Abstract
Objective
The emerging renal complications in beta-thalassemia patients have raised the global exchange of views. Despite better survival due to blood transfusion and iron chelation therapy, the previously unrecognized renal complication remain a burden of disease affecting this population —the primary concern on how iron overload and chelation therapy correlated with renal impairment is still controversial. Early detection and diagnosis is crucial in preventing further kidney damage. Therefore, a systematic review was performed to identify markers of kidney complications in beta thalassemia patients with iron overload receiving chelation therapy.
Methods
Searches of PubMed, Scopus, Science Direct, and Web of Science were conducted according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) to identify studies of literature reporting renal outcome in β-TM patients with iron overload and receiving chelation therapy. The eligible 17 studies were obtained.
Results
uNGAL/NGAL, uNAG/NAG, uKIM-1 are markers that can be used as predictor of renal tubular damage in early renal complications, while Cystatin C and uβ2MG showed further damage at the glomerular level.
Discussion and Conclusion
The renal complication in beta-thalassemia patients with iron overload receiving chelating agent therapy may progress to kidney disease. Early detection using accurate biological markers is a substantial issue that deserves further evaluation to determine prevention and management.
Keywords: thalassemia, iron overload, chelating agent, kidney, health
Introduction
Beta thalassemia is one of the most frequent haemoglobin disorders inherited in an autosomal recessive manner bringing about worldwide health problems, especially in the tropical belt. Southeast Asia accounts for about 50% of thalassemia carriers in the world. Beta-thalassemia results from reduced or lost-globin chain synthesis due to mutations in the beta-globin gene.1,2 Indonesia, with more than 200 million people, has a beta thalassemia carrier frequency of 6–10%, becoming an issue to anticipate by the government. The high demand for blood transfusions and chelating therapy marks their linear effect on the improved prognosis of beta-thalassemia patients.2
Despite a better survival rate due to supportive therapy, the beta-thalassemia population still have an increased risk for various complications and thus remains a challenge in affecting their quality of life. The four most common complications are cardiac, endocrine, hepatic, and renal.3–7 The emerging renal complications reported in beta-thalassemia patients have been correlated with the nature of the disease (eg, chronic anemia, hypoxia), iron overload due to regular blood transfusions, and chelating therapy. Altered vascular resistance and increased renal plasma flow, hyperfiltration, and renal tubular dysfunction were some common mechanisms in diminished renal function of beta-thalassemia patients. Iron overload beta-thalassemia patients were found to have distinct markers of kidney injury such as serum beta2-Μ, urinary calcium/creatinine, urine 2-M/creatinine, urinary NAG, urinary NAGL, urinary a1-microglobulin, and urinary RBP.8–11 Moreover, renal complications also occurred significantly in those who received chelating agent therapy.3,10,12–14 This study aims to identify markers of renal complications in beta-thalassemia patients with iron overload and chelation agent therapy.
Methods
This study was conducted according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
Literature Search
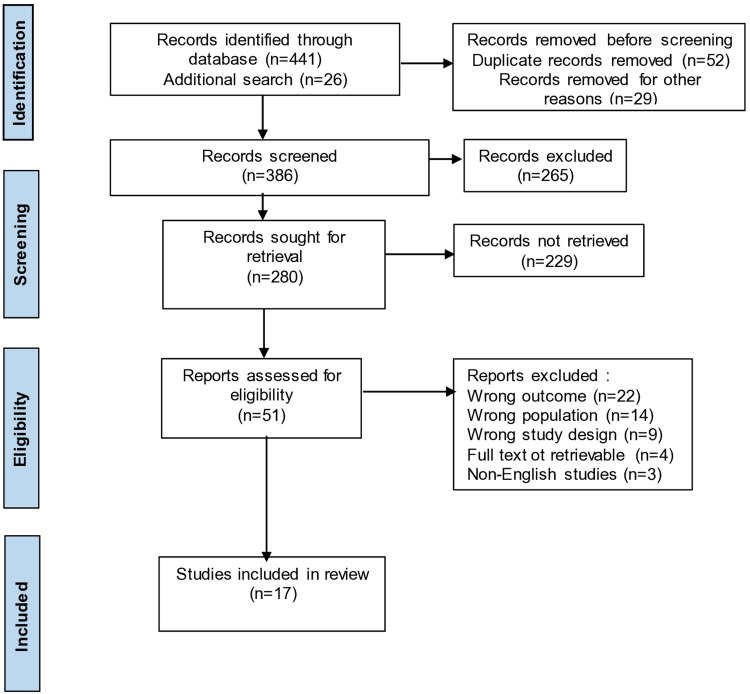
A comprehensive search of the electronic literature was done using the online databases PubMed, Scopus, Science Direct, and Web of Science. We used the following search term: ([MeSH]beta-Thalassemia] OR (thalassemia) OR (thalassemia beta major) AND (renal outcome*) OR (renal complication*) OR (renal function*) OR (renal damage*) OR (kidney injury*) OR (kidney function*) from the Cochrane Collaboration’s search strategy for randomized control trials. Our search approach does not use a filter and has no year restrictions. The first search on each database was done in March, and the final search was done on May 29, 2022. Figure 1 contains the PRISMA flow for literature search.
