Abstract
Gut microbial β-glucuronidases (gmGUS) are involved in the disposition of many endogenous and exogenous compounds. Preclinical studies have shown that inhibiting gmGUS activity affects drug disposition, resulting in reduced toxicity in the G.I. tract and enhanced efficacy systemically. Additionally, manipulating gmGUS activity is expected to be effective in preventing/treating local or systemic diseases. Although results from animal studies are promising, challenges remain in developing drugs by targeting gmGUS. We reviewed the role of gmGUS in the host’s health under physiological and pathological conditions, the impact of gmGUS on the disposition of phenolic compounds, the models to study gmGUS activity, and the perspectives and challenges in developing drugs by targeting gmGUS.
Keywords: Gut microbial β-glucuronidase, druggable target, drug disposition, drug development
Introduction
The gut microbial β-glucuronidase (gmGUS) enzymes are expressed in the gut microbiota and play an important role in the host’s health by disposing drugs, nutritional components, and important endogenous compounds including steroid hormones, neurotransmitters (e.g., dopamine), bile acids, and bilirubin1–3. In the gut, gmGUS catalyzes the hydrolysis of glucuronides to release the aglycones, which is a reverse reaction of glucuronidation mediated by Uridine 5’-diphospho-glucuronosyltransferases (UGTs) expressed in mammalian cells and considered as an “activation” metabolism due to the release of aglycones. These gmGUS enzymes are produced by a broad range of bacterial phyla, such as Firmicutes, Bacteroidetes, Verrucomicrobia, and proteobacteria, which are typically found in human gut microbiota. Among these bacteria, there are at least 279 isoforms of gmGUS that are classified in six unique structural categories with different “loops” in their active site4. The conservation and diversity of gmGUS proteins in function, structures, cellular localization, and expression in bacteria species have been continuously reported since the 1930s. Generally, certain isoforms catalyze small molecular substrates in the intracellular domain, while others hydrolyze glucuronides of large molecules, such as heparan-glucuronides, in the periplasmic space4.
The gmGUS enzymes have attracted researchers’ attention in drug development since the late 1990s5–7. In the past two decades, a range of high-impact studies illustrated the role of gmGUS inhibitors in alleviating drug-induced toxicity in the gastrointestinal (GI) tract and enhancing drug efficacy in chemotherapy8,9. In addition, several publications proposed that gmGUS is involved in hormone-associated cancers by affecting the disposition of hormones10,11, which provides an option for cancer chemoprevention by targeting gmGUS. Due to the promising perspectives, specific probes to determine gmGUS activity were synthesized and simple and rapid spectrophotometric and fluorogenic assays were developed for gmGUS inhibition studies. In addition, fluorescence-based assays were developed to determine in vivo gmGUS inhibition. Furthermore, many specific or broad-spectrum gmGUS inhibitors, which was concepted by Bin Wei and Yan Ru et al12, have been identified from natural products, approved drugs, or newly synthesized small molecules, as summarized in a recent review paper13.
Inhibiting gmGUS using small molecular inhibitors to prevent or treat certain diseases, especially local diseases in the colon, seems promising as supported by many studies using preclinical models. Clinical studies in humans are highly expected to validate the results afforded from preclinical models. In this review paper, we briefly outline the current findings on gmGUS structures and the mutual-relationship between gmGUS with host’s health, summarize the role of gmGUS in drug disposition and the in vitro and in vivo assays for gmGUS inhibitor screening, and review the possibility and challenges of implicating gmGUS as a druggable target in drug development.
The gmGUS and host’s health
The human gut microbiota plays an important role in the host’s physiological function. Microbial enzymes, including GUS have been involved in their metabolic processes. For example, in Phase II metabolism in the mammalian liver, UGTs facilitate the conjugation of hydrophobic compounds to glucuronic acid to increase its water solubility and promotes excretion. While some of the glucuronidated metabolites dissolve in blood and are excreted through urine, a significant proportion is released via bile into the gut which houses a reservoir of microbial-derived enzymes. One of these enzymes is gmGUS which removes the glucuronic acid from the conjugated compounds. This de-glucuronidation reaction regenerates parent compounds, promotes cycling, and delays eliminating different types of substances.
The gmGUS enzymes can hydrolyze glucuronides of endogenous and/or exogenous compounds to release the aglycones. In many cases, aglycones released by gmGUS may affect various gut pathologies, namely drug-induced intestinal toxicities such as inflammation, diarrhea, and constipation, increasing incidences of hormone-driven cancers such as breast, and prostate cancer, and inflammatory bowel diseases (IBD). Several factors such as age, diet, species, genetics, chronic drug use, and disease states dictate the change in gmGUS expression, primarily due to changes in the occurrence of GUS-producing microbial species. Modulation of the gmGUS activities primarily due to changes in gut microbiome or inhibition of microbial GUS enzymes can lead to altered systemic and intestinal exposure of xenobiotics and endogenous compounds, such as bilirubin, steroids hormones (e.g., estrone, estradiol, testosterone, and androstanediol), and neurotransmitters (dopamine, and serotonin), disturbing the homeostasis of endogenous substrates, and affecting the efficacy and toxicity of drugs (Figure 1).
Figure 1.
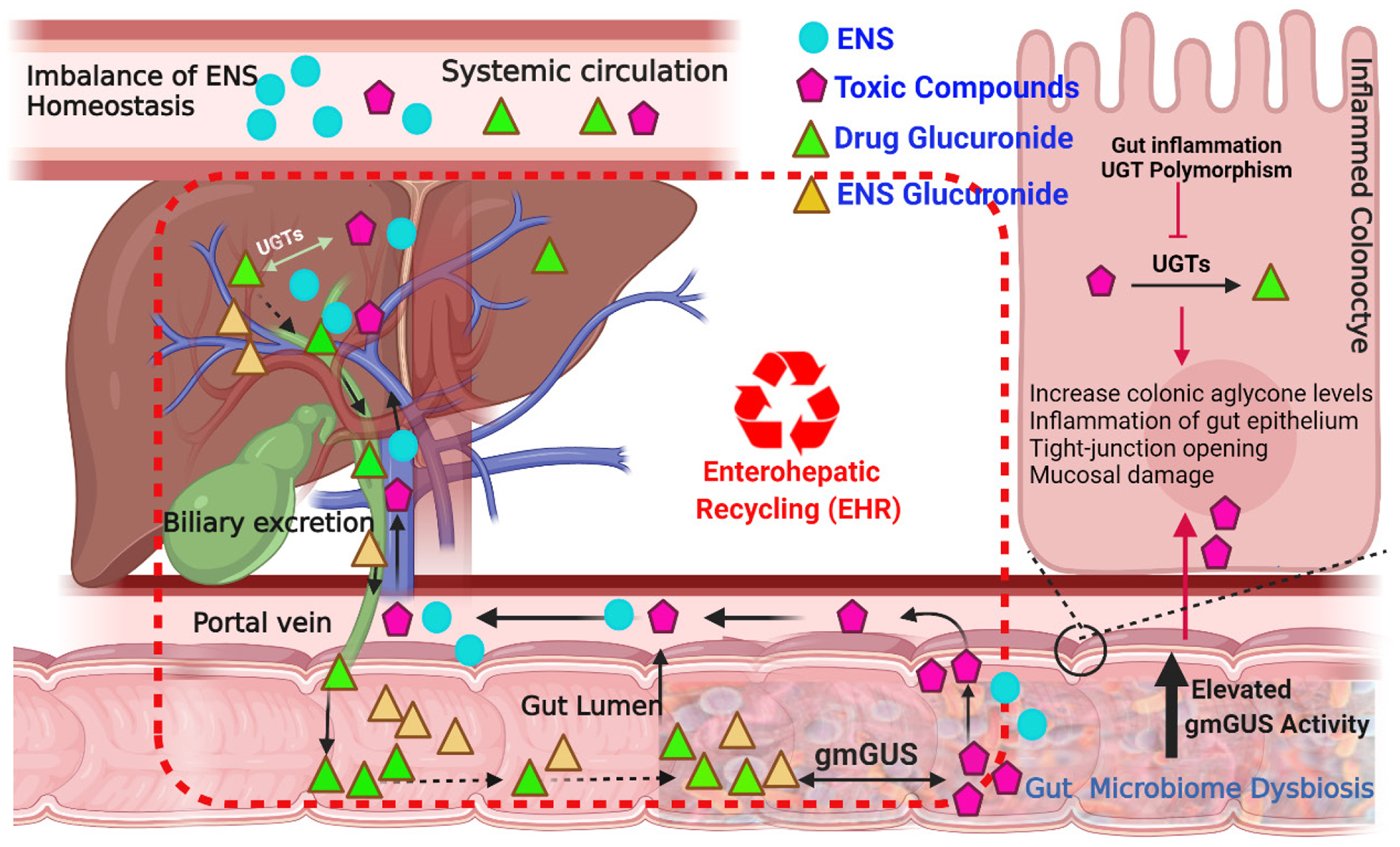
Schematic representation of microbial β-glucuronidases (gmGUS) Pathophysiology. gmGUS plays critical role in the enterohepatic recycling of toxic substances (e.g, anticancer drugs, opioids, dietary carcinogens) and endogenous substrates (ENS) (e.g, estrogen, androgen, serotonin, bilirubin, and dopamine etc.). Elevated gmGUS activity due to dysbiosis of the gut microbiome associated with colon cancer, inflammatory bowel disease, and drug-induced intestinal injury led to increase systemic and gut levels of ENS and increase toxic compounds exposure to colonocytes and subsequent epithelial damage.
Among the thousands of species present in the human gut microbiome, at least 50 species carry genes encoding gmGUS14–16. However, gmGUS from different bacterial phyla shows distinct differences in catalytic efficiency, substrate binding, reaction rates, regulation of GUS expression, and response to GUS inhibitors13,17,18. For instance, only 4 gmGUS (Rg3GUS, Rh2GUS, Fp2GUS and H11G11- BG) displayed deconjugation activity on regorafenib-N-glucuronide19, as compared to 31 gmGUS that could hydrolyze the standard GUS substrate 4-Methylumbelliferone-O-glucuronide13. Moreover, there exists a vast diversity in the function, structure, and cellular localization of gmGUS4. In addition, the high inter-individual variability in the gmGUS composition and activities has been reported in rodents and humans4,20–22. Therefore, the pathogenesis due to imbalance of overall or specific gmGUS activity is a complex equation with multiple factors involved making it very difficult to delineate the effect of changes in microbiota structure on gmGUS activity.
