Abstract
Background
ENPP1 Deficiency—caused by biallelic variants in ENPP1—leads to widespread arterial calcification in early life (Generalized Arterial Calcification of Infancy, GACI) or hypophosphatemic rickets in later life (Autosomal Recessive Hypophosphatemic Rickets type 2, ARHR2). A prior study using the Exome Aggregation Consortium (ExAC)—a database of exomes obtained from approximately 60,000 individuals—estimated the genetic prevalence at approximately 1 in 200,000 pregnancies.
Methods
We estimated the genetic prevalence of ENPP1 Deficiency by evaluating allele frequencies from a population database, assuming Hardy–Weinberg equilibrium. This estimate benefitted from a comprehensive literature review using Mastermind (https://mastermind.genomenon.com/), which uncovered additional variants and supporting evidence, a larger population database with approximately 140,000 individuals, and improved interpretation of variants as per current clinical guidelines.
Results
We estimate a genetic prevalence of approximately 1 in 64,000 pregnancies, thus more than tripling the prior estimate. In addition, the carrier frequency of ENPP1 variants was found to be highest in East Asian populations, albeit based on a small sample.
Conclusion
These results indicate that a significant number of patients with ENPP1 Deficiency remain undiagnosed. Efforts to increase disease awareness as well as expand genetic testing, particularly in non-European populations are warranted, especially now that clinical trials for enzyme replacement therapy, which proved successful in animal models, are underway.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13023-022-02577-2.
Keywords: ENPP1 deficiency, Generalized arterial calcification of infancy (GACI), Autosomal recessive hypophosphatemic rickets type 2 (ARHR2), Population database, Prevalence
Introduction
Ectonucleotide pyrophosphatase/phosphodiesterase 1 (ENPP1; OMIM #173,335) is a transmembrane protein responsible for cleaving ectonucleotides, predominantly adenosine triphosphate (ATP), to generate adenosine monophosphate (AMP) and pyrophosphate (PPi). ENPP1 represents the main contributor to systemic and local concentrations of PPi, a potent inhibitor of hydroxyapatite deposition [1, 2]. ENPP1 Deficiency can result in Generalized Arterial Calcification of Infancy (GACI; OMIM #208,000) which is characterized by ectopic mineralization, particularly along the internal elastic lamina of large and medium-sized arteries, as well as periarticular calcification. Additional arterial involvement stems from intimal proliferation, with consequent luminal narrowing. The constellation of vascular findings leads to organ ischemia, with cardiac failure a common presentation of the disease. Mortality is high, with approximately half of all infants dying from the disease within the first few months of life [2]. In addition, ENPP1 Deficiency can result in Autosomal Recessive Hypophosphatemic Rickets Type 2 (ARHR2; OMIM #613,312) in those who survive GACI, or in individuals who never had clinical cardiovascular manifestations. This form of hypophosphatemic rickets is mediated by FGF23 (OMIM #605,380), a hormone that increases renal losses of phosphate, but the etiology of FGF23 increase remains unknown as of yet [3]. Finally, adult patients present with musculoskeletal symptoms, representing a major cause of morbidity in later life [4].
ENPP1 Deficiency as a whole is known to be a rare disease—defined as a disease affecting less than 200,000 individuals in the United States—but arriving at an accurate estimate of the prevalence is challenging. It is crucial that these estimates are as accurate as possible in order to appropriately assess disease burden as well as the number of patients who may benefit from newly developed therapies. Traditional methods of estimating prevalence rely on clinical data, the accuracy of which can be negatively impacted by diagnostic difficulties, especially for rare diseases with nonspecific and/or heterogeneous phenotypes. For ENPP1 Deficiency, clinical heterogeneity, low clinical awareness, and a high infant mortality rate all contribute to the difficulty associated with estimating the disease prevalence, as clinical data can be unreliable and result in an estimate that is insufficiently representative of the actual patient population.
One method to overcome these diagnostic challenges in estimating prevalence would be to base the estimate on genetic, rather than clinical, data. One such technique based on genetic data involves evaluating the frequency of pathogenic variants in population databases, and then estimating the genetic prevalence of the disease assuming Hardy–Weinberg equilibrium [5–7]. The accuracy of this technique relies on the completeness of the knowledgebase of causative variants as well as the number of healthy individuals contained within the population database that is utilized. A prior study using this technique estimated the genetic prevalence of ENPP1 Deficiency at approximately 1 in 200,000 [3].
