Abstract
Introduction
A potential precision medicine approach to smoking cessation is tailoring pharmacotherapy to a biomarker known as the nicotine metabolite ratio (NMR). Little is known about the potential impact and acceptability of this approach for American Indian (AI) persons.
Aims and Methods
Tribal-academic collaboration was formed and during 2019–2020 AI adults who smoke(N = 54) were recruited to (1) examine correlations between NMR, dependence, and smoking exposure; (2) assess the extent to which pharmacotherapy preference aligned with NMR-informed recommendations; (3) explore acceptability of NMR-informed pharmacotherapy selection. Participants provided samples for assessment of salivary NMR and urinary total nicotine equivalents (TNE) and completed a questionnaire that assessed cigarettes per day (CPD), Fagerstrom Test for Cigarette Dependence (FTCD), pharmacotherapy preference, and perceptions of NMR-informed pharmacotherapy selection.
Results
Significant positive correlations were observed between NMR and FTCD (r = 0.29;p = .0383) and its abbreviated version Heaviness of Smoking Index (HIS) (r = 0.28;p =.0426). Post-hoc analyses suggest that relationships between dependence and NMR were driven by time to first cigarette. Nonsignificant, but directionally consistent, relationships were observed between NMR and CPD (r = 0.21; p =0.1436) and TNE (r = 0.24;p = .2906). Most participants preferred nicotine replacement therapy (71%) over varenicline (29%) and preference for pharmacotherapy matched NMR-based recommendations in 54% of participants. NMR-informed pharmacotherapy selection was supported by 62% of participants.
Conclusion
In a sample of AI adults who smoke, NMR was related to cigarette dependence and about one-half of participants’ pharmacotherapy preference matched their NMR-informed recommendation. There was lower acceptability of NMR-informed approach in this sample of AI adults than prior studies among white or black/African American people who smoke.
Implications
Relationships between NMR, dependence, and self-preference for pharmacotherapy suggest that NMR-informed pharmacotherapy selection may have potential for enhancing smoking quitting success in this Tribe. Lower acceptability of NMR-informed pharmacotherapy in this Tribe suggests that this approach may not be equitably utilized. Future work could include identifying community-driven solutions to mitigate precision medicine concerns.
Introduction
The use of commercially-produced tobacco contributes to significant health disparities among Indigenous persons. For example, in northern plains states in the United States an estimated 2 out of 3 adults who identify as American Indian (AI) or Alaska Native (AN) smoke cigarettes daily, or on some days, and consequently lung cancer rates are two-fold higher among AIAN persons than white persons.1,2 Although the proportion of AIAN persons who are interested in quitting smoking is similar to white persons, quit rates tend to be lower.3,4 Lack of access to smoking cessation approaches that are effective and culturally appropriate has been suggested as the main reason for lower quit rates among AIAN persons.
In the era of personalized medicine, one approach to smoking cessation with the potential to enter clinical practice is tailoring smoking cessation pharmacotherapy to a biomarker known as the nicotine metabolite ratio (NMR).5–11 NMR is the ratio of two metabolites of nicotine (i.e. 3-hydroxycotinine [3-HCOT] to cotinine [COT]), which reflects the enzymatic activity of the primary gene involved in nicotine metabolism (CYP2A6) whereby a lower NMR reflects a slower nicotine metabolism, and can be measured in the blood or saliva of people who regularly smoke.12,13 An NMR-prospectively randomized clinical trial among people who smoke and identified primarily of white race (56%), followed by black race (37%), found that treating participants who had a low NMR with a transdermal nicotine patch and participants who had a high NMR with the prescription medication varenicline increased quit rates and/or minimized side effects.7 Possible mechanisms by which NMR-informed pharmacotherapy selection can help optimize quit rates have been suggested and include mediation by the heaviness of smoking and cigarette dependence. For example, numerous prior studies have found that in people who smoke and are predominately of white race, a higher NMR is associated with more cigarettes per day (CPD) and greater levels of smoking exposure such as the urine biomarker total nicotine equivalents (TNE) as well as higher levels of toxicant and inflammatory biomarkers.14–19 NMR’s relationship with various cigarette dependence scales, such as the Fagerström Test for Cigarette Dependence (TCD), has been mixed.8–10,20
Despite having potentially the most to gain from NMR-informed care because of a high prevalence of commercial cigarette use, the AIAN population has been underrepresented in studies examining this approach. To date, a few studies with AIAN persons on NMR’s relationship with smoking exposure have been conducted. In one study with AN Yup’ik people who smoke (N = 141), individuals with a slower nicotine metabolism had significantly lower nicotine intake measured by the biomarker TNE (56.3 versus 79.2 nmol/mg creatinine, respectively; p = .006).21 Studies with AI persons from Arizona (n = 48), South Dakota (n = 108), and Oklahoma (N = 27) have not observed a relationship between NMR and smoking exposure22–24; which may reflect poor measures of smoking intake (i.e. using CPD versus TNE), or that nicotine metabolism has a smaller impact on smoking in AI persons versus White persons. Taken together these findings raise the question of whether an NMR-informed approach to pharmacotherapy selection would benefit AI persons. Furthermore, there are 574 tribes recognized by the Bureau of Indian Affairs Federal Registry and numerous state- recognized tribes and so diversity in tribal cultures, social norms, and beliefs has important implications for generalizing research findings from prior studies.25 Specifically, relationships between NMR and smoking observed in one tribe may not extend to others.
