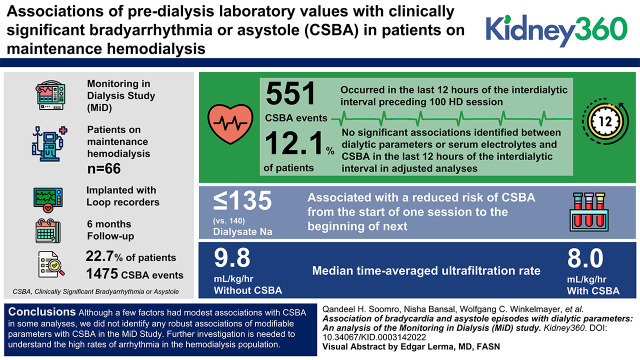
Key Points
There were 551 cases of clinically significant bradyarrhythmia or asystole (CSBA) that occurred in the last 12 hours of the interdialytic interval preceding 100 of 4424 dialysis sessions.
We did not find significant associations between dialytic parameters or electrolytes and CSBA in the last 12 hours of the interdialytic period.
Association of laboratory and dialytic factors with CSBA from one session to the next were not significant except dialysate sodium concentrations <135 mEq/L.
Keywords: dialysis, arrhythmia, asystole, bradycardia, dialysis
Visual Abstract
Abstract
Background
Bradycardia and asystole events are common among patients treated with maintenance hemodialysis. However, triggers of these events in patients on maintenance hemodialysis (HD), particularly during the long interdialytic period when these events cluster, are uncertain.
Methods
The Monitoring in Dialysis Study (MiD) enrolled 66 patients on maintenance HD who were implanted with loop recorders and followed for 6 months. We analyzed associations of predialysis laboratory values with clinically significant bradyarrhythmia or asystole (CSBA) during the 12 hours before an HD session. Associations with CSBA were analyzed with mixed-effect models. Adjusted negative binomial mixed-effect regression was used to estimate incidence rate ratios (IRR) for CSBA. We additionally evaluated associations of CSBA at any time during follow-up with time-averaged dialytic and laboratory parameters and associations of peridialytic parameters with occurrence of CSBA from the start of one HD session to the beginning of the next.
Results
There were 551 CSBA that occurred in the last 12 hours of the interdialytic interval preceding 100 HD sessions in 12% of patients and 1475 CSBA events in 23% of patients overall. We did not identify significant associations between dialytic parameters or serum electrolytes and CSBA in the last 12 hours of the interdialytic interval in adjusted analyses. Median time-averaged ultrafiltration rate was significantly higher in individuals without CSBA (9.8 versus 8, P=0.04). Use of dialysate sodium concentrations ≤135 (versus 140) mEq/L was associated with a reduced risk of CSBA from the start of one session to the beginning of next.
Conclusions
Although a few factors had modest associations with CSBA in some analyses, we did not identify any robust associations of modifiable parameters with CSBA in the MiD Study. Further investigation is needed to understand the high rates of arrhythmia in the hemodialysis population.
Introduction
Sudden cardiac or arrhythmic death (SCD) accounts for 44% of deaths with a known cause in patients on maintenance hemodialysis (HD) (1). Several unique risk factors have been identified that could contribute to high risk such as uremic toxins, cardiovascular autonomic dysfunction, and repetitive volume overload (2). Besides the risk factors, mechanisms triggering arrhythmia in the setting of hemodialysis may differ from the underlying causes in the general population. Some of these triggers such as the long interdialytic period, rapid and cyclic electrolyte and fluid shifts, and repetitive dialysis induced myocardial stunning have been identified as potential factors underlying the high incidence of SCD and arrhythmia in the hemodialysis population (3,4). Of note, defects in conduction including bradycardia and asystole as opposed to ventricular tachycardia or fibrillation appear to be the predominant arrhythmias underlying hemodialysis-associated SCD (5–9). However, the triggers underlying bradyarrhythmia in the hemodialysis population remain incompletely understood, and analysis of data collected around these events could help in identifying and modifying dialysis practices that may prevent the risk of SCD (2,10).
We used data from the Monitoring in Dialysis Study (MiD) (5) to analyze highly granular electrolyte, dialysis, and arrhythmia data from loop recorder monitoring to test for associations of potentially modifiable, predialytic parameters with clinically significant arrhythmia (CSA) episodes with bradycardic and asystole (CSBA).
Materials and Methods
Study Population
The MiD (NCT01779856) was a multicenter, prospective cohort study. The design, objectives, and primary outcomes have been reported in detail (5,11). Briefly, implantable loop recorders (ILR; Medtronic Reveal XT or LINQ) were implanted in 66 individuals on maintenance in-center hemodialysis three times a week. Individuals with an existing permanent pacemaker or implantable defibrillator were excluded. ILR were utilized for continuous cardiac rhythm monitoring to detect the occurrence of CSA for 6 months and were interrogated at each HD session during the 6-month primary outcome period and after every session with protocol-mandated phlebotomy. Vital signs and dialytic parameters were recorded at each dialysis session. Serum chemistries were tested before and after dialysis twice weekly for 4 weeks and then weekly through 6 months.
CSA was prospectively defined as (1) ventricular tachycardia ≥115 beats per minute (bpm) lasting ≥30 seconds, but subsequently changed to ≥130 bpm with a protocol amendment; (2) bradycardia with a rate of ≤40 bpm for ≥6 seconds; (3) asystole for ≥3 seconds; or (4) patient-marked (symptomatic) events with electrocardiogram-confirmed clinically relevant arrhythmia. This was derived using standard arrhythmia definitions (11,12), device capabilities, and expert opinion.
Primary Analysis
The temporal patterns of CSBA in the MiD were strongly associated with the dialytic cycle occurring with a markedly higher incidence rate during the last 12 hours of each interdialytic interval (13). For the current study, our primary question of interest is how volume status and ambient electrolytes—potentially modifiable parameters—relate to the occurrence of serious arrhythmias. We postulated that electrolytes and weight parameters measured at the beginning of a session (primary exposures) are representative surrogates of the ambient electrolytes and volume status during the last few hours of the preceding interdialytic interval (i.e., the previous 12 hours). Thus, our primary outcome for this analysis leveraged the concurrence of a period of maximally elevated risk of CSBA and the ability to infer the ambient levels of blood chemistries and volume status to analyze the association of predialysis laboratory parameters with the occurrence of a CSBA during the final 12 hours before a HD session. In secondary analyses, we analyzed associations of baseline or time-updated serum and dialytic parameters with any occurrence of CSBA during the 6-month study period, and association of laboratory and dialytic parameters with the occurrence of CSBA occurring from the start of one dialysis session to the beginning of the next dialysis session.