There are about 100 drugs whose metabolism has been reported to be affected by the gmGUS activities21. Among the largest class of drugs were eight opioids (including morphine, codeine, hydromorphone, naltrexone, nalmefene, tapentadol, ketobemidone, and buprenorphine) and 6 non-steroidal anti-inflammatory drugs (NSAIDs, ibuprofen, flurbiprofen, suprofen, naproxen, zaltoprofen, and etodolac)21. Most of these drugs are metabolized in the liver and excreted, mainly as O-glucuronides for opioids whereas acyl/ether glucuronides for NSAIDs in the bile, which transverse through the gastrointestinal tract and reach the distal intestine, where gmGUS convert them to respective aglycone leading to GI side effects associated with these class of drugs, such as opioid-induced constipation (experienced by ~40–60% opioid users), and NSAIDs-induced gastritis (~50–70% long-term NSAID users experience increase in intestinal permeability and inflammation, and 30~40% experience mucosal damage)13,23,24.
Microbial conversion of biliary excreted SN-38 glucuronide to SN-38, the active metabolite of anticancer irinotecan, by gmGUS leads to increase colonic exposure to SN-38 and subsequent gut epithelium damage. SN-38 mediated clinically significant diarrhea is one of the major dose-limiting toxicity of irinotecan, which affects up to 87% of patients, with grade 3 or 4 diarrhea (as per National Cancer Institute Common Toxicity Criteria) ranging between 30–40%. As a results, patients on irinotecan therapy often require dose delays, omissions, and dose reductions that impact the drugs’ efficacy, apart from poor quality of life during cancer treatment25. Similarly, chemotherapy-induced diarrheal toxicity mediated by gmGUS activity has been seen with other anticancer agents, such as, tyrosine kinase inhibitors regorafenib and sorafenib19,26.
The action of gmGUS also dictates the conversion of 7-O-glucuronide of mycophenolic acid (MPA) to MPA. MPA is the active moiety of the immunosuppressant drug mycophenolate mofetil (MMF) used in kidney, liver, or heart transplantation, responsible for persistent afebrile diarrhea (daily fecal output of 200 gm) in ~40% MMF-treated transplant patients after one month of transplantation27. In addition, the MPA treatment can cause erosive enterocolitis morphological changes in colonic mucosa with a Crohn’s-like pattern28,29. Recently, colonic reactivation of triclosan, an antimicrobial found in toothpaste, toys, and thousands of other consumer products, from the triclosan-glucuronide by Loop 1 and flavin mononucleotide (FMN)-binding gmGUS has been implicated in chronic gut inflammation and colon damage, leading to IBD30. In several cases, the severity of drug-induced gut toxicities was positively correlated with intestinal β-glucuronidase activity, and selective gmGUS inhibitors or antibiotics have been shown to alleviate drug-induced gut toxicities19,23,31–34.
The morphine dependence model of mice showed an increase in the ratio of concentrations of morphine-3-glucuronide to morphine in serum and feces, indicating a decrease in morphine-3-glucuronide deconjugation within the gut lumen, which was correlated with a decrease in GUS-producing Bacteroidales. These changes in microbiota structure were also associated with a decrease in secondary bile acid, deoxycholic acid, and an increase in phosphatidylethanolamines levels on day 3 in the metabolomic analysis of feces35. A decrease in gmGUS activity on morphine treatment is also expected to impact the colonic exposure to morphine-6-glucuronide, which is >100 times more selective and potent agonist of μ-receptor than morphine36. This further contributes to the decrease in intestinal secretion and inhibition of propulsive colonic motility with chronic use of morphine37.
Among the endogenous substrates, elevated levels of unconjugated bilirubin in circulation are associated with infant hyperbilirubinemia, jaundice, and enteropathy. Probiotics such as (e.g., Bifidobacterium, Lactobacillus, and Saccharomyces boulardii) and prebiotics (e.g., short-chain galacto-oligosacarids and long-chain fructooligosacarids that increases stool frequency and growth of Lactobacillus and Bifidobacterium) have been shown to increase stool frequency, inhibition of gmGUS activity, decrease in the intestinal pH, and decrease in the enterohepatic circulation of bilirubin by promoting intestinal maturity and enhancing tight junction protein of intestinal epithelium38. The gut homeostasis of endogenous neurotransmitters, dopamine, norepinephrine, and serotonin are responsible for physiological GI motility1. The hydrolysis of glucuronides of these compounds by the gmGUS39 is an important process in the metabolism and recirculation of the compounds toward maintaining healthy bowel movements40. Levels of dopamine, norepinephrine, and serotonin in normal mice was found to be several folds higher than those in the germ-free mice due to absence of gmGUS39. An increase in circulating serotonin levels due to increased activity of gmGUS leads to diarrhea because of increased gastrointestinal motor and secretory functions1. Similarly, increased dopamine and norepinephrine levels increase gut motility and IBD40.
The gmGUS activities in stool samples of children with IBD were ~2.5 times lower than those samples from healthy children as determined by the standard pNPG assay (see assay description later) using fecal suspension prepared from patients stool samples22. On the other hand, Gloux and Anba-Mondoloni proposed a microbiota-pathology hypothesis in which this unique β-glucuronidase locus (GUS/FGDFGND C7D2 transporter/AFTR) may contribute to an increased risk of crohn’s disease due to higher exposure to toxic substances causing injury and inflammation. The reduction of gmGUS activity in children with IBD could result from loss of secretory cell function probably due to chronic inflammation or/and drugs22. In addition, gmGUS activity was a prime cause of inflammation-mediated colon cancer41,42.
The colonic and systemic exposure of DNA-adduct forming environmental and dietary carcinogens 2-Amino-3-methylimidazo[4,5-f]quinoline (IQ), 2-Amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP), and azoxymethane was significantly increased due to hydrolysis of N-glucuronides of IQ, PhIP, and N-hydroxy PhIP and O-glucuronide of methylazoxymethanol, and their entry into enterohepatic recirculation. Colon carcinogenesis of IQ and PhIP was positively correlated with fecal gmGUS activities13. Many dietary (e.g., lemongrass, ginger, luteolin) and synthetic (e.g., C-GAL) gmGUS inhibitors suppressed azoxymethane (AOM)-induced colonic DNA-adduct formation. Similarly, probiotics (e.g., Bifidobacteriurn longum) and prebiotics (e.g., inulin) treatment also prevented PhIP and IQ -induced DNA damage, respectively13.
An increase in GUS-producing microbes and gmGUS activities leads to increase estrogen circulating and tissue levels and is considered an important mediator for microbiota–host interaction correlating gut microbiome and breast cancer43. Long-Term administration of Estrogen Replacement Therapy, i.e., a combination of conjugated estrogen and bazedoxifene, for the management of menopause-associated symptoms, including hot flashes and osteoporosis was shown to decrease gmGUS activity in mice, which was positively correlated with the abundance of Lactobacillaceae in the gut microbiome44. Despite thenon-conclusive clinical evidence regarding the positive association of testosterone serum levels with the development of prostate cancer45, the imbalance of testosterone due to changes in gmGUS activity seems to have a significant role in the progression of prostate cancer. The androgen deprivation therapy in combination with enzalutamide or docetaxel can successfully inhibit the development of androgen-sensitive metastatic prostate cancers46,47.
Category of gmGUS and the impact on the disposition of phenolic drugs/compounds.
Type and structure of gmGUS.
Protein X-ray crystal structures have been resolved for microbial GUS protein family homologs from at least 11 species: Escherichia coli8, Lachnospira eligens31, Streptococcus agalactiae17, Clostridium perfringens17, Lactobacillus rhamnosus48, Ruminococcus gnavus48, Faecalibacterium prausnitzii48, Bacteroides fragilis49, Bacteroides dorei48, Bacteroides uniformis4, and Parabacteroides merdae50. Emerging structural data and bioinformatic analyses have yielded significant progress in the understanding of GUS enzyme substrate specificity. A large portion of the core domains in the GUS protein family are structurally conserved, including the amino acid residues in the active site and the α/β hydrolase fold motif. These motifs also have highly conserved sequences as illustrated in Figures 2A and 2B. The main variations among GUS homologs occur in two loop regions adjacent to the active site, denoted as the loop 1 (L1) and loop 2 (L2) regions. Based on the number of residues in these two loop regions, GUS enzymes were classified into 6 subgroups: L1, mini-loop 1 (mL1), L2, mini-loop 2 (mL2), mini-loop 1,2 (mL1,2), and no loop (NL)4. The number of residues in each loop region, the sequence of the loop regions, and the composition of residues in the loop regions vary between subgroups; as shown in Figure 2C, the 6 subgroups can be clearly distinguished based on the sequence identity of residues in the two loop regions. Previous studies suggest that the two loop regions contribute significantly to substrate specificity8,48. For instance, L1 GUS enzymes exhibit stronger activity on the glucuronide substrate p-nitrophenol-β-D-glucuronide (pNPG) compared to GUS enzymes of the other loop subgroups4. Additionally, the GUS inhibitor UNC10201652 strongly inhibits L1 GUS enzymes but is generally less potent toward non-L1 members31,48. Some GUS homologs possess additional peripheral domains that may also play a part in substrate recognition. For example, the protein X-ray crystal structure of Bacteroides dorei GUS (NL class) reveals a non-conserved C-terminal region that contains a Carbohydrate Binding Module (CBM) and a domain with unknown function, in which the CBM domain contains a loop that extends into the active site, suggesting that the CBM may interfere with the substrate specificity48. This example supports the possibility that other structural differences outside of the two loop regions contribute to the specificity or accessibility of the active site in GUS enzymes, but more investigation of non-conserved domains in GUS enzymes is needed.
Figure 2. Structure and sequence homology of GUS enzymes.
