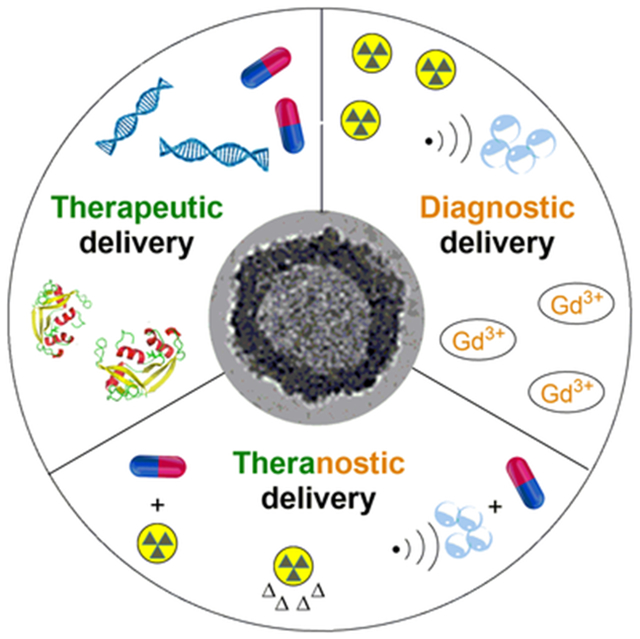
Abstract
Crosslinked polymer nanocapsules (CPNCs) are hollowed nanoparticles with network-like polymeric shells stabilized by primary bonds. CPNCs have drawn broad and significant interests as nanocarriers for biomedical applications in recent years. As compared with conventional polymeric nanoparticles systems without cavity and/or crosslinking architectures, CPNCs possess significant biomedical relevant advantages, including 1) superior structural stability against environmental conditions, 2) high loading capacity and ability for region-specific loading of multiple cargos, 3) tuneable cargo release rate via crosslinking density, and 4) high specific surface area to facilitate surface adsorption, modification, and interactions. With appropriate base polymers and crosslinkages, CPNCs can be biocompatible and biodegradable. While CPNC-based biomedical nanoplatforms can possess relatively stable physicochemical properties owing to their crosslinked architectures, various biomedically relevant stimuli-responsivities can be incorporated with them through specific structural designs. CPNCs have been studied for the delivery of small molecule drugs, genes, proteins, and other therapeutic agents. They have also been investigated as diagnostic platforms for magnetic resonance imaging (MRI), ultrasound imaging, and optical imaging. Moreover, CPNCs have been utilized to carry both therapeutics and bioimaging agents for theranostic applications. This article reviews the therapeutic, diagnostic and theranostic applications of CPNCs, as well as the preparation of these CPNCs, reported in the past decade.
Keywords: nanocapsule, drug delivery, gene delivery, bioimaging, theranostics
Graphical/Visual Abstract and Caption
As a unique class of nanocarriers with inner cavities enclosed by network-like polymer shells, crosslinked polymer nanocapsules have been broadly studied as the platforms for therapeutic delivery, bioimaging, and theranostic applications.
INTRODUCTION
Nanoscopic dimensions of biomaterials are critically relevant to their biomedical applications, especially these via systemic circulations (Farokhzad, & Langer, 2006). To integrate nano-sized biomaterials with medical technologies for achieving improved medical outcomes and reduced side effects, a broad variety of nanocarriers derived from assemblies of small molecules (Kraft, Freeling, Wang, & Ho, 2014), polymer nanostructures (Duncan, 2011), inorganic nanoparticles (Liong et al, 2008), and inorganic-organic/polymer hybrid nanomaterials (Taylor-Pashow, Della Rocca, Huxford, & Lin, 2010) have been developed and studied for therapeutic and diagnostic applications. Among them, assemblies of small molecules typically have relatively limited structural stability and nanomaterials with inorganic nano-sized components often lack effective pathways for eventual elimination from in vivo biological systems. Accordingly, polymer nanocarriers have attracted increasing interest for biomedical applications because of their tailorable structures and versatile properties, which can be tuned for application requirements (Elsabahy & Wooley, 2012).
As an important type of polymer nanocarrier, polymer nanocapsules (PNCs) are hollow polymer particles with dimensions in the submicrometer range (Meier, 2000). As compared to other types of polymer nanostructures (such as regular polymer nanoparticles, polymer micelles, polymer-drug conjugates, dendrimers and brush polymers), PNCs have unique structural features which favor their delivery-based biomedical applications. With inner cavities, PNCs can not only encapsulate cargoes of larger quantities and/or sizes than conventional nanostructures with the same outer dimensions, but also keep these cargoes protected by the polymer shells. Moreover, relative to other types of polymer nanostructures, PNCs also have unusually high specific surface areas, which can facilitate surface loading of biomedical agents (through physical adsorption or chemical conjugation) and also promote the interactions of ligand-modified PNC-based nano-objects with cell surface receptors and other signalling molecules for active targeting. PNCs for biomedical applications have been previously reviewed (Mora-Huertas, Fessi, & Elaissari, 2012).
Despite their favorable structural features for encapsulation and surface interaction, typical PNCs are susceptible to environmental conditions because of their aggregated or self-assembled structures. Therefore, the study of crosslinked PNCs (CPNCs) with robust architectures stabilized by primary bonds is of significance. Relative to their non-crosslinked analogy, CPNCs are more suitable for applications requiring nanocarriers with more persistent shapes and dimensions. Specifically, CPNCs may readily maintain structural integrity during systemic circulation against a number of destabilizing factors including shear stress in the bloodstream, interaction with the serum proteins, and dilution, to suppress premature cargo release (Wang, Ye, Xie, & Gong, 2019). CPNCs can also be engineered with stimuli-responsive properties to enable efficient intracellular release of the payloads triggered by certain stimuli such as redox and adenosine triphosphate (ATP) (Wang et al., 2018; Yoshinaga et al., 2017). Moreover, cleavable structures of CPNCs can be designed to facilitate their ultimate elimination and minimize their long-term side effects for in vivo applications. Thus, CPNCs represent an important class of polymer nanocarriers with promising biomedical applications. CPNCs have been studied for not only therapeutic applications, including drug delivery, gene delivery, drug-gene co-delivery and the delivery of other therapeutic agents, but also diagnostic bioimaging applications. Moreover, CPNCs have been utilized as scaffolds for theranostic applications in which therapeutics and diagnostics are integrated in all-in-one single platforms. The aim of this review is to present and discuss the state-of-the-art therapeutic, diagnostic, and theranostic applications of CPNCs.
SYNTHETIC APPROACHES OF CPNCS
CPNCs can be prepared by template synthesis via cavitation of shell-crosslinked nanostructures, vesicle-based crosslinking, or emulsion-based interfacial crosslinking (Figure 1; Sun, Chen, Cui, & Cheng, 2016). Accuracy of template synthesis essentially is determined by template stability under reaction conditions. Among the synthetic templates used for CPNCs preparation, their stability is typically in the order of shell-crosslinked nanostructures > vesicles > emulsions. However, synthesis of CPNCs via vesicles or emulsions generally is less complicate, and may allow in situ encapsulation of cargoes. Preparation of CPNCs by cavitation of shell-crosslinked nanostructures often involves higher synthetic complexity and cargo loading needs to be performed after core removal (cavitation) step. An exception to this approach critically important for biomedical application of CPNCs is the direct use of nano-sized biomedical cargoes as the core templates for the fabrication of CPNCs (Yan et al., 2010; Chen et al., 2019). In such a case, shell-crosslinking and cargo encapsulation are in the same step, and therefore, core removal step is not required.
FIGURE 1.
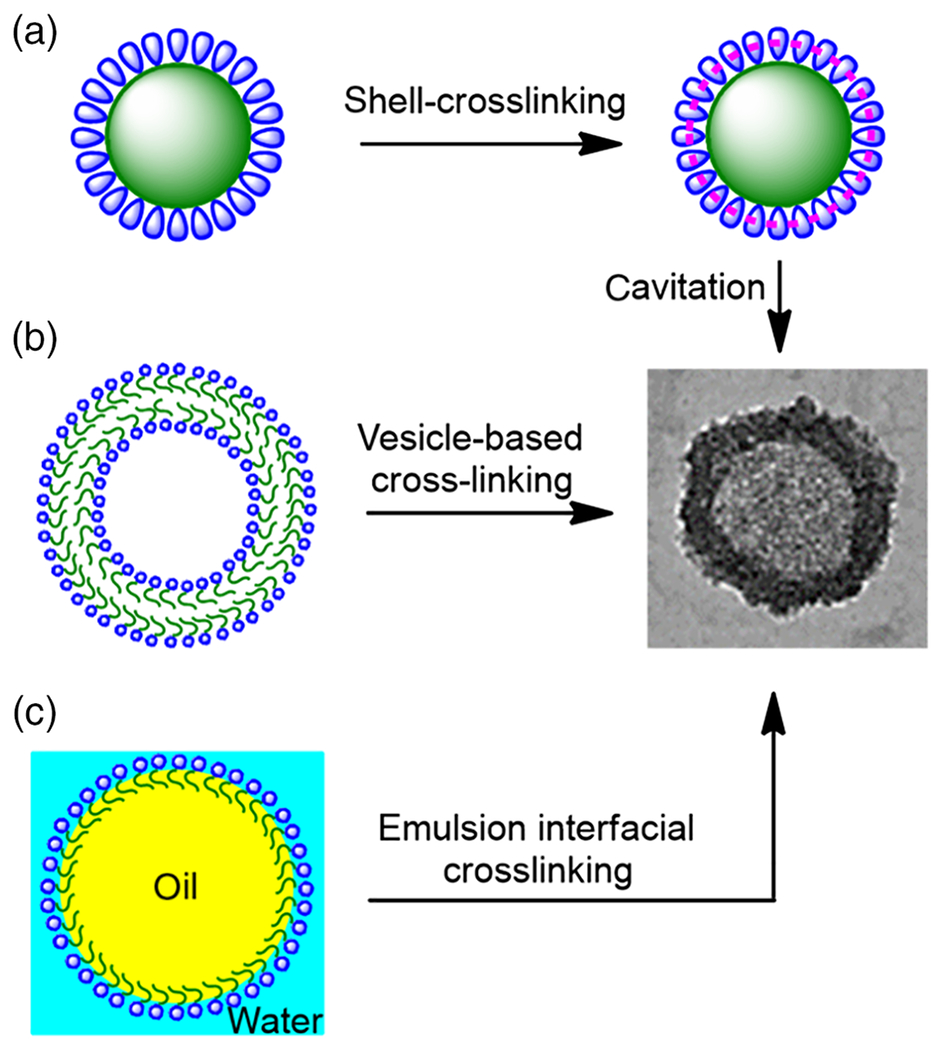
a) Schematic illustration of synthetic approaches of CPNCs: a) cavitation of shell-crosslinked nanostructures, b) vesicle-based crosslinking, and c) emulsion-based interfacial crosslinking.
