Abstract
Interleukin-12 (IL-12) production by human monocytes stimulated with mannoproteins (MPs) of Cryptococcus neoformans was investigated. The results reported show that secreted or cell-associated MPs induce an early and significant production of IL-12. MPs show different capabilities to quantitatively affect IL-12 production; MP2, an 8.2-kDa MP purified from the culture supernatant of C. neoformans, appears to be the most potent stimulator. Cytochalasin B inhibits both internalization and IL-12 induction by MP. In addition, a drastic reduction of IL-12 was observed when monocytes were cultured in the absence of normal human serum or treated with soluble mannan. Early production of IL-12 promotes early secretion of gamma interferon by T cells but does not influence the magnitude of the MP-induced lymphoproliferative response. Overall our results identify cryptococcal antigens responsible for rapid and potent induction of IL-12 in monocytes. MPs appear to regulate IL-12 secretion by internalization via the endocytic pathway and by interaction with monocyte receptors or serum factors.
Cryptococcus neoformans is an opportunistic pathogenic yeast responsible for life-threatening infection in patients with AIDS (12). The host immune response to C. neoformans infection is the result of a complex interplay between cellular and humoral immunity that often guarantees the control of infection in the immunocompetent host (36).
Interleukin-12 (IL-12), produced by a variety of cells including antigen-presenting cells, dendritic cells, B cells, and phagocytic cells, favors Th1 cell generation (29) and orchestrates Th1-dependent resistance to infections caused by bacteria, fungi, virus, and intracellular parasites (6, 29, 32).
IL-12 has been shown to be important for the development of an effective immune response against C. neoformans in mice (14, 42). Studies of human T cells demonstrated that IL-12 facilitates the generation of a Th1 response with high gamma interferon (IFN-γ) production (21). In particular, in humans it has been documented that (i) after C. neoformans infection, IL-12 is produced by monocytes primarily in a T-cell-dependent pathway (27); (ii) peripheral blood mononuclear cells from healthy human immunodeficiency virus-seronegative donors express IL-12 p40 mRNA but are unable to secrete appreciable levels of IL-12 bioactive p70 when primed with C. neoformans (13); (iii) and IL-12 production by monocytes exposed to C. neoformans is indirectly mediated by the interaction of the receptor-ligand pair CD40-CD40 ligand expressed on monocytes and activated T cells (27).
The cell envelope of C. neoformans contains capsular polysaccharides, glucuronoxylomannan (GXM), galactoxylomannan, and mannoproteins (MPs) (3, 5, 16). These components can be isolated from the culture supernatants of the yeast. GXM and MP have separate effects on the immune system (5). The immunosuppressive properties of GXM have been extensively documented (18, 23, 28, 38, 39), and MP plays a role as an immunopotentiating antigen involved in the cell-mediated immune response (5, 23, 24, 26; L. Pitzurra, G. Teti, and A. Vecchiarelli, Abstr. 97th Gen. Meet. Am. Soc. Microbiol. 1997, p. 279, 1997). In particular, several authors have reported the involvement of purified secreted or cell-associated MP in human lymphoproliferation and induction of tumor necrosis factor alpha (TNF-α) secretion by monocytes (1, 2, 19).
In this study, we explored the potential involvement of C. neoformans MPs in IL-12 p40 and bioactive p70 secretion by human monocytes. The results demonstrate that MPs are responsible for rapid IL-12 induction and that both ligand-specific receptor and nonspecific endocytosis by monocytes may be dominant signals.
MATERIALS AND METHODS
C. neoformans.
In this study, C. neoformans acapsular strain NIH B-4131 was used. The morphological characteristics and growth conditions of the C. neoformans isolate have been described elsewhere (27). Cultures were maintained by serial passage on Sabouraud agar (Bio Merieux, Lyon, France) and harvested by suspending a single colony in RPMI 1640. The cells were washed twice, counted on a hematocytometer, and adjusted to the desired concentration. C. neoformans cells were killed by autoclaving.
Reagents.
Mannan from Saccharomyces cerevisiae, cytochalasin B from Helminthosporium dematioideum, and polymyxin B and lipopolysaccharide (LPS) from Escherichia coli O55:135 were obtained from Sigma (St. Louis, Mo.). Human recombinant IL-12 was from Genetics Institute (Cambridge, Mass.).
Purification of cryptococcal MPs.
C. neoformans-secreted MP1 and MP2 were purified as described previously (1, 2, 4, 15, 24, 34). Briefly, secreted MPs were purified from culture supernatants of C. neoformans NIH B-4131. Purification was performed by a combination of ultrafiltration affinity chromatography (concanavalin A), anion-exchange chromatography (DEAE), and gel permeation chromatography. Anion-exchange chromatography of the MPs yielded two fractions, MP1 (35.6 kDa) and MP2 (8.2 kDa) (2). MP1 and MP2 contained 7 and 13% protein and 79 and 43% neutral sugars, respectively. Cell-associated MP105 was purified as described elsewhere (26). MPs did not contain endotoxin, since no 2-keto-3-deoxyoctulosonic acid, determined by the thiobarbituric acid method of Weissbach and Hurwitz (40) and by the semicarbazide method of MacGee and Douoroff (20), could be detected in the sample. Furthermore, no differences in IL-12 induction were observed in the absence or presence of polymyxin B on titration of the fractions (data not shown). Contamination of MPs with cryptococcal capsular polysaccharide (GXM) was excluded by monosaccharide composition analysis and the latex agglutination test, which is specific for GXM. MPs and other reagents, tested for endotoxin contamination by the Limulus amebocyte lysate assay (Sigma), were below the detection limit of the assay, which had a sensitivity of approximately of 0.05 to 0.1 ng of E. coli LPS/ml.
