Abstract
Group A Streptococcus (GAS) is a human pathogen that commonly infects the upper respiratory tract. GAS serotype M1 strains are frequently isolated from human infections and contain the gene encoding the hypervariable streptococcal inhibitor of complement protein (Sic). It was recently shown that Sic variants were rapidly selected on mucosal surfaces in epidemic waves caused by M1 strains, an observation suggesting that Sic participates in host-pathogen interactions on the mucosal surface (N. P. Hoe, K. Nakashima, S. Lukomski, D. Grigsby, M. Liu, P. Kordari, S.-J. Dou, X. Pan, J. Vuopio-Varkila, S. Salmelinna, A. McGeer, D. E. Low, B. Schwartz, A. Schuchat, S. Naidich, D. De Lorenzo, Y.-X. Fu, and J. M. Musser, Nat. Med. 5:924–929, 1999). To test this idea, a new nonpolar mutagenesis method employing a spectinomycin resistance cassette was used to inactivate the sic gene in an M1 GAS strain. The isogenic Sic-negative mutant strain was significantly (P < 0.019) impaired in ability to colonize the mouse mucosal surface after intranasal infection. These results support the hypothesis that the predominance of M1 strains in human infections is related, in part, to a Sic-mediated enhanced colonization ability.
Group A Streptococcus (GAS) is a gram-positive bacterial pathogen that causes human disease globally. The organism is responsible for many diverse infection types, including pharyngitis, acute rheumatic fever, and invasive diseases such as bacteremia and necrotizing fasciitis. A resurgence of invasive disease episodes in recent years has been documented in many countries, including those in North America, Europe, and the Far East (reviewed in reference 23). Extensive epidemiological studies have found that in most localities, strains expressing serotype M1 protein predominate (23). The M protein is a highly polymorphic surface protein that is an important virulence factor of GAS, in part because it is antiphagocytic (8). More than 80 M protein types have been recognized on the basis of serologic studies conducted over decades and more recently DNA sequencing investigations (2). The molecular explanation for the abundant representation of serotype M1 strains among invasive isolates is unknown.
Molecular characterization of serotype M1 strains cultured from patients with invasive infections has shown that in general, there is relatively restricted genetic diversity among most strains (22, 23). For example, analysis of variation in 10 genes in large samples of M1 GAS from several countries failed to identify sequence diversity in virtually all genes studied (22, 23). Although most M1 strains are closely related in overall genomic character, recently it has been shown that a protein that inhibits the normal cytolytic effect of the C5b-C9 membrane attack complex of human complement is remarkably hypervariable (1, 10, 29). This protein, known as the streptococcal inhibitor of complement (Sic), is encoded by the sic gene that is located in the Mga virulence regulon of GAS (1, 14). Initial DNA sequence analysis of sic variation among 165 M1 strains identified 62 alleles, virtually all of which would encode distinct protein variants (29). The observation that most gene alleles would encode distinct protein variants, together with a lack of synonymous (silent, not resulting in amino acid replacements) nucleotide substitutions strongly suggested that the Sic protein is under strong natural selection pressure. This idea is supported by the preponderance of radical amino acid replacements (polar-nonpolar replacements or those resulting in charge changes) found among the Sic variants (10, 29).
Serotype M1 strains also have the ability to undergo rapid changes in disease frequency and severity (19, 21, 23). Epidemic waves of serotype M1 strains have been documented in several countries (23). Although the exact molecular explanation for the ability of M1 strains to cause epidemic waves is unknown, recent analysis of the molecular population genetics of epidemic waves of serotype M1 organisms in Finland; Ontario, Canada; and four regions in the United States found that in contrast to the prevailing idea that these epidemic waves are mono- or pauciclonal, they are composed of M1 strains with a highly diverse array of Sic variants that are rapidly selected in the course of the epidemic waves (10). Study of M1 strains from humans with pharyngitis and analysis of organisms recovered from the nasopharynx of persistently colonized mice discovered that new Sic variants are selected on the mucosal surface.
Taken together, the data suggest that Sic participates in host-pathogen interaction on the mucosal surface. To directly test this idea, we constructed an M1 isogenic mutant strain in which the sic gene was inactivated by a new nonpolar mutagenesis strategy. The nonpolar mutagenesis approach was necessary because the sic gene is located in the Mga regulon and hence is located close to genes encoding important GAS virulence factors, including emm and scpA, encoding M1 protein and a protease that cleaves and inactivates complement factor C5a, respectively. The isogenic mutant strain had significantly decreased ability to colonize the nasopharynx of mice. We hypothesize that the predominance of M1 strains in human infections is related, in part, to a Sic-mediated enhanced colonization ability.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains and plasmids used in this study are described in Table 1. GAS strain MGAS 1600 was used for cloning the sic1.07 allele, which contains a naturally occurring T→C mutation located in the −10 region of the presumed sic promoter. This mutation is associated with decreased Sic expression by the source strain. The strain (MGAS 5005) used to generate the isogenic Sic-deficient mutant contains the most frequently identified sic allele (sic1.01) and produces abundant levels of secreted Sic protein in vitro. This strain also has the emm1.0 gene allele and genes encoding SpeA and SpeC exotoxins.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| Strains | ||
| GAS | ||
| MGAS 5005 | sic1.01; wild-type strain | J. Vuopio-Varkila |
| MGAS 5005 sic | sic-inactivated isogenic mutant strain | This study |
| MGAS 1600 | sic1.07; wild-type strain | K. Johnston |
| MGAS 1600 sic1 | sic::spc1; in-frame insertion | This study |
| MGAS 1600 sic2 | sic::spc2; out-of-frame insertion | This study |
| E. coli XL1-Blue | Cloning strain | Stratagene |
| Plasmids | ||
| pBC KS− | Cmr; E. coli cloning vector | Stratagene |
| pEU904 | Cmr Spr; source of aad gene | J. R. Scott |
| pSL60-1 to -3 | Cmr (Spr)a; spc1- to 3 cassettes in pBC KS− | This study |
| pFW12 | Spr; E. coli cloning vector | 27 |
| pFW14 | Cmr; source of cat gene | 27 |
| pLEX5B | Apr; ori ColE1; high-copy-number E. coli vector | 6 |
| pSL97 | Apr; oriR1; low-copy-number E. coli vector | This study |
| pSL99 | Apr; oriR1; sic1.07 | This study |
| pSL114 | Cmr; oriR1; sic1.07 | This study |
| pSL123 | Spr; 3′ flanking region of sic1.01 in pFW12 | This study |
| pSL124 | Spr; 5′ flanking region of sic1.01 in pSL123 | This study |
| pSL127 | Spr; sic1.01::spc2 in pSL124 | This study |
| pSL128-1 | Cmr; oriR1; sic1.07::spc1 | This study |
| pSL128-2 | Cmr; oriR1; sic1.07::spc2 | This study |
(Spr), spectinomycin resistance upon induction with IPTG.
