Abstract
BACKGROUND
The incidence of anal cancer is substantially higher among persons living with the human immunodeficiency virus (HIV) than in the general population. Similar to cervical cancer, anal cancer is preceded by high-grade squamous intraepithelial lesions (HSILs). Treatment for cervical HSIL reduces progression to cervical cancer; however, data from prospective studies of treatment for anal HSIL to prevent anal cancer are lacking.
METHODS
We conducted a phase 3 trial at 25 U.S. sites. Persons living with HIV who were 35 years of age or older and who had biopsy-proven anal HSIL were randomly assigned, in a 1:1 ratio, to receive either HSIL treatment or active monitoring without treatment. Treatment included office-based ablative procedures, ablation or excision under anesthesia, or the administration of topical fluorouracil or imiquimod. The primary outcome was progression to anal cancer in a time-to-event analysis. Participants in the treatment group were treated until HSIL was completely resolved. All the participants underwent high-resolution anoscopy at least every 6 months; biopsy was also performed for suspected ongoing HSIL in the treatment group, annually in the active-monitoring group, or any time there was concern for cancer.
RESULTS
Of 4459 participants who underwent randomization, 4446 (99.7%) were included in the analysis of the time to progression to cancer. With a median follow-up of 25.8 months, 9 cases were diagnosed in the treatment group (173 per 100,000 person-years; 95% confidence interval [CI], 90 to 332) and 21 cases in the active-monitoring group (402 per 100,000 person-years; 95% CI, 262 to 616). The rate of progression to anal cancer was lower in the treatment group than in the active-monitoring group by 57% (95% CI, 6 to 80; P = 0.03 by log-rank test).
CONCLUSIONS
Among participants with biopsy-proven anal HSIL, the risk of anal cancer was significantly lower with treatment for anal HSIL than with active monitoring. (Funded by the National Cancer Institute; ClinicalTrials.gov number, NCT02135419.)
Like cervical cancer, anal cancer is caused by human papillomavirus (HPV) infection, particularly HPV16,1 and is preceded by a high-grade squamous intraepithelial lesion (HSIL), a precancerous growth that is also known as anal intraepithelial neoplasia (AIN) 2 or 3.2 Although anal cancer is rare in the general population, the incidence, percentage of patients presenting with advanced disease, and associated mortality have been increasing in the United States and other developed countries since the 1970s.3 The risk of anal cancer varies widely among different population groups, with the highest risk seen in persons living with the human immunodeficiency virus (HIV).4 Other high-risk groups include persons with immunosuppression for solid-organ transplantation and women with a history of vulvar or cervical HSIL or cancer.4,5 Additional risk factors include a history of receptive anal sexual intercourse, a history of genital warts, anal fissures or fistulas, and smoking.6
The incidence of anal cancer among men who have sex with men and are living with HIV is estimated to be 89 per 100,000 person-years.4,7 Among women living with HIV, the incidence ranges from 18.6 to 35.6 per 100,000 person-years.7 In comparison, the incidence of anal cancer in the general population is 1.6 per 100,000 person-years,3 whereas that of cervical cancer is 7.5 per 100,000 person-years8 in the general population of U.S. women.
Persons living with HIV have a high prevalence and incidence of anal HPV infection and anal HSIL,9 findings consistent with their elevated risk of anal cancer. In a meta-analysis, the risk of progression from anal HSIL to cancer was estimated to be 265 per 100,000 person-years10 among men who have sex with men and are living with HIV. However, reported risk estimates in some cohorts of persons living with HIV or transplant recipients11–14 are even higher, and Danish data show a 5-year incidence of progression to cancer of 14.1% among persons living with HIV with anal intraepithelial neoplasia.15
Efforts to prevent anal cancer in at-risk groups have been modeled after programs for the secondary prevention of cervical cancer.16 These efforts consist of anal HSIL screening with the use of cytologic analysis, identification of HSIL through high-resolution anoscopy in those who are screen-positive, and HSIL removal through ablation, surgical excision, or other treatments. The prevention of anal cancer is desirable because anal cancer is associated with poor survival when detected at late stages,17,18 and treatment of cancers at all stages with standard chemoradiation therapy is associated with acute and chronic adverse effects.
Current recommendations for anal HSIL screening and treatment are based on expert opinion and lack rigorous evidence-based support.19,20 Formal inclusion of anal-cancer prevention programs in standard-of-care guidelines has awaited direct evidence that treatment for anal HSIL reduces the risk of progression to anal cancer. The purpose of the Anal Cancer–HSIL Outcomes Research (ANCHOR) trial was to determine whether treating anal HSIL is effective and safe in reducing progression to anal cancer among persons living with HIV as compared with active monitoring of HSIL without treatment.
