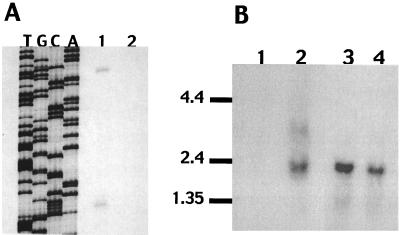
Primer extension analysis of RNA from V. vulnificus MO6-24 grown under low-iron conditions was done by using a synthetic oligonucleotide complementary to the DNA sequence located 36 bp upstream of the initiating codon (Fig. 3, bases 279 through 299). Two primer extension products corresponding to bases 91 and 149 of the sequence were identified in three separate experiments (Fig. 3 and 4A). Potential −35 and −10 boxes are identified upstream of the transcriptional start sites (Fig. 3).
FIG. 4.
(A) Primer extension analysis of RNA from V. vulnificus vuuA. Lanes T, G, C, and A, lanes of the DNA sequencing ladder. Lane 1, primer extension reaction mixture with V. vulnificus RNA prepared from a low-iron culture; lane 2, primer extension reaction mixture without V. vulnificus RNA. (B) Northern blot analysis of RNA prepared from V. vulnificus MO6-24 after growth in high-iron medium (lane 1) and low-iron medium (lane 2) and V. vulnificus CML17 after growth in high-iron medium (lane 3) and low-iron medium (lane 4). The blot was probed with a MscI-NruI fragment internal to vuuA. The positions of RNA standards (in kilobases) are shown on the left.
Homology of the vuuA promoter region to Fur binding consensus sequences.
A 27-bp interrupted dyad symmetric sequence overlapping the upstream −35 box (bases 38 to 64) was located which was identical to a 27-bp upstream dyad symmetric sequence found in the V. cholerae viuA promoter region (11). The 27-bp upstream dyad similarly overlaps the proposed −35 box of the V. cholerae viuA promoter. A Fur box was identified in the central portion of this 27-bp dyad sequence, identical to the upstream viuA Fur box (Fig. 5). Similarity to the downstream viuA Fur box was not identified within the nucleotide sequence of vuuA. The V. vulnificus vuuA Fur box shares 12 of 19 nucleotides with the consensus sequence of the E. coli Fur box.
FIG. 5.
Homology between the vuuA Fur box and Fur boxes from other iron-regulated genes. The vuuA Fur box was compared with the V. cholerae viuA Fur boxes, the Fur box from V. vulnificus hupA, and the E. coli Fur box consensus sequence. The boldface letters indicate the bases that each Fur box shares with the predicted vuuA Fur box. The number of identical nucleotides that each sequence shares with the vuuA Fur box is indicated in parentheses.
Northern blot analysis of the vuuA transcript in V. vulnificus.
Northern blot analysis was performed with RNA prepared from V. vulnificus MO6-24 and Fur mutant CML17 grown in low- and high-iron media. The blot was probed with the MscI-NruI fragment contained in the V. vulnificus vuuA gene. One apparent transcript of approximately 2.3 kb was seen under only low-iron conditions for MO6-24 (Fig. 4B), consistent with the size predicted by the DNA sequence information. Transcripts were seen under both high- and low-iron conditions for the Fur mutant CML17, indicating that regulation of transcription by iron takes place via the fur gene. Resolution of two transcripts on the Northern blot (based on the evidence of two start sites of transcription) was not possible due a size difference of only 58 bases between the transcripts. These data indicate that vuuA is monocistronic.
Homology of V. vulnificus VuuA to V. cholerae ViuA and other iron-regulated outer membrane proteins.
The amino acid sequence of V. vulnificus VuuA compared with that of V. cholerae ViuA shows 74% homology and 86% similarity. Comparison of the nucleotide sequence of V. vulnificus vuuA with that of V. cholerae viuA shows 71% identity. V. vulnificus ViuA also shows significant homology with a number of iron-regulated outer membrane proteins. Between 20 and 22% identity and 53 and 59% similarity were observed with the following siderophore receptors: Bordetella bronchiseptica BfrA (5), Yersinia enterocolitica FoxA (4), Yersinia pestis FyuA (49), and Pseudomonas aeruginosa FiuA (46). The major iron-regulated outer membrane protein of V. cholerae, IrgA (21), also had 22% identity and 55% similarity to V. vulnificus VuuA. The regions of greatest homology with VuuA were located in the amino termini of these proteins.