No matter which approach is used, crosslinking is essential for the synthesis of CPNCs. Covalent crosslinking chemistries are typically used to achieve CPNCs with superior structural stability. Among them, thermal- or photo-initiated radical vinyl polymerization via multivinyl crosslinker in the presence of initiator is the most commonly used crosslinking approach (Odian, 2014; Chen et al., 2019). Various click chemistries (Iha et al., 2009; Hoyle, Lowe, & Bowman, 2010), such as thiol-ene/yne coupling (Zou et al., 2011) and alkene-azide cycloaddition (Siebert, Baier, Musyanovych, & Landfester, 2012), have also been utilized to enable highly efficient crosslinking reactions under mild conditions. Moreover, other chemistries, such as amidation (Huang, Remsen, Kowalewski, & Wooley, 1999), urethane-forming reaction (Jagielski et al., 2007) and radical coupling reaction (Yassin, Appelhans, Mendes, Rümmeli, & Voit, 2012), have been employed in the crosslinking preparation of CPNCs. When base polymers are non-degradable, crosslinkers without cleavable structures generally lead to CPNCs that can maintain integrated crosslinked structures under typical application conditions. On the other hand, crosslinkers with cleavable structures (such as hydrolysable and pH-sensitive ester or acetal group, reduction-sensitive disulfide group, etc.) result in degradable CPNCs that can become uncrosslinked under specific conditions (Chen, Wang, et al., 2014; Kim et al, 2010). These degradable CPNCs may have significant applicability in biomedical areas because their long-term side effects can be minimal and they may enable controlled cargo release under triggered conditions. In addition to covalent crosslinking, ionic crosslinking has also employed for CPNCs, especially these biodegradable ones derived from alginate (Lertsutthiwong, Rojsitthisak, & Nimmannit, 2009; Jaromin, Zarnowski, Piętka-Ottlik, Andes, & Gubernator, 2018).
GENERAL STRUCTURAL DESIGN CONSIDERATION OF CPNCS
According to the needs of biomedical applications (typically via systemic circulation), their structures are designed with the following principal considerations:
The base polymers of CPNCs should be biocompatible, without considerable cytotoxicity. Their biodegradability is also preferred to allow eventual elimination and to minimize their long-term side effects. Accordingly, biopolymers with intrinsic degradability, such as alginate (Nguyen et al., 2015), chitosan (Chen, Wang, et al., 2014; Yin et al., 2010), hyaluronic acid (Yi, Ma, Kang, & Gu, 2018), and starch (Baier et al., 2012), have been employed as the base polymers of CPNCs. A broad variety of CPNCs derived from synthetic polymers have also been studied, and in principle, their non-cleavable segments should have molecular weight (MW) less than 40 kDa to facilitate renal clearance (Tong, & Cheng, 2007).
CPNCs should possess significant water dispersibility, and therefore, their shells should be hydrophilic or amphiphilic. While typically biopolymers are hydrophilic or amphiphilic, synthetic polymers are often hydrophobic and require modification with hydrophilic structures to gain amphiphilicity. Poly(ethylene glycol) (PEG) is a commonly used shell-attached polymer of CPNCs for water-dispersibility enhancement because it is water-soluble, biocompatible and can promote blood circulation time (Knop, Hoogenboom, Fischer, & Schubert, 2010). Recently, polymer modification using superhydrophilic zwitterionic moieties has also attracted noticeable interests, and they can lead to increased blood circulation time and reduced immune responses of biomedical cargos (Liang et al, 2016; Sun et al., 2019; Zhang et al., 2015).
For biomedical applications through systemic circulation, CPNCs should have appropriate nanoscopic hydrodynamic sizes for elongated blood circulation. The preferred hydrodynamic size range of CPNCs is 10-200 nm to avoid fast systemic clearance through kidney or by the reticuloendothelial system (Petros & DeSimone, 2010). Specifically, nanocarriers with sizes of 10-100 nm have been considered to be ideal for passive targeting of tumor tissues owing to enhanced permeability and retention (EPR) effect (Davis, Chen & Shin, 2008). The sizes of CPNCs can be effectively controlled through the synthetic templates. Even if the average hydrodynamic size of CPNCs is somewhat higher than the preferred size range, based on their size distributions, there can still be significant portions of CPNCs within the preferred size range.
Crosslinking endows CPNCs with remarkable structural stability which helps to maintain appropriate physicochemical properties of CPNCs and avoid the major occurrence of premature therapeutic release during systemic circulation. Covalent crosslinking is commonly used for CPNCs; when the base polymer is non-degradable, the crosslinkage needs to be cleavable to promote the eventual clearance of CPNCs. Ionic crosslinking is also employed for CPNCs, especially these derived from alginate (Lertsutthiwong, Rojsitthisak, & Nimmannit, 2009; Jaromin, Zarnowski, Piętka-Ottlik, Andes, & Gubernator, 2018).
CPNCs should allow for effective loading of biomedical-related moieties or agents through physical and/or chemical approaches. While the inner cavities are commonly utilized to encapsulate biomedical agents, the presence of reactive groups on shells can allow for biomedical-related modification of CPNCs.
To exert control over release process of therapeutic agent, it is preferred to incorporate CPNCs with stimuli-responsive structures, including exogenous stimuli-responsive structures (responsive to changes of temperature, magnetic fields, ultrasounds, light and electric fields), and endogenous stimuli-responsive structures (responsive to variations in pH, redox potential, and the concentrations of enzymes) (Mura, Nicolas & Couvreur, 2013; Kwon, Ko, You, Kataoka, & Park, 2019).
In addition to the above design principles, specific designs of CPNCS for biomedical applications will be discussed in next sections, together with the results of the preparation, characterization, and biomedical studies of the CPNCS.
THERAPEUTIC APPLICATIONS OF CPNCS
Drug Delivery
CPNCs have been widely studied as the nanocarriers for the delivery of small molecule drugs. Besides the general structural design considerations of CPNCs, their structural features to facilitate drug loading and controlled drug release should be carefully considered. Typically drug encapsulation within the inner cavities, which may couple with some extent of drug adsorption by polymer shells, is applied for drug loading of CPNCs; conjugation of drug with polymer shells of CPNCs is rarely employed because it may lead to considerably lower drug loadings and requires more substantial synthetic efforts. Most of the small molecule drugs are hydrophobic and have low aqueous solubility. Loading of these drugs into the inner cavities of CPNCs can greatly increase the amounts of drugs to be transported into biological systems. Hydrophobic drugs can be readily encapsulated by CPNCs prepared from normal emulsion-based interfacial crosslinking via an in situ process in which these drugs are dissolved in oil phase during crosslinking. Hydrophilic drugs, on the other hand, can be readily in situ encapsulated by vesicle-based crosslinking or inverse emulsion-based interfacial crosslinking. For example, nearly quantitative encapsulation efficiency (~96%) and significant loading capacity (~12 wt%) of gemcitabine (GEM), a water-soluble hydrophilic anti-cancer drug, were achieved by in situ drug encapsulation during an inverse miniemulsion-based synthesis of CPNCs (Utama, Jiang, Zetterlund, & Stenzel, 2015). Depending on their chemical structures, CPNCs made by cavitation of shell-crosslinked nanostructures can also be loaded with hydrophobic or hydrophilic drugs, but typically drug loading needs to be performed after the preparation of CPNCs. Because the release of physically loaded drugs essentially is a diffusion process with concentration difference as the driving force, burst release of drug from nanocarriers is a critical concern and may lower bioavailability and increase side effects of drugs.
Controlled release of loaded drugs from CPNCs, together with the suppression of burst release, should also be addressed through structural design of CPNCs. Burst release effects can be effectively suppressed by increasing the diffusion resistance within the polymer shells of CPNCs through the increase of crosslinking density or thickness of the polymer shells. Moreover, the incorporation of stimuli-responsive structures with the crosslinked polymer shell domain of CPNCs can further tune the shell permeability and facilitate drug release triggered by specific internal or external environmental stimuli, including the variations of temperature, pH, light wavelength, and concentration of reducing agent or enzyme. As reported by Chen et al., the CPNCs with pH-responsive poly(methacrylic acid-co-divinylbenzene) (P(MAA-co-DVB)) inner shell and temperature-responsive poly(N-isopropylacrylamide) (PNIPAm) surface brushes showed the decrease of permeability of polymer shells of CPNCs with the increase of inner shell thickness and the brush length (Chen, Peng, et al., 2014). Voit and co-workers reported polymersome-based CPNCs based on photo-crosslinkable thermo-responsive and pH-sensitive block copolymer, poly(ethylene glycol)-block-poly[2-(diethylamino) ethyl methacrylate-stat-2-hydroxy-4-(methacryloyloxy) benzophenone] (PEG-b-P(DEAEMA-stat-BMA)) (Yassin, Appelhans, Mendes, Rümmeli, & Voit, 2012). While the loaded drug showed good retention behavior under the storage conditions (pH 7.4, 4 °C), effective drug release can be realized at physiological temperature (37 °C), with drug release rates increasing with the decrease of crosslinking density of the polymer shell, as well as the decrease of solution pH.
