Abstract
Purpose
The study aimed to investigate the ability of inflammation-immunity-nutrition score (IINS) and inflammatory burden index (IBI), individually or in combination, to predict prognosis of hepatocellular carcinoma (HCC) patients after hepatectomy.
Methods
A total of 701 patients who underwent HCC resection at Guangxi Medical University Cancer Hospital were enrolled in the study. An IINS ranging from 0 to 3 was defined based on preoperative C-reactive protein (CRP), lymphocyte count, and serum albumin level, while an IBI was based on CRP and neutrophil-to-lymphocyte ratio. The prognostic value of IINS and IBI was assessed using univariate and multivariate Cox regression and Kaplan–Meier survival curves. The concordance index and calibration curve were used for internal validation of models. Decision curve analysis, net reclassification index and integrated discrimination improvement were used to compare the predictive performance of the models with traditional staging systems.
Results
IINS and IBI were able to predict poor prognosis in HCC patients after hepatectomy, and a nomogram based on the IINS predicted survival at 1, 3, and 5 years better than other models or traditional staging systems.
Conclusion
IINS may be accurate predictors of survival in HCC patients after hepatectomy, with potentially greater prognostic value than conventional markers.
Keywords: hepatocellular carcinoma, inflammation-immunity-nutrition score, inflammatory burden index, prognosis, hepatectomy
Introduction
Hepatocellular carcinoma (HCC) is the sixth most frequent cancer yet third most frequent cause of cancer-related death worldwide.1 Although the indications for surgical HCC resection have expanded, postoperative survival remains short due to the high rate of tumor recurrence, which exceeds 60% within 5 years.2,3 To improve the survival of HCC patients who undergo hepatic resection, new biomarkers are needed to identify patients who may be at higher risk of recurrence and who therefore may benefit from more intense monitoring and management.
Several clinical markers are used to classify HCC and select treatments; these include serum alpha fetoprotein (AFP) level, Barcelona Clinic Liver Cancer (BCLC) stage, and TNM stage. However, these markers are generally poor at predicting prognosis after surgery, since they may not reflect chronic inflammation that can promote tumor cell proliferation and help tumors evade immune surveillance.4 The markers also do not reflect malnutrition, which has been associated with worse prognosis of HCC patients.5,6
Various inflammation- or nutrition-related indicators have been proposed in order to improve prediction of patients with HCC or other cancers. These indicators include C-reactive protein/albumin ratio (CAR), platelet–lymphocyte ratio (PLR), systemic immune inflammation index (SII), neutrophil-to-lymphocyte ratio (NLR), prognostic nutritional index (PNI), prognostic index (PI), Glasgow prognostic score (GPS), and modified Glasgow prognostic score (mGPS).7–11 The inflammation-immunity-nutrition score (IINS), a combination of C-reactive protein (CRP), lymphocyte (LYM) count and serum albumin (ALB) level,12 has shown promising prognostic performance in colorectal cancer,12 endometrial cancer,13 and HCC treated with therapy targeting programmed cell death protein 1.14 Similarly, the inflammatory burden index (IBI) is also a potential inflammatory biomarker that can be used to predict prognosis in patients with colorectal cancer, esophageal cancer, gastric cancer,15 pancreatic cancer16 or bladder cancer.17
To our knowledge, neither the IINS nor IBI has been assessed for its ability to predict prognosis of HCC patients after hepatectomy. Here, we assessed the indices, alone and in combination, benchmarking them against conventional inflammation and nutrition indicators as well as conventional staging systems. Defining new predictors will allow a better selection of patients for surgery and spare resources.
Material and Methods
Patient Enrollment
This study included 921 patients with primary HCC who underwent R0 surgical resection at Guangxi Medical University Cancer Hospital, Nanning, China, from January 2014 to December 2017. Patients were included if they (1) underwent R0 resection, defined as complete resection of the tumor with a negative microscopic margin; (2) had HCC confirmed by postoperative pathology; and (3) no previous anti-cancer treatment, including transcatheter arterial chemoembolization (TACE), radiation or targeted therapy. Patients were excluded if (1) their pathology data were missing, (2) they survived fewer than 30 days after surgery, or (3) they had other concurrent malignancies.
The study was performed in accordance with the principles of the Declaration of Helsinki and was approved by the Ethics Committee of Guangxi Medical University Cancer Hospital (KY2020155). All patients, upon admission, consented to the analysis and release of their anonymized medical data for research purposes.
Clinicopathological Characteristics
The following clinicopathological data were collected within 24 h of admission: sex, age, BCLC stage, liver cirrhosis, tumor number, tumor size, presence or absence of a tumor capsule, and presence or absence of microvascular invasion (MVI). Serological data were collected on hepatitis B surface antigen, HBV-DNA load, AFP, total peripheral neutrophil (NEU) count, total peripheral LYM count, platelet (PLT) count, CRP, ALB, IINS, IBI, NLR, PLR, SII, and CAR.
Follow-Up and Survival
After liver resection, the following examinations were performed every two months for the first two years and every three months for the next 3–5 years: physical examination, liver function test, kidney function test, serum laboratory tests as well as imaging, such as abdominal ultrasonography, enhanced computed tomography, or magnetic resonance imaging. Patients were followed up from admission until death or September 2021.
Disease-free survival (DFS) was defined as the interval between surgery and definitive diagnosis of HCC recurrence. Overall survival (OS) was defined as the interval between hepatectomy and death or the end of follow-up.
Calculations
The inflammation and nutrition indicators were calculated using the following equations:
IBI = CRP × (NEU/LYM), NLR = NEU/LYM; PLR = PLT/LYM, SII = NEU × PLT/LYM, CAR = CRP/ALB, and PNI = ALB + (LYM count × 5). IINS, which could range from 0 to 3, was defined as the sum of the CRP score (0 or 1), LYM count (0 or 1), and ALB (0 or 1). Patients with CRP score 0 had CRP ≤ 4.93 mg/L, while those with CPR score 1 had CRP >4.93 mg/L. LYM score 0 indicated LYM count >1.22 × 109/L, while score 1 indicated LYM ≤1.22 × 109 /L. Patients with ALB score 0 had an ALB level >37.5 g/L, and those with ALB score 1 had ALB ≤37.5 g/L (Figure S1). The optimal cut-off values for each laboratory index were then calculated based on each patient’s OS using X-tile software (version 3.6.1; https://medicine.yale.edu/lab/rimm/research/software/; Figure S1).10,12,15 The scores of PI, GPS, and mGPS were calculated as shown in Table S1.11
Figure 1.