Figure 1.
PRISMA flow chart.
Eligibility and Study Selection
All randomized control trials, cohort studies, case-control studies, and cross-sectional studies reporting renal outcome in patients with TM connected to chelation therapy were considered. We only list publications written in English. Unrecoverable full text studies and duplicate research were eliminated. Authors EP and EDA carried out the search. The full text of any articles that satisfied the inclusion criteria was retrieved by three writers (PZR, SDS, and AEP) after they separately screened the title and abstracts. The eligibility of full text articles was examined by both writers. The Mendeley program, a free online tool for managing references, was used during the selection process to eliminate duplicate studies and analyze the abstract and full text.
Data Extraction
Data extraction was carried out by two reviewers (BAM, CW), and any differences were settled by team consensus. Using an excel predesign table, the studies that satisfied the relevance and eligibility of our aforementioned criteria were extracted. All of collected data were: 1) A summary of the studies that were included, including methodological information about the site, sample, interventions, outcomes, and results. 2) Baseline features of the studies. 3) Renal outcome or function related to the previously described use of chelation therapy.
Results
We finally screened and obtained 17 studies of literature comparing β-TM with iron overload and with chelation therapy. We summarize them in Tables 1 and 2. From this table, 3 studies stated that there were significant differences in uNGAL/NGAL,10,11,15 and 5 studies stated that there were significant differences in uNAG/NAG,11,16–19 2 studies revealed significant differences in uKIM-1,3,10 5 studies stated significant differences in Cystatin C,20–24 3 studies showed a significant difference in uβ2MG20,23,25 in beta thalassemia patients with iron overload and receiving chelating agents. Increase in uNGAL/NGAL; uNAG/NAG; uKIM-1; Cystatin C; This uβ2MG/β2MG is associated with early tubular and glomerular dysfunction. Three studies suggest that deferasirox is a chelating agent that causes an increase in uKIM-1, uNGAL, and u uβ2MG.3,10,23
Table 1.
Chelation Therapy and Marker Reported from Study of Beta Thalassemia
| No. | Authors | Year of Publication | Study Type | Age (Years) | Chelation of Therapy (Number of Patients) | Markers Reported |
|---|---|---|---|---|---|---|
| 1 | Aldudak 200017 | 2000 | Cross-sectional | 9.6 ± 5.0; 10 ± 4.7 | Deferoxamine (70) | BUN |
| S Cr | ||||||
| S Na | ||||||
| S K | ||||||
| S Ua | ||||||
| S Phosp | ||||||
| Ccr | ||||||
| TRP | ||||||
| uVolume | ||||||
| uOsmolality | ||||||
| uP/Cr | ||||||
| uNAG/Cr | ||||||
| uMDA/Cr | ||||||
| 2 | Smolkin 200816 | 2008 | Cross-sectional | 10.8 ± 6.2; 11.2 ± 3.4; 10.2±4.5 | Deferoxamine (n/a) | BUN |
| Serum creatinine | ||||||
| Serum uric acid | ||||||
| GFR | ||||||
| FENa | ||||||
| FEK | ||||||
| Urine Ca/Cr | ||||||
| Uric acid excretion | ||||||
| Tubular phosphorus reab | ||||||
| Urine osmolality | ||||||
| uNAG | ||||||
| uNAG/Cr | ||||||
| 3 | Economou 201025 | 2010 | Case-control | 13.66 ± 5.11; 12.66 ± 4.2 | Deferasirox (28) group A | Serum creatinine |
| Deferiprone + deferoxamine (14) group B | Urea | |||||
| Cystatin-C | ||||||
| U Protein | ||||||
| U Ca | ||||||
| U FeNa | ||||||
| u Phoshor, rabsorbtion | ||||||
| uβ2 MG | ||||||
| CrC | ||||||
| eGFR | ||||||
| 4 | Hamed 20105 | 2010 | Case-control | 8.72±3.7; 8.4±4.1 | Deferoxamine (34); | Cystatin-C |
| w/o chelation (35) | Creatinine | |||||
| eGFRSchwartz | ||||||
| Uric acid | ||||||
| Calcium | ||||||
| Inorganic phosphate | ||||||
| Total antioxidant capacity | ||||||
| Albumin/Cr | ||||||
| NAG/Cr | ||||||
| β2MG/Cr | ||||||
| Inorganic phosphorus | ||||||
| MDA/Cr | ||||||
| 5 | Mansi, 2013(belum ada) | 2013 | Case-control | Deferoxamine (42) | Glucose | |
| Urea | ||||||
| Creatinine | ||||||
| Uric acid | ||||||
| Sodium | ||||||
| Potassium | ||||||