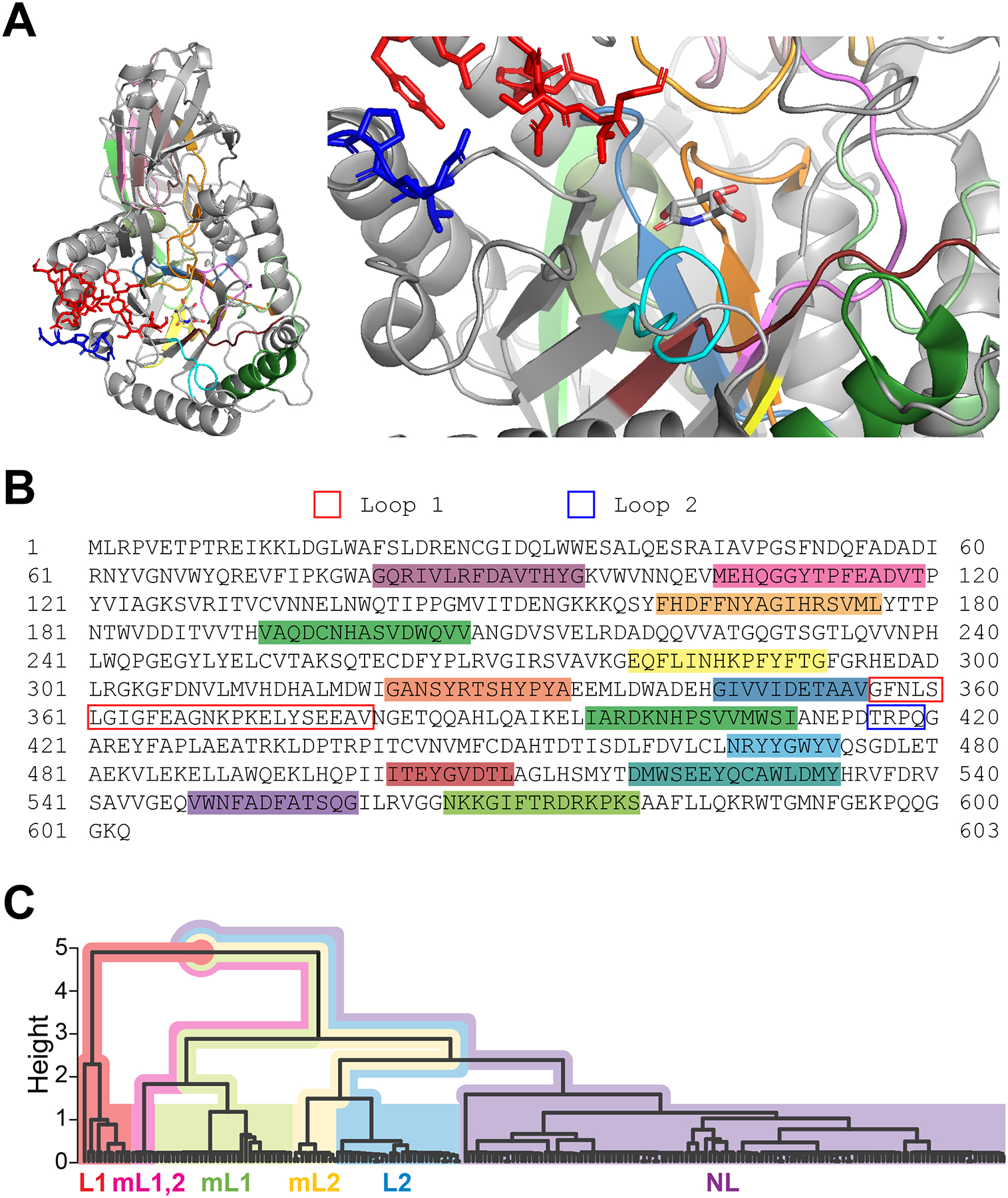
(A) Structure of E. coli GUS with an inhibitor, glucaro-D-lactam. This protein formed a homotetramer in the original X-ray crystal structure (PDB: 3K4D). A monomer is selected to illustrate the tertiary structure of this protein (left) and its active site (right). The parts illustrated as red and blue sticks represent the Loop 1 and Loop 2 regions, respectively. E. coli GUS belongs to the L1 subgroup and its Loop 1 region is proximate to the inhibitor molecule. Other colored parts in cartoon mode have ≥ 90% sequence identity among GUS homologs from a modified version of the HMGC279 GUS data set curated from the Human Microbiome Project (HMP) Stool Sample Catalog4. Samples in the HMGC279 dataset that were described as fragments or had undetermined taxonomy were removed, resulting in a subset of 229 GUS enzyme amino acid sequences (GUS229) that we used for identifying conserved regions. Many of these conserved regions form the active site and interact with the inhibitor. (B) Amino acid sequence of E. coli GUS. Highlighted regions of this sequence (SRS017191.56930) correspond to the conserved motifs colorized in Figure 1A. Loop region sequences are outlined (Loop 1, blue; Loop 2, red). (C) Hierarchical clustering dendrogram of GUS homologs from the GUS229 dataset. Inter-motif regions containing Loop 1 and Loop 2 (from GUS229) were each aligned in UGENE (v41.0)99 using MUSCLE (with default parameters). A sequence identity distance matrix for the GUS229 alignment was calculated in R (v4.1.2) using the R package seqinr (v4.2–8) to evaluate sequence identity, and a custom Python (v3.9) script to weigh the length of the two loop regions. The aggregate distance matrix was used for agglomerative hierarchical clustering (“mcquitty” method) in R. Loop subgroups are distinguished by color as indicated on the dendrogram. Homologs from the same subgroup (L1, mL1,2, mL1, mL2, L2, and NL) clearly clustered together.
Enzyme and substrate specificities.
An N-21 terminal signal sequence in the mL1 BfGUS, L2 BuGUS2, mL2 PmGUS, and NL BdGUS enables the periplasmic translocation of these enzymes and, consequently, favors processing larger polysaccharide substrates, such as heparan monosaccharide4. On the other hand, L1 enzymes contain no signal sequences and are maintained intracellularly, limiting their access to smaller glucuronides that could reach the cytoplasmic space51. Substrate specificity is mainly affected by its physicochemical properties. Several factors such as molecular size, hydrophilicity/lipophilicity, electric properties of the substrates can affect glucuronides’ transport across the membrane barriers and binding with GUS enzymes in the bacteria. For example, EcGUS displayed higher hydrolytic efficacy towards hydrolysis of 3- than 6- morphine glucuronide52, because glucuronic acid at position-3 bound to an electron-rich phenol thus is more prone to hydrolysis than that of position-6. Another example is that the preference of EeGUS, RhGUS, FpGUS, EcGUS, and SaGUS for estrone-3-glucuronide over estradiol-17-glucuronide can be explained by the aromatic ring adjacent to a glucuronic acid moiety and a distant methyl group in estrone-3-glucuronide which allow catalytic residues to interact with the ether linkage10. In addition, the “loop” of the active site in the gmGUS is also a dominant factor in selecting favorite substrates. For example, the N-glucuronide of regorafenib can only be catalyzed by no loop gmGUSs, which can be attributed to the less accessibility to the enzymes caused by the steric hindrance and different electronic and structural characteristics of this unusual “central” N- linked glucuronide as well as the more open catalytic sites offered by this active no loop GUS proteins. Sporadical data from in vitro studies suggested that O-glucuronides could be more favorite substrates for gmGUS compared to N-glucuronides as shown in the regorafenib-glucuronide hydrolysis study. Further studies using substrates with similar aglycone structures support this conclusion. For example, Zenser et al reported that hydrolysis rate of N’-Hydroxy-N-Acetylbenzidine N-glucuronide is about 7-fold higher than that of N’-Hydroxy-N-Acetylbenzidine O-glucuronide mediated by EcGUS53. In this case, different electronic characteristics of N- and O-glucuronide could be a better explanation compared to that of steric hindrance because the structures of the substrates are very similar. More studies using substrates with similar structures are expected to further confirm the conclusion and develop structure-activity relationships. X-ray single crystal analysis using glucuronide-gmGUS co-crystals may be able to find out the reason directly.
GmGUS expression level and isoform composition.
The gut microbiota expresses diverse gmGUS isoforms responsible for metabolizing xeno- and endo-biotics. In view of its significant role, factors that modulate gmGUS expression are interesting. GmGUS expression is affected by host phenotype including sex and age. Elmassry et al. showed that gmGUS was higher in males compared to females, which may be associated to sexual dimorphic response to drugs21. They also estimated gmGUS expression using the human metagenome data and found out that newborn and one-year-old infants might have higher gmGUS expression than their mothers, possibly related to breast-feeding and the introduction of solid food21. Also, a study in mice suggested a female-specific positive association between age and gmGUS54. Various drugs and dietary components also influence gmGUS expression or activity. For instance, long-term supplementation of conjugated estrogens and bazedoxigene significantly reduced gmGUS activity without affecting the overall cecal or fecal microbiome community in ovariectomized mice44. Taylor et al. also reported that an immunosuppressive drug mycophenolate mofetil induces drug toxicity through increasing gmGUS expression and activity, while vancomycin could reverse the effect by abolishing GUS-producing bacteria33. In terms of diet, Reddy et al. suggested dietary modulation on gmGUS by showing an elevated gmGUS expression in individuals on high meat diet compared to those on a nonmeat diet55. Later work in the field also associates upregulated gmGUS expression or activity with high fat, high protein and low fiber diet15,56,57. This modulation may mediate changes in host metabolism and enterohepatic circulation. For example, women who consume vegetarian diet have elevated estrogen fecal excretion and lower plasma and urinary estrogen, as well as reduced gmGUS activity58.
Disposition of Phenolics via Recycling facilitated by gmGUS.
Orally administered compound with hydroxyl moieties often undergo first-pass metabolism, mainly via the glucuronidation pathway, with sulfonation sometimes acting as a substantial secondary pathway59. For many of the glucuronide metabolites, biliary and intestinal excretion are the major elimination pathways, which will eventually force the glucuronides to the terminal ileum and colon, where they will have good chance to be reactivated by gmGUS into aglycones for reabsorption and subsequent disposition. The reactivation followed by re-absorption in the colon into the blood is called recycling or recirculation.
Recycling is classified into three different types, enterohepatic recycling (EHR), enteroenteric recycling (EER) and hepatoenteric recycling (HER), depending on the formation site of glucuronides and their excretion pathways (Figure 3). For glucuronides generated in the enterocytes during the first-pass metabolism of orally administered phenolics, they could be excreted into the lumen directly, and then recycled in the terminal ileum and colon via the action of gmGUS, and this recycling is called EER, which was first termed as enteric recycling in 2003 by Hu and his co-workers60. For the glucuronides that were not excreted back into the lumen but are taken up by the hepatocytes (likely via OATPs) and excreted into the bile, and they will participate in HER. For phenolics undergoing first-pass metabolism mainly in the liver, followed by biliary excretion of the glucuronides, they will participate in EHR.
Figure 3.
