Abstract
An effector strain has been constructed for use in the replacement therapy of dental caries. Recombinant DNA methods were used to make the Streptococcus mutans supercolonizing strain, JH1140, lactate dehydrogenase deficient by deleting virtually all of the ldh open reading frame (ORF). To compensate for the resulting metabolic imbalance, a supplemental alcohol dehydrogenase activity was introduced by substituting the adhB ORF from Zymomonas mobilis in place of the deleted ldh ORF. The resulting clone, BCS3-L1, was found to produce no detectable lactic acid during growth on a variety of carbon sources, and it produced significantly less total acid due to its increased production of ethanol and acetoin. BCS3-L1 was significantly less cariogenic than JH1140 in both gnotobiotic- and conventional-rodent models. It colonized the teeth of conventional rats as well as JH1140 in both aggressive-displacement and preemptive-colonization models. No gross or microscopic abnormalities of major organs were associated with oral colonization of rats with BCS3-L1 for 6 months. Acid-producing revertants of BCS3-L1 were not observed in samples taken from infected animals (reversion frequency, <10−3) or by screening cultures grown in vitro, where no revertants were observed among 105 colonies examined on pH indicator medium. The reduced pathogenic potential of BCS3-L1, its strong colonization potential, and its genetic stability suggest that this strain is well suited to serve as an effector strain in the replacement therapy of dental caries in humans.
A successful effector strain for replacement therapy of a bacterial disease must have the following basic properties. It must not cause disease itself or otherwise predispose the host to other disease states by disrupting the ecosystem in which it resides. It must persistently colonize the host tissue at risk and thereby prevent colonization or outgrowth of the pathogen to levels necessary for it to exert its pathogenic potential. In situations where the pathogen is itself a member of the indigenous flora, an effector strain should aggressively displace the resident pathogen, enabling replacement therapy of the population at large rather than limiting its use to infants who have not yet acquired the pathogen as part of their indigenous flora. Finally, an effector strain should possess a high degree of genetic stability.
Past studies have demonstrated the potential usefulness of replacement therapy for the prevention of dental caries. Since acid production by mutans streptococci has long been known to be integral to the pathogenic process, effector strains with low acid-producing capabilities have been sought (1, 8, 29). Lactate dehydrogenase (LDH)-deficient mutants of Streptococcus rattus were shown to have little or no cariogenic potential in vitro and in various rodent models (18).
The ability of an effector strain to persistently and preemptively colonize the human oral cavity was initially thought to be a complex phenomenon dependent on a large number of phenotypic properties. Numerous studies (19, 21, 27, 28; J. M. Tanzer, B. Krasse, and M. Svanberg, J. Dent. Res. 61:334) documented the difficulty of persistently introducing mutans streptococci into the mouths of humans, particularly if they already harbored an indigenous strain of this organism. This difficulty was surmounted by the discovery that a single phenotypic property could provide an adequate selective advantage to enable mutans streptococci to persistently and preemptively colonize the human oral cavity. A naturally occurring strain (JH1000) of Streptococcus mutans was isolated that produces a lantibiotic called mutacin 1140 capable of killing virtually all other strains of mutans streptococci against which it was tested (9). Mutants that produced no detectable mutacin 1140 or that produced approximately threefold-elevated amounts were isolated. The mutants were used to correlate mutacin production to preemptive colonization and aggressive displacement in a rat model. A correlation was also made between mutacin production and the ability of this strain to persistently colonize the oral cavities of human subjects and aggressively displace their indigenous mutans streptococci (10, 12).
Based on these results, we predicted that an S. mutans strain possessing the combination of LDH deficiency and mutacin 1140 production would satisfy the prerequisites for an effector strain in replacement therapy of dental caries. However, both genetic and physiologic methods (2) clearly established the fact that LDH deficiency is lethal in the S. mutans strains tested, including JH1140, probably due to the accumulation of toxic intermediates or to NAD-NADH imbalance. We subsequently used a temperature-sensitive ldh mutation to demonstrate that this lethal effect could be overcome by augmenting the indigenous S. mutans alcohol dehydrogenase (ADH) activity by expression of a cloned Zymomonas mobilis adhB gene (15).
In the present report, we describe the construction of a recombinant effector strain called BCS3-L1 that combines LDH deficiency with mutacin 1140 production. The in vitro growth and fermentation properties of this strain are presented. We also present the results of animal studies designed to test its cariogenic and colonization properties, its genetic stability, and its long-term effect on the general health of the host.
MATERIALS AND METHODS
Strains, cultivation conditions, and plasmids.
S. mutans JH1140 is a spontaneous mutant of S. mutans JH1001 (9) that produces two- to threefold-increased amounts of mutacin 1140. S. mutans Ingbritt (resistant to 1 mg of streptomycin sulfate/ml) and S. rattus BHT-2 (resistant to 1 mg of streptomycin sulfate/ml) have been described previously (9). Escherichia coli DH5α was obtained from Bethesda Research Laboratories, Gaithersburg, Md. Streptococcus strains were grown in brain heart infusion (BHI) broth, Todd-Hewitt broth (THB), or minimal salts medium (25) supplemented with 0.5% yeast extract and carbohydrates (50 mM), or on plates of the same media containing 1.5% agar. Glucose tetrazolium plates were prepared by the method of Lederberg (22). Mitis-Salivarius agar (MS) with bacitracin (MSB) plates were prepared as described by Gold et al. (6). E. coli strains were grown with aeration in Luria-Bertani (LB) broth or on plates of the same medium containing 1.5% agar. Ampicillin (50 μg/ml), tetracycline (15 μg/ml), and streptomycin sulfate (100 μg/ml) were added to the plates as required. Plasmid p10-5, containing the ldh gene from JH1001, has been described previously (13). Plasmid pLOI286, containing the cloned Z. mobilis ADH II (adhB) gene (3), was provided by L. O. Ingram. pVA981, containing the cloned tetracycline resistance gene from S. mutans (30), was provided by F. L. Macrina. Chemicals and antibiotics were from Sigma Chemical Co. (St. Louis, Mo.).
