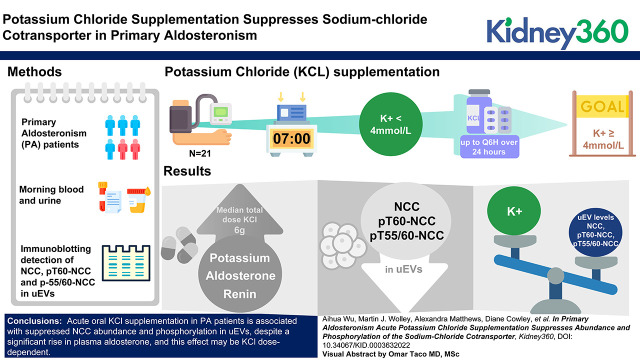
Key Points
Potassium chloride intake induced a reduction in sodium-chloride cotransporter (NCC) and phosphorylated NCC in urinary extracellular vesicles from patients with primary aldosteronism during a significantly raised level of endogenous aldosterone.
Low plasma potassium (secondary to aldosterone excess) may dominate in terms of NCC regulation in the setting of primary aldosteronism.
Keywords: hypertension, blood pressure, NCC, potassium, primary aldosteronism, urinary extracellular vesicles
Visual Abstract
Abstract
Background
Elevated abundance of sodium-chloride cotransporter (NCC) and phosphorylated NCC (pNCC) are potential markers of primary aldosteronism (PA), but these effects may be driven by hypokalemia.
Methods
We measured plasma potassium in patients with PA. If potassium was <4.0 mmol/L, patients were given sufficient oral potassium chloride (KCl) over 24 hours to achieve as close to 4.0 mmol/L as possible. Clinical chemistries were assessed, and urinary extracellular vesicles (uEVs) were examined to investigate effects on NCC.
Results
Among 21 patients with PA who received a median total dose of 6.0 g (2.4–16.8 g) of KCl, increases were observed in plasma potassium (from 3.4 to 4.0 mmol/L; P<0.001), aldosterone (from 305 to 558 pmol/L; P=0.01), and renin (from 1.2 to 2.5 mIU/L; P<0.001), whereas decreases were detected in uEV levels of NCC (median fold change(post/basal) [FC]=0.71 [0.09–1.99]; P=0.02), pT60-NCC (FC=0.84 [0.06–1.66]; P=0.05), and pT55/60-NCC (FC=0.67 [0.08–2.42]; P=0.02). By contrast, in 10 patients with PA who did not receive KCl, there were no apparent changes in plasma potassium, NCC abundance, and phosphorylation status, but increases were observed in plasma aldosterone (from 178 to 418 pmol/L; P=0.006) and renin (from 2.0 to 3.0 mU/L; P=0.009). Plasma potassium correlated inversely with uEV levels of NCC (R2=0.11; P=0.01), pT60-NCC (R2=0.11; P=0.01), and pT55/60-NCC (R2=0.11; P=0.01).
Conclusions
Acute oral KCl loading replenished plasma potassium in patients with PA and suppressed NCC abundance and phosphorylation, despite a significant rise in plasma aldosterone. This supports the view that potassium supplementation in humans with PA overrides the aldosterone stimulatory effect on NCC. The increased plasma aldosterone in patients with PA without KCl supplementation may be due to aldosterone response to posture challenge.
Introduction
Primary aldosteronism (PA) is a common and potentially curable form of hypertension. Hypokalemia was considered as a mandatory feature of PA until the more recent recognition of the high prevalence of PA among normokalemic hypertensives (1–4). The thiazide-sensitive sodium-chloride (Cl–) cotransporter (NCC) is the major transporter on the apical surface of epithelial cells for electroneutral Na+/Cl– reabsorption in the early distal convoluted tubule (DCT). Aldosterone and its analogs were thought to be major regulators of NCC through the mineralocorticoid receptor (5–9), but whether these effects in humans are primarily driven by mineralocorticoid-induced hypokalemia is unclear.
Urinary extracellular vesicles (uEVs) are frequently used as a noninvasive source of renal biomarker discovery (10, 11) and are a reliable tool to monitor specific physiologic responses and disease mechanisms (12). In humans, PA is associated with increased abundance of phosphorylated NCC (pNCC) in uEVs (13). Examination of uEVs from patients with PA undergoing 4 days co-administration of fludrocortisone acetate and oral NaCl loading, with oral potassium chloride (KCl) supplements to correct or prevent hypokalemia during testing, provided evidence that NCC is mineralocorticoid sensitive (14). However, recent studies demonstrated potassium (K+) supplementation reduced the degree of upregulation of NCC abundance induced by mineralocorticoids in the murine kidney and in uEVs from patients with PA (14–16), suggesting that higher plasma K+ induced by higher KCl intake may counterbalance mineralocorticoid-induced NCC upregulation.
In the Hypertension Unit of Princess Alexandra Hospital, Brisbane, Australia, before undergoing seated saline suppression testing (SSST) as a means of confirming or excluding PA, patients whose plasma K+ are <4.0 mmol/L are given oral potassium supplements (in the form of KCl) in variable amounts to achieve or maintain normokalaemia during SSST the next day. By taking advantage of the clinical SSST protocol, the current study aimed to utilize uEVs to address the hypothesis that oral KCl supplementation in patients with PA with elevated endogenous aldosterone will suppress NCC abundance and phosphorylation. Concomitantly, we examined the renal outer medullary potassium channel (ROMK) to assess whether altering plasma K+ in a setting of aldosterone excess affected K+ excretion through ROMK.
Materials and Methods
Ethical Issues
The clinical procedures of posture responsiveness testing and seated saline testing were performed in the Hypertension Unit of the Princes Alexandra Hospital, Brisbane, Australia. The laboratory investigations were performed in the Endocrine Hypertension Research Centre, The University of Queensland Diamantina Institute, Brisbane, Australia. Ethical approval was granted by the Metro South Human Research Ethics Committee (HREC/18/QPAH/103).
Recruitment
Hypertensive patients with raised plasma aldosterone-to-renin ratios (ARRs) who were admitted for SSST were invited to participate and provided informed written consent. A total of 38 (24 women/14 men) patients were invited, and all agreed to participate. All patients completed the KCl replacement experiment before SSST.
KCl Replacement
At least 4 weeks before admission, medications affecting plasma aldosterone and renin levels were withdrawn and replaced by other antihypertensive medications (e.g., verapamil, prazosin, doxazosin, moxonidine, and/or hydralazine). Patients were admitted to hospital to ensure the dietary (normal hospital diet) and posture requirements were met and to facilitate monitoring of plasma K+ levels and other parameters. Detailed clinical routine procedures and sampling time points for the current study are listed in Table 1. In brief, before SSST, patients commenced a 24-hour urine collection at home in the morning of the day of admission. The next day (day 0), aldosterone responsiveness to upright posture was determined by measuring plasma aldosterone at 7:00am after overnight recumbency and again at 10:00am after 3 hours of upright posture. On the day of SSST (day 1), an infusion of 2 L 0.9% saline over 4 hours was commenced at 8:00am, at least 2 hours after rising from bed and at least 30 minutes after assuming a seated position.
Table 1.
Detailed clinical procedures and sampling time point for hypertensive patients with raised plasma ARRs who were admitted for SSST
| Time Point | Admission Day | Day of Posture Responsiveness Testing (Day 0) | Day of SSST (Day 1) |
|---|---|---|---|
| 6:00am | 1. Urine for uEVs was collected after getting up (post-test spot urine and uEV for the current study) | ||
| 7:00am | Home 24-hour urine test started | 1. Home 24-hour urine test ended 2. Blood was collected after overnight recumbency (results were adopted as baseline blood measurements for the current study) 3. Urine for uEVs was collected after bleeding (baseline spot urine and uEV for the current study) |
2. Before SSST, blood was collected 30 minutes after assuming a seated posture (results were adopted as post-test blood measurements for the current study) 3. SSST commenced at 8:00am |
| 10:00am | 4. Blood results at 7:00am were available For the current study, if plasma [K+] <4.0 mmol/L, participants were given sufficient oral KCl up to Q6h to achieve as close to 4.0 mmol/Las possible by the next morning at 7:00am 5. Blood was collected after 3 hours of upright posture |
||
| 12:00pm | SSST completed | ||
| 1:00pm | Participants discharged | ||
| 3:00pm | Patients were invited and consented to participate in the study |
At least 4 weeks before admission, medication affected plasma aldosterone or renin levels was replaced by other antihypertension drugs, e.g., verapamil, prazosin, moxonidine, and/or hydralazine. Patients were admitted to hospital to ensure the dietary (hospital normal diet) and posture requirements were met and to facilitate measurement of plasma K+ levels and other parameters. Oral KCl supplementation: if participants’ plasma K+ level at 7:00am on day 0 was <4 mmol/L, participants were given sufficient slow-release KCl (Span-K) up to four times per day (Q6h) to achieve as close to 4 mmol/L as possible by the next morning at 7:00am. ARR, aldosterone-to-renin ratio; SSST, seated saline suppression testing; uEV, urinary extracellular vesicles; KCl, potassium chloride, given as slow-released KCl (Span-K).
The baseline (7:00am) plasma K+ results were available at 10:00am on day 0. If plasma K+ concentration at 7:00am was <4.0 mmol/L, participants were given sufficient slow-release KCl (Span-K) up to four times a day (Q6H) in an attempt to achieve as close to 4 mmol/L as possible by the next morning (day 1) before SSST.