In this study, we improve on the previous estimate by performing a more comprehensive literature review using Mastermind to identify additional variants as well as supporting evidence, by using clinical standard variant interpretation to assess the level of evidence supporting the inclusion of variants in the estimate, and by using a larger population database [8].
Results
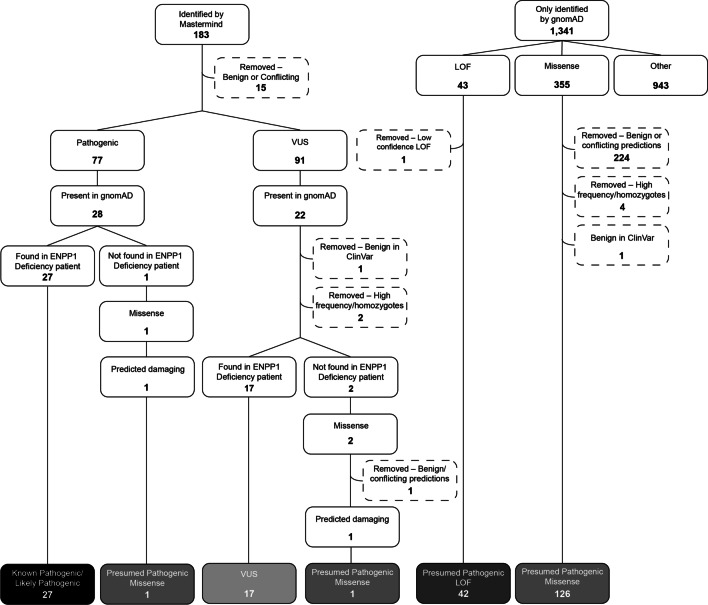
To begin determining ENPP1 Deficiency prevalence estimates, ENPP1 variants were identified through a comprehensive literature review performed using the Mastermind Genomic Search Engine [8]. A total of 183 ENPP1 variants were identified and interpreted according to the American College of Medical Genetics and Association of Molecular Pathologists (ACMG/AMP) variant interpretation guidelines [9]. Of these variants, 77 were classified as Pathogenic/Likely Pathogenic, 91 as Variants of Undetermined Significance (VUS), 13 as Benign/Likely Benign and 2 as conflicting (having sufficient evidence to meet both a Benign and Pathogenic classification). All Benign/Likely Benign and conflicting variants were excluded from the proceeding analysis.
Variants classified as Pathogenic/Likely Pathogenic or VUS that had a non-zero allele frequency in the Genome Aggregation Database (gnomAD) were included or excluded as described in Additional file 1: Figs. S1 and S2, respectively [10]. This selection process considered the variant’s presence in ENPP1 Deficiency patients, allele frequency and presence in homozygotes in gnomAD, classification in ClinVar, effect type, and predictions from computational algorithms [11]. Following this process, a total of 27 Pathogenic/Likely Pathogenic variants and 17 VUSs were included in the prevalence calculation as Known Pathogenic/Likely Pathogenic variants and VUS variants, respectively (Fig. 1; Additional file 2: Table S1). Notably, 49 of the total Pathogenic/Likely Pathogenic variants (64%) and 69 of the total VUS (76%) were excluded solely as a result of not being present in gnomAD.
Fig. 1.
Inclusion/Exclusion of Variants in ENPP1. The number of variants included or excluded, along with the reason, is displayed. Loss of function (LOF) variants include start loss, nonsense, frameshift, and canonical splice site variants. VUS Variant of Undetermined Significance
In addition, there were variants in the gnomAD database that were not identified in the published literature, which were included or excluded as described in Additional file 1: Fig. S3. This selection process considered the variant’s allele frequency and presence in homozygotes in gnomAD, classification in ClinVar, effect type, and predictions from computational algorithms. Following this process, a total of 42 Presumed Pathogenic Loss of Function (LOF) variants and 128 Presumed Pathogenic missense variants were included in the prevalence calculation (Fig. 1; Additional file 2: Table S1).
The overall allele frequency in gnomAD was summed across the specific groups of included variants—Known Pathogenic/Likely Pathogenic, VUS, Presumed Pathogenic LOF, and Presumed Pathogenic Missense variants. The carrier frequency and genetic prevalence was then calculated using the Hardy–Weinberg equilibrium equation for these groups to showcase the range of estimates that result from different levels of stringency in the variant selection process.