Flowchart of patient enrollment.
Abbreviations: IINS, inflammation-immunity-nutrition score; IBI, inflammatory burden index; CRP, C-reactive protein; ALB, albumin; LYM, lymphocyte; H, high; L, low; N, number.
Statistical Analysis
Continuous variables were presented as median [interquartile range (IQR)] or mean ± standard deviation (SD). Intergroup differences in normally distributed continuous variables were assessed for significance using Student’s t-test; differences in skewed continuous variables, using the Mann–Whitney test; and differences in categorical variables, using chi-squared or Fisher’s exact tests. Differences between Kaplan–Meier survival curves were assessed using the Log rank test. Univariate and multivariate Cox proportional hazard models were used to detect potential associations between certain variables or indicators and survival. Associations were expressed as hazard ratios (HRs) and 95% confidence intervals (CIs).
The ability of a given index or model to predict survival was assessed in terms of the consistency index (C-index), decision curve analysis (DCA), net reclassification index (NRI) and integrated discrimination improvement (IDI). NRI is used mainly to evaluate how likely the proposed model will predict results better than the old model. NRI > 0 indicates a positive improvement, indicating that the new model is better at predicting events than the old model, while NRI < 0 indicates the opposite. NRI = 0 means that the old and new models have similar predictive power. IDI values are interpreted in similar ways.
Variables that independently predicted risk in multivariate regression were used to construct a nomogram with the rms package in R. The ability of individual variables or models to predict prognosis was evaluated by 1000 bootstrapping replications, and their performance at 1, 3, and 5 years post-surgery was assessed using calibration plots. Time-AUC, C-index and decision curves were also generated using the timeROC, pec and ggDCA packages in R 4.1.2 (http://www.r-project.org/). Other statistical analyses were performed using SPSS 24.0 (IBM, Chicago, IL, USA) and GraphPad Prism version 9.3.1 (GraphPad, San Diego, CA, USA). All statistical tests were two-sided, and differences associated with P < 0.05 were considered statistically significant.
Results
A total of 701 patients (601 men, 85.7%) who underwent hepatectomy were enrolled (Figure 1). Most patients were positive for hepatitis B surface antigen (99.0%), 384 patients were MVI-positive (54.8%), and 60.2% had a tumor diameter >5 cm (Table 1).
Table 1.
Characteristics of Enrolled Patients with Resectable Hepatocellular Carcinoma
| Characteristics | Overall (n = 701) |
|---|---|
| Sexa | |
| Male | 601(85.7) |
| Female | 100(14.3) |
| Age(years)a | |
| <50 | 399(56.9) |
| ≥50 | 302(43.1) |
| BCLC Stagea | |
| 0-A | 343(48.9) |
| B-C | 358(51.1) |
| HBsAga | |
| Negative | 7(1.0) |
| Positive | 694(99.0) |
| HBV-DNA (IU/mL)a | |
| <5×102 | 207(29.5) |
| ≥5×102 | 494(70.5) |
| Liver cirrhosisa | |
| Negative | 295(42.1) |
| Positive | 406(57.9) |
| Tumor numbera | |
| Single | 488(69.6) |
| Multiple | 213(30.4) |
| Tumor size (cm)a | |
| ≤5 | 279(39.8) |
| >5 | 422(60.2) |
| Tumor capsulea | |
| Complete | 685(97.7) |
| Incomplete | 16(2.3) |
| AFP (ng/mL)a | |
| ≤400 | 351(50.1) |
| >400 | 350(49.9) |
| MVIa | |
| Negative | 317(45.2) |
| Positive | 384(54.8) |
| Neu (×109/L)b | 3.61[2.73,4.70] |
| Lym (×109/L)b | 1.70[1.33, 2.12] |
| Plt (×109/L)b | 204.43[158, 264] |
| CRP (mg/L)b | 3.60[1.37,9.75] |
| Alb (g/L)b | 39.40[36.5,42.1] |
| IBIb | 6.44[2.36,24.47] |
| NLRb | 2.09[1.54,2.68] |
| PLRb | 118.37[85.37,166.30] |
| SIIb | 427.30[263.66,675.27] |
| CARb | 0.09[0.03,0.26] |
| PNIb | 48.19[43.95, 52.05] |
| IINSa | |
| 0 | 276(39.4) |
| 1 | 255(36.4) |
| 2 | 124(17.7) |
| 3 | 46(6.5) |
| PIa | |
| 0 | 510(72.8) |
| 1 | 174(24.8) |
| 2 | 17(2.4) |
| GPSa | |
| 0 | 468(66.8) |
| 1 | 189(26.9) |
| 2 | 44(6.3) |
| mGPSa | |
| 0 | 534(76.2) |
| 1 | 123(17.5) |
| 2 | 44(6.3) |
Notes: aCategorical variables are presented as number (percentage). bContinuous variables are presented as median[interquartile range].
Abbreviations: BCLC, Barcelona Clinic Liver Cancer; HBsAg, hepatitis B surface antigen; HBV-DNA, hepatitis B virus DNA; AFP, alpha-fetoprotein; MVI, microvascular invasion; Neu, neutrophil; Lym, lymphocyte; Plt, platelet; CRP, C reactive protein; Alb, albumin; IBI, inflammatory burden index; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet- lymphocyte ratio; SII, systemic immune inflammation index; CAR, C-reactive protein/albumin ratio, PNI, prognostic nutritional index; IINS, inflammation-immunity-nutrition score; PI, prognostic index; GPS, Glasgow Prognostic Score; mGPS, modified Glasgow Prognostic Score.
Association of IINS and IBI with Prognosis
To identify the optimal predictive inflammatory indicators for prognosis in HCC patients after hepatectomy, we calculated the AUC values for IINS, IBI, CAR, PLR, SII, NLR, PNI, PI, GPS, and mGPS. Among them, IINS and IBI had the highest AUC values for DFS and OS (Figure S2 and Figure 2).
Figure 2.
Receiver operating characteristic curves of the following indices for overall survival in patients with hepatocellular carcinoma: (A) IINS, (B) IBI, (C) CAR, (D) PLR, (E) SII, (F) NLR, (G) PNI, (H) PI, (I) GPS, and (J) mGPS.