GAS strains were grown in Todd-Hewitt broth (Difco Laboratories, Detroit, Mich.) supplemented with 0.2% yeast extract (THY medium). Brain-heart infusion agar (Difco Laboratories) or tryptose agar with 5% sheep blood (Becton Dickinson, Cockeysville, Md.) was used as solid medium. For mutant selection, brain heart infusion agar supplemented with spectinomycin (150 μg/ml) was used. The GAS strains were incubated at 37°C in 5% CO2–20% O2 atmosphere.
Cloning experiments were performed with Escherichia coli XL-1 Blue (Stratagene, La Jolla, Calif.).
Construction of nonpolar spc cassettes.
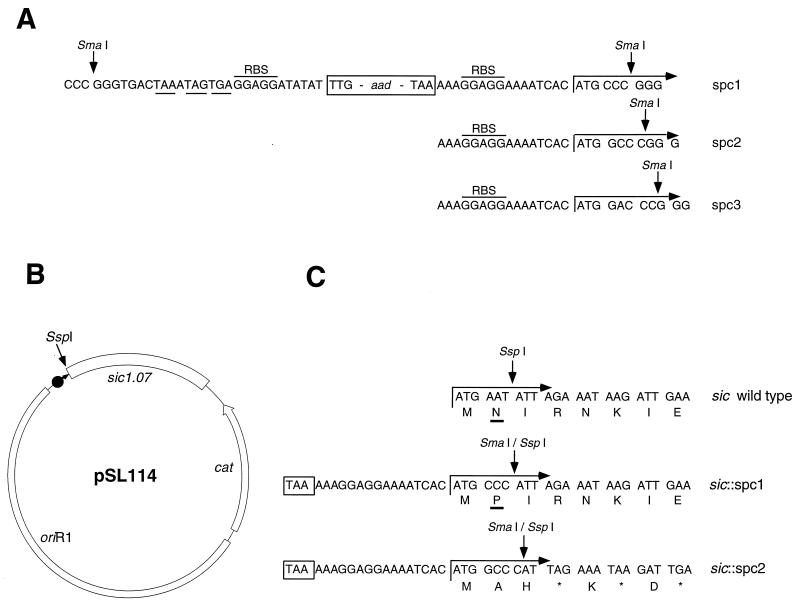
A nonpolar spectinomycin resistance (spc) cassette was developed to inactivate the sic gene (Fig. 1A). The cassette contains the promoterless spectinomycin resistance marker encoded by the aad gene (16). The 5′ region of the cassette upstream of the aad gene has stop codons in all three reading frames. A consensus ribosome-binding site (GGAGG) followed by the ATG start codon is located at the 3′ end. To avoid a polarity effect on downstream genes, the spc cassette is cloned so that the ATG start codon is in frame with the downstream mutated gene, in this case sic. Construction of the three cassettes allows one of them to be used at any restriction site available in the target gene. This strategy was based on similar aph-3 (20) and cat (chloramphenicol resistance) (17) cassettes used successfully for nonpolar gene inactivation in gram-negative bacteria.
FIG. 1.
Construction and testing of the spc cassettes. (A) Schematic representation of the promoterless spc cassettes. spc1- to 3 cassettes are flanked by SmaI sites and contain the aad gene conferring resistance to spectinomycin. The promoterless aad gene was amplified with its original ribosome-binding site (RBS). At the 5′ synthetic end, upstream of the aad gene, there are stop codons in all three reading frames (underlined). At the 3′ synthetic end, downstream of the aad gene, the spc1- to 3 cassettes contain the GGAGG consensus ribosome-binding sites and the ATG start codons in all three reading frames. (B) Construction of the suicide plasmid pSL114 containing sic1.07. pSL114 has a temperature-sensitive origin of replication (oriR1) which maintains low copy number at 30°C. It carries the cat gene. (C) Construction of the polar and nonpolar mutations within sic1.07. The first eight amino acids of the Sic signal peptide are shown. The SspI cleaves in frame after the sixth base pair of the wild-type sic sequence. The spc1 and spc2 cassettes were cloned at the SspI site of pSL114, resulting in plasmids pSL128-1 and 128-2, respectively. After passing the TAA stop codon (boxed) of the aad gene, translation is restored at the ATG start codon located at the 3′ end of the cassettes. An insertion of the spc1 cassette is in frame and results in a single amino acid N→P (underlined) substitution within the Sic signal peptide. An out-of-frame insertion of the spc2 cassette generates multiple translational stop codons (asterisks).
The aad gene contained on plasmid pEU904 (provided by J. R. Scott, Emory University) and synthetic regulatory sequences described above which flank the aad gene were amplified by PCR with three sets of primers. One forward primer (spcF [GGACCCGGGTGACTAAATAGTGAGGAGGATATATTTG]) complementary to the 5′ region of the aad gene and three different reverse primers (spcR1 [CCTCCCGGGCATGTGATTTTCCTCCTTTTTATAATTTTTTTAATCTGTTA], spcR2 [CCTCCCGGGCCATGTGATTTTCCTCCTTTTTATAA], and spcR3 [CCTCCCGGGTCCATGTGATTTTCCTCCTTTTTATAA]) were used. These three PCR products are flanked by SmaI sites and were back cloned into the E. coli vector pBC KS− (Stratagene) to obtain cassettes spc1 to 3, contained on plasmids pSL60-1 to -3, respectively (Table 1). The sequences of the spc cassettes were confirmed to rule out spurious mutations. All three cassettes conferred spectinomycin resistance to E. coli in the presence of isopropyl-β-d-thiogalactopyranoside (IPTG).
spc cassettes are nonpolar in GAS: proof of principle.