Since many iron uptake systems are dependent upon TonB, VuuA was examined for the presence of an amino acid sequence which had been shown in other genes to be associated with TonB dependence. Analysis of the amino acid sequence of VuuA did not reveal a region which was highly homologous to the consensus E. coli TonB box sequence. However, a region of homology with the putative TonB boxes of HutA of V. cholerae and HupA of V. vulnificus was identified within the amino terminus end of the VuuA sequence. Figure 6B shows a comparison between the TonB boxes of the E. coli vitamin B12 receptor BtuB, the V. cholerae heme receptor HutA, the V. cholerae iron-regulated gene A product IrgA, the V. vulnificus heme receptor HupA, the Y. enterocolitica heme receptor HemR, and a homologous region in VuuA. Although no TonB box was identified in the vibriobactin receptor ViuA (11), a region showing the most homology with the putative TonB boxes of V. cholerae HutA and V. vulnificus HupA is also shown in Fig. 6B. The most highly conserved amino acids among the TonB boxes as described by Nau and Konisky (43) are shown in the BtuB, HupA, and HemR sequences. In both VuuA and ViuA, the conserved threonine (the second residue) is replaced by a proline residue, and in HutA and HupA, the threonine is replaced by a glutamic acid residue. In VuuA, ViuA, and HutA, the conserved alanine is replaced by an isoleucine. These data show that the putative TonB boxes of V. cholerae and V. vulnificus have some homology with other TonB-dependent outer membrane proteins. The TonB boxes of Vibrio species, however, differ with respect to the highly conserved threonine and alanine residues.
Additional regions of VuuA were also examined for similarities among TonB-dependent proteins. Lundrigan and Kadner (37) described four regions as highly homologous among four TonB-dependent receptors (FepA, BtuB, FhuA, and IutA). Even though FhuA showed an overall identity of 22% and homology of 56% with VuuA, much higher degrees of homology with FhuA were identified in two of the four regions (designated I to IV) described as highly homologous among TonB-dependent receptors. Only 29% homology was seen with region I (residues 34 to 50 of unprocessed FhuA), and 47% homology was seen with region III (residues 107 to 144), but region II (residues 711 to 722) showed 83% homology and region IV (residues 154 to 194) showed 64% homology. Region II, near the carboxyl terminus, has been described as the most highly conserved of the four homology regions (37).
Construction of a mutant of V. vulnificus with an internal deletion of viuA (strain CML57).
To introduce an internal deletion of vuuA into the chromosome of V. vulnificus by marker exchange, we constructed plasmid pCML61, a suicide vector containing the cloned fragment of the vuuA gene from pCML59 with a 1,300-bp internal deletion from the MscI site to the NruI site within vuuA. Plasmid pCML61 was transferred by conjugation into V. vulnificus MO6-24, with selection on medium containing ampicillin and polymixin for the merodiploid state in which pCML61 had integrated into the chromosomal vuuA by homologous recombination. The resulting merodiploid strain was grown without selection to late logarithmic phase, spread on plates containing 10% sucrose, and incubated overnight at 30°C. Thirty-four of 313 sucrose-resistant colonies were sensitive to ampicillin, suggesting that vector sequences were lost.
(i) Verification of strain CML57 by Southern blot analysis.
Of the 34 sucrose-resistant, ampicillin-sensitive colonies, 1 colony had a vuuA gene sequence with the internal deletion. The genetic construction of strain CML57 was confirmed by Southern hybridization of XhoI-EcoRV-digested chromosomal DNA, probing with the XhoI-EcoRV fragment of the vuuA gene, and comparing the Southern blotting results with the wild-type V. vulnificus DNA and the XhoI-EcoRV vuuA fragment containing the internal deletion in pCML60. On the Southern blot, the wild-type V. vulnificus showed a 2.5-kbp hybridizing band and strain CML57 showed a 1.3-kbp hybridizing band (data not shown).
(ii) Verification of strain CML57 by analysis of iron-regulated outer membrane proteins.
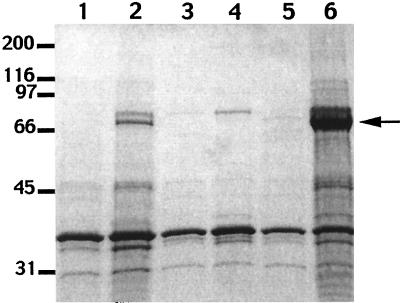
We additionally confirmed the vuuA phenotype of CML57 by comparing the outer membrane proteins of wild-type V. vulnificus and strain CML49 after growth in low- and high-iron media (Fig. 7). In wild-type V. vulnificus the two proteins with apparent molecular masses of 72 and 77 kDa appear after growth under low-iron conditions. Mutant CML57 showed loss of expression of the 72-kDa iron-regulated protein.
FIG. 7.