Statistical Analyses
Baseline demographics, dialysis parameters, and laboratory characteristics are presented as the mean±SD or median interquartile range (IQR) for continuous variables and percent (n/N) for categorical variables. Time-averaged serum electrolyte concentrations or dialysis prescription parameters are presented as the mean or median of all sessions, depending on normality. Characteristics between patients with and without at least one CSBA were compared using unpaired t tests or Wilcoxon rank sum tests for continuous variables and Fisher’s exact tests for categorical variables. Where three or more groups were assessed, ANOVA and Kruskal–Wallis tests were used for continuous variables and Fisher’s exact tests were used for categorical variables.
Unadjusted associations between serum chemistries and weight parameters and the occurrence of CSBA in the final 12 hours of the interdialytic interval were analyzed with mixed-effect models, accounting for repeated measures per patient. In secondary analyses, negative binomial mixed-effect regression was used to analyze associations of electrolytes, dialysis prescription, and interdialytic changes in fluid or electrolytes with CSBA rate as the incidence rate ratio (IRR) for the final 12 hours of the interdialytic interval and in a secondary analysis during the interval beginning at the end of each dialysis session through the next dialysis session—the same interval utilized for the primary analysis of the MiD study (5). The latter interval was chosen because causal associations between dialytic parameters and chemistries measured at the time of dialysis were felt to be unlikely to extend beyond the next dialysis session.
Given the limited sample size, a base-model was created incorporating age, sex, race, dialysis vintage, and vascular access. This model has been previously utilized in the primary and secondary outcome analyses on the basis of known associations with cardiovascular outcomes or mortality (5). All analyses were completed using SAS v9.4 (SAS Institute, Cary, NC), with P<0.05 considered significant.
Results
Baseline Characteristics and CSA
Overall, the study included 66 patients (4761 dialysis sessions): 15 with CSBA and 51 without CSBA (Table 1). In the analysis restricted to 12 hours before the start of dialysis, 62 patients and 4424 sessions were included. Most study participants were men (70%) with a median age of 58 years (IQR 49.2–66.1 years). The most common causes of ESKD were diabetes (42%) and hypertension (38%). Individuals with CSBA were older (67 years) than those without CSBA (57 years). The distribution of comorbidities was similar, although a numerically higher proportion of patients with CSBA had a history of heart failure (40%) or arrhythmia (40%) than patients without CSBA during follow-up (22% and 29%, respectively).
Table 1.
Baseline characteristics of the study population
| Characteristics | All Subjects (N=66) | No Clinically Significant Arrhythmia | Clinically Significant Arrhythmia |
|---|---|---|---|
| Brady/Asystole (N=51) | Brady/Asystole (N=15) | ||
| Age (yr) | 57.8 (49.2–66.1) | 57.2 (44.7–63.4) | 66.6 (54.2–69.6) |
| Age ≥70 yr | 12 (8/66) | 10 (5/51) | 20 (3/15) |
| Women | 30 (20/66) | 28 (14/51) | 40 (6/15) |
| Race | |||
| Asian | 35 (23/66) | 35 (18/51) | 33 (5/15) |
| Black | 53 (35/66) | 53 (27/51) | 53 (8/15) |
| Other | 2% (1/66) | 2 (1/51) | 0 (0/15) |
| White | 11 (7/66) | 10 (5/51) | 13 (2/15) |
| Hispanic ethnicity | 0 (0/66) | 0 (0/51) | 0 (0/15) |
| Cause of ESKD | |||
| Diabetes | 42 (28/66) | 43 (22/51) | 40 (6/15) |
| GN | 9 (6/66) | 8 (4/51) | 13 (2/15) |
| Hypertension | 38 (25/66) | 37 (19/51) | 40 (6/15) |
| Other | 11 (7/66) | 12 (6/51) | 7 (1/15) |
| ESKD vintage (yr) | 65 | 50 | 15 |
| 2.4 (1.2–5.3) | 2.5 (1.2–5.7) | 1.3 (1–3.9) | |
| Prior kidney transplant | 14 (9/66) | 14 (7/51) | 13 (2/15) |
| Previous peritoneal dialysis | 11 (7/66) | 14 (7/51) | 0 (0/15) |
| Vascular access | |||
| AV fistula | 69 (45/65) | 66 (33/50) | 80 (12/15) |
| AV graft | 26 (17/65) | 28 (14/50) | 20 (3/15) |
| Catheter | 5 (3/65) | 6 (3/50) | 0 (0/15) |
| Diabetes | 64 (42/66) | 63 (32/51) | 67 (10/15) |
| Diabetes duration (yr) | 37 | 30 | 7 |
| 17.9±12.8 | 17.6±13.6 | 19±9.3 | |
| Hyperlipidemia | 61 (40/66) | 61 (31/51) | 60 (9/15) |
| Hypertension | 85 (56/66) | 84 (43/51) | 87 (13/15) |
| Ischemic heart disease | 49 (32/66) | 47 (24/51) | 53 (8/15) |
| Myocardial infarction | 9 (6/66) | 6 (3/51) | 20 (3/15) |
| Congestive heart failure | 26 (17/66) | 22 (11/51) | 40 (6/15) |
| Coronary artery bypass surgery | 14 (9/66) | 12 (6/51) | 20 (3/15) |
| Arrhythmia | 32 (21/66) | 29 (15/51) | 40 (6/15) |
| Smoking | |||
| Current | 8 (5/66) | 10 (5/51) | 0 (0/15) |
| Never | 70 (46/66) | 71 (36/51) | 67 (10/15) |
| Past | 23 (15/66) | 20 (10/51) | 33 (5/15) |
| Weight (kg) | 66 | 51 | 15 |
| 81.7 (68.2–95.2) | 82 (67.3–94.5) | 81.3 (68.9–108.2) | |
| BMI | 66 | 51 | 15 |
| 27.2 (24.3–32.5) | 26.6 (23.7–32.5) | 27.6 (25.9–34.1) | |
| Systolic BP | 66 | 51 | 15 |
| 140.8±23.4 | 138.2±23.7 | 149.8±20.4 | |
| Diastolic BP | 66 | 51 | 15 |
| 80 (70–84) | 80 (66–83) | 80 (76–85) | |
| LVEF | 65 | 50 | 15 |
| 55 (55–60) | 55.3 (55–61) | 55 (55–60) | |
| Medication | |||
| β blocker | 58 (38/66) | 55 (28/51) | 67 (10/15) |
| Calcium channel blocker | 58 (38/66) | 59 (30/51) | 53 (8/15) |
| ACE or ARB | 33 (22/66) | 31 (16/51) | 40 (6/15) |
| Antilipidemic | 49 (32/66) | 43 (22/51) | 67 (10/15) |
| Calcium binder | 35 (23/66) | 37 (19/51) | 27 (4/15) |
Binary data are presented as % (n/N). Continuous data are presented as n with median (interquartile range) or median±SD. BMI, body mass index; AV, arteriovenous; LVEF, left ventricular ejection fraction; ACE, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker.