In the aforementioned two examples, the stimuli-responsivity of CPNCs was essentially introduced through stimuli-responsive precursor polymers. On the other hand, crosslinker can also be utilized to introduce stimuli-responsivity to the resulting CPNCs. For instance, Tian et al. synthesized pH and reduction dual-responsive poly(ethylene glycol)-block-poly(acrylic acid)-based CPNCs in which pH-sensitivity was from polymeric structures and reduction responsivity was from the disulfide-functionalized crosslinker, N,N-bis(acryloyl) cystamine (Tian et al., 2015). Drug release from these CPNCs became fast at pH 5.0 relative to pH 7.4, and could be critically triggered with the presence of glutathione (GSH) as the reducing agent to cleave the disulfide-based crosslinkages of the CPNCs. Li et al. also used N,N-bis(acryloyl)cystamine in cross-linked polymerization of N-isopropylacrylamide (NIPAM) and acrylamide around magnetic Fe3O4 cores (Li, Ma, Feng, & Liu, 2016). In addition to thermal-responsivity just above body temperature provided by the polymers themselves and the ability of Fe3O4 particles to provide magnetic targeting, these CPNCs were also shown to release cargo in a reducing environment.
Furthermore, multifunctionalization to incorporate targeting ligands and/or cell-penetrating peptides with CPNCs may increase the bioavailability of the loaded drugs, and therefore is of importance for CPNC-based drug delivery systems. Functionalization of hydroxyethyl starch (HES)-based CPNCs with targeting ligands, including folic acid (FA) and (oligo)mannose, has been studied. Conjugation of FA moieties onto the surface of crosslinked polymer shells enabled a specific cellular uptake of the resulting CPNCs into HeLa cancer cells with folate-receptor, confirming receptor-mediated targeting activities (Baier et al., 2012). Similarly, surface conjugation of (oligo)mannose also led to an enhancement of uptake of the (oligo)mannose-functionalized CPNCs into dendritic cells, indicating the targeting properties of the CPNCs (Freichels et al., 2013).
With few exceptions (Belbekhouche, Mansour, & Carbonnier, 2018; Landis et al., 2017; Pejawar-Gaddy, Kovacs, Barouch, Chen, & Irvine, 2014), anti-cancer drugs have been commonly used as small molecule drugs in the drug delivery studies using CPNCs as nanocarriers, because cancer is a major public health issue and one of the leading causes of death worldwide (Siegel, Miller, & Jemal, 2019). The structural design of these CPNCs should also be made with the consideration of the specific cancer environment. For instance, because cancer tissue is generally more acidic than normal tissue and a cancer cell also has a more reducing environment than a normal cell, acid- and/or reduction-sensitive structures of crosslinked polymer shells of CPNCs may be favorable for anti-cancer drug delivery. Moreover, functionalization of CPNCs with cancer-specific targeting ligands may also enhance the overall efficacy of the anti-cancer drug delivery systems. By employing commonly used anti-cancer drugs, such as doxorubicin (DOX), PTXL and GEM as the model drugs, CPNCs also showed some significant merits as drug nanocarriers through biomedical assessments.
A number of in vitro studies have indicated that relative to free anti-cancer drugs, CPNC-based formations may enhance the therapeutic effects of drugs against cancer cells. Reduction-responsive CPNCs with disulfide-containing crosslinked polymer shells and size of around 200 nm for the delivery of GEM was studied (Utama, Jiang, Zetterlund, & Stenzel, 2015). As illustrated by cell viability assessment, the cytotoxicity of GEM-loaded CPNCs against AsPC-1 pancreatic cancer cell line (IC50 = 136 nM) was increased by 2-fold as compared with free GEM (IC50 = 270 nM), mainly due to the prolonged drug bioavailability by using the CPNC-based formulation and the higher accumulation of the drug-loaded CPNCs within the cancer cells. Moreover, functionalization of drug-loaded CPNCs with cell-penetrating peptides or targeting ligands (such as FA) is also an effective strategy to increase the efficacy of CPNC-based formulations through enhanced drug delivery to cancer cells for improving therapeutic efficacy (Lee, Bae, Kim, Nam, & Park, 2011; Fu et al., 2018; Yang et al., 2017).
CPNC-based formulations can be particularly effective for the delivery of anti-cancer drugs into cancer cells with multi-drug resistance (MDR). As demonstrated by Cheng and co-workers, CPNCs can be utilized to carry DOX for overcoming MDR of cancer cells (Chen, Law, et al., 2014). The DOX-loaded cationic polylactide (CPLA)-based CPNCs were prepared by miniemulsion interfacial crosslinking of allyl-functionalized CPLA using UV-induced thiol-ene chemistry, with in situ encapsulation of 11.5 wt% of DOX and a loading efficiency of 76% (Figure 2a). By using MCF7/ADR cell line as the stable MDR model with high expression of P-glycoprotein (Pgp), which can recognize and export DOX, in vitro study revealed that DOX-loaded CPLA-based CPNCs led to 3.6 and 3.2 fold enhanced concentrations of DOX in the cytoplasm of the MDR cells at 4 and 8 h post treatment, respectively, compared to free DOX alone, indicating that the Pgp efflux pump can be successfully evaded through encapsulation of DOX into the CPNCs (Figure 2b). Furthermore, the DOX-loaded CPNCs exhibited more significant anti-proliferation effect and 15-20% lower cell viability than free DOX at all concentrations of DOX studied (Figure 2c). Because intrinsic and acquired MDR by cancer cells contributes to most cancer treatment failures, the remarkable effectiveness of drug-loaded CPNCs in evading MDR may suggest their promising potential applications in cancer chemotherapy.
FIGURE 2.
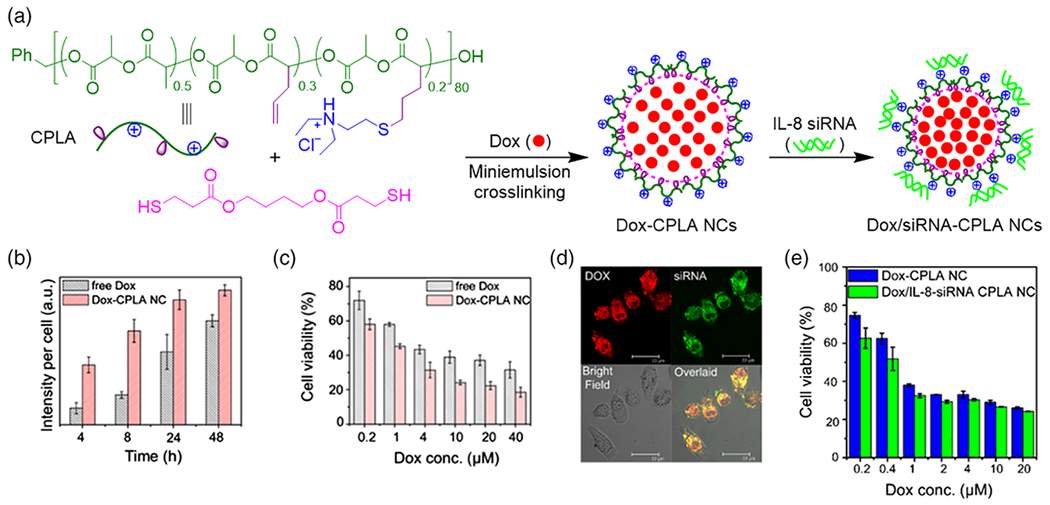
a) Schematic illustration of the preparation of DOX-loaded and DOX/siRNA-loaded CPLA nanocapsules (NCs, i.e. CPNCs). b) Fluorescence quantification of DOX uptake by MCF7/ADR cells via free DOX and DOX-CPLA NCs. c) MTS cell viability assay of MCF7/ADR cells after 48 h incubation with free DOX and DOX-CPLA NCs. d) Confocal images of PC3 cells after incubation with DOX/IL-8-siRNAFAM-CPLA NCs for 4 h. (e) MTS cell viability assay of PC3 cells after incubation with DOX-CPLA NCs and DOX/IL-8-siRNA CPLA NCs for 72 h ([siRNA]0 = 0.1 μM; WDOX/WNCs = 0.116). (Adapted with permission from Chen, Law, et al. (2014)).
Some promising in vivo results in CPNC-based systems for the delivery of anti-cancer drugs have also been obtained. The merits of a CPNC-based formation for intracellular delivery using PTXL as a model drug have been demonstrated (Lee, Bae, Kim, Nam, & Park, 2011). The PTXL-loaded CPNCs with average hydrodynamic diameter (Dh) of ~280 nm were prepared by emulsion-based interfacial crosslinking through amidation reaction of human serum albumin (HSA) with amine-reactive six-arm-branched PEG, with in situ encapsulation of PTXL (loading capacity: 9.58 wt%). As shown by in vivo studies using MCF-7 tumor-bearing mice as the animal model, the accumulation of HSA/PEG CPNCs was eight times higher in tumor than liver on day 14 post-injection, indicating effective EPR effects and the stealthy properties of these CPNCs. The PTXL-loaded CPNCs also effectively suppressed tumor growth, and only 1.7-fold increase of tumor volume in 14 days was observed for the mice treated with a single intravenous administration of CPNC-based formulation, while untreated control mice had about 8-fold increase in tumor volume in the same period.