Genetic methods.
Restriction enzymes and DNA-modifying enzymes were obtained from New England BioLabs, Inc. (Beverly, Mass.), and were used according to the manufacturer's directions. PCR primers were supplied by National BioSciences Inc. (Plymouth, Minn.). DNA sequencing was performed by the University of Florida's Interdisciplinary Center for Biotechnology Research. Southern hybridizations were performed with the enhanced chemiluminescence gene detection system and Hyperfilm-ECL from Amersham International PLC (Amersham, England) according to the manufacturer's directions. Chromosomal DNA was isolated from S. mutans by a modification of the method of Marmur as previously described (7). DNA fragments from PCR amplification and restriction enzyme digestions were excised from agarose gels (0.7%) and purified by the Prep-A-Gene protocol (Bio-Rad Laboratories, Inc., Hercules, Calif.). Transformation of S. mutans was performed as described by Perry and Kuramitsu (26). Other DNA manipulations were performed as described by Maniatis et al. (23).
Enzyme assays.
Cells for enzyme assays were grown in BHI broth supplemented with 0.5% (wt/vol) glucose. ADH and LDH activities were measured in cell extracts as previously described (13, 15). The protein concentrations of the cell extracts were determined by the bicinchoninic acid method. One unit of activity was defined as 1 μmol of NADH metabolized per min per mg of protein. Specific activities are expressed as the mean of four trials from each of two independent cultures.
Growth and fermentation end product analysis.
Overnight cultures of strains grown in minimal medium supplemented with 0.5% yeast extract and 50 mM glucose were subcultured 1:50 in fresh medium. Optical densities at 550 nm were recorded during incubation, aerobic standing at 37°C, with a Spectronic 20D spectrophotometer (Milton Roy Co.). Generation times were calculated by linear regression analysis of log (optical density) plotted as a function of time. After 72 h of incubation, the cells were removed by centrifugation at 14,000 × g for 15 min. The pH values of the cell-free liquors were determined. Pyruvate, lactate, formate, acetate, ethanol, and acetoin were determined by high-performance liquid chromatography (LKB, Bromma, Sweden) with an Aminex HPX 87H organic column (Bio-Rad) and a 2142 refractive index detector coupled to an SP4270 integrator. The data presented are the averages of three independent experiments.
Detection of mutacin 1140 production.
Mutacin 1140 production was assayed by a modification of the overlay technique of Fredericq (5). Single colonies of the strains to be tested were stab inoculated into THB plates and incubated anaerobically at 37°C for 24 h. Three milliliters of molten (42°C) BHI top agar containing streptomycin sulfate and 105 cells from an overnight THB culture of S. rattus BHT-2 was spread evenly over the surface of the plate. After an additional 24 h of incubation, the diameters of clear zones surrounding the test strain stabs were measured.
In vitro plaque accumulation assay.
Plaque accumulation was measured as previously described (8). Sterile, preweighed microscope slides were immersed in THB supplemented with 5% (wt/vol) sucrose and inoculated 1:100 from overnight cultures grown in THB. After 24 h of incubation at 37°C, the slides were removed, gently washed in distilled water to remove any tenuously attached cells, and dried at 42°C for 24 h. The change in weight of the slide was taken to be the amount of plaque accumulation. The results are the averages of three independent experiments.
Animal cariogenicity studies.
The cariogenic potentials of JH1140 and BCS3-L1 were measured with a gnotobiotic Fischer rat model (24). Two groups of 10 germfree animals were housed separately and fed diet 305 (5% sucrose; Harlan-Techlad, Madison, Wis.) and fluoride-free water ad libitum. At 21 days of age, each animal was inoculated with a cotton-tipped applicator saturated with an overnight culture of the appropriate bacterial strain. The applicator was placed in the oral cavity and moved side to side for 15 s. This procedure was repeated twice at 24-h intervals. Three weeks postinfection, four animals from each group were sacrificed, and their left mandibular teeth were removed with sterile rongeurs. The teeth were immediately placed in 3 ml of phosphate-buffered saline and adherent plaque was dispersed by sonication on ice for 10 s. Serially diluted samples were spread on duplicate MS plates, and colonies which arose following 2 days of incubation were counted. Seven weeks postinfection, the remaining animals were sacrificed. The left maxillary and mandibular molars were extracted and treated as described above to enumerate adherent bacteria. The right maxillary and mandibular molars were stained with murexide and scored for carious lesions by the methods of Keyes (20). Differences between group mean bacterial counts and caries scores were tested for significance by an independent t test.