Incidence
As reported previously (5), there were 1461 CSA bradycardia events detected in 13 (20%) patients and 14 CSA asystole events detected in six (9%) patients, which occurred at a rate of 0.74 and 0.002 events per patient month, respectively. Of these, 551 CSBA occurred in the last 12 hours of the interdialytic interval preceding 100 of 4424 dialysis sessions. Events occurred in eight (12%) patients with an expected event rate per patient per month of three (confidence interval [CI], 0.7 to 12.9).
Time-Averaged Parameters and Bradycardia
In analyses including the entire duration of follow-up regardless of timing relative to dialysis, there was only modest evidence of an association between time-averaged dialytic parameters and the occurrence of CSBA (Table 2). The median time-averaged ultrafiltration rate (UFR) was significantly higher (9.8 ml/kg per hour; IQR 7.8–12.7) in individuals without than those with CSBA (8 ml/kg per hour; IQR 5.8–9.6; P=0.04). However, there were no significant differences in dialytic parameters such as dialysate temperature, dialysate potassium concentration, dialysate calcium, or dialysate bicarbonate between the two groups. Time-averaged serum chemistries, including serum potassium, calcium, and magnesium, were also similar in those with and without CSBA during follow-up.
Table 2.
Time-averaged characteristics by presence of clinically significant arrhythmia bradycardia or asystole during 6-month follow-up
| Characteristics | No Clinically Significant Arrhythmia | Clinically Significant Arrhythmia | P Value |
|---|---|---|---|
| Brady/Asystole (N=51) | Brady/Asystole (N=15) | ||
| Median (Median Interquartile Range) | Median (Median Interquartile Range) | ||
| Duration of dialysis (h) | 4 (3.8–4) | 4 (3.5–4) | 0.54 |
| Dry weight target (kg) | 80.5 (66–92) | 79 (65.5–107.5) | 0.64 |
| kg over dry weight target (kg) | 3 (2.6–3.7) | 3 (1.7–3.7) | 0.5 |
| Ultrafiltration rate (ml/kg per h) | 9.8 (7.8–12.7) | 8 (5.8–9.6) | 0.04 |
| Interdialytic weight change (kg) | 2.6 (2.1–3.1) | 2.1 (1.6–3.2) | 0.18 |
| Dialysate temperature (°C) | 37 (37–37) | 37 (37–37) | 0.97 |
| Dialysate potassium (mEq/L) | 2 (2–2) | 2 (2–2) | 0.9 |
| Dialysate calcium (mEq/L) | 2.5 (2.5–3.) | 2.5 (1.6–2.5) | 0.31 |
| Dialysate sodium (mEq/L)a | 140 (140–140) | 140 (138–140) | 0.42 |
| Laboratory values | |||
| BUN (mg/dl) | 52.0 (46.5–64.7) | 64.4 (53.4–67.9) | 0.2 |
| Creatinine (mg/dl) | 9.6 (7.3–12.5) | 8.1 (7.8–10.8) | 0.33 |
| Sodium (mEq/L) | 137 (134–139) | 139 (134–142) | 0.12 |
| Potassium (mEq/L) | 4.8 (4.4–5.1) | 4.7 (4.3–5.2) | 0.59 |
| Bicarbonate (mEq/L) | 22.6 (19.9–24.5) | 24.4 (19.6–25.4) | 0.6 |
| Calcium (mg/dl) | 8.6 (8.2–9.2) | 9 (8.4–9.4) | 0.36 |
| Corrected calcium (mg/dl) | 8.8 (8.4–9.2) | 9 (8.6–9.3) | 0.17 |
| Magnesium (mg/dl) | 2.3 (2–2.5) | 2.4 (2–2.7) | 0.99 |
| Phosphorous (mg/dl) | 5.1 (4.5–5.9) | 5.7 (3.9–6.8) | 0.46 |
| Hemoglobin (g/dl) | 10.7 (10.1–11.4) | 10.4 (9.7–11) | 0.33 |
| Albumin (g/dl) | 4.0 (3.7–4.2) | 3.9 (3.7–4.1) | 0.43 |
| Predialysis systolic BP (sitting) | 147 (134–151) | 150 (138–155) | 0.47 |
| Post-dialysis systolic BP (sitting) | 140 (120–150) | 140 (128–149) | 0.62 |
| Nadir intradialytic systolic BP | 120 (107–135) | 120 (110–130) | 0.98 |
Dialysate sodium. CSA brady/asystole N=46 (missing in five individuals). Corrected calcium (mg/dl)=[0.8×(normal albumin–Pt’s albumin)+serum Ca]; normal albumin=4 g/dl. Interdialytic weight change is the weight gain or loss from the end of one dialysis session to the beginning next consecutive one in kg.
Associations of Dialytic Parameters and Serum Chemistry with CSBA during the last 12 Hours of the Interdialytic Interval
In unadjusted analyses, (Table 3), there were no significant differences in the predialysis serum chemistries or dialytic parameters in sessions with versus without CSBA during the last 12 hours of the interdialytic period. Mean±SD predialysis serum potassium (4.7±0.7 versus 4.9±0.8), calcium (8.8±0.8 versus 8.7±0.9), magnesium (2±0.3 versus 2.4±0.5), and bicarbonate (24.7±3.1 versus 22.3±4) levels were similar in sessions with and without CSBA.
Table 3.
Laboratory and weight parameters in sessions with or without clinically significant bradycardia or asystole in the last 12 hours of the interdialytic interval
| Characteristics | Sessions without Clinically Significant Arrhythmia Brady/Asystole | Sessions with Clinically Significant Arrhythmia Brady/Asystole | P Valuea |
|---|---|---|---|
| Predialysis potassium (per mEq/L) | 1523 | 38 | 0.86 |
| 4.9±0.8 | 4.7±0.7 | ||
| Calcium predialysis (per mg/dl) | 1532 | 38 | 0.22 |
| 8.7±0.9 | 8.8±0.8 | ||
| Corrected calcium | 1532 | 38 | 0.86 |
| 8.8±0.8 | 8.9±0.8 | ||
| Sodium predialysis (per mEq/L) | 1532 | 38 | 0.23 |
| 136.9±4.1 | 139.3±2.8 | ||
| Bicarbonate predialysis (per mEq/L) | 1531 | 38 | 0.67 |
| 22.3±4 | 24.7±3.1 | ||
| Magnesium predialysis (mg/dl) | 1532 | 38 | 0.43 |
| 2.4±0.5 | 2±0.3 | ||
| Phosphorus predialysis (mg/dl) | 1530 | 38 | 0.92 |
| 5.4±1.7 | 4.5±1.2 | ||
| Interdialytic weight change (kg) | 4273 | 98 | 0.31 |
| 2.7±1.5 | 1.7±1.2 |
P values are from mixed-effect models, accounting for repeated measures per subject. Corrected calcium (mg/dl)=[0.8×(normal albumin–Pt’s albumin)+serum Ca]; normal albumin=4 g/dl. Interdialytic weight change is the weight gain or loss from the end of one dialysis session to the beginning next consecutive one in kg.