METHODS
TRIAL DESIGN AND OVERSIGHT
This phase 3 randomized, controlled trial was performed at 25 sites in the United States. The trial was approved by the institutional review boards at all the participating clinical sites, and an independent data safety and monitoring board was appointed by the National Cancer Institute to monitor the trial. The authors performed the statistical analysis and wrote the first draft of the manuscript. The final submitted manuscript incorporated changes recommended by the co-authors and was reviewed and approved by all the authors, who vouch for the accuracy and completeness of the data and for adherence of the trial to the protocol. No one who is not an author contributed to the manuscript. No commercial support was provided for trial planning and execution. Additional details of the trial design have been published previously21 and are also provided in the Supplementary Appendix and protocol, available with the full text of this article at NEJM.org.
TRIAL POPULATION
Persons living with HIV who were 35 years of age or older were invited for anal HSIL screening. After written informed consent was provided, an anal swab was collected for liquid-based anal cytologic analysis. A complete physical examination and high-resolution anoscopy were performed. Lesions suspicious for HSIL or cancer were biopsied according to published standards22 by clinicians certified for competence in high-resolution anoscopy (Fig. S1 in the Supplementary Appendix).
Participants were eligible for the trial if HSIL (AIN3 or p16-positive AIN2)23 had been diagnosed in at least one anal-canal or perianal biopsy sample by a local pathology laboratory. Those with a history of anal cancer or anal cancer detected at screening were ineligible and were referred for therapy as appropriate. A complete list of inclusion and exclusion criteria is provided in the trial protocol.
RANDOMIZATION, STRATIFICATION, AND MASKING
Eligible participants returned within 12 weeks for randomization, at which time blood samples were collected for the measurement of plasma HIV-1 RNA and CD4 levels. High-resolution anoscopy was repeated to confirm the presence of HSIL. Randomization was stratified according to trial site, the nadir CD4 count (≤200 vs. >200 cells per cubic millimeter), and lesion size at randomization (≤50% vs. >50% of the anal canal or perianal region). Participants were randomly assigned in a 1:1 ratio to the treatment group or the active-monitoring group with the use of a permuted random block design.
TRIAL PROCEDURES
Participants who were assigned to the treatment group underwent immediate treatment with the aim of eradicating all HSIL. Clinicians selected the method of treatment from a list of protocol-defined options, in accordance with clinician and participant preference, using method-specific algorithms (Fig. S2A and S2B). These treatments included ablative procedures (infrared coagulation, electrocautery, and laser), ablation or excision under anesthesia, and topical treatments (imiquimod and fluorouracil). Participants in the treatment group returned for repeat high-resolution anoscopy in accordance with the treatment protocol and at least every 6 months after randomization after all HSIL had been fully treated. Lesions suspicious for HSIL were biopsied at any visit, and any recurrence was treated. Participants in the active-monitoring group underwent high-resolution anoscopy every 6 months after randomization. Visible lesions were biopsied annually to confirm ongoing HSIL and the absence of cancer. Participants in either group with lesions arousing concern for imminent progression to cancer could be seen every 3 months. Lesions suspicious for cancer could be biopsied at any time. Participants who received a diagnosis of biopsy-proven cancer were immediately removed from the trial and referred for appropriate therapy.
OUTCOMES
The primary outcome was progression to anal cancer in a time-to-event analysis. A secondary outcome was the safety of treatments for anal HSIL.
STATISTICAL ANALYSIS
Sample-size estimates were based on a log-rank test to compare the time to progression to anal cancer between the treatment group and the active-monitoring group under the following assumptions: rates of progression to cancer in both groups were constant over time, with a 3-year accrual period and 5 years of follow-up, a 5% annual dropout rate in both groups, and a 7% annual nonadherence rate in the active-monitoring group.24,25 Participants in the active-monitoring group who received treatment for HSIL after randomization were classified as having been nonadherent to the trial protocol. Participants in either group who withdrew informed consent or who died during the trial were defined as having dropped out. Data were censored at the time of nonadhererence or dropout. The trial had a power of 90% to detect a difference between a rate of progression to cancer of 50 per 100,000 person-years in the treatment group and a rate of 200 per 100,000 person-years in the active-monitoring group (75% lower rate in the treatment group) at a two-sided 0.05 significance level; this required 2529 participants per group for a total of 5058 participants to detect 31 anal cancers.
Two interim analyses of the primary outcome were conducted after 50% and 75% of the projected cancer cases had been observed to assess the futility of detecting a significant between-group difference and to evaluate efficacy before full enrollment had been completed. A final analysis was performed when 100% of the projected cases had been observed. The Lan and DeMets spending function was used to specify the O’Brien–Fleming boundaries on the basis of a two-sided log-rank test (alpha level, 0.05).26 At the final test, an overall two-sided alpha level of 0.05 and 90% power were maintained.