Another question is whether an NMR-informed approach would be equitably utilized if implemented in the real world. Data from among people who smoke predominately of white race (67.9%) followed by black race (28.4%) suggest that high levels of acceptability of an NMR-informed approach with approximately 90% of participants in favor.5 Other research with African American participants found somewhat lower acceptability with 70% in favor.26 Prior studies on attitudes towards personalized medicine in other applications have shown that concerns about improper use of data, privacy, and costs and fear of discrimination likely contribute to greater concerns with personalized medicine approaches among AIAN persons and other racial and ethnic minoritized groups.27–29 Importantly, these concerns are justifiable given both historical and present-day malpractices involving exploitation of racial and ethnic minoritized groups in medicine and research; and unfortunately this experience extends to some AIAN tribes.30,31
To ensure that AI persons, who can have some of the highest smoking rates, are not left behind in the personalized medicine era, a Tribal-academic collaboration was formed to pursue the following objectives: First, to quantify NMR among AI adults who smoke and examine correlations with smoking exposure and cigarette dependence. Second, to assess preferences for pharmacotherapy for smoking cessation and the extent to which self-preference aligns with NMR-informed recommendations. Third, to assess attitudes towards NMR-informed pharmacotherapy selection and more generally personalized medicine among AI adults who smoke. The results may be used to guide the development of smoking cessation approaches for the Tribe.
Methods
Community-Based Participatory Rresearch Approach
The study was conducted via a Tribal-academic collaboration at a rurally-located tribal clinic in Minnesota. To protect the identity of the Tribe and the study participants, the specific name of the Tribe is not mentioned in this manuscript. We have previously described the use of community-based participatory research (CBPR) practices to conduct this study.32 In brief, multiple years of relationship building and planning the study resulted in signing of a Memorandum of Understanding (MOU) between the Tribe and the University of Minnesota in 2018. The MOU delineated the study goals, intended outcomes, responsibilities of key personnel, and plans for managing the biological and survey data resulting from the study. Tribal representatives were involved in all aspects of planning the study and an elder was hired as the study coordinator. To honor the Tribe’s wishes, the participants’ biological samples were stored and regulated by the Tribe. All participants provided written informed consent before enrollment in the study. In addition to being reviewed by Tribal Representatives for feasibility, and ethical and cultural considerations, the study was reviewed and approved by the institutional review board at the University of Minnesota and the Cancer Protocol Review Committee at the Masonic Cancer Center. All dissemination activities, including this manuscript, were reviewed and approved by Tribal representatives.
Participants
Recruitment of participants occurred primarily through study advertisements posted throughout the Tribe’s health clinics and community buildings. In addition to coming to the Tribe’s health clinic to participate, all participants self-reported AI race with at least one biological parent and two biological grandparents of AI descent. Similar to prior studies examining NMR and its role in smoking behaviors and cessation, additional participant inclusion criteria included the following: (1) being of legal age to purchase tobacco which was ≥ 18 years of age at the time of the study (confirmed by photo identification); (2) if female, not currently pregnant or breastfeeding; (3) self-report smoking ≥ 5 cigarettes per day (CPD) on average for the past 3 months; (4) biochemically confirmed smoking status via a carbon monoxide (CO) test in exhaled breath ≥ 10 parts per million (ppm) or if < 10 ppm a urinary cotinine level of greater than 1000 ng/mL (i.e. NicAlert urine test of 6 to confirm smoking); (5) if using other commercial tobacco products, using a combined total of ≤9 days in the past month with the exception of e-cigs. If using e-cigs, using ≤14 days in the past month to maintain the focus on people who smoke cigarettes; (6) not currently taking medications known to impact nicotine metabolism; and (7) no organ failure or history of liver disease, cancer, or transplant as nicotine is primarily metabolized in the liver.
Study Procedures
Interested individuals were screened for initial eligibility via telephone or in-person at the Tribal clinic. If eligible based on the initial screen, potential participants were invited to attend a visit at the clinic to complete informed consent and to assess additional eligibility criteria, and if consented and were eligible were enrolled into the study. Participants completed questionnaires on smoking history and dependence, attitudes towards NMR-informed care for smoking cessation, and personalized medicine in general and provided urine and saliva samples via standardized methods. Samples were stored at the Tribe’s clinic in a locked freezer until shipped to the University of Minnesota for analysis of the below measures.
Measures
NMR
Prior studies examining NMR, have largely used the ratio of 3-HCOT to COT in whole blood, plasma, or saliva which are all strongly correlated.13 A saliva sample for measurement of NMR was recommended over a blood sample by the Tribe given the ease of collecting, storing, and processing saliva over blood and for maximizing participant recruitment.