Abbreviations: IINS, inflammation-immunity-nutrition score; IBI, inflammatory burden index; CAR, C-reactive protein/albumin ratio; PLR, platelet–lymphocyte ratio; SII, systemic immune inflammation index; NLR, neutrophil-to-lymphocyte ratio; PNI, prognostic nutritional index; PI prognostic index; GPS, Glasgow prognostic score; mGPS, modified Glasgow prognostic score; AUC, area under the curve; HCC, hepatocellular carcinoma.
The optimal cut-off value of IBI was determined to be 7.20, such that patients with a greater IBI were assigned to the “high IBI” and other patients to the “low IBI” group (Table 2). IBI was significantly associated with sex, BCLC stage, HBV-DNA load, tumor number, tumor size, serum AFP, and MVI; IINS showed significant associations with age, BCLC stage, HBV-DNA load, liver cirrhosis, tumor number, tumor size, serum AFP, and MVI (Table 3).
Table 2.
Optimal Cut-Off Values for Inflammatory and Nutritional Indicators
| Variable | High | N% | Low | N% |
|---|---|---|---|---|
| IBI | >7.20 | 334(47.7) | ≤7.20 | 367(52.3) |
| CAR | >0.12 | 281(40.0) | ≤0.12 | 420(60.0) |
| PLR | >148.43 | 225(32.0) | ≤148.43 | 476(68.0) |
| SII | >1038.54 | 87(12.4) | ≤1038.54 | 614(87.6) |
| NLR | >2.58 | 223(31.8) | ≤2.58 | 478(68.2) |
| PNI | >43.95 | 525(74.8) | ≤43.95 | 176(25.2) |
Abbreviations: IINS, inflammation-immunity-nutrition score; IBI, inflammatory burden index; CAR, C-reactive protein/albumin ratio; PLR, platelet–lymphocyte ratio; SII, systemic immune inflammation index; NLR, neutrophil-to-lymphocyte ratio; PNI, prognostic nutritional index.
Table 3.
Associations of IINS and IBI with Clinicopathological Characteristics in Patients with Hepatocellular Carcinoma
| Variable | IINS | IBI | ||||||
|---|---|---|---|---|---|---|---|---|
| 0 (N = 276) | 1 (N = 255) | 2 (N = 124) | 3 (N = 46) | P | Low (N = 367) | High (N = 334) | P | |
| Sex | 0.156 | 0.021 | ||||||
| Male | 228 | 226 | 105 | 42 | 304 | 297 | ||
| Female | 48 | 29 | 19 | 4 | 63 | 37 | ||
| Age (years) | <0.001 | 0.866 | ||||||
| ≤50 | 181 | 142 | 53 | 23 | 210 | 189 | ||
| >50 | 95 | 113 | 71 | 23 | 157 | 145 | ||
| BCLC stage | <0.001 | <0.001 | ||||||
| 0-A | 183 | 105 | 40 | 15 | 233 | 110 | ||
| B-C | 93 | 150 | 84 | 31 | 134 | 224 | ||
| HBsAg | 0.634 | 0.613 | ||||||
| Negative | 3 | 4 | 0 | 0 | 3 | 4 | ||
| Positive | 273 | 251 | 124 | 46 | 364 | 330 | ||
| HBV-DNA (IU/mL) | 0.025 | <0.001 | ||||||
| <5×102 | 98 | 71 | 27 | 11 | 129 | 78 | ||
| ≥5×102 | 178 | 184 | 97 | 35 | 238 | 256 | ||
| Liver cirrhosis | 0.034 | 0.208 | ||||||
| Negative | 130 | 106 | 44 | 15 | 146 | 149 | ||
| Positive | 146 | 149 | 80 | 31 | 221 | 185 | ||
| Tumor number | <0.001 | 0.001 | ||||||
| Single | 214 | 174 | 68 | 32 | 275 | 213 | ||
| Multiple | 62 | 81 | 56 | 14 | 92 | 121 | ||
| Tumor size (cm) | <0.001 | <0.001 | ||||||
| ≤5 | 149 | 98 | 29 | 3 | 213 | 66 | ||
| >5 | 127 | 157 | 95 | 43 | 154 | 268 | ||
| Tumor capsule | 0.931 | 0.087 | ||||||
| Complete | 274 | 247 | 119 | 45 | 362 | 323 | ||
| Incomplete | 2 | 8 | 5 | 1 | 5 | 11 | ||
| AFP (ng/mL) | 0.033 | 0.045 | ||||||
| ≤400 | 153 | 126 | 56 | 16 | 197 | 154 | ||
| >400 | 123 | 129 | 68 | 30 | 170 | 180 | ||
| MVI | 0.034 | <0.001 | ||||||
| Negative | 138 | 118 | 44 | 17 | 191 | 126 | ||
| Positive | 138 | 137 | 80 | 29 | 176 | 208 | ||
Abbreviations: HCC, hepatocellular carcinoma; BCLC, Barcelona Clinic Liver Cancer; HBsAg, hepatitis B surface antigen; HBV-DNA, hepatitis B virus DNA; AFP, alpha-fetoprotein; MVI, microvascular invasion; Neu, neutrophil; Lym, lymphocyte; IINS, inflammation-immunity-nutrition; IBI, inflammatory burden index.
Kaplan–Meier survival analysis revealed that patients with higher IINS and IBI had worse DFS and OS than patients with lower IINS and IBI (Figure 3). Associations of survival with other inflammatory and nutritional indicators are shown in Figures S3–S4.
Figure 3.
Kaplan–Meier survival curves of HCC patients after hepatectomy, stratified by (A and B) inflammation-immunity-nutrition score (IINS) or (C and D) inflammatory burden index (IBI).
Prognostic Model Based on IINS
Univariate analysis showed that sex, HBV-DNA, tumor number, tumor size, serum AFP, MVI, IINS and IBI were significantly associated with poor DFS and OS in patients with primary HCC after partial hepatectomy (Table 4).
Table 4.