The carrier frequency for ENPP1 variants associated with ENPP1 Deficiency was estimated to be 1 in 509 to 1 in 127 in the general population which corresponds to a genetic prevalence of 1 in 1,033,927 to 1 in 64,035 pregnancies (Table 1).
Table 1.
Genetic Prevalence of ENPP1 Deficiency
| Known pathogenic/Likely pathogenic | Known pathogenic/Likely pathogenic and LOF presumed pathogenic | Known pathogenic/Likely pathogenic and all presumed pathogenic | |
|---|---|---|---|
| VUS excluded | 1/1,033,927 | 1/334,177 | 1/82,620 |
| VUS included | 1/471,585 | 1/206,123 | 1/64,035 |
The genetic prevalence was calculated using the Hardy–Weinberg equilibrium equation and the sum of the allele frequencies of the specified variants. The ratio represents the proportion of pregnancies that have an ENPP1 Deficiency associated genotype
Known Pathogenic/Likely Pathogenic variants that were classified as Pathogenic/Likely Pathogenic by ACMG/AMP
LOF Presumed Pathogenic start loss, nonsense, frameshift, and canonical splice site variants that were found in gnomAD but not the published literature
All Presumed Pathogenic LOF Presumed Pathogenic variants in addition to predicted damaging missense variants that were found in gnomAD but not the published literature
There was a 1,515% difference between the lowest, most conservative prevalence estimate, which included only variants known to be Pathogenic/Likely Pathogenic by ACMG/AMP, and the highest, most inclusive prevalence estimate, which included all Presumed Pathogenic variants as well as VUS. Notably, the inclusion of Presumed Pathogenic LOF and Presumed Pathogenic Missense variants had a large impact on the estimated prevalence. Inclusion of Presumed Pathogenic LOF variants increased the estimate (excluding VUSs) by 209% which increased by another 304% with inclusion of Presumed Pathogenic missense variants. In contrast, inclusion of VUS had a comparatively minor impact on the estimated prevalence—a 29–119% increase.
Our estimated genetic prevalence was also 211% higher than a previous study which estimated it to be ~ 1 in 200,000 pregnancies, including all presumed Pathogenic variants [3]. This study had several methodological differences including the use of a smaller population database (the Exome Aggregation Consortium (ExAC)), not using Mastermind to complete a literature review, and no use of ACMG/AMP interpretation [12]. Because of the greater number of individuals represented in gnomAD as compared to ExAC, the number of carriers identified was likely increased based on a more representative statistical sampling for these rare variants. Moreover, a significant number of new variants and patients were reported since the prior publication, resulting in both a greater number of Known Pathogenic/Likely Pathogenic variants (27 compared to 17) as well as an increased number of Presumed Pathogenic variants (170 compared to 96).
Specifically pertaining to the Known Pathogenic/Likely Pathogenic variants, there were 8 variants that were presumed to be Pathogenic in the previous study by assessment of computational predictions but confirmed to be Pathogenic/Likely Pathogenic by ACMG/AMP standards in this study, 3 variants that were classified as Pathogenic/Likely Pathogenic in both studies but were present only in gnomAD, 3 variants that were classified as Pathogenic in the previous study but were VUS by ACMG/AMP in this study, 2 that were presumed to be Benign in the previous study but were found to be Likely Pathogenic in this study, and 1 variant that was not identified in the previous study but was found to be Pathogenic/Likely Pathogenic in this study.
Overall, the assessment of complete literature evidence along with ACMG/AMP interpretation was instrumental in ensuring the precision of the genetic prevalence estimate. If the estimate relied solely on classifications in ClinVar, but otherwise used the same methodology, a total of 15 variants would have been excluded on the basis of conflicting classifications and/or conflicting computational predictions.