In crude count analyses before dialysis, calcium concentration was associated with a higher incidence of CSBA (IRR=1.38 per 1 mg/dl; 95% CI, 1 to 1.91; P=0.05), although the result was not significant for analyses using the albumin-corrected calcium level, whereas predialysis BUN levels were associated with a lower incidence rate of CSA (IRR=0.96 per 1 mg/dl; 95% CI, 0.94 to 0.99; P=0.007). There were no significant associations between the remaining serum chemistries as continuous variables (Table 4). Further, in models adjusting for age, race, sex, current vascular access, and dialysis vintage (Table 4), there were no significant associations between potassium, bicarbonate, phosphorous, or sodium concentrations and the incidence of CSBA, and the association of predialysis calcium concentration with CSA bradycardia was consistent with lower risk at higher serum calcium levels but was no longer significant (IRR=0.62; 95% CI, 0.36 to 1.08; P=0.09). A qualitatively similar association was seen for the albumin-corrected calcium level (P=0.02). In contrast, the pre-BUN concentration remained significantly associated with a lower incidence rate of CSBA in the preceding 12 hours (IRR=0.95; 95% CI, 0.93 to 0.98; P≤0.001).
Table 4.
Unadjusted and adjusted associations with the rate of clinically significant bradycardia/asystole in the 12 hours before dialysis
| Characteristic | Unadjusted Incidence Rate Ratios (95% Confidence Interval) | P Value | Adjusted Incidence Rate Ratios (95% Confidence Interval) | P Value |
|---|---|---|---|---|
| Interdialytic weight change (kg) | 0.66 (0.4 to 1.09) | 0.1 | 0.71 (0.46 to 1.07) | 0.1 |
| Predialysis BUN (mg/dl) | 0.96 (0.94 to 0.99) | 0.01 | 0.95 (0.93 to 0.98) | <0.001 |
| Predialysis calcium (per mg/dl) | 1.38 (1 to 1.91) | 0.05 | 0.62 (0.36 to 1.08) | 0.09 |
| Corrected calcium | 1.12 (0.79 to 1.59) | 0.54 | 0.47 (0.24 to 0.9) | 0.02 |
| Predialysis bicarbonate (per mg/dl) | 0.99 (0.81 to 1.2) | 0.89 | 0.85 (0.32 to 2.27) | 0.74 |
| Predialysis phosphorus (mg/dl) | 0.76 (0.38 to 1.5) | 0.42 | 0.57 (0.24 to 1.33) | 0.19 |
| Predialysis potassium (per mEq/L) | 1.59 (0.76 to 3.31) | 0.22 | 1.07 (0.49 to 2.35) | 0.86 |
| Predialysis sodium (per mEq/L) | 0.99 (0.85 to 1.16) | 0.89 | 0.92 (0.8 to 1.04) | 0.19 |
Each row represents a separate model with multivariate models adjusted for age, race, sex, current vascular access, and years on dialysis. The univariate model for predialysis magnesium concentration would not converge. Corrected calcium (mg/dl)=[0.8×(normal albumin–Pt’s albumin)+serum Ca]; normal albumin=4 g/dl. The univariate model for predialysis magnesium would not converge. In the adjusted model predialysis magnesium was not associated with CSBA rate (IRR=0.22, 95% CI, 0.01 to 7.98, P=0.41). Interdialytic weight change is the weight gain or loss from the end of one dialysis session to the beginning next consecutive one in kg.
Associations of Dialytic Parameters and Serum Chemistry with CSBA from Start of One Session to the Beginning of the Next
In unadjusted count analyses (Table 5), higher dialysate calcium (>2.5 versus 2.5) was associated with an increased risk of CSBA (IRR=2.38; 95% CI, 1.39 to 4.06; P=0.001). Conversely, a lower dialysate sodium concentration (≤135 versus 140 mEq/L) was associated with a lower risk (IRR=0.27, 95% CI: 0.10–0.73; P=0.01), and greater interdialytic weight change was associated with reduced risk of CSBA (IRR=0.77; 95% CI, 0.63 to 0.95; P=0.02). There were no signification associations between serum chemistries and CSBA.
Table 5.
