Abstract
Purpose
To evaluate the relationships of the triglyceride-glucose (TyG) index with pregnancy-related complications (PRCs) and to clarify the predictability of the TyG index for PRCs.
Patients and Methods
Totally of 11,387 women with a singleton pregnancy were prospectively followed until after delivery. Maternal fasting lipids and glucose concentration were measured in the first trimester (11 weeks gestation on average). The TyG index was calculated as ln [triglyceride (mg/dL) × fasting plasma glucose (mg/dL)/2]. We used generalized linear models to calculate the relative risks and 95% confidence intervals. Receiver-operating characteristic curve analysis was employed to assess the ability of the TyG index to predict the risks of PRCs.
Results
Smooth spline reveals that the probability of gestational diabetes mellitus (GDM) is intensified with the increasing TyG index. Multivariate logistic regression adjusted for risk factors demonstrates a 1-unit and a 1-SD increment in the TyG index raises the risk of GDM by 3.63 and 1.57 times, respectively. Identically, the risk of GDM maximizes in the TyG quintile 5 (OR: 3.14; 95% CI: 2.55~3.85) relative to the lowest TyG index group. However, no association between TyG index and the risk of other PRCs was observed after full adjustment. The area under receiver operating characteristic curves is 0.647 (95% CI: 0.632–0.66) for GDM, and the optimal predictive cut-off is 8.55, with a specificity of 0.679 and sensitivity of 0.535.
Conclusion
The first-trimester TyG index is significantly associated with the risk of incident GDM, while the relationships between the TyG index and other PRCs need further exploration.
Keywords: first-trimester, triglyceride-glucose index, pregnancy-related complications, gestational diabetes mellitus, singleton pregnancy
Plain Language Summary
Pregnancy-related complications (PRCs) can reach approximately 5% to 30% in mainland China, particularly gestational diabetes mellitus. It is critical to identify high-risk individuals more accurately and earlier. Our results showed that the probability of gestational diabetes mellitus is intensified with the increasing first-trimester TyG index in southeast China, and the optimal predictive cut-off is around 8.55.
Introduction
Pregnancy-related complications (PRCs) are well-known causes of significant morbidity and mortality in mothers and their families and can recover slowly, with long-lasting sequelae.1–3 Common PRCs include gestational diabetes mellitus (GDM), gestational hypertension, fetal distress, preeclampsia, placental abruption, and premature rupture of membranes. Reportedly, the total incidence of PRCs can reach approximately 5% to 30% in mainland China.4–6 PCRs (eg, preeclampsia and GDM) not only affect direct adverse pregnancy outcomes such as placental disease, preterm delivery, fetal distress, and intrauterine growth retardation,7,8 but also contribute to maternal postpartum cardiovascular and metabolic morbidity.9 Thus, more data are needed to identify the clinical risk factors of PRCs as early as possible.
The triglyceride-glucose (TyG) index, computed as ln[fasting triglycerides (mg/dl) ×fasting blood glucose (mg/dl)/2], is put forward as a useful surrogate marker for recognizing insulin resistance (IR).10,11 This index is feasible for stratifying the risks of metabolic disorders,12 atherosclerosis,13 and cardiovascular diseases.14 In pregnant women, several suggested mechanisms contribute to the state of IR, which is prone to generate metabolic disorders, chronic inflammation, and related disorders (eg, diabetes, hypertension, dyslipidemia).15,16 Therefore, it is essential to be aware of complications during pregnancy. Identifying PRCs is insufficient but urgent and depends on physicians’ feasible, available, cost-effective, and high-performance approaches. The TyG index, which can be easily obtained and calculated in clinical practice or large-scale epidemiologic investigations,12,17 may be used to assess the risks of PRCs. Indeed, several descriptions of the TyG index are available for recognizing women susceptible to GDM in China,18,19 Korea,20 Iran,21 and Mexico.22 However, the associations of the TyG index with PRCs beyond GDM have not yet been widely validated in China. Besides, the existing research on pregnant women is constrained by many methodological limitations, including small sample groups and subgroups, inappropriate control of confounding factors, lack of dose-response analysis, and no widely persuasive cut-off point for the TyG index established in pregnant women.
We hypothesize that the maternal first-trimester TyG index may be valuable for predicting complications in later pregnancy. Our study aims to investigate relationships between the TyG index and PRCs, and to clarify the predictability of the TyG index for PRCs. If the TyG index is verified as a valuable predictor for PRCs, we can establish a scheme of close outpatient follow-up in high-TyG pregnant women, so as to early and promptly detect the contributors to an unfavorable pregnant outcome and to make appropriate solutions to better pregnancy outcomes.
Materials and Methods
Data Source
This study was based on the Fujian Birth Cohort Study (FJBCS), which is a large, comprehensive, ongoing study designed to examine the effects of prenatal exposure on adverse pregnancy outcomes and the healthy development of children in Southeast China. Continuous registration of pregnant women was prospectively carried out in the prenatal clinic of the Fujian provincial Maternity and Child Health Hospital. Pregnant women were invited to participate at their first visit to the center; women ≥18 years old and ≤14 weeks gestation were eligible. Women with severe liver or kidney disease, serious cerebrovascular disease, serious psychiatric disorders, intellectual disability, or unable to complete the study independently were excluded. Each participant was contacted during pregnancy to collect questionnaire data, and blood and urine samples. Gestational age and date of delivery were calculated using the last menstrual period date or ultrasound to estimate if their menstruation were irregular. Detailed clinical data were collected for review in the postpartum chart. Between Jan 2019 and September 2020, the FJBCS recruited 15,818 pregnant women attending their first-trimester prenatal health visit and attending their first-trimester prenatal health visit and undergoing early pregnancy examinations. We first excluded these participants who lacked information on pregnancy outcomes, abortion, and multiple births, yielding a sample of 11,566 women. In addition, we excluded participants who were diagnosed with diabetes or hypertension before conception or in the first-trimester. A total of 11,387 women who gave singleton birth were eligible for the final analysis. The participant flowchart was presented in Supplementary Figure 1. The studies involving human participants were reviewed and approved by the Research Ethics Committee of Fujian Maternity and Child Health Hospital (approval number: 2017KR-030) and complied with the Declaration of Helsinki. The participants provided their written informed consent to participate in this study.