SDS-PAGE of outer membrane proteins. Lane 1, wild-type V. vulnificus grown in high-iron medium; lane 2, wild-type V. vulnificus grown in low-iron medium; lane 3, strain CML57 grown in high-iron medium; lane 4, strain CML57 grown in low-iron medium; lane 5, strain CML57(pCML63) grown in high-iron medium; lane 6, strain CML57(pCML63) grown in low-iron medium. The numbers on the left indicate the positions of protein standards (in kilodaltons). The arrow indicates the position of VuuA.
Characterization of vuuA mutant CML57.
The vuuA deletion mutant, CML57, was tested for its ability to use ferric vulnibactin, transferrin, hemin, hemoglobin, and FeSO4 as iron sources. As shown in Table 2, CML57 was unable to use transferrin and ferric vulnibactin as sources of iron. CML57 was also unable to grow around MO6-24 and CML57 spotted cultures.
TABLE 2.
Stimulation of growth of V. vulnificus strains by various iron sources and producer strains
| Producer strain or iron compound | Diameter of zone of growth (mm) of indicator straina
|
||
|---|---|---|---|
| Wild-type MO6-24 | CML57 | CML57(pCML63) | |
| Hemoglobin (10 μM) | 21 | 15 | 23 |
| Hemin (20 μM) | 20 | 11 | 20 |
| Transferrin (2.6 mM) | 24 | 0 | 25 |
| FeSO4 (10 mM) | 26 | 15 | 28 |
| Vulnibactin (2 mM) | 25 | 0 | 25 |
| MO6-24 | 21 | 0 | 21 |
| CML57 | 21 | 0 | 20 |
Cultures were seeded into LB agar containing 75 μg of ethylenediamine-di(o-hydroxyphenyl) acetic acid and 5 μl of various iron-containing compounds or overnight growth of a bacterial strain was spotted onto the medium or onto sterile disks placed on the medium. Strains MO6-24 and CML57 were used as producer strains to detect any alteration in the ability to produce siderophores (e.g., vulnibactin) and the ability of the indicator strain to use siderophores as iron sources. The zones of growth around the spots or the disks were measured after 18 to 24 h. Diameter measurements include the size of the disks or spots except in instances of no growth around the disks. The measurements represent the averages of three experiments.
Complementation of CML57 with pCML63.
The entire vuuA gene, including the promoter from EcoRI to EcoRV restriction sites (2.8 kb), was subcloned into pLAFR3 (pCML63). When pCML63 was introduced into strain CML57, the ability to use ferric vulnibactin and transferrin iron was restored, indicating that vuuA cloned on a plasmid could reconstitute the vulnibactin receptor (Table 2). Mutant CML57 containing pCML63 showed an apparent 72-kDa outer membrane protein after growth in high-iron medium (Fig. 7). A large amount of a 72-kDa protein was expressed under low-iron conditions, indicating iron-regulated synthesis of the VuuA protein.
Virulence assay.
To determine whether vuuA has a role in the virulence of V. vulnificus, LD50 assays of the MO6-24 wild type and mutant CML57 were performed. The LD50 measurements represent the means of replicate determinations. The LD50 of the mutant strain CML57 was 1.1 × 104, compared with 2.75 × 103 for the parental strain, MO6-24. The increase in LD50 of 1 order of magnitude suggests that vuuA is important for virulence in this animal model.
DISCUSSION
The acquisition of iron is an important adaptive response that bacteria have evolved to allow competitive growth and survival in the host. Many iron transport systems in gram-negative bacteria involve iron-regulated outer membrane receptors. The genes that encode iron-regulated receptors in E. coli and Vibrio species are controlled at the transcriptional level by the Fur repressor proteins. In this study, the V. vulnificus gene, vuuA, encoding a 72-kDa iron-regulated outer membrane protein, was sequenced and its regulation by iron was characterized.
The fur mutant of V. vulnificus has been very useful for studying iron acquisition proteins in this pathogen. SDS-PAGE analysis of the V. vulnificus fur mutant showed the constitutive expression of at least two outer membrane proteins of approximately 72 and 77 kDa which are normally regulated by iron in wild-type V. vulnificus (33). Previously, using N-terminal sequence analysis, we were able to deduce an oligonucleotide to clone the gene, hupA encoding the 77-kDa protein, which is required for heme utilization (32). Using the same techniques as for hupA, we cloned the gene vuuA, encoding the 72-kDa protein, the outer membrane vulnibactin receptor. Comparable to our experience with cloning hupA (32), we found that clones expressing the full-length vuuA were somewhat unstable and grew more slowly. The original clone could be maintained only in E. coli ABLE K, which reduces the copy number approximately 10-fold, thus decreasing the amount of expressed cloned protein product. Successful complementation of the mutant CML57 could be accomplished only by subcloning the vuuA gene and its promoter into a pLAFR3 plasmid, which has a much lower copy number. Even in a low-copy-number plasmid, the outer membrane protein is expressed in much greater amounts under low-iron conditions than the wild-type V. vulnificus outer membrane protein (Fig. 7).