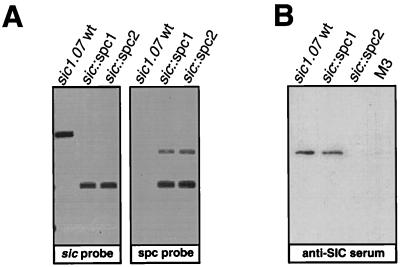
To test the utility of the nonpolar mutagenesis strategy, two sic mutants were generated with the spc1 and spc2 cassettes by allelic replacement in the chromosome of GAS. Initial data indicated that sic cloned into E. coli was unstable, perhaps due to a toxic effect of the Sic protein. Hence, a strain (MGAS 1600) with a mutation in the −10 region of the presumed sic promoter that is associated with decreased Sic production by the parental GAS strain was used as the source organism for the cloned sic allele (sic1.07). The strategy for the cloning of the sic1.07 gene and insertion of the spc1 and spc2 cassettes is shown in Fig. 1B and C. The sic1.07 allele was amplified with sic-specific primers (sic1 [TAAGGAGAGGTCACAAACTA] and sic2 [TTACGTTGCTGATGGTGTAT]) and cloned in a promoterless low-copy-number plasmid vector pSL97 (Table 1). This vector was based on plasmid pLEX5B (6), in which the ColE1 origin of replication (ori) was replaced with a temperature-sensitive oriR1 which allows the plasmid to be maintained at low copy number (15). Next, the β-lactamase gene was replaced with the cat gene from pFW14 (27), resulting in plasmid pSL114; plasmid pSL114 cannot replicate in GAS. This cloned sic1.07 allele was then interrupted by insertion of the spc1 and spc2 cassettes after the sixth base pair of the sic coding sequence (Fig. 1C). The spc1 cassette was inserted in frame with the downstream sic region (pSL128-1), whereas the spc2 cassette insertion (pSL128-2) was out of frame. These two constructs were used to generate the sic mutants of MGAS 1600 by allelic replacement (Fig. 2A).
FIG. 2.
Nonpolar mutagenesis of the sic gene by using the spc cassette. (A) Southern blot analysis of the genomic DNAs isolated from the MGAS 1600 (sic1.07) wild-type strain and from MGAS 1600 sic1 (sic::spc1, in-frame insertion) and MGAS 1600 sic2 (sic::spc2, out-of-frame insertion) mutants. DNAs were digested with BanII, which has a single recognition site within the aad gene of the spc cassettes but not in the sic sequence. Only one mutated sic copy can be detected in the mutant strains by using the sic-specific probe homologous to the 3′ end of the sic gene. As expected, the spc probe hybridized only to the DNA of the mutants. (B) Immunoblot detection of the Sic protein in GAS culture supernatants. Sic1.07 was produced by the MGAS 1600 wild-type strain and by the in-frame sic::spc1 mutant. No Sic could be detected in the culture supernatant of the sic::spc2 out-of-frame mutant. The identically processed culture supernatant of a GAS serotype M3 strain lacking the sic gene is shown as a negative control.
Since insertion in the sic::spc1 mutant resulted in only one amino acid change in the signal peptide of the Sic protein, production of secreted Sic by this mutant was expected. In contrast, the out-of-frame sic::spc2 insertion should cause early translation termination, thereby resulting in lack of Sic secretion. As expected, the in-frame mutant (MGAS 1600 sic1 [sic::spc1]) produced extracellular Sic, whereas the out-of-frame mutant (MGAS 1600 sic2 [sic::spc2]) did not (Fig. 2B). These results indicated that the spc cassettes can be used successfully for nonpolar mutagenesis in GAS.
Construction of the sic-inactivated isogenic mutant strain.
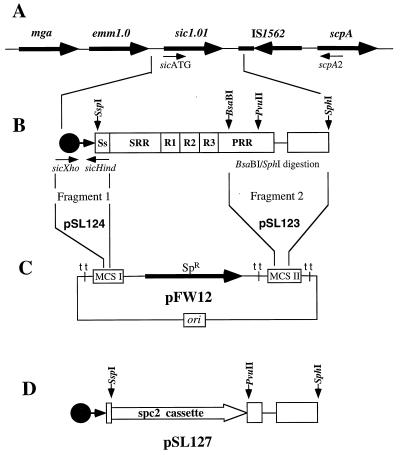
An analogous strategy was used to generate a nonpolar sic1.01 knockout isogenic strain of MGAS 5005. We selected the sic1.01 allele for inactivation because this is the most common sic allele found in clinical isolates of GAS serotype M1. For example, the sic1.01 allele was found in 36% of M1 GAS isolates recovered in Finland from 1988 to 1998 (10). Suicide plasmid pSL127 was constructed (detailed cloning strategy is shown in Fig. 3) such that an internal part of the sic gene (bp 6 to 811) was replaced with the spc2 cassette (in-frame insertion). This construct was introduced by electroporation into the wild-type MGAS 5005 organism to obtain a nonpolar isogenic sic-inactivated mutant derivative. After initial screening, the MGAS 5005 sic strain was analyzed by PCR, Southern and Western blotting, and sequencing.
FIG. 3.
Construction of the suicide plasmid pSL127, used to generate an sic1.01-inactivated MGAS 5005. All primers and restriction sites used in the cloning strategy are shown. (A) Schematic representation of the Mga regulon with the sic gene in MGAS 5005 (not to scale). The map was drawn based on PCR analyses using different combinations of primers specific for the emm1.0, sic1.01, IS1562, and scpA genes. (B) DNA region containing the sic gene and part of the IS1562 used in plasmid construction. Major parts of the sic sequence are shown: Ss, signal sequence; SRR, short repeat region; R1 to R3, repeat regions 1 to 3; PRR, proline-rich region. Two chromosomal fragments from MGAS 5005 were amplified. Fragment 1 (∼150 bp), flanked by the PCR-generated XhoI (sicXho [TCGACTCGAGGTTAAGGAGAGGTCAC]) and HindIII (sicHind [TTTTCAAGCTTATTTCTAATATTC]) sites, contained the sic promoter region and first 26 bp of the coding region. Fragment 2 (∼830 bp), obtained by restriction digestion of the PCR product (sicATG [GGAGAGAATACTAATGAATATTAG] and scpA2 [CTGGTGTATCAGCAGTTTTAGC]) with restriction enzymes BsaBI and SphI, contained a 3′ end of the sic proline-rich region and adjusted part of IS1562. (C) First, fragment 2 was cloned into multiple cloning site (MCS) II of the E. coli vector pFW12, generating plasmid pSL123. Next, fragment 1 was cloned into MCS I of the pSL123, resulting in construct pSL124. (D) In the last step, the nonpolar spc2 cassette was cloned in frame between the SspI and PvuII sites of the sic coding sequence, replacing the original spectinomycin resistance marker (SpR) of the vector. In this plasmid, designated pSL127, the spc2 insertion limits the amount of the sic coding region to 6 bp (2 amino acids) at the 5′ end and 129 bp (43 residues) at the 3′ end. Suicide plasmid pSL127 was used to generate an sic-inactivated isogenic variant of MGAS 5005.