The primary analysis population was the intention-to-treat population, which included all the eligible participants who had undergone randomization. Poisson regression models were used to compare the two groups with respect to dropout rates and serious adverse events. For each participant, the time until cancer detection was defined as the time from randomization to diagnosis of anal cancer, with data collection censored at the date of the last follow-up or time of nonadherence. A confirmatory analysis was performed without censoring data for the participants in the active-monitoring group at the time of nonadherence. The log-rank test was used to compare the treatment group and the active-monitoring group with respect to the time to detection of anal cancer. The Cox proportional-hazards model was used to assess the association of lesion size and nadir CD4 count with the time to cancer detection. Poisson rates and 95% confidence intervals were used to describe cancer risk.
The AdvantageEDC electronic data-capture system, which had been developed and was maintained by the Emmes Company, was used to collect trial data. Sample-size estimates were generated with the use of PASS software. Analyses were performed with the use of SAS software, version 9.4.
RESULTS
PARTICIPANTS
From September 24, 2014, to August 5, 2021, a total of 10,723 participants underwent screening (Figs. 1 and S3). Biopsy-confirmed HSIL was diagnosed in 4257 of 7729 men (55.1%), in 860 of 1822 women (47.2%), and in 188 of 280 transgender persons (67.1%) on the basis of complete results from cytologic and histologic analyses and high-resolution anoscopy. A total of 17 participants (0.2%) received a diagnosis of anal cancer. The primary reason for screening failure was the absence of biopsy-proven HSIL (Table S2). Table 1 and Table S1 show the demographic characteristics of the participants who underwent randomization according to trial group. A total of 4459 participants underwent randomization, 2237 to the treatment group and 2222 to the active-monitoring group. A total of 4446 participants had at least one follow-up visit after randomization and were included in the analysis. Overall, the characteristics of the participants were well balanced between the two groups and were similar to those of the overall U.S. population of persons living with HIV, with some small differences (Table S3).
Figure 1. Screening, Randomization, and Follow-Up.
Of the six participants in the treatment group who discontinued the intervention for “other reason,” two received a diagnosis of anal cancer but were ineligible for analysis and were discontinued from the trial because they were determined to have had anal cancer before randomization, three relocated to a nontrial site, and one received immunomodulatory agents after a kidney transplantation. Of the two participants in the active-monitoring group who discontinued the intervention for “other reason,” both relocated to a nontrial site. A total of 25 of 2219 participants (1.1%) in the active-monitoring group were considered to be nonadherent because they had received treatment for anal high-grade squamous intraepithelial lesions at some time after randomization.
Table 1.
Demographic and Clinical Characteristics of the Participants at Baseline.*
| Characteristic | Treatment Group (N = 2227) | Active-Monitoring Group (N = 2219) |
|---|---|---|
| Median age (IQR) — yr | 51 (44–57) | 51 (44–57) |
| Median time since HIV diagnosis (IQR) — yr | 17 (10–24) | 17 (10–25) |
| Median follow‑up (IQR) — mo | 25.3 (11.7–42.0) | 27.2 (12.0–42.1) |
| Gender identity — no. (%) | ||
| Male | 1793 (80.5) | 1782 (80.3) |
| Female | 346 (15.5) | 365 (16.4) |
| Transgender | 85 (3.8) | 68 (3.1) |
| Nonbinary | 2 (0.1) | 2 (0.1) |
| Declined to answer | 1 (<0.1) | 2 (0.1) |
| Race or ethnic group — no. (%)† | ||
| Black | 935 (42.0) | 939 (42.3) |
| Non-Hispanic White | 695 (31.2) | 737 (33.2) |
| Non-Black Hispanic | 381 (17.1) | 339 (15.3) |
| Asian or Pacific Islander | 27 (1.2) | 29 (1.3) |
| Other or unknown | 189 (8.5) | 175 (7.9) |
| CDC criterion for risk of HIV infection — no. (%)‡ | ||
| Male-to-male sexual contact | 1716 (77.1) | 1717 (77.4) |
| Heterosexual | 532 (23.9) | 510 (23.0) |
| Injection-drug use | 152 (6.8) | 177 (8.0) |
| Transfusion | 53 (2.4) | 47 (2.1) |
| Hemophilia | 2 (0.1) | 4 (0.2) |
| Other | 34 (1.5) | 27 (1.2) |
| Smoking history — no. (%) | ||
| Current smoker | 710 (31.9) | 743 (33.5) |
| Smoked >100 cigarettes over lifetime§ | 1268 (56.9) | 1353 (61.0) |
| History of HSIL treatment ≥6 mo before randomization — no. (%)¶ | 228 (10.2) | 215 (9.7) |
| Plasma HIV-1 RNA copies/ml — no./total no. (%) | ||
| <50 | 1853/2213 (83.7) | 1800/2201 (81.8) |
| 51–199 | 155/2213 (7.0) | 160/2201 (7.3) |
| 200–1000 | 83/2213 (3.8) | 93/2201 (4.2) |
| >1000 | 122/2213 (5.5) | 148/2201 (6.7) |
| Median CD4 count (IQR) — cells/mm3‖ | 602 (393–827) | 607 (410–837) |
| Nadir CD4 count — no. (%)** | ||
| ≤200 cells/mm3 | 1130 (50.7) | 1121 (50.5) |
| >200 cells/mm3 | 1097 (49.3) | 1098 (49.5) |
| HSIL size at screening — no. (%)** | ||
| >50% of anal canal or perianal region | 285 (12.8) | 282 (12.7) |
| ≤50% of anal canal or perianal region | 1942 (87.2) | 1937 (87.3) |
Percentages may not total 100 because of rounding. CDC denotes Centers for Disease Control and Prevention, HIV human immunodeficiency virus, and IQR interquartile range.