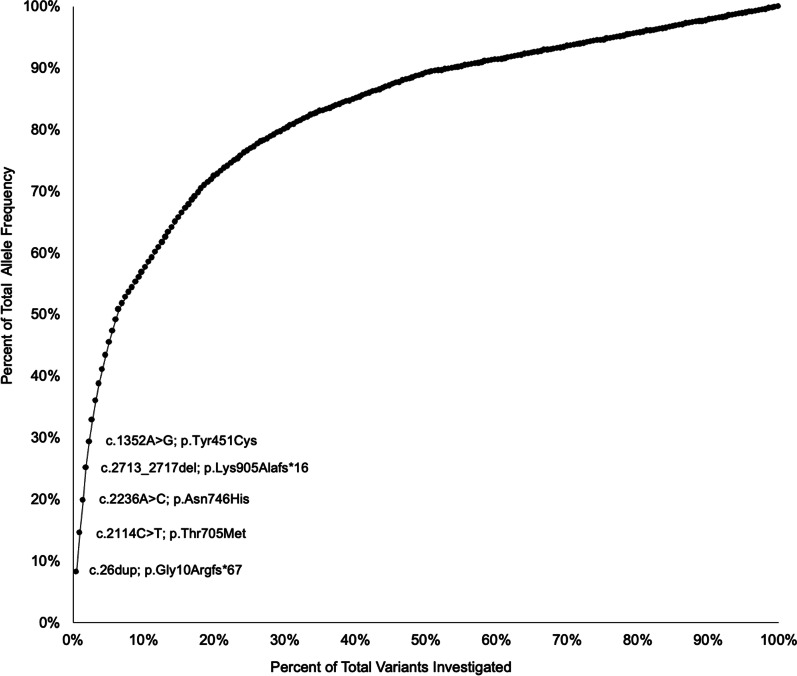
Assessing the contribution of individual ENPP1 variants to the genetic prevalence estimate revealed that only 13 variants accounted for 50% of the total allele frequency included in the prevalence calculation (Fig. 2). The top five most frequent variants alone—c.26dup; p.Gly10ArgfsTer67 (0.032% allele frequency), c.2114C > T; p.Thr705Met (0.025% allele frequency), c.2236A > C; p.Asn746His (0.021% allele frequency), c.2713_2717del; p.Lys905AlafsTer16 (0.021% allele frequency), c.1352A > G; p.Tyr451Cys (0.016% allele frequency)—accounted for 30% of the total.
Fig. 2.
Contribution of Individual ENPP1 Variants to the Genetic Prevalence Estimate
The most common variant (c.26dup; p.Gly10ArgfsTer67) is a previously unpublished variant that is presumed to be pathogenic as a result of it being a frameshift variant at the 5’ end of the gene but may have an inflated allele frequency due to the small number of captured alleles (3,086 overall). The remaining high frequency variants were unaffected by this issue. p.Thr705Met is an unpublished variant that is predicted to be pathogenic by computational algorithms, p.Asn746His has previously been found in four probands initially suspected of having X-Linked Hypophosphatemia (XLH) but is considered a VUS by both our interpretation and interpretations in ClinVar, and p.Lys905AlafsTer16 and p.Tyr451Cys have been found in multiple patients with GACI/ARHR2 and are interpreted as Pathogenic and Likely Pathogenic, respectively [13–16].
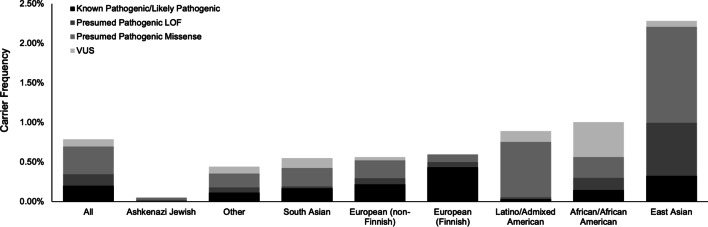
In addition, the estimated heterozygous carrier frequency of ENPP1 variants was found to vary between specific populations in gnomAD, with the East Asian population having a significantly higher carrier frequency than other populations (2.3%; Fig. 3). The most frequent variant in the East Asian population is c.26dup; p.Gly10Argfs*67 (0.32% allele frequency in the East Asian population). As noted previously, this variant was captured in a low number of alleles overall, and 1/310 alleles in this population, which could have artificially inflated the allele frequency, and subsequently the carrier frequency. Removing this variant from consideration, the carrier frequency of ENPP1 variants in the East Asian population is 1.6%, which remains higher than other populations in gnomAD.
Fig. 3.