Measurements and Definitions
At the first-trimester prenatal visit (about 11 weeks gestation), anthropometric and biochemical measurements were assessed. Blood pressure was measured with an upper arm oscillometric device sitting, taken three or more readings, and the average was determined. Fasting plasma glucose (FPG), triglyceride (TG), total cholesterol (TC), Low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), hemoglobin (Hb), uric acid (UA), creatinine (Cr), alanine aminotransferase (ALT), and aspartate aminotransferase (AST) levels were tested after more than 8 hours of fasting. The TyG index was calculated as previously described.11
In China, at 24–28 weeks of gestation (if women have pre-existing risk factors such as familial history of diabetes/GDM, history of GDM, and obesity, early screening is also possible), women underwent a “one-step” approach to GDM screening with a standardized 75 g oral glucose tolerance test (OGTT). Venous blood samples were collected at 0, 1, and 2 h after a glucose load. The diagnosis of GDM was made according to the International Association of the Diabetes and Pregnancy Study Group (IADPSG):23 fasting plasma glucose ≥ 5.1 mmol/L, plasma glucose at 1h ≥ 10 mmol/L, or plasma glucose at 2h ≥ 8.5 mmol/L. GDM was defined as data under the International Classification of Diseases (ICD)-10 codes of O24.4 or O24.9 during prenatal care. Other PRCs were defined as claims of the corresponding ICD-10 code from medical reports. After delivery, information regarding pregnancy outcomes was collected.
Other Covariates
In this study, the following covariates were also assessed: maternal age, pre-pregnancy body mass index (BMI), assisted reproduction, abortion history, gravidity, parity, as well as gestational weight gain (GWG), and delivery gestations. Participants’ weight and height were determined using a scale and stadiometer. Pre-pregnancy BMI was calculated as pre-pregnancy weight (kg) divided by the square of height (m2) and was further categorized into three groups: underweight (<18.50 kg/m2), normal weight (18.50–24.99 kg/m2), overweight and obese (≥25.00 kg/m2). GWG was calculated as the difference between the mother’s pre-pregnancy weight and her weight before delivery, and was assessed in the three following categories, according to the Institute of Medicine (IOM) recommendations from 2009:24 (1) below the recommendation; (2) within the recommendation; (3) above the recommendation.
Statistical Analysis
Descriptive statistics are shown as mean ± standard deviation or medians (25th percentile, 75th percentile) for continuous variables with regard to the normality of distribution, while frequency and percentage were used for categorical variables. For clinical characteristics analysis, the statistical differences among TyG index quintiles were tested with one-way analysis of variance (ANOVA) or Kruskal–Wallis rank-sum test for continuous variables and the Chi-square test for categorical variables.
The relationships between the TyG index and the occurrence of pregnancy-related complications were explored using smoothing splines generated in generalized additive models by R package mgcv.25 Subsequently, unconditional binary logistic regressions were performed to determine the associations of the TyG index (per unit, per SD, and quintiles-the lowest quintile was used as reference) with pregnancy-related complications adjusted for potential confounders. Tests of linear trends across increasing TyG index quintiles were performed by assigning the median value to each category and treating it as a continuous variable.
The regression models were also used to evaluate the odds ratios and 95% confidence intervals (CI) of having GDM for TyG index (per unit) by maternal age (<30y, ≥30y), pre-pregnancy BMI (underweight, normal weight, overweight and obese), assisted reproduction (no, yes), abortion history (no, yes), gravidity (1, 2, 3, ≥4), and GWG (<recommendation, within recommendation, >recommendation). Interaction across subgroups was tested using the likelihood ratio test. Receiving-operating characteristic (ROC) curves and areas under the curve (AUC) were employed to evaluate the ability of the indices to detect PRCs.
To replace missing covariate data with statistical estimates of the missing data, we used mean or median as imputation value for continuous indices and made dummy level-9 for categorical indices. Thereby, we can use the data acquired from an incomplete dataset. Robustness of results was tested via sensitivity analysis after excluding women with covariates, missing data, non-Han ethnicity, or baseline TG ≥ 5.1 mmol/L.
Statistical significance was defined as a two-tailed P ≤ 0.05. Data management and all analyses were conducted using R software, version 4.0.2 (http://www.R-project.org).
Results
Baseline Characteristics
Overall, most women were Han (98.7%) with maternal age of 30.0 ± 4.0 years, and 43.0% were the first gravidity. Maternal age, pre-pregnancy BMI, assisted reproduction, gravidity, parity, gestational week at the examination, systolic BP, diastolic BP, FPG, TG, TC, LDL, hemoglobin, uric acid, and ALT increased with TyG index quintile increment, while HDL, delivery gestations, and GWG showed the contrary trend (P value <0.001). No significant differences in creatinine and AST were found across TyG index quintiles (Table 1).
Table 1.