Race and ethnic group were reported by the participant.
Percentages exceed 100% because some participants classified themselves in more than one category.
Data were missing for 64 participants in the treatment group and 53 participants in the active-monitoring group.
Previous treatment of anal high-grade squamous intraepithelial lesions (HSILs) was an exclusion criterion until the adoption of version 8 of the protocol in December 2017.
Data were missing for 6 participants in the treatment group and 12 participants in the active-monitoring group.
This characteristic was a stratification factor at randomization.
Central pathological review of screening biopsies from 4257 participants who had received a diagnosis of HSIL from the local pathology laboratory confirmed HSIL in 4113 participants (96.6%); participants without confirmed HSIL remained in the trial and were included in the intention-to-treat analysis. Of the 4446 participants followed after randomization, 4151 (93.4%) remained in the trial at the time of trial closure. Reasons for trial discontinuation are shown in Figure 1.
INTERVENTIONS AND FOLLOW-UP
In the treatment group, the initial treatment was office-based electrocautery ablation (primarily hyfrecation) in 1862 of 2227 participants (83.6%), infrared coagulation in 107 (4.8%), ablation or excision under anesthesia in 52 (2.3%), topical fluorouracil in 100 (4.5%), and topical imiquimod in 12 (0.5%). More than one method of treatment was used initially in 46 participants (2.1%), and 48 participants (2.2%) were not treated initially. Over the course of the trial, 1921 participants (86.3%) were treated with one therapeutic method, 233 (10.5%) with two methods, 33 (1.5%) with three methods, and 1 (<0.1%) with four methods.
The data and safety monitoring board was notified when 32 cases of anal cancer had been observed; on data cleaning, 2 cases were determined to be ineligible (Fig. 1). In consultation with the data and safety monitoring board, we proceeded with the analysis with 30 cases. Participants in the treatment group and the active monitoring group contributed a median of 25.3 and 27.2 months of follow-up, respectively (Table 1); the overall median follow-up time was 25.8 months. The cumulative dropout rates were 2.8 per 100 person-years in the treatment group and 2.2 per 100 person-years in the active-monitoring group. The cumulative nonadherence rate in the active-monitoring group was 0.5 per 100 person-years.