For the current study, blood collected at 7:00am during recumbent posture on day 0 were adopted as the baseline blood measurement (basal), and blood collected on day 1 just before commencement of SSST at 8:00am during seated posture was adopted as the post-test blood measurement.
Urine Collection and uEV Isolation
Two sterilized 200-ml containers were given to the 38 participants for urine collection. Urine collection time points are listed in Table 1. Briefly, 25–200 ml of midstream morning urine was collected after baseline blood collection and before KCl supplementation on day 0 (baseline urine), and at 6:00–7:00am on day 1 before commencement of SSST (post-test urine). On day 0, 26 participants who were unable to produce enough urine volume (≥25 ml) at 7:00am collected urine between 7:00am and 10:00am but before KCl replacement commenced; two participants (patients 26 and 34) were on low-dose (1.2 g) KCl supplementation before baseline urine collection. Collected urine samples were immediately treated with protease inhibitor cocktail (Roche cOmplete, EDTA-free; Roche, Basel, Switzerland; 1 tablet per 50 ml urine) before aliquoting and freezing at –80°C. uEVs were isolated using progressive ultracentrifugation techniques with dithiothreitol (DTT) treatment as previously described (14). Obtained uEV pellets were re-suspended in 70–110 μl 1× PBS containing 0.1% v/v SDS, followed by on-ice sonicating with approximately 5–10 cycles at 50% power (10 seconds on/off on Bioruptor Pico; Diagenode, Denville, NJ) and centrifugation for 10 minutes at 17,000 g to pellet insoluble residues. Total protein concentration of the obtained supernatant was measured by spectrophotometer (NanoDrop Lite; Thermo Fisher Scientific, Waltham, MA).
Sample Measurements
Measurements of blood and 24-hour urine were performed by Pathology Queensland Laboratory at Princess Alexandra Hospital immediately after collection was completed. Plasma aldosterone was determined by liquid chromatography with tandem mass spectrometry (17), direct renin concentration was determined by chemiluminescent immunoassay (18), and plasma cortisol was determined by immunoassay. Spot urine creatinine concentration was measured using a creatinine urinary detection assay kit (EIACUN; Invitrogen, Waltham, MA) at the Endocrine Hypertension Research Centre. Spot urinary Na+ and K+ were measured by Pathology Queensland Chemistry Department.
Immunoblotting
uEVs were treated with 5× Laemmli buffer (1/4, v/v) and incubated at 60°C for 10 minutes before SDS-PAGE. Ten micrograms of each sample were loaded and separated on 4%–15% Criterion TGX Precast Midi Protein gels (5671083 or 56710985 depending on loading volume; Bio-Rad, Hercules, CA) and were transferred to Turbo Polyvinyl Difluoride Midi Membrane (1704157; Bio-Rad) under 2.5 A and 25 V for 7 minutes on the Bio-Rad Turbo transferring system. Each blot was duplicated for proteins with similar size. Blots were then blocked followed by overnight 4°C incubation with the following antibodies: rabbit anti-NCC (1/1000; AB3553; Merck Millipore, Billerica, MA), rabbit anti-T55/60pNCC (1/1000) (19), rabbit anti-T60pNCC (1/2500) (19), rabbit anti-ROMK (1/500) (20), rabbit anti-ALG-2-interacting protein X (ALIX; 1/2000; ABC40; Merck Millipore), rabbit anti-tumor susceptibility gene 101 (TSG101; 1/2000; MASBC649; Merck Millipore), and rabbit anti-tetraspanin CD9 (CD9; 1/1000; ab92726; Abcam, Cambridge, United Kingdom). NCC and pNCCs were measured by the dominant band between 100 and 150 kDa. ROMK was measured by a complex glycosylated band detected between 50 and 55 kDa, core-glycosylated band at 40 kDa, and unglycosylated band at 37 kDa (20). uEV marker proteins ALIX, TSG101, and CD9 were measured by the dominant bands at 96, 45, and 25 kDa, respectively. Horseradish peroxidase conjugated goat antirabbit IgG antibody (12–348; Merck Millipore) was used as the secondary antibody at 1/20,000 and 1/500 dilutions, respectively, for luminol-based enhanced chemiluminescence (1705061; Bio-Rad) before exposure in configuring signal accumulation mode by Bio-Rad ChemiDoc XRS+ Imager with Image Lab software. Images that did not exceed saturation were exported for analyses.
Analyses Inclusion Criteria
Due to the different physiologic states, only patients with confirmed PA were included in all analyses. Immunoblotting detection of at least two EV-enriched proteins (ALIX, TSG101, or CD9) in each sample was considered successful isolation of uEVs, and patients in whom uEVs were successfully isolated from both basal and post-test samples were included in the comparison analyses. Samples included in uEV protein analyses are listed in Supplemental Table 1.
Statistical Analyses
Calculations were processed with R (The R Foundation for Statistical Computing, Vienna, Austria). Due to blood at baseline being hemolyzed, patient 10 was excluded from the comparisons of blood biochemical parameters. Therefore, comparisons of blood biochemical parameters were performed in a total of 37 patients. Because the volume of spot urine collected from patients 15, 18, 29, 31, and 35 remained inadequate after uEV isolation, measurements of creatinine, Na+, and K+, the comparisons of spot urinary electrolytes-to-creatinine ratios were performed in a total of 32 patients. Paired Wilcoxon tests were performed to compare the differences of biochemical parameters in blood samples collected at 7:00am on day 0 and 8:00am on day 1 and in spot urine electrolytes-to-creatinine (Na+/creatinine and K+/creatinine) at basal and post test. In immunoblotting analyses, absolute abundances of analyzed proteins were analyzed with ImageJ (National Institutes of Health, Bethesda, MD). To minimize errors due to alterations in actual uEV biogenesis or excretion rates, relative protein abundances (derived by dividing the absolute abundances of proteins of interest by the sum abundance of ALIX, TSG101 and CD9 in each patient’s sample) were used for comparisons. Wilcoxon tests were performed to assess baseline differences in analyzed proteins. Paired t tests were performed to assess the changes in the relative abundances of analyzed proteins (log10 transformed). Pearson’s correlations were sought between log10 transformed relative abundances of analyzed proteins and biochemical parameters. If baseline urines for uEV were collected after 10:00am, 10:00am biochemical results were used. P<0.05 was considered statistically significant. Data are presented as median (range) unless stated otherwise.
Results
Participants’ Characteristics
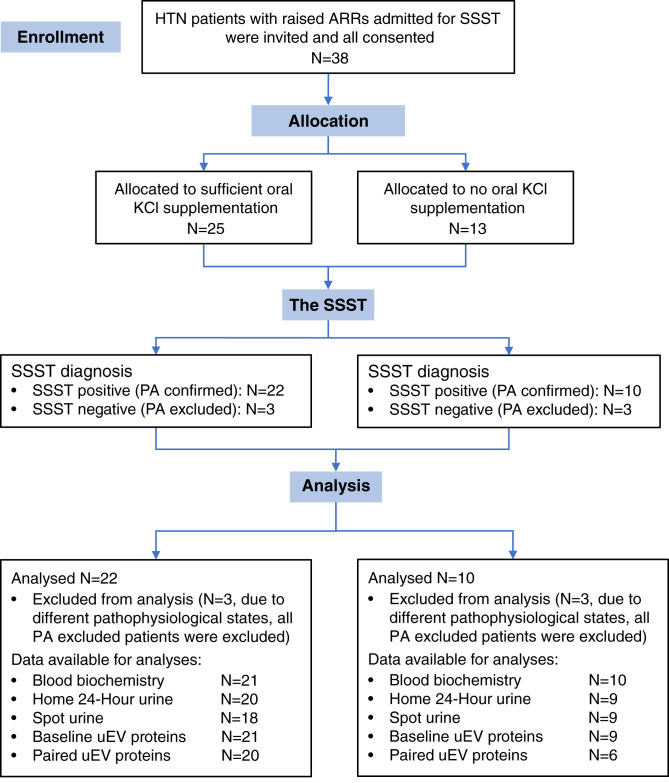
A total of 38 (24 women and 14 men) hypertensive patients with repeatedly raised ARRs were recruited, and all agreed to participate (Table 2). SSST was positive in 32, thereby confirming the diagnosis of PA, and negative in six, in whom PA was excluded (designated low renin hypertension [LRH]). Due to the different physiologic states, only patients with confirmed PA were included in the analyses. The report numbers of participants at each stage of study are depicted in Figure 1. Among the 32 (19 women/12 men) patients with PA, the median age was 45 (25–70) years old, with a median body mass index of 30.3 (20.8–47.5) kg/m2, median blood pressure of 146 (115–174)/89 (68–110) mm Hg, and were on a median of 2 (0–4) antihypertensive medications without affecting plasma aldosterone and renin levels.
Table 2.