As shown in Fig. 7, the vuuA DNA and upstream DNA cloned on a plasmid (pCML63) was highly regulated by iron, resulting in expression of a 72-kDa protein under low-iron conditions in V. vulnificus. This indicates that a Fur box is present in the cloned sequence. In a previous study, we found a high degree of homology between the proposed hupA Fur box and the E. coli consensus Fur box (Fig. 5) (32). Analysis of the promoter region of vuuA revealed one region that was similar to the E. coli Fur binding consensus sequence. The proposed Fur box of vuuA was identical to the upstream Fur box of viuA, including the 27-bp interrupted dyad symmetric sequence encompassing this region. Two contiguous Fur binding sites were identified in the viuA V. cholerae promoter region (11). Only one Fur binding site, however, was identified in vuuA. The proposed Fur box of vuuA showed identity with only 12 of 19 nucleotides of the consensus Fur box. Most “iron boxes” of E. coli show homology with this consensus sequence that ranges from 13 to 17 base pair matches (12, 13, 15, 17). There have been other proposed Fur boxes reported, however, that also share only 12 of 17 base pair matches with the consensus Fur box, including fyuA of Y. enterocolitica (49). Although the proposed Fur box of V. vulnificus is less homologous to the consensus Fur box, vuuA is tightly regulated by iron. Additional sequence information from iron-regulated Vibrio genes may reveal slight differences in Fur binding regions from those in E. coli Fur boxes.
Studies with E. coli indicate that transport of iron across iron uptake receptors requires a TonB system to allow passage of the iron ligand into the periplasm (10, 29). A TonB box has been proposed to be the site of the interaction of the iron transport receptor with TonB (25). A consensus heptapeptide (D/ETMVVTA) near the amino terminus has been proposed by Nau and Konisky (43). The proposed TonB box of VuuA has identity with only two of the seven residues of the consensus TonB box. The sequence suggested for the VuuA TonB box is based on homology with the proposed HutA TonB box of V. cholerae and the proposed HupA box of V. vulnificus. Henderson and Payne (26) noted a similar difference of the purported TonB box of V. cholerae HutA, in that it shares identity with only three of the seven residues of the consensus TonB box.
Recently, Occhino et al (45) described two TonB systems for V. cholerae. They postulated that there might be differences in the ways these two TonB proteins interact with specific outer membrane proteins. This is based on the evidence that TonB2 was able to complement an E. coli TonB mutation but TonB1 was not (26). Some distinct similarities are noted when the putative TonB boxes of V. cholerae and V. vulnificus are compared (Fig. 6B). In VuuA and HupA of V. vulnificus and ViuA and HutA of V. cholerae, the highly conserved threonine in the TonB box is replaced by a proline or glutamic acid. In VuuA, ViuA, and HutA, all of the alanines in the TonB box are replaced with an isoleucine. The low degree of homology of the V. vulnificus TonB boxes with the E. coli consensus sequence and the high degree of homology with V. cholerae TonB boxes suggest that the TonB protein or proteins of V. vulnificus may recognize sequences different from that of E. coli TonB. An alternative explanation could be that this particular region may not be as important for interaction with TonB as previously proposed.
The amino acid sequence of VuuA showed similarity to those of other iron-regulated siderophore outer membrane receptors, including a high degree of similarity with the 74-kDa iron-regulated vibriobactin receptor ViuA of V. cholerae. The vuuA mutant failed to used Fe-vulnibactin as a source of iron. There was no defect in the ability to use heme, hemoglobin, or FeSO4 as an iron source. Analysis of the proteins of the mutant revealed the absence of the 72-kDa, iron-regulated outer membrane protein present in wild-type V. vulnificus. The introduction of a plasmid containing the wild-type vuuA gene restored the ability of the mutant to use Fe-vulnibactin as an iron source. Because the loss of expression of the 72-kDa protein correlated with the failure to use vulnibactin, we propose that this iron-regulated outer membrane protein of V. vulnificus is the vulnibactin receptor.