Clinically significant bradycardia or asystole in 6 months: multiple regression models (clinically significant arrhythmia brady/asystole from one session to the next)
| Characteristic | Unadjusted Incidence Rate Ratios (95% Confidence Interval) | P Value | Adjusted Incidence Rate Ratios (95% Confidence Interval) | P Value |
|---|---|---|---|---|
| Dialysate potassium, mEq/L | 0.88 | 0.73 | ||
| 1 | NE | NE | 0.53 (0 to 80.94) | 0.8 |
| 2 | 1 (referent) | 1 (referent) | ||
| 3 | 0.81 (0.05 to 12.1) | 0.88 | 2.24 (0.09 to 53.54) | 0.62 |
| 4 | NE | NE | 0.13 (0 to 7.58) | 0.32 |
| Dialysate calcium, mEq/L | 0.001 | 0.13 | ||
| <2 | 8.91 (0.17 to 469.34) | 0.28 | NE | NE |
| 2–<2.5 | NE | NE | 0.09 (0.01 to 1.52) | 0.09 |
| 2.5 | 1 (referent) | 1 (referent) | ||
| >2.5 | 2.39 (1.4 to 4.08) | 0.001 | NE | NE |
| Dialysate sodium, mEq/L | <0.001 | <0.001 | ||
| ≤135 | 0.27 (0.1 to 0.73) | 0.01 | 0.22 (0.11 to 0.44) | <0.001 |
| >135–<140 | 0.09 (0 to 3.1) | 0.18 | 0.86 (0.04 to 19.74) | 0.92 |
| 140 | 1 (referent) | 1 (referent) | ||
| >140 | NE | NE | 0.18 (0.01 to 4.26) | 0.29 |
| Dialysate bicarbonate, mEq/L | 0.86 | 0.47 | ||
| ≤28 | 0.68 (0.07 to 6.97) | 0.74 | NE | NE |
| >28–<35 | 2.05 (0.22 to 18.74) | 0.53 | 2.68 (0.15 to 49.24) | 0.51 |
| 35 | 1 (referent) | 1 (referent) | ||
| >35 | 1.32 (0.21 to 8.42) | 0.77 | 2.71 (0.25 to 29.73) | 0.42 |
| Interdialytic weight change (≥median [≥2.5 kg]) | 0.48 (0.26 to 0.87) | 0.02 | 0.49 (0.29 to 0.83) | 0.01 |
| Intradialytic weight change (≥median [≥2.6 kg]) | 0.43 (0.21 to 0.9) | 0.03 | 0.55 (0.32 to 0.95) | 0.03 |
| Predialysis sitting systolic BP | 1.01 (1 to 1.03) | 0.15 | 1.02 (0.96 to 1.07) | 0.6 |
| Postdialysis sitting systolic BP | 1 (0.97 to 1.04) | 0.94 | 1 (0.98 to 1.01) | 0.76 |
| Nadir systolic BP | 1 (0.98 to 1.01) | 0.7 | 1 (0.97 to 1.04) | 0.83 |
| Laboratory results, serum | ||||
| Predialysis BUN (per mg/dl) | 0.99 (0.97 to 1.01) | 0.25 | 1 (0.98 to 1.03) | 0.73 |
| Predialysis calcium (per mg/dl) | 1.5 (0.79 to 2.87) | 0.21 | 0.96 (0.49 to 1.9) | 0.91 |
| Predialysis bicarbonate (per mEq/L) | 1.01 (0.9 to 1.12) | 0.88 | 1.02 (0.87 to 1.19) | 0.82 |
| Predialysis magnesium (per mg/dl) | 1.56 (0.48 to 5.07) | 0.46 | 0.07 (0 to 1.1) | 0.06 |
| Predialysis phosphorus (per mg/dl) | 1.04 (0.71 to 1.54) | 0.83 | 1.2 (0.66 to 2.19) | 0.54 |
| Predialysis potassium (per mEq/L) | 0.7 (0.4 to 1.23) | 0.21 | 0.97 (0.58 to 1.62) | 0.91 |
| Predialysis sodium (per mEq/L) | 1.04 (0.94 to 1.14) | 0.46 | 1.06 (0.91 to 1.21) | 0.44 |
Models include ge, race, sex, current vascular access, and years on dialysis. For dialysate potassium, there were 0 events in the 1 or 4 mEq/L groups. For DialysateCaCat1, there were 0 events in the 2–2.5 group. For dialysate calcium, there were no events in the 2–2.5 mEq/L group. There were no events in the dialysate sodium >140 group. The model for interdialytic weight change would not converge. NE, not estimable.
Discussion
To obtain a broader picture of the patient and dialytic characteristics associated with the occurrence of serious bradycardia and asystole events—the rhythms that appear to underlie the majority of SCD—we analyzed data from 66 hemodialysis patients enrolled in the MiD study. Although the highest risk period of CSBA is the 12 hours before HD, our analysis did not identify any significant differences in chemistries or dialytic parameters, including predialysis sodium, potassium, magnesium, calcium, or interdialytic weight change, between individuals with and without CSBA in this period, and in a multivariable model adjusting for key factors, only predialysis BUN was associated with the rate of CSA during the last 12 hours of the interdialytic period, with a decreased incidence rate in those with higher predialysis BUN. We additionally explored other intervals and similarly found no significant differences in time-averaged electrolytes and dialytic parameters between individuals with and without any CSBA during follow-up, with the exception of a higher UFR in those without CSBA during follow-up and associations of greater interdialytic weight gain and greater intradialytic weight loss with a reduced incidence rate of CSBA between the start of dialysis and the beginning of the next dialysis session. Associations of other chemistries and dialytic factors with the occurrence of CSBA from one session to the beginning of next were not significant, with the exception of a lower risk following sessions using dialysate sodium concentrations <135 (versus 140) mEq/L.
To our knowledge, this is the first study to examine the association of dialysate and serum potassium concentrations with the occurrence of serious bradycardia and asystole—arrhythmias that seem to underlie many instances of SCD in patients on maintenance HD. Notably, neither predialytic serum potassium nor dialysate potassium levels were associated CSBA in any of our analyses. Our findings are consistent with a recent international study including 55,183 patients on HD evaluating the association between serum and dialysate potassium and an arrhythmia composite (arrhythmia-related hospitalizations and sudden death). In that study, there was no association between dialysate potassium and the arrhythmia composite outcome in any of the serum potassium subgroups (<4 to >6 mEq/L). In addition, there was no effect modification of dialysate potassium by serum potassium for either all-cause mortality or the arrhythmia composite. Only serum potassium concentrations >6 mEq/L were associated with the arrhythmia composite, whereas a U-shaped association was found with serum potassium and all-cause mortality in adjusted analyses (14). In contrast, in another study including 830,471 paired dialysate and serum potassium values, a higher serum dialysate potassium gradient at the start of dialysis was associated with greater risk of all-cause hospitalizations and emergency department visits; however, no association was found with mortality (15). Similarly, in an analysis of 43,200 individuals dialyzed as outpatients within a single large dialysis organization, dialysate potassium concentrations <2 mEq/L were associated with an increased risk of sudden death. Most patients in our analysis were dialyzed against a dialysate potassium concentration ≥2 mEq/L (98% versus dialysate of 1 K=2%) and only a small minority of serum potassium values were >6 mEq/L (8%). Thus, one possible explanation of these discrepant findings is that neither serum nor dialysate potassium concentration is a trigger of CSBA within the generally nonextreme values seen in the MiD.
We did not find a consistent association of other electrolytes with CSBA. Although there was some evidence of an association between dialysate or serum calcium concentration and arrhythmia risk in unadjusted analyses, none of these were confirmed after adjustment for relevant confounders. For example, predialysis serum calcium, magnesium levels, and dialysate calcium were not independently associated with CSBA during the last 12 hours of the interdialytic interval, neither were time-averaged analyses looking at CSBA occurring at any time during follow-up, nor models looking at CSBA occurring from the start of one dialysis session to the beginning of the next. In aggregate, these analyses do not suggest that the risk of SCD is likely to be prevented entirely by interventions designed to modify excursions in serum calcium or magnesium or the choice of the dialysate calcium and magnesium baths.