Detailed participants’ clinical features
| Patient No. | Sex | Age (yr) | BMI (kg/m2) | SBP/ DBP (mm Hg) | eGFRa | Plasma [Creatinine] (μmol/L)a | Plasma [K+] (mmol/L)a | No. of Anti- HTN Drugs | Anti-HTN Drugs before SSST (per Day) | Total Dose of Span-K (g) before SSST (per Day) | Response to Upright Posture | SSST Dx | Included in Analyses | Group | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Moxo (μg) | Prazo (mg) | Vera (mg) | Hydra (mg) | ||||||||||||||
| 1 | F | 54 | 27.3 | 164/92 | >90 | 61 | 3.0 | 2 | — | — | 180 | 25 | 12 | U | PA | Included | KCl |
| 2 | F | 64 | 47.1 | 170/82 | 82 | 68 | 3.0 | 3 | 600 | — | 240 | 100 | 14.4 | R | PA | Included | KCl |
| 3 | M | 25 | 25.9 | 145/95 | >90 | 75 | 2.8 | 2 | — | — | 240 | 25 | 16.8 | U | PA | Included | KCl |
| 4 | M | 29 | 47.3 | 174/110 | >90 | 66 | 3.1 | 2 | — | — | 480 | 100 | 10.8 | R | PA | Included | KCl |
| 5 | M | 43 | 25.9 | 140/95 | >90 | 69 | 3.3 | 3 | 200 | — | 240 | 25 | 10.8 | R | PA | Included | KCl |
| 6 | F | 70 | 39 | 154/69 | 77 | 69 | 3.4 | 4 | 600 | 4 | 60 | 100 | 6 | R | PA | Included | KCl |
| 7 | F | 54 | 31.1 | 136/96 | >90 | 59 | 3.5 | 2 | — | — | 240 | 50 | 6 | R | PA | Included | KCl |
| 8 | F | 52 | 25.6 | 150/80 | >90 | 55 | 3.9 | 0 | — | — | — | — | 0 | U | PA | Included | Non-KCl |
| 9 | F | 59 | 43.3 | 155/84 | >90 | 62 | 3.8 | 4 | 600 | 1.5 | 240 | 100 | 2.4 | U | PA | Included | KCl |
| 10 | F | 39 | 25.6 | 115/78 | 84 | 77 | 3.8b | 0 | — | — | — | — | 3.6 | — | PA | Included | KCl |
| 11 | F | 45 | 38.1 | 164/71 | >90 | 57 | 3.9 | 2 | 400 | — | 360 | — | 3.6 | R | PA | Included | KCl |
| 12 | F | 43 | 20.8 | 166/88 | >90 | 56 | 2.9 | 2 | — | — | 480 | 50 | 6 | U | PA | Included | KCl |
| 13 | F | 32 | 22.4 | 140/80 | >90 | 53 | 2.9 | 1 | — | — | 180 | — | 13.2 | U | PA | Included | KCl |
| 14 | M | 39 | 47.3 | 165/80 | >90 | 89 | 3.3 | 1 | — | — | 240 | — | 9.6 | U | LRH | Excluded | — |
| 15 | F | 54 | 28.7 | 146/82 | >90 | 38 | 3.8 | 1 | — | — | 90 | — | 6 | R | PA | Included | KCl |
| 16 | F | 72 | 28 | 130/80 | >90 | 52 | 3.7 | 0 | — | — | — | — | 2.4 | R | LRH | Excluded | — |
| 17 | F | 53 | 30.9 | 142/88 | >90 | 63 | 3.6 | 0 | — | — | — | — | 4.8 | R | LRH | Excluded | — |
| 18 | M | 61 | 38.9 | 152/98 | >90 | 73 | 3.0 | 4 | 600 | 4.5 | 240 | 200 | 7.2 | R | PA | Included | KCl |
| 19 | M | 35 | 35.9 | 162/100 | >90 | 81 | 3.0 | 3 | — | 5 | 480 | 100 | 9.6 | R | PA | Included | KCl |
| 20 | F | 45 | 31.4 | 136/96 | >90 | 63 | 4.4 | 0 | — | — | — | — | 0 | R | PA | Included | Non-KCl |
| 21 | M | 38 | 31.8 | 160/110 | >90 | 77 | 4.0 | 4 | 400 | 6 | 240 | 100 | 0 | R | PA | Included | Non-KCl |
| 22 | F | 34 | 27.9 | 140/92 | >90 | 42 | 3.9 | 1 | — | — | 240 | — | 0 | R | LRH | Excluded | — |
| 23 | M | 63 | 30.6 | 149/95 | 67 | 103 | 4.3 | 1 | — | — | 240 | — | 0 | R | PA | Included | Non-KCl |
| 24 | F | 30 | 29.2 | 140/78 | >90 | 54 | 3.4 | 2 | — | — | 180 | 150 | 4.8 | U | PA | Included | KCl |
| 25 | M | 43 | 31.3 | 144/91 | >90 | 79 | 3.5 | 2 | — | — | 480 | 50 | 7.2 | U | PA | Included | KCl |
| 26 | F | 31 | 23.7 | 128/86 | >90 | 63 | 3.5 | 2 | — | — | 240 | 25 | 6 | U | PA | Included | KCl |
| 27 | F | 36 | 27 | 123/78 | >90 | 68 | 3.6 | 2 | — | — | 240 | 25 | 6 | R | PA | Included | KCl |
| 28 | F | 51 | 24.9 | 125/68 | >90 | 59 | 4.1 | 2 | — | — | 240 | 150 | 0 | U | PA | Included | Non-KCl |
| 29 | F | 36 | 32.8 | 129/72 | >90 | 59 | 4.0 | 1 | — | — | 180 | — | 0 | R | LRH | Excluded | — |
| 30 | M | 44 | 28.5 | 145/95 | >90 | 73 | 4.1 | 2 | — | — | 240 | 37.5 | 0 | — | PA | Included | Non-KCl |
| 31 | F | 49 | 39.9 | 142/86 | >90 | 68 | 3.9 | 2 | — | 2 | 180 | — | 0 | U | PA | Included | Non-KCl |
| 32 | F | 57 | 26.9 | 140/102 | >90 | 62 | 3.1 | 1 | — | — | 120 | — | 0 | R | PA | Included | Non-KCl |
| 33 | F | 63 | 30 | 144/84 | 90 | 64 | 3.9 | 1 | — | — | 180 | — | 0 | R | PA | Included | Non-KCl |
| 34 | M | 55 | 33.7 | 170/106 | >90 | 61 | 3.3 | 4 | 200 | 2 | 240 | 150 | 4.8 | U | PA | Included | KCl |
| 35 | M | 52 | 24.8 | 116/76 | 82 | 93 | 3.6 | 1 | — | — | 240 | — | 5.4 | U | PA | Included | KCl |
| 36 | F | 37 | 42.4 | 164/96 | >90 | 74 | 3.7 | 4 | 400 | 2 | 240 | 150 | 3.6 | R | PA | Included | KCl |
| 37 | M | 39 | 36.8 | 170/98 | 75 | 108 | 4.2 | 4 | 600 | 4 | 360 | 75 | 0 | R | PA | Included | Non-KCl |
| 38 | M | 45 | 48.8 | 160/100 | 86 | 92 | 4.3 | 4 | 400 | 4 | 240 | 25 | 0 | U | LRH | Excluded | — |
BMI body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; plasma [K+], plasma potassium concentration; anti-HTN, antihypertensive; SSST, seated saline suppression testing; Moxo, moxonidine; Prazo, prazosin; Vera, verapamil; Hydralazine; Span-K, slow-release potassium chloride; Dx, diagnosis of primary aldosteronism; F, women; M, men; U, posture-unresponsive; R, posture-responsive; PA, primary aldosteronism; LRH, low renin essential hypertension. KCl, the group of patients who received KCl supplementation; Non-KCl, the group of patients who did not receive KCl.
Bloods for eGFR and plasma creatinine were collected on the day of admission; bloods for plasma K+ were collected in a recumbent posture at 7:00am on day 0.
The 7:00am blood sample was hemolyzed, and the plasma K+ level at 10:00am was 3.8 mmol/L. Hence, KCl supplementation was given and commenced at midday.
Figure 1.
Flow diagram of report numbers of participants at each stage of study. Comparisons of blood biochemical parameters were performed between 21 (13 women/eight men) in the KCl group (excluding patient 10) and ten (six women/four men) in the non-KCl group. Home 24-hour urinary parameters were compared between 20 (12 women/eight men) in the KCl group (excluding patients 7 and 9) and nine (five women/four men) in the non-KCl group (excluding patient 28). Spot urinary parameters were compared between 18 (12 women/six men) in the KCl group (excluding patients 15, 18, and 35) and nine (five women/four men) in the non-KCl group (excluding patient 31). A total of 30 (18 women/12 men) patients with PA were included in the baseline analyses of NCC abundance and phosphorylation, and 26 (16 women/ten men) patients with PA were included in paired comparisons. NCC, sodium-chloride cotransporter; HTN, hypertension; ARRs, aldosterone-to-renin ratios; SSST, seated saline suppression testing; N, sample size; KCl, potassium chloride; PA, primary aldosteronism; uEV, urinary extracellular vesicles.
The baseline blood sample from patient 10 was hemolyzed, but her plasma K+ at 10:00am on day 0 was 3.8 mmol/L. Hence, oral KCl supplementation was given and commenced at midday on the same day. Of the remaining 31 (18 women/12 men) patients with PA, 21 (13 women/eight men) whose baseline plasma K+ was <4 mmol/L were given KCl supplementation (hereafter referred to as the KCl group), and ten (six women/four men) did not receive KCl (hereafter referred to as the non-KCl group). Among the ten in the non-KCl group, nine had baseline K+ levels that were either ≥4 mmol/L (n=6) or only just below 4 mmol/L (n=3), whereas the remaining patient (patient 32) had a baseline K+ of 3.1 but was not given KCl, despite meeting the protocol criteria.