In addition to the inability to use Fe-vulnibactin as an iron source, the V. vulnificus vuuA mutant was also unable to use transferrin iron. We previously isolated a V. vulnificus TnphoA mutant that was unable to produce vulnibactin or to acquire iron from transferrin (34). There are several mechanisms which bacteria have evolved to use transferrin and lactoferrin iron. Many bacteria secrete siderophores which can compete with transferrin and lactoferrin to mobilize iron for bacterial use. Transport of the iron-siderophore complexes into the bacterium requires iron-repressible outer membrane proteins that serve as receptors for the iron complexes (44). Other bacterial species, such as Neisseria gonorrhoeae and Haemophilus influenzae, are able to use transferrin and lactoferrin iron directly without mediation of siderophores via outer membrane receptors (6, 8, 36, 56). Still other pathogenic bacteria secrete reductants (14), which remove ferric iron from transferrin. In this study, a mutation in the ability to produce the 72-kDa outer membrane protein resulted in a defect in the ability to acquire ferric vulnibactin and use transferrin iron. This evidence, coupled with the data from a previous study in which a V. vulnificus siderophore synthesis mutant could not use transferrin iron (34), suggests that the main mechanism for the acquisition of transferrin iron is via siderophore secretion and iron-siderophore uptake by a specific outer membrane protein.
Considering the high degree of homology between VuuA and ViuA, it is not surprising that the structures of their cognate siderophores, vulnibactin and vibriobactin, are quite similar. Vulnibactin contains one residue of 2,3-dihydroxybenzoic acid and one residue of threonine (47), whereas vibriobactin receptor contains three 2,3-dihydroxybenzoic acid residues and two residues of threonine (22). Both vulnibactin and vibriobactin have norspermidine backbones.
The contribution of siderophore-dependent iron uptake systems to the virulence of pathogenic bacteria appears to vary according to the pathogen. The siderophore aerobactin appears to be a more important virulence factor for enteric bacteria than the siderophore enterobactin (16). Siderophores are reported not to be essential for shigellae to survive within the intestinal lumen or for invasion of intestinal epithelial cells and intracellular survival and multiplication (48). The ferric vibriobactin transport system of V. cholerae is not required for the virulence of the pathogen (57). The ferric vulnibactin transport system of V. vulnificus, however, appears to be important for the virulence of the organism. In a study of clinical and environmental isolates, Stelma et al. (60) found that virulent V. vulnificus produced a catechol siderophore and used transferrin-bound iron. Avirulent isolates were unable either to use transferrin-bound iron or to produce catechol siderophores. We previously showed that a mutant in a gene responsible for catechol synthesis in V. vulnificus exhibited reduced virulence in a mouse model of infection (34). The increase of the LD50 of the vuuA mutant of V. vulnificus compared to that of the wild-type MO6-24 in the infant mouse animal model provides further evidence of the importance of siderophore-dependent iron uptake to the virulence of the organism.
ACKNOWLEDGMENTS
We gratefully acknowledge Bob Schackman of the Huntsman Cancer Institute for providing synthetic oligonucleotides and N-terminal amino acid sequence analysis (NCI CA42014).
This work was supported by Public Health Service grant AI40067 from the National Institute of Allergy and Infectious Diseases to C.M.L.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J L. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Bagg A, Neilands J B. Ferric uptake regulation protein acts as a repressor, employing iron (II) as a cofactor to bind the operator of an iron transport operon in Escherichia coli. Biochemistry. 1987;26:5471–5477. doi: 10.1021/bi00391a039. [DOI] [PubMed] [Google Scholar]
- 3.Bagg A, Neilands J B. Molecular mechanism of regulation of siderophore-mediated iron assimilation. Microbiol Rev. 1987;51:509–518. doi: 10.1128/mr.51.4.509-518.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baumler A J, Hantke K. Ferrioxamine uptake in Yersinia enterocolitica: characterization of the receptor protein FoxA. Mol Microbiol. 1992;6:1309–1321. doi: 10.1111/j.1365-2958.1992.tb00852.x. [DOI] [PubMed] [Google Scholar]