PRIMARY OUTCOME
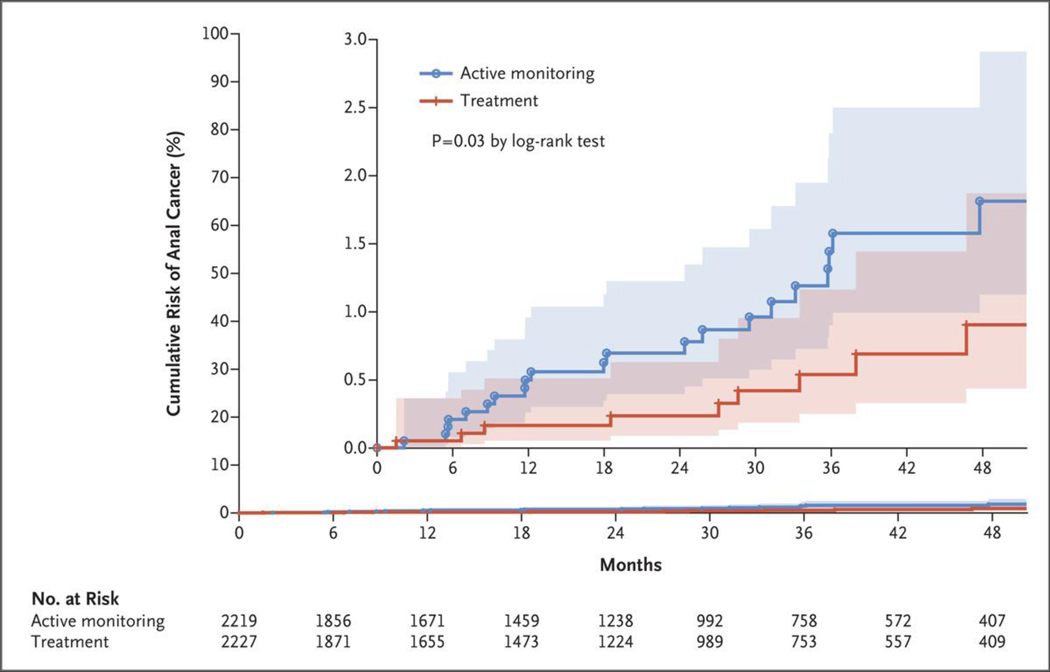
A total of 9 participants in the treatment group received a diagnosis of invasive anal cancer, as did 21 participants in the active-monitoring group. All cases were confirmed on central pathological review. The observed rate of progression to cancer in the treatment group was 173 per 100,000 person-years (95% confidence interval [CI], 90 to 332) of follow-up, as compared with 402 per 100,000 person-years (95% CI, 262 to 616) in the active-monitoring group. The rate was lower in the treatment group than in the active-monitoring group by 57% (95% CI, 6 to 80; P = 0.03 by log-rank test). The cumulative incidence of progression to anal cancer at 48 months was 0.9% in the treatment group and 1.8% in the active-monitoring groups. Kaplan–Meier curves of the time to cancer detection in both groups are shown in Figure 2.
Figure 2. Kaplan–Meier Curve of the Time to Progression to Anal Cancer.
The inset shows the data on an expanded y axis. The shaded areas represent 95% confidence intervals.
A total of 25 participants in the active-monitoring group were nonadherent, and the median time to nonadherence was 9.0 months (interquartile range, 1.8 to 17.9). An analysis in which these participants’ time to cancer detection was not censored at the time of nonadherence confirmed the findings that treatment reduced anal cancer (P = 0.03 by log-rank test).
The characteristics of the participants in whom cancer developed are shown in Table S4. A total of 8 of 9 participants (89%) who had progression to cancer in the treatment group were treated with electrocautery and 1 of 9 (11%) was treated with infrared coagulation. The proportional-hazards model showed that the time to progression to anal cancer was associated with lesion size (hazard ratio, 5.26; 95% CI, 2.54 to 10.87) but not with nadir CD4 count (hazard ratio, 1.93; 95% CI, 0.88 to 4.23). The rate of progression to anal cancer was 1047 per 100,000 person-years among participants with a lesion size of more than 50% of the anal canal or perianal region and 185 per 100,000 person-years among those with a lesion size of 50% or less of the anal canal or perianal region.
ADVERSE EVENTS
Seven serious adverse events that were considered by the investigators to be related to a trial intervention were reported in the treatment group, and one such event was reported in the active-monitoring group (Table 2). Death occurred in 55 participants in the treatment group and 48 participants in the active-monitoring group. No deaths were determined by the investigators to be related to trial interventions. The causes of death are shown in Table S5.
Table 2.
Adverse Events.
| Events | Treatment Group | Active-Monitoring Group |
|---|---|---|
| number | ||
| Adverse events | 683 | 635 |
| Serious adverse events* | 586 | 568 |
| Trial-related adverse events† | 43 | 4 |
| Trial-related serious adverse events‡ | 7 | 1 |
| Skin ulceration due to fluorouracil | 1 | 0 |
| Anal abscess due to electrocautery | 1 | 0 |
| Pain due to electrocautery | 1 | 0 |
| Pain due to treatment under anesthesia | 1 | 0 |
| Pain due to infrared coagulation | 1 | 0 |
| Infection or abscess due to anal biopsy | 2 | 1 |
Shown are all serious adverse events regardless of intervention, as determined by the investigators. P = 0.61 for the between-group difference.
Shown are adverse events with a possible, probable, or definite relationship to trial interventions, as determined by the investigators.
Shown are serious adverse events with a possible, probable, or definite relationship to trial interventions, as determined by the investigators. P = 0.07 for the between-group difference.