Population-Specific Carrier Frequencies of ENPP1 Variants. The carrier frequency was calculated using the Hardy–Weinberg equilibrium equation and the sum of the allele frequencies of the specified variants. Known Pathogenic/Likely Pathogenic: variants that were classified as Pathogenic/Likely Pathogenic by ACMG/AMP. Presumed Pathogenic LOF: start loss, nonsense, frameshift, and canonical splice site variants that were found in gnomAD but not the published literature. Presumed Pathogenic Missense: predicted damaging missense variants that were found in gnomAD but not the published literature
Notably, considering only the Known Pathogenic/Likely Pathogenic variants, the carrier frequency of ENPP1 variants was highest in the Finnish population at 0.43%, which the next highest being the East Asian population at 0.32% (Fig. 3). The most frequent pathogenic variant in the Finnish population is c.2713_2717del; p.Lys905Alafs*16 (0.18% allele frequency in the Finnish population), which has previously been published in three Caucasian siblings with GACI as well as three unrelated patients with GACI/ARHR2, two of which were American (unspecified ethnicity), and one of which was Finnish [3, 14, 16].
Discussion
An adequate assessment of the prevalence of rare diseases is crucial for estimating the burden of disease as well as assessing the number of individuals who might benefit from potential new therapies. Rare diseases are often difficult to diagnose which limits the accuracy of epidemiological assessment that is based solely on clinical reports. One recently developed technique that can overcome this limitation involves evaluating the frequency of pathogenic variants in population databases, and then estimating the genetic prevalence of the disease assuming Hardy–Weinberg equilibrium [5–7].
Prior work estimated the genetic prevalence of ENPP1 Deficiency as 1 in 200,000 pregnancies, 3.1-fold less frequent than the current estimate of 1 in 64,000 pregnancies [3]. This discrepancy can be attributed to the identification of additional variants since the prior publication, the use of a 2.3-fold larger population database (gnomAD having 141,456 individuals compared to 60,706 in ExAC), and the use of clinical standard variant interpretation [10, 12]. Thus, our numbers should not be considered definitive, but rather an improved estimate of genetic prevalence based on increased availability of population data and published reports of ENPP1 Deficiency in the five intervening years between these two analyses. Our genetic prevalence estimates could be further improved in coming years, especially with inclusion of data from larger population studies, such as the UK Biobank [17].
This genetic prevalence estimate also has several important caveats that should be considered in terms of its accuracy and impact on diagnosis and therapeutic development for ENPP1 Deficiency. (1) This estimate cannot predict the phenotypic presentation of the disease, given the clinical heterogeneity and a lack of documented genotype–phenotype correlation, and should be interpreted as an estimate of all phenotypes associated with ENPP1 Deficiency; (2) This estimate is only representative of the number of pregnancies with a disease-associated genotype and not a birth prevalence, given the possibility of in utero lethality; (3) A significant number of patients with ENPP1 Deficiency present with novel variants not previously reported in the literature and/or not captured in a population database; (4) A small proportion of ENPP1 Deficiency cases result from structural variants; however, gnomAD lacks complete data on these variants and so they were not considered in our estimate; and (5) Our method of estimating genetic prevalence only takes into account biallelic inheritance (homozygous or compound heterozygous), while recent literature suggests that certain variants can lead to disease even in a heterozygous state [13, 18]. All of these aforementioned factors, as well as additional diagnostic challenges, could lead to a discrepancy between the estimated genetic prevalence and the observed clinical prevalence of ENPP1 Deficiency.
The current genetic prevalence estimate of 1 in 64,000 pregnancies indicates that ENPP1 Deficiency is likely significantly under-diagnosed clinically. In addition, our results indicate that ENPP1 Deficiency may be more common in certain populations, particularly East Asian populations. This estimate, however, is complicated by the relative lack of sequencing coverage in these populations, with only 9,977 genomes or exomes available in gnomAD compared to 64,603 for Non-Finnish European populations. Expanded sequencing efforts in East Asia would be required to confirm this finding.
These results, overall, indicate that expanded genetic testing, especially in non-European populations, will be crucial for ensuring comprehensive diagnosis of patients with ENPP1 Deficiency. The emerging use of Newborn Screening by Next-Generation Sequencing will support this effort by ensuring early diagnosis and mitigating the challenges presented by the clinical heterogeneity and low clinical awareness of the disease.
These efforts to expand sequencing are particularly important now that an enzyme replacement therapy (ERT) is being developed and has been shown to be beneficial in mouse models of ENPP1 Deficiency [4, 13, 18–20]. Specifically, ERT has been shown to be effective in preventing mortality and vascular calcification, improving blood pressure and cardiovascular function, suppressing vascular cell proliferation and intimal hyperplasia, preventing osteomalacia, and partially preventing musculoskeletal comorbidities such as enthesis calcification. Clinical trials of ERT in patients with ENPP1 Deficiency are also currently underway. Accurate and timely diagnosis of patients with ENPP1 Deficiency will be crucial to ensure that patients receive appropriate treatment, should this therapy be approved for clinical use.