Baseline Biochemical Differences
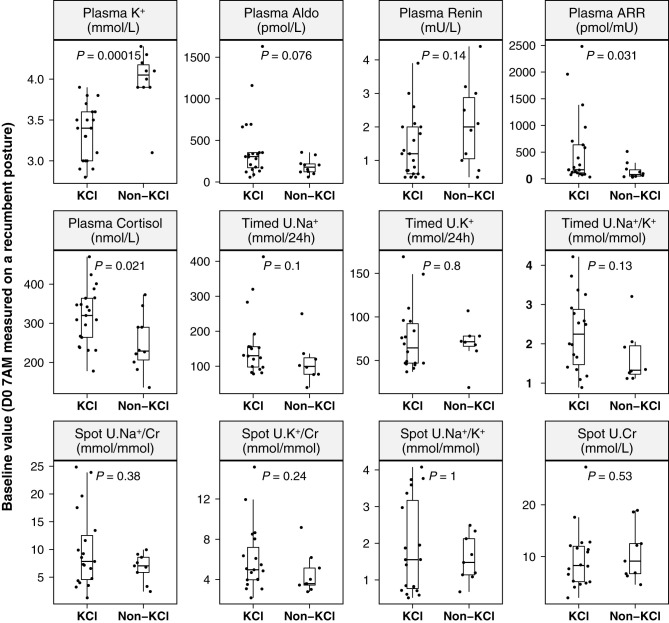
Baseline plasma K+ was significantly higher (P<0.001) in the non-KCl group (4.0 [3.1–4.4] mmol/L) than in the KCl group (3.4 [2.8–3.9] mmol/L; Figure 2), whereas plasma levels of aldosterone (178 [59–356] versus 305 [57–1630] pmol/L; P=0.08), ARR (81 [31–513] versus 171 [34–2480] pmol/mIU; P=0.03), and cortisol (229 [136–373] versus 320 [178–470] nmol/L; P=0.02) were relatively lower in the non-KCl group than in the KCl group. There were no statistical differences in 24-hour urinary levels of Na+, K+, and Na+/K+, and in spot urinary Na+/creatinine, K+/creatinine, and ratio of Na+/K+ and creatinine between the two groups.
Figure 2.
Baseline (7:00am) biochemical differences between PA in the KCl group and those in the non-KCl group. KCl, patients with PA who received oral KCl (Span-K) supplementation; non-KCl, patients with PA who did not receive oral KCl supplementation; K+, potassium; Aldo, aldosterone; Timed U.Na+/Cr, 24-hour urinary sodium-to-creatinine ratio; Timed U.K+/Cr, 24-hour urinary potassium-to-creatinine ratio; Timed U.Na+/K+, 24-hour urinary sodium-to-potassium ratio. Spot U.Na+/Cr, spot urinary sodium-to-creatinine ratio; Spot U.K+/Cr, spot urinary potassium-to-creatinine ratio; Spot U.Na+/K+, spot urinary sodium-to-potassium ratio; P values on the basis of unpaired Wilcoxon test; median, the median value of each parameter in each group.
Physiologic Effects of KCl Supplementation in PA
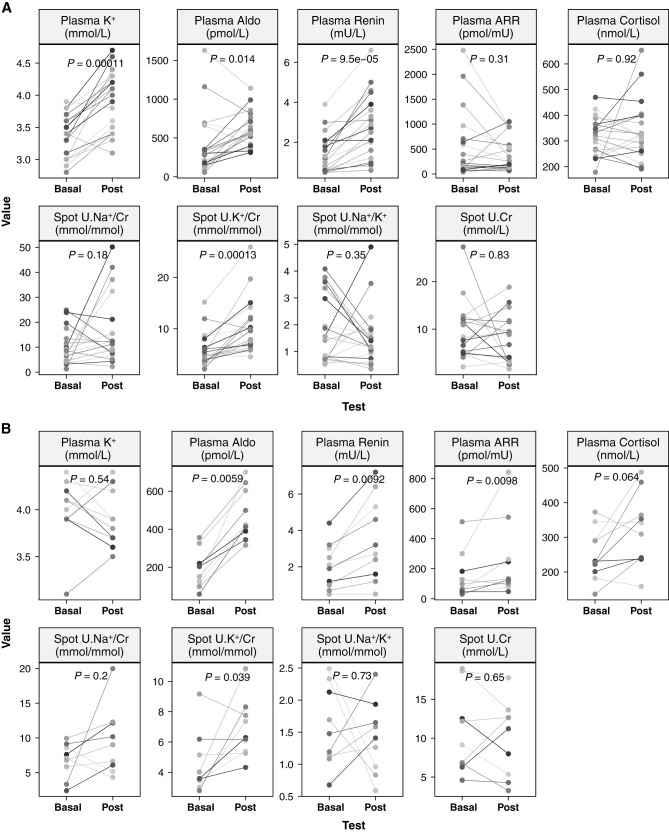
In the KCl group (Figure 3A), plasma K+ increased (P<0.001) from a median of 3.4 (2.8–3.9) to 4 (3.1–4.7) mmol/L in response to a median of 6.0 (2.4–16.8) g total dose of KCl, although plasma K+ fell in two patients (from 3.9 to 3.8 mmol/L in patient 11, despite 3.6 g KCl, and from 3.4 to 3.1 mmol/L in patient 24, despite 4.8 g KCl). Spot urine K+/creatinine increased from 5.0 (2.2–15.2) to 9.8 (4.5–25.9) mmol/mmol (P<0.001). Increases were also detected in plasma aldosterone (from 305 [57–1630] to 558 [313–1140] pmol/L; P=0.01) and direct renin concentration (from 1.2 [0.5–3.9] to 2.5 [0.6–6.6] mIU/L; P<0.001). There were no apparent changes in plasma levels of ARR (P=0.31) and cortisol (P=0.92), spot urine Na+/creatinine (P=0.25), spot urine Na+/K+ (P=0.39), and spot urine creatinine concentration (P=0.83).
Figure 3.
Physiological effects of KCl supplementation in PA. Physiologic changes within the 25-hour experimental period in the KCl group (A) and the non-KCl group (B). Basal, baseline values measured at 7:00am on day 0 in a recumbent posture; Post, post-test values measured at 8:00am on day 1 during seated posture; P values on the basis of paired Wilcoxon test.
Among the 10 patients (six women/four men) in the non-KCl group (Figure 3B), there were no apparent changes in plasma K+ (basal 4.0 [3.1–4.4] versus post 3.8 [3.5–4.4] mmol/L; P=0.54), but increases in the spot urine K+/creatinine were observed, from 3.6 [2.8–9.2] to 6.3 [4.3–10.8] mmol/mmol (P=0.04). Like those in the KCl group, there were increases in plasma levels of aldosterone (from 178 [59–356] to 418 [316–700] pmol/L; P=0.006) and renin (from 2.0 [0.5–4.4] to 3 [0.5–7.2] mIU/L; P=0.009), and an additional increase in plasma ARR (from 81 [31–513] to 131 [48–843] pmol/mIU; P=0.01). There were no apparent changes in plasma cortisol (P=0.06), spot urine Na+/creatinine (P=0.2), spot urine Na+/K+ (P=0.73), and spot urine creatinine (P=0.65).
uEV Characterization
There was insufficient sample quantity to allow for characterization of the uEVs by transmission electron microscopy and nanoparticle tracking analysis, but we have previously used these approaches to examine uEV pools isolated from humans by the same protocol and seen very clear uEV structures, and the uEVs’ size distribution was within the EVs appropriate range (30–1000 nm in diameter) (21, 22). Immunoblotting detection of at least two EV-enriched proteins (ALIX, TSG101, or CD9) in each sample was considered successful isolation of uEVs, and patients in whom uEVs were successfully isolated from both basal and post-test samples were included in the comparison analyses (Supplemental Figure 1, Supplemental Table 1). The observation of a shift in the ALIX bands in multiple samples may be due to ALIX truncation by the endosomal sorting complex required for transport machinery (23) or related to the presence of Tamm Horsfall protein (24). During the test, no apparent changes were observed in spot urine creatinine concentration (Figure 3) and in absolute abundance of the EV-enriched proteins (Supplemental Figure 2), rendering an effect of potassium on uEV concentration less likely (12, 24).