DISCUSSION
Anal cancer is among the limited types of cancers that are potentially preventable through treatment of known cancer precursors. Treating cervical HSIL is effective in reducing progression to cervical cancer, although data from randomized clinical trials are lacking.15 The Minnesota Colon Cancer Control Study showed a 67% reduction in colon cancer over a period of 24 years with polypectomy.27 In this trial, we found that participants who had undergone treatment for anal HSIL had a rate of progression to anal cancer that was nearly 60% lower than those who had undergone active monitoring and not received treatment. The trial was not designed to compare the efficacy of different methods of treatment of HSIL; however, most participants were treated with electrocautery (primarily hyfrecation), an office-based procedure that is quick and generally has an acceptable adverse-event profile.
The rate of progression to cancer among the participants in the active-monitoring group, at 402 per 100,000 person-years, was higher than expected on the basis of published estimates from cancer–HIV registry matches,6 even after accounting for all the trial participants having HSIL. This finding may in part reflect early cancer detection in our trial; in the absence of screening, anal cancer is usually diagnosed after the development of symptoms. Consistent with this finding, the percentage of stage I or II cancers that were diagnosed in the active-monitoring group was higher than that reported in national data.3 This result may also partially reflect the higher proportion of smokers and participants reporting male-to-male sexual contact than in the overall U.S. population of persons living with HIV.
Not all anal cancers were prevented through HSIL treatment. Similarly, treatment of cervical HSIL or colon polyps does not completely eliminate the risk of progression to cervical cancer28–31 or colon cancer,27 respectively. In the cervix, larger lesion size and positive margins are associated with an increased risk of recurrent HSIL after treatment, particularly among women living with HIV,32 and progression to cervical cancer.33 Similarly, a larger lesion size was associated with an increased risk of progression to anal cancer in our trial. Treatment of anal HSIL is particularly challenging in persons living with HIV owing to a large lesion burden and number as reflected by high rates of HSIL recurrence or metachronous disease with currently available methods of treatment.34–36 The high rate of anal cancer in the treatment group highlights the need for more effective HSIL treatment approaches and for close follow-up after HSIL treatment. Smoking was common in our population and is a modifiable risk factor for anal cancer.
Strengths of the trial include a large, multicenter, diverse population of participants with characteristics that were similar to those of the overall U.S. population of persons living with HIV.37 Trial procedures were performed by clinicians who were well trained in high-resolution anoscopy with pretrial qualification and ongoing quality assurance. Central pathological review was performed on almost all biopsy samples with positive results for HSIL during screening and all cases of anal cancer during the trial. The treatment approaches in the trial were guided by high-resolution anoscopy and selected to replicate those currently used in the community, maximizing generalizability. We had high trial retention with low nonadherence and dropout rates. A limitation of the trial is that our results may not be replicated if high-resolution anoscopy and treatment are performed by clinicians with less training and clinical support.
The high rate of progression from HSIL to cancer among persons living with HIV in our trial highlights the need for strong prevention efforts. HPV vaccination effectively prevents the initial acquisition of anal HPV38 and the development of anal HSIL in young persons living with HIV.39 However, secondary prevention programs including treatment of anal HSIL are urgently needed for those already exposed to anal HPV. Additional research is needed to improve screening algorithms to identify anal HSIL. High-resolution anoscopy is not a feasible screening tool given its cost and limited availability, and both anal cytologic analysis and anal HPV testing have limitations.40 Expansion of diagnostic and therapeutic training programs in the use of high-resolution anoscopy is also needed.
Our data show that treatment of anal HSIL, primarily with office-based electrocautery, significantly reduced the risk of progression to anal cancer among persons living with HIV who were 35 years of age or older. Such treatment was associated with a low incidence of serious adverse events. Our data provide support for the use of screening and treatment for anal HSIL as the standard of care for persons living with HIV who are 35 years of age or older. Additional considerations should include assessment of the effect on quality of life and other risk–benefit measures. Our data may also be relevant for other groups at increased risk for anal cancer.
Supplementary Material
Acknowledgments
The views expressed in this article are those of the authors and do not necessarily represent the official views of the National Cancer Institute or the National Institutes of Health.
Supported by the National Cancer Institute (award number 2 UM1 CA121947). In-kind support was provided by Hologic (ThinPrep vials) and Bausch Health (fluorouracil cream).
