Key Points
In patients with atrial fibrillation on dialysis, the incidence of stroke was similar with apixaban or no anticoagulation, regardless of P2Y12 prescription.
In patients with atrial fibrillation on dialysis who were on a P2Y12 inhibitor, apixaban increased the risk of bleeding, compared with no anticoagulation.
The incidence of myocardial infarction or ischemic stroke was similar with apixaban or no anticoagulation, regardless of P2Y12 prescription status.
Keywords: dialysis, apixaban, atrial fibrillation, bleeding, dialysis, P2Y12 inhibitor, prescriptions, stroke
Atrial fibrillation is common among patients on maintenance dialysis, but antithrombotic strategies for stroke prevention are not well established in this population (1). We recently reported that apixaban was not associated with a lower incidence of stroke, transient ischemic attack, or systemic thromboembolism compared with no anticoagulation in patients with incident atrial fibrillation on maintenance dialysis, but was associated with a higher incidence of fatal or intracranial bleeding (2). In this report, we present the incidence of thrombotic and bleeding outcomes by P2Y12 inhibitor prescription status at baseline.
We conducted a retrospective cohort study of patients with incident nonvalvular atrial fibrillation who were undergoing long-term dialysis between 2012 and 2015, using United States Renal Data System (USRDS) data (3). Study design and main results have been reported elsewhere (2). In brief, we matched patients who were alive at 30 days post–atrial fibrillation diagnosis and were treated with apixaban with patients without any anticoagulant prescription, using a propensity score. The primary outcome was hospital admission for a new stroke (ischemic or hemorrhagic), transient ischemic attack, or systemic thromboembolism. Secondary outcomes included fatal or intracranial bleeding and ischemic stroke or myocardial infarction. Condition-specific diagnostic codes are presented in Supplemental Table 1. Cox proportional hazard models were used to examine the association between apixaban prescription and the clinical outcomes, considering death from any cause as a competing risk. We censored patients using apixaban at the date of the last available prescription and all patients when Medicare Part A, B, or D coverage was lost. Hazard ratios (HRs) were adjusted for congestive heart failure, hypertension, age, diabetes, stroke, vascular history (CHA2DS2-Vasc) score for the primary outcome; modified hypertension, kidney disease, liver disease, stroke, prior bleeding or predisposition to bleeding, labile international normalized ratios, age, medication, alcohol use (HAS-BLED) score for fatal or intracranial bleeding; and hypertension, diabetes, dyslipidemia, or myocardial infarction for the outcome of ischemic stroke or myocardial infarction. Statistical analyses were performed in Stata (version 17 SE; College Station, TX). The Partners Healthcare institutional review board approved the study (2016P001613/BWH) and ruled that informed consent was not needed.
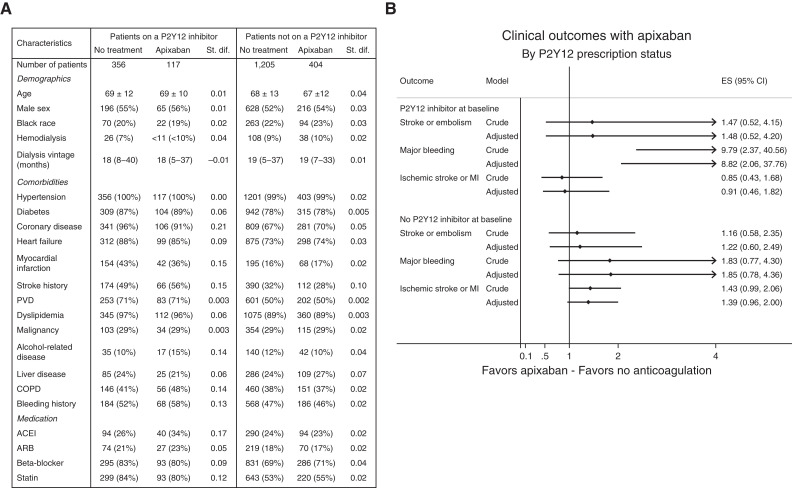
Our propensity score–matched cohort included 2082 patients. Among them, 473 were on a P2Y12 inhibitor (clopidogrel, prasugrel, or ticagrelor) at baseline (117 also received apixaban, whereas 356 did not receive any anticoagulant agents) and 1609 were not on a P2Y12 inhibitor at baseline (404 received apixaban whereas 1205 did not receive any anticoagulant agents). Aspirin prescription was not available in this dataset. Baseline characteristics by P2Y12 prescription status are shown in Figure 1A.