MAbs.
Two anti-IL-12 monoclonal antibodies (MAbs), C11.79 (anti-IL-12 p40/p70) and C11.5.14 (anti-IL-12 p70), were used in this study. MAbs were purified from hybridomas (kindly provided by G. Trinchieri) as previously described (7). Mouse immunoglobulin G1 kappa (unrelated MAb) was purchased from Sigma.
Isolation of PBM and T(E+) cells.
Heparinized venous blood from healthy donors was diluted in RPMI 1640 (Gibco BRL, Life Technologies, San Giuliano Milanese, Italy), and the mononuclear cells were separated by density gradient centrifugation on Ficoll-Hypaque (27). The mononuclear cells were washed twice in RPMI 1640 plus 5% heat-inactivated fetal calf serum (cRPMI) and incubated for 1 h at a concentration of 2 × 106 to 3 × 106/ml in 100-mm-diameter polystyrene tissue culture plates (Corning Glass Works, Corning, N.Y.). After 1 h at 37°C in 5% CO2, nonadherent cells were removed by washing four times and E rosetted as previously described (28). The cells recovered [T(E+) cells] were >98% CD3+, as evaluated by flow cytometry analysis. The remaining adherent cells were refrigerated for 15 min in Ca+-Mg2+-free phosphate-buffered saline and collected by scraping with a rubber policeman (Costar, Cambridge, Mass.). The adherent cells were washed twice with cRPMI and resuspended in RPMI supplemented with 2 mM l-glutamine, penicillin-streptomycin (100 IU/ml and 100 μg/ml, respectively), and 10% normal human serum (NHS; Sigma) (NHS-RPMI). The adherent cells were >98% viable as evaluated by trypan blue dye exclusion, and at least 95% were peripheral blood monocytes (PBM) as determined by Wright-Giemsa staining (Diff-Quik stain; Baxter Scientific Products, McGaw Park, Ill.). Each experiment was performed with monocytes or T cells isolated from a single donor.
PBM stimulation.
Isolated PBM were distributed in 2-ml volumes of NHS-RPMI into 24-well flat-bottom tissue culture plates (Falcon, Becton Dickinson, Oxnard, Calif.) at 2.5 × 106 cells per ml and incubated for different times at 37°C under a 5% CO2 atmosphere in the absence or presence of heat-inactivated C. neoformans (at an effector-to-target [E:T] ratio of 1:1) or decreasing doses, from 25 to 0.25 μg of MP (MP1, MP2, or MP105) per ml. At different time intervals, fluid supernatants were harvested for IL-12 determination. In selected experiments, IL-12 p40 mRNA expression in unstimulated or stimulated PBM was determined by reverse transcriptase (RT)-mediated PCR (RT-PCR), and IL-12 p70 secretion was determined by capture assay with purified MAb C11.5.14 as described below.
Coculture of PBM and T lymphocytes.
Monolayers of PBM (2 × 104) adhered in 96-well flat-bottom plates were incubated with or without heat-inactivated C. neoformans (2 × 104) or 25 μg of MP1, MP2, or MP105 per ml. Incubation was performed in NHS-RPMI at 37°C in a 5% CO2 atmosphere. After 6 h of incubation, PBM monolayers were washed to remove stimuli, and then autologous T(E+) cells (105) in NHS-RPMI were added. At different times, IL-12 and IFN-γ content of fluid supernatants and T-cell lymphoproliferation were determined as described below. In selected experiments, cells were cultured in the absence or presence of various doses (from 1 to 0.1 μg/ml) of anti-IL-12 MAb C11.79 or unrelated MAb.
Cytokine determination.
Cytokine levels in culture supernatants were measured by enzyme-linked immunosorbent assay (ELISA) based on the antibody sandwich principle for human IFN-γ and IL-12 (Biosource International). The IL-12 kit is a solid-phase ELISA based on the antibody sandwich principle; its sensitivity is <0.8 pg/ml. The substances tested were human IL-1β, IL-2, IL-3, IL-4, IL-5, IL-7, IL-8, IFN-γ, granulocyte-macrophage colony-stimulated factor, LIF stem cell factor, TNF-α, IL-10, IL-13, and IL-15; cross-reactivity was not present. The assay recognizes both natural and recombinant human IL-12, as well as the free p40 subunit. Selected samples (Fig. 2) were tested for human IL-12 p70 heterodimer by capture bioassay as previously described (7). Briefly, supernatants (100 μl) from unstimulated or 6-h-stimulated PBM were dispensed in 96-well flat-bottom plates coated with sterile purified antibody C11.5.14 (20 μg/ml) diluted in 0.1 M sodium carbonate-bicarbonate buffer (pH 9.5). After 5 h at room temperature, plates were washed four times with phosphate-buffered saline. Then experimental wells were filled with 100 μl of phytohemagglutinin (PHA) blast cell suspension (5 × 106/ml) in cRPMI with 50 U of IL-2 per ml. PHA blast cells were from 6-day peripheral blood cell cultures (106 cells/ml) containing 1% PHA (Sigma). These preparations were composed of >98% T cells, as determined by immunofluorescence with MAb to CD3. After 48 h of incubation, IFN-γ production was evaluated as secreted cytokine in cell-free supernatants. Each supernatant was tested in triplicate in parallel with serial dilutions of recombinant IL-12 (from 3 ng to 0.3 ng/ml), using medium as the background. Results are the means for two donors calculated against a standard curve obtained with serial dilutions of recombinant human IL-12 (kindly provided by Genetics Institute).