Smoking Exposure and Cigarette Dependence
Prior to the assessment of self-report smoking measures, participants were told to think about their use of commercial tobacco and not a traditional use of tobacco during ceremony and prayer which is common among the Tribe. Cigarettes per day (CPD) were assessed by the question “On the average, about how many cigarettes do you now smoke each day?.” Urinary TNE (molar sum of total nicotine, total nicotine N-oxide, total COT, and total 3-HCOT) was quantified using methods previously described33 and expressed per mg creatinine to account for differences in urine dilution between subjects. We utilized two common measures of cigarette dependence: The Fagerström Test for Cigarette Dependence (FTCD) and Heaviness of Smoking Index (HSI).34–36 The FTCD includes six measures that are scored 0 to 10 with higher scores indicative of greater cigarette dependence. Two of the FTCD measures, cigarettes per day (responses: 10 or less = 0, 11–20 = 1, 21–30 = 2, >30 = 3) and time to first cigarette of the day (TTFC; 0 to 5 minutes = 3, 6 to 30 minutes = 2, 31 to 60 minutes = 1, >60 minutes = 0), comprise the Heaviness of Smoking Index (HSI) and are scored 0–6 with higher scores indicative of greater cigarette dependence.
Preference for Smoking Cessation Pharmacotherapy
During a structured interview, participants were asked if they ever plan to quit smoking. If yes, the following statement was read to participants: Please indicate your preference for the following treatments to help quit smoking: (1) nicotine replacement therapy such as the nicotine patch, nicotine gum, or nicotine lozenge, or (2) medicine in the form of tablets such as Chantix or Zyban. Preference for pharmacotherapy was reported by participants without any knowledge of their NMR values.
Perceptions of NMR-Informed Pharmacotherapy Selection
A questionnaire on perceptions toward NMR-informed care and more generally, personalized medicine, reported by Wells et al.5 was utilized. Three of the six measures underwent minor word changes for increased comprehension based on feedback from the Tribe’s representatives. See Supplementary Table 1 for questions used in the present study and their modifications from the Wells et al. version. All items were rated on a 5-point Likert scale ranging from strongly disagree to strongly agree.
Statistical Analysis
Means and standard deviations (STD) or proportions were calculated to describe characteristics of the study sample. As in prior studies,22 only participants with saliva COT > 10 (n = 52) were included in analyses involving NMR. Also similar to prior studies, NMR was not normally distributed and therefore either log-transformed and modeled as a continuous variable or as a bivariate variable (saliva NMR <0.31 vs. ≥0.31) which is based on a 0.31 cutpoint to identify slow versus fast nicotine metabolizers in prior studies.5,7,37 Relationships between NMR, smoking exposure measures, dependence, and preference for pharmacotherapy were examined via chi-square tests for association or linear regression models. Responses to measures on attitudes towards personalized medicine were collapsed to strongly agree or agree, neutral, and disagree or strongly disagree and summarized via proportions. P-values of < .05 were considered statistically significant. Analyses were performed using SAS 9.2.
Results
Characteristics of the Sample
A total of 54 participants completed the study. Table 1 shows the characteristics of the study sample. The sample was 50% male with mean age of 51.0 years. The vast-majority (92.6%) of participants self-identified as being AI race only and 7.4% of the sample self-identified as being AI and white race. Regarding educational attainment, 51.8% of participants reported being a high school graduate, receiving a GED, or having less education. The average CPD was 14.1 and most participants (83.3%) smoked nonmenthol cigarettes. The mean saliva NMR was 0.34 (STD: 0.22) with a median of 0.28 and a range of 0.07–1.29. Average scores on the FTCD (4.3; STD: 2.3) and HSI (2.9; STD: 1.5) were indicative of moderate cigarette dependence. Mean level of interest in quitting was 5.2 out of 10 and slightly more than three-fourths (77.8%) of the sample indicated that they plan to quit smoking cigarettes. Regarding traditional tobacco, 96% of participants reported using traditional tobacco, and use without burning or smoking was the method of use among the vast majority of participants.
Table 1.
Characteristics of the Sample (N = 54)
| Mean (std) or N (%) | |
|---|---|
| Age | 51.0 years (10.7) |
| Male gender | 27 (50.0%) |
| Hispanic ethnicity | 3 (5.6%) |
| Race | |
| American Indian or Alaska Native only | 50 (92.6%) |
| American Indian or Alaska Native and White | 4 (7.4%) |
| Educational attainment | |
| ≤ High school graduate or GED | 28 (51.8%) |
| Some college or Associates degree | 22 (40.7%) |
| ≥ Bachelors degree | 4 (7.4%) |
| Cigarettes per day | 14.1 (7.1) |
| Expired CO (ppm) | 20.1 (11.2) |
| Usually smokes menthol cigarettes | 9 (16.7%) |
| Age first smoked a cigarette | 11.9 y (3.3) |
| Age first smoked fairly regularly | 16.8 y (4.7) |
| Age started smoking daily | 18.5 y (5.3) |
| Duration smoking | 34.2 y (10.9) |
| Fagerström Test for Cigarette Dependence (FTCD) | 4.3 (2.3) |
| Heaviness of smoking index (HSI) | 2.9 (1.5) |
| Urinary TNE (nmol/mg creatinine) | 74.9 (40.4) |
| Saliva NMRa | 0.34 (0.22) |
| Saliva NMR <0.31(“slow nicotine metabolizers”) | 28 (54%) |
| Cessation interest(1 not at all interested to 10 extremely interested) | 5.2 (3.4) |
| Plan to ever quit smoking | 42 (77.8%) |
| Cessation timeframe among those planning to ever quit | |
| Next 6 mo | 15 (35.7%) |
| 6 mo+ | 27 (64.3%) |
| Past month binge drinking | 10 (18.5%) |
| Past 14 d cannabis use | 9 (16.7%) |
Among participants with saliva COT > 10 ng/mL (N = 52)
Relationships Between NMR, Smoking Exposure, and Cigarette Dependence
As shown in Table 2, smoking exposure measures CPD, expired CO, and TNE as well as dependence were consistently higher with higher values of NMR. However of note, this reached significance for both FTCD and HSI, both as correlations with log-transformed NMR (FTCD r = 0.29, p = .0383; HSI r = 0.28, p = .0426) and with a 0.31 NMR split (FTCD 5.17 vs. 3.61, p = .0139; HSI 2.43 versus 3.42, p = .0156). Defining NMR-based on a median split produced similar results. Regardless of how NMR was modeled, NMR was not significantly associated with age, sex, menthol use, duration of smoking, past month binge drinking, and past 14 days of cannabis use.