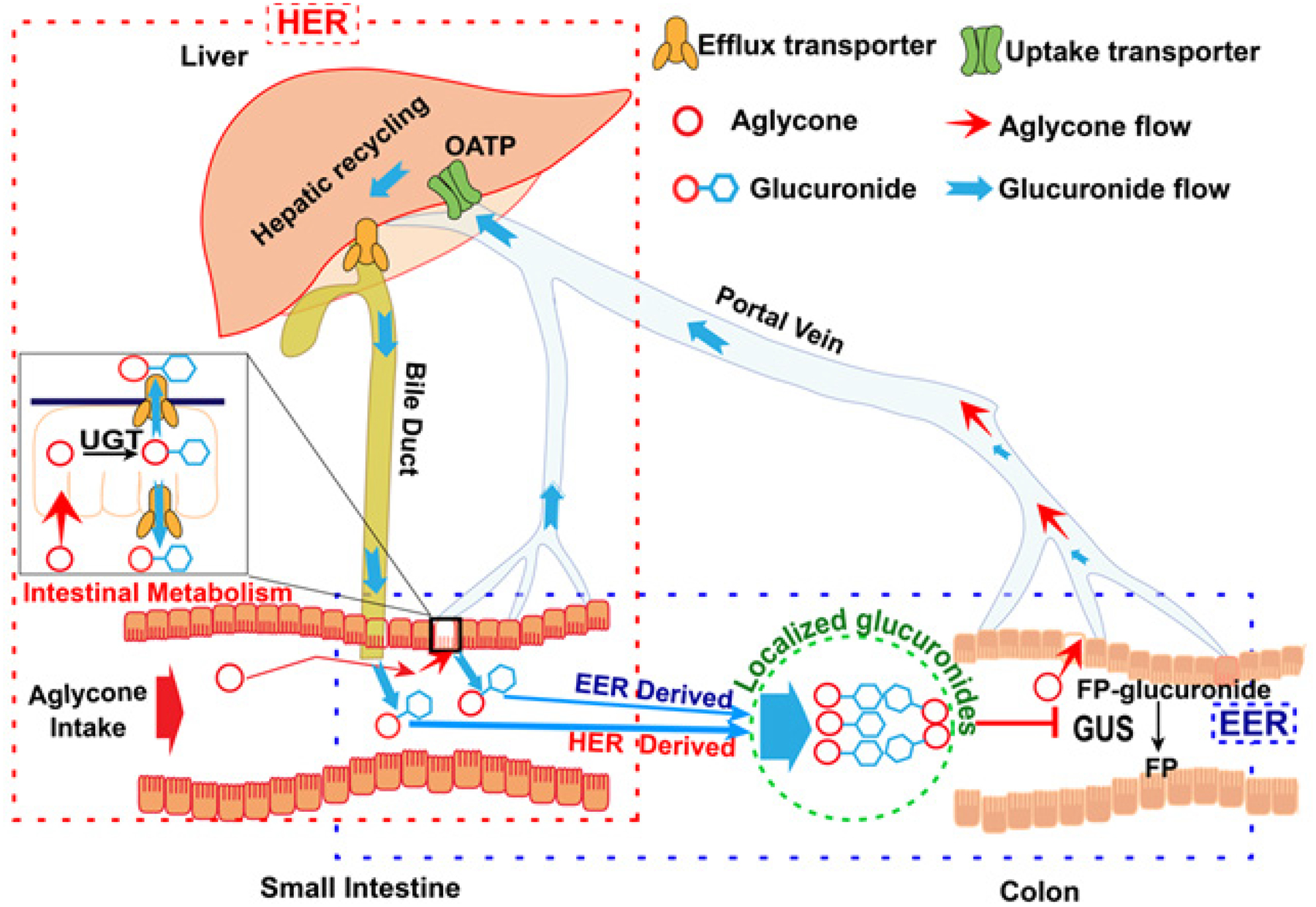
HER, EER and Glucuronide Localization to the Lower Gut.
HER and EER are especially important for determining colon biodistribution of oral phenolics that undergo extensive gut glucuronidation by UGTs (Fig.3, Intestinal Metabolism insert). Once glucuronidated during the HER process (Fig.2, dashed red rectangle), glucuronides are excreted into mesentery blood, taken up by hepatocytes, excreted into the bile (via BCRP and MRP2), not absorbed as glucuronides (too hydrophilic), and then guided into the lower gut to be hydrolyzed by gmGUS to aglycones for reabsorption. For EER (Fig.2, dashed blue rectangle), gut-produced glucuronides are excreted into the lumen, not absorbed, and then be guided into the colon to be hydrolyzed by gmGUS. HER and EER are different from the classical EHR (known since 1958 for bile acid recycling61), because intestine is the metabolism organ. In EHR, the metabolites are generated in the liver. On the other hand, HER and EHR share an important similarity in that biliary excreted glucuronides are involved in the recycling, whereas EER only involves intestinal excreted phase II metabolites. Regardless of how glucuronides reach terminal ileum or colon, gmGUS is essential in their recycling.
Glucuronides hydrolysis mediated by gmGUS in the gut is one of the critical steps in EHR, HER, and EER. Since hydrolysis reaction are usually very rapid as reported in in vitro studies, it is speculated that hydrolysis mediated by gmGUS might not be the rate-limiting step in these three recyclings. However, the microenvironment in the gut could be affected by physiological or pathological factors and activity of gmGUS may be altered and the efficiency of EHR, HER, and EER could be affected significantly, which will consequentially affect local or even systemic exposure of certain drugs. More in vivo studies with different in vivo conditions (e.g., food effects, inflammation in the gut) should be conducted to further elucidate the contribution of gmGUS activity to the efficiency of these three recyclings.
Assays and models to study gmGUS activity
In vitro assays to determine the activity of gmGUS.
The gmGUS activity evaluation is extensively performed in various fields with different requirements. For example, In vitro gmGUS assays are routinely used for diagnostic purposes for detecting E. coli and Shigella species in clinics, water, food and environmental samples62,63. In this regard, simple, quick, and high-throughput assays in vitro have been developed using synthetic substrates of glucuronic acid linked to a chromophore (e.g., 4-nitrophenol) or fluorophore (e.g., 4-methylumbelliferone). Chromogenic substrates are usually phenol-based, water-soluble, thermostable and specific and occur in a wide range of wavelengths and the results are typically read using UV-spectrometers64. The commonly used chromogenic substrate for gmGUS activity is 4-nitrophenyl β-D-glucopyranoside (4-pNPG)20, a small substrate that can be rapidly hydrolyzed by gmGUS to release the aglycone 4-nitrophenol. Typically, a mM range of 4-pNPG is incubated with gmGUS at μM ranges for 30 min in 96-well plates, followed by reading the absorbance at 405 nm using a plate reader. The whole assay needs about one hour or less.
Although a few chromogenic substrates are available to determine activity gmGUS, the use of fluorogenic substrates is more appealing due to their higher sensitivity. A common fluorogenic substrate is 4-methyl-umbelliferyl-β-D-glucuronide (4-MUG), which releases the fluorescent aglycone 4-methyl umbelliferone (4-MU) upon hydrolysis for fluorescence detection in plate readers. This assay is usually more sensitive than those above-mentioned chromogenic substrates-based assay. However, the major drawback of 4-MUG is that its pKa value is 7.8, which causes only partial dissociation at the optimum pH requested by gmGUS (<7.0). To overcome this issue, a novel method called the “discontinuous method ” was tested by conducting the hydrolysis at different temperatures to afford kinetic data to enable prompt evaluation of gmGUS activity65. Other than 4-MUG, 3-carboxy-umbelliferyl-β-D-glucuronide was also turned out to be a good substrate because this compound has higher solubility compared with 4-MU and been recently applied for the detection of E. coli GUS, one of the isoform in gmGUS family, in a rugged in situ optical sensor66. In addition, 6-chloro-4-methylumbelliferyl-β-D-glucuronide, which enabled higher sensitivity at physiological pH, was also reported in the literature to be used to detect E. coli in a culture based assay67. Besides plate readers, fluorogenic assays can also be measured using microchip-based capillary electrophoresis and Q-PCR instrumentation68,69. For example, a novel method to detect E. coli GUS activity using a microbially-modified glassy carbon electrode was developed and applied for fecal pollution monitoring70.
Because certain bacterial species are be culture, fecal lysates are usually used to determine the activity of gmGUS even through lysate prepared from feces may not be able to reflect in vivo gmGUS activity. So far, two methods have been used to prepare fecal lysates: fecal suspension and fecal S9 fraction20. With fecal suspension, fresh feces are homogenized and suspended in phosphate buffer, which will be incubated with substrate directly to determine the hydrolysis rates. With fecal S9 fraction, the fecal suspension in buffer is sonicated to break the cells to release the contain inside the cells and then centrifuge at 9,000 ×g at 4 °C for 15 min to afford a fraction called fecal S9 fraction. This is a traditional method used to prepare tissue S9 fraction in drug metabolism studies. We previously found out that total protein concentration in fecal S9 fraction is higher than that in fecal suspension. In addition, the gmGUS activity determined using fecal S9 fraction is higher than those using fecal suspension20. Additionally, time of feces collection also affected GUS activity. These findings suggested that fecal S9 fraction prepared using fresh feces could be a better enzyme source when determine gmGUS activity in vitro.
In vivo assays to determine the activity of gmGUS.
In vivo activity of gmGUS can be determined using fluorogenic assays, where fluorescence is generated upon hydrolysis of glucuronides to release fluorogenic aglycones that can be detected outside of the animal body. For example, fluorescein di-β-D-glucuronide, shows a high level of fluorescence intensity (Ex: 480 nm, Em: 514 nm) after removing of the mono-glucuronide by GUSs with a detection limit of 104 CFU cells per well. Furthermore, in an in vivo study, the fluorescence of the released fluorescein fluxes in the abdomens reached the maximum value at 3 hours after fluorescein di-β-D-glucuronide is administered through oral gavage at 7.3 μmol/kg in mice71,72. Other example specific probes using in in vivo studies include 7-hydroxy-9H-(1,3-dichloro-9,9-dimethylacridin-2-one) glucuronide (DDAO-glu, Ex: 633 nm, Em: 645–690 nm)73, and hemicyanine glucuronide, which have been successfully used to in the determination of gmGUS activity in mice and zebrafish74.
Factors affecting gmGUS activities in in vitro and in vivo assays.
The enzyme-mediated reaction is a well-accepted in vitro model to evaluate GUS activity. Theoretically, in vivo model assay seems to be the best approach to study the total activity of GUS in animals, but a specific probe is still understudied. Thus, total proteins prepared from feces (e.g., fecal S9 fractions and fecal suspensions) are usually used as the enzyme sources within in vitro studies or recombinant microbial GUS from the bacterial culture reported in the literature to determine glucuronide hydrolysis in the gut75. Therefore, the preparation process of the fecal S9 fractions and reaction conditions (e.g., pH values and Mg2+ concentrations) could highly affect the readout. In addition, anaerobic conditions may also have some impact on gmGUS activity since gut microbe grow under an anerobic condition in vivo. We previously found that buffer pH values and Mg2+ concentrations in the buffer could affect gmGUS activity significantly20. Additionally, MU also has spectroscopic characteristics strictly dependent on the pH of the assay solution: in particular, its absorption spectrum varies in the transition between the undissociated, at acidic pH, and dissociated form at alkaline pH76. The pH of the assay solution affected the substrate structure and substrate interactions with the enzyme.
GmGUS as a therapeutic target
As described above, although gmGUS is not expressed in any organs of the host, this bacterial enzyme family participates in the disposition of endogenous compounds (e.g., neurotransmitters, hormones..) and drugs (e.g., SN-38, NSAID), which could significantly impact systemic or local exposure of these substrates, resulting in local or remote beneficial or harmful biological effects. Therefore, manipulating gmGUS activity could affect local or systemic exposure of certain drugs for reduced toxicity in the gut and/or enhanced efficacy in the disease sites (Fig. 1). Advantages of targeting gmGUS to achieve desired pharmacological efficacy have been demonstrated in animal models including (1) gmGUS modulators (e.g., inhibitor) do not purposely kill bacteria as antibiotics do so that the risk of dysbiosis is limited with gmGUS modulators; (2) systemic side effects can be limited when the inhibitors are localized in the gut; (3) targeted or onset drug delivery is easier compared to delivering drugs to other organs; and (4) Oral administration is the appropriate drug administration route and many dosage form options are available. Towards these points, gmGUS inhibitors, especially those from dietary components, are highly appreciated and it would be more beneficial if the inhibitors could be localized in the colon as initiated by the authors of this paper77.