Conclusion
We provided a more accurate genetic prevalence estimate of ENPP1 Deficiency benefiting from a comprehensive literature review using Mastermind, a larger population database, and improved interpretation of variants as per current clinical guidelines. The current estimate of 1 in 64,000 pregnancies suggests that ENPP1 Deficiency is likely significantly underdiagnosed. In addition, the higher carrier frequency observed in East Asian populations illustrates a need for expanded sequencing in non-European populations both to confirm this finding and to ensure maximal and accurate diagnosis.
Methodology
Aggregation, classification, and selection of ENPP1 variants for inclusion in genetic prevalence calculation
Published variants in ENPP1, including single-nucleotide variants (SNVs) and indels, along with their corresponding references, were automatically identified and extracted from the medical and scientific literature using Mastermind, a database that is assembled by Genomenon, Inc. [8]. This analysis included systematic review of 1,547 articles describing variants in ENPP1 published on or before May 23, 2022.
Each variant was standardized using the GRCh37/hg19 genome build and the canonical transcript—NM_006208.3—as well as the nomenclature guidelines set by the Human Genome Variation Society (HGVS) [21]. These variants were then manually interpreted according to the standards set by the American College of Medical Genetics and Association of Molecular Pathologists (ACMG/AMP) [9]. This interpretation process considered clinical and functional studies from the literature, population frequencies derived from gnomAD v2.1.1, computational predictions of the effect of missense variants derived from PolyPhen-2, MutationTaster2, and SIFT, and computational predictions of splicing defects for single nucleotide variants derived from dbscSNV [10, 22–25].
Variants identified from the literature were selected for inclusion in the prevalence calculation through consideration of its presence or absence in published ENPP1 Deficiency patients, the classification by ACMG/AMP, the allele frequency in gnomAD, and the classification in ClinVar—a crowdsourced database of variant classifications [11].
Variants that were Benign/Likely Benign by ACMG/AMP and/or were Benign/Likely Benign in ClinVar were excluded. Variants that had conflicting classifications, including those that had sufficient evidence to simultaneously meet both a Benign and Pathogenic classification by ACMG/AMP were also excluded. Remaining variants were included/excluded as specified in Additional file 1: Figs. S1–S2.
Variants that were not found in a patient with ENPP1 Deficiency or were found in gnomAD but not the published literature were also selected for inclusion in the prevalence calculation through consideration of the variant effect type, assessment by Loss-Of-Function Transcript Effect Estimator (LOFTEE), allele frequency in gnomAD, and computational predictions from PolyPhen-2 and SIFT (for missense variants) [10, 22, 25]. The inclusion/exclusion process is specified in Additional file 1: Fig. S3.
Calculation of genetic prevalence for ENPP1 deficiency
All variants in ENPP1 were downloaded from gnomAD v2.1.1 along with their overall and population-specific allele frequencies [10]. gnomAD v2.1.1 contains 125,748 exome sequences and 15,708 whole-genome sequences from 141,456 unrelated individuals, which are selected for absence of early-onset disease. Because ENPP1 Deficiency manifests with significant morbidity, we presumed that there are no ENPP1 Deficiency patients represented in the gnomAD dataset.
The overall (or population-specific) allele frequency was summed across all selected variants and then used within the Hardy–Weinberg equation to calculate the carrier frequency (2pq) and the frequency of a disease-causing genotype (q2) [26]. This calculation was performed for different sets of variants, segregated by classification and presence in the literature.
Supplementary Information
Additional file 1. Selection Process for ENPP1 Variants in Genetic Prevalence Estimates. Description: Processes for determining whether individual ENPP1 variants will be included or excluded from the genetic prevalence estimates
Additional file 2. ENPP1 Variants Analyzed in Genetic Prevalence Estimates. Description: ENPP1 Variants analyzed in the genetic prevalence estimates along with their respective allele frequencies, classifications by Genomenon/ClinVar, and reason for inclusion
Acknowledgements
Not applicable.