Anthropometric and Biochemical Characteristics of the Participants According to Triglyceride-Glucose (TyG) Index Quintiles
| Variables | Overall | TyG Index Quintiles | P value | ||||
|---|---|---|---|---|---|---|---|
| Total (n = 11,387) | Q1 (n = 2278) | Q2 (n = 2277) | Q3 (n = 2277) | Q4 (n = 2276) | Q5 (n = 2279) | ||
| Race-Han, n (%) | 11,234 (98.7) | 2238 (98.2) | 2250 (98.8) | 2246 (98.6) | 2244 (98.6) | 2256 (99) | 0.029 |
| Maternal age, years | 30.0 ± 4.0 | 29.2 ± 3.7 | 29.5 ± 3.8 | 30.0 ± 3.9 | 30.3 ± 4.0 | 31.2 ± 4.1 | < 0.001 |
| Pre-pregnancy BMI, kg/m2 | 21.1 ± 3.0 | 20.3 ± 2.6 | 20.6 ± 2.7 | 20.9 ± 2.7 | 21.4 ± 3.0 | 22.5 ± 3.2 | < 0.001 |
| Assisted reproduction, n (%) | 603 (5.3) | 67 (2.9) | 84 (3.7) | 106 (4.7) | 147 (6.5) | 199 (8.8) | < 0.001 |
| Abortion history, n (%) | 4173 (36.7) | 695 (30.5) | 760 (33.4) | 809 (35.5) | 914 (40.2) | 995 (43.7) | < 0.001 |
| Gravidity, n (%) | < 0.001 | ||||||
| 1 | 4902 (43.0) | 1242 (54.5) | 1108 (48.7) | 1000 (43.9) | 851 (37.4) | 701 (30.8) | |
| 2 | 3423 (30.1) | 564 (24.8) | 645 (28.3) | 681 (29.9) | 759 (33.3) | 774 (34) | |
| 3 | 1862 (16.4) | 286 (12.6) | 335 (14.7) | 364 (16) | 402 (17.7) | 475 (20.8) | |
| ≥4 | 1200 (10.5) | 186 (8.2) | 189 (8.3) | 232 (10.2) | 264 (11.6) | 329 (14.4) | |
| Parity, n (%) | < 0.001 | ||||||
| 0 | 6646 (58.4) | 1581 (69.4) | 1457 (64) | 1350 (59.3) | 1217 (53.5) | 1040 (45.6) | |
| 1 | 4278 (37.6) | 632 (27.8) | 731 (32.1) | 837 (36.8) | 964 (42.4) | 1114 (48.9) | |
| ≥2 | 463 (4.0) | 64 (2.8) | 89 (3.9) | 90 (4) | 95 (4.2) | 125 (5.5) | |
| Gestational week at examination, weeks | 11.3 ± 1.1 | 11.0 ± 1.2 | 11.2 ± 1.2 | 11.3 ± 1.1 | 11.4 ± 1.0 | 11.5 ± 1.1 | < 0.001 |
| SBP, mmHg | 113.8 ± 10.9 | 111.6 ± 10.9 | 112.9 ± 10.7 | 113.5 ± 10.6 | 114.8 ± 11.1 | 116.1 ± 10.5 | < 0.001 |
| DBP, mmHg | 68.6 ± 8.6 | 66.8 ± 8.5 | 67.9 ± 8.5 | 68.4 ± 8.4 | 69.4 ± 8.5 | 70.2 ± 8.5 | < 0.001 |
| FPG, mg/dl | 85.7 ± 6.4 | 83.4 ± 6.1 | 84.8 ± 5.9 | 85.5 ± 5.9 | 86.4 ± 6.1 | 88.3 ± 7.0 | < 0.001 |
| TG, mg/dl | 111.6 (90.3, 139.9) | 75.3 (68.2, 81.5) | 94.7 (89.4, 100.1) | 111.6 (105.4, 117.8) | 132.8 (124.0, 140.8) | 174.4 (158.5, 201.9) | < 0.001 |
| Total cholesterol, mg/dl | 179.3 ± 28.3 | 166.4 ± 24.6 | 174.2 ± 25.7 | 179.2 ± 26.2 | 185.1 ± 27.6 | 191.4 ± 30.5 | < 0.001 |
| LDL-C, mg/dl | 92.3 ± 23.3 | 81.9 ± 20.0 | 88.1 ± 20.8 | 92.2 ± 21.5 | 97.1 ± 22.9 | 102.1 ± 25.5 | < 0.001 |
| HDL-C, mg/dl | 65.0 ± 11.8 | 67.0 ± 11.3 | 66.8 ± 11.7 | 65.8 ± 11.6 | 64.7 ± 11.4 | 60.6 ± 11.5 | < 0.001 |
| Hemoglobin, g/L | 127.4 ± 9.7 | 125.9 ± 9.7 | 126.9 ± 9.5 | 127.4 ± 9.7 | 128.0 ± 9.5 | 129.0 ± 9.9 | < 0.001 |
| Uric acid, μmoI/L | 232.0 (203.3, 265.0) | 224.6 (197.6, 253.9) | 227.4 (199.4, 260.5) | 231.2 (203.3, 261.0) | 232.7 (204.6, 268.1) | 244.8 (213.0, 280.8) | < 0.001 |
| Creatinine, μmoI/L | 43.0 (38.9, 47.2) | 42.8 (38.6, 47.0) | 43.1 (39.0, 47.3) | 43.0 (38.9, 47.1) | 43.2 (39.0, 47.6) | 43.1 (38.9, 47.2) | 0.505 |
| ALT, U/L | 12.6 (9.7, 17.9) | 11.9 (9.5, 16.2) | 12.2 (9.5, 16.9) | 12.3 (9.5, 17.4) | 12.9 (9.9, 18.9) | 13.9 (10.4, 20.5) | < 0.001 |
| AST, U/L | 16.4 (14.3, 19.1) | 16.4 (14.3, 18.9) | 16.3 (14.3, 18.9) | 16.3 (14.2, 18.9) | 16.4 (14.3, 19.4) | 16.5 (14.2, 19.6) | 0.345 |
| Delivery gestations, weeks | 39.2 ± 1.5 | 39.3 ± 1.5 | 39.2 ± 1.6 | 39.2 ± 1.4 | 39.2 ± 1.5 | 39.0 ± 1.6 | < 0.001 |
| Gestational weight gain, kg | 13.5 ± 5.0 | 14.3 ± 4.6 | 14.0 ± 4.5 | 13.6 ± 4.7 | 13.3 ± 5.3 | 12.4 ± 5.5 | < 0.001 |
| Gestational weight gain, n (%) | < 0.001 | ||||||
| <recommendation | 3634 (32.3) | 618 (27.4) | 621 (27.6) | 747 (33.2) | 768 (34.2) | 880 (39.3) | |
| Within recommendation | 4415 (39.3) | 916 (40.7) | 955 (42.5) | 873 (38.8) | 867 (38.6) | 804 (35.9) | |
| >recommendation | 3187 (28.4) | 719 (31.9) | 672 (29.9) | 631 (28) | 612 (27.2) | 553 (24.7) | |
Note: Data are presented as mean ± standard deviation, median (interquartile range) or n (%).
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; DBP, diastolic blood pressure; HDL-C, high density lipoprotein cholesterol; LDL-C, low density lipoprotein cholesterol; SBP, systolic blood pressure; TC, total cholesterol.