- 5.Beall B, Hoenes T. An iron-regulated outer-membrane protein specific to Bordetella bronchiseptica and homologous to ferric siderophore receptors. Microbiology. 1997;143:135–145. doi: 10.1099/00221287-143-1-135. [DOI] [PubMed] [Google Scholar]
- 6.Biswas G D, Sparling P F. Characterization of lbpA, the structural gene for a lactoferrin receptor in Neisseria gonorrhoeae. Infect Immun. 1995;63:2958–2967. doi: 10.1128/iai.63.8.2958-2967.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blake P A, Merson M H, Weaver R E, Hollis D G, Heublein P C. Disease caused by a marine Vibrio. Clinical characteristics and epidemiology. N Engl J Med. 1979;300:1–5. doi: 10.1056/NEJM197901043000101. [DOI] [PubMed] [Google Scholar]
- 8.Blanton K J, Biswas G D, Tsai J, Adams J, Dyer D W, Davis S M, Koch G G, Sen P K, Sparling P F. Genetic evidence that Neisseria gonorrhoeae produces specific receptors for transferrin and lactoferrin. J Bacteriol. 1990;172:5225–5235. doi: 10.1128/jb.172.9.5225-5235.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blomfield I C, Vaughn R, Rest R F, Eisenstein B I. Allelic exchange in Escherichia coli using the Bacillus subtilis sacB gene and a temperature-sensitive pSC101 replicon. Mol Microbiol. 1991;5:1447–1457. doi: 10.1111/j.1365-2958.1991.tb00791.x. [DOI] [PubMed] [Google Scholar]
- 10.Bradbeer C. The proton motive force drives the outer membrane transport of cobalamin in Escherichia coli. J Bacteriol. 1993;175:3146–3150. doi: 10.1128/jb.175.10.3146-3150.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butterton J R, Stoebner J A, Payne S M, Calderwood S B. Cloning, sequencing, and transcriptional regulation of viuA, the gene encoding the ferric vibriobactin receptor of Vibrio cholerae. J Bacteriol. 1992;174:3729–3738. doi: 10.1128/jb.174.11.3729-3738.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calderwood S B, Mekalanos J J. Confirmation of the Fur operator site by insertion of a synthetic oligonucleotide into an operon fusion plasmid. J Bacteriol. 1988;170:1015–1017. doi: 10.1128/jb.170.2.1015-1017.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calderwood S B, Mekalanos J J. Iron regulation of Shiga-like toxin expression in Escherichia coli is mediated by the fur locus. J Bacteriol. 1987;169:4759–4764. doi: 10.1128/jb.169.10.4759-4764.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cowart R E, Foster B G. Differential effects of iron on the growth of Listeria monocytogenes: minimum requirements and mechanism of acquisition. J Infect Dis. 1985;151:721–730. doi: 10.1093/infdis/151.4.721. [DOI] [PubMed] [Google Scholar]
- 15.De Grandis S, Ginsberg J, Toone M, Climie S, Friesen J, Brunton J. Nucleotide sequence and promoter mapping of the Escherichia coli Shiga-like toxin operon of bacteriophage H-19B. J Bacteriol. 1987;169:4313–4319. doi: 10.1128/jb.169.9.4313-4319.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Lorenzo V, Martinez J L. Aerobactin production as a virulence factor: a reevaluation. Eur J Clin Microbiol Infect Dis. 1988;7:621–629. doi: 10.1007/BF01964239. [DOI] [PubMed] [Google Scholar]
- 17.de Lorenzo V, Wee S, Herrero M, Neilands J B. Operator sequences of the aerobactin operon of plasmid ColV-K30 binding the ferric uptake regulation (fur) repressor. J Bacteriol. 1987;169:2624–2630. doi: 10.1128/jb.169.6.2624-2630.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donnenberg M S, Kaper J B. Construction of an eae deletion mutant of enteropathogenic Escherichia coli by using a positive-selection suicide vector. Infect Immun. 1991;59:4310–4317. doi: 10.1128/iai.59.12.4310-4317.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferguson A D, Hofmann E, Coulton J W, Diederichs K, Welte W. Siderophore-mediated iron transport: crystal structure of FhuA with bound lipopolysaccharide. Science. 1998;282:2215–2220. doi: 10.1126/science.282.5397.2215. [DOI] [PubMed] [Google Scholar]
- 20.Gish W, States D J. Identification of protein coding regions by database similarity search. Nat Genet. 1993;3:266–272. doi: 10.1038/ng0393-266. [DOI] [PubMed] [Google Scholar]
- 21.Goldberg M B, DiRita V J, Calderwood S B. Identification of an iron-regulated virulence determinant in Vibrio cholerae, using TnphoA mutagenesis. Infect Immun. 1990;58:55–60. doi: 10.1128/iai.58.1.55-60.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Griffiths G L, Sigel S P, Payne S M, Neilands J B. Vibriobactin, a siderophore from Vibrio cholerae. J Biol Chem. 1984;259:383–385. [PubMed] [Google Scholar]