Author contributions
LMC performed the genetic prevalence calculations and analysis of the population frequency database and assisted in writing the manuscript. SAM performed the literature review and ACMG interpretation with assistance from LMC, JB, and SVH JB, MJK, and CRF assisted in writing the manuscript. JB, CRF, FR, and MJK supervised the data collection and evidence interpretation process. All authors have reviewed and approved of the manuscript and its contents and conclusions.
Funding
This work was supported in part by the Intramural Research Program of the National Human Genome Research Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Availability of data and materials
All data analyzed during this study are available in gnomAD (https://gnomad.broadinstitute.org/) and this published article/its supplementary file.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
L.M.C., J.B, S.V.H., S.A.M., and M.J.K are all full-time employees of Genomenon, Inc. C.R.F. reports a collaboration with Inozyme Pharma Inc. as part of a Cooperative Research and Development Agreement (CRADA).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lomashvili KA, Narisawa S, Millán JL, O’Neill WC. Vascular calcification is dependent on plasma levels of pyrophosphate. Kidney Int. 2014;85(6):1351–1356. doi: 10.1038/ki.2013.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Villa-Bellosta R, Wang X, Millán JL, Dubyak GR, O’Neill WC. Extracellular pyrophosphate metabolism and calcification in vascular smooth muscle. Am J Physiol Heart Circ Physiol. 2011;301(1):H61–H68. doi: 10.1152/ajpheart.01020.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferreira CR, Hackbarth ME, Ziegler SG, Pan KS, Roberts MS, Rosing DR, et al. Prospective phenotyping of long-term survivors of Generalized Arterial Calcification of Infancy (GACI) Genet Med Off J Am Coll Med Genet. 2021;23(2):396–407. doi: 10.1038/s41436-020-00983-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferreira CR, Ansh AJ, Nester C, O’Brien C, Stabach PR, Murtada SI, et al. Musculoskeletal comorbidities and quality of life in enpp1-deficient adults and the response of enthesopathy to enzyme replacement therapy in murine models. J Bone Miner Res Off J Am Soc Bone Miner Res. 2022;37(3):494–504. doi: 10.1002/jbmr.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cross JL, Iben J, Simpson CL, Thurm A, Swedo S, Tierney E, et al. Determination of the allelic frequency in Smith-Lemli-Opitz syndrome by analysis of massively parallel sequencing data sets. Clin Genet. 2015;87(6):570–575. doi: 10.1111/cge.12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wassif CA, Cross JL, Iben J, Sanchez-Pulido L, Cougnoux A, Platt FM, et al. High incidence of unrecognized visceral/neurological late-onset Niemann-Pick disease, type C1, predicted by analysis of massively parallel sequencing data sets. Genet Med Off J Am Coll Med Genet. 2016;18(1):41–48. doi: 10.1038/gim.2015.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xiao Q, Lauschke VM. The prevalence, genetic complexity and population-specific founder effects of human autosomal recessive disorders. Npj Genomic Med. 2021;6(1):1–7. doi: 10.1038/s41525-021-00203-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chunn LM, Nefcy DC, Scouten RW, Tarpey RP, Chauhan G, Lim MS, et al. Mastermind: a comprehensive genomic association search engine for empirical evidence curation and genetic variant interpretation. Front Genet. 2020 [cited 2022 Jul 12];11. Available from: https://www.frontiersin.org/articles/10.3389/fgene.2020.577152 [DOI] [PMC free article] [PubMed]
- 9.Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med Off J Am Coll Med Genet. 2015;17(5):405–424. doi: 10.1038/gim.2015.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karczewski KJ, Francioli LC, Tiao G, Cummings BB, Alföldi J, Wang Q, et al. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature. 