TyG Index and Risk of Incident GDM
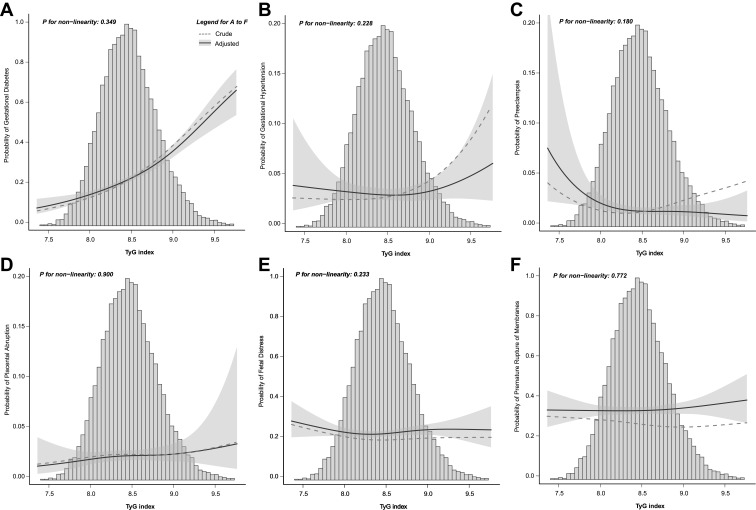
The curvilinear relationship between the TyG index and the probability of GDM is shown in Figure 1A. With or without adjusted confounding factors, the probability of GDM is intensified with the increasing TyG index, P for non-linearity = 0.349 (adjusted model).
Figure 1.
The risk of pregnancy-related complications with increasing triglyceride-glucose (TyG) index. The solid line represents adjusted probability and shadow parts 95% confidence intervals. Adjusted for race, pre-pregnancy BMI, maternal age, assisted reproduction, abortion history, gravidity, parity, gestational weight gain, delivery gestations, gestational week at the examination, systolic blood pressure, diastolic blood pressure, total cholesterol, low density lipoprotein cholesterol, high density lipoprotein cholesterol, hemoglobin, uric acid, creatinine, alanine aminotransferase, and aspartate aminotransferase. (A), Gestational diabetes mellitus; (B), Gestational hypertension; (C), Preeclampsia; (D), Placental abruption; (E), Fetal distress; (F), Premature rupture of membranes.
Table 2 shows the risk of GDM according to the TyG index in logistic regression analysis. After adjusting for race, pre-pregnancy BMI, maternal age, assisted reproduction, mother DM history, abortion history, gravidity, parity, delivery gestations, and GWG (Model 1), a 1-unit and a 1-SD increase in the TyG index increased the risk of GDM by 3.12 and 1.48 times, respectively. Identically, the risk of GDM was highest in the TyG quintile 5 group (OR: 2.74; 95% CI: 2.35~3.19) compared with that in the lowest TyG index group. Even after further adjustment for gestational week at the examination, SBP, DBP, TC, LDL-C, HDL-C, HB, UA, Cr, ALT, and AST, these findings remained unchanged (Model 2). Moreover, the risk of GDM showed a stepwise increase with the quintiles of the TyG index in all models (P for linear trend <0.001).
Table 2.
Association of Triglyceride-Glucose (TyG) Index with Gestational Diabetes Mellitus (GDM)
| Predictor | Number | GDM, n (%) | Crude | P value | Model 1a | P value | Model 2b | P value |
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | ||||||
| TyG index, per unit | 11,387 | 2787 (24.5) | 4.31 (3.79~4.90) | <0.001 | 3.12 (2.72~3.59) | <0.001 | 3.49 (2.87~4.25) | <0.001 |
| TyG index, per SD | 11387 | 2787 (24.5) | 1.66 (1.59~1.73) | <0.001 | 1.48 (1.41~1.56) | <0.001 | 1.54 (1.44~1.65) | <0.001 |
| TyG index Quintile 1 | 2278 | 335 (14.7) | 1 (Ref) | 1 (Ref) | 1 (Ref) | |||
| Quintile 2 | 2275 | 410 (18.0) | 1.28 (1.09~1.49) | 0.003 | 1.22 (1.04~1.44) | 0.015 | 1.20 (1.02~1.41) | 0.031 |
| Quintile 3 | 2279 | 506 (22.2) | 1.66 (1.42~1.93) | <0.001 | 1.46 (1.25~1.71) | <0.001 | 1.41 (1.20~1.66) | <0.001 |
| Quintile 4 | 2276 | 637 (28.0) | 2.25 (1.94~2.61) | <0.001 | 1.90 (1.63~2.22) | <0.001 | 1.81 (1.54~2.14) | <0.001 |
| Quintile 5 | 2279 | 899 (39.4) | 3.78 (3.27~4.36) | <0.001 | 2.74 (2.35~3.19) | <0.001 | 2.52 (2.09~3.05) | <0.001 |
| Trend test | <0.001 | <0.001 | <0.001 |
Notes: aModel 1 adjusted for race, pre-pregnancy BMI, maternal age, assisted reproduction, abortion history, gravidity, parity, delivery gestations, and gestational weight gain; bModel 2 further adjusted for gestational week at the examination, systolic blood pressure, diastolic blood pressure, total cholesterol, low density lipoprotein cholesterol, high density lipoprotein cholesterol, hemoglobin, uric acid, creatinine, alanine aminotransferase, and aspartate aminotransferase. The bolded P value indicates statistical significance.
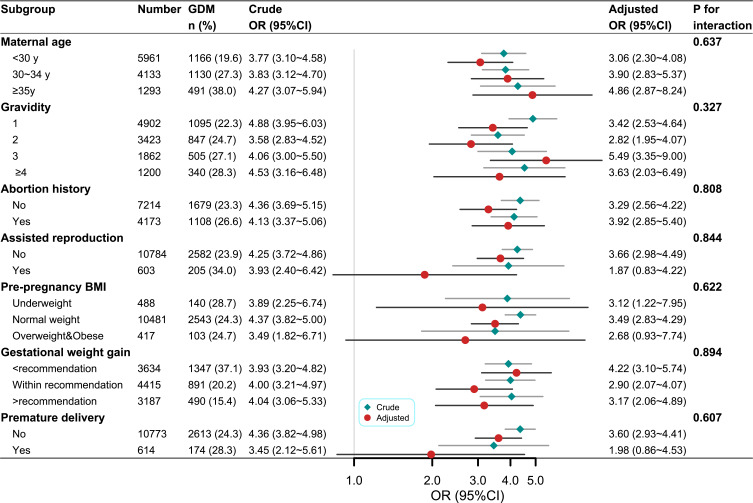
In addition, multiple analyses stratified by age, BMI, assisted reproduction, abortion history, gravidity, parity, and GWG were performed (Figure 2). The TyG index (1-unit increase) was associated with a higher risk for GDM in all subgroups, and no interaction was found between subgroup variables and the association of the TyG index with the risk of GDM.