| Chloride | ||||||
| 6 | Ali 201421 | 2014 | Cross-sectional | 9.67± 1.35; n/a | Deferoxamine (62) group IA | Cystatin-C |
| No chelating (38) group IB | Serum creatinine | |||||
| Control (50) group II | eGFRSchwartz | |||||
| CrC | ||||||
| Albumin/Cr | ||||||
| 7 | Sen 201511 | 2015 | Cross-sectional | 9.14 ± 4.4; 8.8 ± 4.0 | Deferasirox (59) | U Na/Cr |
| U K/Cr | ||||||
| U Ca/Cr | ||||||
| U P/Cr | ||||||
| U Mg/Cr | ||||||
| U Protein/Cr | ||||||
| U Uric acid/Cr | ||||||
| uNAG/Cr | ||||||
| uNGAL/Cr | ||||||
| uKIM1/Cr | ||||||
| uL-FABP/Cr | ||||||
| 8 | Behairy 201724 | 2017 | Case-control | 10.41±3.86, 8.6±3.47 | Deferoxamine (60%) | Urea |
| Deferasirox (32.9%) | Creatinine | |||||
| Hydra (7.1%) | Urea/Cr | |||||
| Urine volume | ||||||
| CrC | ||||||
| Cystatin-C | ||||||
| β2MG | ||||||
| uAlbumin/Cr | ||||||
| eGFRSchwartz | ||||||
| 9 | Bekhit 201719 | 2017 | Case-control | 8.5 ± 3.5; n/a | Deferasirox (21) | S Urea |
| Deferiprone (8) | S Cr | |||||
| Deferoxamine (3) | U Creatinine | |||||
| U Ca/Cr | ||||||
| U uric acid | ||||||
| GFR | ||||||
| uNAG | ||||||
| 10 | Annayev 201823 | 2018 | Case-control | 18.4 ± 11.8; n/a | Deferasirox (38) | FENa |
| Deferiprone (8) | Na | |||||
| None (4) | K | |||||
| Ca | ||||||
| P | ||||||
| Mg | ||||||
| U Ca/Cr | ||||||
| Urea | ||||||
| Creatinine | ||||||
| Albumin | ||||||
| GFR based on age | ||||||
| SCystatin-C | ||||||
| uβ2 MG | ||||||
| 11 | ElAlfy 201820 | 2018 | Cross-sectional | 12.8 ± 3.2 | Deferoxamine (2) | Urea |
| Deferasirox (9) | Uric acid | |||||
| Deferiprone (3) | Serum creatinine | |||||
| Combined (36) | Cystatin-C | |||||
| Urinary β2 microglobulin | ||||||
| uAlbumin/Cr | ||||||
| uβ2 M/albumin | ||||||
| 12 | Badelli 201910 | 2019 | Case-control | n/a | Deferoxamine (19) | GFR |
| Deferasirox (21) | BUN | |||||
| Creatinine | ||||||
| Cystatin-C | ||||||
| GFR based on Cystatin | ||||||
| uNGAL | ||||||
| uKIM1 | ||||||
| uIL-18 | ||||||
| uCreatinine | ||||||
| uAlbumin | ||||||
| uNGAL/Cr | ||||||
| uIL-18/Cr | ||||||
| uKIM1/Cr | ||||||
| uAlbumin/Cr | ||||||
| 13 | Fouad 201915 | 2019 | Case-control | 29±9; 28±5 | Deferasirox (11), | Albumin/Cr |
| Deferipone(3) | eGFR | |||||
| NGAL | ||||||
| uNGAL/Cr | ||||||
| 14 | Nafea 20193 | 2019 | Cross-sectional | 14.7 ± 4.3 | Deferasirox (26) | Serum K |
| Desferoxamine (14) | Serum Na | |||||
| Deferiprone(26) | Serum Ca total | |||||
| Seurm P | ||||||
| Serum Mg | ||||||
| Serum creatinine | ||||||
| U K/Cr | ||||||
| U Na/Cr | ||||||
| U P/Cr | ||||||
| U Mg/Cr | ||||||
| Urinary creatinine | ||||||
| eGFR | ||||||
| U KIM-1/Cr | ||||||
| 15 | Bilir 202022 | 2020 | Cross-sectional | 12.63 ± 4.58; 11.44 ± 4.37 | Deferasirox (47) | Creatinin |
| S Sodium | ||||||
| S Potassium | ||||||
| S Calcium | ||||||
| S Phosphorus | ||||||
| eGFR | ||||||
| Cystatin-C | ||||||
| Total antioxidant capacity | ||||||
| Total oxidant capacity | ||||||
| U Ca/Cr | ||||||
| U Protein/Cr | ||||||
| uβ2MG/Cr | ||||||
| uRBP/Cr | ||||||
| uNAG/Cr | ||||||
| MDA/Cr | ||||||
| 16 | Capolongo 202045 | 2020 | Case-control | 34 ± 12; 33 ± 14 | Deferoxamine (40) | Serum creatinine |
| eGFR | ||||||
| S Na | ||||||
| S K | ||||||
| S Ca | ||||||
| S Chlorine | ||||||
| S Bicarbonate | ||||||
| FENa | ||||||
| U Ca | ||||||
| U Phosp | ||||||
| uAlbumin/Cr | ||||||
| uOsmolarity | ||||||
| uOsmolality | ||||||
| pH | ||||||
| 17 | Mahmoud 202142 | 2021 | Case-control | 9.58±4.07 | Deferasirox (100) | Urea |
| Creatinine | ||||||
| eGFR | ||||||
| U protein/Cr | ||||||
| UNa/Cr | ||||||
| UK/Cr | ||||||
| UCa/Cr | ||||||
| Uuric acid/Cr | ||||||
| uNAG | ||||||
| uNAG/Cr | ||||||
| uKIM1 | ||||||
| uKIM1/Cr | ||||||
| SCystatin-C | ||||||
| 18 | Tanous 202118 | 2021 | Cross-sectional | 20.92± 9.7 | Deferasirox (26) | Serum creatinine |
| Deferiprone + deferoxamine (6) | Serum Na | |||||
| Deferoxamine (4) | Serum K | |||||
| Serum uric acid | ||||||
| eGFR | ||||||
| uNAG <12 | ||||||
| Abnormal uNAG (>12) | ||||||
| Abnormal urine Ca/Cr (>0.14) | ||||||
| Urine osmolality |
Table 2.