DNA methods.
Standard methods (28) were used to manipulate DNA. Plasmid DNA was prepared with a QIAGEN-tip (Qiagen, Valencia, Calif.). Chromosomal DNA was isolated from GAS as described previously (22); 5 to 6 μg was used for Southern blotting. Hybridization was performed with a nonradioactive labeling and detection system (ECL [enhanced chemiluminescence]; Amersham Pharmacia Biotech, Inc., Piscataway, N.J.). DNA amplification was performed with Taq polymerase (Perkin-Elmer). When PCR was performed directly from E. coli or GAS colonies, cells were transferred to the reaction mixture on a disposable tip. The Taq DyeDeoxy terminator cycle sequencing kit (Applied Biosystems, Inc., Foster City, Calif.) and an ABI 377 instrument were used to obtain DNA sequence data.
RNA methods.
Total RNA was prepared from GAS cultures (10 ml) grown in THY medium to mid-log phase (optical density at 600 nm [OD600] of ∼0.4). Cell pellets were suspended in 500 μl of TE buffer (10 mM Tris [pH 7.0], 1 mM EDTA) and treated at 37°C for 5 min with 6 μl of mutanolysin (1 mg/ml), 60 μl of lysozyme (10 mg/ml), and 25 μl of the RNase inhibitor aurintricarboxylic acid (100 mM stock solution; Sigma, St. Louis, Mo.). GAS cells were lysed with 60 μl of 20% sodium dodecyl sulfate and 600 μl of acid-phenol–chloroform (5:1 mix, pH 4.5; Ambion, Austin, Tex.) at 65°C for 5 min. Samples were extracted with equal volumes of acid-phenol–chloroform until no debris was seen at the interface. The RNA contained in 400 μl was mixed with 15 μl of 5 M NaCl and precipitated with 2 volumes of ethanol. DNA was removed by DNase I digestion followed by an acid-phenol–chloroform extraction. The RNA was precipitated as described above. Usually, 250 to 300 μg of total RNA was obtained from a 10-ml culture by this method. The RNA showed good sample integrity when analyzed in an agarose gel.
Samples (15 μg) of total RNA were denatured and separated in a 0.8% formaldehyde gel, blotted to a Nytran SuPerCharge membrane (Schleicher & Schuell, Keene, N.H.), and cross-linked. RNA transfer, hybridization (42°C), and posthybridization washes were performed with NorthernMax reagents (Ambion). Biotinylated molecular weight markers (Millenium; Ambion) were used to evaluate transcript sizes.
Genomic DNA purified from the homologous strain (MGAS 5005) was used to amplify DNA probes with primers specific for the emm (9), sic (1), scpA (4), and recA (30) genes (Table 2). DNA probes (500 to 600 bp) corresponding to gene regions encoding the amino-terminal parts of the mature proteins were biotinylated with BrightStar labeling reagents (Ambion). Hybridizing bands were detected with streptavidin-POD conjugate (Roche Molecular Biochemicals, Indianapolis, Ind.) and visualized with an ECL reagent.
TABLE 2.
Primers used to amplify DNA probes for Northern hybridization
| Gene | Primer designation | Primer sequence | Probe size (bp) | Reference |
|---|---|---|---|---|
| emm | emmFor | CACGAATAGACACTATTCGCTTAG | 629 | 9 |
| emmRev | CAAGTTCAAGTTTTGCATTGCC | |||
| sic | sicFor | CCGAAACGTATACATCACGC | 494 | 1 |
| sicRev | CTCAGGTTTAGGAATATGTCC | |||
| scpA | scpAFor | CTGTGACAGAAGACACTCC | 500 | 4 |
| scpARev | CCCTGACACGTGTGTGCC | |||
| rec | recFor | CGTTCAGGAAGTCTAGCTC | 553 | 30 |
| recRev | CATCCAGCCGAACAGAAGC |
The transcripts were quantitated by densitometry with the BioImage Whole Band Analysis computer program (BioImage, Ann Arbor, Mich.). The amounts of the transcripts were normalized by comparison with the amount of the recA transcript in the same sample.
Protein methods.
Sic expression was assayed in the culture supernatants of GAS grown to exponential phase (OD600 of ∼0.5) in THY medium. Proteins were precipitated with 30% ammonium sulfate and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by staining with Coomassie brilliant blue or by Western blotting with rabbit polyclonal serum raised against purified Sic1.01 (10). An ECL visualization protocol was used.
Measurement of anti-Sic immunoglobulin G (IgG) in mouse and human sera.
Mouse sera were obtained on day 24 from the blood of 34 animals that survived 21 days after intranasal inoculation with the MGAS 5005 wild-type strain. Eleven mice were persistently colonized in the throat with GAS, and 23 were not. Serum samples from 15 of the mice that were not colonized on day 24 and all 11 samples from persistently colonized mice were assayed. Human sera from 198 healthy individuals with no history of invasive GAS disease were used for the analysis.