Figure 2.
Subgroup analysis of the association between triglyceride-glucose (TyG) index and risk of gestational diabetes mellitus (GDM). Models adjusted for race, pre-pregnancy BMI, maternal age, assisted reproduction, abortion history, gravidity, parity, gestational weight gain, delivery gestations, gestational week at the examination, systolic blood pressure, diastolic blood pressure, total cholesterol, low density lipoprotein cholesterol, high density lipoprotein cholesterol, hemoglobin, uric acid, creatinine, alanine aminotransferase, and aspartate aminotransferase (not for adjusted variables stratified analysis).
TyG Index and Risk of Incident Other Pregnancy-Related Complications
Figure 1B–F shows the predictive probability of other pregnancy-related complications according to the TyG index. No noticeable affiliated trend was observed with or without adjusted confounding factors, and all P for non-linearity > 0.05 (adjusted model). Likewise, although a 1-unit in the TyG index increment was associated with a 106% higher risk of gestational hypertension, an 84% higher risk of preeclampsia, and a 26.0% lower risk of premature rupture of membranes were found in the univariate logistic regression analysis, no association between TyG index and the risk of other PRCs remained significantly after fully adjusted (Table 3).
Table 3.
Association of Triglyceride-Glucose (TyG) Index with Other Pregnancy-Related Complications
| Predictor | Number | Pregnancy-Related Complications, n (%) | Crude | P value | Model 1a | P value | Model 2b | P value |
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | ||||||
| Gestational hypertension | 11,387 | 387 (3.4) | 2.06 (1.56~2.73) | <0.001 | 1.86 (1.38~2.5) | <0.001 | 1.07 (0.7~1.66) | 0.746 |
| Preeclampsia | 11,387 | 196 (1.7) | 1.84 (1.25~2.73) | 0.002 | 1.26 (0.83~1.9) | 0.281 | 0.56 (0.30~1.01) | 0.054 |
| Placental abruption | 11,387 | 257 (2.3) | 1.16 (0.82~1.66) | 0.399 | 1.20 (0.82~1.77) | 0.341 | 1.33 (0.78~2.29) | 0.294 |
| Fetal distress | 11,387 | 2152 (18.9) | 0.97 (0.84~1.11) | 0.631 | 1.12 (0.97~1.29) | 0.127 | 1.04 (0.85~1.27) | 0.721 |
| Premature rupture of membranes | 11,387 | 3014 (26.5) | 0.84 (0.74~0.95) | 0.005 | 0.98 (0.86~1.12) | 0.797 | 1.05 (0.88~1.26) | 0.609 |
Notes: aModel 1 adjusted for race, pre-pregnancy BMI, maternal age, assisted reproduction, abortion history, gravidity, parity, delivery gestations, and gestational weight gain; bModel 2 further adjusted for gestational week at the examination, systolic blood pressure, diastolic blood pressure, total cholesterol, low density lipoprotein cholesterol, high density lipoprotein cholesterol, hemoglobin, uric acid, creatinine, alanine aminotransferase, and aspartate aminotransferase. The bolded P value indicates statistical significance.
ROC Curve Analyses of TyG Index to Predict Pregnancy-Related Complications
The ROC curve analyses for predicting PRCs by the TyG index are shown in Table 4. As for detecting GDM, gestational hypertension, and fetal distress, the TyG index showed significant areas under the ROC curve (P < 0.05 for all). Among them, the AUC value of the TyG index for GDM was the highest (AUC: 0.637, 95% CI: 0.625~0.649), and the most optimal predictive cut-off was 8.549, with the sensitivity of 0.553 and specificity of 0.649. Moreover, the cut-off values of the TyG index for other PRCs ranged from 8.4 to 8.6, respectively. Further, the difference in AUC values between the TyG index and FPG was also compared (Supplementary Figure 2). According to the method described by DeLong, the TyG index shows a better predictive capability than FPG (AUC: 0.637 vs 0.613, P = 0.002).
Table 4.
ROC Analysis of the Triglyceride-Glucose (TyG) Index for Detecting Pregnancy-Related Complications
| Predictor | AUC (95% CI), % | Threshold | Sensitivity | Specificity | PPV | NPV | Accuracy | Precision |
|---|---|---|---|---|---|---|---|---|
| Gestational diabetes mellitus | 63.74 (62.55~64.93) | 8.549 | 0.553 | 0.649 | 0.338 | 0.817 | 0.625 | 0.338 |
| Gestational hypertension | 56.41 (53.36~59.46) | 8.547 | 0.512 | 0.601 | 0.043 | 0.972 | 0.598 | 0.043 |
| Preeclampsia | 56.19 (51.87~60.52) | 8.560 | 0.515 | 0.614 | 0.023 | 0.986 | 0.613 | 0.023 |
| Placental abruption | 51.24 (47.65~54.83) | 8.680 | 0.300 | 0.732 | 0.025 | 0.978 | 0.722 | 0.025 |
| Fetal distress | 50.36 (48.99~51.73) | 8.411 | 0.448 | 0.568 | 0.194 | 0.815 | 0.545 | 0.194 |
| Premature rupture of membranes | 51.82 (50.62~53.02) | 8.574 | 0.652 | 0.381 | 0.275 | 0.753 | 0.453 | 0.275 |
Note: The bolded AUC (95% CI) indicates statistical significance.
Abbreviations: AUC: area under curve; PPV positive predictive value; NPV, negative predictive value.
Sensitivity Analysis
The associations between the TyG index and PRCs were not essential attenuated after excluding women with covariates missing data, not the Han ethnicity, or with baseline TG ≥ 5.1 mmol/L (n=10,836, Supplementary Tables 1–4, Supplementary Figure 3).