Table 2.
Saliva NMR’s Relationship With Smoking Exposure and Dependence Measures
| NMR continuous (log-transformed) n = 52 r (P-value) |
NMR bivariate | |||
|---|---|---|---|---|
| Slow NMR (<0.31) n = 28 Mean (STD) |
Fast NMR (≥0.31) n = 24 Mean (STD) |
P-value | ||
| Cigarettes per day | 0.21 (0.1436) | 13.07 (5.52) | 15.45 (8.69) | 0.2361 |
| Expired CO (ppm) | 0.26 (0.0658) | 17.68 (10.02) | 22.87 (11.97) | 0.0945 |
| Urine TNE (nmol/mg creatinine) | 0.24 (0.2906) | 70.80 (38.13) | 79.72 (44.67) | 0.4408 |
| Fagerström Test for Cigarette Dependence (FTCD; 0 to 10) | 0.29 (0.0383) | 3.61 (1.93) | 5.17 (2.48) | 0.0139 |
| Heaviness of smoking index (HIS; 0 to 6) | 0.28 (0.0426) | 2.43 (1.34) | 3.42 (1.50) | 0.0156 |
| Bolded p-value indicates statistically significant | ||||
Because of observing a relationship between NMR and FTCD, relationships between NMR and each of the FTCD factors were explored (Supplementary Table 2). Time to first cigarette based on the ordinal measure (0 to 3 where 3 indicates shorter time and thus greater dependence) was higher among fast versus slow nicotine metabolizers (2.46 vs. 1.75 respectively; p = .0048). Similar findings were observed when time to first cigarette was modeled as a categorical variable (p = .0087). Fast versus slow metabolizers were also significantly more likely to report “yes” to the following FTCD measure “Do you smoke when you are so ill that you are in bed most of the day?” (25.0% versus 3.6% respectively; p = 0.0396).
Agreement between participant preferences for pharmacotherapy and NMR-informed pharmacotherapy recommendations
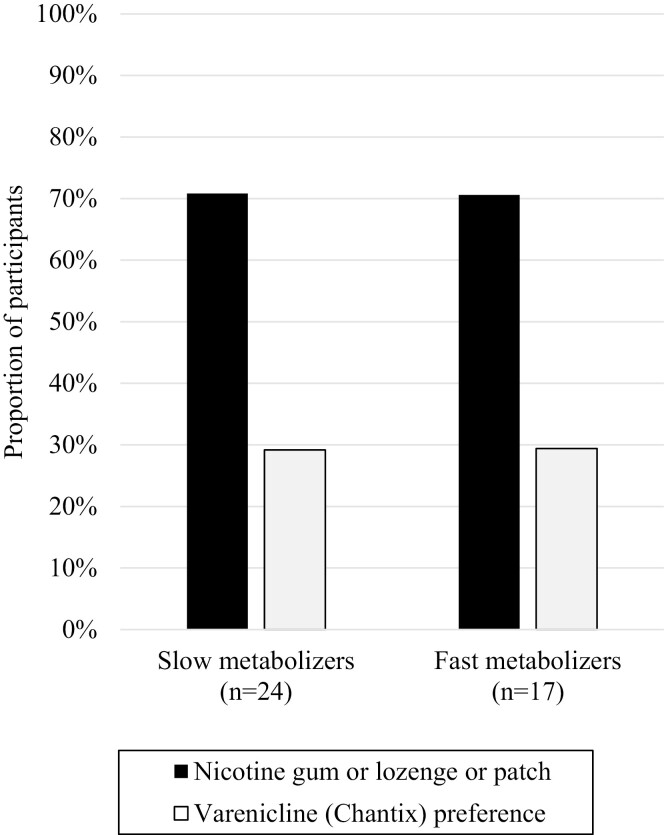
Slightly more than three-fourths of the sample (n = 41; 78%) reported they plan to quit smoking and only these participants were asked about their preference for pharmacotherapy. Most participants, reported preference for NRT (n = 29; 71%) over varenicline (n = 12, 29%). As shown in Figure 1, 71% of slow metabolizers selected the pharmacotherapy that matched their NMR-informed recommendation versus only 29% of fast metabolizers. Thus, 54% of all participants’ preferences aligned with their NMR-informed recommendation
Figure 1.