Result and conclusion from Study of Beta Thalassemia with Iron Overload and Receiving Chelation Agent Therapy
| No | Authors | Results-Conclusions |
|---|---|---|
| 1 | Aldudak 2000 | Administration of chelating agents in beta thalassemia patients actuates a damage to the proximal tubular kidney. |
| 2 | Smolkin 2008 | In beta thalassemia patients, UNAG and UNAG/creatinine ratio markers can be used as markers to determine the frequency of transfusion and the effect of chelating agent therapy on ferritin levels. |
| 3 | Economou 2010 | Glomerular and tubular dysfunction in iron overload beta thalassemia patients receiving chelating agents was observed through the elevation of cystatin C, proteinuria, hypercalciuria and beta2-microglobulin. |
| 4 | Hamed 2010 | Chelating agents in beta thalassemia patients have been shown stimulate glomerular and tubular dysfunction. |
| 5 | Smolkin 2008 | In beta thalassemia patients, UNAG and UNAG/creatinine ratio markers can be used as markers to determine the frequency of transfusion and the effect of chelating agent therapy on ferritin levels. |
| 6 | Mansi 2013 | Kidney damage occurs in beta thalassemia patients receiving chelating agents characterized by increased urea, creatinine, uric acid, urinary sodium and potassium. |
| 7 | Ali 2014 | Elevated cystatin C, serum creatinine, and serum ferritin and high uACR indicate kidney damage in beta thalassemia |
| 8 | Sen 2015 | NAG and NGAL can be used as markers of renal injury in beta thalassemia patients experiencing iron overload and receive therapeutic chelating agents. |
| 9 | Behairy 2017 | Cystatin-C and beta-2 microglobulin are specific and sensitive early biomarkers for monitoring glomerular and tubular dysfunction in children with beta-TM. |
| 10 | Bekhit 2017 | NAG is excreted following renal damage, hence used as marker of the toxicity index in beta thalassemia patients receiving chelating agents. |
| 11 | Annayev 2018 | Kidney damage was concluded after an increase in Cystatin C and beta microglobulin in beta thalassemia patients receiving iron chelating agents. |
| 12 | ElAlfy 2018 | Iron overload in beta thalassemia patients with long-term use of chelating agents revealed differences in serum cystatin C, uACR, and urinary B2 microglobulin, and B2 microglobulin/albumin ratio values. |
| 13 | Badeli 2019 | Administration of deferasirox led to kidney damage, characterized by an increase in uNGAL, inflammatory factors, sort of IL18, in beta thalassemia patients. |
| 14 | Fouad 2019 | uNGAL levels, uNGAL/creatinine ratio, eGFR and urinary albumin creatinine ratio were distinctive between group with iron overload and added chelating agents. |
| 15 | Nafea 2019 | UKIM-1 is a biomarker used to detect worsening eGFR in beta-thalassemia patients with iron overload and routine deferasirox. |
| 16 | Bilir 2020 | Iron overload marked by high serum ferritin, is associated with proteinuria, increased cystatin C, and urinary protein/Cr in beta thalassemia patients routinely receiving chelating agents. |
| 17 | Capolongo 2020 | The administration of chelating agents in patients with beta thalassemia iron overload induced renal tubular damage and required early detection to prevent further kidney damage. |
| 18 | Mahmoud 2021 | Serum cystatin-C is a good predictive marker in the evaluation of glomerular dysfunction. Urine concentrations of NAG and KIM-1 represent sensitive, specific, and highly predictive early biomarkers for acute renal injury in patients with beta TM when subclinical kidney injury or dysfunction is expected before serum creatinine increases. |
| 19. | Tanous 2021 | The use of deferasirox for approximately 10 years induced the decline of eGFR and was negatively correlated with uNAG in beta thalassemia patients with iron overload. |
Discussion
uNGAL/NGAL
The proximal tubule, distal tubule, and loop of Henle segments all contain epithelial cells that express the 25-kDa lipocalin iron-carrying protein known as neutrophil gelatinase-associated lipocalin (NGAL), which is released by active neutrophils. Detection of NGAL can be done through serum or urine withdrawal. However, in renal tubular damage, NGAL is often upregulated.26 NGAL is a low-concentration protein that binds iron-siderophore complexes and is found in a variety of cell types. NGAL is discharged in urine when proximal tubular damage inhibits the reabsorption or increase the synthesis. An abrupt increase in NGAL is brought on by acute kidney injury (AKI). High NGAL levels are also present in patients with other abnormalities, such as lupus nephritis, immunoglobulin A nephropathy, and urinary tract infections.27,28 Significant relationships between urinary NGAL (uNGAL) and proteinuria were documented in chronic kidney disease.29