Discussion
This study comprehensively evaluates the associations between maternal first-trimester TyG index and the risk of PRCs among Chinese pregnant women. We demonstrate the TyG index is significantly and linearly linked with the risk of incident GDM in singleton pregnant women, but is not independently associated with the risk of other PRCs. Based on the ROC analysis, the AUC (95% CI) for GDM is 0.637 (0.625–0.649), and the optimal predictive cut-off is 8.549, with a sensitivity of 0.553 and specificity of 0.649. Furthermore, the TyG index can better predict GDM than FPG. These results imply that the TyG index is a valuable predictor for GDM and thus may be a screening marker to decrease the demand for many oral glucose tolerance assays.
The impaired insulin secretion or sensitivity is commonly known as the primary pathophysiology of GDM, and women with GDM and dominant IR are at a severer risk for adverse outcomes. Euglycemic-hyperinsulinemic clamp and HOMA-IR, the conventional indicators of IR, are limited by invasiveness and complexity in the clinic, questionable accessibility, and lack of cut-off value. Besides, GDM is generally diagnosed at 24–28 weeks of gestation with nearly no time to avoid its onset and harmful effects. Thus, identifying women prone to GDM at early pregnancy is crucial, which will reduce the morbidity by a surrogate marker of IR. Many observations suggest that the TyG index incorporates TG and FPG as potentially useful indicators of IR and shows its importance in forecasting the onset and development of DM among common people. The present study exhibits that the risk of definite GDM is intensified with an increment in first-trimester TyG index in a large Chinese population, regardless of known risk factors. The analyses stratified by maternal age, pre-pregnancy BMI, assisted reproduction, abortion history, gravidity, or GWG does not alter our main results. Similarly, studies involving 954 Iranian21 and 352 Chinese18 women demonstrated that mothers in the top tertile versus the bottom tertile of the first-trimester TyG index are 4.91 and 3.54 times more susceptible to GDM, respectively. A study using a Korean National Health Screening Exam20 indicates that a 1-SD increase in the TyG index two years before pregnancy raises 33% risk of incident GDM among 380,208 Korean primipara women. A meta-analysis further verified these results.26 Given the finding that the TyG index is a simple way of IR evaluation, using it to recognize high-risk women may be a valuable alternative to the universal identification of people at high risk for GDM, and may reduce the number of women in demand for oral glucose tolerance assay.
Epidemiological research also suggests IR as an initiation factor of preeclampsia and hypertensive disorders of pregnancy.27–31 A study shows a four-fold increase in the risk of hypertensive disorders in insulin-resistant pregnant women.27 The underlying mechanism may be as follows:32,33 over-activation of IR during pregnancy leads to placental hypoxia and ischemia, reduced carbon monoxide (NO), increased oxidative stress, discorded lipid metabolism, decreased prostaglandin E2 synthesis, damaged vascular endothelial cells, and promoted endothelial dysfunction, that results in the characteristic symptoms of preeclampsia (eg, hypertension, proteinuria, and edema). Although our univariate analysis shows the TyG index is related to the risk of hypertension during pregnancy, this association was not significant after possible confounders were adjusted. In addition, we cannot observe an independent association of TyG index with preeclampsia, placental abruption, fetal distress, or premature membrane rupture, which is similar to the results from a previous small-size study.22 There is still relatively little research on the relationships between the TyG index and other PRCs beyond GDM, which calls for confirmation by more studies.
The predicting ability of the TyG index for type 2 diabetes mellitus (T2DM) or GDM has already been addressed, and the appropriate thresholds for the TyG index are comparable across most studies.18,20,22,34–38 A 15-year prospective study in a Chinese population by Wang et al indicates the threshold impact between the TyG index around 8.51 and incident T2DM with a nadir of risk.37 Lee et al35 demonstrated the cut-off point of the TyG index in forecasting T2DM among 7708 Koreans aged 40–69 years is 8.52. Zhang et al36 revealed that the optimal TyG index for T2DM in women is 8.76 (sensitivity 65.0%, specificity 70.2%). Kim et al20 reported that the cut-off point of the TyG index in 2 years before pregnancy for GDM was 8.15 (sensitivity 47.0%, specificity 68.2%, AUC 0.60). As for the diagnostic behavior of the TyG index to recognize GDM at 24–28 weeks of pregnancy, Sanchez-Garcia et al found a relatively low TyG index cut-off of 4.69 (sensitivity 89.0%, specificity 50%).22 In addition, Liu et al18 investigated the diagnostic ability of the first prenatal visit TyG index to predict the risk of GDM, of which AUC was 0.686 (95% CI: 0.615–0.756), but no best threshold value was reported. Here, our results are consistent with most of the previous studies, as the predictive capability of the TyG index qualified for GDM has an AUC of 63.74%, and the optimal predictive cut-off value of most PRCs is around 8.549. Noteworthy, the TyG index is a more robust predictor of GDM compared to FPG. Given the growing shreds of evidence, first-trimester maternal TyG index is a potential early biomarker of GDM as it reflects the degree of IR, and the population quartile of 75% to 80% (approximately TyG index= 8.5) can be used as a threshold value in clinical practice.
The merits of our research include the prospective design, large sample size, multi-study outcomes, and cautious recording of obstetric complications by researchers. Blood samples and routine maternal care in China were detected in the first trimester of pregnancy, which may provide earlier data and more time for intervention to avoid or minimize the onset of PRCs. Moreover, to mitigate bias, mothers with undiagnosed DM can be excluded from the first-trimester FPG levels. Several limitations should be considered. Firstly, we failed to collect data on some potential confounders, such as the family history of hypertension or diabetes, dietary, physical activity, and sleep status. Nevertheless, the absence of these factors is unable to substantially alter our results since we have already adjusted many essential confounders. Secondly, our study lacks biomarkers (eg, β-cell function, insulin, NO) to explore the mechanisms underlying the effect of the TyG index on PRCs. Lastly, only women in southeast China were enrolled, so our results cannot be generalized to be clinically effective for the overall population and call for parallel investigations into other people.