Footnotes
REFERENCES
- 1.Hoots BE, Palefsky JM, Pimenta JM, Smith JS. Human papillomavirus type distribution in anal cancer and anal intraepithelial lesions. Int J Cancer 2009; 124:2375–83. [DOI] [PubMed] [Google Scholar]
- 2.Berry JM, Jay N, Cranston RD, et al. Progression of anal high-grade squamous intraepithelial lesions to invasive anal cancer among HIV-infected men who have sex with men. Int J Cancer 2014;134:1147–55. [DOI] [PubMed] [Google Scholar]
- 3.Deshmukh AA, Suk R, Shiels MS, et al. Recent trends in squamous cell carcinoma of the anus incidence and mortality in the United States, 2001–2015. J Natl Cancer Inst 2020;112:829–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clifford GM, Georges D, Shiels MS, et al. A meta-analysis of anal cancer incidence by risk group: toward a unified anal cancer risk scale. Int J Cancer 2021;148:38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sand FL, Munk C, Jensen SM, Svahn MF, Frederiksen K, Kjær SK. Long-term risk for noncervical anogenital cancer in women with previously diagnosed high-grade cervical intraepithelial neoplasia: a Danish nationwide cohort study. Cancer Epidemiol Biomarkers Prev 2016;25:1090–7. [DOI] [PubMed] [Google Scholar]
- 6.Holly EA, Whittemore AS, Aston DA, Ahn DK, Nickoloff BJ, Kristiansen JJ. Anal cancer incidence: genital warts, anal fissure or fistula, hemorrhoids, and smoking. J Natl Cancer Inst 1989;81:1726–31. [DOI] [PubMed] [Google Scholar]
- 7.Colón-López V, Shiels MS, Machin M, et al. Anal cancer risk among people with HIV infection in the United States. J Clin Oncol 2018;36:68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cancer stat facts: cervical cancer. Bethesda, MD: National Cancer Institute; (https://seer.cancer.gov/statfacts/html/cervix.html). [Google Scholar]
- 9.Wei F, Gaisa MM, D’Souza G, et al. Epidemiology of anal human papillomavirus infection and high-grade squamous intraepithelial lesions in 29 900 men according to HIV status, sexuality, and age: a collaborative pooled analysis of 64 studies. Lancet HIV 2021; 8(9): e531–e543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Machalek DA, Poynten M, Jin F, et al. Anal human papillomavirus infection and associated neoplastic lesions in men who have sex with men: a systematic review and meta-analysis. Lancet Oncol 2012;13:487–500 [DOI] [PubMed] [Google Scholar]
- 11.Watson AJ, Smith BB, Whitehead MR, Sykes PH, Frizelle FA. Malignant progression of anal intra-epithelial neoplasia. ANZ J Surg 2006;76:715–7. [DOI] [PubMed] [Google Scholar]
- 12.Cajas-Monson LC, Ramamoorthy SL, Cosman BC. Expectant management of high-grade anal dysplasia in people with HIV: long-term data. Dis Colon Rectum 2018;61:1357–63. [DOI] [PubMed] [Google Scholar]
- 13.McCutcheon T, Hawkins AT, Muldoon RL, Hopkins MB, Geiger TM, Ford MM. Progression of anal intraepithelial neoplasia in HIV-positive individuals: predisposing factors. Tech Coloproctol 2019;23:325–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee GC, Kunitake H, Milch H, et al. What is the risk of anal carcinoma in patients with anal intraepithelial neoplasia III? Dis Colon Rectum 2018;61:1350–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faber MT, Frederiksen K, Palefsky JM, Kjaer SK. Risk of anal cancer following benign anal disease and anal cancer precursor lesions: a Danish nationwide cohort study. Cancer Epidemiol Biomarkers Prev 2020;29:185–92. [DOI] [PubMed] [Google Scholar]
- 16.Bray F, Loos AH, McCarron P, et al. Trends in cervical squamous cell carcinoma incidence in 13 European countries: changing risk and the effects of screening. Cancer Epidemiol Biomarkers Prev 2005;14:677–86. [DOI] [PubMed] [Google Scholar]
- 17.Ludmir EB, Kachnic LA, Czito BG. Evolution and management of treatment-related toxicity in anal cancer. Surg Oncol Clin N Am 2017;26:91–113. [DOI] [PubMed] [Google Scholar]
- 18.Benson AB, Venook AP, Al-Hawary MM, et al. Anal carcinoma, version 2.2021, NCCN Clinical Practice Guidelines in Oncology. National Comprehensive Cancer Network, 2021. (https://www.nccn.org/professionals/physician_gls/pdf/anal.pdf). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson MA, Horberg MA, Agwu AL, et al. Primary care guidance for persons with Human Immunodeficiency Virus: 2020 update by the HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis 2021;73(11):e3572-e3605. [DOI] [PubMed] [Google Scholar]
- 20.Stewart DB, Gaertner WB, Glasgow SC, Herzig DO, et al. The American Society of Colon and Rectal Surgeons clinical practice guidelines for anal squamous cell cancers (revised 2018). Dis Colon Rectum 2018;61:755–74. [DOI] [PubMed] [Google Scholar]