Agreement between participant preferences for pharmacotherapy for smoking cessation and NMR-informed recommendations.
Perceptions of personalized medicine and NMR-informed pharmacotherapy selection
As shown in Table 3, slightly more than one-half (53.7%) of participants reported approval (i.e. response of strongly agree or agree) for personalized medicine. When asked about approval of NMR-informed pharmacotherapy selection, slightly less than two-thirds of participants (62.0%) approved. Likewise, 58% of participants approved of their doctors knowing how their body breaks down nicotine. Approximately one-fourth (26.0%) of the sample reported worrying about the consequences of knowing what their nicotine metabolism status means in terms of quitting and 18.0% of these participants expressed fear of knowing about their nicotine metabolism.
Table 3.
Perceptions of NMR-Informed Care for Smoking Cessation
| Survey question | Response | Present study | Wells et al. (N = 81) |
|---|---|---|---|
| I think that the development of tests to help match patients with the drugs that might work best for them is a positive medical progressa (n = 54) | Strongly Agree or Agree | 53.7% | 91.4% |
| Neutral | 27.8% | 3.7%b | |
| Disagree or Strongly Disagree | 18.2% | ||
| I approve of using tests to determine how my body breaks down nicotine to help choose medications to help me quit smokinga(n = 50) | Strongly Agree or Agree | 62.0% | 97.5% |
| Neutral | 28.0% | 2.5%b | |
| Disagree or Strongly Disagree | 10.0% | ||
| I would want to know if the way my body breaks down nicotine makes it harder for me to stop smoking compared to some other people(n = 50) | Strongly Agree or Agree | 72.0% | 92.6% |
| Neutral | 18.0% | 6.2%b | |
| Disagree or Strongly Disagree | 10.0% | ||
| I do not want my doctor to know how my body breaks down nicotine (n = 50) | Strongly Agree or Agree | 10.0% | NA |
| Neutral | 32.0% | ||
| Disagree or Strongly Disagree | 58.0% | ||
| If a test on my own biological sample (urine, saliva, or blood) told me that I might have a more difficult time quitting smoking than some other people I wouldn’t even bother trying to quita (n = 50) | Strongly Agree or Agree | 22.0% | 8.6% |
| Neutral | 28.0% | 87.7%b | |
| Disagree or Strongly Disagree | 58.0% | ||
| I worry about the consequences of knowing how my body breaks down nicotine for the chances of quitting smoking.(n = 50) | Strongly Agree or Agree | 26.0% | 14.8% |
| Neutral | 24.0% | 84.0%b | |
| Disagree or Strongly Disagree | 50.0% | ||
| The idea of knowing how my body breaks down nicotine frightens me.(n = 50) | Strongly Agree or Agree | 18.0% | 11.1% |
| Neutral | 26.0% | 86.4%b | |
| Disagree or Strongly Disagree | 56.0% |
Question includes minor modifications when compared to the Wells et al version of the survey question;
Wells et al responses were dichotomized and included a don’t know or decline response that was not provided in the present study (i.e. strongly agree/agree vs. neutral/disagree/strongly disagree versus do not know/decline)
Discussion
The present study examined three questions within the context of generalizing NMR-informed care to a Tribe with a high prevalence of commercial smoking. First, we explored relationships between NMR, dependence, and smoking exposure and observed significant positive correlations between NMR and dependence scales FTCD and HSI and non-significant, but directly consistent, relationships between NMR, and the smoking exposure measures cigarettes per day (CPD) and TNE. Second, we assessed agreement between self-preference for pharmacotherapy and NMR-informed recommendations and observed agreement among slightly more than one-half (54%) of the sample. Finally, while not formally tested, we observed lower acceptability of NMR-informed care in this sample versus prior studies among participants who identified as either white or Black or African American race.
Studies predominately among persons who identify as white race suggests that faster nicotine metabolizers clear nicotine more quickly and therefore, compensate by smoking more intensely.12,20,38 In the present study, we did not observe significant relationships between NMR and TNE (r = 0.24; p = .2906) or CPD (r = 0.21; p = 0.1436); however, these relationships trended in the expected direction and the lack of significance may reflect low statistical power. Our findings on NMR and TNE are directionally consistent with prior data from a large sample of white adults who smoke (r = 0.33; p < .0001; N = 1422). An additional interpretation of the data is that perhaps nicotine metabolism plays a smaller role in influencing smoking behavior in this sample compared to white populations; which has been speculated to be the case for other AI tribes and African American persons.17,23,24,39 Other risk factors for smoking among AI persons, such as historical trauma and stress may attenuate the impact of nicotine metabolism on smoking behavior in AI persons. For these reasons, NMR-informed care may need to be augmented with a more holistic, culturally-tailored approach to maximize quit rates in AI persons.