The cariogenic potentials of strains were also measured with conventional, pathogen-free Sprague-Dawley rats (Harlan-Techlad). The animals were housed in metal wire cages without bedding and maintained on MIT 200 (67% sucrose) and fluoride-free water ad libitum. Oral-swab samples taken when the animals were 18 days old were streaked on MSB medium to detect the presence of mutans streptococci in their normal flora. At 21 days of age, groups of 20 animals were orally infected with a micropipettor with 0.1 ml of an overnight culture of JH1140 or BCS3-L1. Twenty animals in a third group were sham treated with sterile BHI. The infection regimen was repeated twice at 24-h intervals. Three weeks postinfection, the molar teeth of two male and two female animals from each group were analyzed as described above to determine the level of colonization by the test microorganism. Ten weeks postinfection, the remaining animals were sacrificed. The left maxillary and mandibular molars were extracted and treated as described above to enumerate adherent bacteria. The right maxillary and mandibular molars were stained with murexide and scored for carious lesions by the methods of Keyes (20). Differences between group mean bacterial counts and caries scores were tested for significance by an independent t test.
Animal colonization studies.
The ability of BCS3-L1 to preemptively colonize the teeth of conventional, S. mutans-free Sprague-Dawley rats was compared to that of its parent by previously reported methods (9). Three groups of 12 animals were housed in separate wire cages and maintained on Diet 305. At 21 days of age, two groups were orally infected with 0.1 ml of an overnight BHI culture of JH1140 or BCS3-L1. The third group was sham treated with sterile BHI. The infection regimen was repeated 24 h later. Three weeks postinfection, subgroups of three animals were challenged with serial 10-fold dilutions of an overnight BHI culture of strain Ingbritt. Three weeks postchallenge, the animals were sacrificed and their molars were extracted with sterile rongeurs. The teeth were suspended in 2 ml of sterile BHI and sonicated for 10 s on ice, and serial dilutions were spread on MSB medium with or without streptomycin to quantify Ingbritt and the test strains. As defined for this study, superinfection occurred when Ingbritt constituted 1% or more of the S. mutans colonies recovered.
The ability of BCS3-L1 to aggressively displace an indigenous S. mutans strain from the teeth of conventional, S. mutans-free Sprague-Dawley rats was compared to that of its parent, JH1140, by a previously published method (9). Three groups of 12 animals were housed in separate wire cages and maintained on Diet 305. At 21 days of age, all of the rats were infected by pipetting 0.1 ml of an overnight BHI broth culture of strain Ingbritt into their oral cavities. The infection regimen was repeated 24 h later. Three weeks postinfection, subgroups of three animals were challenged once orally by pipetting 0.1 ml of a 10-fold serial dilution of overnight BHI broth cultures of JH1140 or BCS3-L1. Animals in the third group were sham treated with sterile BHI. Three weeks postchallenge, the animals were sacrificed and their molars were extracted with sterile rongeurs. Ingbritt and the challenge strains were quantified by spreading samples on medium with and without streptomycin as described above. As defined for this study, superinfection occurred when the challenge strain constituted 1% or more of the S. mutans colonies recovered.
Long-term animal toxicity and safety study.
Three groups of three conventional, S. mutans-free rats were housed in separate wire cages and maintained on Diet 305 and fluoride-containing (1 ppm) water ad libitum. At 21 days of age, two groups were orally infected with 0.1 ml of an overnight BHI culture of JH1140 or BCS3-L1. The third group was sham treated with sterile BHI. The infection regimen was repeated 24 h later. Three weeks postinfection, oral-swab samples from each animal were streaked on MSB medium to verify colonization by the infecting strains. Six months postinfection, the left maxillary and mandibular molars were collected and analyzed for the infecting strains as described above. Necropsy was performed, and organs were fixed in 10% buffered formalin for histologic preparations, which were performed by American Histology Laboratories (Gaithersburg, Md.). Histopathologic analyses were performed by Paul Hildebrandt, Pathco, Inc. (Ijamsville, Md.).
RESULTS
Effector strain construction.
S. mutans JH1140 was chosen as the starting strain for effector strain construction because it is a spontaneous variant of a clinical isolate which produces two- to three-fold-elevated levels of a lantibiotic bacteriocin called mutacin 1140. Production of this lantibiotic was shown to inhibit the growth of essentially all other mutans streptococci strains (9), and it promoted colonization in both experimental animals and human subjects (12).