For detection of anti-Sic1.01 IgG in mouse and human sera, plate wells were coated with purified Sic1.01 (10 μg/ml in carbonate buffer, pH 9.6) at room temperature overnight. The wells were washed three times with phosphate-buffered saline (PBS) containing 0.1% Tween 20 (TPBS) and blocked with 5% gelatin in TPBS. For detection of anti-Sic IgG in mouse sera, twofold dilutions of each serum sample were prepared in PBS from a starting dilution of 1:20. One hundred microliters of each dilution was added to test wells. Mouse serum that did not react with Sic1.01 was used as a negative control. The plate blank consisted of 100 μl of PBS. For detection of anti-Sic IgG in human sera, microtiter plates were coated with Sic1.01 and washed as described above. Human sera were used at a single dilution of 1:200. Human sera that did not react with Sic1.01 were used as negative controls. The plate blank consisted of 100 μl of PBS. For both mouse and human sera, the plates were incubated at 37°C for 2 h. The plates were washed three times with TPBS, and 100 μl of horseradish peroxidase-conjugated goat antibody directed against mouse or human IgG (whole molecule, 1:2,000; Bio-Rad, Hercules, Calif.) was added to all wells. The plates were then incubated for 2 h at 37°C, washed three times as described above, and incubated with 2,2′-azino-bis-[3-ethylbenzthiazoline-6-sulfonic acid] (ABTS; Roche Molecular Biochemicals) as the development agent for 20 min at room temperature in the dark. Absorbance was measured at 405 nm with a Spectramax PLUS instrument (Molecular Dynamics, Sunnyvale, Calif.). Titers of anti-Sic IgG in mouse sera were calculated with SOFTmax PRO software version 2.6.1. Human serum samples were considered to have specific Sic antibody if absorbance readings were greater than the average of the negative controls (i.e., human sera that did not react with Sic1.01) plus 3 standard deviation values.
Mouse colonization experiments.
Isogenic GAS wild-type and sic mutant strains were grown in THY medium and harvested in the logarithmic phase (OD600 of ∼0.5 to 0.6), when abundant Sic production occurs. The cells were washed once and resuspended in sterile ice-cold, pyrogen-free PBS to give the required inoculum. Colony counts were performed to determine the actual number of CFU used in each experiment. Control animals inoculated with PBS were always culture negative for GAS. The animals were anesthetized by inhalation of Metofane (Mallinckrodt Veterinary, Mundelein, Ill.) prior to experimental procedures.
Mouse throat colonization studies were conducted with adult (18- to 20-g) male outbred CD-1 Swiss mice (Harlan Sprague-Dawley Inc., Houston, Tex.) as described previously (18). The mice were inoculated in the nares with 50 μl of GAS cell suspensions. The mouse throats were swabbed periodically for 3 weeks. GAS cells present on swabs were suspended in 1 ml of PBS, and 0.1 ml from these samples was cultured on blood agar. Blood was collected from dead animals by cardiac puncture and cultured on blood agar. All blood samples from dead animals grew GAS. In addition, sic mutant colonies recovered from both dead and colonized mice were tested for reversion by streaking on medium with spectinomycin. All 400 colonies tested (one-half isolated from the blood and one-half from the throat) maintained the spectinomycin-resistant phenotype, indicating that the mutant was stable in the host without antibiotic selection.
Statistical analysis.
Fisher's exact two-tailed test was used to assess statistical differences in mouse mortality and throat colonization between the animal groups infected with the wild-type GAS or the isogenic sic mutant strain. A logistic regression model was used to compare mouse throat colonization by the wild-type and mutant strains. Statistical calculations were performed with SAS software (SAS Institute Inc., Cary, N.C.).
RESULTS
Anti-Sic immune response in mice and humans.
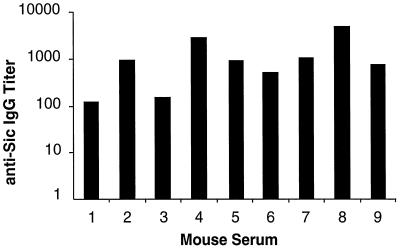
Recent investigation discovered that in contrast to other genes analyzed (including emm1, encoding M1 protein), the sic gene and Sic protein are highly variable among serotype M1 GAS recovered in epidemic waves in all geographic localities studied (10). In addition, Sic structural variants were selected after intranasal inoculation of mice but not during in vitro cultivation (10, 29). These observations imply that Sic is made in vivo, but this aspect of GAS pathogenesis has not been studied. If Sic is made in vivo, we expected that infected mammalian hosts would make antibody against this protein. To test this issue, we first analyzed the anti-Sic serum antibody response in mice inoculated intranasally with wild-type MGAS 5005. Nine of 11 animals (82%) persistently colonized in the throat on day 24 after intranasal inoculation had specific anti-Sic1.01 IgG antibodies as assessed by enzyme-linked immunosorbent assay (ELISA) (Fig. 4). In contrast, animals that were not persistently colonized after intranasal inoculation lacked anti-Sic serum antibodies.
FIG. 4.
Graph of anti-Sic1.01 IgG titers in serum from mice persistently colonized in the throat with MGAS 5005. Serum samples were obtained on day 24 postinfection and tested in an ELISA against purified Sic1.01 at a starting dilution of 1:20. Titers were calculated with SOFTmax PRO software version 2.6.1.
These data suggested that Sic was expressed in the course of GAS infection of the human mucosal surface. To address this possibility, we tested serum obtained from 198 randomly selected children between 6 months and 18 years of age for anti-Sic serum IgG by ELISA. These children had no known history of invasive GAS disease. Approximately one-fourth (46 of 198 [23%]) has serum antibody against Sic, a result indicating that this protein is also made during human mucosal infection. Detailed analysis of the human serologic response to Sic will be presented elsewhere.
Construction and characterization of the Sic1.01-negative nonpolar isogenic mutant.
The presence of anti-Sic antibody in experimental mice and humans indicated that Sic was expressed during mucosal infection. In addition, interaction with the host mucosal surface has been shown to result in a high frequency of selection of new Sic variants (10). Inasmuch as both seroconversion to Sic and selection of Sic variants have been observed in mice and humans, we investigated if Sic participates in mouse throat colonization. A nonpolar sic- inactivated isogenic mutant strain was constructed to test this idea.
Strain MGAS 5005 contains the most frequently occurring sic1.01 gene allele (10, 29) and was used to generate an isogenic sic-inactivated mutant derivative. This strain also has the emm1.0 allele and genes encoding SpeA and SpeC exotoxins. To generate the Sic-negative nonpolar isogenic strain of MGAS 5005, an internal part of the cloned sic gene located between bp 6 to 811 was replaced with the spc2 cassette (in-frame insertional replacement). After allelic replacement, the MGAS 5005 sic mutant was confirmed by PCR, Southern blotting, and sequencing. Since insertion of the spc2 cassette removed most of the sic coding sequence (270 of the 313 amino acid residues), the mutant strain did not produce extracellular Sic protein (data not shown).