Recent case-control studies revealed that β -TM patients receiving deferasirox and regular blood transfusions had considerably greater levels of uNGAL and NGAL.9 It is interesting to note that in β-TM patients, combined values of albumin/creatinin ratio and the uNGAL/creatinin ratio may be a more accurate predictor of kidney impairment and taken into account as potential indicators of renal failure. Additionally, as previously demonstrated, uNGAL somehow can predict renal function since it can estimate eGFR, evaluate the course of CKD, and serve as a surrogate measure for baseline eGFR.30
NGAL is highly associated with early renal complication regardless of the creatinine level, both in major and intermediate thalassemia.31 In both young and adult beta-thalassemia patients, NGAL level elevates due to iron overload and prolonged use of chelating agents. Since renal injury might elevate NGAL to a significant level, it is wisely advised to routinely monitor the NGAL level. Another highlighted feature of NGAL is that it immediately elevates right after kidney injury, even before serum creatinine, urinary N-acetyl glucosaminidase, and 2-microglobulin levels are detected.
uNAG/NAG
Tubular damage is indicated by an increase in the level of N-acetyl-beta-d-glucosamine (NAG), a well-known biomarker for proximal tubular injury.32 According to Aldudak et al, people with beta thalassemia had higher levels of the tubular damage indicators, such as NAG, malondialdehyde, and b2-microglobulin excreted in their urine.17 In addition, Jalali et al showed that b-TM patients had significantly higher urine NAG levels than controls, with high NAG levels being the norm in most cases.33 Besides, participants in Sen et al’s study who had renal proximal tubular damage also showed significantly elevated levels of uNAG/Cr and uNGAL/Cr.11
The proximal tubular dysfunction that results from thalassemia itself may be caused by chronic hypoxia brought on by persistent anemia, by iron deposition, or by iron chelation.4,19,23,34 According to some studies, the absence of a link between urine indicators and hemoglobin, haematocrit and ferritin levels may be due to very early tubular dysfunction, as seen by the rise of only NAG and NGAL, but not KIM-1, L-FABP, or urinary electrolytes.11,19,34,35
uKIM-1
A transmembrane protein called urinary human kidney injury molecule-1 (uKIM-1) is found in the proximal renal tubules within 24 hours of acute tubular necrosis after renal ischemia. Even while serum creatinine concentrations are unaffected by exposure to certain nephrotoxic drugs, urinary KIM-1 may still be detected.36
According to the findings of the Nafea et al study, young thalassemia patients receiving deferasirox therapy had evident subclinical nephrotoxicity when compared to patients receiving other chelation therapies. This was demonstrated by the statistically significantly higher levels of serum creatinine, eGFR, and UKIM-1/Cr. The serum levels of phosphorus, magnesium, creatinine, and blood sugar all showed a significant positive association with UKIM-1/Cr, however the blood hemoglobin level showed a significant negative correlation.3
According to Badeli et al’s study results, the deferasirox group had significantly greater levels of uIL-18, uNGAL, uNGAL/CREA, uKIM-1/CREA, and BUN than the control group.10 Deferasirox treatment led to partial necrosis in the renal tubules and increased urinary NGAL, Cystatin C, KIM-1, protein, and glucose production, as demonstrated by Sánchez González et al in an animal study.37
Cystatin C and uβ2MG
Cystatin C, not excreted by the renal tubules or reabsorbed into the serum, is a sensitive biomarker for glomerular filtration rate (GFR). All cells in the body continuously produce cystatin C and its production is unaffected by changes in age, sex, gender, or muscle mass. Cystatin C is a reliable and early marker of glomerular dysfunction in the pediatric population.22,36,38 A low-molecular-weight protein called urinary β2 microglobulin (u β2MG) is freely filtered by glomeruli, reabsorbed by renal tubules, and then eliminated. Due to its continual production, both are thought to be a more reliable endogenous measure of early glomerular filtration rate (GFR) affection than creatinine.39 For monitoring glomerular and tubular dysfunction in kids with β -TM, β2MG is a sensitive early biomarker.23,24 β2MG levels are very low in healthy people; it rises in inflammatory, immunologic, and cancerous conditions.36 Other than that, Cystatin C and β2MG have strong correlation with age and creatinine clearance.40 Early detection of glomerular disease will decrease the rate of renal failure and mortality.