Baseline uEV Protein Differences
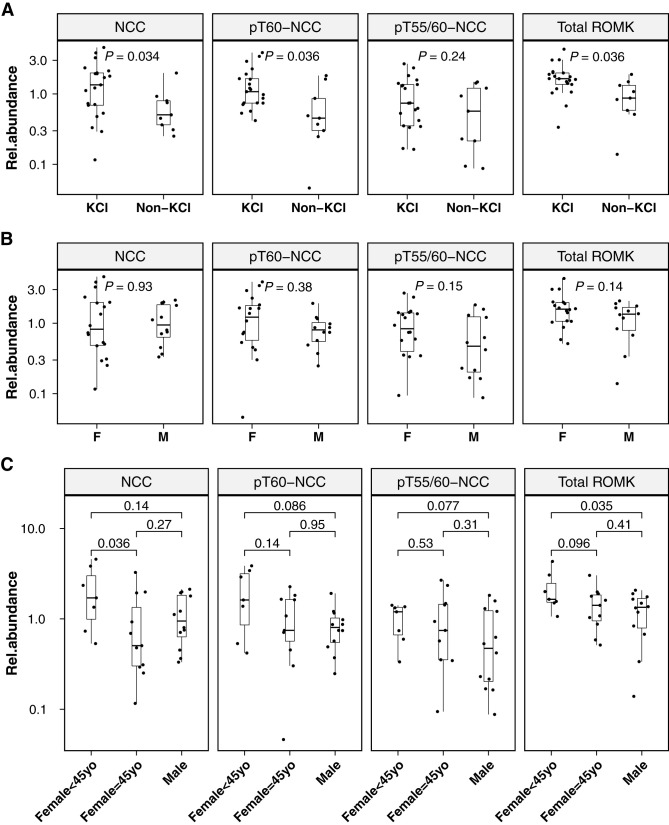
Baseline proteins were compared by allocation of oral KCl supplementation and sex (Figure 4). PA in the KCl group (N=21; 13 women/eight men) had higher abundances of NCC (1.35 [0.12–4.57]; P=0.03), pT60-NCC (1.08 [0.42–3.86], P=0.04), and total ROMK (1.65 [0.34–4.32], P=0.04) than PA in the non-KCl group (N=9; five women/four men; NCC: 0.5 [0.25–1.98]; pT60-NCC: 0.45 [0.05–1.82]; total ROMK: 0.87 [0.14–1.9]; Figure 4A). No sex-related differences were detected in analyzed proteins when total women were compared with total men (Figure 4B). By dividing women into two groups on the basis of the age of 45 into “likely premenopausal” and “likely postmenopausal” (25), women who were <45 years old exhibited a significantly higher level of NCC (1.71 [0.53–4.57], P=0.04) compared with women who were ≥45 years of age (0.5 [0.12–3.26]) and a higher level of ROMK (1.65 [1.06–4.32], P=0.04) compared with that in men (1.34 [0.14–2.07]; Figure 4C).
Figure 4.
Baseline uEV protein differences. Baseline differences in uEV levels of analyzed proteins in participants with PA according to allocation of oral KCl supplementation (A) and sex (B and C). KCl, participants in the KCl group; non-KCl, participants in the non-KCl group; F, women; M, men; Female<45yo, women who were <45 years old; Female≥45yo, women who were ≥45 years old; Rel.abundance, relative protein abundance (the ratio of protein absolute abundance to the sum abundance of ALIX, TSG101, and CD9); P values on the basis of unpaired Wilcoxon test.
Acute KCl Reduces NCC Abundance and Phosphorylation
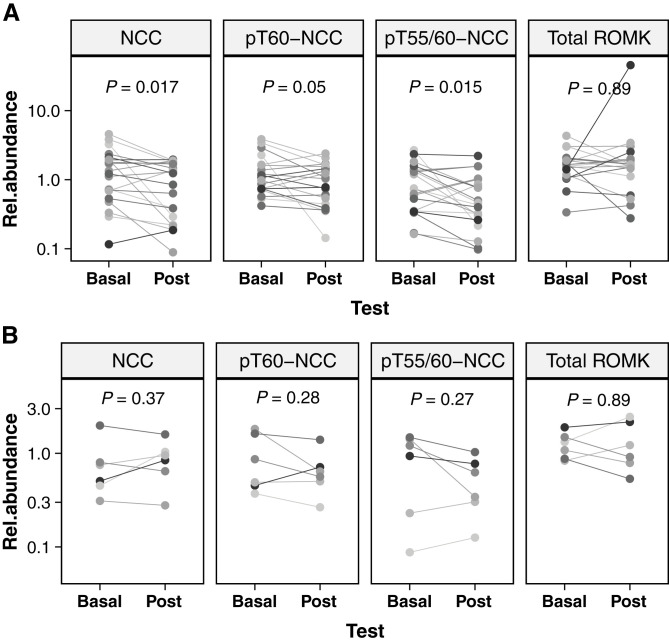
In the KCl group, there were significant decreases in the abundances of NCC (median fold change(post/basal) [FC]=0.71 [0.09–1.99]; P=0.02), pT60-NCC (FC=0.84 [0.06–1.66]; P=0.05), and pT55/60-NCC (FC=0.67 [0.08–2.42]; P=0.02). By contrast, in the non-KCl group, no significant changes in NCC, pT60-NCC, and pT55/60-NCC were seen. There were no significant changes in uEV levels of ROMK in either the KCl or non-KCl group (Figure 5, Supplemental Figure 2).
Figure 5.
Acute KCl supplementation reduces NCC and pNCC in PA. Changes of relative abundances of analyzed proteins in participants with PA (A) who received and (B) who did not receive oral KCl supplementation. Basal, baseline; Post, post-test; P values on the basis of paired t test (log10 transformed relative protein abundance was applied).
K+ Inversely Correlates with NCC Abundance and Phosphorylation
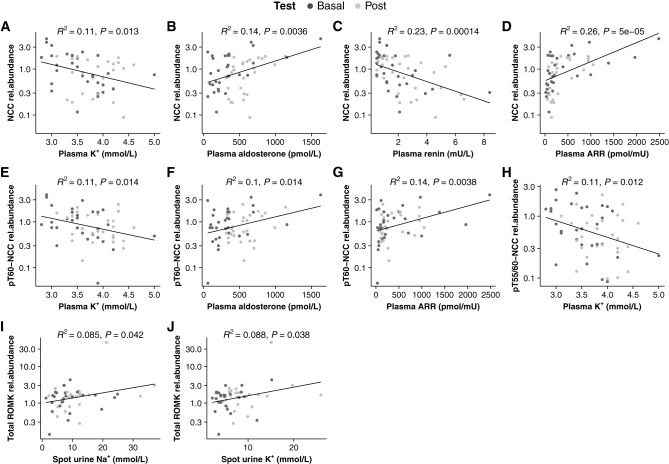
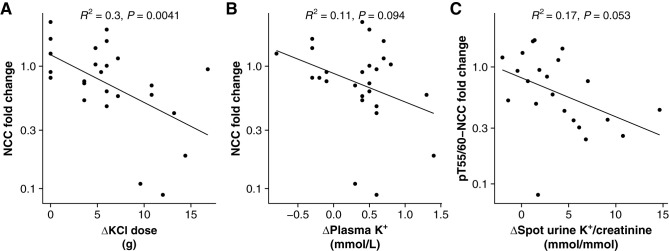
The uEV levels of NCC (R2=0.11; P=0.01), pT60-NCC (R2=0.11; P=0.01), and pT55/60 (R2=0.11; P=0.01) were negatively correlated with plasma [K+] among all PA participants during the test (Figure 6, A, E, and H). In addition, NCC positively correlated with plasma aldosterone (R2=0.14; P=0.004) and ARR (R2=0.26; P<0.001) and negatively correlated with plasma renin (R2=0.23; P<0.001; Figure 6, B–D). pT60-NCC positively correlated with plasma aldosterone (R2=0.1; P=0.01) and ARR (R2=0.14; P=0.004; Figure 6, F and G). Furthermore, ROMK weakly positively correlated with spot urine Na+/creatinine (R2=0.09; P=0.04) and K+/creatinine (R2=0.09; P=0.04; Figure 6, I and J). ΔKCl dose strongly negatively correlated with fold change of NCC (R2=0.3; P=0.004; Figure 7A). There were no significant associations between Δplasma K+ and fold change of NCC (R2=0.11; P=0.09) and between Δspot urine K+/creatinine and fold change of pT55/60-NCC (R2=0.17; P=0.05; Figure 7, B and C). There were no additional associations of the analyzed proteins with other biochemical parameters (Supplemental Figure 3).
Figure 6.
Notable correlations among all participants with PA during the test. Basal, baseline values (colored in dark gray), biochemical parameter measured at 7:00am on day 0 in a recumbent posture; Post, post-test values (colored in silver), biochemical parameter measured at 8:00am on day 1 during a seated posture; rel.ab, relative protein abundance (the ratio of protein absolute abundance to the sum abundance of ALIX, TSG101, and CD9).
Figure 7.
ΔKCl dose strongly negatively correlated with fold change of NCC. Correlations of ΔKCl dose (A), Δplasma [K+] (B), and Δspot urine K+/creatinine (C) with fold changes of NCC abundance and phosphorylation in participants with PA.
Discussion
Aggressively correcting hypokalemia by 24-hour oral KCl supplementation before SSST increased median plasma [K+] in the KCl group, whereas in the non-KCl group, plasma [K+] remained unchanged. However, during the 24-hour period, increases in plasma aldosterone were observed in both KCl (1.8-fold) and non-KCl (2.2-fold) groups, which is likely due to difference in posture between baseline (recumbent) and post-test (upright) sampling as evidenced by the observed rises in direct renin concentration in both groups (26). Upright posture has a significant stimulatory effect on renin beginning at 15 minutes and peaking between 60 and 120 minutes, and aldosterone secretion directly correlates with the elevation in the renin activity during the 120 minutes in an upright posture (27–30). Although there are posture (or angiotensin II)-unresponsive forms of PA, in which adrenocorticotropic hormone assumes a dominant role over angiotensin II in regulating aldosterone production (31), in the current study, plasma aldosterone levels in most patients in the non-KCl group were responsive to the upright posture (Table 2). Furthermore, blood samples were collected at 7:00am at baseline and just before 8:00am post test, and there were no statistically changes in plasma cortisol in either group, making it unlikely that the changes in plasma aldosterone reflected its circadian rhythm. In the KCl group, the rise in plasma K+ may also have contributed to the rise in plasma aldosterone, but no apparent correlation was detected (Supplemental Figure 4).