Figure 1.
Apixaban increased the risk of bleeding in patients who were on a P2Y12 inhibitor, compared with no anticoagulation. (A) Baseline characteristics by P2Y12 prescription status at baseline (matched cohort). Results are presented as mean±SD, median (interquartile range), or number (percentage). (B) Clinical outcomes with apixaban by P2Y12 prescription status at baseline. Effect size (ES) is presented as hazard ratio with 95% CI. AC, anticoagulation; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; COPD, chronic obstructive pulmonary disease; MI, myocardial infarction; PVD, peripheral vascular disease; St. dif., standardized difference.
In the subgroup of patients who were on a P2Y12 inhibitor, apixaban use was not associated with lower risk of stroke (ischemic or hemorrhagic), transient ischemic attack, or systemic thromboembolism: 8.2 versus 9.1 events per 100 patient-years in patients treated with apixaban and in patients who did not receive any anticoagulant, respectively (HR, 1.48; 95% CI, 0.52 to 4.20; P=0.46). Similarly, in the subgroup of patients who were not on a P2Y12 inhibitor, the incidence of the primary outcome was similar in patients treated with apixaban and in patients who did not receive any anticoagulant (7.3 versus 6.4 events per 100 patient-years): HR, 1.22; 95% CI, 0.60 to 2.49; P=0.58. There was no interaction between P2Y12 prescription at baseline and the effect of apixaban on stroke (ischemic or hemorrhagic), transient ischemic attack, or systemic thromboembolism (P for interaction=0.66).
The incidence of fatal or intracranial bleeding was higher among patients treated with apixaban compared with patients who did not receive any anticoagulant in the subgroup of patients who were on a P2Y12 inhibitor at baseline (4.9 versus 1.0 events per 100 patient-years): HR, 8.82; 95% CI, 2.06 to 37.76; P=0.003; Figure 1B). In contrast, the incidence of fatal or intracranial bleeding did not differ between the treatment groups among patients who were not on a P2Y12 inhibitor at baseline (2.3 versus 1.8 events per 100 patient-years): HR, 1.85; 95% CI, 0.78 to 4.36; P=0.16; Figure 1B). A significant interaction was detected between P2Y12 prescription status at baseline and the effect of apixaban on fatal or intracranial bleeding: bleeding risk with apixaban was more important among patients with a P2Y12 inhibitor at baseline (P for interaction=0.03).
In the subgroup of patients who were on a P2Y12 inhibitor, the incidence of ischemic stroke or myocardial infarction was similar in both the apixaban and the no anticoagulation groups (incidence of 29.0 versus 38.9 events per 100 patient-years, respectively; HR, 0.91; 95% CI, 0.46 to 1.82; P=0.80). Similarly, the incidence of ischemic stroke or myocardial infarction was similar in both treatment groups among patients who were not on a P2Y12 inhibitor at baseline (incident rate of 27.2 versus 21.5 events per 100 patient-years; HR, 1.39; 95% CI, 0.96 to 2.00; P=0.08). There was no interaction between P2Y12 prescription at baseline and the effect of apixaban on ischemic stroke or myocardial infarction (P for interaction=0.22).
Up to 30% of patients with atrial fibrillation also have coronary disease (4). These patients are frequently treated with a combination of anticoagulant and antiplatelet agents. The atrial fibrillation and ischemic events with rivaroxaban in patients with stable coronary artery disease (AFIRE) trial recently assessed antithrombotic strategies in patients with stable coronary disease and atrial fibrillation (5). The study showed that rivaroxaban monotherapy was superior to the combination of rivaroxaban with an antiplatelet agent with respect to major bleeding and noninferior for the composite efficacy outcome of death from any cause, myocardial infarction, unstable angina requiring revascularization, stroke, or systemic embolism. In this report, we showed that the association of apixaban with a P2Y12 inhibitor significantly increased the risk of fatal or intracranial bleeding, compared with no anticoagulation. In contrast, the incidence of major bleeding was similar in both treatment groups for patients who were not receiving a P2Y12 inhibitor. The incidence of stroke or embolism or the composite outcome of myocardial infarction and ischemic stroke were similar in both treatment groups (apixaban or no anticoagulation), regardless of P2Y12 prescription status at baseline.