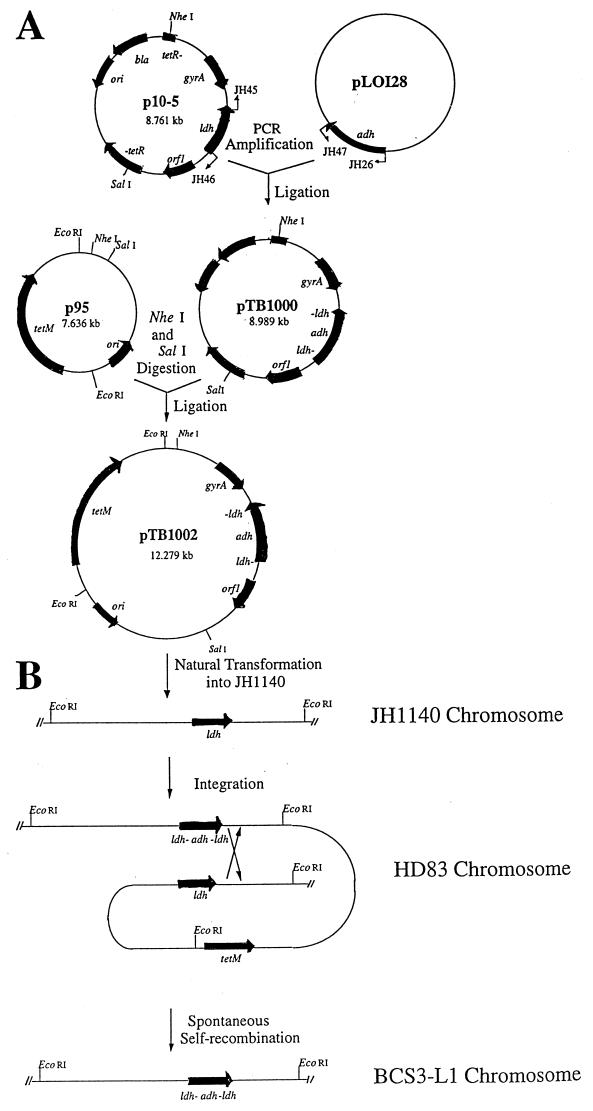
A defect in the lactic acid synthesis pathway due to a defect in the gene encoding LDH was introduced into JH1140 by recombinant DNA techniques. Because LDH deficiency was found to be a lethal mutation in S. mutans (15), an auxiliary ADH activity from Z. mobilis was simultaneously introduced to circumvent this problem. To accomplish this, essentially the entire cloned S. mutans ldh open reading frame (ORF) in the pBR322-based plasmid, p10-5, was deleted by circle PCR mutagenesis with primers JH45 and JH46 (Fig. 1A). Only the first 28 bases, including the translation (ATG) start codon and the last 9 bases prior to the translation stop codon (TAA), were retained. PCR with primers JH26 and JH47 was used to amplify the entire ORF of the Z. mobilis adhB gene present in pLOI128 except for its translation start codon (ATG) plus one additional (G) base. Following agarose gel purification, the p10-5 fragment and the adh fragment were ligated and transformed into E. coli DH5α. Transformants were selected on LB plates containing ampicillin. Restriction enzyme digestion and sequence analysis were used to confirm the size (9.0 kbp) and proper orientation of the insert in the resulting plasmid, pTB1000 (Fig. 1A), which has the Z. mobilis adh gene fused in frame to the start and end of the ldh ORF. This recombinant ORF was subcloned into p95, a suicide vector with suitable antibiotic resistance markers for cloning in S. mutans. This yielded the final plasmid construct, pTB1002.
FIG. 1.
Construction of BCS3-L1. (A) Circle PCR mutagenesis was used to delete the ldh ORF from p10-5. The resulting fragment was ligated with the adh ORF to create pTB1000. The recombinant fragment was subcloned into p95 to create the suicide vector, pTB1002, for cloning into S. mutans. (B) Transformation of JH1140 with pTB1002 yielded the heterodiploid intermediate, HD83. Spontaneous resolution of the heterodiploid intermediate yielded the isogenic mutant, BCS3-L1.
The recombinant ORF was introduced into JH1140 to replace the wild-type ldh gene via a heterodiploid intermediate (Fig. 1B). Transformation with 1 μg of purified pTB1002 DNA resulted in four transformants that arose on medium containing tetracycline. This result accords with previous studies which demonstrated the difficulty of transforming JH1001 and its derivatives (14, 15). Following purification, the clones were shown by Southern analysis to be heterodiploids containing chromosomal copies of both the wild-type and mutant ldh genes separated only by the p95 vector (data not shown).
One of these clones, HD83, was grown for ca. 20 generations in the absence of tetracycline to enable a second, spontaneous recombination event to eliminate p95 and either the wild-type or mutant copy of the ldh gene (Fig. 1B). Eleven tetracycline-sensitive clones were identified by replica patching from approximately 16,000 colonies screened. Of these 11 clones, 2 were found which were less acidogenic than the parent strain, JH1140, when grown for 48 h in BHI broth supplemented with 0.5% glucose. Enzyme analysis of cell lysates of these clones indicated that they both had no detectable LDH activity and 10-fold-elevated levels of ADH activity relative to JH1140 (Table 1). One of these clones, called BCS3-L1, was chosen for further study. Southern blot analysis with probes including an internal fragment of the S. mutans ldh gene, the PCR-generated Z. mobilis adh insert, and a pVA981 fragment was used to confirm the deletion of the native ldh gene, incorporation into the chromosome of the Z. mobilis adh gene, and loss of the p95 vector backbone, including its tetracycline resistance gene.
TABLE 1.
Phenotypes of wild-type and effector strains
| Strain | Generation timea (h) | Final pHa | Sp actb (μmol/min/mg of protein)
|
Mutacin productionc (mm diam) | Plaque productiond (mg dry wt) | |
|---|---|---|---|---|---|---|
| LDH | ADH | |||||
| JH1140 | 1.0 | 4.3 | 2.098 | 0.002 | 18.4 | 12.2 |
| BCS3-L1 | 1.1 | 5.1 | NDe | 0.025 | 18.9 | 25.3 |
Growth in minimal medium supplemented with 0.5% yeast extract and 50 mM glucose; aerobic standing at 37°C.
Growth in BHI broth supplemented with 0.5% glucose; aerobic standing at 37°C.
Growth on THB agar and overlaid with S. rattus BHT-2.
Growth in THB containing 5% sucrose; aerobic standing at 37°C.
ND, not detectable.
In vitro characterization of the BCS3-L1 effector strain.