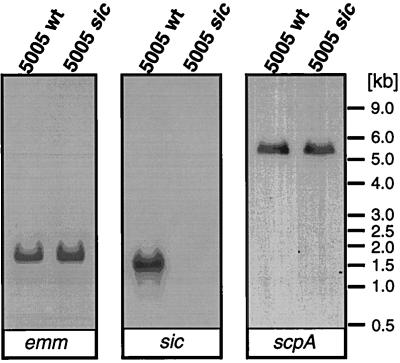
To confirm that sic inactivation did not have a polar effect on flanking genes, expression of the emm and scpA genes was studied (Fig. 5). This analysis was particularly important since both genes encode documented GAS virulence factors. Single transcripts of the predicted molecular size were identified for emm, sic, and scpA genes in the wild-type MGAS 5005 organism. No sic-specific transcript was detected in the MGAS 5005 sic mutant. In addition, there was no difference in the amount of the emm-specific transcripts made by the wild-type and sic mutant strains. Moreover, there was no significant difference in the amount of the scpA-specific transcript quantified in RNA samples isolated from both strains in three independent experiments. Hence, the genetic strategy ablated Sic production without altering expression of the genes flanking sic. sic inactivation did not alter the growth rate or colony morphology of the mutant (data not shown).
FIG. 5.
Northern blot analysis of emm, sic, and scpA gene expression by wild-type strain MGAS 5005 (5005 wt) and by the isogenic 5005 sic mutant. No sic transcript could be detected in the mutant strain. In addition, transcription of the emm and scpA genes was unaffected. Biotinylated DNA probes were used for hybridization. The biotinylated molecular weight RNA marker was used to evaluate transcript sizes.
sic inactivation decreases mouse throat colonization.
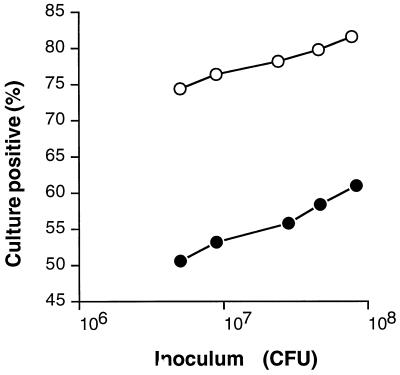
To test the hypothesis that inactivation of sic altered mouse throat colonization, we used a logistic regression analysis to compare colonization by the wild-type GAS serotype M1 and the isogenic sic-inactivated mutant (Fig. 6). Five dilutions (twofold dilutions ranging from ∼8 × 107 to 0.5 × 107 CFU) of the wild-type and mutant strains were used to inoculate groups of 10 mice intranasally. Mouse throats were swabbed periodically, and the bacteria were cultured on a blood agar. In addition, blood samples from dead animals were cultured. There was no significant difference in the number of dead mice or Kaplan-Meier survival curves on day 21 after infection with the wild-type (16 of 50 mice on day 21) or mutant (14 of 50 mice) strain. In contrast, we observed a significant difference in colonization of the mouse mucosa. Significantly more mice inoculated with the wild-type GAS strain were culture positive than mice infected with the sic mutant strain (P < 0.019) (Fig. 5). The results of a logistic regression model using GAS colonization data obtained from only the mice which survived were also significantly different (P = 0.023), as observed with the data obtained from the model which included dead mice (analysis described above). The odds ratio for the wild-type strain was 2.81, which indicated that the MGAS 5005 parental organism had a much higher incidence of throat colonization. However, the level of inoculum was not significant, indicating that the incidence of throat colonization was not related to the amount of inoculum given to the mice. Therefore, experiments with animals infected with all five inocula of the wild-type strain or the sic mutant were combined. A significant group difference was found (P < 0.035; Fisher's exact two-tailed test). We note that the difference in colonization by the wild-type and mutant strains was statistically significant during the first 4 days postinoculation. These results suggested that Sic plays a significant role at the early stages of infection. Because the large number of the mice died during the study period, the number of surviving animals in each experimental group was not adequate for statistical comparison. Taken together, our results indicate that Sic participated in GAS-host interactions by enhancing early colonization of the mucosal surfaces.
FIG. 6.
Logistic regression analysis of death and throat colonization at 72 h after intranasal inoculation with the 5005 wild-type strain (open circles) or with the 5005 sic mutant (solid circles). Each data point represents 10 mice. The y axis represents estimated percent culture-positive animals. The data fit the model (P = 0.021); the group difference was significant (P < 0.019); the dose-dependent parameter was not significant.
DISCUSSION
GAS causes epidemic waves of pharyngitis and severe invasive infections worldwide (23). GAS strains of serotype M1 have been predominantly responsible for countrywide epidemics for reasons that are not known. The observations that the Sic protein was hypervariable in serotype M1 strains recovered from epidemic waves and that new structural Sic variants were selected on mucosal surfaces in the course of epidemic waves suggested that Sic could contribute to host colonization (10).
We constructed a sic-inactivated nonpolar isogenic mutant strain to directly assess the contribution of Sic to mucosal colonization of a mammalian host. Construction of gene-inactivated GAS isogenic strains has mainly used the conjugative transposon Tn916 (3, 24, 32) or plasmid-directed insertional mutagenesis (25, 26, 31). Despite many successful applications, both methods have several disadvantages, including uncontrolled chromosomal insertions resulting in the need for laborious screening, presence of foreign vector sequences in the GAS genome, or existence of two copies of the mutated gene, thereby creating potential substrates for recombination. Both conjugative transposition and plasmid-directed insertional mutagenesis have the drawback of insertion instability and potential polar effects of the integron on neighboring genes. This is an especially problematic issue in assessing the contribution of Sic to host-pathogen interactions because the gene is located in the Mga regulon containing proven virulence genes. Therefore, we developed a new strategy that used a nonpolar spc cassette to inactivate the sic gene. Analogous aph-3 and cat cassettes have been used successfully to generate nonpolar mutants in Shigella flexneri (20) and in E. coli (17) and Pasteurella haemolytica (7), respectively. Two important features of our nonpolar spc cassette are shown in this study: (i) it restores the expression of a downstream gene, and (ii) since it is promoterless, it does not alter the level of transcription of a downstream gene. In our study, we showed that insertion of the spc cassette within sic did not alter the transcriptional pattern of genes in the Mga regulon. Specifically, the isogenic mutant did not express a sic transcript, and transcription of the flanking emm and scpA genes was not affected. Hence, the data indicate that this spc cassette can be used to conduct nonpolar GAS mutagenesis.