In the present study, cigarette dependence, measured by FTCD and its shortened version HSI, was significantly higher among fast versus slow nicotine metabolizers. In prior studies, the relationship between NMR or CYP2A6 nicotine metabolism gene and FTCD or HSI has been mixed.8–10,14,40–43 When significant,14,40 the relationships seem to reflect differences in CPD and thus heaviness of smoking versus broader metrics of dependence such as time to first cigarette. In the present study, time to first cigarette was significantly shorter, indicating greater dependence, in faster versus slower metabolizers (p = .0048). Interestingly, in prior clinical trials testing NMR’s utility in predicting treatment response NMR was not associated with FTCD and yet NMR-informed pharmacotherapy selection increased quit rates.8-10 Thus, NMR’s association with dependence in the present raises the question of how NMR-informed care would impact quitting outcomes among AI persons. Another potential implication of this work is whether time to first cigarette instead of NMR could be used to guide pharmacotherapy selection, especially if there are concerns regarding using a biological sample for NMR-informed care and/or feasibility issues. However, based on the sample herein, using time to first cigarette (≤ 5 minutes vs. > 5 minutes) would result in approximately 18% of slow metabolizers and 38% of fast metabolizers being mismatched based on their NMR-informed pharmacotherapy. Future comparative effectiveness research that compares the benefits and tradeoffs of using NMR versus time to first cigarette to inform pharmacotherapy selection may be considered.
Similar to a prior study5 and without any knowledge of their NMR-informed treatment recommendations, more than one-half of participants in the present study selected the pharmacotherapy that matched recommendations based on NMR-informed care and therefore suggests room for improvement. While not formally tested, the acceptability of NMR-informed care among our sample seems lower than reported in prior studies. For example, 62% of participants in the present study approved NMR-informed care versus 97.5% of participants in a prior study who identified as either white or black race.5 Thus, future qualitative work is recommended with AI persons who smoke, as well as their health care providers, to identify solutions, such as patient education and explicit policies on how biological samples are stored and shared, to mitigate concerns with this precision medicine approach.
There are limitations to the present study. The sample size in the present study is relatively small which potentially resulted in the lack of a relationship between NMR and smoking exposure measures. Second, and related to the first limitation, is that this was a convenience sample at one Tribe and may not be representative of all AI adults who smoke within the Tribe or to other tribes. While not always the case, many studies and the resulting scientific papers with AI communities involve smaller sample sizes and are limited to a single tribe. As discussed previously by others,25 limitations of generalizability are likely balanced by potential public health impact given the lower representation of AI persons in this area of study. Third, the acceptability of NMR-informed care among this sample may be an over-estimation given that participants of this study consented to provide biological samples and therefore were likely less concerned with providing a biological sample which is a requirement for NMR-informed care. Fourth, it is likely that previous use of smoking cessation pharmacotherapy influenced preference reported in the present study. Despite these limitations, this study identified a unique relationship between NMR and cigarette dependence and lays important groundwork for future exploration of the effectiveness and feasibility of NMR-informed care in the Tribe.
Supplementary Material
A Contributorship Form detailing each author’s specific involvement with this content, as well as any supplementary data, are available online at https://academic.oup.com/ntr.
Contributor Information
Dana Mowls Carroll, Division of Environmental Health Sciences, School of Public Health, University of Minnesota, Minneapolis, MN, USA.
Sharon Murphy, Department of Biochemistry, Molecular Biology, and Biophysics, University of Minnesota, Minneapolis, MN, USA.
Ellen Meier, Department of Psychology, University of Wisconsin Stevens Point, Stevens Point, WI, USA.
Kristine Rhodes, American Indian Cancer Foundation and Asemaake, LLC, Minneapolis, MN, USA.
Casey Dorr, Department of Nephrology, Hennepin Healthcare Research Institute; Department of Medicine, University of Minnesota, Minneapolis, MN, USA.
Greg Braaten, Minnesota Cancer Clinical Trials Network, Minneapolis, MN, USA.
Pamala A Jacobson, Department of Experimental and Clinical Pharmacology, University of Minnesota, Minneapolis, MN, USA.
Linda Frizzell, Division of Environmental Health Sciences, School of Public Health, University of Minnesota, Minneapolis, MN, USA.
Rachel F Tyndale, Department of Pharmacology & Toxicology, and Psychiatry, University of Toronto and Centre for Addiction and Mental Health, Toronto, ON, Canada.
Dorothy Hatsukami, Department of Psychiatry and Behavioral Sciences, University of Minnesota, Minneapolis, MN, USA.
Carol Hernandez, Minnesota Cancer Clinical Trials Network, Minneapolis, MN, USA.
Funding
The research was supported by the National Institute On Minority Health And Health Disparities of the NIH under award number K01MD014795 and by the NIH National Research Service Award from the National Institute on Drug Abuse under award number T32 DA007097. Research was also supported by the Forster Family Chair for Cancer Prevention, Minnesota Cancer Clinical Trials Network, Minnesota’s Discovery, Research and InnoVation Economy, and University of Minnesota Grand Challenge Award for Advancing Health for Tailored Solutions through Precision Medicine. It was also supported in part through the Canada Research Chairs program (RFT CRC in Pharmacogenomics) and the Center for Addiction and Mental Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or any of the other funding entities.