- 23.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 24.Hantke K. Regulation of ferric iron transport in Escherichia coli K12: isolation of a constitutive mutant. Mol Gen Genet. 1981;182:288–292. doi: 10.1007/BF00269672. [DOI] [PubMed] [Google Scholar]
- 25.Heller K J, Kadner R J, Gunther K. Suppression of the btuB451 mutation by mutations in the tonB gene suggests a direct interaction between TonB and TonB-dependent receptor proteins in the outer membrane of Escherichia coli. Gene. 1988;64:147–153. doi: 10.1016/0378-1119(88)90488-x. [DOI] [PubMed] [Google Scholar]
- 26.Henderson D P, Payne S M. Characterization of the Vibrio cholerae outer membrane heme transport protein HutA: sequence of the gene, regulation of expression, and homology to the family of TonB-dependent proteins. J Bacteriol. 1994;176:3269–3277. doi: 10.1128/jb.176.11.3269-3277.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hofmann K, Stoffel W. TMBASE-A database of membrane spanning protein segments. Biol Chem Hoppe-Seyler. 1993;374:166. [Google Scholar]
- 28.Johnston J M, Becker S F, McFarland L M. Vibrio vulnificus. Man and the sea. JAMA. 1985;253:2850–2853. doi: 10.1001/jama.253.19.2850. [DOI] [PubMed] [Google Scholar]
- 29.Klebba P E, Rutz J M, Liu J, Murphy C K. Mechanisms of TonB-catalyzed iron transport through the enteric bacterial cell envelope. J Bioenerg Biomembr. 1993;25:603–611. doi: 10.1007/BF00770247. [DOI] [PubMed] [Google Scholar]
- 30.Klontz K C, Lieb S, Schreiber M, Janowski H T, Baldy L M, Gunn R A. Syndromes of Vibrio vulnificus infections. Clinical and epidemiologic features in Florida cases, 1981–1987. Ann Intern Med. 1988;109:318–323. doi: 10.7326/0003-4819-109-4-318. [DOI] [PubMed] [Google Scholar]
- 31.Litwin C M, Boyko S A, Calderwood S B. Cloning, sequencing, and transcriptional regulation of the Vibrio cholerae fur gene. J Bacteriol. 1992;174:1897–1903. doi: 10.1128/jb.174.6.1897-1903.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Litwin C M, Byrne B L. Cloning and characterization of an outer membrane protein of Vibrio vulnificus required for heme utilization: regulation of expression and determination of the gene sequence. Infect Immun. 1998;66:3134–3141. doi: 10.1128/iai.66.7.3134-3141.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Litwin C M, Calderwood S B. Cloning and genetic analysis of the Vibrio vulnificus fur gene and construction of a fur mutant by in vivo marker exchange. J Bacteriol. 1993;175:706–715. doi: 10.1128/jb.175.3.706-715.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Litwin C M, Rayback T W, Skinner J. Role of catechol siderophore synthesis in Vibrio vulnificus virulence. Infect Immun. 1996;64:2834–2838. doi: 10.1128/iai.64.7.2834-2838.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Locher K P, Rees B, Koebnik R, Mitschler A, Moulinier L, Rosenbusch J P, Moras D. Transmembrane signaling across the ligand-gated FhuA receptor: crystal structures of free and ferrichrome-bound states reveal allosteric changes. Cell. 1998;95:771–778. doi: 10.1016/s0092-8674(00)81700-6. [DOI] [PubMed] [Google Scholar]
- 36.Loosmore S M, Yang Y P, Coleman D C, Shortreed J M, England D M, Harkness R E, Chong P S, Klein M H. Cloning and expression of the Haemophilus influenzae transferrin receptor genes. Mol Microbiol. 1996;19:575–586. doi: 10.1046/j.1365-2958.1996.406943.x. [DOI] [PubMed] [Google Scholar]
- 37.Lundrigan M D, Kadner R J. Nucleotide sequence of the gene for the ferrienterochelin receptor FepA in Escherichia coli. Homology among outer membrane receptors that interact with TonB. J Biol Chem. 1986;261:10797–10801. [PubMed] [Google Scholar]
- 38.Miller V L, Mekalanos J J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988;172:2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morris J G., Jr Vibrio vulnificus—a new monster of the deep? Ann Intern Med. 1988;109:261–263. doi: 10.7326/0003-4819-109-4-261. [DOI] [PubMed] [Google Scholar]
- 40.Morris J G, Jr, Black R E. Cholera and other vibrioses in the United States. N Engl J Med. 1985;312:343–350. doi: 10.1056/NEJM198502073120604. [DOI] [PubMed] [Google Scholar]