The merits of CPNCs for in vivo drug delivery using DOX as a model drug have also been demonstrated. With appropriate nano-sizes to capitalize EPR effects, CPNCs may increase the circulation time of the encapsulated drugs, leading to higher drug bioavailability (Radhakrishnan et al, 2015). Moreover, studies have shown that enhanced tumor-specificity and efficacy of DOX-loaded CPNCs can be achieved by incorporating targeting moieties onto the surface of such nanocarriers. In the study of Zhang et al., cRGD peptide was used as the targeting moiety because it is able to interact with αvβ3 surface integrins typically overexpressed in squamous cells, and promote receptor-mediated endocytosis (Zhang et al., 2017). The CPNC itself consists of a four-armed, poly(ethylene glycol)-poly(propylene sulfide)-cRGD (PEG-PPS-cRGD) self-crosslinked copolymer with DOX encapsulated via a solvent-antisolvent method (Figure 3a), having an average diameter of 255 nm and 3.6 wt% DOX loading. In vitro studies showed a 1.8-fold higher uptake of PEG-PPS-cRGD DOX-loaded CPNCs in SCC-15 cells than those without cRGD targeting; relative to free DOX, DOX-loaded PEG–PPS and DOX-loaded PEG–PPS–cRGD CPNCs resulted in a remarkably enhanced cytotoxicity with 33.2% and 18.1% living SCC-15 cells at the DOX concentration of 18 μg mL−1 (Figure 3b). In vivo studies revealed that DOX-loaded CPNCs led to significantly elongated blood circulation than free DOX. Moreover, DOX concentrations in tumor were 2.9- and 1.7-fold higher under treatment with PEG-PPS-cRGD DOX-loaded CPNCs than with free DOX and PEG-PPS DOX-loaded CPNCs, respectively (Figure 3c). Furthermore, dual redox-responsiveness to higher concentrations of both reactive oxygen species (ROS; an oxidizing agent) and glutathione (a reducing agent) in cancer cells resulted in triggered release of DOX and an excellent antitumor effect. Using FA as tumor-targeting moiety, Yi et al. demonstrated that hyaluronic acid-based, DOX-encapsulated CPNCs with FA-terminated zwitterionic tentacles exhibited both a higher cytotoxicity, more efficient in vitro cellular uptake by 4T1 cells, and more significant antitumor efficacy towards tumor-bearing BALB/c mouse, as compared to the control CPNCs without FA modification (Yi, Ma, Kang, & Gu, 2018).
Figure 3.
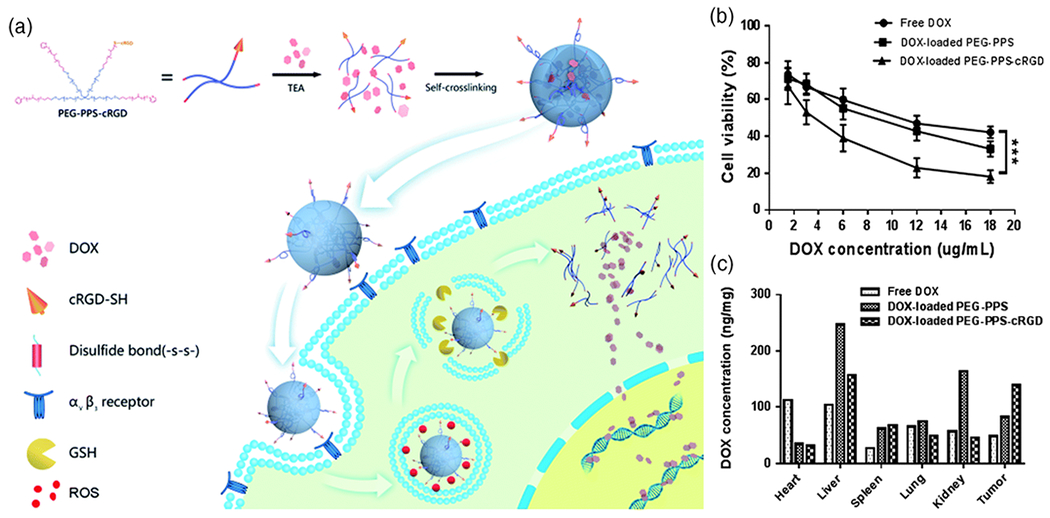
a) Schematic illustration of the synthesis and cellular uptake of DOX-loaded PEG-PPS-cRGD CPNCs. b) MTT assay of SCC-15 cells after incubation with for 24 h (***P < 0.001). c) DOX concentrations in various organs and tumor tissue. (Reproduced with permission from Zhang et al. (2017). Copyright 2017, The Royal Society of Chemistry).
Gene Delivery
CPNCs have also been used as the nanocarriers for the delivery of various genetic materials. Because free gene can be rapidly degraded by nucleases in serum, the utilization of viral or non-viral vectors is necessary for effective gene delivery. Viral vectors may lead to suitable transfection efficiencies but can cause serious concerns including cytotoxicity, cargo capacity, immunogenicity, and difficulty in vector scale-up (Yin et al., 2014). These concerns may be circumvented by the exploitation of non-viral vectors (Jones, Chen, Ravikrishnan, Rane, & Pfeifer, 2013; Jin, Zeng, Liu, Deng, & He, 2014). As an important type of non-viral vectors, polymeric vectors have been broadly studied, and their structures can be tailored to tune biomedical-related properties as required in gene delivery applications. Representing a novel category of polymeric vectors, CPNCs can be employed to load genetic materials with two major methods, including 1) using gene-containing cores for gene loading before the crosslinking step and 2) using CPNCs with surface charge for surface adsorption of genes or nanoplex. Accordingly, some promising gene delivery results have been obtained from the resulting systems.
Genes can be loaded into the inner cavity of CPNCs by using gene-containing cores for template synthesis of CPNCs. Nanoplexes of genes with cationic polymers (Gu, Yuan, He, Zhang, & Ni, 2009; Haladjova, Kyulavska, Doumanov, Topouzova-Hristova, & Petrov, 2017) and gene-adsorbed silica particles (Zelikin, Becker, Johnston, Wark, Turatti, & Caruso 2007) have been utilized as the gene-containing cores for the multistep preparation of gene-loaded capsules. However, how to reach high gene loading amounts and how to remove or eliminate the assisted core materials (i.e. polymers of nanocomplexes and silica particles) without causing reduced bioactivity of genes or side effects present as challenges for these approaches. Addressing these concerns, studies using gene-based cores for CPNCs have attracted significant interests.
Chen and co-workers developed an innovative gene encapsulation strategy directly using individual molecules of genetic materials as cores for template synthesis of CPNCs (Yan et al., 2012). Because CCR5 is a major silencing target of HIV therapy, CCR5–siRNA was chosen as the core gene in their initial study. The surface of the negatively charged CCR5–siRNA molecules was assembled with hydrophilic and positively-charged monomers and acid-cleavable crosslinker through electrostatic interaction and hydrogen bonding. CCR5–siRNA-encapsulated CPNCs (~20 nm) were obtained by subsequent in situ polymerization, and in vitro study showed that they were highly effective in silencing CCR5–mCherry expression. Control experiments illustrated that the CPNCs provide more sufficient protection and stabilization to siRNAs against human serum nucleases as compared with lipofectamine, a commercial transfection agent.
Moreover, the research team further demonstrated the encapsulation of RNA-encoded DNAs using CPNCs (Yan et al, 2015). To overcome the limitation of direct delivery of RNAs, DNA molecules encoding RNAs, including short hairpin RNAs (shRNAs), guide RNAs (gRNAs) and microRNAs (miRNAs), were utilized as DNA expression cassettes for RNAs. CPNCs (~30 nm) with a thin crosslinked polymer shell covering the DNA cassettes were obtained by subsequent in situ polymerization of monomers and crosslinkers enriched on the surface of DNA molecules through electrostatic interactions and hydrogen bonding (Figure 4a). Via CPNCs, the encapsulated DNA cassettes can be delivered effectively to various normal cell lines, as well as primary human hematopoietic stem cells. Specifically, in vitro study showed that the DNA cassette encoding an shRNA transcriptional unit for downregulating CCR5 was successfully delivered by CPNCs and could enable prolonged knocking down of CCR5 to block HIV-1 infection. Relative to siRNA1005 formulated with lipofectamine, the sh1005DNA cassette showed more than 200-fold effectiveness at downregulating CCR5 on a molar basis (Figure 4b). The delivery of DNA cassette-encoded gRNA via CPNCs was also illustrated in CRISPR/Cas9 system for the mutation of integrated HIV-1 (Figure 4c). Furthermore, they utilized the CPNCs encapsulating the miRNA-125b-encoded DNA cassette for regulation of the phenotype of HSPCs, and an improved survival rate (Figure 4d) and enhanced expansion ability of CD34+ HSPC cells were observed.
FIGURE 4.