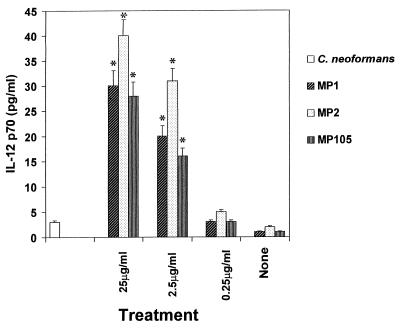
FIG. 2.
IL-12 p70 secretion by PBM stimulated with various doses of MP. IL-12 p70 production was evaluated by capture bioassay with MAb to IL-12 (C11.5.14) as described in Materials and Methods. IL-12 p70 was evaluated in supernatants from PBM unstimulated or stimulated for 6 h with C. neoformans (E:T = 1:1) or decreasing doses (from 25 to 0.25 μg/ml) of MP1, MP2, and MP105. Values are means ± SE of three separate experiments. ∗, P < 0.001 (treated versus untreated cells).
Lymphocyte proliferation assays.
T(E+) cell proliferation was measured by [3H]thymidine (0.5 μg/ml; Amersham International, Aylesbury, United Kingdom) incorporation after 3 and 7 days of coculture. Cells were collected onto filter paper by using a cell harvester (Flow Labs, McLean, Va.), and the dried filters were counted directly in a β counter (Packard Instruments Inc., Downers Grove, Ill.). Proliferation was expressed as the mean of indicated replicates ± standard error (SE).
RNA extraction and RT-PCR.
Total RNA was isolated from PBM by established procedures (22). RNA integrity was assessed by denaturing gel electrophoresis. Only samples with intact 28S and 18S rRNA were used for the RT reaction. For each experiment, equivalent amounts of intact RNA (5 μg) were reverse transcribed as described elsewhere (22). Equivalent amounts of cDNA in 5-μl aliquots were amplified by PCR (Perkin-Elmer Cetus DNA thermal cycler) in a reaction mixture containing PCR buffer (Pharmacia), MgCl2 (2 mM), deoxynucleoside triphosphates (1.25 mM), specific primers (0.4 mM), and 1 U of Taq polymerase (Pharmacia). All samples were denatured at 96°C for 2 min and cycled with the following log-linear parameters for 32 cycles of denaturation (94°C for 45 s), annealing (60°C for 45 s), and extension (72°C for 90 s). Positive cDNA controls and negative controls (RT-diethylpyrocarbonate-treated H2O) were included. Ten microliters of the PCR amplification products was separated in ethidium bromide-stained 1.5% agarose gel and visualized by UV transillumination. Aliquots of 0.05 μg of φX174 DNA HaeIII fragments (New England BioLabs, Beverley, Mass.) were run in parallel as molecular size markers. The amplified bands showed predicted sizes. Primers were DNA specific and nonreactive with RNA. The primer sequences were as follows: for glyceraldehyde-3-phosphate dehydrogenase (358 bp), 5′ TTCTTCAACCCCGAGGAGT 3′ (sense) and 5′ GGGAAGGAGGGTGGCCGTG 3′ (antisense) (17); for IL-12 p40 (373 bp), 5′ GGACCAGAGCAGTGAGGTCTT 3′ (sense) and 5′ CTCCTTGTTCCCCTCTGA 3′ (antisense) (33).
Cell viability assay.
Cell viability was measured with a colorimetric reaction based on the capacity of mitochondrial dehydrogenase of living cells to reduce 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Aldrich Chemical Co., Milan, Italy) to formazan. The quantity of formazan produced and measured at an optical density of 540 nm in a Sorin Bomedica microplate reader correlated with the number of living cells (25).
Statistical analysis.
Statistical significance was calculated by Student's paired t test.
RESULTS
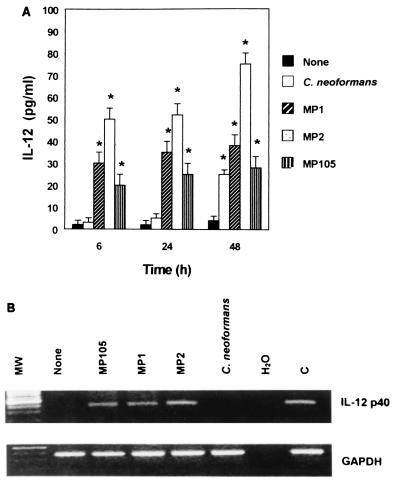
To evaluate the involvement of secreted or cell-associated C. neoformans MPs in IL-12 secretion by PBM, kinetic experiments were performed with cells from healthy donors. In our experimental conditions, PBM exposed to C. neoformans yeast cells produced appreciable levels of IL-12 after 48 h (Fig. 1A). Differences in kinetics and the amount of MP-induced IL-12 secretion relative to C. neoformans-induced secretion were observed. All MPs induced IL-12 after 6 h of stimulation, MP2 being the best stimulator. In contrast, no IL-12 production was observed for C. neoformans whole cells. Early induction of IL-12 was consistent with the analysis of IL-12 p40 mRNA showing that MP1, MP2, and MP105 induced IL-12 gene expression after 6 h of stimulation (Fig. 1B). Time course experiments to determine IL-12 p70 showed that MPs induced biologically active IL-12 levels similar to those reported in Fig. 1.
FIG. 1.