Conclusion
The first-trimester TyG index is intimately related to the risk of incident GDM. It may be an early beneficial screening and monitoring tool to reduce the onset and progression of GDM in the clinic. Definitely, the relationships between the TyG index and other PRCs need further research. More research is demanded to repeat our findings and to explore the cause-effect of the relationships, the concrete mechanisms, and the possibility for public health interventions.
Acknowledgments
The authors are grateful to all of the participants, the staff, and the other study investigators for their valuable contributions. Additionally, we thank the Free Statistics team (Beijing, China) for providing technical assistance and practical data analysis and visualization tools. Additionally, we thank Dr. Xiao, National Center for Children’s Health, Beijing Children’s Hospital, for the assistance in revising the manuscript.
Funding Statement
This work was supported by the Natural Science Foundation of Fujian Province of China for Youths (Grant No. 2021J05076) (Haibo Li), the Key Project on Science and Technology Program of Fujian Health Commission (Grant No. 2021ZD01002) (Hua Cao), the provincial-level special subsidy funds for health in Fujian Province of China (Grant No. Fujian Finance Index (2019) 827) (Yibing Zhu), and the Scientific Research Foundation of Fujian Maternity and Child Health Hospital (Grant No. YCXY20-04) (Haibo Li). The funder did not contribute to the study’s design, collection, analysis, and interpretation of data.
Ethical Approval and Informed Consent
The studies involving human participants were reviewed and approved by the Research Ethics Committee of Fujian Maternity and Child Health Hospital (approval number: 2017KR-030) and complied with the Declaration of Helsinki. The participants provided their written informed consent to participate in this study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Luscher TF. Forgotten cardiovascular risk factors: pregnancy complications and preterm birth, bullying, periodontal disease, and hypoxic burden. Eur Heart J. 2019;40:1093–1096. doi: 10.1093/eurheartj/ehz171 [DOI] [PubMed] [Google Scholar]
- 2.Chu AHY, Yuan WL, Loy SL, et al. Maternal height, gestational diabetes mellitus and pregnancy complications. Diabetes Res Clin Pract. 2021;178:108978. doi: 10.1016/j.diabres.2021.108978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perak AM, Lancki N, Kuang A, et al. Associations of maternal cardiovascular health in pregnancy with offspring cardiovascular health in early adolescence. JAMA. 2021;325:658–668. doi: 10.1001/jama.2021.0247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu W, Liu Q, Liu W, Qiu C. Maternal risk factors and pregnancy complications associated with low birth weight neonates in preterm birth. J Obstet Gynaecol Res. 2021;47(9):3196–3202. doi: 10.1111/jog.14830 [DOI] [PubMed] [Google Scholar]
- 5.Song C, Xu Y, Ding Y, et al. The rates and medical necessity of cesarean delivery in China, 2012–2019: an inspiration from Jiangsu. BMC Med. 2021;19:14. doi: 10.1186/s12916-020-01890-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang M, Hu RY, Gong WW, et al. Trends in prevalence of gestational diabetes mellitus in Zhejiang Province, China, 2016–2018. Nutr Metab. 2021;18:12. doi: 10.1186/s12986-020-00539-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Minschart C, Beunen K, Benhalima K. An update on screening strategies for gestational diabetes mellitus: a narrative review. Diabetes Metab Syndr Obes. 2021;14:3047–3076. doi: 10.2147/DMSO.S287121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Korzeniewski SJ, Sutton E, Escudero C, Roberts JM. The global pregnancy collaboration (CoLab) symposium on short- and long-term outcomes in offspring whose mothers had preeclampsia: a scoping review of clinical evidence. Front Med. 2022;9:984291. doi: 10.3389/fmed.2022.984291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christensen MH, Rubin KH, Petersen TG, et al. Cardiovascular and metabolic morbidity in women with previous gestational diabetes mellitus: a nationwide register-based cohort study. Cardiovasc Diabetol. 2022;21:179. doi: 10.1186/s12933-022-01609-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guerrero-Romero F, Simental-Mendia LE, Gonzalez-Ortiz M, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95:3347–3351. doi: 10.1210/jc.2010-0288 [DOI] [PubMed] [Google Scholar]
- 11.Simental-Mendia LE, Rodriguez-Moran M, Guerrero-Romero F. The product of fasting glucose and triglycerides as surrogate for identifying insulin resistance in apparently healthy subjects. Metab Syndr Relat Disord. 2008;6:299–304. doi: 10.1089/met.2008.0034 [DOI] [PubMed] [Google Scholar]
- 12.Yu X, Wang L, Zhang W, et al. Fasting triglycerides and glucose index is more suitable for the identification of metabolically unhealthy individuals in the Chinese adult population: a nationwide study. J Diabetes Investig. 2019;10:1050–1058. doi: 10.1111/jdi.12975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lambrinoudaki I, Kazani MV, Armeni E, et al. The TyG index as a marker of subclinical atherosclerosis and arterial stiffness in lean and overweight postmenopausal women. Heart Lung Circ. 2018;27:716–724. doi: 10.1016/j.hlc.2017.05.142 [DOI] [PubMed] [Google Scholar]
- 14.Barzegar N, Tohidi M, Hasheminia M, Azizi F, Hadaegh F. The impact of triglyceride-glucose index on incident cardiovascular events during 16 years of follow-up: Tehran lipid and glucose study. Cardiovasc Diabetol. 2020;19:155. doi: 10.1186/s12933-020-01121-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poveda NE, Garces MF, Darghan AE, et al. Triglycerides/glucose and triglyceride/high-density lipoprotein cholesterol indices in normal and preeclamptic pregnancies: a longitudinal study. Int J Endocrinol. 2018;2018:8956404. doi: 10.1155/2018/8956404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newbern D, Freemark M. Placental hormones and the control of maternal metabolism and fetal growth. Curr Opin Endocrinol Diabetes Obes. 2011;18:409–416. doi: 10.1097/MED.0b013e32834c800d [DOI] [PubMed] [Google Scholar]