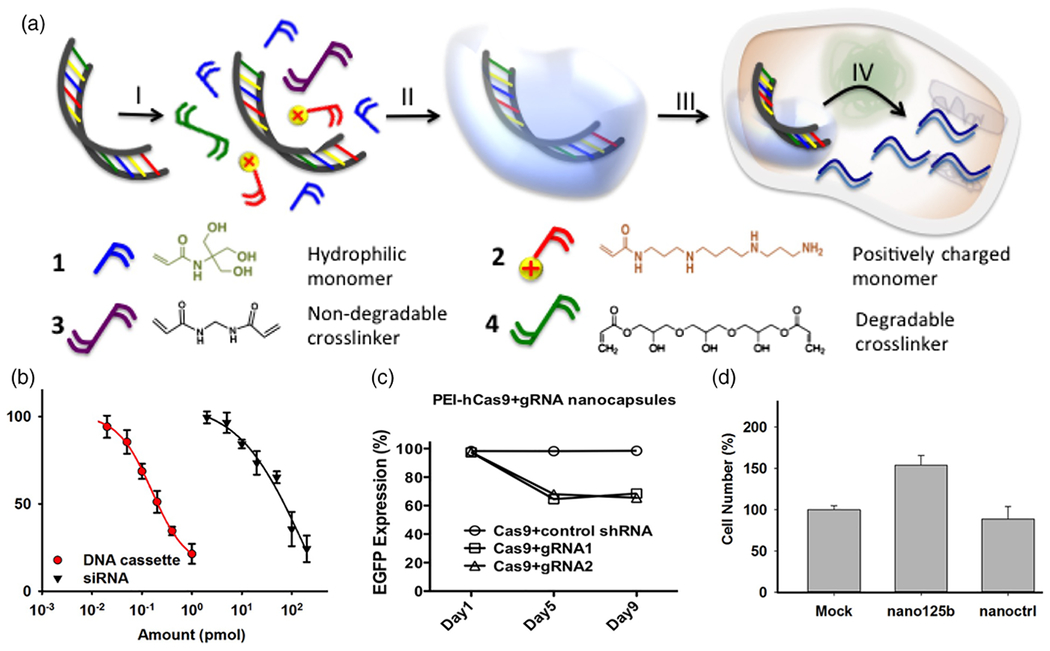
a) Schematic illustration of CPNCs as DNA cassettes. b) sh1005 DNA cassette versus siRNA 1005 lipofectamine complex in the knockdown of CCR5-Luciferase in HEK-293T cells. c) A time profile for knockout of EGFP expression by DNA cassette-encoded gRNA as determined by flow cytometry. d) Ex-vivo culture of cytokine-mobilized CD34+cells for 6 days after delivery of miR-125b DNA cassette versus controls. (Adapted with permission from Yan et al. (2015). Copyright 2015, Public Library of Science).
Very recently, Gong and co-workers reported GSH-degradable CPNCs encapsulating preassembled Cas9 ribonucleoprotein (RNP) complex for both in vitro and in vivo genome editing (Chen et al., 2019). By using the RNP complex consisting of a Cas9 nuclease and a single-guide RNA (sgRNA) as the cores, the CPNCs were prepared by in situ polymerization of a mixture of monomers and a GSH-degradable crosslinker adsorbed on the RNP surface via electrostatic interactions, hydrogen bonding and van der Waals interactions (Figure 5a). In vitro experiments using a human embryonic kidney (HEK 293) cell line revealed critical factors to optimize the formulations of CPNCs for promoting gene editing efficiency. High gene editing levels were exhibited for the formulations fabricated under the following conditions: 1) using cationic and anionic monomers at 1:1 ratio due to heterogeneous surface charge of RNP; 2) with appropriate amount of imidazole-containing monomer to facilitate endosomal escape of NCs via proton sponge effect; 3) employing sufficient amount of biodegradable crosslinker to enable crosslinking of CPNC and intracellular release of RNP; 4) with 2:1 mass ratio of CPNC/RNP (Figure 5b). The CPNC obtained using the optimized formulation (Dh = 25 nm, ξ = −4 mV) showed the capability of escaping from the endosomes, GSH-triggered degradability and payload release, and freezing and lyophilization stability. The CPNC led to higher gene editing efficiency but lower cytotoxicity towards HEK 293 cells than lipofectamine 2000 (Lipo), a commercially available transfection agent for RNP (Figure 5c). Surface modification of the CPNCs with cell-penetrating peptides could further boost gene editing efficiency twofold for HEK 293 cells (Figure 5d) and threefold for human embryonic stem cells (hESCs). Finally, gene editing experiments in eyes and muscles of transgenic Ai14 mice illustrated that the CPNCs are effective in producing in vivo gene edits, with extents that can be modulated by surface modification of CPNCs with targeting ligands (Figure 5e). Collectively, this comprehensive study elucidates the promising potentials of gene editing via Cas9 RNP-encapsulated CPNCs.
FIGURE 5.
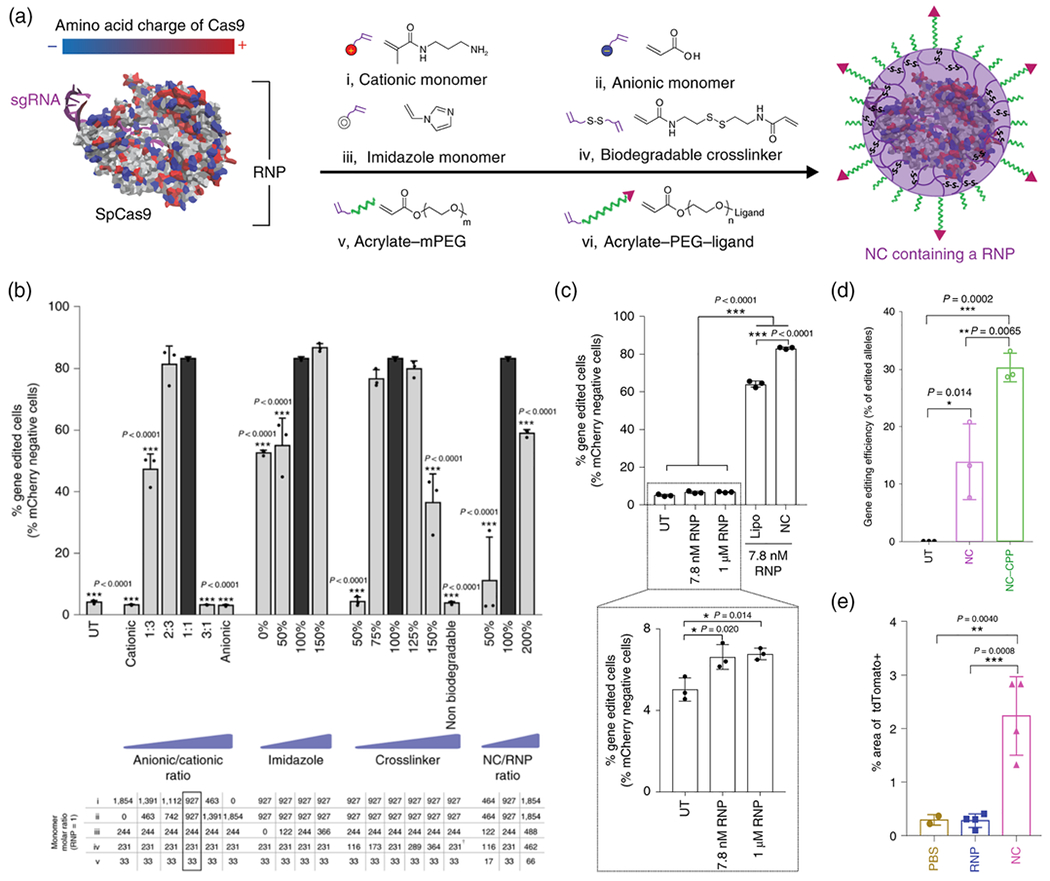
a) Synthesis of Cas9 RNP-encapsulating NCs (i.e. CPNCs). b) Optimizing the NC formulation in vitro using mCherry-expressing HEK 293 (mCherry-HEK 293) cells. c) Gene editing in mCherry-HEK 293 cells with RNP-encapsulating NCs, as compared to unencapsulated RNP, optimized Lipofectamine (Lipo) system and untransfected control sample (UT). d) Deep sequencing of HEK 293 cells to examine in vitro gene editing efficiency with RNP-encapsulating NCs with and without decoration using cell-penetrating peptide (CPP). e) In vivo gene editing efficiency expressed as the percentage area of whole muscle tissue showing confocal fluorescent signal for a genome editing reporter (tdTomato+) 12 days after the intramuscular injection of genome editors. (Adapted with permission from Chen et al., 2019).
Surface adsorption of genes or gene-containing polyplex with CPNCs through electrostatic interactions is also a general approach to load genes for controlled release via CPNCs. Having high specific surface area due to their capsular structures, cationic CPNCs may carry significant amounts of genetic materials through surface adsorption. Besides drug delivery studies, Cheng and co-workers also utilized the aforementioned CPLA-based cationic CPNCs (Dh = 22 nm, ξ = 45 mV) for gene delivery studies (Chen, Law, et al., 2014). Interleukin-8 (IL-8) siRNA, which can suppress tumor growth through the RNA-interference (RNAi) mechanism, was selected as the model genetic material. IL-8 siRNA was loaded on CPLA NCs simply by mixing their aqueous solutions, and electrophoresis gel retardation assay showed strong binding of siRNA with CPLA NCs. Confocal imaging study verified the successful delivery of IL-8 siRNA via CPLA NCs into the PC3 prostate cancer cells. Moreover, IL-8-siRNA-loaded CPLA NCs were effective in gene silencing of IL-8 in the PC3 cells. Under optimal conditions for siRNA delivery (WNCs/WsiRNA of 30), CPLA NCs led to the gene silencing efficiency comparable to commercial transfection agents, such as Mirus TransIT (MT) and lipofectamine. Similarly, CPLA NCs were also studied for the delivery of a specific siRNA targeting the mutant K-Ras gene in PANC-1 pancreatic carcinoma cells, and transfection efficiency of over 90% and the knockdown efficiency of about 50% were achieved after 48 hours of treatment by using the optimized formulation (Lin et al., 2013).