Declaration Of Interests
RF Tyndale has consulted for Quinn Emanuel and Ethismos Research Inc. All other authors have no conflict to disclose.
Data Availability
Data is owned by the Tribe and has not made publicly available.
References
- 1. Forster J. Cigarette smoking among urban American Indian Adults—Hennepin and Ramsey Counties, Minnesota, 2011. MMWR Morb Mortal Wkly Rep. 2016;65(21):534–537. [DOI] [PubMed] [Google Scholar]
- 2. American Lung Association. State of Lung Cancer. 2020; https://www.lung.org/research/state-of-lung-cancer. Accessed April 20, 2021.
- 3. General S. The health consequences of smoking—50 years of progress: a report of the surgeon general. Paper presented at: US Department of Health and Human Services, 2014. [Google Scholar]
- 4. Carroll DM, Cole A.. Racial/ethnic group comparisons of quit ratios and prevalences of cessation-related factors among adults who smoke with a quit attempt. Am J Drug Alcohol Abuse. 2022;48(1):58–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wells QS, Freiberg MS, Greevy JRA, et al. Nicotine metabolism-informed care for smoking cessation: a pilot precision RCT. Nicotine Tob Res. 2018;20(12):1489–1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lerman C, Jepson C, Wileyto E, et al. Genetic variation in nicotine metabolism predicts the efficacy of extended-duration transdermal nicotine therapy. Clin Pharmacol Therap. 2010;87(5):553–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lerman C, Schnoll RA, Hawk LW, et al. Use of the nicotine metabolite ratio as a genetically informed biomarker of response to nicotine patch or varenicline for smoking cessation: a randomised, double-blind placebo-controlled trial. Lancet Respir Med. 2015;3(2):131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lerman C, Tyndale R, Patterson F, et al. Nicotine metabolite ratio predicts efficacy of transdermal nicotine for smoking cessation. Clin Pharmacol Ther. 2006;79(6):600–608. [DOI] [PubMed] [Google Scholar]
- 9. Patterson F, Schnoll RA, Wileyto EP, et al. Toward personalized therapy for smoking cessation: a randomized placebo-controlled trial of bupropion. Clin Pharmacol Ther. 2008;84(3):320–325. [DOI] [PubMed] [Google Scholar]
- 10. Schnoll RA, Patterson F, Wileyto EP, et al. Nicotine metabolic rate predicts successful smoking cessation with transdermal nicotine: a validation study. Pharmacol Biochem Behav. 2009;92(1):6–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schnoll RA, Wileyto EP, Leone FT, Tyndale RF, Benowitz NL.. High dose transdermal nicotine for fast metabolizers of nicotine: a proof of concept placebo-controlled trial. Nicotine Tob Res. 2013;15(2):348–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dempsey D, Tutka P, Jacob P, et al. Nicotine metabolite ratio as an index of cytochrome P450 2A6 metabolic activity. Clin Pharmacol Ther. 2004;76(1):64–72. [DOI] [PubMed] [Google Scholar]
- 13. St Helen G, Novalen M, Heitjan DF, et al. Reproducibility of the nicotine metabolite ratio in cigarette smokers. Cancer Epidemiol Biomarkers Prev. 2012;21(7):1105–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Carroll DM, Murphy SE, Benowitz NL, et al. Relationships between the nicotine metabolite ratio and a panel of exposure and effect biomarkers: findings from two studies of U.S. Commercial Cigarette Smokers. Cancer Epidemiol Biomarkers Prev. 2020;29(4):871–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Strasser AA, Benowitz NL, Pinto AG, et al. Nicotine metabolite ratio predicts smoking topography and carcinogen biomarker level. Cancer Epidemiol Biomarkers Prev. 2011;20(2):234–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pianezza ML, Sellers EM, Tyndale RF.. Nicotine metabolism defect reduces smoking. Nature. 1998;393(6687):750–750. [DOI] [PubMed] [Google Scholar]
- 17. Ross KC, Gubner NR, Tyndale RF, et al. Racial differences in the relationship between rate of nicotine metabolism and nicotine intake from cigarette smoking. Pharmacol Biochem Behav. 2016;148:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. West O, Hajek P, McRobbie H.. Systematic review of the relationship between the 3-hydroxycotinine/cotinine ratio and cigarette dependence. Psychopharmacology (Berl). 2011;218(2):313–322. [DOI] [PubMed] [Google Scholar]
- 19. Park SL, Tiirikainen MI, Patel YM, et al. Genetic determinants of CYP2A6 activity across racial/ethnic groups with different risks of lung cancer and effect on their smoking intensity. Carcinogenesis. 2016;37(3):269–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Siegel SD, Lerman C, Flitter A, Schnoll RA.. The use of the nicotine metabolite ratio as a biomarker to personalize smoking cessation treatment: current evidence and future directions. Cancer Prev Res (Phila). 2020;13(3):261–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhu AZ, Binnington MJ, Renner CC, et al. Alaska Native smokers and smokeless tobacco users with slower CYP2A6 activity have lower tobacco consumption, lower tobacco-specific nitrosamine exposure and lower tobacco-specific nitrosamine bioactivation. Carcinogenesis. 