Referring to a study by ElAllfy et al, thalassemia patients had significantly greater serum levels of cystatin C than healthy controls.20 Positive correlations were found between serum cystatin C and indirect bilirubin, LDH and serum ferritin. Additionally, there was no connection between any of these indicators and the kidney function tests (serum creatinine, urea, and uACR) or between serum cystatin C and u2MG.20 Elbedewy et al41 and Mahmoud et al42 showed a significant positive correlation between cystatin-C and serum ferritin and negative correlation with eGFR.
Behairy et al discovered that serum cystatin C and uβ2MG were negatively correlated with creatinine clearance, hemoglobin, and estimated GFR in children with β -TM while both markers were positively correlated with urea, creatinine, serum ferritin, UACR, duration of chelation therapy, and frequency of blood transfusion per year. As indicators for glomerular and tubular dysfunction, cystatin C and β2MG have good sensitivity and good specificity.24
Cystatin C and β2M, as well as serum ferritin and liver iron deposition were found to be significantly positively correlated, according to Kacar et al.43 Serum ferritin levels were discovered to be associated with cystatin C and β2M levels by Uzun et al44 found that serum ferritin is correlated with level of cystatin C and β2 M. The risk of glomerular and tubular dysfunction may increase with iron buildup in the body.
Conclusion
The renal complication in beta-thalassemia patients with iron overload receiving chelating agent therapy may progress to kidney disease. Early detection using accurate biological markers is a substantial issue that deserves further evaluation to determine prevention and management.
Ethics
This study was approved by Airlangga Hospital’s ethical board with certificate number 024/KEP/2022. All analyses for the present study were based on previous published research, thus no patient consent was required.
Disclosure
The authors report no financial or other conflicts of interest in this review article.
References
- 1.Andani CN, Aman AK, Hariman H, Lubis B. Cytogenetic mutation in a family with sickle-cell beta-thalassemia in North Sumatera, Medan, Indonesia: a preliminary study. Bali Med J. 2019;8(2):623. doi: 10.15562/bmj.v8i2.1417 [DOI] [Google Scholar]
- 2.Setianingsih I, Harahap A, Nainggolan IM. Alpha thalassaemia in Indonesia: phenotypes and molecular defects. Adv Exp Med Biol. 2003;531:47–56. doi: 10.1007/978-1-4615-0059-9_4 [DOI] [PubMed] [Google Scholar]
- 3.Nafea OE, Zakaria M, Hassan T, El Gebaly SM, Salah HE. Subclinical nephrotoxicity in patients with beta-thalassemia: role of urinary kidney injury molecule. Drug Chem Toxicol. 2022;45(1):93–102. doi: 10.1080/01480545.2019.1660362 [DOI] [PubMed] [Google Scholar]
- 4.Demosthenous C, Vlachaki E, Apostolou C, et al. Beta-thalassemia: renal complications and mechanisms: a narrative review. Hematology. 2019;24(1):426–438. doi: 10.1080/16078454.2019.1599096 [DOI] [PubMed] [Google Scholar]
- 5.Bakr A, Al-Tonbary Y, Osman G, El-Ashry R. Renal complications of beta-thalassemia major in children. Am J Blood Res. 2014;4(1):1–6. [PMC free article] [PubMed] [Google Scholar]
- 6.Mehrvar A, Azarkeivan A, Faranoush M, et al. Endocrinopathies in patients with transfusion-dependent beta-thalassemia. Pediatr Hematol Oncol. 2008;25(3):187–194. doi: 10.1080/08880010801938207 [DOI] [PubMed] [Google Scholar]
- 7.Cappellini MD. Coagulation in the pathophysiology of hemolytic anemias. Hematol Am Soc Hematol Educ Prog. 2007;2007:74–78. doi: 10.1182/asheducation-2007.1.74 [DOI] [PubMed] [Google Scholar]
- 8.Cetinkaya PU, Azik FM, Karakus V, Huddam B, Yilmaz N. β2-microglobulin, neutrophil gelatinase-associated lipocalin, and endocan values in evaluating renal functions in patients with β-thalassemia major. Hemoglobin. 2020;44(3):147–152. doi: 10.1080/03630269.2020.1766486 [DOI] [PubMed] [Google Scholar]
- 9.Karaman K, Şahin S, Geylan H, et al. Evaluation of renal function disorder with urinary neutrophil gelatinase-associated lipocalin level in patients with β-thalassemia major. J Pediatr Hematol Oncol. 2019;41(7):507–510. doi: 10.1097/MPH.0000000000001577 [DOI] [PubMed] [Google Scholar]