Targeting gmGUS for attenuated drug-induced toxicity in the gut and enhanced therapeutic efficacy.
The impacts of microbiota on drug-induced toxicity or efficacy have been recognized for decades. For example, antibiotics such as cefpodoxime and streptomycin have been tested to suppress gmGUS activity for diarrhea attenuation in chemotherapy78,79. Furthermore, a high-impact study published in Science in 2010 demonstrated that gmGUS inhibitors successfully alleviated diarrhea induced by irinotecan, a prodrug of SN-38 used for the treatment of different types of cancers, in mice8. The underlying mechanism is that the inhibitors can effectively inhibit gmGUS activity towards SN-38G hydrolysis to release the toxic form SN-38 in the colon, leading to decreased colonic exposure of SN-38, which is correlated with diarrheal severity80. Interestingly, the identified gmGUS inhibitors only inhibited gmGUS without killing the bacteria as many antibiotics do. Other than irinotecan, it has been reported that inhibiting gmGUS could also protect against gut damage and diminish dose-limiting toxicity induced by other drugs such as regorafenib19 and mycophenolic acid33 in animals due to reduced drug exposure with gmGUS inhibition. These interesting studies demonstrated that gmGUS is a druggable target for attenuation dose-limiting adverse effect (e.g., diarrhea, constipation, mucositis) in the GI tract. The mechanism is reducing glucuronides hydrolysis to release the toxic compounds (i.e., aglycones) in the gut due to gmGUS inhibition. However, local drug exposure may be affected by different factors, such as efflux/uptake transporters and UGTs, and only inhibiting gmGUS may or may not be effectively reduce local drug exposure. In addition, different mechanisms (e.g., inflammation81) could be involved in drug-induced toxicity and only reduce local drug exposure sometimes may not be able to significantly attenuate tissue damage and symptoms.
Other than alleviating drug-induced toxicity in the gut, inhibiting gmGUS can enhance irinotecan efficacy in chemotherapy as demonstrated in mice in 2020 in a paper published in PNAS9. This study is from the same group as the paper in Science, indicating that gmGUS inhibition alleviates gut damage, improves survival, and does not alter the gut microbial composition. In addition, due to gut protection, inhibiting gmGUS enables irinotecan dose escalation, which significantly improve therapeutic effectiveness due to gut protection. The mechanism of gut protection was not entirely elucidated in this paper and SN-38 exposure was not determined. The author of this paper proposed that reduced SN-38G hydrolysis in the gut due to gmGUS inhibition is likely the mechanism described above. This fascinating study provides an option of developing combination therapy targeting gmGUS for better anticancer efficacy, especially for the treatment of relapsed and refractory cancers, by enhancing systemic drug exposure.
Targeting gmGUS to prevent or treat local and remote diseases.
Glucuronidation of toxic chemicals (e.g., carcinogens) is a detoxification procedure because glucuronides are usually easier to be eliminated through urine or feces when compared to aglycones. However glucuronides of toxic compounds can be efficiently hydrolyzed by gmGUS in the gut to release the toxic compounds to cause certain diseases in the gut such as colitis and colorectal cancer30. In addition, aberrant expression/ activity of gmGUS could disturb the harmoniousness of certain hormones (e.g., estradiol) and neurotransmitters (e.g., dopamine, santonin) as these endogenous compounds undergo efficient HER or EER, leading to remote diseases such as breast cancer or mental disorders. Treating diseases by targeting gmGUS has not been quite success in humans, but some related research has been published in high-impact journals including Science and Nature, suggesting that targeting gmGUS is a currently a pioneer work.
Inhibiting gmGUS could reduce the risk of local diseases.
It is known that gmGUS facilitates initiation or progression of certain diseases in the gut because gmGUS could reactivate toxic exogenous compounds. The well-studied case probably is that gmGUS could cause certain carcinogens in the gut to cause colorectal cancer. The host body processes exogenous carcinogens such as PhIP, NNK (Nicotine-derived nitrosamine ketone) through glucuronidation in the liver to afford glucuronides, which could be excreted through the bile into the gut for elimination82,83. GmGUS could hydrolyze these non-toxic “wastes” to reactivate the carcinogens in the colon. The impact of gmGUS on the disposition of these carcinogens such as PhIP has been demonstrated in mouse models and approaches targeting gmGUS to prevent colorectal cancer have been proposed83. Other than colorectal cancer, gmGUS is also associated with colitis. A recent study showed that gmGUS reactivates triclosan, a compound used in thousands of consumer products, to cause colitis and colitis-associated colorectal tumorigenesis in animal models30. These examples showed that excessive activity of gmGUS at the functional level reactivates toxic compounds and harmer elimination. Therefore, inhibiting gmGUS activity was effective in treating the related diseases.
Inhibiting gmGUS holds the promise to prevent remote diseases.
Since signaling endogenous compounds participate in 3Rs, aberrant gmGUS activity in the gut could significantly impact the harmony of these compounds to cause disease in the other organs. For example, estradiol and estrone are hormones associated with hormone-related breast cancer11,84,85. These two compounds undergo enterohepatic recycling through glucuronidation in the liver and hydrolysis by the gmGUS in the gut. It was proposed that in case the activity of gmGUS is aberrantly elevated, more estradiol could be generated in the gut from glucuronide, resulting in hormone disorder to increase the risk of breast cancer86–88. However, controversial results were observed in a recent study showing that although gmGUS participate in hormone disposition, breast cancer risk was not reduced by gmGUS inhibitors, suggesting that estrobolome disposition in the host is a multidimensional process10. The challenge in this type of study is how gmGUS inhibition could quantitatively affect hormone exposure. Although deconjugation is an essential step in estradiol and estrone reactivation, systemic exposure to these two compounds is also impacted by other factors including glucuronidation in the liver and efflux of the glucuronide’s conjugates. More accurate quantification analysis is expected to determine the impact of gmGUS inhibition on hormone disorder to reveal the role of gmGUS in remote cancer entirely.
Other than hormones, some neurotransmitters including dopamine and serotonin, also undergo recycling via glucuronidation, where reactivation by gmGUS is also an important step in recycling to affect their bioavailability. Direct evidence linking gmGUS with neurotransmitter-related diseases has not been reported. However, it has been demonstrated that gut microbiota play an important role in regulating neurotransmitters in gut-brain axis89. Therefore, it is plausible that gmGUS could be one of the important players in neurotransmitters’ bioavailability, which may indirectly affect the function of gut-brain axis. If so, gmGUS could be tested as a target to affect neurotransmitters’ bioavailability to treat certain medical conditions such as depression and Parkinson’s diseases and maintain normal physical and mental wellbeing such as mood, sleep memory, learning, and motor control. More studies on how gmGUS affecting intestinal luminal and systemic exposure of these neurotransmitters needs to be conducted since the storage, release and transport of neurotransmitters are complex90,91.
GmGUS is involved in processing nutritional and herbal components.
The majority of research on treating and preventing diseases is gmGUS inhibition. For nutritional and herbal components processing, activation of gmGUS may be beneficial because many nutritional and active herbal components exist naturally as glucuronides or glycosides forms, which have high polarity and are difficult to be absorbed. GmGUS could hydrolyze a wide range of sugars to release the aglycones to facilitate absorption. For example, wogonoside and baicalin are two glucuronides in Scutellaria baicalensis, an herbal material used in many traditional medicines. GmGUS activates wogonoside and baicalin via hydrolysis to release wogonin and baicalein, two absorbable aglycones having multiple pharmacological functions including cancer prevention92. In this regard, boosting or enhancing gmGUS activity could facilitate beneficial aglycones’ release. More basic research on boosting and enhancing gmGUS expression and activity, and the impact of enhancing gmGUS on the disposition of endogenous compounds should be conducted.
Gut microbial GUS inhibitors.
Natural products and chemically synthesized small molecular gmGUS inhibitors have been continuously reported since the connection between gmGUS with certain diseases was established. In addition, some approved drugs were also reported to be active in gmGUS inhibition. A recent review paper summarized gmGUS inhibitors reported from these three categories13. For example, Scutellarein, a naturally occurring flavonoid, inhibited hydrolysis of pNPG, a standard GUS substrate, with IC50 at 5.76 μM against EcGUS93. Another example showed that palbociclib, an approved drug used to treat breast cancer, inhibited hydrolysis of pNPG by EcGUS with IC50 at 3.5 μM against EcGUS hydrolysis31. Interestingly, some glucuronides also showed gmGUS inhibition. For example, wogonoside, a naturally occurring glucuronide of wogonin, inhibited hydrolysis of SN-38G with Ki at 4–16 μM against EcGUS. Other than inhibition of a specific GUS isoform, many potent broad-spectrum gmGUS inhibitors were also identified. For instance, a recent publication reported amentoflavone as a potent inhibitor that can inhibit hydrolysis of pNPG against multiple gmGUS isoforms including EcGUS, CpGUS, and SpasGUS94. These examples suggested that dietary components or approved drugs may interact with microbiota via gmGUS inhibition to exert certain pharmacological efficacy.
Challenges and Perspectives of targeting gmGUS in drug development
Although the function of gmGUS has been reported back to the 1930s5, the role of gmGUS in drug development has only been appreciated for less than two decades, and many challenges still exist. Although preclinical studies have demonstrated the efficacy of modulating gmGUS in treating and preventing diseases and drug toxicity, there are currently no drugs targeting gmGUS approved in human. In other words, using gmGUS as a druggable target needs to be validated in humans in drug development.
One of the significant challenges is that the homeostasis of endogenous could be broken when gmGUS is inhibited. As described above, potent and broad-spectrum gmGUS inhibitors have been identified, and these inhibitors can decrease gmGUS activity to reduce local exposure to toxic compounds to alleviate local toxicities. However, gmGUS activity inhibition may disturb recyclings of certain important endogenous compounds (e.g., bilirubin, bile acids, dopamine), which may cause severe consequences. It is well-known that dysbiosis of gut microbiota could cause severe impact of host health. Whether inhibiting gmGUS could cause any a negative impact on the host’s health needs to be further evaluated. For example, dysfunction of dopamine homeostasis is associated with abnormal brain functions including Parkinson’s disease95, and severe consequences may occur if dopamine recyclings were significantly disturbed via gmGUS inhibition. Homeostasis of phenolic compounds is maintained by different factors such as UGTs, uptake/efflux transporters with complex interplays. Inhibiting gmGUS may break these interplays to cause severe pathological effects. In additional to mammalian cells in the host, gut bacterial may also expressed enzymes, such as UDP-glycosyltransferases, that may have potential impact on disposition of phenolics and glucuronides. More studies are expected to address these concerns.