Our analysis has important limitations. It is an observational, retrospective study using diagnostic codes. Residual confounding may explain part of the results. The crude number of events for each outcome, provided in Supplemental Table 2, was small for most subgroups. In addition, patients were not matched by P2Y12 prescription status at baseline. Nevertheless, use of competing risk models and similar results in interaction terms and stratified analyses represent important strengths of the study.
In conclusion, the association of apixaban with a P2Y12 inhibitor may be used with caution in patients on maintenance dialysis.
Disclosures
D.M. Charytan reports receiving personal fees and grants from Amgen, Gilead, Janssen, Medtronic, and Novo Nordisk; and receiving personal fees from AstraZeneca, Boehringer Ingelheim/Eli Lilly, Fresenius GlaxoSmithKline, Merck, and Zoll Medical, outside the submitted work. T.A. Mavrakanas reports serving on advisory boards for Boehringer Ingelheim; and receiving honoraria from BMS Canada, Daiichi Sankyo, Janssen, and Pfizer, outside the submitted work.
Funding
T.A. Mavrakanas is supported by a Fonds de Recherche du Québec – Santé Junior 1 Clinician Scholar Award grant 298742, and he has received operating funds from the Quebec Society of Nephrology grant 309790.
Acknowledgments
The data reported here have been supplied by the USRDS. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US Government.
Author Contributions
D.M. Charytan reviewed and edited the manuscript, provided supervision, and was responsible for methodology, project administration, and resources. T.A. Mavrakanas conceptualized the study, wrote the original draft, and was responsible for data curation and formal analysis.
Data Sharing Statement
Data cannot be shared. Restrictions apply to the availability of these data, which were used under license for this study, and, therefore, are not publicly available.
Supplemental Material
This article contains supplemental material online at http://kidney360.asnjournals.org/lookup/suppl/doi:10.34067/KID.0003002022/-/DCSupplemental.
Condition-specific ICD-9 and ICD-10 diagnostic codes used in this study. Download Supplemental Table 1, PDF file, 68 KB (67.6KB, pdf) .
Crude number of events. Download Supplemental Table 2, PDF file, 68 KB (67.6KB, pdf) .
References
- 1.Mavrakanas TA: Should oral anticoagulation be used in ESKD patients on hemodialysis with atrial fibrillation? Commentary. Kidney360 2: 1412–1414, 2021. 10.34067/KID.0001372021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mavrakanas TA, Garlo K, Charytan DM: Apixaban versus no anticoagulation in patients undergoing long-term dialysis with incident atrial fibrillation. Clin J Am Soc Nephrol 15: 1146–1154, 2020. 10.2215/CJN.11650919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.United States Renal Data System : 2021 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2021. Available at https://adr.usrds.org/2021. Accessed on July 6, 2022 [Google Scholar]
- 4.Verheugt FWA, Ten Berg JM, Storey RF, Cuisset T, Granger CB: Antithrombotics: From aspirin to DOACs in coronary artery disease and atrial fibrillation (part 3/5). J Am Coll Cardiol 74: 699–711, 2019. 10.1016/j.jacc.2019.02.080 [DOI] [PubMed] [Google Scholar]
- 5.Yasuda S, Kaikita K, Akao M, Ako J, Matoba T, Nakamura M, Miyauchi K, Hagiwara N, Kimura K, Hirayama A, Matsui K, Ogawa H; AFIRE Investigators : Antithrombotic therapy for atrial fibrillation with stable coronary disease. N Engl J Med 381: 1103–1113, 2019. 10.1056/NEJMoa1904143 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Condition-specific ICD-9 and ICD-10 diagnostic codes used in this study. Download Supplemental Table 1, PDF file, 68 KB (67.6KB, pdf) .
Crude number of events. Download Supplemental Table 2, PDF file, 68 KB (67.6KB, pdf) .