(A) IL-12 production by PBM unstimulated or exposed to C. neoformans whole cells or purified MPs. IL-12 levels in culture supernatants of human PBM unstimulated (None) or exposed for 6, 24, and 48 h to C. neoformans (E:T = 1:1), MP1 (25 μg/ml), MP2 (25 μg/ml), or MP105 (25 μg/ml) were evaluated by ELISA. The results are means ± SE of three separate experiments. ∗, P < 0.001 (treated versus untreated cells). (B) RT-PCR detection of IL-12 p40 mRNA from PBM unstimulated or exposed to purified MPs was evaluated after 6 h of exposure to indicated stimuli as described in Materials and Methods. MW, molecular weight markers; H2O, negative control; C, positive control. The results are from one representative experiment of three performed with similar results.
To evaluate whether MPs stimulate bioactive IL-12 p70 secretion by PBM, a capture bioassay using purified anti-IL-12 MAb was performed. The results showed that supernatants from 6-h MP-stimulated PBM induced IL-12 p70 secretion, whereas unstimulated or C. neoformans stimulated cells did not (Fig. 2). The effect was dose dependent and was maximum in supernatant fluids of MP2-treated PBM (Fig. 2).
Previous studies indicated the involvement of monocyte mannose receptors or serum mannose binding proteins (MBP) in MP-mediated immunopotentiation, such as cytokine (i.e., TNF-α) secretion or lymphoproliferation (2). Furthermore, internalization of particulate antigen has been proposed as a potent stimulus for monocyte IL-12 expression and secretion (11). To evaluate the potential involvement of MBP, specific receptors, and the endocytic pathway in MP-induced IL-12 secretion, experiments were performed (i) in the absence of NHS to exclude MBP involvement, (ii) in the presence of mannose to block or reduce the potential ligation of MPs to specific receptors, or (iii) in the presence of cytochalasin B, which inhibits microtubule polymerization and blocks MP internalization. The results showed that the omission of NHS from cell culture as well as pretreatment of PBM with mannan or cytochalasin B significantly reduced MP-induced IL-12 secretion (Table 1). In particular, mannan and cytochalasin B reduced the IL-12 response by 35 and 39%, respectively. To exclude possible nonspecific cytotoxicity, preliminary experiments were performed to determine the dose of cytochalasin B that did not affect cell viability by the MTT reduction test, and subsequently a dose of 5.0 μg/ml was used.
TABLE 1.
Effects of NHS mannan, and cytochalasin B treatment on early (6 h) induction of MP2-mediated IL-12 secretion by PBM
PBM were exposed to MP2 (25 μg/ml) in the presence of 10% NHS.
PBM were exposed to MP2 (25 μg/ml) in the absence of NHS.
PBM were treated with mannan (125 μg/ml) and then with MP2 (25 μg/ml) in the presence of 10% NHS.
PBM were treated with cytochalasin B (5.0 μg/ml) and then with MP2 (25 μg/ml) in the presence of 10% NHS.
Mean ± SE of three separate experiments. *, P < 0.01 (treated versus untreated PBM).
PBM cultured in the presence or absence of 10% NHS stimulated with LPS (1 μg/ml) produced similar levels of IL-12 (70 ± 10 pg/ml).
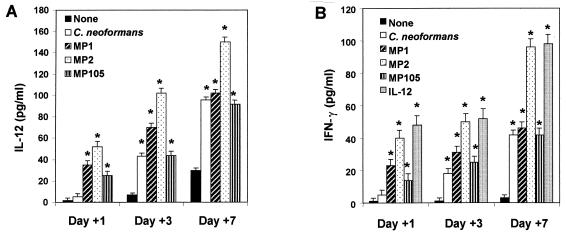
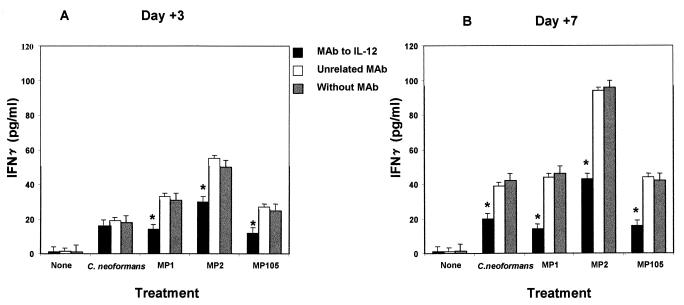
It is well known that IL-12 directly regulates IFN-γ production (14, 31, 33), raising the possibility that early secretion of IL-12 could promote early induction of IFN-γ. For this purpose, IL-12 and IFN-γ production were determined at days 1, 3, and 7 in cocultures of monocytes treated with MP plus autologous T cells. The results reported in Fig. 3A show that MPs induce high levels of IL-12, reaching a maximum after 7 days of incubation. It should be noted that MPs, in contrast to C. neoformans whole cells, were able to induce early production of IFN-γ (Fig. 3B). MP2 was a better stimulator of IFN-γ than MP1 or MP105. The kinetics and amount of MP2-induced IFN-γ secretion were similar to those observed in assays using recombinant IL-12 (0.3 ng/ml) as the stimulus (Fig. 3B), raising the possibility that in our experimental system early IL-12 induction involves IFN-γ production. Further evidence that IFN-γ was produced in an IL-12-dependent manner was provided by assessment of IL-12 and IFN-γ production by the same supernatant fluids from MP-stimulated PBM after 6 h of incubation. The results showed that a significant amount of IL-12 was produced while only negligible levels of IFN-γ were observed (data not shown). In addition, blocking experiments were performed with a MAb to IL-12, C11.79, that specifically recognizes both IL-12 p40 and p70 (7), thus showing that neutralization of MP-induced endogenous IL-12 drastically reduced early secretion of IFN-γ (Fig. 4A). This inhibitory effect was most pronounced (50% reduction) in the presence of 0.1 μg of MAb to IL-12 per ml (Fig. 4B). The specificity of MAb to IL-12 inhibitory activity was confirmed by the inability of an unrelated MAb to affect IFN-γ secretion. In addition, the MAb to IL-12 inhibited IFN-γ secretion in C. neoformans-stimulated cells at 7 days of incubation (Fig. 4).