Univariate Analysis to Identify Clinicopathological Characteristics Associated with Poor DFS and OS
| Variable | Disease-Free Survival | Overall Survival | ||||
|---|---|---|---|---|---|---|
| HR | 95CI% | P | HR | 95CI% | P | |
| Sex (M) | 1.559 | 1.161–2.093 | 0.003 | 1.365 | 1.015–1.835 | 0.039 |
| Age (>50 years) | 1.100 | 0.913–1.324 | 0.317 | 1.057 | 0.869–1.284 | 0.580 |
| HBsAg (Positive) | 0.631 | 0.282–1.412 | 0.263 | 0.675 | 0.280–1.632 | 0.383 |
| HBV-DNA (≥5×102 IU/mL) | 1.368 | 1.107–1.691 | 0.004 | 1.452 | 1.158–1.820 | 0.001 |
| Liver cirrhosis (Positive) | 1.114 | 0.923–1.346 | 0.260 | 0.994 | 0.818–1.209 | 0.955 |
| Tumor number (Multiple) | 1.797 | 1.482–2.178 | <0.001 | 1.557 | 1.272–1.906 | <0.001 |
| Tumor size (>5cm) | 1.921 | 1.573–2.344 | <0.001 | 2.316 | 1.864–2.876 | <0.001 |
| Tumor capsule (Incomplete) | 1.281 | 0.704–2.332 | 0.417 | 1.561 | 0.857*2.844 | 0.146 |
| AFP (>400 ng/mL) | 1.431 | 1.188–1.723 | <0.001 | 1.455 | 1.198–1.768 | <0.001 |
| MVI (Positive) | 1.873 | 1.546–2.270 | <0.001 | 2.077 | 1.696–2.544 | <0.001 |
| IINS | ||||||
| 1 | 1.554 | 1.239–1.949 | <0.001 | 2.344 | 1.827–3.007 | <0.001 |
| 2 | 2.727 | 2.110–3.525 | <0.001 | 4.118 | 3.109–5.456 | <0.001 |
| 3 | 4.051 | 2.811–5.838 | <0.001 | 7.023 | 4.900–10.067 | <0.001 |
| IBI (H) | 1.960 | 1.624–2.366 | <0.001 | 2.799 | 2.288–3.425 | <0.001 |
Abbreviations: DFS, disease-free survival; OS, overall survival; M, male; HBsAg, hepatitis B surface antigen; HBV-DNA, hepatitis B virus DNA; AFP, alpha-fetoprotein; MVI, microvascular invasion; IINS, inflammation-immunity-nutrition score; IBI, inflammatory burden index; H, high.
Multivariate analysis of IINS and clinicopathological indicators identified the following independent prognostic factors for poor DFS (Table 5): sex, tumor number, tumor size, serum AFP, MVI and IINS. Therefore, these factors were used to construct Model 1 to predict DFS (Figure S5A).
Table 5.
Multivariate Analysis of the Three Models to Predict DFS and OS
| Variable | Model1 | Model2 | Model3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95CI% | P | HR | 95CI% | P | HR | 95CI% | P | |
| Model for disease-free Survival | |||||||||
| Sex (M) | 1.485 | 1.099–2.005 | 0.010 | 1.522 | 1.128–2.053 | 0.006 | 1.478 | 1.094–1.997 | 0.011 |
| Tumor number (Multiple) | 1.506 | 1.236–1.835 | <0.001 | 1.530 | 1.257–1.862 | <0.001 | 1.505 | 1.235–1.833 | <0.001 |
| Tumor size (>5cm) | 1.493 | 1.212–1.839 | <0.001 | 1.511 | 1.219–1.871 | <0.001 | 1.454 | 1.170–1.807 | 0.001 |
| AFP (>400 ng/mL) | 1.346 | 1.113–1.628 | 0.002 | 1.361 | 1.125–1.646 | 0.001 | 1.350 | 1.116–1.634 | 0.002 |
| MVI (Positive) | 1.593 | 1.309–1.939 | <0.001 | 1.569 | 1.290–1.908 | <0.001 | 1.586 | 1.304–1.930 | <0.001 |
| IINS | |||||||||
| 1 | 1.370 | 1.088–1.724 | 0.006 | 1.318 | 1.029–1.687 | 0.028 | |||
| 2 | 2.170 | 1.666–2.827 | <0.001 | 2.034 | 1.503–2.754 | <0.001 | |||
| 3 | 3.064 | 2.098–4.475 | <0.001 | 2.843 | 1.877–4.306 | <0.001 | |||
| IBI (H) | 1.524 | 1.245–1.865 | <0.001 | 1.111 | 0.873–1.413 | 0.391 | |||
| Model for Overall Survival | |||||||||
| Tumor number (Multiple) | 1.231 | 1.001–1.513 | 0.048 | 1.327 | 1.081–1.629 | 0.007 | 1.236 | 1.005–1.519 | 0.044 |
| Tumor size (>5cm) | 1.625 | 1.296–2.037 | <0.001 | 1.580 | 1.252–1.993 | <0.001 | 1.496 | 1.181–1.895 | 0.001 |
| AFP (>400 ng/mL) | 1.321 | 1.081–1.614 | 0.006 | 1.348 | 1.104–1.647 | 0.003 | 1.334 | 1.091–1.630 | 0.005 |
| MVI (Positive) | 1.804 | 1.466–2.221 | <0.001 | 1.750 | 1.424–2.151 | <0.001 | 1.789 | 1.454–2.202 | <0.001 |
| IINS | |||||||||
| 1 | 2.081 | 1.615–2.680 | <0.001 | 1.848 | 1.406–2.430 | <0.001 | |||
| 2 | 3.328 | 2.493–4.443 | <0.001 | 2.752 | 1.979–3.827 | <0.001 | |||
| 3 | 5.118 | 3.519–7.443 | <0.001 | 4.116 | 2.718–6.232 | <0.001 | |||
| IBI (H) | 2.173 | 1.750–2.699 | <0.001 | 1.361 | 1.052–1.761 | 0.019 | |||
Notes: Model 1, prognostic model based on IINS; Model 2, prognostic model based on IBI; Model 3, prognostic model based on IINS and IBI.
Abbreviations: DFS, disease-free survival; OS, overall survival; M, male; AFP, alpha-fetoprotein; MVI, microvascular invasion; IINS, inflammation-immunity-nutrition score; IBI, inflammatory burden index; H, high.