Our study showed that inactivation of sic decreased colonization of the host mucosal surface. Although the molecular mechanism by which Sic contributes to throat colonization is not yet known, several possibilities can be envisioned. Akesson et al. (1) reported that in vitro, Sic binds to the human plasma components clusterin and histidine-rich glycoprotein and interferes with formation of the membrane attack complex of complement. It is possible that Sic-mediated impairment of complement function enhances survival of M1 GAS strains in the host. In this regard, we note that Ji et al. (11–13) reported that inactivation of the gene (scpA) encoding the peptidase that cleaves complement protein C5a reduced the capacity of GAS to colonize the throats of mice following intranasal inoculation. Immunization of mice with purified streptococcal C5a peptidase also reduced colonization capacity. It is also possible that Sic exerts other mucosal effects that create local conditions that enhance colonization. Clearly, additional studies are required to delineate the exact mechanism of Sic action at the mucosal surfaces.
The humoral immune response induced in the host to microbial infection commonly results in clearance of the organism. Many microbes escape antibody-mediated clearance by positive selection of allelic variants with altered affinity to host antibodies. Recent results have shown that GAS strains expressing new Sic variants were selected in mice following long-term throat colonization (10), but only after a colonization time sufficient to generate an anti-Sic antibody response. In the present study we showed that 82% of the persistently colonized mice raised specific serum IgGs directed against the Sic protein. Anti-Sic antibodies were also identified in the serum of human subjects. These two observations suggest that host antibody may select new Sic variants, an idea postulated in earlier studies (10, 29).
Our results demonstrate that in a mouse model Sic contributes to colonization of the mucosal surface by serotype M1 GAS. We have also shown that colonized mice, and humans with no known history of invasive GAS disease, have anti-Sic antibody, which means that Sic is expressed in the context of mucosal interaction. The results of epidemiologic studies in the United States and Finland indicate that colonization of the human mucosal surface is a critical step in determining the frequency of occurrence of invasive episodes. For example, Cockerill et al. (5) studied an outbreak of invasive disease episodes caused by a clone of serotype M3 GAS and found that the outbreak-associated strain was the most common organism recovered from nasopharyngeal carriers in the same locality. Similarly, Muotiala et al. (21) reported that in Finland between 1988 and 1992, the proportions of serotype M1 isolates among invasive and pharyngitis isolates were identical. Taken together, the results of these studies suggest that expression of a protein capable of enhancing the rate or efficiency of GAS colonization of the host mucosal surface is likely to increase the frequency of invasive disease. In this regard, it is noteworthy that expression of the Sic protein may be restricted to relatively few clonal lineages of GAS, most notably M1 organisms (1). We believe it reasonable to hypothesize that the predominance of M1 strains in human infections and the capability of M1 strains to cause epidemic waves are related in part to Sic-mediated enhanced colonization. Additional studies are under way to test this idea.
ACKNOWLEDGMENTS
This work was supported by Public Health Service grant AI-33119 to J.M.M.
We thank J. Scott for supplying plasmid pEU904.
REFERENCES
- 1.Akesson P, Sjoholm A G, Bjorck L. Protein SIC, a novel extracellular protein of Streptococcus pyogenes interfering with complement function. J Biol Chem. 1996;271:1081–1088. doi: 10.1074/jbc.271.2.1081. [DOI] [PubMed] [Google Scholar]
- 2.Beall B, Facklam R, Hoenes T, Schwartz B. Survey of emm-gene sequences and T-antigen types from systemic Streptococcus pyogenes infection isolates collected in San Francisco, California; Atlanta, Georgia; and Connecticut in 1994 and 1995. J Clin Microbiol. 1997;35:1231–1235. doi: 10.1128/jcm.35.5.1231-1235.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Betschel S D, Borgia S M, Barg N L, Low D E, de Azavedo J C S. Reduced virulence of group A streptococcal Tn916 mutants that do not produce streptolysin S. Infect Immun. 1998;66:1671–1679. doi: 10.1128/iai.66.4.1671-1679.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen C C, Cleary P P. Complete nucleotide sequence of the streptococcal C5a peptidase gene of Streptococcus pyogenes. J Biol Chem. 1990;265:3161–3167. [PubMed] [Google Scholar]
- 5.Cockerill F R, III, MacDonald K L, Thompson R L, Roberson F, Kohner P C, Besser-Wiek J, Manahan J M, Musser J M, Schlievert P M, Talbot J, Frankfort B, Steckelberg J M, Wilson W R, Osterholm M T the Investigation Team. An outbreak of invasive group A streptococcal disease associated with high carriage rates of the invasive clone among school-aged children. JAMA. 1997;277:38–43. [PubMed] [Google Scholar]
- 6.Diederich L, Roth A, Messer W. A versatile plasmid vector system for the regulated expression of genes in Escherichia coli. BioTechniques. 1994;16:916–923. [PubMed] [Google Scholar]
- 7.Fedorova N D, Highlander S K. Generation of targeted nonpolar gene insertions and operon fusions in Pasteurella haemolytica and creation of a strain that produces and secretes inactive leukotoxin. Infect Immun. 1997;65:2593–2598. doi: 10.1128/iai.65.7.2593-2598.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fischetti V A. Streptococcal M protein: molecular design and biological behavior. Clin Microbiol Rev. 1989;2:285–314. doi: 10.1128/cmr.2.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harbaugh M P, Podbielski A, Hugl S, Cleary P P. Nucleotide substitutions and small-scale insertion produce size and antigenic variation in group A streptococcal M1 protein. Mol Microbiol. 1993;8:981–991. doi: 10.1111/j.1365-2958.1993.tb01642.x. [DOI] [PubMed] [Google Scholar]