The growth properties of BCS3-L1 closely resemble those of its parent, JH1140 (Table 1). Fermentation end product analysis showed that BCS3-L1 makes no detectable lactic acid (Table 2). Instead, much of the metabolized carbon is converted to the nonacidic end products, ethanol and acetoin. Both of these are normal end products for S. mutans growing under certain cultivation conditions (11). Thus, metabolism in BCS3-L1 is not changed qualitatively but does distribute carbon differently over known catabolic routes. The product profile, as measured, was balanced with respect to carbon utilized but led to an apparent surplus of NADH formed during catabolism. The reason for this observation is currently unknown. Under various cultivation conditions, including growth on a variety of sugars and polyols, such as sucrose, fructose, lactose, mannitol, and sorbitol, BCS3-L1 yielded final pH values that were at least 0.4 pH units higher than those of JH1140. Other features of the BCS3-L1 phenotype that may affect its ability to serve as an effector strain in replacement therapy of dental caries were also tested. BCS3-L1 formed plaque on glass surfaces when grown in the presence of sucrose as well or better than JH1140 (Table 1). BCS3-L1 was also found to produce mutacin 1140 in amounts comparable to JH1140.
TABLE 2.
Fermentation end products of wild-type and effector strainsa
| Strain | Glucose metabolized (mmol) | Fermentation end products (mmol)
|
Carbon balance (%) | |||||
|---|---|---|---|---|---|---|---|---|
| Lactate | Pyruvate | Formate | Acetate | Ethanol | Acetoin | |||
| JH1140 | 25.6 | 43.7 | <0.05 | 5.0 | 4.4 | 1.7 | <0.05 | 97.3 |
| BCS3-L1 | 20.0 | <0.05 | 6.1 | 19.3 | 6.9 | 13.0 | 7.1 | 100.5 |
Grown anaerobically on minimal medium supplemented with 0.5% yeast extract and 50 mM glucose.
The genetic stability of BCS3-L1 could be assessed in vitro, since this strain produces red colonies when grown on glucose tetrazolium medium (8) whereas wild-type S. mutans, including JH1140, produces white colonies on this medium. No white colonies were observed among over 100,000 independent colonies of BCS3-L1 screened, indicating a spontaneous reversion frequency of <1 × 10−5.
In vivo cariogenicity studies.
The efficacy of BCS3-L1 was evaluated in an animal model with two groups of 10 21-day-old, germfree Fischer rats. The groups of animals were monoinfected with either JH1140 or BCS3-L1. The appropriate challenge strains were found in fecal samples recovered 1 week after infection. Three weeks after infection, before the appearance of gross carious lesions, four animals in each group were killed to determine the level of dental colonization by JH1140 and BCS3-L1. The mean (± standard deviation) number of cultivable organisms recoverable per set of left mandibular molars was (5.99 ± 1.20) × 106 and (2.41 ± 1.83) × 106 CFU for JH1140 and BCS3-L1, respectively. This difference was significant (P < 0.01). As illustrated in Table 3, rats infected with the parent strain for 8 weeks had significantly (P < 0.0001) higher caries scores than did animals infected with BCS3-L1. The differences were observable for all types of lesions, sulcal, interproximal, and buccal.
TABLE 3.
Caries scores of gnotobiotic rats infected with wild-type or effector strain
| Strain | Caries score (mean ± standard deviation)
|
|||
|---|---|---|---|---|
| Sulcal | Proximal | Buccal | Total | |
| JH1140 | 35.5 ± 6.1 | 4.7 ± 2.4 | 15.5 ± 3.7 | 57.3 ± 9.4 |
| BCS3-L1 | 15.8 ± 3.0 | 0.5 ± 1.2 | 4.2 ± 1.9 | 20.5 ± 3.5 |
In a similarly designed experiment, conventional (S. mutans-free) Sprague-Dawley rats were used to assess the cariogenic potential of BCS3-L1 in the presence of a mixed natural flora. In this model, no significant difference was detected between the mean levels of colonization by JH1140 [(3.13 ± 0.79) × 103 CFU/three molars] and BCS3-L1 [(2.46 ± 0.45) × 103 CFU/three molars] 3 weeks after infection. As shown in Table 4, rats infected with the parent strain for 8 weeks had significantly (P < 0.0001) higher caries scores than did animals infected with BCS3-L1 or uninfected control animals. The differences were observable for all types of lesions. Animals infected with BCS3-L1 had a slightly lower mean caries score than S. mutans-free control animals, but this difference was not significant.
TABLE 4.
Caries scores of conventional rats infected with wild-type or effector strain
| Strain | Caries score (mean ± standard deviation)
|
|||
|---|---|---|---|---|
| Sulcal | Proximal | Buccal | Total | |
| JH1140 | 85.2 ± 12.4 | 28.5 ± 8.5 | 13.7 ± 8.4 | 126.7 ± 23.6 |
| BCS3-L1 | 44.3 ± 8.0 | 14.8 ± 7.4 | 7.0 ± 4.4 | 66.1 ± 16.4 |
| Control | 51.3 ± 9.5 | 13.8 ± 8.9 | 7.1 ± 4.7 | 70.7 ± 19.5 |
In vivo colonization studies.
BCS3-L1 was compared to JH1140 for its ability to preemptively colonize the S. mutans niche in the oral cavity and thereby protect a host against colonization by naturally occurring strains of this bacterium. As shown in Table 5, prior establishments of JH1140 and BCS3-L1 on the teeth of Sprague-Dawley rats were equally effective in preventing persistent colonization by strain Ingbritt even when the animals were challenged with as many as 1010 CFU. By comparison, S. mutans-free control animals could be infected by as few as 106 CFU.