Although genetic materials are less protected by shells of CPNCs through surface adsorption relative to encapsulation, the CPNCs with surface-adsorbed genes possess their inner cavities which can be utilized for encapsulating other biomedical-related agents and promoting the overall biomedical outcomes. For instance, pluronic/polyethylenimine-based CPNCs with embedded magnetite nanocrystals have been reported for magnetically-triggered delivery of siRNA (Lee et al., 2010). The novel CPNCs with sizes of ~500 nm were prepared by the emulsion-based interfacial crosslinking reaction of polyethylenimine with pluronic F-127 triblock copolymer preactivated at the terminal group using p-nitrophenyl chloroformate, with the presence of oleic acid-coated magnetite nanocrystals with sizes of ~15 nm for in situ encapsulation. Polyelectrolyte nanocomplexes were subsequently formed by the electrostatic interactions of the CPNCs with a siRNA-PEG conjugate with a cleavable disulfide linkage (siRNA-s-s-PEG). With encapsulated magnetite nanocrystals, the CPNC-based nanocomplexes were efficiently taken up by cancer cells under exposure to a magnetic field, leading to enhanced silencing effect of siRNA.
Moreover, as reported and reviewed by Rouge and co-workers recently (Awino et al., 2017; Santiana, Sui, Gomez, & Rouge, 2017; Santiana, Gudipati, Hartmann, & Rouge, 2019), nucleic acid-conjugated CPNCs were prepared by using enzyme cleavable crosslinkers, and they may have remarkable potentials in gene regulation and enzyme-triggered drug release according to proof-of-principle studies.
Co-Delivery of Drug and Gene
Because of the synergistic effects of drugs and therapeutic genes in disease treatment, drug-gene co-delivery has become an emerging research topic in biomedical field (Khan, Ong, Wiradharma, Attia, & Yang, 2012). Specifically, co-delivery of anticancer drugs and siRNA has drawn remarkable attention in the treatment of drug-resistant cancers (Sun, Yarovoy, Capeling, & Cheng, 2017). Based on their ability of region-specific loading of multiple cargos, CPNCs have been studied for co-delivery of drug and gene. As demonstrated by Cheng and co-workers (Chen, Law, et al., 2014), the aforementioned CPLA-based CPNCs can serve as the nanoplatform of drug-gene co-delivery because their inner cavities can encapsulate drug and their cationic shells can bind gene via electrostatic adsorption. The CPNCs co-loaded with DOX (11.6 wt%) in cavities and IL-8 siRNA (3.3 wt%) on shells were prepared. Confocal imaging showed co-localization of fluorescence from both DOX and FAM-labeled siRNA in the same PC3 cancer cells within 4 h of incubation (Figure 2d). The therapeutic effect of DOX and IL-8 siRNA co-delivered via CPLA NCs was further studied at a constant siRNA concentration (0.1 μM). Relative to CPNCs loaded with only DOX, the CPNCs loaded with both DOX and IL-8 siRNA exhibited noticeably higher cytotoxicity towards PC3 cells at low DOX concentrations (≤1 μM), and this may be attributed to the silencing of IL-8 gene (a tumor growth factor) which made the treated cells more vulnerable to DOX (Figure 2e). Such encouraging in vitro results suggested that these CPNCs with cationic polymer shells hold promise for subsequent in vivo studies for drug-gene co-delivery. Based on the broad studies of individual deliveries of drug and gene using CPNCs, there should be a remarkable design space for other types of CPNCs for co-delivery of drug and gene, although they have not been reported in literature.
Protein Delivery
Therapeutic proteins and peptides have been widely studied for the treatment of different diseases including cancers. Owing to their nanoscopic dimensions, proteins are not suitable for encapsulation by CPNCs after crosslinking step. Accordingly, protein-encapsulated CPNCs are typically prepared by crosslinking of preassembled protein-containing systems.
CPNCs prepared by vesicle-based crosslinking has been utilized for protein delivery. Zhong and co-workers reported reduction and temperature dual-responsive crosslinked polymersomes for encapsulation of proteins (Cheng et al, 2011). In the presence of proteins, thermosensitive PEG-PAA-PNIPAM triblock copolymers were used to generate robust polymersomes, in which the PAA segment was crosslinked with cystamine (Cys) via carbodiimide chemistry. A variety of proteins (serum albumin, lysozyme, cytochrome C (CC), and ovalbumin) were loaded into the polymersomes with protein loading efficiencies of 60~100% at theoretical 10~50 wt% protein loading. In vitro release studies showed a fast protein release under intracellular mimicking reductive environment. Cellular uptake study showed that the Cys-crosslinked polymersomes loaded with fluorescein isothiocyanate (FITC)-conjugated CC (FITC-CC) delivered FITC-CC into the cytosol of MCF-7 cells with high efficiency compared with MCF-7 cells treated with free FITC-CC after 12 h incubation. In addition, compared with free CC and the reduction-insensitive control, CC loaded Cys-crosslinked polymersomes induced highly enhanced apoptosis of MCF-7 cells. A similar polymersome system developed by PEG-PAA-PNIPAM could also be used for triggered drug release (Xu, Meng, & Zhong, 2009).
CPNCs encapsulating single protein molecules were precisely prepared directly using proteins as synthetic templates. In the pioneering study to demonstrate the preparation principles reported by Lu and co-workers (Yan et al., 2010; Wu et al., 2019), monomer-modified protein was prepared, and subsequent polymerization with neutral monomer, positively-charged monomer, and non-degradable or pH-sensitive degradable crosslinker in aqueous solutions resulted in quantitative wrapping of protein molecules with either non-degradable or degradable CPNCs. The non-degradable CPNCs showed structural stability and preserved protein bioactivity under changed environmental conditions and under in vivo conditions, whereas the degradable ones could release the core protein under acidic conditions, including acidic intercellular environment. The versatility of this protein encapsulation approach and its applicability in protein delivery were illustrated by using a variety of proteins, multiple cell lines, and a mice model. When a disulfide-containing crosslinker was used in this approach, the resulting redox-responsive single-protein CPNCs could efficiently internalized into a variety of human cells (HeLa, MCF-7 and U-87 MG) and then release the protein in the reducing cytosol (Zhao et al., 2011).
This approach has been extended to develop zwitterionic CPNCs for the encapsulation of individual protein molecules. Lu and co-workers used a zwitterionic monomer, methacryloyloxyethyl phosphorylcholine (MPC), together with a conventional crosslinker in the formulation to encapsulate individual protein molecules with phosphorylcholine-containing zwitterionic CPNCs (Liang et al., 2016). Due to the biomedical merits of zwitterionic polymers, these zwitterionic CPNCs can prevent the encapsulated protein to be phagocytosed by macrophages and to be identified by immune cells. By employing both monomer and crosslinker with a carboxybetaine functionality in this approach, Jiang and co-workers obtained uricase encapsulated by carboxybetaine-containing zwitterionic CPNCs (Zhang et al., 2015). The encapsulated uricase showed exceptional stability and led to a greatly prolonged circulation half-life. Moreover, the pharmacokinetic profile was unchanged and no immunogenic response were detected after three weekly injections of the encapsulated uricase in a rat model. Such results indicate that protein encapsulation by zwitterionic CPNCs is promising for the development of novel protein therapies with various biomedical merits.
The single protein encapsulation approach has also been employed to rebuild cells and tissues, as demonstrated in a recent work in which tunable release of growth factor BMP-2 for bone regeneration was enabled (Tian et al., 2016). CPNCs were prepared by in situ polymerization of N-(3-aminopropyl) methacrylamide (APm), 2-(dimethylamino)ethyl methacrylate (DMA), acrylamide, and glycerol dimethacrylate (GDMA) crosslinker around BMP-2 (Figure 6a). Agarose gel electrophoresis results displayed that not only the GDMA crosslinker degrades under alkaline conditions, which mimic bone repair conditions in vivo, but also the subsequent protein release kinetics can be tuned by varying the ratio of APm and DMA monomer used in the polymerization process. As compared with the treatment with native BMP-2 alone, BMP-2 nanocapsules (nBMP-2) provided sustained BMP-2 release in vivo with higher bone quality and a smaller inflammatory response, according to histological examination and MRI results (Figure 6b–d). These results demonstrate the significant advantages of using CPNCs for controlled protein delivery versus direct protein administration.
FIGURE 6.
a) Schematic illustration of preparing protein-encapsulated CPNCs with sustained release capability. b) In vivo fusion score using a rat spinal fusion model at 8 weeks (nMBP-2: BMP-2-encapsulated CPNC with BMP-2 concentration equivalent to 1.5 μg BMP-2). c) Quantified relative bone volume (BV/TV) resulted from the nBMP-2 versus BMP-2. d) Quantified inflammatory reaction volume and area caused by nBMP-2 versus BMP-2, as measured by MRI and histology, respectively. **p < 0.01, ***p < 0.001. (Adapted with permission from Tian et al. (2016). Copyright 2016, American Chemical Society).
NOTE: For Figure 6a, 6b, 6c, and 6d please refer to Scheme 1, Figure 3a, Figure 3c, and Figure 3g, respectively, of the manuscript by Tian et al.:
Tian, H., Du, J., Wen, J., Liu, Y., Montgomery, S. R., Scott, T. P., … Lu, Y. (2016). Growth-factor nanocapsules that enable tunable controlled release for bone regeneration. ACS Nano, 10(8), 7362-7369.
Because the aforementioned single protein encapsulation approach requires free radical polymerization to form CPNCs over the protein surface, it may be problematic for proteins with structures sensitive to radicals. To address this restriction, a radical-free assembling process to form CPNCs encapsulating protein was also developed by Lu and co-workers (Li et al., 2016). A cationic self-crosslinkable polymer was formed by the reactions of poly(allylamine hydrochloride) with the N-hydroxysuccinimide ester of PEG (PEG-NHS) and 2-iminothiolane hydrochloride. BSA-encapsulated CPNCs were formed by covering negatively-charged BSA with the polymer, followed by self-crosslinking of the polymer. The resulting CPNCs with disulfide-containing crosslinkages were largely non-toxic to cells, exhibited improved cellular uptake over native protein alone, and could release BSA under cytosol-mimicking GSH concentrations. Moreover, thanks to their radical-free preparation, encapsulation by CPNCs resulted in more than 86% of preserved protein activity for all five proteins tested.