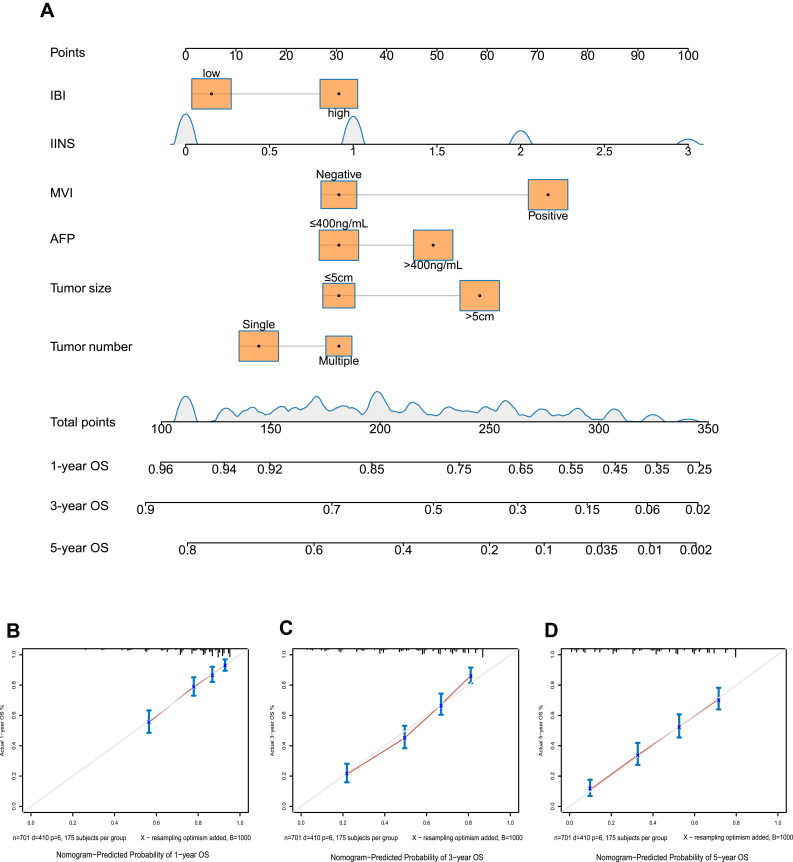
The following independent risk factors were identified for poor OS (Table 5): tumor number, tumor size, serum AFP, MVI and IINS. These factors were used to construct Model 1 for predicting OS (Figure 4A). The calibration curves showed strong agreement between predicted and observed values of 1-, 3- and 5-year OS (Figure 4B–D) and DFS (Figure S5B–D).
Figure 4.
Model 1 for predicting OS of HCC patients after hepatectomy. (A) Nomogram of the model. (B–D) Calibration curves for OS at (B) 1 year, (C) 3 years and (D) 5 years. The light gray line indicates the ideal, where predictions match observations. The red dots were calculated by bootstrapping (1000 resamplings) and describe the performance of the nomogram: the closer the solid red line is to the light gray line, the more accurately the model predicts survival.
Note: Model 1, prognostic model based on IINS.
Abbreviations: IINS, inflammation-immunity-nutrition score; MVI, microvascular invasion; AFP, alpha-fetoprotein; OS, overall survival.
Prognostic Model Based on IBI
Multivariate analysis of IBI and clinicopathological indicators identified the following independent prognostic factors for poor DFS (Table 5): sex, tumor number, tumor size, serum AFP, MVI and IBI. Therefore, these indicators were used to construct Model 2 to predict DFS (Figure S6A).
The following independent risk factors were identified for poor OS (Table 5): tumor number, tumor size, serum AFP, MVI and IBI. These factors were included in Model 2 for predicting OS (Figure 5A). In addition, the calibration curves showed strong agreement between predicted and observed values of 1-, 3- and 5-year DFS (Figure S6B–D) and OS (Figure 5B–5D).
Figure 5.
Model 2 for predicting OS of HCC patients after hepatectomy. (A) Nomogram of the model. (B–D) Calibration curves for OS at (B) 1 year, (C) 3 years and (D) 5 years.
Note: Model 2, prognostic model based on IBI.
Abbreviations: IBI, inflammation-immunity-nutrition score; MVI, microvascular invasion; AFP, alpha-fetoprotein; OS, overall survival.
Combined Model
To investigate the prognostic implication of the combination of the IINS and IBI, we also generated a combined prediction model as Model 3. Sex, tumor number, tumor size, serum AFP level, MVI, IINS and IBI were used to construct Model 3 for DFS (Table 5, Figure S7A). Furthermore, tumor number, tumor size, AFP level, MVI, IINS and IBI were incorporated into Model 3 as a prognostic model for OS (Table 5, Figure 6A). The calibration curves showed strong agreement between predicted and observed values of 1-, 3- and 5-year DFS and OS (Figures S7B–7D and Figures 6B–D).
Figure 6.
Model 3 to predict OS of HCC patients after hepatectomy. (A) Nomogram of the model. (B–D) Calibration curves for OS at (B) 1 year, (C) 3 years and (D) 5 years.
Note: Model 3, prognostic model based on IINS and IBI.
Abbreviations: IBI, inflammation-immunity-nutrition score; IINS, inflammation-immunity-nutrition score; MVI, microvascular invasion; AFP, alpha-fetoprotein; OS, overall survival.
Model Comparison
Comparison of the prognostic efficacy of inflammatory and nutritional indicators using time–AUC curves indicated that IINS performed best at predicting DFS and OS at 1, 3, and 5 years (Figure S8 and Table S2).