- 10.Badeli H, Baghersalimi A, Eslami S, et al. Early kidney damage markers after deferasirox treatment in patients with thalassemia major: a case-control study. Oxid Med Cell Longev. 2019;2019:5461617. doi: 10.1155/2019/5461617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Şen V, Ece A, Uluca Ü, et al. Urinary early kidney injury molecules in children with beta-thalassemia major. Ren Fail. 2015;37(4):607–613. doi: 10.3109/0886022X.2015.1007871 [DOI] [PubMed] [Google Scholar]
- 12.Pinto VM, Forni GL. Management of iron overload in beta-thalassemia patients: clinical practice update based on case series. Int J Mol Sci. 2020;21(22):8771. doi: 10.3390/ijms21228771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casale M, Picariello S, Corvino F, et al. Life-threatening drug-induced liver injury in a patient with β-thalassemia major and severe iron overload on polypharmacy. Hemoglobin. 2018;42(3):213–216. doi: 10.1080/03630269.2018.1503187 [DOI] [PubMed] [Google Scholar]
- 14.Yii E, Doery JC, Kaplan Z, Kerr PG. Use of deferasirox (Exjade) for iron overload in peritoneal dialysis patients. Nephrology. 2018;23(9):887–889. doi: 10.1111/nep.13389 [DOI] [PubMed] [Google Scholar]
- 15.Fouad IZ, ElNahid MS, Youssef MF, Amroussy YM. Urinary neutrophil gelatinase-associated lipocalin as a marker of kidney injury in Egyptian patients with thalassemia. Egypt J Intern Med. 2019;31(3):343–352. doi: 10.4103/ejim.ejim_114_18 [DOI] [Google Scholar]
- 16.Smolkin V, Halevy R, Levin C, et al. Renal function in children with β-thalassemia major and thalassemia intermedia. Pediatr Nephrol. 2008;23(10):1847–1851. doi: 10.1007/s00467-008-0897-8 [DOI] [PubMed] [Google Scholar]
- 17.Aldudak B, Karabay Bayazit A, Noyan A, et al. Renal function in pediatric patients with beta-thalassemia major. Pediatr Nephrol. 2000;15(1–2):109–112. doi: 10.1007/s004670000434 [DOI] [PubMed] [Google Scholar]
- 18.Tanous O, Azulay Y, Halevy R, et al. Renal function in β-thalassemia major patients treated with two different iron-chelation regimes. BMC Nephrol. 2021;22(1):418. doi: 10.1186/s12882-021-02630-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bekhit OE, El Dash HH, Ahmed MS. Early detection of kidney dysfunction in Egyptian patients with beta-thalassemia major. Egypt Pediatr Assoc Gazette. 2017;65(3):85–89. doi: 10.1016/j.epag.2017.02.002 [DOI] [Google Scholar]
- 20.ElAlfy MS, Khalil Elsherif NH, Ebeid FSE, et al. Renal iron deposition by magnetic resonance imaging in pediatric β-thalassemia major patients: relation to renal biomarkers, total body iron and chelation therapy. Eur J Radiol. 2018;103:65–70. doi: 10.1016/j.ejrad.2018.04.007 [DOI] [PubMed] [Google Scholar]
- 21.Ali BA, Mahmoud AM. Frequency of glomerular dysfunction in children with Beta thalassaemia major. Sultan Qaboos Univ Med J. 2014;14(1):e88–94. doi: 10.12816/0003341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arman Bilir Ö, Kirkiz S, Fettah A, et al. Renal function and the oxidative status among children with thalassemia major and healthy controls: a cross-sectional study. Transfus Apher Sci. 2020;59(4):102746. doi: 10.1016/j.transci.2020.102746 [DOI] [PubMed] [Google Scholar]
- 23.Annayev A, Karakaş Z, Karaman S, Yalçıner A, Yılmaz A, Emre S. Glomerular and tubular functions in children and adults with transfusion-dependent thalassemia. Turk J Haematol. 2018;35(1):66–70. doi: 10.4274/tjh.2017.0266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Behairy OG, Abd Almonaem ER, Abed NT, et al. Role of serum cystatin-C and beta-2 microglobulin as early markers of renal dysfunction in children with beta thalassemia major. Int J Nephrol Renovasc Dis. 2017;10:261–268. doi: 10.2147/IJNRD.S142824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Economou M, Printza N, Teli A, et al. Renal dysfunction in patients with beta-thalassemia major receiving iron chelation therapy either with deferoxamine and deferiprone or with deferasirox. Acta Haematol. 2010;123(3):148–152. doi: 10.1159/000287238 [DOI] [PubMed] [Google Scholar]
- 26.Schrezenmeier EV, Barasch J, Budde K, Westhoff T, Schmidt-Ott KM. Biomarkers in acute kidney injury - pathophysiological basis and clinical performance. Acta Physiologica. 2017;219(3):554–572. doi: 10.1111/apha.12764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kashani K, Cheungpasitporn W, Ronco C. Biomarkers of acute kidney injury: the pathway from discovery to clinical adoption. Clin Chem Lab Med. 2017;55(8):1074–1089. doi: 10.1515/cclm-2016-0973 [DOI] [PubMed] [Google Scholar]