Association of low calcium concentrations with the risk of QT prolongation, myocardial stunning, and risk of SCD have been previously reported in observational studies (16–18). For example, in a matched case-control study designed to identify modifiable risk factors for SCD, a dialysate calcium concentration of <2.5 mEq/L was significantly associated with risk of sudden cardiac arrest, despite similar serum calcium levels between cases and controls (19). Similarly, low magnesium levels have been associated with risk of ventricular arrhythmia and prolonged QT (20). As with the analysis of potassium, the absence of an association in our study and variation from prior findings could reflect the more specific end point examined: actual confirmed serious arrhythmia versus sudden death or hospitalization, which may not always reflect a primary arrhythmia. Sample size and limited variation in dialytic/electrolyte parameters may also have limited ability to detect biologically important associations, particularly as recent literature has raised concerns over use of low dialysate potassium or calcium baths and may have changed practice patterns, with avoidance of more extreme values. Another possibility is that other causes of arrhythmia and bradycardia such as cardiovascular autonomic dysfunction and myocardial stunning in this population might be contributing to the arrhythmia burden (21,22).
Interestingly, we found that a higher time-averaged UFR and, in other analyses, higher interdialytic or intradialytic weight change was associated with a lower risk of CSBA. The nature of this association is unclear and may be spurious, given multiple comparisons in our analysis. In particular, it is counterintuitive, given prior literature associating higher UFRs with increased risk of death and suggesting a need for extended and frequent dialysis when intradialytic gains are excessive (23). The association in our analysis may reflect the severity of the underlying cardiovascular comorbidities in the group with CSBA. Similarly, the association of lower dialysate sodium concentrations with CSBA risk of from the start of one HD session to the beginning of next might reflect patients at higher risk of CSBA requiring higher dialysate sodium concentration to tolerate ultrafiltration. These latter findings were not consistent among the various analyses. In aggregate, our observations raise the possibility of manipulating UFR and dialysate sodium in patients to prevent triggering arrhythmia while highlighting that the recommendations regarding avoidance of higher UFR or use of high sodium dialysate on the basis of observational studies need to be further evaluated in randomized controlled trials (23,24).
The high incidence of SCD in the hemodialysis population has led to an interest in better understanding the sequence of events and ambient physiologic parameters in individuals before an arrhythmic event, with a goal of moving toward individualizing the dialysis prescription to reduce the incidence of procedure-related or triggered adverse events. Our findings reinforce the idea that arrhythmia in individuals requiring maintenance dialysis is a multifactorial process. Several triggers may transpire in a sequential or concurrent manner in the existing environment of cardiac and neural remodeling for arrhythmia to ensue. Furthermore, the level of tolerance to electrolyte values outside the normal range or a shift in the concentration during dialysis likely differs in individual patients and depends on physiologic adaptation at a higher steady state and underlying comorbidities. There is a potential role for identifying and doing point-of-care testing in high-risk patients before each dialysis and adjusting the prescription accordingly—although this is not possible in the United States at this time because laboratory parameters are only checked in most outpatient centers once a month. However, the lack of an association of common serum and dialysate electrolyte concentrations with CSBA in our analysis raises concerns that this approach may not reduce sudden death risk, despite the intuitive appeal of this approach and support the need for larger, better-powered monitoring studies or randomized clinical trials before advocating for changing the current standard of care.
There are several limitations to consider. First, the sample size was modest, and our power to detect small effects of dialytic parameters and electrolyte concentrations on the occurrence of arrhythmia was limited. Second, the majority of patients in the study were on β blockers, which have strong nodal and conduction effects and might have attenuated associations of other factors with the occurrence of CSBA. There is a possibility of residual confounding accounting for the observed findings. Furthermore, although the various electrolytes and dialytic parameters are likely to influence each other rather than operating in isolation, we were unable to analyze their joint effects. Additionally, our data should be generalized cautiously and reflect risk in a cohort of patients willing to undergo research implantation of a loop recorder and with a high median dialysis vintage. Data on dialysate magnesium were not routinely collected, limiting the ability to analyze this critical parameter. The overall incidence of bradyarrhythmia and asystole was slightly higher in our study than in several other studies using ILRs in patients on dialysis (8). The higher incidence rate may have increased our power to detect associations but suggests that our results should be generalized cautiously, although the sample size of all studies was small, with a wide confidence interval around the estimates. Our analysis of associations of CSBA with volume status and electrolytes purposely focused on the final 12 hours before each dialysis treatment because we were able to impute the likely value of those parameters during this interval, during which the rate of CSBA peaked. However, CSBA during this period was frequently preceded by CSBA earlier in the interdialytic interval (data not shown). Additional analyses and protocols measuring interdialytic laboratory parameters could be informative. Lastly, we did not correct for multiple comparisons, given the post hoc/exploratory nature of this analysis. The possibility of type 2 error should be considered, and our few positive associations should be interpreted with appropriate caution in this context.
In summary, our study differs from prior studies that have focused primarily on sudden death, all-cause mortality, or hospitalization as outcomes instead of specific arrhythmic events. We did not confirm associations with the dialytic factors and electrolyte shifts previously suggested as the potential perpetrators of significant asystole or bradycardia. Our analysis highlights the need for research to understand these and other overlooked factors and suggests that in addition to the electrolyte and weight changes that occur in patients on maintenance dialysis, other modifiable factors are likely to be at play and require study in larger prospective studies to gain a more comprehensive view of the triggers for arrhythmia and SCD.