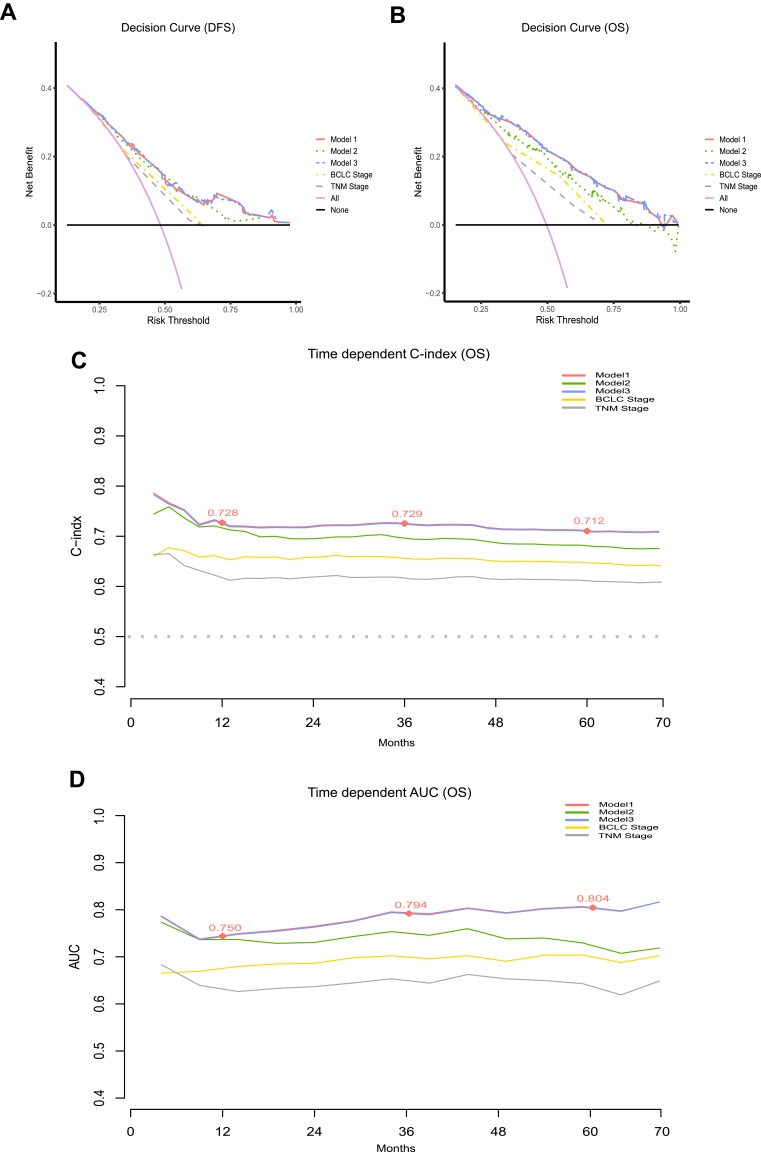
Moreover, the predictive performance of the three models with traditional clinical staging systems was compared using DCA (Figure 7A and B), C‐index, NRI, and IDI. The results indicated no difference between Models 1 and 3 in their ability to predict DFS (NRI = 0, P = 0.998; IDI = 0, P = 0.925) and OS (NRI = 0.023, P = 0.225; IDI = 0.005, P = 0.078). Both models showed better predictive performance than Model 2, BCLC staging, and AJCC TNM staging (Table 6).
Figure 7.
Comparison of models and traditional clinical staging systems for predicting prognosis. (A and B) Decision curve analysis (DCA) of nomogram models. The horizontal solid black line represents the hypothesis that no patients reached the endpoint, and the solid pink line represents the hypothesis that all patients met the endpoint. Model 1 showed the highest area under the curve and emerged as the optimal decision-making tool for maximal net benefit in HCC patients after hepatectomy. (C) Time-dependent C -index. (D) Time-dependent AUCs. The red nodes represent C-index or AUC values of Model 1 for 1 year, 3 years and 5 years.
Notes: Model 1, prognostic model based on IINS; Model 2, prognostic model based on IBI; Model 3, prognostic model based on IINS and IBI.
Abbreviations: BCLC, Barcelona Clinic Liver Cancer; AJCC TNM, American Joint Committee on Cancer/Tumor-Node-Metastasis; AUC, area under the curve; IINS, inflammation-immunity-nutrition score; IBI, inflammatory burden index; OS, overall survival.
Table 6.
Comparison of Concordance Indices for Different Models to Predict Disease-Free Survival and Overall Survival
| Model | Disease-Free Survival | Overall Survival | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C-Index (95% CI) | NRI | P | IDI | P | C-Index (95% CI) | NRI | P | IDI | P | |
| Model 1a | 0.692 (0.665–0.719) | 0.717 (0.692–0.742) | ||||||||
| Model 2 | 0.677 (0.652–0.702) | −0.059 | 0.013 | −0.020 | <0.001 | 0.701 (0.676–0.726) | −0.176 | <0.001 | −0.046 | <0.001 |
| Model 3 | 0.692 (0.665–0.719) | 0 | 0.998 | 0 | 0.925 | 0.722 (0.698–0.746) | 0.023 | 0.225 | 0.005 | 0.078 |
| BCLC stage | 0.628 (0.603–0.653) | −0.205 | <0.001 | −0.088 | <0.001 | 0.645 (0.619–0.670) | −0.324 | <0.001 | −0.116 | <0.001 |
| AJCC TNM (8th) | 0.613 (0.588–0.638) | −0.225 | <0.001 | −0.105 | <0.001 | 0.611 (0.583–0.366) | −0.332 | <0.001 | −0.156 | <0.001 |
| Model 2b | ||||||||||
| Model 3 | 0.059 | 0.013 | 0.020 | <0.001 | 0.198 | <0.001 | 0.050 | <0.001 | ||
| BCLC stage | −0.145 | <0.001 | −0.068 | <0.001 | −0.245 | <0.001 | −0.070 | <0.001 | ||
| AJCC TNM (8th) | −0.166 | <0.001 | −0.063 | <0.001 | −0.273 | <0.001 | −0.110 | <0.001 | ||
| Model 3c | ||||||||||
| BCLC stage | −0.205 | <0.001 | −0.088 | <0.001 | −0.346 | <0.001 | −0.120 | <0.001 | ||
| AJCC TNM (8th) | −0.225 | <0.001 | −0.083 | <0.001 | −0.355 | <0.001 | −0.161 | <0.001 | ||
Notes: aComparison of NRI and IDI values between the Model 1 and other models; bComparison of NRI and IDI values between the Model 2 and other models; cComparison of NRI and IDI values between the Model 3 and other models; Model 1, prognostic model based on IINS; Model 2, prognostic model based on IBI; Model 3, prognostic model based on IINS and IBI.
Abbreviations: IINS, inflammation-immunity-nutrition score; IBI, inflammatory burden index; BCLC, Barcelona Clinic Liver Cancer; AJCC TNM, American Joint Committee on Cancer/Tumor-Node-Metastasis; NRI, net reclassification index; IDI, integrated discrimination improvement.
C-indexes for nomograms to predict OS based on Models 1–3 were, respectively, 0.717, 0.701 and 0.722 (Table 6). Further comparison of their prognostic performance at different time points based on time-dependent C-index and AUC suggested that Model 1 was superior to other models and traditional clinical staging systems for predicting survival at 1, 3 and 5 years after hepatectomy (Figure S9, Figure 7C and D and Table S3).