FIG. 3.
IL-12 and IFN-γ levels in supernatant fluids of PBM plus autologous T lymphocytes stimulated with MPs. PBM plus T lymphocytes were unstimulated or stimulated with C. neoformans (E:T = 1:1) or with MPs (25 μg/ml). After 1, 3, and 7 days of coculture, IL-12 (A) and IFN-γ (B) levels were evaluated in cell-free supernatants by ELISA. Human recombinant IL-12 (0.3 ng/ml) was used as positive control to induce IFN-γ secretion. Values are means ± SE of three separate experiments. ∗, P < 0.01 (treated versus untreated cells).
FIG. 4.
Effect of MAb to IL-12 (C11.79) on IFN-γ secretion by cocultures of PBM plus autologous T lymphocytes were cocultured with C. neoformans or MPs in the absence or presence of MAb to IL-12 (0.1 μg/ml) or unrelated MAb (0.1 μg/ml). IFN-γ levels were determined by ELISA in supernatant fluids collected after 3 (A) and 7 (B) days of incubation. Values are means ± SE of three separate experiments. ∗, P < 0.01 (MAb to IL-12-treated versus untreated cells).
Having established that MP-induced IL-12 promotes early secretion of IFN-γ, we examined the possibility that endogenous IL-12 could affect C. neoformans or MP-induced lymphoproliferation. To this end, MAb to IL-12 was incorporated in the mixture of MPs or C. neoformans-treated PBM and T cells. The results reported in Table 2 show that the MAb to IL-12 did not modify the T-cell proliferative response. Furthermore, the use of increasing concentrations of MAb to IL-12 (from 0.01 to 1 μg/ml) did not modify the results (data not shown).
TABLE 2.
Effect of addition of MAb to IL-12 (C11.79) on MP-induced T-lymphocyte proliferative response
| PBM treatmenta | MAbb
|
T-cell proliferationc (cpm [103])
|
||
|---|---|---|---|---|
| To IL-12 | Unrelated | 3 days | 7 days | |
| None | − | − | 2 ± 0.3 | 5 ± 0.3 |
| + | − | 3 ± 0.5 | 4 ± 0.6 | |
| − | + | 3 ± 0.3 | 4 ± 0.4 | |
| C. neoformans | − | − | 7 ± 0.8 | 13 ± 1.2 |
| + | − | 7 ± 0.3 | 13 ± 0.9 | |
| − | + | 7 ± 0.5 | 13 ± 0.6 | |
| MP1 | − | − | 6 ± 0.6 | 19 ± 0.5* |
| + | − | 7 ± 0.5 | 19 ± 0.6 | |
| − | + | 7 ± 0.7 | 19 ± 1.2 | |
| MP2 | − | − | 8 ± 0.3 | 25 ± 2.3* |
| + | − | 8 ± 0.5 | 27 ± 2.8 | |
| − | + | 8 ± 0.6 | 26 ± 3.0 | |
| MP105 | − | − | 6 ± 0.6 | 18 ± 1.2* |
| + | − | 7 ± 0.8 | 18 ± 0.9 | |
| − | + | 6 ± 0.3 | 18 ± 0.5 | |
PBM were stimulated for 6 h with heat-inactivated C. neoformans (E:T = 1:1), MP1 (25 μg/ml), MP2 (25 μg/ml), or MP105 (25 μg/ml), washed, and cocultured with autologous T lymphocytes.
PBM were treated with MAb to IL-12 or unrelated MAb (0.1 μg/ml).
Proliferation was measured by [3H]thymidine incorporation after 3 and 7 days of coculture. Values represent means ± SE of four replicates from three separate experiments. *, P < 0.001 (MP-treated versus C. neoformans-treated cells.).
DISCUSSION
The data from this study show that C. neoformans MPs are involved in early induction of both IL-12 p40 subunit and p70 heterodimer secretion by human monocytes. While IL-12 was produced by monocytes in response to C. neoformans whole cells and MP stimulation, profound differences were observed in both the magnitude and timing of the IL-12 response. MPs are early and strong inducers of IL-12 compared to C. neoformans whole cells. MP-induced IL-12 is mediated by signals involving MP ligation, possibly through mannan MP recognition by monocyte receptors or by serum factors, and the nonspecific endocytic pathway. Early secretion of MP-induced IL-12 strongly influences the early production of IFN-γ by T cells but is unable to affect MP-induced T-cell proliferation.
Monocytes and macrophages play a critical role in the protective cellular immune response to C. neoformans (37, 39). Furthermore, they are possible major physiological producers of IL-12, as suggested by in vivo and in vitro studies in various infectious disease models (32). Studies of experimental murine models emphasize the pivotal role of IL-12 in the induction of protective responses against C. neoformans (8, 42). Recently we reported that IL-12 produced by human monocytes responding to C. neoformans whole cells is secreted late, predominantly in a T-cell-dependent mechanism (27). In addition, an immunosuppressive role for C. neoformans in IL-12 production has been reported (17). Here we identify MP antigens of the cryptococcal envelope responsible for early and massive induction bioactive IL-12 by human monocytes.