By using uEV, we observed a significant suppressive effect of oral KCl supplementation on NCC abundance and phosphorylation (pT55/60-NCC and pT60-NCC) in patients with PA, despite an increase in endogenous aldosterone, whereas no obvious changes were detected in patients with PA who did not receive KCl. uEV analysis is thought to offer only an indirect way to assess NCC abundance in the DCT and might reflect changes in NCC trafficking rather than changes in NCC expression. A recent large-scale unbiased analysis demonstrated that uEV proteins track the abundance of the parent protein in the kidney, thus supporting the use of uEV protein changes to monitor specific physiologic responses and disease mechanisms (12). Moreover, human uEVs reproduced the inverse correlations of plasma K+ and the positive association of plasma aldosterone with kidney NCC and pT60-NCC that have been observed in in vivo studies and clinical studies of NCC in uEVs (14, 21, 32–34). The negative correlation between ΔKCl dose and NCC fold change suggests that at least part of the variance in NCC may be a result of the KCl dose. And this variance has also been observed in two groups of patients with PA who underwent 4-day administration of exogenous mineralocorticoid (14, 15). The negative correlation between ΔKCl dose and NCC fold change, the greater change of spot urine K+/creatinine in the KCl group, and negative correlations of plasma K+ with NCC abundance and phosphorylation infer that reduced NCC phosphorylation may be related to promoting kaliuresis to avoid acute hyperkalemia induced by KCl supplementation (35–38).
Interestingly, patients with PA in the non-KCl group exhibited relatively low baseline plasma aldosterone (approximately half of that in PA in the KCl group) and doubled their aldosterone levels post test. However, the significant rise in plasma aldosterone in non-KCl patients with PA did not lead to overall increased NCC abundance and phosphorylation. In contrast, baseline NCC and pT60-NCC were significantly higher in patients with PA in the KCl group than those in the non-KCl group in association with relatively low plasma K+. These observations support the hypothesis that elevated endogenous aldosterone per se does not result in increased NCC abundance and phosphorylation.
In the current study, alteration of plasma K+ level was achieved by oral KCl supplementation, which leaves a question as to whether plasma K+ independently suppresses NCC abundance and phosphorylation or whether it acts concurrently with Cl–. Our recent study demonstrated that significantly increased plasma Cl– in patients with PA during acute saline infusion appeared only to cause changes in NCC and pNCC due to urine dilution (21). Therefore, it is less likely that plasma Cl– contributes to reduction in uEV levels of NCC abundance and phosphorylation. The putative renal “K+ switch” mechanism is a relatively cohesive model that may provide a mechanism linking K+ intake and NCC regulation (14, 32, 39–41). The “switch” activates NCC in response to low K+ intake, and “turns off” NCC in response to high K+ intake. Hence, although aldosterone stimulates ENaC to promote distal Na+ reabsorption and K+ excretion in the aldosterone-sensitive distal nephron, the K+ concentration itself regulates NCC activity to alter the Na+ amount delivered downstream to ENaC, at least in the conventional model (42, 43).
The current study provides further evidence that K+ regulates NCC activity during a state of increased endogenous aldosterone in patients with PA. Although other interventions, including adrenalectomy and spironolactone, reduced NCC in adrenal-intact animals (44, 45), and conditional tubule knockdown of the mineralocorticoid receptor also reduces NCC (46), these effects might also be attributable to plasma K+. Because this was an observational study undertaken opportunistically while patients were admitted for a clinical procedure (SSST), hospital 24-hour urine measurements at both baseline and completion of the test period (which would have meant delaying SSST and therefore extending the hospital admission for a further 24 hours) were not clinically feasible. We instead used the creatinine normalized spot urine values of Na+ and K+ as indicators of changes in distal nephron function. The significant increase in urinary K+/creatinine accompanied by the trend to an increase of urinary Na+/creatinine in patients who received oral KCl is in accordance with the phenomenon of K+-induced natriuresis. However, we were unable to assess if the kaliuresis induced by oral KCl supplementation was ENaC dependent, as reported by others (47), because ENaC (which has low abundance and is difficult to measure in uEVs) was not measured in this study.
ROMK weakly positively correlated with spot urinary Na+/creatinine and spot urinary K+/creatinine, but we detected no overall significant changes in ROMK among patients who received KCl. The visible reductions in ROMK upon KCl replacement seen in some patients were not accompanied by reduced spot urinary K+/creatinine. Because ROMK is also expressed in the thick ascending limb of Henle’s loop, it may be hard to detect K+-dependent and aldosterone effects on ROMK, which are restricted to the aldosterone-sensitive segments. Although there is a ROMK isoform that is predominantly expressed in the distal nephron (48), the commercially unavailable antibody specific to the ROMK isoform makes it infeasible to minimize the interference with the thick ascending limb of Henle’s loop expressed ROMK. Furthermore, animal studies have revealed a separate, flow-dependent BK channel that can partially compensate for inactive ROMK (49), but this was not assessed in the current study due to the limited availability of uEV material.
Estrogen in women may protect from cardiovascular and renal diseases before menopause (50, 51). Although the sex dimorphic regulation of NCC is disputed, it has been observed that estrogen restores NCC abundance in ovariectomized female rats and stimulates NCC abundance and phosphorylation (52–54). In the current study, the lack of sex-related differences in NCC and its phosphorylated forms at baseline may be related to the inclusion of women who were ≥45 years of age. By dividing women into two groups on the basis of age into “likely premenopausal” and “likely postmenopausal,” we were able to demonstrate differences, possibly sex hormone related, in NCC.
In conclusion, the current study reports for the first time that acute oral KCl supplementation in patients with PA is associated with suppressed NCC abundance and phosphorylation in uEVs, despite a significant rise in plasma aldosterone, and this effect may be KCl dose dependent. These observations support (1) the speculation that elevation of endogenous aldosterone per se does not result in increased NCC abundance and phosphorylation and (2) that in PA, the effects of low plasma K+ (secondary to aldosterone excess) may dominate in terms of NCC regulation.
Disclosures
R.A. Fenton reports an advisory or leadership role for the American Journal of Physiology Renal (associate editor) and JASN (editorial board member). P.A. Welling reports an advisory or leadership role for the American Journal of Physiology (renal editorial board) and the American Society of Physiology (chair, finance committee, and council member). All remaining authors have nothing to disclose.
Funding
This work was supported by a grant from the Leducq Foundation (Potassium in Hypertension Network).
Acknowledgments
A. Wu was supported by an Australian Government Research Training Program scholarship.
Footnotes
See related editorial, “Potassium Homeostasis and WNK Kinases in the Regulation of the Sodium-Chloride Cotransporter: Hyperaldosteronism and Its Metabolic Consequences,” on pages 1823–1828.
Author Contributions
D. Cowley, A. Matthews, and A. Wu were responsible for the methodology; R.A. Fenton, M. Stowasser, P.A. Welling, M.J. Wolley, and A. Wu reviewed and edited the manuscript; R.A. Fenton, P.A. Welling, and M.Stowasser were responsible for funding acquisition; M. Stowasser and M.J. Wolley were responsible for supervision; M. Stowasser, M.J. Wolley, and A. Wu were responsible for the conceptualization; M.J. Wolley and A. Wu were responsible for the investigation; A. Wu was responsible for data curation, formal analysis, project administration, resources, software, validation, and visualization, and wrote the original draft of the manuscript.
Data Sharing Statement
All data are included in the manuscript and/or supporting information.
Supplemental Material
This article contains the following supplemental material online at http://kidney360.asnjournals.org/lookup/suppl/doi:10.34067/KID.0003632022/-/DCSupplemental.
Immunoblots of analyzed proteins in all participants. Download Supplemental Figure 1, PDF file, 6.2 MB (6.1MB, pdf) .
Changes of absolute abundances of uEV proteins among the 26 patients with PA within the 24-hour experiment period. Download Supplemental Figure 2, PDF file, 6.2 MB (6.1MB, pdf) .
Correlations of the analyzed proteins with biochemical parameters. Download Supplemental Figure 3, PDF file, 6.2 MB (6.1MB, pdf) .
Correlation between changes in plasma K+ and plasma aldosterone in patients with PA receiving KCl replacement. Download Supplemental Figure 4, PDF file, 6.2 MB (6.1MB, pdf) .
Detection of EV-enriched proteins in each uEV isolate, and sample inclusion in uEV analyses. Download Supplemental Table 1, PDF file, 6.2 MB (6.1MB, pdf) .