2020;581(7809):434–443. doi: 10.1038/s41586-020-2308-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Landrum MJ, Chitipiralla S, Brown GR, Chen C, Gu B, Hart J, et al. ClinVar: improvements to accessing data. Nucleic Acids Res. 2020;48(D1):D835–D844. doi: 10.1093/nar/gkz972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karczewski KJ, Weisburd B, Thomas B, Solomonson M, Ruderfer DM, Kavanagh D, et al. The ExAC browser: displaying reference data information from over 60 000 exomes. Nucleic Acids Res. 2017;45(D1):D840–D845. doi: 10.1093/nar/gkw971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kato H, Ansh AJ, Lester ER, Kinoshita Y, Hidaka N, Hoshino Y, et al. Identification of ENPP1 haploinsufficiency in patients with diffuse idiopathic skeletal hyperostosis and early-onset osteoporosis. J Bone Miner Res Off J Am Soc Bone Miner Res. 2022;37(6):1125–1135. doi: 10.1002/jbmr.4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ralph D, Nitschke Y, Levine MA, Caffet M, Wurst T, Saeidian AH, et al. ENPP1 variants in patients with GACI and PXE expand the clinical and genetic heterogeneity of heritable disorders of ectopic calcification. PLoS Genet. 2022;18(4):e1010192. doi: 10.1371/journal.pgen.1010192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rush ET, Johnson B, Aradhya S, Beltran D, Bristow SL, Eisenbeis S, et al. Molecular diagnoses of X-linked and other genetic hypophosphatemias: results from a sponsored genetic testing program. J Bone Miner Res Off J Am Soc Bone Miner Res. 2022;37(2):202–214. doi: 10.1002/jbmr.4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rutsch F, Böyer P, Nitschke Y, Ruf N, Lorenz-Depierieux B, Wittkampf T, et al. Hypophosphatemia, hyperphosphaturia, and bisphosphonate treatment are associated with survival beyond infancy in generalized arterial calcification of infancy. Circ Cardiovasc Genet. 2008;1(2):133–140. doi: 10.1161/CIRCGENETICS.108.797704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.UK Biobank. 2022 [cited 2022 Jul 13]. Available from: https://www.ukbiobank.ac.uk/
- 18.Oheim R, Zimmerman K, Maulding ND, Stürznickel J, von Kroge S, Kavanagh D, et al. Human heterozygous ENPP1 deficiency is associated with early onset osteoporosis, a phenotype recapitulated in a mouse model of enpp1 deficiency. J Bone Miner Res Off J Am Soc Bone Miner Res. 2020;35(3):528–539. doi: 10.1002/jbmr.3911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Albright RA, Stabach P, Cao W, Kavanagh D, Mullen I, Braddock AA, et al. ENPP1-Fc prevents mortality and vascular calcifications in rodent model of generalized arterial calcification of infancy. Nat Commun. 2015;1(6):10006. doi: 10.1038/ncomms10006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan T, Sinkevicius KW, Vong S, Avakian A, Leavitt MC, Malanson H, et al. ENPP1 enzyme replacement therapy improves blood pressure and cardiovascular function in a mouse model of generalized arterial calcification of infancy. Dis Model Mech. 2018;11(10):dmm035691. doi: 10.1242/dmm.035691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.den Dunnen JT, Dalgleish R, Maglott DR, Hart RK, Greenblatt MS, McGowan-Jordan J, et al. HGVS recommendations for the description of sequence variants: 2016 update. Hum Mutat. 2016;37(6):564–569. doi: 10.1002/humu.22981. [DOI] [PubMed] [Google Scholar]
- 22.Adzhubei I, Jordan DM, Sunyaev SR. Predicting functional effect of human missense mutations using PolyPhen-2. Curr Protoc Hum Genet. 2013 doi: 10.1002/0471142905.hg0720s76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jian X, Boerwinkle E, Liu X. In silico prediction of splice-altering single nucleotide variants in the human genome. Nucleic Acids Res. 2014;42(22):13534–13544. doi: 10.1093/nar/gku1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwarz JM, Cooper DN, Schuelke M, Seelow D. MutationTaster2: mutation prediction for the deep-sequencing age. Nat Methods. 2014;11(4):361–362. doi: 10.1038/nmeth.2890. [DOI] [PubMed] [Google Scholar]
- 25.Sim NL, Kumar P, Hu J, Henikoff S, Schneider G, Ng PC. SIFT web server: predicting effects of amino acid substitutions on proteins. Nucleic Acids Res. 2012;40((Web Server issue)):W452–457. doi: 10.1093/nar/gks539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hardy GH. Mendelian proportions in a mixed population. Science. 1908;28(706):49–50. doi: 10.1126/science.28.706.49. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Selection Process for ENPP1 Variants in Genetic Prevalence Estimates. Description: Processes for determining whether individual ENPP1 variants will be included or excluded from the genetic prevalence estimates
Additional file 2. ENPP1 Variants Analyzed in Genetic Prevalence Estimates. Description: ENPP1 Variants analyzed in the genetic prevalence estimates along with their respective allele frequencies, classifications by Genomenon/ClinVar, and reason for inclusion
Data Availability Statement
All data analyzed during this study are available in gnomAD (https://gnomad.broadinstitute.org/) and this published article/its supplementary file.