- 41.Morris J G, Jr, Wright A C, Roberts D M, Wood P K, Simpson L M, Oliver J D. Identification of environmental Vibrio vulnificus isolates with a DNA probe for the cytotoxin-hemolysin gene. Appl Environ Microbiol. 1987;53:193–195. doi: 10.1128/aem.53.1.193-195.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mouzin E, Mascola L, Tormey M P, Dassey D E. Prevention of Vibrio vulnificus infections. Assessment of regulatory educational strategies. JAMA. 1997;278:576–578. [PubMed] [Google Scholar]
- 43.Nau C D, Konisky J. Evolutionary relationship between TonB-dependent outer membrane transport proteins: nucleotide and amino acid sequences of Escherichia coli colicinI receptor gene. J Bacteriol. 1989;171:1041–1047. doi: 10.1128/jb.171.2.1041-1047.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neilands J B, Bindereif A, Montgomerie J Z. Genetic basis of iron assimilation in pathogenic Escherichia coli. Curr Top Microbiol Immunol. 1985;118:179–195. doi: 10.1007/978-3-642-70586-1_10. [DOI] [PubMed] [Google Scholar]
- 45.Occhino D A, Wyckoff E E, Henderson D P, Wrona T J, Payne S M. Vibrio cholerae iron transport: haem transport genes are linked to one of two sets of tonB, exbB, exbD genes. Mol Microbiol. 1998;29:1493–1507. doi: 10.1046/j.1365-2958.1998.01034.x. [DOI] [PubMed] [Google Scholar]
- 46.Ochsner U A, Vasil M L. Gene repression by the ferric uptake regulator in Pseudomonas aeruginosa: cycle selection of iron-regulated genes. Proc Natl Acad Sci USA. 1996;93:4409–4414. doi: 10.1073/pnas.93.9.4409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Okujo N, Saito M, Yamamoto S, Yoshida T, Miyoshi S, Shinoda S. Structure of vulnibactin, a new polyamine-containing siderophore from Vibrio vulnificus. Biometals. 1994;7:109–116. doi: 10.1007/BF00140480. [DOI] [PubMed] [Google Scholar]
- 48.Payne S M. Iron and virulence in Shigella. Mol Microbiol. 1989;3:1301–1306. doi: 10.1111/j.1365-2958.1989.tb00281.x. [DOI] [PubMed] [Google Scholar]
- 49.Rakin A, Saken E, Harmsen D, Heesemann J. The pesticin receptor of Yersinia enterocolitica: a novel virulence factor with dual function. Mol Microbiol. 1994;13:253–263. doi: 10.1111/j.1365-2958.1994.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 50.Reed I J, Muench H A. A simple method of estimating fifty percent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 51.Rogers H J. Iron-binding catechols and virulence in Escherichia coli. Infect Immun. 1973;7:445–456. doi: 10.1128/iai.7.3.445-456.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rost B, Sander C. Combining evolutionary information and neural networks to predict protein secondary structure. Proteins. 1994;19:55–72. doi: 10.1002/prot.340190108. [DOI] [PubMed] [Google Scholar]
- 53.Rost B, Sander C. Prediction of protein secondary structure at better than 70% accuracy. J Mol Biol. 1993;232:584–599. doi: 10.1006/jmbi.1993.1413. [DOI] [PubMed] [Google Scholar]
- 54.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 55.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schryvers A B, Gray-Owen S. Iron acquisition in Haemophilus influenzae: receptors for human transferrin. J Infect Dis. 1992;165:S103–S104. doi: 10.1093/infdis/165-supplement_1-s103. [DOI] [PubMed] [Google Scholar]
- 57.Sigel S P, Stoebner J A, Payne S M. Iron-vibriobactin transport system is not required for virulence of Vibrio cholerae. Infect Immun. 1985;47:360–362. doi: 10.1128/iai.47.2.360-362.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Simpson L M, Oliver J D. Ability of Vibrio vulnificus to obtain iron from transferrin and other iron-binding proteins. Curr Microbiol. 1987;15:155–157. [Google Scholar]
- 59.Southern E M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975;98:503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- 60.Stelma G N, Jr, Reyes A L, Peeler J T, Johnson C H, Spaulding P L. Virulence characteristics of clinical and environmental isolates of Vibrio vulnificus. Appl Environ Microbiol. 1992;58:2776–2782. doi: 10.1128/aem.58.9.2776-2782.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stookey L L. Ferrozine—a new spectrophotometric reagent for iron. Anal Chem. 1970;42:779–781. [Google Scholar]
- 62.Tacket C O, Brenner F, Blake P A. Clinical features and an epidemiological study of Vibrio vulnificus infections. J Infect Dis. 1984;149:558–561. doi: 10.1093/infdis/149.4.558. [DOI] [PubMed] [Google Scholar]
- 63.Wright A C, Simpson L M, Oliver J D. Role of iron in the pathogenesis of Vibrio vulnificus infections. Infect Immun. 1981;34:503–507. doi: 10.1128/iai.34.2.503-507.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]