Disclosures
N. Bansal reports an advisory or leadership role for Kidney360 (associate editor). D.M. Charytan reports consultancy for Amgen, Allena Pharmaceuticals (DSMB), AstraZeneca, CSL Behring, Eli Lilly/Boehringer Ingelheim, Fresenius, Gilead, GSK, Janssen (steering committee), Medtronic, Merck, Novo Nordisk, PLC medical (clinical events committee), Renalytix, and Zogenix; research funding from Amgen, Bioporto (clinical trial support), Gilead, Medtronic (clinical trial support), and NovoNordisk; an advisory or leadership role for CJASN; and other interests or relationships as an expert witness (fees related to proton pump inhibitors). A.I. Costea reports consultancy for Biosense Webster and Biotronik; research funding from Biosense Webster and Biotronik; honoraria from Biosense Webster and Biotronik; an advisory or leadership role for the Journal of Cardiovascular Electrophysiology; and participation in a speakers’ bureau for Biosense Webster and Biotronik. S. Pokhariyal reports ownership interest in VitusCare Medlife PVT Ltd. P. Roy-Chaudhury reports consultancy for Akebia, Bayer, BD-Bard, Cormedix, Humacyte, InRegen, Medtronic/Covidien, Reata, Vifor-Relypsa, and WL Gore; ownership interest in Inovasc LLC (chief scientific officer and founder); research funding in the form of NIH small business grants as the MPI or site PI with Adgero, Cylerus, Eko, and Inovasc; honoraria from Akebia, Bayer, BD-Bard, Chugai Pharmaceuticals, Cormedix, Humacyte, InRegen, Medtronic/Covidien, Reata, Vifor-Relypsa, and WL Gore; an advisory or leadership role for Akebia, ASN, Bayer, BD-Bard, Cardiorenal Society, Cormedix, Humacyte, InRegen, Medtronic/Covidien, Reata, the Vascular Access Society of the Americas, Vifor-Relypsa, and WL Gore; being on the editorial board for the Journal of Vascular Access; and other interests or relationships with Elucid Bio, Outset Medical, the University of Arizona with Kidney Research Institute, Seattle (research contract), and Vasbio. J.A. Tumlin reports consultancy for Abbott Pharmaceuticals, Abbvie Pharmaceuticals, Achillion Pharmaceuticals, Alexion Pharmaceuticals, AstraZeneca Pharmaceuticals, Epizon Pharmaceuticals, Gilead Aurinia Pharmaceuticals, Johnson& Johnson, LaJolla Pharmaceuticals, Liliy Pharmaceuticals, Mallinckrodt Pharmaceuticals, NephroNet Clinical Trials Consortium, Relypsa Pharmaceuticals, and ZS Pharmaceuticals; research funding from Abbvie Pharmaceuticals, Achillion Pharmaceuticals, Akebia Pharmaceuticals, AstraZeneca, Epizon Pharmaceuticals, Gilead Pharmaceuticals, Johnson& Johnson, LaJolla Pharmaceuticals, Mallinckrodt Pharmaceuticals, NephroNet Clinical Trials Consortium, Relypsa Pharmaceuticals, Vinterra Pharmaceuticals, and ZS Pharmaceuticals; honoraria from Alexion Pharmaceuticals, AstraZeneca Pharmaceuticals, Aurinia Pharmaceuticals, Bayer Pharmaceuticals, Genentech, Genzyme Corp., and Mallinckrodt Pharmaceuticals; patents or royalties from HIBAR Microsciences LLC; an advisory or leadership role for Achillion Pharmaceuticals, Alexion Pharmaceuticals, Bayer Corp., Bayer Pharmaceuticals, Chemocentryx, Epizon Pharmaceuticals, Gilead Pharmaceuticals, KBP Pharmaceuticals, and Relypsa Pharmaceuticals; participation in a speakers’ bureau for Alexion Pharmaceuticals, AstraZeneca Pharmaceuticals, Aurinia Pharmaceuticals, Bayer Corp., Bayer Pharmaceuticals, LaJolla Pharmaceuticals, and Mallinckrodt; and other interests or relationships with NephroNet, Inc. (CEO and board member). D.E. Williamson reports consultancy for American Renal Associates; ownership interest in American Renal Associates; research funding from Medtronic, Inc.; and an advisory or leadership role for American Renal Associates, CloudCath, and the New England NKF Medical Advisory Board. W.C. Winkelmayer reports consultancy for Akebia, AstraZeneca, Bayer, Boehringer Ingelheim, GlaxoSmithKline, Eli Lilly, Merck, Otsuka, Pharmacosmos, and Reata; honoraria from Akebia, AstraZeneca, Bayer, Boehringer Ingelheim, GlaxoSmithKline, Eli Lilly, Merck, Otsuka, Pharmacosmos, and Reata; and an advisory or leadership role for the American Journal of Kidney Diseases, the American Journal of Nephrology, CJASN, the Journal of the American Medical Association (associate editor; with stipend), Kidney Disease: Improving Global Outcomes (KDIGO; co-chair), and Seminars in Dialysis (editorial boards). All remaining authors have nothing to disclose.
Funding
The MiD study was sponsored by Medtronic. All of the authors with the exception of Drs. Soomro and Bansal received research support and/or consulting fees from Medtronic in relationship to the design and execution of the study.
Author Contributions
N. Bansal, D.M. Charytan, A.I. Costea, V. Kher, B.A. Koplan, S. Pokhariyal, P. Roy-Chaudhury, Q.H. Soomro, J.A. Tumlin, and D.E. Williamson were responsible for the methodology; N. Bansal, D.M. Charytan, V. Kher, S. Pokhariyal, P. Roy-Chaudhury, J.A. Tumlin, D.E. Williamson, and W.C. Winkelmayer were responsible for supervision; D.M. Charytan, B.A. Koplan, P. Roy-Chaudhury, Q.H. Soomro, and W.C. Winkelmayer were responsible for the conceptualization; D.M. Charytan and C.K. McClure were responsible for the formal analysis; A.I. Costea and B.A. Koplan were responsible for data curation; Q.H. Soomro wrote the original draft of the manuscript; and all authors reviewed and edited the manuscript.
Data Sharing Statement
All data are included in the manuscript and/or supporting information.
Supplemental Material
This article contains the following supplemental material online at http://kidney360.asnjournals.org/lookup/suppl/doi:10.34067/KID.0003142022/-/DCSupplemental.
Monitoring in Dialysis investigators and committees. Download Supplemental Summary 1, PDF file, 95 KB (99.1KB, pdf) .