MPs from C. neoformans are responsible for T-cell proliferation and are strong inducers of proinflammatory cytokines such as IL-1β (Pitzurra et al., Abstr. 97th Gen. Meet. Am. Soc. Microbiol. 1997) and TNF-α (1, 2). A relationship between TNF-α production and IL-12 has been observed in murine macrophages stimulated with bacillus Calmette-Guérin (10). Consequently, the early and massive induction of IL-12 observed by us could be related to the ability of MPs to favor TNF-α secretion by human monocytes.
Monocytes alone are poor producers of IL-12 when stimulated with encapsulated C. neoformans, but an appreciable increase of IL-12 secretion was observed when an acapsular mutant was used (27). Our present observation that MPs induce an early and high release of IL-12 suggests that MPs of the acapsular cryptococcal envelope could be responsible, at least in part, for the significant production of IL-12 by monocytes. This is consistent with the increase of MP fractions recovered from acapsular C. neoformans with respect to encapsulated strains (26, 35). It is likely that MP antigen can be masked by capsular material in the encapsulated strains, or MP may compete with GXM to bind to the same receptors on monocytes since GXM binding to phagocytic cells has been observed (9).
Importantly, the early presence of endogenous IL-12 appears to be a promoter of early and consistent production of IFN-γ by T cells. In contrast to C. neoformans whole cells, which primarily induce IL-12 in a T-cell-dependent mechanism, MPs induce IL-12 by monocytes through direct interaction in a T-cell-independent mechanism. While endogenous IL-12 is a strong promoter of IFN-γ, it is unable to affect the magnitude of MP-induced lymphoproliferation. This is consistent with previous observations showing the inability of recombinant IL-12 to influence the proliferative response to C. neoformans (13).
As described for MP2-induced TNF-α, MP2-induced IL-12 is moderately reduced by pretreatment of monocytes with mannan. A possible explanation is that the same signal transduction promotes the synthesis of TNF-α and IL-12. Alternatively, the synthesis and secretion of the two cytokines could be mutually regulated in an autocrine manner. In addition, we demonstrate that, other than MP ligation on monocyte receptors, the endocytic pathway that allows MP internalization plays an important role in MP2-induced IL-12 induction. However, we cannot exclude the possibility that cytochalasin B influences the adherence of MP to monocytes. In addition, mannan and cytochalasin B never completely blocked IL-12 production; thus, the stimulation of IL-12 through alternative mechanisms or the use of an insufficient amount of these substances can be considered.
Previous results by Harrison and Levitz (13) showed that in spite of the undetectable levels of IL-12, IL-12 p40 mRNA message was detected in monocytes responding to C. neoformans after 6 h of stimulation. The ability of MP to induce IL-12 p40 mRNA within 6 h of stimulation correlated with IL-12 secretion.
Overall our results show that MPs are cryptococcal envelope antigens responsible for early production of IL-12 by phagocytic cells. Secretion requires two steps, the first likely involving the mannan part of MPs, which can be recognized by monocyte receptors or serum factors, and the second requiring MP2 internalization via the endocytic pathway.
Early and significant IL-12 production in response to MPs suggests that purified MP antigen favors the development of a prompt and strong protective Th1 cellular response. Consequently, MPs that positively influence the cellular immune response to C. neoformans could be useful for developing a rational strategy to prevent cryptococcosis.
ACKNOWLEDGMENTS
We are grateful to Eileen Zannetti for excellent editorial and secretarial assistance and Genetics Institute for human recombinant IL-12.
This study was supported by the National Research Program on AIDS “Opportunistic Infections and Tuberculosis” contract 50A.0.35.Italy.
REFERENCES
- 1.Chaka W, Verheul A F, Vaishnav V V, Cherniak R, Scharringa J, Verhoef J, Snippe H, Hoepelman I M. Cryptococcus neoformans and cryptococcal glucuronoxylomannan, galactoxylomannan, and mannoprotein induce different levels of tumor necrosis factor alpha in human peripheral blood mononuclear cells. Infect Immun. 1997;65:272–278. doi: 10.1128/iai.65.1.272-278.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chaka W, Verheul A F, Vaishnav V V, Cherniak R, Scharringa J, Verhoef J, Snippe H, Hoepelman I M. Induction of TNF-alpha in human peripheral blood mononuclear cells by the mannoprotein of Cryptococcus neoformans involves human mannose binding protein. J Immunol. 1997;159:2979–2985. [PubMed] [Google Scholar]
- 3.Cherniak R, Reiss E, Slodki M E, Plattner R D, Blumer S O. Structure and antigenic activity of the capsular polysaccharide from Cryptococcus neoformans serotype A. Mol Immunol. 1980;17:1025–1032. doi: 10.1016/0161-5890(80)90096-6. [DOI] [PubMed] [Google Scholar]