Another concern is inhibitor selectivity. Besides gut microbiota, mammalian cells also express beta-glucuronidases abbreviated as “GUSB”96. It was reported that GUSB is involved in the degradation of glucuronate-containing glycosaminoglycan, which is an essential process in the human brain and deficiency of GUSB could cause mucopolysaccharidosis type VII (MPSVII), leading to lysosomal storage in the brain. In case GUSB were inhibited, a severe side effect could occur. In addition, gmGUS inhibition could potentially affect drug efficacy. For example, many cancer cells stably expressed GUSB97, theoretically SN-38G could be hydrolyzed by GUSB to release the active form SN-38. In case the activity of GUSB were inhibited in cancer cells, exposure of SN-38 could be potentially decreased, which may cause negative impact on therapeutic efficacy. Currently, inhibitor selectivity is understudied, and more studies should be conducted to determine whether gmGUS and GUSB share same inhibitors and what is the impact on drug exposure in the diseases tissue or organ in case both gmGUS and GUSB were inhibited.
Inhibitors identified using in vitro models may not function in in vivo studies. Gut microbiota is a mixture of trillions of bacteria, and it has been discovered that at least 279 gmGUS isoforms are expressed in human microbiota. These enzymes may share substrates even though they may have different favorite substrates. To develop inhibitors for decreased glucuronides hydrolysis in the gut, broad-spectrum inhibitors may be needed. Many studies used a single EcGUS as the enzyme source for inhibitor screening. Whether the inhibitors identified using a single enzyme could inhibit glucuronide hydrolysis in vivo need further evaluation. Different isoforms were used to address this concern, but it is still not comfortable to state that these enzymes can represent the entire gmGUS as evidences have shown that high potent EcGUS inhibitor (i.e., Amoxapine) showed incomplete inhibition of gmGUS activity from human gut microbiota98. Fecal enzymes could be a better option in this regard. However, when we prepare fecal enzymes from feces, it is unclear whether some isoforms were lost during preparation because anaerobic bacteria, which may express gmGUS, could not survive in feces unless the preparation is in anaerobic condition.
Additionally, in many in vitro and in vivo studies, the general gmGUS activity was evaluated using a chromogenic (e.g., 4-nitrophenyl β-D-glucopyranoside) or fluorogenic substrates (e.g., 4-methyl-umbelliferyl-β-D-glucuronide). These probes are sensitive, and the methods are simple and rapid. However, the aglycones of these two glucuronides are small and activity determined using these substrates may not reflect the activity for a specific substrate. Unfortunately, these is no studies reporting the correlation between “general activity” determined using gmGUS probes with the activity to hydrolyze a specific substrate. Additionally, structure-activity relationship study is rare and the relationship between general gmGUS activity and activity towards a specific substate is unclear. To address this concern, a probe cocktail containing glucuronides with structural diversity might be a better option. Additionally, it would be appreciated if the glucuronides and/or their metabolites (i.e., aglycone) in the cocktail could be sensitively detectable using UV or florescence detectors. How to select glucuronides to make a cocktail probe depends on our knowledges on further understanding the substrate-enzyme binding and the structure-activity relationships (SAR).
Individual variation of gmGUS expression levels and activity are also concerned. We previously found that fecal gmGUS activity toward hydrolysis of wogonoside, a naturally occurring flavonoid glucuronide were highly different (up to 6-fold) in individual rats20, suggesting that gmGUS expression in an individual could be highly different. More studies should be conducted to verify the results in humans.
Finally, a significant concern is the impact of gmGUS inhibition on the microbiome composition. One of the advantages of developing gmGUS inhibitors is that gmGUS inhibition will not kill the cells to cause microbiome dysbiosis, which is a common challenge for most approved antibiotics. In vitro study showed that inhibiting gmGUS using selected approved drugs did not kill E. coli94. In addition, preliminary in vivo studies also suggested that certain synthesized gmGUS inhibitor did not alter the microbiome composition significantly9. In vitro model has been used to address this concern is to determine the IC50 of the inhibitors against E. Coli94, which is a pretty simple and decent assay for primary evaluation and in vivo assays (e.g., 16S rRNA gene sequencing, shotgun metagenomics) to determine microbiome composition are commonly used in many labs or available in many facility cores. Determining the impact of gmGUS inhibitors on the microbiome is expected to be carefully studied in developing gmGUS inhibitors to treat/prevent diseases. In addition, many gmGUS inhibitors have been identified for the purpose of treating certain diseases or drug-induced toxicity, however the downside of on inhibiting gmGUS is not well studied. Since gmGUS enzymes are also involved in the disposition of many important endogenous compounds, the impact of inhibiting gmGUS on the disposition of these compounds and their physiological function should be considered and monitored when gmGUS is used as the target in drug development.
Concluding remarks
It has been demonstrated that gmGUS plays an essential role in the disposition of many endogenous and exogenous compounds. Many gmGUS inhibitors have been identified from natural products, approved drugs, and newly synthesized compounds using in vitro and in vivo models. Based on the findings from animal studies, it is expected that gmGUS can be used as a druggable target to treat gut or remote diseases. However, challenges remain and studies in humans are highly expected to be conducted to validate the findings from animal studies. In addition, since gmGUS is involved in the disposition of many important endogenous compounds, more studies should be conducted to determine the short-term and long-term impact of gmGUS manipulation on the disposition of the endogenous compounds, whose homeostasis is essential to the host’s health.
Acknowledgment:
This work was supported by a grant from The Cancer Prevention Research Institute of Texas, USA (CPRIT, RP190672) for Song Gao, a grant from NIH (R01CA246209) for Ming Hu, grants from NIH (R35GM142421 and R15GM135813) for Clement T. Y. Chan, grants from NIH (NIDDK P30DK56338, R01DK130517, NIAID R01AI10091401, U01AI24290, P01AI152999, NINR R01NR013497) for Tor Savidge. This work was also made possible, in part, by the GCC Center for Comprehensive PK/PD and Formulation (CCPF) with CPRIT (RP180748) and RCMI grant with the National Institute of Minority Health and Health Disparity, USA (U54MD007605). We appreciate Imoh Etim and Katherine Beigel’s help in final editing.
Abbreviations
- gmGUS
gut microbial beta-glucuronidases
- UGT
Uridine 5’-diphospho-glucuronosyltransferases
- 4-MUG
4-Methylumbelliferone-O-glucuronide
- 4-MU
4-methyl umbelliferone
- pNPG
p-nitrophenol-β-D-glucuronide
- EHR
Enterohepatic recycling
- HER
hepatoenteric recycling
- EER
enteroenteric recycling
References
- 1.Pellock SJ, Redinbo MR. Glucuronides in the gut: Sugar-driven symbioses between microbe and host. J Biol Chem; 292 (2017) 8569–8576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Winter J, Bokkenheuser VD. Bacterial metabolism of natural and synthetic sex hormones undergoing enterohepatic circulation. J Steroid Biochem; 27 (1987) 1145–1149. [DOI] [PubMed] [Google Scholar]
- 3.Alpuim Costa D, Nobre JG, Batista MV, Ribeiro C, Calle C, Cortes A, et al. Human Microbiota and Breast Cancer-Is There Any Relevant Link?-A Literature Review and New Horizons Toward Personalised Medicine. Front Microbiol; 12 (2021) 584332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pollet RM, D’Agostino EH, Walton WG, Xu Y, Little MS, Biernat KA, et al. An Atlas of beta-Glucuronidases in the Human Intestinal Microbiome. Structure; 25 (2017) 967–977 e965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masamune H BIOCHEMICAL STUDIES ON CARBOHYDRATES: IV. On an Enzyme which Catalyses the Hydrolysis of Biosynthetic Osides of Glucuronic Acid. J Biochem; 19 (1934) 353–375. [Google Scholar]
- 6.Kim DH, Hong SW, Kim BT, Bae EA, Park HY, Han MJ. Biotransformation of glycyrrhizin by human intestinal bacteria and its relation to biological activities. Arch Pharm Res; 23 (2000) 172–177. [DOI] [PubMed] [Google Scholar]
- 7.McBain AJ, Macfarlane GT. Ecological and physiological studies on large intestinal bacteria in relation to production of hydrolytic and reductive enzymes involved in formation of genotoxic metabolites. J Med Microbiol; 47 (1998) 407–416. [DOI] [PubMed] [Google Scholar]
- 8.Wallace BD, Wang H, Lane KT, Scott JE, Orans J, Koo JS, et al. Alleviating cancer drug toxicity by inhibiting a bacterial enzyme. Science; 330 (2010) 831–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bhatt AP, Pellock SJ, Biernat KA, Walton WG, Wallace BD, Creekmore BC, et al. Targeted inhibition of gut bacterial beta-glucuronidase activity enhances anticancer drug efficacy. Proc Natl Acad Sci U S A; 117 (2020) 7374–7381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ervin SM, Li H, Lim L, Roberts LR, Liang X, Mani S, et al. Gut microbial beta-glucuronidases reactivate estrogens as components of the estrobolome that reactivate estrogens. J Biol Chem; 294 (2019) 18586–18599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Starek-Swiechowicz B, Budziszewska B, Starek A. Endogenous estrogens-breast cancer and chemoprevention. Pharmacol Rep; 73 (2021) 1497–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wei Bin Y W, Yan Zhi-Xiang, Zhang Qing-Wen, Yan Ru. Prenylflavonoids sanggenon C and kuwanon G from mulberry (Morus alba L.) as potent broad-spectrum bacterial β-glucuronidase inhibitors: Biological evaluation and molecular docking studies. Journal of Functional Foods; 48 (2018) 210–219. [Google Scholar]