- 28.Goldstein SL, Devarajan P. Progression from acute kidney injury to chronic kidney disease: a pediatric perspective. Adv Chronic Kidney Dis. 2008;15(3):278–283. doi: 10.1053/j.ackd.2008.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paragas N, Qiu A, Hollmen M, Nickolas TL, Devarajan P, Barasch J. NGAL-Siderocalin in kidney disease. Biochim Biophys Acta. 2012;1823(9):1451–1458. doi: 10.1016/j.bbamcr.2012.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patel ML, Sachan R, Verma A, Kamal R, Gupta KK. Neutrophil gelatinase-associated lipocalin as a biomarker of disease progression in patients with chronic kidney disease. Indian J Nephrol. 2016;26(2):125–130. doi: 10.4103/0971-4065.157799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohammed M, Mohammad J, Fathi Z, Al-Hamdany M, Alkazzaz N. Comparative evaluation of cystatin C and neutrophil gelatinase-associated lipocalin in patients with thalassemia major versus thalassemia intermedia. Pharmacia. 2021;68(4):741–746. doi: 10.3897/pharmacia.68.e71475 [DOI] [Google Scholar]
- 32.Mårtensson J, Martling CR, Bell M. Novel biomarkers of acute kidney injury and failure: clinical applicability. Br J Anaesth. 2012;109(6):843–850. doi: 10.1093/bja/aes357 [DOI] [PubMed] [Google Scholar]
- 33.Jalali A, Khalilian H, Ahmadzadeh A, et al. Renal function in transfusion-dependent pediatric beta-thalassemia major patients. Hematology. 2011;16(4):249–254. doi: 10.1179/102453311X12953015767662 [DOI] [PubMed] [Google Scholar]
- 34.Musallam KM, Taher AT. Mechanisms of renal disease in β-thalassemia. J Am Soc Nephrol. 2012;23(8):1299–1302. doi: 10.1681/ASN.2011111070 [DOI] [PubMed] [Google Scholar]
- 35.Tantawy AA, El Bablawy N, Adly AA, Ebeid FS. Early predictors of renal dysfunction in Egyptian patients with β-thalassemia major and intermedia. Mediterr J Hematol Infect Dis. 2014;6(1):e2014057. doi: 10.4084/mjhid.2014.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Waring WS, Moonie A. Earlier recognition of nephrotoxicity using novel biomarkers of acute kidney injury. Clin Toxicol. 2011;49(8):720–728. doi: 10.3109/15563650.2011.615319 [DOI] [PubMed] [Google Scholar]
- 37.Sánchez-González PD, López-Hernandez FJ, Morales AI, Macías-Nuñez JF, López-Novoa JM. Effects of deferasirox on renal function and renal epithelial cell death. Toxicol Lett. 2011;203(2):154–161. doi: 10.1016/j.toxlet.2011.03.018 [DOI] [PubMed] [Google Scholar]
- 38.Tantawy AA, Adly AA, Ismail EA, Aly SH. Endothelial nitric oxide synthase gene intron 4 variable number tandem repeat polymorphism in β-thalassemia major: relation to cardiovascular complications. Blood Coagulat Fibrinolysis. 2015;26(4):419–425. doi: 10.1097/MBC.0000000000000277 [DOI] [PubMed] [Google Scholar]
- 39.Randers E, Erlandsen EJ, Pedersen OL, Hasling C, Danielsen H. Serum cystatin C as an endogenous parameter of the renal function in patients with normal to moderately impaired kidney function. Clin Nephrol. 2000;54(3):203–209. [PubMed] [Google Scholar]
- 40.Hashemieh M, Radfar M, Azarkeivan A, et al. Renal hemosiderosis among Iranian transfusion dependent β-thalassemia major patients. Int J Hematol Oncol Stem Cell Res. 2017;11(2):133–138. [PMC free article] [PubMed] [Google Scholar]
- 41.Elbedewy T, Gawaly A, Abd El-Naby A. Serum cystatin-C and urinary N-acetyl-glucosaminidase as biomarkers for early renal dysfunction in adult Egyptian patients with thalassemia major. Tanta Med J. 2015;43(1):28–35. doi: 10.4103/1110-1415.154563 [DOI] [Google Scholar]
- 42.Mahmoud AA, Elian DM, Abd El Hady NM, et al. Assessment of subclinical renal glomerular and tubular dysfunction in children with beta thalassemia major. Children. 2021;8(2):100. doi: 10.3390/children8020100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kacar A. Levels of beta-2 microglobulin and cystatin C in beta thalassemia major patients. J Clin Analyt Med. 2015;6:269–273. [Google Scholar]
- 44.Uzun E, Balcı YI, Yüksel S, Aral YZ, Aybek H, Akdağ B. Glomerular and tubular functions in children with different forms of beta thalassemia. Ren Fail. 2015;37(9):1414–1418. doi: 10.3109/0886022X.2015.1077314 [DOI] [PubMed] [Google Scholar]
- 45.Capolongo G, Zacchia M, Beneduci A, et al. Urinary metabolic profile of patients with transfusion-dependent β-thalassemia major undergoing deferasirox therapy. Kidney Blood Press Res. 2020;45(3):455–466. doi: 10.1159/000507369 [DOI] [PubMed] [Google Scholar]