- 4.Cherniak R, Reiss E, Turner S H. A galactoxylomannan antigen of Cryptococcus neoformans serotype A. Carbohydr Res. 1982;103:239–250. [Google Scholar]
- 5.Cherniak R, Sundstrom J B. Polysaccharide antigens of the capsule of Cryptococcus neoformans. Infect Immun. 1994;62:1507–1512. doi: 10.1128/iai.62.5.1507-1512.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coutelier J P, Van Broeck J, Wolf S F. Interleukin-12 gene expression after viral infection in the mouse. J Virol. 1995;69:1955–1958. doi: 10.1128/jvi.69.3.1955-1958.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D'Andrea A, Regaraju M, Valiante N M, Chehimi J, Kubin M, Aste M, Chan S H, Kobayashi M, Young D, Nickbarg E, Chizzonte R, Wolf S F, Trinchieri G. Production of natural killer cell stimulatory factor (interleukin 12) by peripheral blood mononuclear cells. J Exp Med. 1992;176:1387–1398. doi: 10.1084/jem.176.5.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Decken K, Kohler G, Palmer-Lehmann K, Wunderlin A, Mattner F, Magram J, Gately M K, Alber G. Interleukin 12 is essential for a protective Th1 response in mice infected with Cryptococcus neoformans. Infect Immun. 1998;66:4994–5000. doi: 10.1128/iai.66.10.4994-5000.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong Z M, Murphy J W. Cryptococcal polysaccharides bind to CD18 on human neutrophils. Infect Immun. 1997;65:557–563. doi: 10.1128/iai.65.2.557-563.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flesch I E, Hess J H, Huang S, Auget M, Rothe J, Bluethmann H, Kaufmann S H E. Early interleukin 12 production by macrophages in response to mycobacterial infection depends on interferon-γ and tumor necrosis factor α. J Exp Med. 1995;181:1615–1621. doi: 10.1084/jem.181.5.1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fulton S A, Johnson J M, Wolf S F, Sieburth D S, Boom W H. Interleukin-12 production by human monocytes infected with Mycobacterium tuberculosis: role of phagocytosis. Infect Immun. 1996;64:2523–2531. doi: 10.1128/iai.64.7.2523-2531.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Good C B, Coax W A. Cryptococcal infection in patients with AIDS. N Engl J Med. 1990;322:701–702. doi: 10.1056/nejm199003083221017. [DOI] [PubMed] [Google Scholar]
- 13.Harrison T S, Levitz S M. Role of IL-12 in peripheral blood mononuclear cell responses to fungi in persons with and without HIV infection. J Immunol. 1996;156:4492–4497. [PubMed] [Google Scholar]
- 14.Hoag K A, Lipscomb M F, Izzo A A, Street N E. IL-12 and IFN-γ are required for initiating the protective Th1 response to pulmonary cryptococcosis in resistant C.B-17 mice. Am J Respir Cell Mol Biol. 1997;17:733–739. doi: 10.1165/ajrcmb.17.6.2879. [DOI] [PubMed] [Google Scholar]
- 15.James P G, Cherniak R. Galactoxylomannan of Cryptococcus neoformans. Infect Immun. 1992;60:1084–1088. doi: 10.1128/iai.60.3.1084-1088.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.James P G, Cherniak R, Jones R G, Stortz C A, Reiss E. Cell wall glucans of Cryptococcus neoformans Cap67. Carbohydr Res. 1990;188:23–38. doi: 10.1016/0008-6215(90)84273-w. [DOI] [PubMed] [Google Scholar]
- 17.Kawakami K, Qureshi M H, Koguchi Y, Nakajima K, Saito A. Differential effect of Cryptococcus neoformans on the production of IL-12p40 and IL-10 by murine macrophages stimulated with lipopolysaccharide and gamma interferon. FEMS Microbiol Lett. 1999;175:87–94. doi: 10.1111/j.1574-6968.1999.tb13605.x. [DOI] [PubMed] [Google Scholar]
- 18.Kozel T R, Gotschlich E C. The capsule of Cryptococcus neoformans passively inhibits phagocytosis of the yeast macrophages. J Immunol. 1982;129:1675–1680. [PubMed] [Google Scholar]
- 19.Levitz S M, North E A. Lymphoproliferation and cytokine profiles in human peripheral blood mononuclear cells stimulated by Cryptococcus neoformans. J Med Vet Mycol. 1997;35:229–236. doi: 10.1080/02681219780001201. [DOI] [PubMed] [Google Scholar]
- 20.MacGee J, Doudoroff M. A new phosphorylated intermediate in glucose oxidation. J Biol Chem. 1954;210:617–629. [PubMed] [Google Scholar]
- 21.Manetti R, Gerosa F, Giudizi M G, Biagiotti R, Parronchi P, Piccinini M P, Sampognaro S, Maggi S, Romagnani S, Trinchieri G. Interleukin-12 induces stable priming for interferon γ (IFN-γ) production during differentiation of human T helper (Th) cells and transient IFN-γ production in established Th2 cell clones. J Exp Med. 1994;179:1273–1283. doi: 10.1084/jem.179.4.1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Monari C, Retini C, Palazzetti B, Bistoni F, Vecchiarelli A. Regulatory role of exogenous IL-10 in the development of immune response versus Cryptococcus neoformans. Clin Exp Immunol. 1997;109:242–247. doi: 10.1046/j.1365-2249.1997.4021303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Murphy J W. Influence of Cryptococcus antigens on cell-mediated immunity. Rev Infect Dis. 1988;10:S432–S435. doi: 10.1093/cid/10.supplement_2.s432. [DOI] [PubMed] [Google Scholar]