- 13.Wang P, Jia Y, Wu R, Chen Z, Yan R. Human gut bacterial beta-glucuronidase inhibition: An emerging approach to manage medication therapy. Biochem Pharmacol; 190 (2021) 114566. [DOI] [PubMed] [Google Scholar]
- 14.Gloux K, Anba-Mondoloni J. Unique beta-Glucuronidase Locus in Gut Microbiomes of Crohn’s Disease Patients and Unaffected First-Degree Relatives. PLoS One; 11 (2016) e0148291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McIntosh FM, Maison N, Holtrop G, Young P, Stevens VJ, Ince J, et al. Phylogenetic distribution of genes encoding beta-glucuronidase activity in human colonic bacteria and the impact of diet on faecal glycosidase activities. Environ Microbiol; 14 (2012) 1876–1887. [DOI] [PubMed] [Google Scholar]
- 16.Gloux K, Berteau O, El Oumami H, Beguet F, Leclerc M, Dore J. A metagenomic beta-glucuronidase uncovers a core adaptive function of the human intestinal microbiome. Proc Natl Acad Sci U S A; 108 Suppl 1 (2011) 4539–4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wallace BD, Roberts AB, Pollet RM, Ingle JD, Biernat KA, Pellock SJ, et al. Structure and Inhibition of Microbiome beta-Glucuronidases Essential to the Alleviation of Cancer Drug Toxicity. Chem Biol; 22 (2015) 1238–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Little MS, Pellock SJ, Walton WG, Tripathy A, Redinbo MR. Structural basis for the regulation of beta-glucuronidase expression by human gut Enterobacteriaceae. Proc Natl Acad Sci U S A; 115 (2018) E152–E161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ervin SM, Hanley RP, Lim L, Walton WG, Pearce KH, Bhatt AP, et al. Targeting Regorafenib-Induced Toxicity through Inhibition of Gut Microbial beta-Glucuronidases. ACS Chem Biol; 14 (2019) 2737–2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ebuzoeme C, Etim I, Ikimi A, Song J, Du T, Hu M, et al. Glucuronides Hydrolysis by Intestinal Microbial beta-Glucuronidases (GUS) Is Affected by Sampling, Enzyme Preparation, Buffer pH, and Species. Pharmaceutics; 13 (2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elmassry MM, Kim S, Busby B. Predicting drug-metagenome interactions: Variation in the microbial beta-glucuronidase level in the human gut metagenomes. PLoS One; 16 (2021) e0244876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mroczynska M, Galecka M, Szachta P, Kamoda D, Libudzisz Z, Roszak D. Beta-glucuronidase and Beta-glucosidase activity in stool specimens of children with inflammatory bowel disease. Pol J Microbiol; 62 (2013) 319–325. [PubMed] [Google Scholar]
- 23.Boelsterli UA, Redinbo MR, Saitta KS. Multiple NSAID-induced hits injure the small intestine: underlying mechanisms and novel strategies. Toxicol Sci; 131 (2013) 654–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Davis MP. The opioid bowel syndrome: a review of pathophysiology and treatment. J Opioid Manag; 1 (2005) 153–161. [DOI] [PubMed] [Google Scholar]
- 25.Swami U, Goel S, Mani S. Therapeutic targeting of CPT-11 induced diarrhea: a case for prophylaxis. Curr Drug Targets; 14 (2013) 777–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Edginton AN, Zimmerman EI, Vasilyeva A, Baker SD, Panetta JC. Sorafenib metabolism, transport, and enterohepatic recycling: physiologically based modeling and simulation in mice. Cancer Chemother Pharmacol; 77 (2016) 1039–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arslan H, Inci EK, Azap OK, Karakayali H, Torgay A, Haberal M. Etiologic agents of diarrhea in solid organ recipients. Transpl Infect Dis; 9 (2007) 270–275. [DOI] [PubMed] [Google Scholar]
- 28.Dalle IJ, Maes BD, Geboes KP, Lemahieu W, Geboes K. Crohn’s-like changes in the colon due to mycophenolate? Colorectal Dis; 7 (2005) 27–34. [DOI] [PubMed] [Google Scholar]
- 29.Maes BD, Dalle I, Geboes K, Oellerich M, Armstrong VW, Evenepoel P, et al. Erosive enterocolitis in mycophenolate mofetil-treated renal-transplant recipients with persistent afebrile diarrhea. Transplantation; 75 (2003) 665–672. [DOI] [PubMed] [Google Scholar]
- 30.Zhang J, Walker ME, Sanidad KZ, Zhang H, Liang Y, Zhao E, et al. Microbial enzymes induce colitis by reactivating triclosan in the mouse gastrointestinal tract. Nat Commun; 13 (2022) 136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pellock SJ, Creekmore BC, Walton WG, Mehta N, Biernat KA, Cesmat AP, et al. Gut Microbial beta-Glucuronidase Inhibition via Catalytic Cycle Interception. ACS Cent Sci; 4 (2018) 868–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kong R, Liu T, Zhu X, Ahmad S, Williams AL, Phan AT, et al. Old drug new use--amoxapine and its metabolites as potent bacterial beta-glucuronidase inhibitors for alleviating cancer drug toxicity. Clin Cancer Res; 20 (2014) 3521–3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Taylor MR, Flannigan KL, Rahim H, Mohamud A, Lewis IA, Hirota SA, et al. Vancomycin relieves mycophenolate mofetil-induced gastrointestinal toxicity by eliminating gut bacterial beta-glucuronidase activity. Sci Adv; 5 (2019) eaax2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saitta KS, Zhang C, Lee KK, Fujimoto K, Redinbo MR, Boelsterli UA. Bacterial beta-glucuronidase inhibition protects mice against enteropathy induced by indomethacin, ketoprofen or diclofenac: mode of action and pharmacokinetics. Xenobiotica; 44 (2014) 28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang F, Meng J, Zhang L, Johnson T, Chen C, Roy S. Morphine induces changes in the gut microbiome and metabolome in a morphine dependence model. Sci Rep; 8 (2018) 3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frances B, Gout R, Campistron G, Panconi E, Cros J. Morphine-6-glucuronide is more mu-selective and potent in analgesic tests than morphine. Prog Clin Biol Res; 328 (1990) 477–480. [PubMed] [Google Scholar]
- 37.Nelson AD, Camilleri M. Chronic opioid induced constipation in patients with nonmalignant pain: challenges and opportunities. Therap Adv Gastroenterol; 8 (2015) 206–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen K, Yuan T. The role of microbiota in neonatal hyperbilirubinemia. Am J Transl Res; 12 (2020) 7459–7474. [PMC free article] [PubMed] [Google Scholar]
- 39.Sudo N Biogenic Amines: Signals Between Commensal Microbiota and Gut Physiology. Front Endocrinol (Lausanne); 10 (2019) 504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Asano Y, Hiramoto T, Nishino R, Aiba Y, Kimura T, Yoshihara K, et al. Critical role of gut microbiota in the production of biologically active, free catecholamines in the gut lumen of mice. Am J Physiol Gastrointest Liver Physiol; 303 (2012) G1288–1295. [DOI] [PubMed] [Google Scholar]
- 41.Jahrig K [Analysis of health care systems]. Arztl Jugendkd; 81 (1990) 2–3. [PubMed] [Google Scholar]
- 42.Hemminki K, Li X, Sundquist J, Sundquist K. Cancer risks in Crohn disease patients. Ann Oncol; 20 (2009) 574–580. [DOI] [PubMed] [Google Scholar]
- 43.Sui Y, Wu J, Chen J. The Role of Gut Microbial beta-Glucuronidase in Estrogen Reactivation and Breast Cancer. Front Cell Dev Biol; 9 (2021) 631552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen KLA, Liu X, Zhao YC, Hieronymi K, Rossi G, Auvil LS, et al. Long-Term Administration of Conjugated Estrogen and Bazedoxifene Decreased Murine Fecal beta-Glucuronidase Activity Without Impacting Overall Microbiome Community. Sci Rep; 8 (2018) 8166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Michaud JE, Billups KL, Partin AW. Testosterone and prostate cancer: an evidence-based review of pathogenesis and oncologic risk. Ther Adv Urol; 7 (2015) 378–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Armstrong AJ, Shore ND, Szmulewitz RZ, Petrylak DP, Holzbeierlein J, Villers A, et al. Efficacy of Enzalutamide plus Androgen Deprivation Therapy in Metastatic Hormone-Sensitive Prostate Cancer by Pattern of Metastatic Spread: ARCHES Post Hoc Analyses. J Urol; 205 (2021) 1361–1371. [DOI] [PubMed] [Google Scholar]
- 47.Hamid AA, Huang HC, Wang V, Chen YH, Feng F, Den R, et al. Transcriptional profiling of primary prostate tumor in metastatic hormone-sensitive prostate cancer and association with clinical outcomes: correlative analysis of the E3805 CHAARTED trial. Ann Oncol; 32 (2021) 1157–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Biernat KA, Pellock SJ, Bhatt AP, Bivins MM, Walton WG, Tran BNT, et al. Structure, function, and inhibition of drug reactivating human gut microbial beta-glucuronidases. Sci Rep; 9 (2019) 825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.R UA, R M, T R, H S, M M, G T, et al. Data from: Crystal structure of putative beta-galactosidase from Bacteroides fragilis. 2008. Protein Data Bank. doi: 10.2210/pdb3cmg/pdb [DOI] [Google Scholar]
- 50.Little MS, Ervin SM, Walton WG, Tripathy A, Xu Y, Liu J, et al. Active site flexibility revealed in crystal structures of Parabacteroides merdae beta-glucuronidase from the human gut microbiome. Protein Sci; 27 (2018) 2010–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pellock SJ, Walton WG, Biernat KA, Torres-Rivera D, Creekmore BC, Xu Y, et al. Three structurally and functionally distinct beta-glucuronidases from the human gut microbe Bacteroides uniformis. J Biol Chem; 293 (2018) 18559–18573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Romberg RW, Lee L. Comparison of the hydrolysis rates of morphine-3-glucuronide and morphine-6-glucuronide with acid and beta-glucuronidase. J Anal Toxicol; 19 (1995) 157–162. [DOI] [PubMed] [Google Scholar]
- 53.Zenser TV, Lakshmi VM, Davis BB. Human and Escherichia coli beta-glucuronidase hydrolysis of glucuronide conjugates of benzidine and 4-aminobiphenyl, and their hydroxy metabolites. Drug Metab Dispos; 27 (1999) 1064–1067. [PubMed] [Google Scholar]
- 54.Walsh J, Olavarria-Ramirez L, Lach G, Boehme M, Dinan TG, Cryan JF, et al. Impact of host and environmental factors on beta-glucuronidase enzymatic activity: implications for gastrointestinal serotonin. Am J Physiol Gastrointest Liver Physiol; 318 (2020) G816–G826. [DOI] [PubMed] [Google Scholar]