References
- 1.Conn JW, Cohen EL, Rovner DR, Nesbit RM: Normokalemic primary aldosteronism. A detectable cause of curable “essential” hypertension. JAMA 193: 200–206, 1965. 10.1001/jama.1965.03090030022005 [DOI] [PubMed] [Google Scholar]
- 2.Mulatero P, Stowasser M, Loh KC, Fardella CE, Gordon RD, Mosso L, Gomez-Sanchez CE, Veglio F, Young WF Jr: Increased diagnosis of primary aldosteronism, including surgically correctable forms, in centers from five continents. J Clin Endocrinol Metab 89: 1045–1050, 2004. 10.1210/jc.2003-031337 [DOI] [PubMed] [Google Scholar]
- 3.Stowasser M, Gordon RD, Gunasekera TG, Cowley DC, Ward G, Archibald C, Smithers BM: High rate of detection of primary aldosteronism, including surgically treatable forms, after “non-selective” screening of hypertensive patients. J Hypertens 21: 2149–2157, 2003. 10.1097/00004872-200311000-00025 [DOI] [PubMed] [Google Scholar]
- 4.Burrello J, Monticone S, Losano I, Cavaglià G, Buffolo F, Tetti M, Covella M, Rabbia F, Veglio F, Pasini B, Williams TA, Mulatero P: Prevalence of hypokalemia and primary aldosteronism in 5100 patients referred to a tertiary hypertension unit. Hypertension 75: 1025–1033, 2020. 10.1161/HYPERTENSIONAHA.119.14063 [DOI] [PubMed] [Google Scholar]
- 5.Rozansky DJ, Cornwall T, Subramanya AR, Rogers S, Yang YF, David LL, Zhu X, Yang CL, Ellison DH: Aldosterone mediates activation of the thiazide-sensitive Na-Cl cotransporter through an SGK1 and WNK4 signaling pathway. J Clin Invest 119: 2601–2612, 2009. 10.1172/JCI38323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arroyo JP, Lagnaz D, Ronzaud C, Vázquez N, Ko BS, Moddes L, Ruffieux-Daidié D, Hausel P, Koesters R, Yang B, Stokes JB, Hoover RS, Gamba G, Staub O: Nedd4-2 modulates renal Na+-Cl– cotransporter via the aldosterone-SGK1-Nedd4-2 pathway. J Am Soc Nephrol 22: 1707–1719, 2011. 10.1681/ASN.2011020132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lagnaz D, Arroyo JP, Chávez-Canales M, Vázquez N, Rizzo F, Spirlí A, Debonneville A, Staub O, Gamba G: WNK3 abrogates the NEDD4-2-mediated inhibition of the renal Na+-Cl– cotransporter. Am J Physiol Renal Physiol 307: F275–F286, 2014. 10.1152/ajprenal.00574.2013 [DOI] [PubMed] [Google Scholar]
- 8.McCormick JA, Bhalla V, Pao AC, Pearce D: SGK1: A rapid aldosterone-induced regulator of renal sodium reabsorption. Physiology (Bethesda) 20: 134–139, 2005. 10.1152/physiol.00053.2004 [DOI] [PubMed] [Google Scholar]
- 9.Ronzaud C, Loffing-Cueni D, Hausel P, Debonneville A, Malsure SR, Fowler-Jaeger N, Boase NA, Perrier R, Maillard M, Yang B, Stokes JB, Koesters R, Kumar S, Hummler E, Loffing J, Staub O: Renal tubular NEDD4-2 deficiency causes NCC-mediated salt-dependent hypertension. J Clin Invest 123: 657–665, 2013. 10.1172/JCI61110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salih M, Zietse R, Hoorn EJ: Urinary extracellular vesicles and the kidney: Biomarkers and beyond. Am J Physiol Renal Physiol 306: F1251–F1259, 2014. 10.1152/ajprenal.00128.2014 [DOI] [PubMed] [Google Scholar]
- 11.Merchant ML, Rood IM, Deegens JKJ, Klein JB: Isolation and characterization of urinary extracellular vesicles: Implications for biomarker discovery. Nat Rev Nephrol 13: 731–749, 2017. 10.1038/nrneph.2017.148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu Q, Poulsen SB, Murali SK, Grimm PR, Su XT, Delpire E, Welling PA, Ellison DH, Fenton RA: Large-scale proteomic assessment of urinary extracellular vesicles highlights their reliability in reflecting protein changes in the kidney. J Am Soc Nephrol 32: 2195–2209, 2021. 10.1681/ASN.2020071035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.van der Lubbe N, Jansen PM, Salih M, Fenton RA, van den Meiracker AH, Danser AH, Zietse R, Hoorn EJ: The phosphorylated sodium chloride cotransporter in urinary exosomes is superior to prostasin as a marker for aldosteronism. Hypertension 60: 741–748, 2012. 10.1161/HYPERTENSIONAHA.112.198135 [DOI] [PubMed] [Google Scholar]
- 14.Wolley MJ, Wu A, Xu S, Gordon RD, Fenton RA, Stowasser M: In primary aldosteronism, mineralocorticoids influence exosomal sodium-chloride cotransporter abundance. J Am Soc Nephrol 28: 56–63, 2017. 10.1681/ASN.2015111221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wu A, Wolley MJ, Wu Q, Gordon RD, Fenton RA, Stowasser M: The Cl–/HCO3– exchanger pendrin is downregulated during oral co-administration of exogenous mineralocorticoid and KCl in patients with primary aldosteronism. J Hum Hypertens 35: 837–848, 2021. 10.1038/s41371-020-00439-7 [DOI] [PubMed] [Google Scholar]
- 16.Xu N, Hirohama D, Ishizawa K, Chang WX, Shimosawa T, Fujita T, Uchida S, Shibata S: Hypokalemia and pendrin induction by aldosterone. Hypertension 69: 855–862, 2017. 10.1161/HYPERTENSIONAHA.116.08519 [DOI] [PubMed] [Google Scholar]
- 17.Thuzar M, Young K, Ahmed AH, Ward G, Wolley M, Guo Z, Gordon RD, McWhinney BC, Ungerer JP, Stowasser M: Diagnosis of primary aldosteronism by seated saline suppression test—Variability between immunoassay and HPLC-MS/MS. J Clin Endocrinol Metab 105: dgz150, 2020. 10.1210/clinem/dgz150 [DOI] [PubMed] [Google Scholar]
- 18.Guo Z, Poglitsch M, McWhinney BC, Ungerer JPJ, Ahmed AH, Gordon RD, Wolley M, Stowasser M: Measurement of equilibrium angiotensin II in the diagnosis of primary aldosteronism. Clin Chem 66: 483–492, 2020. 10.1093/clinchem/hvaa001 [DOI] [PubMed] [Google Scholar]
- 19.Pedersen NB, Hofmeister MV, Rosenbaek LL, Nielsen J, Fenton RA: Vasopressin induces phosphorylation of the thiazide-sensitive sodium chloride cotransporter in the distal convoluted tubule. Kidney Int 78: 160–169, 2010. 10.1038/ki.2010.130 [DOI] [PubMed] [Google Scholar]
- 20.Wade JB, Fang L, Coleman RA, Liu J, Grimm PR, Wang T, Welling PA: Differential regulation of ROMK (Kir1.1) in distal nephron segments by dietary potassium. Am J Physiol Renal Physiol 300: F1385–F1393, 2011. 10.1152/ajprenal.00592.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu A, Wolley MJ, Wu Q, Cowley D, Palmfeldt J, Welling PA, Fenton RA, Stowasser M: Acute intravenous NaCl and volume expansion reduces NCC abundance and phosphorylation in urinary extracellular vesicles. Kidney360 3: 910–921, 2022. 10.34067/KID.0000362022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huebner AR, Cheng L, Somparn P, Knepper MA, Fenton RA, Pisitkun T: Deubiquitylation of protein cargo is not an essential step in exosome formation. Mol Cell Proteomics 15: 1556–1571, 2016. 10.1074/mcp.M115.054965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Odorizzi G: The multiple personalities of Alix. J Cell Sci 119: 3025–3032, 2006. 10.1242/jcs.03072 [DOI] [PubMed] [Google Scholar]
- 24.Blijdorp CJ, Tutakhel OAZ, Hartjes TA, van den Bosch TPP, van Heugten MH, Rigalli JP, Willemsen R, Musterd-Bhaggoe UM, Barros ER, Carles-Fontana R, Carvajal CA, Arntz OJ, van de Loo FAJ, Jenster G, Clahsen-van Groningen MC, Cuevas CA, Severs D, Fenton RA, van Royen ME, Hoenderop JGJ, Bindels RJM, Hoorn EJ: Comparing approaches to normalize, quantify, and characterize urinary extracellular vesicles. J Am Soc Nephrol 32: 1210–1226, 2021. 10.1681/ASN.2020081142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yap S, Vassallo A, Goldsbury DE, Salagame U, Velentzis L, Banks E, O’Connell DL, Canfell K, Steinberg J: Accurate categorisation of menopausal status for research studies: A step-by-step guide and detailed algorithm considering age, self-reported menopause and factors potentially masking the occurrence of menopause. BMC Res Notes 15: 88, 2022. 10.1186/s13104-022-05970-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stowasser M, Gordon RD: Primary aldosteronism: Changing definitions and new concepts of physiology and pathophysiology both inside and outside the kidney. Physiol Rev 96: 1327–1384, 2016. 10.1152/physrev.00026.2015 [DOI] [PubMed] [Google Scholar]
- 27.Cohen EL, Conn JW, Rovner DR: Postural augmentation of plasma renin activity and aldosterone excretion in normal people. J Clin Invest 46: 418–428, 1967. 10.1172/JCI105543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Balikian HM, Brodie AH, Dale SL, Melby JC, Tait JF: Effect of posture on the metabolic clearance rate, plasma concentration and blood production rate of aldosterone in man. J Clin Endocrinol Metab 28: 1630–1640, 1968. 10.1210/jcem-28-11-1630 [DOI] [PubMed] [Google Scholar]