TABLE 5.
Preemptive colonization by wild-type and effector strains
| Concn of challenge strain Ingbritt (CFU/ml) | Frequency of superinfection in animals colonized by:
|
||
|---|---|---|---|
| JH1140 | BCS3-L1 | Control | |
| 107 | 0/3 | 0/3 | 3/3 |
| 108 | 0/3 | 0/3 | 3/3 |
| 109 | 0/3 | 0/3 | 3/3 |
| 1010 | 0/3 | 0/3 | 3/3 |
BCS3-L1 was also compared to JH1140 for its ability to aggressively displace an established S. mutans strain from its niche in the oral cavities of Sprague-Dawley rats. As in the previous study, BCS3-L1 behaved like its parent with regard to its colonization potential. At challenge concentrations of 107, 108, 109, and 1010 CFU/ml, BCS3-L1 and JH1140 uniformly supercolonized animals previously infected with Ingbritt. At the highest challenge concentration, JH1140 constituted about one-third of all S. mutans organisms recovered from animals initially colonized with Ingbritt, whereas BCS3-L1 constituted approximately 93% of the total (data not shown). At lower challenge concentrations, BCS3-L1 also appeared to perform somewhat better than JH1140.
Long-term animal toxicity and safety study.
After infection for 6 months, the mean weights of animals colonized with BCS3-L1 (398.3 ± 49.9 g) did not differ significantly from those of animals colonized with JH1140 (344.7 ± 13.7 g) or S. mutans-free control animals (377.7 ± 34.2 g). Approximately 1,000 BCS3-L1 colonies recovered on MSB medium all produced red colonies on glucose tetrazolium plates, indicating an in vivo reversion frequency of <10−3. All of these colonies produced mutacin 1140 in amounts comparable to JH1140 as indicated by zone sizes in the deferred antagonism assay with BHT-2 as the target strain. Histopathological examination was performed on all of the major organs from each animal, including liver, spleen, kidney, bladder, adrenal gland, pituitary gland, salivary glands, mandibular and mesenteric lymph nodes, thyroid, parathyroid, trachea, esophagus, heart, thymus, lungs, stomach, pancreas, intestines, testes, prostate, skin, mammary gland, tongue, palate, brain, bone and eyes. No treatment-related lesions were observed in this study.
DISCUSSION
For many years, replacement therapy has been considered an appealing approach for the prevention of certain microbial infections. However, there has been no instance where a particular effector strain has embodied all of the necessary traits to enable it to serve reliably in the prevention of a clinical disease. In the case of dental caries, mutations that affect acid production by mutans streptococci have long been known to reduce their cariogenicity (8, 29). It has also been known for some time that increased production of a mutacin by a particular strain of S. mutans could enable it to successfully displace indigenous mutans streptococci and persistently colonize the human oral cavity (12). However, in order to combine these traits in a genetically stable effector strain it was first necessary to determine the reason why LDH-deficient mutants could not be found for most strains of S. mutans (14). Using an allele of ldh that expressed a thermolabile LDH activity, it was possible to demonstrate that LDH deficiency is a lethal mutation in S. mutans and that a cloned, heterologous ADH could overcome the resulting metabolic defect (15). The application of recombinant DNA methods to solving these problems yielded the effector strain BCS3-L1 described here. In addition to enabling us to combine the low acid production and mutacin overproduction traits, recombinant methods provided considerable assurance against the occurrence of cryptic mutations. The recombinant methods also provided assurance against reversion of the ldh mutation, since essentially all of the ORF was deleted. Reversion by transformation is also unlikely, since JH1000 and its derivatives are, under optimal conditions, poorly transformable. Thus, BCS3-L1 is expected to be genetically very stable. Strain construction employed Campbell insertion of the suicide vector, pTB1002, and spontaneous resolution of the heterodiploid intermediate resulted in allelic exchange. This strategy was necessary because transformation with linear DNA has not been achieved in JH1001 or its derivatives (14). In addition to elimination of the wild-type gene, this strategy had the added advantage of eliminating all of the vector DNA, including its antibiotic resistance genes. Thus, a truly isogenic mutant was generated.
Southern blot analysis of BCS3-L1 confirmed the exchange of the Z. mobilis adh gene for the native ldh gene and the concomitant loss of vector sequences. Enzyme assays of BCS3-L1 cell extracts showed no detectable LDH activity and 10-fold increase in ADH activity relative to that of its parent. The ldh promoter appears to be transcribed at more or less constant levels regardless of cultivation conditions, as indicated by measurements of β-galactoside activity in a JH1140 derivative containing an ldh::lacZ transcriptional fusion (data not shown). Thus, constant high levels of ADH should be constitutively available to BCS3-L1. In support of this hypothesis, BCS3-L1 grew well on a variety of carbon sources. Supernatants from these cultures contained greatly elevated ethanol concentrations and no detectable lactic acid. Formate, acetate, and acetoin concentrations were also significantly elevated relative to those of JH1140, indicating increased dissimilation of pyruvate by pyruvate dehydrogenase and pyruvate-formate lyase pathways. When BCS3-L1 was grown in the presence of various carbon sources, the final pH reached was at least 0.4 pH units higher than that of JH1140. Although there are no direct measurements to demonstrate that the difference in terminal pH observed in vitro is exactly reflected in plaque containing wild-type S. mutans versus plaque containing BCS3-L1, the results of animal models described below indicate that BCS3-L1 is less able to reduce the pH below the critical threshold required for initiation and progression of caries lesions.