References
- 1.United States Renal Data System : USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2020 [Google Scholar]
- 2.Turakhia MP, Blankestijn PJ, Carrero JJ, Clase CM, Deo R, Herzog CA, Kasner SE, Passman RS, Pecoits-Filho R, Reinecke H, Shroff GR, Zareba W, Cheung M, Wheeler DC, Winkelmayer WC, Wanner C; Conference Participants : Chronic kidney disease and arrhythmias: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Eur Heart J 39: 2314–2325, 2018. 10.1093/eurheartj/ehy060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Makar MS, Pun PH: Sudden cardiac death among hemodialysis patients. Am J Kidney Dis 69: 684–695, 2017. 10.1053/j.ajkd.2016.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tereshchenko LG, Posnack NG: Does plastic chemical exposure contribute to sudden death of patients on dialysis? Heart Rhythm 16: 312–317, 2019. 10.1016/j.hrthm.2018.08.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roy-Chaudhury P, Tumlin JA, Koplan BA, Costea AI, Kher V, Williamson D, Pokhariyal S, Charytan DM; MiD Investigators and Committees : Primary outcomes of the Monitoring in Dialysis Study indicate that clinically significant arrhythmias are common in hemodialysis patients and related to dialytic cycle. Kidney Int 93: 941–951, 2018. 10.1016/j.kint.2017.11.019 [DOI] [PubMed] [Google Scholar]
- 6.Sacher F, Jesel L, Borni-Duval C, De Precigout V, Lavainne F, Bourdenx JP, Haddj-Elmrabet A, Seigneuric B, Keller A, Ott J, Savel H, Delmas Y, Bazin-Kara D, Klotz N, Ploux S, Buffler S, Ritter P, Rondeau V, Bordachar P, Martin C, Deplagne A, Reuter S, Haissaguerre M, Gourraud JB, Vigneau C, Mabo P, Maury P, Hannedouche T, Benard A, Combe C: Cardiac rhythm disturbances in hemodialysis patients: Early detection using an implantable loop recorder and correlation with biological and dialysis parameters. JACC Clin Electrophysiol 4: 397–408, 2018. 10.1016/j.jacep.2017.08.002 [DOI] [PubMed] [Google Scholar]
- 7.Roberts PR, Zachariah D, Morgan JM, Yue AM, Greenwood EF, Phillips PC, Kalra PA, Green D, Lewis RJ, Kalra PR: Monitoring of arrhythmia and sudden death in a hemodialysis population: The CRASH-ILR Study. PLoS One 12: e0188713, 2017. 10.1371/journal.pone.0188713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roberts PR, Stromberg K, Johnson LC, Wiles BM, Mavrakanas TA, Charytan DM: A systematic review of the incidence of arrhythmias in hemodialysis patients undergoing long-term monitoring with implantable loop recorders. Kidney Int Rep 6: 56–65, 2020. 10.1016/j.ekir.2020.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rautavaara J, Kerola T, Kaartinen K, Vilpakka M, Aitkoski A, Anttonen O, Ahvonen J, Koistinen J, Vääräniemi K, Miettinen M, Ylitalo A, Laine K, Ojanen S, Nieminen T. Asystole episodes and bradycardia in patients with end-stage renal disease. Nephrol Dial Transplant 37: 575–583, 10.1093/ndt/gfab023 [DOI] [PubMed] [Google Scholar]
- 10.Jadoul M, Thumma J, Fuller DS, Tentori F, Li Y, Morgenstern H, Mendelssohn D, Tomo T, Ethier J, Port F, Robinson BM: Modifiable practices associated with sudden death among hemodialysis patients in the Dialysis Outcomes and Practice Patterns Study. Clin J Am Soc Nephrol 7: 765–774, 2012. 10.2215/CJN.08850811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Charytan DM, Foley R, McCullough PA, Rogers JD, Zimetbaum P, Herzog CA, Tumlin JA; MiD Investigators and Committees : Arrhythmia and sudden death in hemodialysis patients: Protocol and baseline characteristics of the Monitoring in Dialysis Study. Clin J Am Soc Nephrol 11: 721–734, 2016. 10.2215/CJN.09350915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Echt DS, Liebson PR, Mitchell LB, Peters RW, Obias-Manno D, Barker AH, Arensberg D, Baker A, Friedman L, Greene HL, Huther ML, Richardson DW; CAST Investigators : Mortality and morbidity in patients receiving encainide, flecainide, or placebo—The Cardiac Arrhythmia Suppression Trial. N Engl J Med 324: 781–788, 1991. 10.1056/NEJM199103213241201 [DOI] [PubMed] [Google Scholar]
- 13.Tumlin JA, Roy-Chaudhury P, Koplan BA, Costea AI, Kher V, Williamson D, Pokhariyal S, Charytan DM; MiD investigators and Committees : Relationship between dialytic parameters and reviewer confirmed arrhythmias in hemodialysis patients in the monitoring in dialysis study. BMC Nephrol 20: 80, 2019. 10.1186/s12882-019-1212-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karaboyas A, Zee J, Brunelli SM, Usvyat LA, Weiner DE, Maddux FW, Nissenson AR, Jadoul M, Locatelli F, Winkelmayer WC, Port FK, Robinson BM, Tentori F: Dialysate potassium, serum potassium, mortality, and arrhythmia events in hemodialysis: Results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis 69: 266–277, 2017. 10.1053/j.ajkd.2016.09.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brunelli SM, Spiegel DM, Du Mond C, Oestreicher N, Winkelmayer WC, Kovesdy CP: Serum-to-dialysate potassium gradient and its association with short-term outcomes in hemodialysis patients. Nephrol Dial Transplant 33: 1207–1214, 2018. 10.1093/ndt/gfx241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Näppi SE, Virtanen VK, Saha HH, Mustonen JT, Pasternack AI: QTc dispersion increases during hemodialysis with low-calcium dialysate. Kidney Int 57: 2117–2122, 2000. 10.1046/j.1523-1755.2000.00062.x [DOI] [PubMed] [Google Scholar]
- 17.Brunelli SM, Sibbel S, Do TP, Cooper K, Bradbury BD: Facility dialysate calcium practices and clinical outcomes among patients receiving hemodialysis: A retrospective observational study. Am J Kidney Dis 66: 655–665, 2015. 10.1053/j.ajkd.2015.03.038 [DOI] [PubMed] [Google Scholar]
- 18.McGill RL, Weiner DE: Dialysate composition for hemodialysis: Changes and changing risk. Semin Dial 30: 112–120, 2017. 10.1111/sdi.12573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pun PH, Lehrich RW, Honeycutt EF, Herzog CA, Middleton JP: Modifiable risk factors associated with sudden cardiac arrest within hemodialysis clinics. Kidney Int 79: 218–227, 2011. 10.1038/ki.2010.315 [DOI] [PubMed] [Google Scholar]
- 20.Millane TA, Ward DE, Camm AJ: Is hypomagnesemia arrhythmogenic? Clin Cardiol 15: 103–108, 1992. 10.1002/clc.4960150210 [DOI] [PubMed] [Google Scholar]
- 21.Rogovoy NM, Howell SJ, Lee TL, Hamilton C, Perez-Alday EA, Kabir MM, Zhang Y, Kim ED, Fitzpatrick J, Monroy-Trujillo JM, Estrella MM, Sozio SM, Jaar BG, Parekh RS, Tereshchenko LG: Hemodialysis procedure-associated autonomic imbalance and cardiac arrhythmias: Insights from continuous 14-day ECG monitoring. J Am Heart Assoc 8: e013748, 2019. 10.1161/JAHA.119.013748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McIntyre CW: Effects of hemodialysis on cardiac function. Kidney Int 76: 371–375, 2009. 10.1038/ki.2009.207 [DOI] [PubMed] [Google Scholar]
- 23.Flythe JE, Kimmel SE, Brunelli SM: Rapid fluid removal during dialysis is associated with cardiovascular morbidity and mortality. Kidney Int 79: 250–257, 2011. 10.1038/ki.2010.383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Movilli E, Gaggia P, Zubani R, Camerini C, Vizzardi V, Parrinello G, Savoldi S, Fischer MS, Londrino F, Cancarini G: Association between high ultrafiltration rates and mortality in uraemic patients on regular haemodialysis. A 5-year prospective observational multicentre study. Nephrol Dial Transplant 22: 3547–3552, 2007. 10.1093/ndt/gfm466 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Monitoring in Dialysis investigators and committees. Download Supplemental Summary 1, PDF file, 95 KB (99.1KB, pdf) .