Discussion
Recent studies have shown that inflammatory biomarkers are closely associated with postoperative prognosis in patients with cancer, including HCC.14,18–20 However, the value of IINS and IBI in predicting prognosis in HCC patients after hepatectomy has not been explored. Here, we investigated IINS and IBI as potential predictors of poor DFS and OS and compared the performance of models based on IINS, IBI or both, in combination with other inflammation and nutrition markers and clinical staging systems. Our results suggest that both IINS and IBI are independent risk factors for poor outcomes and that IINS can successfully predict prognosis in patients with HCC after hepatectomy.
Previous studies have shown that systemic infections interact with tumors to form an immunosuppressive microenvironment that promotes tumor proliferation and metastasis, contributing to high mortality.21,22 Several inflammation-related models have been developed to predict prognosis of cancer patients, and these models involve CRP, LYM count, NEU count, and serum ALB. CRP is a non-invasive biomarker that the liver produces in response to acute infection,23 and it may activate complement and promote inflammation, leading to more severe disease.24 CRP may facilitate early diagnosis of colorectal and lung cancer25,26 as well as prediction of prognosis.27 LYM are immune defense cells that inhibit tumor cell growth and proliferation by enhancing immune surveillance,28 and their increased infiltration into tumor microenvironments has been associated with better outcomes.29 NEU exert pro-tumor effects by recruiting immunosuppressive cells such as macrophages and Treg cells to the tumor microenvironment.30 Besides, tumor-associated neutrophils (TAN) protect tumor cells from toxic killing by cytotoxic lymphocyte (CTL) releasing neutrophil extracellular traps (NET), promoting tumor growth and angiogenesis.31 Low serum ALB levels reflect malnutrition,18 which can suppress immune reactions, including against tumors.14,32 In addition, higher values of IBI are strongly associated with poor physical status, high mortality, and advanced tumor stage, making it a reliable serum biomarker for predicting prognosis in various cancers.15–17 Consistent with these results, we found that high IINS and IBI values were strongly associated with progressive BCLC staging, tumor multi-nodularity, tumor diameter ˃ 5 cm, serum AFP > 400 ng/mL, positive MVI, and worse prognosis. Our results are consistent with the idea that an immunosuppressive nutritional imbalance or more severe inflammatory state are associated with worse prognosis in HCC patients after hepatectomy.
To the best of our knowledge, our study is the first to compare the prognostic value of a combined IINS and IBI model or individual with other indicators in patients after HCC resection. Our results showed that the prognostic performance of the individual model (Model 1) was no worse than that of the combined model (Model 3), which contains two overlapping indicators: CRP and LYM. Therefore, we conclude that the prognostic model based on IINS (Model 1) has the best predictive performance.
Most HCC patients are in a severe inflammatory state due to chronic viral infection, imbalance between pro- and anti-inflammatory immune cells, and injuries or hematologic alterations as a result of surgical resection. Our nomogram models may be useful for identifying patients at higher risk of poor prognosis after hepatectomy. Such patients may benefit from efforts to improve nutritional status as well as early interventions such as therapy targeting programmed cell death protein 1 or other molecules.
Despite its strengths, our study has certain limitations. First, its retrospective, single-center design limits the quality of data and may reflect population bias. In addition, some inflammatory indicators may change in the postoperative period and affect prognosis, but our study assessed only the preoperative phase and did not evaluate outcomes over time. Indeed, most of our patients had chronic hepatitis B infection, so our results need to be verified in patients with HCC induced by other factors.
Conclusion
IINS and IBI are independent predictors of survival of HCC patients after hepatic resection. A concise model that includes IINS may predict survival better than other models, and it may also outperform traditional staging systems. IINS is an easily accessible scoring tool that may reliably identify patients at high risk of postoperative recurrence or mortality and help individualize treatment.
Funding Statement
This work was supported by grants from the National Natural Science Foundation of China (81960450), the High-Level Innovation Team and Outstanding Scholar Program in Guangxi Colleges and Universities, the “139” Projects for Training of High-level Medical Science Talents from Guangxi, the Key Research and Development Project of Guangxi (AB20297009, AA18221001, AB18050020), the Key Laboratory of Early Prevention and Treatment for Regional High-Frequency Tumors of the Ministry of Education, the Guangxi Independent Research Project (GKE2019-ZZ07, GKE-ZZ202005, GKE-ZZ202111), and the Development and Application of Medical and Health Appropriate Technology in Guangxi (S2019039).
Disclosure
The authors report no conflicts of interest.
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 2.European Association For The Study Of The Live. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi: 10.1016/j.jhep.2018.03.019 [DOI] [PubMed] [Google Scholar]
- 3.Poon RT, Fan ST, Lo CM, Liu CL, Wong J. Long-term survival and pattern of recurrence after resection of small hepatocellular carcinoma in patients with preserved liver function: implications for a strategy of salvage transplantation. Ann Surg. 2002;235(3):373–382. doi: 10.1097/00000658-200203000-00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yu LX, Ling Y, Wang HY. Role of nonresolving inflammation in hepatocellular carcinoma development and progression. NPJ Precis Oncol. 2018;2(1):6. doi: 10.1038/s41698-018-0048-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Dijk AM, Coppens BJP, van Beers MA, et al. Nutritional status in patients with hepatocellular carcinoma: potential relevance for clinical outcome. Eur J Intern Med. 2022;104:80–88. doi: 10.1016/j.ejim.2022.07.002 [DOI] [PubMed] [Google Scholar]
- 6.Qian W, Xiao-Jian J, Jun H, Liang L, Xiao-Yong C. Comparison of the value of multiple preoperative objective nutritional indices for the evaluation of prognosis after hepatectomy for hepatocellular carcinoma. Nutr Cancer. 2022;74:1–11. [DOI] [PubMed] [Google Scholar]