Increased plaque formation during growth in the presence of sucrose as shown here for BCS3-L1 has been previously reported for LDH-deficient mutants of S. rattus (8). This is likely to be a reflection of the pH dependence of glucosyl transferase activities. Since in general S. mutans constitutes a relatively small proportion of the oral flora, it is unlikely that this property, if expressed in vivo, would have a significant effect on the overall amount or quality of plaque that would predispose the host to other diseases, such as gingivitis or periodontitis. Increased dextran production and the clumping caused by dextran may, however, account for the twofold-lower recovery of BCS3-L1 from gnotobiotic rats compared to JH1140. As predicted from its reduced acidogenic potential, both gnotobiotic and conventional rats infected with BCS3-L1 had significantly lower incidences and severity of dental caries than animals infected with JH1140. In the gnotobiotic-rat study, residual caries activity in the mutant infected animals was confined primarily to sulcal areas where, in the weanling rat, the enamel is the thinnest (4) and the conditions for acid accumulation are the greatest. In the case of the conventional-rat model, BCS3-L1 did not add to the background incidence or severity of caries lesions caused by the indigenous flora. Clearly, the 1,000-fold-higher numbers of BCS3-L1 attained in gnotobiotic animals were responsible for the residual pathogenic potential observed in this model compared to that in the conventional-rat model.
In earlier studies (12), we demonstrated that JH1005, equivalent to JH1140 in producing two- to threefold-elevated amounts of mutacin 1140, could persistently colonize the human oral cavity and aggressively displace indigenous strains without causing an observable effect on other plaque species. At least two of the three subjects treated by brushing and flossing approximately 1011 cells onto their cleaned tooth surfaces for 3 min remain colonized almost 15 years later (J. D. Hillman, unpublished observation). Thus, increased production of this lantibiotic appears to provide a selective advantage in colonization suitable for use in replacement therapy of dental caries. In both preemptive-colonization and aggressive-displacement rat model studies, we found that BCS3-L1 performed as well as or better than JH1140. These results strongly suggest that BCS3-L1 should be able to colonize the human oral cavity and occupy the niche normally occupied by wild-type S. mutans.
No adverse effects were observed at the gross or microscopic level in conventional rats colonized for 6 months with BCS3-L1. LDH deficiency requires BCS3-L1 to depend entirely on mixed acid fermentation pathways that yield formate, acetate, ethanol, and acetoin as the principal end products. These are common end products of a variety of microorganisms, including S. mutans growing in the presence of limiting substrate, so they were not expected to result in an observable toxic effect. Sufficient amounts of mutacin 1140 have not yet been purified to be able to directly test its toxicity (16), but it belongs to the same class of antibiotics as nisin, which is used as a food preservative worldwide and which has very low toxicity (17).
From the experiments described in this study, BCS3-L1 appears to be well suited to serve as an effector strain in the replacement therapy of dental caries. It has a significantly reduced pathogenic potential, it has a selective advantage in colonizing the tissues at risk for disease, and it is genetically stable. Additional studies in human clinical trials are planned to further test this hypothesis.
ACKNOWLEDGMENTS
This research was supported in part by Public Health Service Grants DE04529, DE09081, and DE08182 from the National Institute of Dental Research.
REFERENCES
- 1.Abhyankar S, Sandham H J, Chan K H. Serotype c Streptococcus mutans mutable to lactate dehydrogenase deficiency. J Dent Res. 1985;64:1267–1271. doi: 10.1177/00220345850640110201. [DOI] [PubMed] [Google Scholar]
- 2.Chen A, Hillman J D, Duncan M. l-(+)-Lactate dehydrogenase deficiency is lethal in Streptococcus mutans. J Bacteriol. 1994;176:1542–1545. doi: 10.1128/jb.176.5.1542-1545.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Conway T, Sewell G W, Osman Y A, Ingram L O. Cloning and sequencing of the alcohol dehydrogenase II gene from Zymomonas mobilis. J Bacteriol. 1987;169:2591–2597. doi: 10.1128/jb.169.6.2591-2597.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fitzgerald R J, Konig K G. Maturation of dental enamel in germ-free and mono-infected Sprague-Dawley rats. Helv Odont Acta. 1968;12:55–61. [PubMed] [Google Scholar]
- 5.Fredericq P. Colicins. Annu Rev Microbiol. 1957;11:7–22. doi: 10.1146/annurev.mi.11.100157.000255. [DOI] [PubMed] [Google Scholar]
- 6.Gold O G, Jordan H V, van Houte J. A selective medium for Streptococcus mutans. Arch Oral Biol. 1973;20:473–477. doi: 10.1016/0003-9969(73)90109-x. [DOI] [PubMed] [Google Scholar]