- 10.Hoe N P, Nakashima K, Lukomski S, Grigsby D, Liu M, Kordari P, Dou S-J, Pan X, Vuopio-Varkila J, Salmenlinna S, McGeer A, Low D E, Schwartz B, Schuchat A, Naidich S, De Lorenzo D, Fu Y-X, Musser J M. Rapid selection of structural variants of group A Streptococcus complement-inhibiting protein in serotype M1 epidemic waves. Nat Med. 1999;5:924–929. doi: 10.1038/11369. [DOI] [PubMed] [Google Scholar]
- 11.Ji Y, Carlson B, Kondagunta A, Cleary P P. Intranasal immunization with C5a peptidase prevents nasopharyngeal colonization of mice by the group A Streptococcus. Infect Immun. 1997;65:2080–2087. doi: 10.1128/iai.65.6.2080-2087.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ji Y, McLandsborough L, Kondagunta A, Cleary P P. C5a peptidase alters clearance and trafficking of group A streptococci by infected mice. Infect Immun. 1996;64:503–510. doi: 10.1128/iai.64.2.503-510.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ji Y, Schnitzler N, DeMaster E, Cleary P. Impact of M49, Mrp, Enn, and C5a peptidase proteins on colonization of the mouse oral mucosa by Streptococcus pyogenes. Infect Immun. 1998;66:5399–5405. doi: 10.1128/iai.66.11.5399-5405.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kihlberg B M, Cooney J, Caparon M G, Olsen A, Bjorck L. Biological properties of a Streptococcus pyogenes mutant generated by Tn916 insertion in mga. Microb Pathog. 1995;19:299–315. doi: 10.1016/s0882-4010(96)80003-9. [DOI] [PubMed] [Google Scholar]
- 15.Larsen J E L, Gerdes K, Light J, Molin S. Low-copy-number plasmid-cloning vectors amplifiable by derepression of an inserted foreign promoter. Gene. 1984;28:45–54. doi: 10.1016/0378-1119(84)90086-6. [DOI] [PubMed] [Google Scholar]
- 16.LeBlanc D J, Lee L N, Inamine J M. Cloning and nucleotide base sequence analysis of a spectinomycin adenyltransferase Aad(9) determinant from Enterococcus faecalis. Antimicrob Agents Chemother. 1991;35:1804–1810. doi: 10.1128/aac.35.9.1804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lukomski S, Hull R A, Hull S I. Identification of the O antigen polymerase (rfc) gene in Escherichia coli O4 by insertional mutagenesis using a nonpolar chloramphenicol resistance cassette. J Bacteriol. 1996;178:240–247. doi: 10.1128/jb.178.1.240-247.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lukomski S, Montgomery C A, Rurangirwa J, Geske R S, Barrish J P, Adams G J, Musser J M. Extracellular cysteine protease produced by Streptococcus pyogenes participates in the pathogenesis of invasive skin infection and dissemination in mice. Infect Immun. 1999;67:1779–1788. doi: 10.1128/iai.67.4.1779-1788.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin D R, Single L A. Molecular epidemiology of group A streptococcus M type 1 infections. J Infect Dis. 1993;167:1112–1117. doi: 10.1093/infdis/167.5.1112. [DOI] [PubMed] [Google Scholar]
- 20.Menard R, Sansonetti P J, Parsot C. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J Bacteriol. 1993;174:5899–5906. doi: 10.1128/jb.175.18.5899-5906.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muotiala A, Seppala H, Huovinen P, Vuopio-Varkila J. Molecular comparison of group A streptococci of T1M1 serotype from invasive and noninvasive infections in Finland. J Infect Dis. 1997;175:392–399. doi: 10.1093/infdis/175.2.392. [DOI] [PubMed] [Google Scholar]
- 22.Musser J M, Kapur V, Szeto J, Pan X, Swanson D S, Martin D R. Genetic diversity and relationships among Streptococcus pyogenes strains expressing serotype M1 protein: recent intercontinental spread of a subclone causing episodes of invasive disease. Infect Immun. 1995;63:994–1003. doi: 10.1128/iai.63.3.994-1003.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Musser J M, Krause R M. The revival of group A streptococcal diseases with a commentary on staphylococcal toxic shock syndrome. In: Krause R M, Fauci A, editors. Emerging infections. San Diego, Calif: Academic Press; 1998. pp. 185–218. [Google Scholar]
- 24.Norgren M, Caparon M G, Scott J R. A method for allelic replacement that uses the conjugative transposon Tn916: deletion of the emm6.1 allele in Streptococcus pyogenes JRS4. Infect Immun. 1989;57:3846–3850. doi: 10.1128/iai.57.12.3846-3850.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perez-Casal J F, Dillon H F, Husmann L K, Graham B, Scott J R. Virulence of two Streptococcus pyogenes strains (types M1 and M3) associated with toxic-shock-like syndrome depends on an intact mry-like gene. Infect Immun. 1993;61:5426–5430. doi: 10.1128/iai.61.12.5426-5430.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Podbielski A, Pohl B, Woischnik M, Korner C, Schmidt K H, Rozdzinski E, Leonard B A B. Molecular characterization of the group A streptococcal (GAS) oligopeptide permease (Opp) and its effect on cysteine protease production. Mol Microbiol. 1996;21:1087–1099. doi: 10.1046/j.1365-2958.1996.661421.x. [DOI] [PubMed] [Google Scholar]
- 27.Podbielski A, Spellerberg B, Woischnik M, Pohl B, Lutticken R. Novel series of plasmid vectors for gene inactivation and expression analysis in group A streptococci (GAS) Gene. 1996;177:137–147. doi: 10.1016/0378-1119(96)84178-3. [DOI] [PubMed] [Google Scholar]
- 28.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 29.Stockbauer K E, Grigsby D, Pan X, Fu Y-X, Perea Mejia L M, Cravioto A, Musser J M. Hypervariability generated by natural selection in an extracellular complement-inhibiting protein of serotype M1 strains of group A Streptococcus. Proc Natl Acad Sci USA. 1998;95:3128–3133. doi: 10.1073/pnas.95.6.3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tao L, Hollingshead S K, Suvorov A N, Ferretti J J, McShan W M. Construction of a Streptococcus pyogenes recA mutant via insertional inactivation, and cloning and sequencing of the complete recA gene. Gene. 1995;162:59–62. doi: 10.1016/0378-1119(95)00273-9. [DOI] [PubMed] [Google Scholar]
- 31.Tao L, LeBlanc D J, Ferretti J J. Novel streptococcal-integration shuttle vectors for gene cloning and inactivation. Gene. 1992;120:105–110. doi: 10.1016/0378-1119(92)90016-i. [DOI] [PubMed] [Google Scholar]
- 32.Wessels M R, Moses A E, Goldberg J B, DiCesare T J. Hyaluronic acid capsule is a virulence factor for mucoid group A streptococci. Proc Natl Acad Sci USA. 1991;88:8317–8321. doi: 10.1073/pnas.88.19.8317. [DOI] [PMC free article] [PubMed] [Google Scholar]