- 29.Biglieri EG: Effect of posture on the plasma concentrations of aldosterone in hypertension and primary hyperaldosteronism. Nephron 23: 112–115, 1979. 10.1159/000181618 [DOI] [PubMed] [Google Scholar]
- 30.Tuck ML, Dluhy RG, Williams GH: Sequential responses of the renin-angiotensin-aldosterone axis to acute postural change: Effect of dietary sodium. J Lab Clin Med 86: 754–763, 1975 [PubMed] [Google Scholar]
- 31.Guo Z, Nanba K, Udager A, McWhinney BC, Ungerer JPJ, Wolley M, Thuzar M, Gordon RD, Rainey WE, Stowasser M: Biochemical, histopathological, and genetic characterization of posture-responsive and unresponsive APAs. J Clin Endocrinol Metab 105: e3224–e3235, 2020. 10.1210/clinem/dgaa367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Terker AS, Zhang C, Erspamer KJ, Gamba G, Yang CL, Ellison DH: Unique chloride-sensing properties of WNK4 permit the distal nephron to modulate potassium homeostasis. Kidney Int 89: 127–134, 2016. 10.1038/ki.2015.289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Veiras LC, Han J, Ralph DL, McDonough AA: Potassium supplementation prevents sodium chloride cotransporter stimulation during angiotensin II hypertension. Hypertension 68: 904–912, 2016. 10.1161/HYPERTENSIONAHA.116.07389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salih M, Bovée DM, van der Lubbe N, Danser AHJ, Zietse R, Feelders RA, Hoorn EJ: Increased urinary extracellular vesicle sodium transporters in Cushing syndrome with hypertension. J Clin Endocrinol Metab 103: 2583–2591, 2018. 10.1210/jc.2018-00065 [DOI] [PubMed] [Google Scholar]
- 35.Hoorn EJ, Gritter M, Cuevas CA, Fenton RA: Regulation of the renal NaCl cotransporter and its role in potassium homeostasis. Physiol Rev 100: 321–356, 2020. 10.1152/physrev.00044.2018 [DOI] [PubMed] [Google Scholar]
- 36.van Buren M, Rabelink TJ, van Rijn HJ, Koomans HA: Effects of acute NaCl, KCl and KHCO3 loads on renal electrolyte excretion in humans. Clin Sci (Lond) 83: 567–574, 1992. 10.1042/cs0830567 [DOI] [PubMed] [Google Scholar]
- 37.Calò L, Borsatti A, Favaro S, Rabinowitz L: Kaliuresis in normal subjects following oral potassium citrate intake without increased plasma potassium concentration. Nephron 69: 253–258, 1995. 10.1159/000188466 [DOI] [PubMed] [Google Scholar]
- 38.Rabinowitz L, Sarason RL, Yamauchi H: Effects of KCl infusion on potassium excretion in sheep. Am J Physiol 249: F263–F271, 1985. 10.1152/ajprenal.1985.249.2.F263 [DOI] [PubMed] [Google Scholar]
- 39.Terker AS, Ellison DH: Renal mineralocorticoid receptor and electrolyte homeostasis. Am J Physiol Regul Integr Comp Physiol 309: R1068–R1070, 2015. 10.1152/ajpregu.00135.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Czogalla J, Vohra T, Penton D, Kirschmann M, Craigie E, Loffing J: The mineralocorticoid receptor (MR) regulates ENaC but not NCC in mice with random MR deletion. Pflugers Arch 468: 849–858, 2016. 10.1007/s00424-016-1798-5 [DOI] [PubMed] [Google Scholar]
- 41.van der Lubbe N, Moes AD, Rosenbaek LL, Schoep S, Meima ME, Danser AH, Fenton RA, Zietse R, Hoorn EJ: K+-induced natriuresis is preserved during Na+ depletion and accompanied by inhibition of the Na+-Cl– cotransporter. Am J Physiol Renal Physiol 305: F1177–F1188, 2013. 10.1152/ajprenal.00201.2013 [DOI] [PubMed] [Google Scholar]
- 42.Rengarajan S, Lee DH, Oh YT, Delpire E, Youn JH, McDonough AA: Increasing plasma [K+] by intravenous potassium infusion reduces NCC phosphorylation and drives kaliuresis and natriuresis. Am J Physiol Renal Physiol 306: F1059–F1068, 2014. 10.1152/ajprenal.00015.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sorensen MV, Grossmann S, Roesinger M, Gresko N, Todkar AP, Barmettler G, Ziegler U, Odermatt A, Loffing-Cueni D, Loffing J: Rapid dephosphorylation of the renal sodium chloride cotransporter in response to oral potassium intake in mice. Kidney Int 83: 811–824, 2013. 10.1038/ki.2013.14 [DOI] [PubMed] [Google Scholar]
- 44.Ivy JR, Jones NK, Costello HM, Mansley MK, Peltz TS, Flatman PW, Bailey MA: Glucocorticoid receptor activation stimulates the sodium-chloride cotransporter and influences the diurnal rhythm of its phosphorylation. Am J Physiol Renal Physiol 317: F1536–F1548, 2019. 10.1152/ajprenal.00372.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abdallah JG, Schrier RW, Edelstein C, Jennings SD, Wyse B, Ellison DH: Loop diuretic infusion increases thiazide-sensitive Na(+)/Cl(–)-cotransporter abundance: Role of aldosterone. J Am Soc Nephrol 12: 1335–1341, 2001. 10.1681/ASN.V1271335 [DOI] [PubMed] [Google Scholar]
- 46.Terker AS, Yarbrough B, Ferdaus MZ, Lazelle RA, Erspamer KJ, Meermeier NP, Park HJ, McCormick JA, Yang CL, Ellison DH: Direct and indirect mineralocorticoid effects determine distal salt transport. J Am Soc Nephrol 27: 2436–2445, 2016. 10.1681/ASN.2015070815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jensen IS, Larsen CK, Leipziger J, Sørensen MV: Na(+) dependence of K(+)-induced natriuresis, kaliuresis and Na(+)/Cl(–) cotransporter dephosphorylation. Acta Physiol (Oxf) 218: 49–61, 2016. 10.1111/apha.12707 [DOI] [PubMed] [Google Scholar]
- 48.Welling PA, Ho K: A comprehensive guide to the ROMK potassium channel: Form and function in health and disease. Am J Physiol Renal Physiol 297: F849–F863, 2009. 10.1152/ajprenal.00181.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bailey MA, Cantone A, Yan Q, MacGregor GG, Leng Q, Amorim JBO, Wang T, Hebert SC, Giebisch G, Malnic G: Maxi-K channels contribute to urinary potassium excretion in the ROMK-deficient mouse model of Type II Bartter’s syndrome and in adaptation to a high-K diet. Kidney Int 70: 51–59, 2006. 10.1038/sj.ki.5000388 [DOI] [PubMed] [Google Scholar]
- 50.Reckelhoff JF: Gender differences in the regulation of blood pressure. Hypertension 37: 1199–1208, 2001. 10.1161/01.HYP.37.5.1199 [DOI] [PubMed] [Google Scholar]
- 51.Cobo G, Hecking M, Port FK, Exner I, Lindholm B, Stenvinkel P, Carrero JJ: Sex and gender differences in chronic kidney disease: Progression to end-stage renal disease and haemodialysis. Clin Sci (Lond) 130: 1147–1163, 2016. 10.1042/CS20160047 [DOI] [PubMed] [Google Scholar]
- 52.Verlander JW, Tran TM, Zhang L, Kaplan MR, Hebert SC: Estradiol enhances thiazide-sensitive NaCl cotransporter density in the apical plasma membrane of the distal convoluted tubule in ovariectomized rats. J Clin Invest 101: 1661–1669, 1998. 10.1172/JCI601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rojas-Vega L, Reyes-Castro LA, Ramírez V, Bautista-Pérez R, Rafael C, Castañeda-Bueno M, Meade P, de Los Heros P, Arroyo-Garza I, Bernard V, Binart N, Bobadilla NA, Hadchouel J, Zambrano E, Gamba G: Ovarian hormones and prolactin increase renal NaCl cotransporter phosphorylation. Am J Physiol Renal Physiol 308: F799–F808, 2015. 10.1152/ajprenal.00447.2014 [DOI] [PubMed] [Google Scholar]
- 54.Veiras LC, Girardi ACC, Curry J, Pei L, Ralph DL, Tran A, Castelo-Branco RC, Pastor-Soler N, Arranz CT, Yu ASL, McDonough AA: Sexual dimorphic pattern of renal transporters and electrolyte homeostasis. J Am Soc Nephrol 28: 3504–3517, 2017. 10.1681/ASN.2017030295 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Immunoblots of analyzed proteins in all participants. Download Supplemental Figure 1, PDF file, 6.2 MB (6.1MB, pdf) .
Changes of absolute abundances of uEV proteins among the 26 patients with PA within the 24-hour experiment period. Download Supplemental Figure 2, PDF file, 6.2 MB (6.1MB, pdf) .
Correlations of the analyzed proteins with biochemical parameters. Download Supplemental Figure 3, PDF file, 6.2 MB (6.1MB, pdf) .
Correlation between changes in plasma K+ and plasma aldosterone in patients with PA receiving KCl replacement. Download Supplemental Figure 4, PDF file, 6.2 MB (6.1MB, pdf) .
Detection of EV-enriched proteins in each uEV isolate, and sample inclusion in uEV analyses. Download Supplemental Table 1, PDF file, 6.2 MB (6.1MB, pdf) .