- 17.Vasques AC, Novaes FS, de Oliveira Mda S, et al. TyG index performs better than HOMA in a Brazilian population: a hyperglycemic clamp validated study. Diabetes Res Clin Pract. 2011;93:e98–e100. doi: 10.1016/j.diabres.2011.05.030 [DOI] [PubMed] [Google Scholar]
- 18.Liu PJ, Liu Y, Ma L, et al. The predictive ability of two triglyceride-associated indices for gestational diabetes mellitus and large for gestational age infant among Chinese pregnancies: a preliminary cohort study. Diabetes Metab Syndr Obes. 2020;13:2025–2035. doi: 10.2147/DMSO.S251846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, Ge Z, Chen L, et al. Risk prediction model of gestational diabetes mellitus in a Chinese population based on a risk scoring system. Diabetes Ther. 2021;12:1721–1734. doi: 10.1007/s13300-021-01066-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim JA, Kim J, Roh E, et al. Triglyceride and glucose index and the risk of gestational diabetes mellitus: a nationwide population-based cohort study. Diabetes Res Clin Pract. 2021;171:108533. doi: 10.1016/j.diabres.2020.108533 [DOI] [PubMed] [Google Scholar]
- 21.Pazhohan A, Rezaee Moradali M, Pazhohan N. Association of first-trimester maternal lipid profiles and triglyceride-glucose index with the risk of gestational diabetes mellitus and large for gestational age newborn. J Matern Fetal Neonatal Med. 2019;32:1167–1175. doi: 10.1080/14767058.2017.1402876 [DOI] [PubMed] [Google Scholar]
- 22.Sanchez-Garcia A, Rodriguez-Gutierrez R, Saldivar-Rodriguez D, et al. Diagnostic accuracy of the triglyceride-glucose index for gestational diabetes screening: a practical approach. Gynecol Endocrinol. 2020;36:1112–1115. doi: 10.1080/09513590.2020.1742687 [DOI] [PubMed] [Google Scholar]
- 23.Weinert LS. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy: comment to the international association of diabetes and pregnancy study groups consensus panel. Diabetes Care. 2010;33:e97. doi: 10.2337/dc10-0544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Institute of M, National Research Council Committee to Reexamine IOMPWG. The National Academies Collection: reports funded by National Institutes of Health. In: Rasmussen KM, Yaktine AL, editors. Weight Gain During Pregnancy: Reexamining the Guidelines. Washington (DC): National Academies Press (US), National Academy of Sciences; 2009. [PubMed] [Google Scholar]
- 25.Wood SN. Stable and Efficient Multiple Smoothing Parameter Estimation for Generalized Additive Models. J Am Stat Assoc. 2004;99:673–686. doi: 10.1198/016214504000000980 [DOI] [Google Scholar]
- 26.Song T, Su G, Chi Y, Wu T, Xu Y, Chen C. Triglyceride-glucose index predicts the risk of gestational diabetes mellitus: a systematic review and meta-analysis. Gynecol Endocrinol. 2021;38:1–6. [DOI] [PubMed] [Google Scholar]
- 27.Lin J, Jin H, Chen L. Associations between insulin resistance and adverse pregnancy outcomes in women with gestational diabetes mellitus: a retrospective study. BMC Pregnancy Childbirth. 2021;21:526. doi: 10.1186/s12884-021-04006-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Niekerk G, Christowitz C, Engelbrecht AM. Insulin-mediated immune dysfunction in the development of preeclampsia. J Mol Med. 2021;99:889–897. doi: 10.1007/s00109-021-02068-0 [DOI] [PubMed] [Google Scholar]
- 29.Parretti E, Lapolla A, Dalfra M, et al. Preeclampsia in lean normotensive normotolerant pregnant women can be predicted by simple insulin sensitivity indexes. Hypertension. 2006;47:449–453. doi: 10.1161/01.HYP.0000205122.47333.7f [DOI] [PubMed] [Google Scholar]
- 30.D’Anna R, Baviera G, Corrado F, et al. Adiponectin and insulin resistance in early- and late-onset preeclampsia. BJOG. 2006;113:1264–1269. doi: 10.1111/j.1471-0528.2006.01078.x [DOI] [PubMed] [Google Scholar]
- 31.Sierra-Laguado J, Garcia RG, Celedon J, et al. Determination of insulin resistance using the homeostatic model assessment (HOMA) and its relation with the risk of developing pregnancy-induced hypertension. Am J Hypertens. 2007;20:437–442. doi: 10.1016/j.amjhyper.2006.10.009 [DOI] [PubMed] [Google Scholar]
- 32.Lopez-Jaramillo P, Barajas J, Rueda-Quijano SM, Lopez-Lopez C, Felix C. Obesity and preeclampsia: common pathophysiological mechanisms. Front Physiol. 2018;9:1838. doi: 10.3389/fphys.2018.01838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Villalobos-Labra R, Silva L, Subiabre M, et al. Akt/mTOR role in human foetoplacental vascular insulin resistance in diseases of pregnancy. J Diabetes Res. 2017;2017:1–13. doi: 10.1155/2017/5947859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chamroonkiadtikun P, Ananchaisarp T, Wanichanon W. The triglyceride-glucose index, a predictor of type 2 diabetes development: a retrospective cohort study. Prim Care Diabetes. 2020;14:161–167. doi: 10.1016/j.pcd.2019.08.004 [DOI] [PubMed] [Google Scholar]
- 35.Lee JW, Lim NK, Park HY. The product of fasting plasma glucose and triglycerides improves risk prediction of type 2 diabetes in middle-aged Koreans. BMC Endocr Disord. 2018;18:33. doi: 10.1186/s12902-018-0259-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang M, Wang B, Liu Y, et al. Cumulative increased risk of incident type 2 diabetes mellitus with increasing triglyceride glucose index in normal-weight people: the rural Chinese cohort study. Cardiovasc Diabetol. 2017;16:30. doi: 10.1186/s12933-017-0514-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Z, Zhao L, He S. Triglyceride-glucose index as predictor for future type 2 diabetes mellitus in a Chinese population in southwest China: a 15-year prospective study. Endocrine. 2021;72:124–131. doi: 10.1007/s12020-020-02589-7 [DOI] [PubMed] [Google Scholar]
- 38.Wang B, Zhang M, Liu Y, et al. Utility of three novel insulin resistance-related lipid indices for predicting type 2 diabetes mellitus among people with normal fasting glucose in rural China. J Diabetes. 2018;10:641–652. doi: 10.1111/1753-0407.12642 [DOI] [PubMed] [Google Scholar]