- 7.Gutierrez J A, Crowley P J, Brown D P, Hillman J D, Youngman P, Bleiweis A S. Insertional mutagenesis and recovery of interrupted genes of Streptococcus mutans using transposon Tn917: preliminary characterization of mutants displaying acid sensitivity and nutritional requirements. J Bacteriol. 1996;178:4166–4175. doi: 10.1128/jb.178.14.4166-4175.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hillman J D. Lactate dehydrogenase mutants of Streptococcus mutans: isolation and preliminary characterization. Infect Immun. 1978;21:206–212. doi: 10.1128/iai.21.1.206-212.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hillman J D, Johnson K P, Yaphe B I. Isolation of a Streptococcus mutans strain producing a novel bacteriocin. Infect Immun. 1984;44:141–144. doi: 10.1128/iai.44.1.141-144.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hillman J D, Yaphe B I, Johnson K P. Colonization of the human oral cavity by a strain of Streptococcus mutans. J Dent Res. 1985;64:1272–1274. doi: 10.1177/00220345850640110301. [DOI] [PubMed] [Google Scholar]
- 11.Hillman J D, Andrews S W, Dzuback A L. Acetoin production by wild-type strains and a lactate dehydrogenase-deficient mutant of Streptococcus mutans. Infect Immun. 1987;55:1399–1402. doi: 10.1128/iai.55.6.1399-1402.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hillman J D, Dzuback A L, Andrews S W. Colonization of the human oral cavity by a Streptococcus mutans mutant producing increased bacteriocin. J Dent Res. 1987;66:1092–1094. doi: 10.1177/00220345870660060101. [DOI] [PubMed] [Google Scholar]
- 13.Hillman J D, Duncan M J, Stashenko K P. Cloning and expression of the gene encoding the fructose-1,6-diphosphate-dependent l-(+)-lactate dehydrogenase of Streptococcus mutans. Infect Immun. 1990;58:1290–1295. doi: 10.1128/iai.58.5.1290-1295.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hillman J D, Chen A, Duncan M, Lee S-W. Evidence that l-(+)-lactate dehydrogenase deficiency is lethal in Streptococcus mutans. Infect Immun. 1994;62:60–64. doi: 10.1128/iai.62.1.60-64.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hillman J D, Chen A, Snoep J L. Genetic and physiological analysis of the lethal effect of l-(+)-lactate dehydrogenase deficiency in Streptococcus mutans: complementation by alcohol dehydrogenase from Zymomonas mobilis. Infect Immun. 1996;64:4319–4323. doi: 10.1128/iai.64.10.4319-4323.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hillman J D, Novak J, Sagura E, Gutierrez J A, Brooks T A, Crowley P J, Azziz A, Leung K-P, Cvitkovitch D, Bleiweis A S. Genetic and biochemical analysis of mutacin 1140, a lantibiotic from Streptococcus mutans. Infect Immun. 1998;66:2743–2749. doi: 10.1128/iai.66.6.2743-2749.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hurst A. Nisin. In: Perlman D, Laskin A I, editors. Advances in applied microbiology. Vol. 27. London, United Kingdom: Academic Press; 1981. pp. 85–123. [Google Scholar]
- 18.Johnson C P, Gross S M, Hillman J D. Cariogenic potential in vitro in man and in vivo in the rat of lactate dehydrogenase mutants of Streptococcus mutans. Arch Oral Biol. 1980;25:707–713. doi: 10.1016/0003-9969(80)90124-7. [DOI] [PubMed] [Google Scholar]
- 19.Jordan H V, Englander H R, Engler W O, Kulczyk S. Observations on the implantation and transmission of Streptococcus mutans in humans. J Dent Res. 1972;51:515–518. doi: 10.1177/00220345720510024501. [DOI] [PubMed] [Google Scholar]
- 20.Keyes P S. Dental caries in the molar teeth of rats: a method for diagnosing and scoring several types of lesions simultaneously. J Dent Res. 1958;37:1088–1099. doi: 10.1177/00220345580370060901. [DOI] [PubMed] [Google Scholar]
- 21.Krasse B, Edwardsson S, Svensson I, Trell L. Implantation of caries-inducing streptococci in the human oral cavity. Arch Oral Biol. 1967;12:231–236. doi: 10.1016/0003-9969(67)90042-8. [DOI] [PubMed] [Google Scholar]
- 22.Lederberg J. Detection of fermentative variants with tetrazolium. J Bacteriol. 1948;56:695. doi: 10.1128/jb.56.5.695-695.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1982. [Google Scholar]
- 24.Michalek S M, McGhee J R, Navia J M. Virulence of Streptococcus mutans. A sensitive method for evaluating cariogenicity in gnotobiotic rats. Infect Immun. 1975;12:69–75. doi: 10.1128/iai.12.1.69-75.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Neidhardt F C, Bloch P L, Smith D F. Culture medium for Enterobacteria. J Bacteriol. 1974;119:736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perry D, Kuramitsu H K. Genetic transformation of Streptococcus mutans. Infect Immun. 1981;32:1295–1297. doi: 10.1128/iai.32.3.1295-1297.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ruangsri P, Orstavik D. Effect of the acquired pellicle and of dental plaque on the implantation of Streptococcus mutans on tooth surfaces in man. Caries Res. 1977;11:204–210. doi: 10.1159/000260269. [DOI] [PubMed] [Google Scholar]
- 28.Svanberg M, Krasse B. Oral implantation of saliva-treated Streptococcus mutans in man. Arch Oral Biol. 1981;26:197–201. doi: 10.1016/0003-9969(81)90130-8. [DOI] [PubMed] [Google Scholar]
- 29.Tanzer J M, Freedman M L. Genetic alterations of Streptococcus mutans virulence. Adv Exp Med Biol. 1978;107:661–672. doi: 10.1007/978-1-4684-3369-2_75. [DOI] [PubMed] [Google Scholar]
- 30.Tobian J A, Cline M L, Macrina F L. Characterization and expression of a cloned tetracycline resistance determinant from the chromosome of Streptococcus mutans. J Bacteriol. 1984;160:556–563. doi: 10.1128/jb.160.2.556-563.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]