- 55.Reddy BS, Weisburger JH, Wynder EL. Fecal bacterial beta-glucuronidase: control by diet. Science; 183 (1974) 416–417. [DOI] [PubMed] [Google Scholar]
- 56.Reddy BS, Engle A, Simi B, Goldman M. Effect of dietary fiber on colonic bacterial enzymes and bile acids in relation to colon cancer. Gastroenterology; 102 (1992) 1475–1482. [DOI] [PubMed] [Google Scholar]
- 57.Domellof L, Darby L, Hanson D, Mathews L, Simi B, Reddy BS. Fecal sterols and bacterial beta-glucuronidase activity: a preliminary metabolic epidemiology study of healthy volunteers from Umea, Sweden, and metropolitan New York. Nutr Cancer; 4 (1982) 120–127. [DOI] [PubMed] [Google Scholar]
- 58.Goldin BR, Adlercreutz H, Gorbach SL, Warram JH, Dwyer JT, Swenson L, et al. Estrogen excretion patterns and plasma levels in vegetarian and omnivorous women. N Engl J Med; 307 (1982) 1542–1547. [DOI] [PubMed] [Google Scholar]
- 59.Gao S, Hu M. Bioavailability challenges associated with development of anti-cancer phenolics. Mini Rev Med Chem; 10 (2010) 550–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen J, Lin H, Hu M. Metabolism of flavonoids via enteric recycling: role of intestinal disposition. The Journal of pharmacology and experimental therapeutics; 304 (2003) 1228–1235. [DOI] [PubMed] [Google Scholar]
- 61.Norman A, Sjovall J. On the transformation and enterohepatic circulation of cholic acid in the rat: bile acids and steroids 68. J Biol Chem; 233 (1958) 872–885. [PubMed] [Google Scholar]
- 62.Feng PC, Hartman PA. Fluorogenic assays for immediate confirmation of Escherichia coli. Appl Environ Microbiol; 43 (1982) 1320–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rompre A, Servais P, Baudart J, de-Roubin MR, Laurent P. Detection and enumeration of coliforms in drinking water: current methods and emerging approaches. J Microbiol Methods; 49 (2002) 31–54. [DOI] [PubMed] [Google Scholar]
- 64.Frampton EW, Restaino L. Methods for Escherichia coli identification in food, water and clinical samples based on beta-glucuronidase detection. J Appl Bacteriol; 74 (1993) 223–233. [DOI] [PubMed] [Google Scholar]
- 65.Briciu-Burghina C, Heery B, Regan F. Continuous fluorometric method for measuring beta-glucuronidase activity: comparative analysis of three fluorogenic substrates. Analyst; 140 (2015) 5953–5964. [DOI] [PubMed] [Google Scholar]
- 66.Chen R. Enzyme and microbial technology for synthesis of bioactive oligosaccharides: an update. Appl Microbiol Biotechnol; 102 (2018) 3017–3026. [DOI] [PubMed] [Google Scholar]
- 67.Tung CH, Zeng Q, Shah K, Kim DE, Schellingerhout D, Weissleder R. In vivo imaging of beta-galactosidase activity using far red fluorescent switch. Cancer Res; 64 (2004) 1579–1583. [DOI] [PubMed] [Google Scholar]
- 68.Crow RM, Gartland JS, McHugh AT, Gartland KM. Real-time GUS analysis using Q-PCR instrumentation. J Biotechnol; 126 (2006) 135–139. [DOI] [PubMed] [Google Scholar]
- 69.Starkey DE, Han A, Bao JJ, Ahn CH, Wehmeyer KR, Prenger MC, et al. Fluorogenic assay for beta-glucuronidase using microchip-based capillary electrophoresis. J Chromatogr B Biomed Sci Appl; 762 (2001) 33–41. [DOI] [PubMed] [Google Scholar]
- 70.Togo CA, Wutor VC, Limson JL, Pletschke BI. Novel detection of Escherichia coli beta-D-glucuronidase activity using a microbially-modified glassy carbon electrode and its potential for faecal pollution monitoring. Biotechnol Lett; 29 (2007) 531–537. [DOI] [PubMed] [Google Scholar]
- 71.Cheng KW, Tseng CH, Yang CN, Tzeng CC, Cheng TC, Leu YL, et al. Specific Inhibition of Bacterial beta-Glucuronidase by Pyrazolo[4,3-c]quinoline Derivatives via a pH-Dependent Manner To Suppress Chemotherapy-Induced Intestinal Toxicity. J Med Chem; 60 (2017) 9222–9238. [DOI] [PubMed] [Google Scholar]
- 72.Cheng KW, Tseng CH, Tzeng CC, Leu YL, Cheng TC, Wang JY, et al. Pharmacological inhibition of bacterial beta-glucuronidase prevents irinotecan-induced diarrhea without impairing its antitumor efficacy in vivo. Pharmacol Res; 139 (2019) 41–49. [DOI] [PubMed] [Google Scholar]
- 73.Feng L, Yang Y, Huo X, Tian X, Feng Y, Yuan H, et al. Highly Selective NIR Probe for Intestinal beta-Glucuronidase and High-Throughput Screening Inhibitors to Therapy Intestinal Damage. ACS Sens; 3 (2018) 1727–1734. [DOI] [PubMed] [Google Scholar]
- 74.Feng L, Yang Y, Huo X, Tian X, Feng Y, Yuan H, et al. Correction to Highly Selective NIR Probe for Intestinal beta-Glucuronidase and High-Throughput Screening Inhibitors to Therapy Intestinal Damage. ACS Sens; 4 (2019) 2553. [DOI] [PubMed] [Google Scholar]
- 75.Yeo HK, Hyun YJ, Jang SE, Han MJ, Lee YS, Kim DH. Development of fecal microbial enzyme mix for mutagenicity assay of natural products. J Microbiol Biotechnol; 22 (2012) 838–848. [DOI] [PubMed] [Google Scholar]
- 76.Simone Fior AV, Gerola Paolo D. A novel method for fluorometric continuous measurement of β-glucuronidase (GUS) activity using 4-methyl-umbelliferyl-β-d-glucuronide (MUG) as substrate. Plant Science; 176 (2009) 130. [Google Scholar]
- 77.Hu M, inventor; Locally bioavailable drugs (Patent). USA patent application US11,202,773 B2. 2021.
- 78.Kurita A, Kado S, Matsumoto T, Asakawa N, Kaneda N, Kato I, et al. Streptomycin alleviates irinotecan-induced delayed-onset diarrhea in rats by a mechanism other than inhibition of beta-glucuronidase activity in intestinal lumen. Cancer Chemother Pharmacol; 67 (2011) 201–213. [DOI] [PubMed] [Google Scholar]
- 79.McNall-Knapp RY, Williams CN, Reeves EN, Heideman RL, Meyer WH. Extended phase I evaluation of vincristine, irinotecan, temozolomide, and antibiotic in children with refractory solid tumors. Pediatr Blood Cancer; 54 (2010) 909–915. [DOI] [PubMed] [Google Scholar]
- 80.Sun R, Zhu L, Li L, Song W, Gong X, Qi X, et al. Irinotecan-mediated diarrhea is mainly correlated with intestinal exposure to SN-38: Critical role of gut Ugt. Toxicol Appl Pharmacol; 398 (2020) 115032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lian Q, Xu J, Yan S, Huang M, Ding H, Sun X, et al. Chemotherapy-induced intestinal inflammatory responses are mediated by exosome secretion of double-strand DNA via AIM2 inflammasome activation. Cell Res; 27 (2017) 784–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yuan JM, Knezevich AD, Wang R, Gao YT, Hecht SS, Stepanov I. Urinary levels of the tobacco-specific carcinogen N’-nitrosonornicotine and its glucuronide are strongly associated with esophageal cancer risk in smokers. Carcinogenesis; 32 (2011) 1366–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhang J, Lacroix C, Wortmann E, Ruscheweyh HJ, Sunagawa S, Sturla SJ, et al. Gut microbial beta-glucuronidase and glycerol/diol dehydratase activity contribute to dietary heterocyclic amine biotransformation. BMC Microbiol; 19 (2019) 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Coradini D, Oriana S. Impact of sex hormones dysregulation and adiposity on the outcome of postmenopausal breast cancer patients. Clin Obes; 11 (2021) e12423. [DOI] [PubMed] [Google Scholar]
- 85.Yang Z, Hu Y, Zhang J, Xu L, Zeng R, Kang D. Estradiol therapy and breast cancer risk in perimenopausal and postmenopausal women: a systematic review and meta-analysis. Gynecol Endocrinol; 33 (2017) 87–92. [DOI] [PubMed] [Google Scholar]
- 86.Gorbach SL. Estrogens, breast cancer, and intestinal flora. Rev Infect Dis; 6 Suppl 1 (1984) S85–90. [DOI] [PubMed] [Google Scholar]
- 87.Parida S, Sharma D. The Microbiome-Estrogen Connection and Breast Cancer Risk. Cells; 8 (2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Komorowski AS, Pezo RC. Untapped “-omics”: the microbial metagenome, estrobolome, and their influence on the development of breast cancer and response to treatment. Breast Cancer Res Treat; 179 (2020) 287–300. [DOI] [PubMed] [Google Scholar]
- 89.Hamamah S, Aghazarian A, Nazaryan A, Hajnal A, Covasa M. Role of Microbiota-Gut-Brain Axis in Regulating Dopaminergic Signaling. Biomedicines; 10 (2022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Iversen L Neurotransmitter transporters and their impact on the development of psychopharmacology. Br J Pharmacol; 147 Suppl 1 (2006) S82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Winkler H, Schmidt W, Fischer-Colbrie R, Weber A. Molecular mechanisms of neurotransmitter storage and release: a comparison of the adrenergic and cholinergic systems. Prog Brain Res; 58 (1983) 11–20. [DOI] [PubMed] [Google Scholar]
- 92.Banik K, Khatoon E, Harsha C, Rana V, Parama D, Thakur KK, et al. Wogonin and its analogs for the prevention and treatment of cancer: A systematic review. Phytother Res; (2022) [DOI] [PubMed] [Google Scholar]
- 93.Weng ZM, Wang P, Ge GB, Dai ZR, Wu DC, Zou LW, et al. Structure-activity relationships of flavonoids as natural inhibitors against E. coli beta-glucuronidase. Food Chem Toxicol; 109 (2017) 975–983. [DOI] [PubMed] [Google Scholar]
- 94.Bai Y, Chen L, Wang PP, Tang YQ, Wu DC, Zhang CL, et al. Discovery of a naturally occurring broad-spectrum inhibitor against gut bacterial beta-glucuronidases from Ginkgo biloba. Food Funct; 12 (2021) 11190–11201. [DOI] [PubMed] [Google Scholar]
- 95.Febo M, Blum K, Badgaiyan RD, Baron D, Thanos PK, Colon-Perez LM, et al. Dopamine homeostasis: brain functional connectivity in reward deficiency syndrome. Front Biosci (Landmark Ed); 22 (2017) 669–691. [DOI] [PubMed] [Google Scholar]
- 96.Naz H, Islam A, Waheed A, Sly WS, Ahmad F, Hassan I. Human beta-glucuronidase: structure, function, and application in enzyme replacement therapy. Rejuvenation Res; 16 (2013) 352–363. [DOI] [PubMed] [Google Scholar]
- 97.Wang H, Yang B, Geng T, Li B, Dai P, Chen C. Tissue-specific selection of optimal reference genes for expression analysis of anti-cancer drug-related genes in tumor samples using quantitative real-time RT-PCR. Exp Mol Pathol; 98 (2015) 375–381. [DOI] [PubMed] [Google Scholar]
- 98.Yang W, Wei B, Yan R. Amoxapine Demonstrates Incomplete Inhibition of beta-Glucuronidase Activity from Human Gut Microbiota. SLAS Discov; 23 (2018) 76–83. [DOI] [PubMed] [Google Scholar]
- 99.Okonechnikov K, Golosova O, Fursov M, team U. Unipro UGENE: a unified bioinformatics toolkit. Bioinformatics; 28 (2012) 1166–1167. [DOI] [PubMed] [Google Scholar]