Protein-encapsulated CPNCs were also prepared by using protein-trapped sacrificial cores. For example, layer-by-layer assembled polyaspartamide CPNC synthesized by using protein-trapped silica sphere as cores has been reported for protein delivery (Gu et al., 2013). Such a method may be limited by relatively low protein loading amount and the potential reduction of protein bioactivity resulted from the core removal step.
Delivery of Other Therapeutics
CPNCs have also been utilized to load other therapeutic agents, in additions to drugs, genes or proteins. For example, because silver nanoparticles have proven to be effective for the prevention and treatment of infections owing to their good antibacterial properties (Rai, Yadav, & Gade, 2009), the potato starch-based CPNCs decorated with silver nanoparticles on their shells were prepared by inverse miniemulsion polyaddition (Taheri et al., 2014). The silver nanoparticles were in situ formed via reduction reaction. The use of 2,4-toluene diisocyanate (TDI) as crosslinker allowed for readily tuning of the shell thickness of CPNCs by varying the amount of TDI. Two common microorganisms Staphylococcus epidermidis ATCC 35984 and Escherichia coli ATCC 25922 were used to assess the antibacterial activity. It was demonstrated that the minimum inhibitory concentration (MIC) of the CPNCs was as low as 2.315 μg/mL against both bacteria, while control CPNCs without silver showed no inhibitory effect on bacterial growth. Those antibacterial CPNCs were promising as antibacterial coatings for medical devices. Moreover, their cavities may be loaded with other therapeutics, to achieve synergistic biomedical effects.
DIAGNOSTIC APPLICATIONS OF CPNCS
CPNCs have also been investigated for diagnostic applications based upon the integration of CPNCs with diagnostic agents. Several types of diagnostic agents have been utilized in such studies, including those for optical imaging (using fluorescent dyes or quantum dots), magnetic resonance imaging (MRI; using superparamagnetic materials), and ultrasound imaging (using bubble generators). The loading strategies of diagnostic agents depend on their dimensions and whether they need to be released from the systems. Similar to the cases of drug delivery, small molecules that need to be released for diagnostic purpose, such as bubble generators for ultrasound imaging, can be readily loaded into the cavities of CPNCs by encapsulation and structures of CPNCs can be tailored to tune the release rate of these agents. On the other hand, for the small molecule diagnostic agents or the nano-sized ones that need to be confined with scaffolds during diagnostic periods, diffusion-permitting physical loading would result in unfavorable premature release, and these agents need to be either conjugated with the polymer shells of CPNCs via stable linkages or encapsulated by CPNCs whose crosslinking density is sufficiently high to confine them based on their nanoscopic dimensions.
Among all the aforementioned imaging modalities, MRI showed remarkable advantages because it can provide superior resolution by using nonionizing radiation, yielding physiological information (Shung, Smith, & Tsui, 2012). Landfester and co-workers reported the synthesis of CPNCs containing the hydrophilic gadolinium (Gd) complex contrast agents (Magnevist® and Gadovist®) by miniemulsion technique (Jagielski et al., 2007). The highly water-permeable porous polymeric shells were formed by polyurethane, polyurea and crosslinked dextran. The possible Gd load of a single CPNC could be as high as 10−17 mmol (50 nm) and 10−14 mmol (300 nm), and there were no significant compromises observed in the T1 relaxitivity of the contrast agents. Moreover, the use of CPNCs made contrast agents with high stability during circulation, and also avoided the leakage of Gd complex into the systemic system. This indicated that CPNCs containing contrast agents could be promising for MRI. Wong and co-workers reviewed MRI agents developed by polyamine-salt aggregate (PSA) assembly (Bagaria, & Wong, 2011). Such PSA assemblies stabilized by ionic crosslinking also had other applications, such as photothermal therapy and protease-responsive near-infrared (NIR) imaging.
Ultrasound imaging is also among the most widely used non-ionizing radiation diagnostic imaging modalities, because it is not only noninvasive, relatively low cost and portable, but also provides real-time visualization (Wells, 2006). The application of CPNCs in encapsulating low-boiling point (low-bp) perfluorocarbons (PFCs) at physiological temperatures for ultrasound-triggered vaporization showed promise to improve the contrast of ultrasound imaging, which can be greatly enhanced by gas microbubbles. In the study reported by Huang et al. (Huang et al., 2016), microdroplets containing low-bp PFCs were prepared by using a crosslinkable amphiphilic triblock copolymer to emulsify PFCs, and the subsequent UV-induced thiol-ene crosslinking of the triblock copolymer yielded CPNCs (~180 nm) encapsulating PFCs. While the presence of the crosslinked shells of CPNCs promoted the stabilization of the PFC cores which remain as liquid even at temperatures higher than their boiling points, the vaporization of the encapsulated PFCs at rarefactional pressures relevant for clinical ultrasound was critically demonstrated.
CPNCs possess further applicability in fluorescence imaging of both cancerous and healthy cells. For example, PEGylated, antibody-functionalized polymer nanocapsules containing Ln3+-doped LaVO4 hollowed nanoparticles for fluorescence imaging were reported (Jeyaraman, Shukla, & Sivakumar, 2016) LaVO4:Ln3+ nanoparticles, known for their large Stoke’s shift and upconversion emission, were first formed over a silica template, using a PEG-based internal cross-linking agent. In the layer-by-layer process over the LaVO4:Ln3+ nanoparticles, polyethyleneimine was used as the first layer, followed by coating with alternating layers of poly(sodium 4-styrene-sulfonate) (PSS) and poly(allylamine hydrochloride). The final CPNCs were obtained by further PEGylation of the outer PSS layer, removal of the silica core using HF, and surface functionalization with tumor-targeting antibody. These CPNCs showed excellent biocompatibility and efficient cellular uptake through macropinocytosis, with clear fluorescence observed in six different cell lines. Furthermore, HER-2/Erbb-2 antibody modification enabled 3-fold higher cellular uptake in MCF-7 cancer cells than a HeLa cell control, and EGFR antibody modification enabled 10-fold higher cellular uptake in H460 cancer cells than the control.
More recently, zwitterionic CPNCs were studied for NIR fluorescence cancer imaging (Sun et al., 2019; Figure 7). The PLA-based CPNCs with conjugated sulfobetaine (SB) zwitterions and fluorescent dye Cy5.5 were prepared by miniemulsion interfacial crosslinking of the precursor copolymer using a dithiol cross-linker via UV-induced thiol–ene chemistry. These CPNCs were biodegradable, biocompatible, with high colloidal stability. They could be taken up by MIA PaCa-2 cancer cells. In vivo imaging study demonstrated that, relative to a small molecule dye, these CPNCs led to a longer circulation time, facilitating their accumulation at tumor site for cancer imaging via EPR effect. This work reveals the promising applicability of zwitterionic biodegradable CPNCs in cancer diagnosis.
FIGURE 7.
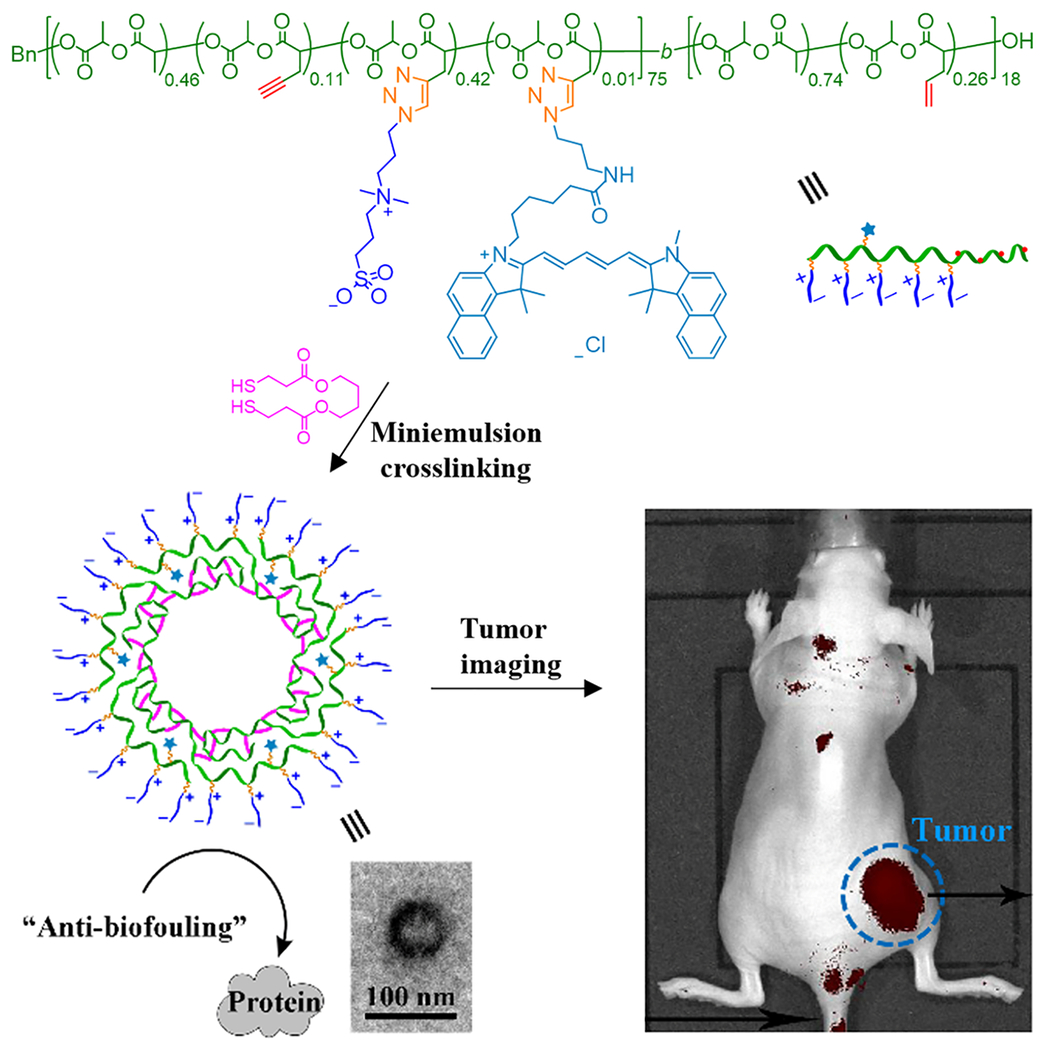
PLA-based zwitterionic CPNCs for NIR fluorescence cancer imaging (Adapted with permission from Sun et al. (2019).