- 21.Lee JY, Lensing SY, Berry-Lawhorn JM, et al. Design of the ANal Cancer/HSIL Outcomes Research study (ANCHOR study): a randomized study to prevent anal cancer among persons living with HIV. Contemp Clin Trials 2022;113:106679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hillman RJ, Cuming T, Darragh T, et al. 2016 IANS international guidelines for practice standards in the detection of anal cancer precursors. J Low Genit Tract Dis 2016;20:283–91. [DOI] [PubMed] [Google Scholar]
- 23.Darragh TM, Colgan TJ, Thomas Cox J, et al. The Lower Anogenital Squamous Terminology Standardization project for HPV-associated lesions: background and consensus recommendations from the College of American Pathologists and the American Society for Colposcopy and Cervical Pathology. Int J Gynecol Pathol 2013;32:76–115. [DOI] [PubMed] [Google Scholar]
- 24.Lakatos E. Sample sizes based on the log-rank statistic in complex clinical trials. Biometrics 1988;44:229–41. [PubMed] [Google Scholar]
- 25.Lakatos E. Designing complex group sequential survival trials. Stat Med 2002;21:1969–89. [DOI] [PubMed] [Google Scholar]
- 26.Lan KKG, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika 1983;70:659–63. [Google Scholar]
- 27.Shaukat A, Shyne M, Mandel JS, Snover D, Church TR. Colonoscopy with polypectomy reduces long-term incidence of colorectal cancer in both men and women: extended results from the Minnesota Colon Cancer Control Study. Gastroenterology 2021;160(4):1397–1399.e3. [DOI] [PubMed] [Google Scholar]
- 28.Soutter WP, Sasieni P, Panoskaltsis T. Long-term risk of invasive cervical cancer after treatment of squamous cervical intraepithelial neoplasia. Int J Cancer 2006;118:2048–55. [DOI] [PubMed] [Google Scholar]
- 29.Melnikow J, McGahan C, Sawaya GF, Ehlen T, Coldman A. Cervical intraepithelial neoplasia outcomes after treatment: long-term follow-up from the British Columbia Cohort Study. J Natl Cancer Inst 2009;101:721–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Massad LS, Hessol NA, Darragh TM, et al. Cervical cancer incidence after up to 20 years of observation among women with HIV. Int J Cancer 2017;141:1561–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCredie MRE, Sharples KJ, Paul C, et al. Natural history of cervical neoplasia and risk of invasive cancer in women with cervical intraepithelial neoplasia 3: a retrospective cohort study. Lancet Oncol 2008;9:425–34. [DOI] [PubMed] [Google Scholar]
- 32.Reimers LL, Sotardi S, Daniel D, et al. Outcomes after an excisional procedure for cervical intraepithelial neoplasia in HIV-infected women. Gynecol Oncol 2010;119:92–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tidbury P, Singer A, Jenkins D. CIN 3: the role of lesion size in invasion. Br J Obstet Gynaecol 1992;99:583–6. [DOI] [PubMed] [Google Scholar]
- 34.Richel O, de Vries HJC, van Noesel CJM, Dijkgraaf MGW, Prins JM. Comparison of imiquimod, topical fluorouracil, and electrocautery for the treatment of anal intraepithelial neoplasia in HIV-positive men who have sex with men: an open-label, randomised controlled trial. Lancet Oncol 2013;14:346–53. [DOI] [PubMed] [Google Scholar]
- 35.Gaisa MM, Liu Y, Deshmukh AA, Stone KL, Sigel KM. Electrocautery ablation of anal high-grade squamous intraepithelial lesions: effectiveness and key factors associated with outcomes. Cancer 2020;126:1470–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Goldstone SE, Lensing SY, Stier EA, et al. A randomized clinical trial of infrared coagulation ablation versus active monitoring of intra-anal high-grade dysplasia in adults with Human Immunodeficiency Virus infection: an AIDS Malignancy Consortium Trial. Clin Infect Dis 2019;68:1204–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention. HIV surveillance supplemental report: estimated HIV incidence and prevalence in the United States, 2015–2019. 2021. (https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-supplemental-report-vol-26-1.pdf).
- 38.Palefsky JM, Giuliano AR, Goldstone S, et al. HPV vaccine against anal HPV infection and anal intraepithelial neoplasia. N Engl J Med 2011;365:1576–85. [DOI] [PubMed] [Google Scholar]
- 39.Palefsky JM, Lensing SY, Belzer M, et al. High prevalence of anal high-grade squamous intraepithelial lesions, and prevention through Human Papillomavirus vaccination, in young men who have sex with men living with Human Immunodeficiency Virus. Clin Infect Dis 2021;73:1388–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clarke MA, Wentzensen N. Strategies for screening and early detection of anal cancers: a narrative and systematic review and meta-analysis of cytology, HPV testing, and other biomarkers. Cancer Cytopathol 2018;126:447–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.