- 24.Orendi J M, Verheul A F M, De Vos N M, Visser M R, Snippe H, Cherniak R, Vaishnav V V, Rijkers G T, Verhoef J. Mannoproteins of Cryptococcus neoformans induce proliferative response in human peripheral blood mononuclear cells (PBM) and enhance HIV-1 replication. Clin Exp Immunol. 1997;107:293–299. doi: 10.1111/j.1365-2249.1997.283-ce1169.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pauwels R, Balzarini J, Baba M, Snoeck R, Schols D, Herdewijn P, Desmyter J, De Clercq E. Rapid and automated tetrazolium-based colorimetric assay for the detection of anti-HIV compounds. J Virol Method. 1988;20:309–321. doi: 10.1016/0166-0934(88)90134-6. [DOI] [PubMed] [Google Scholar]
- 26.Pitzurra L, Vecchiarelli A, Peducci R, Cardinali A, Bistoni F. Identification of a 105 kilodalton Cryptococcus neoformans mannoprotein involved in human cell-mediated immune response. J Med Vet Mycol. 1997;35:299–303. [PubMed] [Google Scholar]
- 27.Retini C, Casadevall A, Pietrella D, Monari C, Palazzetti B, Vecchiarelli A. Specific activated T cells regulate interleukin-12 production by human monocytes stimulated with Cryptococcus neoformans. J Immunol. 1999;162:1618–1623. [PubMed] [Google Scholar]
- 28.Retini C, Vecchiarelli A, Monari C, Bistoni F, Kozel T R. Encapsulation of Cryptococcus neoformans with glucuronoxylomannan inhibits the antigen-presenting capacity of monocytes. Infect Immun. 1998;66:664–669. doi: 10.1128/iai.66.2.664-669.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Romani L, Puccetti P, Bistoni F. Interleukin 12 in infectious diseases. Clin Microbiol Rev. 1997;10:611–636. doi: 10.1128/cmr.10.4.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trinchieri G. Interleukin-12 and its role in the generation of Th1 cells. Immunol Today. 1993;14:335–338. doi: 10.1016/0167-5699(93)90230-I. [DOI] [PubMed] [Google Scholar]
- 31.Trinchieri G. Interleukin-12: a cytokine produced by antigen-presenting cells with immunoregulatory functions in the generation of T-helper cells type 1 and cytotoxic lymphocytes. Blood. 1994;84:4008–4027. [PubMed] [Google Scholar]
- 32.Trinchieri G. Cytokines acting on or secreted by macrophages during intracellular infection (IL-10, IL-12, IFN-γ) Curr Opin Immunol. 1997;9:17–23. doi: 10.1016/s0952-7915(97)80154-9. [DOI] [PubMed] [Google Scholar]
- 33.Trinchieri G. Function and clinical use of interleukin-12. Curr Opin Hematol. 1997;4:59–66. doi: 10.1097/00062752-199704010-00010. [DOI] [PubMed] [Google Scholar]
- 34.Turner S H, Cherniak R, Reiss E. Fractionation and characterization of galactoxylomannan from Cryptococcus neoformans. Carbohydr Res. 1984;125:343–349. doi: 10.1016/0008-6215(84)85172-1. [DOI] [PubMed] [Google Scholar]
- 35.Vartivarian S E, Reyes G H, Jacobson E S, James P G, Cherniak R, Mumaw V R, Tingler M J. Localization of mannoprotein in Cryptococcus neoformans. J Bacteriol. 1989;17:6850–6852. doi: 10.1128/jb.171.12.6850-6852.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vecchiarelli A, Casadevall A. Antibody-mediated effects against Cryptococcus neoformans: evidence for interdependency and collaboration between humoral and cellular immunity. Res Immunol. 1998;149:321–333. doi: 10.1016/s0923-2494(98)80756-6. [DOI] [PubMed] [Google Scholar]
- 37.Vecchiarelli A, Dottorini M, Pietrella D, Monari C, Retini C, Todisco T, Bistoni F. Role of human alveolar macrophages as antigen presenting cells in Cryptococcus neoformans infection. Am J Respir Cell Mol Biol. 1994;11:130–137. doi: 10.1165/ajrcmb.11.2.8049074. [DOI] [PubMed] [Google Scholar]
- 38.Vecchiarelli A, Pietrella D, Dottorini M, Monari C, Retini C, Todisco T, Bistoni F. Encapsulation of Cryptococcus neoformans regulates fungicidal activity and antigen presentation process in human alveolar macrophages. Clin Exp Immunol. 1994;98:217–223. doi: 10.1111/j.1365-2249.1994.tb06128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vecchiarelli A, Retini C, Pietrella D, Monari C, Tascini C, Beccari T, Kozel T R. Downregulation of cryptococcal polysaccharide of tumor necrosis factor alpha and interleukin-1β secretion from human monocytes. Infect Immun. 1995;63:2919–2923. doi: 10.1128/iai.63.8.2919-2923.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weissbach A, Hurwitz J. The formation of 2-keto-3-deoxyheptonic acid in extracts of Escherichia coli B. J Biol Chem. 1959;234:705–709. [PubMed] [Google Scholar]
- 41.Wolf S F, Temple P A, Kobayashi M, Young D, Dicig M, Lowe L, Dzialo R, Fitz L, Ferenz C, Hewick R M, Kelleher K, Hermann S H, Clark S C, Azzoni L, Chan S H, Trinchieri G, Perussia B. Cloning of cDNA for natural killer cell stimulatory factor, a heterodimeric cytokine with multiple biologic effects on T and natural killer cells. J Immunol. 1991;146:3074–3081. [PubMed] [Google Scholar]
- 42.Zhang T, Kawakami K, Qureshi M H, Okamura H, Kurimoto M, Saito A. Interleukin-12 (IL-12) and IL-18 synergistically induce the fungicidal activity of murine peritoneal exudate cells against Cryptococcus neoformans through production of gamma interferon by natural killer cells. Infect Immun. 1997;65:3594–3599. doi: 10.1128/iai.65.9.3594-3599.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]