2013;34(1):93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tanner J-A, Henderson JA, Buchwald D, et al. Variation in CYP2A6 and nicotine metabolism among two American Indian tribal groups differing in smoking patterns and risk for tobacco-related cancer. Pharmacogenet Genomics. 2017;27(5):169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Vogel EA, Benowitz NL, Skan J, Schnellbaecher M, Prochaska JJ.. Correlates of the nicotine metabolite ratio in Alaska Native people who smoke cigarettes. Exp Clin Psychopharmacol. 2021. doi: 10.1037/pha0000461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Carroll DM, Wagener TL, Stephens LD, et al. The relationship between nicotine metabolism and nicotine and carcinogen exposure among American Indian commercial cigarette smokers and electronic nicotine delivery system users. Addict Behav. 2019;92:58–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Walters K, Walls M, Dillard D, Kaur J.. American Indian and Alaska Native research in the health sciences: critical considerations for the review of research applications. Tribal Health Research Office (THRO), National Institutes of Health. 2019. https://usetinc.org/wp-content/uploads/2019/12/Critical-Considerations-for-Reviewing-AIAN-Research.pdf [Google Scholar]
- 26. Senft N, Sanderson M, Selove R, et al. Attitudes toward precision treatment of smoking in the Southern Community Cohort Study. Cancer Epidemiol Biomarkers Prev. 2019;28(8):1345–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Diaz VA, Mainous AG, Gavin JK, Wilson D.. Racial differences in attitudes toward personalized medicine. Public Health Genomics. 2014;17(1):1–6. [DOI] [PubMed] [Google Scholar]
- 28. Glenn BA, Chawla N, Bastani R.. Barriers to genetic testing for breast cancer risk among ethnic minority women: an exploratory study. Ethn Dis. 2012;22(3):267–273. [PubMed] [Google Scholar]
- 29. Hiratsuka VY, Beans JA, Blanchard JW, et al. An Alaska Native community’s views on genetic research, testing, and return of results: results from a public deliberation. PLoS One. 2020;15(3):e0229540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Manson S. Barrow alcohol study: emphasis on its ethical and procedural aspects. Am Indian Alsk Native Ment Health Res. 1989;2(3):5–6. [Google Scholar]
- 31. Sterling RL. Genetic research among the Havasupai: a cautionary tale. Virtual Mentor. 2011;13(2):113–117. [DOI] [PubMed] [Google Scholar]
- 32. Carroll DM, Hernandez C, Braaten G, et al. Recommendations to researchers for aiding in increasing American Indian representation in genetic research and personalized medicine. Per Med. 2021;18(1):67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Murphy SE, Villalta P, Ho SW, von Weymarn LB.. Analysis of [3’,3’-d(2)]-nicotine and [3’,3’-d(2)]-cotinine by capillary liquid chromatography-electrospray tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;857(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Heatherton TF, Kozlowski LT, Frecker RC, Rickert W, Robinson J.. Measuring the heaviness of smoking: using self-reported time to the first cigarette of the day and number of cigarettes smoked per day. Br J Addict. 1989;84(7):791–799. [DOI] [PubMed] [Google Scholar]
- 35. Fagerström K. Determinants of tobacco use and renaming the FTND to the Fagerströöm Test for Cigarette Dependence. Nicotine Tob Res. 2011;14(1):75–78. [DOI] [PubMed] [Google Scholar]
- 36. Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO.. The fagerstrom test for nicotine dependence: a revision of the fagerstrom tolerance questionnaire. Br J Addict. 1991;86(9):1119–1127. [DOI] [PubMed] [Google Scholar]
- 37. Scoville EA, Tindle HA, Wells QS, et al. Precision nicotine metabolism-informed care for smoking cessation in Crohn’s disease: A pilot study. PLoS One. 2020;15(3):e0230656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Allenby CE, Boylan KA, Lerman C, Falcone M.. Precision medicine for tobacco dependence: development and validation of the nicotine metabolite ratio. J Neuroimmune Pharmacol. 2016;11(3):471–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ho MK, Mwenifumbo JC, Al Koudsi N, et al. Association of nicotine metabolite ratio and CYP2A6 genotype with smoking cessation treatment in African-American light smokers. Clin Pharmacol Ther. 2009;85(6):635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schnoll RA, George TP, Hawk L, et al. The relationship between the nicotine metabolite ratio and three self-report measures of nicotine dependence across sex and race. Psychopharmacology (Berl). 2014;231(12):2515–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Johnstone E, Benowitz N, Cargill A, et al. Determinants of the rate of nicotine metabolism and effects on smoking behavior. Clin Pharmacol Ther. 2006;80(4):319–330. [DOI] [PubMed] [Google Scholar]
- 42. Benowitz NL, Pomerleau OF, Pomerleau CS, Jacob P.. Nicotine metabolite ratio as a predictor of cigarette consumption. Nicotine Tob Res. 2003;5(5):621–624. [DOI] [PubMed] [Google Scholar]
- 43. Wassenaar CA, Dong Q, Wei Q, et al. Relationship between CYP2A6 and CHRNA5-CHRNA3-CHRNB4 variation and smoking behaviors and lung cancer risk. J Natl Cancer Inst. 2011;103(17):1342–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is owned by the Tribe and has not made publicly available.