- 7.Chen F, Chen D, Jin L, Xu C, Zhao W, Hu W. Prognostic significance of neutrophil-to-lymphocyte ratio and C-Reactive protein/albumin ratio in luminal breast cancers with HER2-negativity. Front Oncol. 2022;12:845935. doi: 10.3389/fonc.2022.845935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ni HH, Lu Z, Huang X, et al. Combining pre- and postoperative lymphocyte-C-reactive protein ratios can better predict hepatocellular carcinoma prognosis after partial hepatectomy. J Inflamm Res. 2022;15:2229–2241. doi: 10.2147/JIR.S359498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Feng J, Wang L, Yang X, Chen Q. Development and validation of a novel pre-operative comprehensive prognostic score in esophageal squamous cell carcinoma. Bosn J Basic Med Sci. 2022;22(3):460–470. doi: 10.17305/bjbms.2021.6350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang M, Zhang Q, Ge Y, et al. Prognostic roles of inflammation- and nutrition-based indicators for female patients with cancer. J Inflamm Res. 2022;15:3573–3586. doi: 10.2147/JIR.S361300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mei J, Sun XQ, Lin WP, et al. Comparison of the prognostic value of inflammation-based scores in patients with hepatocellular carcinoma after anti-PD-1 therapy. J Inflamm Res. 2021;14:3879–3890. doi: 10.2147/JIR.S325600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li XY, Yao S, He YT, et al. Inflammation-immunity-nutrition score: a novel prognostic score for patients with resectable colorectal cancer. J Inflamm Res. 2021;14:4577–4588. doi: 10.2147/JIR.S322260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jiang P, Wang J, Gong C, Yi Q, Zhu M, Hu Z. A nomogram model for predicting recurrence of stage I-III Endometrial Cancer Based on Inflammation-Immunity-Nutrition Score (IINS) and traditional classical predictors. J Inflamm Res. 2022;15:3021–3037. doi: 10.2147/JIR.S362166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Z, Liang Y, Zhong D, et al. Prognostic value of inflammation-immunity-nutrition score in patients with hepatocellular carcinoma treated with anti-PD-1 therapy. J Clin Lab Anal. 2022;36(5):e24336. doi: 10.1002/jcla.24336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Xie H, Ruan G, Ge Y, et al. Inflammatory burden as a prognostic biomarker for cancer. Clin Nutr. 2022;41(6):1236–1243. doi: 10.1016/j.clnu.2022.04.019 [DOI] [PubMed] [Google Scholar]
- 16.Taniai T, Haruki K, Furukawa K, et al. The novel index using preoperative C-reactive protein and neutrophil-to-lymphocyte ratio predicts poor prognosis in patients with pancreatic cancer. Int J Clin Oncol. 2021;26(10):1922–1928. doi: 10.1007/s10147-021-01964-2 [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Wang K, Ni J, et al. Combination of C-reactive protein and neutrophil-to-lymphocyte ratio as a novel prognostic index in patients with bladder cancer after radical cystectomy. Front Oncol. 2021;11:762470. doi: 10.3389/fonc.2021.762470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sprinzl MF, Kirstein MM, Koch S, et al. Improved prediction of survival by a risk factor-integrating inflammatory score in sorafenib-treated hepatocellular carcinoma. Liver Cancer. 2019;8(5):387–402. doi: 10.1159/000492628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dolan RD, McSorley ST, Park JH, et al. The prognostic value of systemic inflammation in patients undergoing surgery for colon cancer: comparison of composite ratios and cumulative scores. Br J Cancer. 2018;119(1):40–51. doi: 10.1038/s41416-018-0095-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qi WX, Xiang Y, Zhao S, Chen J. Assessment of systematic inflammatory and nutritional indexes in extensive-stage small-cell lung cancer treated with first-line chemotherapy and atezolizumab. Cancer Immunol Immunother. 2021;70(11):3199–3206. doi: 10.1007/s00262-021-02926-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dupré A, Malik HZ. Inflammation and cancer: what a surgical oncologist should know. Eur J Surg Oncol. 2018;44(5):566–570. doi: 10.1016/j.ejso.2018.02.209 [DOI] [PubMed] [Google Scholar]
- 22.Guthrie GJ, Charles KA, Roxburgh CS, Horgan PG, McMillan DC, Clarke SJ. The systemic inflammation-based neutrophil-lymphocyte ratio: experience in patients with cancer. Crit Rev Oncol Hematol. 2013;88(1):218–230. doi: 10.1016/j.critrevonc.2013.03.010 [DOI] [PubMed] [Google Scholar]
- 23.Murata M. Inflammation and cancer. Environ Health Prev Med. 2018;23(1):50. doi: 10.1186/s12199-018-0740-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111(12):1805–1812. doi: 10.1172/JCI200318921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heikkilä K, Ebrahim S, Lawlor DA. A systematic review of the association between circulating concentrations of C reactive protein and cancer. J Epidemiol Community Health. 2007;61(9):824–833. doi: 10.1136/jech.2006.051292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsilidis KK, Branchini C, Guallar E, Helzlsouer KJ, Erlinger TP, Platz EA. C-reactive protein and colorectal cancer risk: a systematic review of prospective studies. Int J Cancer. 2008;123(5):1133–1140. doi: 10.1002/ijc.23606 [DOI] [PubMed] [Google Scholar]
- 27.Diakos CI, Charles KA, McMillan DC, Clarke SJ. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014;15(11):e493–503. doi: 10.1016/S1470-2045(14)70263-3 [DOI] [PubMed] [Google Scholar]
- 28.Heinzel S, Marchingo JM, Horton MB, Hodgkin PD. The regulation of lymphocyte activation and proliferation. Curr Opin Immunol. 2018;51:32–38. doi: 10.1016/j.coi.2018.01.002 [DOI] [PubMed] [Google Scholar]
- 29.Huh JW, Lee JH, Kim HR. Prognostic significance of tumor-infiltrating lymphocytes for patients with colorectal cancer. Arch Surg. 2012;147(4):366–372. doi: 10.1001/archsurg.2012.35 [DOI] [PubMed] [Google Scholar]
- 30.Que H, Fu Q, Lan T, Tian X, Wei X. Tumor-associated neutrophils and neutrophil-targeted cancer therapies. Biochim Biophys Acta Rev Cancer. 2022;1877:188762. doi: 10.1016/j.bbcan.2022.188762 [DOI] [PubMed] [Google Scholar]
- 31.Cristinziano L, Modestino L, Loffredo S, et al. Anaplastic thyroid cancer cells induce the release of mitochondrial extracellular DNA traps by viable neutrophils. J Immunol. 2020;204(5):1362–1372. doi: 10.4049/jimmunol.1900543 [DOI] [PubMed] [Google Scholar]
- 32.Nakanishi Y, Masuda T, Yamaguchi K, et al. Albumin-globulin ratio is a predictive biomarker of antitumor effect of anti-PD-1 antibody in patients with non-small cell lung cancer. Int J Clin Oncol. 2020;25(1):74–81. doi: 10.1007/s10147-019-01539-2 [DOI] [PubMed] [Google Scholar]