CPNCs can be used as heat-sensitive recording material for medicinal images. Crosslinked poly(methylmethacrylate) (PMMA)-based CPNCs with heat-sensitive color-developing properties were prepared by emulsion-based template synthesis (An, Ba, & Lu, 2010). While the shells of CPNCs were crosslinked by using unsaturated hyperbranched poly(amide-ester), leucocompound, an electron-donating dye precursor, was in situ encapsulated in the inner cavities of the CPNCs. When the CPNCs were heated to a temperature above the glass transition temperature of the shell, the transmittance of the shell would increase and the leucocompound would react with the electron-accepting compound dissolved outside of the CPNCs, thereby developing a color.
THERANOSTIC APPLICATIONS OF CPNCS
As versatile nanocarriers, CPNCs have also been employed as the theranostic platforms. Therapeutic application can be integrated with MRI diagnostic application in the corresponding studies. Using superparamagnetic iron oxide (SPIO) as an MRI contrast agent and DOX as a chemotherapeutic agent, Gong and co-workers reported SIPO/DOX-loaded CPNCs. These CPNCs were derived from triblock copolymers. In one study (Yang, Grailer, Rowland, Javadi, Hurley, Steeber, et al., 2010), R (R = methoxy or FA)-PEG114-PLA293-PEG46-acrylate triblock copolymer was prepared, and then SIPO/DOX-loaded wormlike vesicles with FA moieties mostly on outer shell surface and acrylate groups mostly on the inner shell surface were obtained by a double emulsion approach. Subsequently, crosslinking via acrylate groups yielded the corresponding CPNCs with SIPO encapsulated in their inner cavities and DOX loaded in the PLA-based hydrophobic membrane of their shells. Their crosslinked structures led to more sustained DOX release behavior, while the conjugated FA moieties on shell surface facilitated cellular uptake via the folate receptor-mediated endocytosis process and resulted in enhanced cytotoxicity against the HeLa tumor cell line. Moreover, the SPIO/DOX-loaded wormlike CPNCs exhibited a much higher r2 relaxivity value than Feridex®, a clinically used T2 agent, presumably due to the high loading level of SPIO and the SPIO clustering effect. In another study (Yang, Grailer, Rowland, Javadi, Hurley, Matson, et al., 2010), R (methoxy or FA)-PEG114-poly(glutamate hydrazone DOX)-PEG46-acrylate) triblock copolymer was prepared, and the SIPO/DOX-loaded spherical CPNCs were prepared by polymer self-assembly in aqueous solution in the presence of SIPO, followed by crosslinking of inner layers. In addition to the merits similar to the previous system, a major advantage of this system is that the conjugation of DOX to the vesicular middle layers with a pH-sensitive hydrazone bond allowed pH-trigger sustained DOX release. Noteworthily, CPNCs loaded with both magnetic materials and DOX were also investigated by other groups using different polymer precursors, but relaxivity data were not reported in these studies (Ma et al., 2012; Balan et al., 2015).
Co-delivery of ultrasound contrast agent (UCA) and therapeutic agent using CPNCs was also studied. Gas-filled microbubbles have been developed as UCAs to enhance ultrasound signal, but they dissolved and collapsed during diagnostic conditions without an ability to deliver therapeutic agents. Taking advantage of the robust structure and loading capacity of CPNCs, Yang and co-workers studied gas-filled CPNCs as UCAs, which were also loaded with DOX as a chemotherapeutic agent (Hu et al., 2016). Their poly(MAA-co-EGDMA) (HPMAA) CPNCs, approximately 250 nm in diameter and capable of a high DOX loading capacity of 52.4 wt%, were prepared via combination of the sol-gel method and surface-initiated polymerization of methacrylic acid (MAA) monomer and ethylene glycol dimethacrylate (EGDMA) crosslinker, followed by dissolution of the silica core template in 10% HF. The surface was further modified with methoxy polyethylene glycol amine (mPEG) to improve biocompatibility. According to in vitro MTT assay results, DOX-loaded HPMAA-mPEG CPNCs left a 26% HeLa cell viability at the highest DOX concentration of 100 μg/mL after 24 h. HMPAA-mPEG CPNCs were also shown to display high ultrasound resonance both in vitro and in vivo, indicating their potential use as contrast agents in addition to their chemotherapeutic use. In addition, Chen et al. recently reported the development of hydrogen-bonded multilayer capsules for DOX delivery and ultrasound imaging (Chen et al., 2017). The DOX-encapsulated capsules showed longer contrast than commercially available microbubbles, ready modulation of DOX release by dose of ultrasound radiation. In vitro cytotoxicity study showed that they resulted in 99% MCF-7 cell viability in absence of ultrasound irradiation, whereas only 14% of cells remains viable after ultrasound irradiation. Although the capsules were micron-sized and stabilized via hydrogen bonding, the above promising results provided helpful information for the design of CPNCs for ultrasound imaging and guided drug delivery.
Wang and co-workers reported perfluorohexane (PFH)-filled triple-stimuli responsive (ultrasound/pH/GSH) biodegradable CPNCs as novel UCAs, with DOX loaded in their shells, to serve as ultrasound traced and triggered drug delivery system (Yang et al., 2014). The poly(methacrylic acid) (PMAA)-based CPNCs with disulfide-containing crosslinkages in their shells were prepared by radical polymerization of MAA and N,N-bis(acryloyl)cystamine (disulfide crosslinker) using uncrosslinked PMAA nanospheres as seeds, which were subsequently removed by dissolution. These CPNCs with uniform size of 300 nm were loaded with DOX (36 wt% in PMMA shells; 93.5% loading efficiency) and filled with PFH. DOX release from the CPNCs showed triple-stimuli responsivity and could be effectively triggered by ultrasound, low pH, and GSH (Figure 8a). Especially, quick DOX release (< 5 min) was observed under ultrasound conditions. These DOX-loaded CPNCs could be readily taken up by HeLa cells and exhibited significant therapeutic efficacy. As compared with the blank CPNCs (i.e. PMMA-40), the PFH-filled CPNCs gave highly enhanced ultrasound imaging signal via acoustic droplet vaporization (Figure 8b). The integrated DOX-loaded PFH-filled CPNCs showed promising potentials as multifunctional theranostic nanoplatforms for ultrasound diagnostic and image-guided therapeutic applications.
FIGURE 8.
a) Ultrasound (US) triggered pH/redox-responsive DOX release profiles from DOX-loaded PMAA-PFH CPNCs under pH 7.4 (top) and pH 5.0 (bottom). b) In vitro US imaging for PBS control, PMAA-40 CPNCs and PMAA-PFH CPNCs under B-mode and power Doppler mode (Reprinted with permission from Yang et al. (2014). Copyright 2014 Elsevier)
NOTE: For Figure 8a and 8b please refer to Figure 6 and Figure 9, respectively, of the manuscript by Yang et al.:
Yang, P., Li, D., Jin, S., Ding, J., Guo, J., Shi, W., & Wang, C. (2014). Stimuli-responsive biodegradable poly(methacrylic acid) based nanocapsules for ultrasound traced and triggered drug delivery system. Biomaterials, 35(6), 2079-2088.
Co-delivery of fluorescent agents and anticancer drugs has also been studied. Co-encapsulation of pyrene fluorophore and DOX inside microporous CPNCs derived from hypercrosslinked polymers was reported very recently (Razzaque, Cheng, Hussain, & Tan, 2020). The CPNCs were prepared by emulsion polymerization in presence of silica nanoparticles as templates, shell-crosslinking, followed by removal of silica cores. The microporous CPNCs exhibited high surface area of 700 m2/g. Decoration of the surface of the microcapsules with folic acid via EDC/NHS chemistry enhanced their accumulation in MCF-7 breast cancer cells by receptor mediated endocytosis. Incorporation of self-fluorescent pyrene within the microcapsules suggested their application of in diagnostic imaging.
CONCLUSION
Over the past decade, numerous types of CPNCs have been prepared and utilized as the nanocarriers for therapeutic delivery, diagnostic, and theranostic applications. The major structural advantages of CPNCs include: 1) their inner cavities allowing for encapsulation of cargoes of larger quantities and/or sizes; 2) their high specific surface area favoring surface adsorption, modification and interactions; 3) their ability for region-specific loading of multiple cargos; 4) their crosslinked structures providing architectural integrity and relatively stable physicochemical properties to protect cargo and to regulate cargo release. By selecting or designing appropriate base polymers, crosslinkages, and functionalization strategies, CPNCs can possess a variety of preferred biomedically relevant properties (such as biocompatibility, biodegradability, water-dispersibility, stimuli-responsivity, etc.). In general, CPNCs are prepared by using template-assisted synthetic approaches, with their dimensions controlled via templates. Among these approaches, emulsion-based interfacial crosslinking and vesicle-based crosslinking can permit in situ encapsulation of biomedical cargos; core cavitation of shell-crosslinked nanoparticles is less commonly used, and requires further developments to facilitate core removal and cargo loading; direct use of nano-sized biomedical cargos as synthetic templates has emerged as an important method for encapsulation of genetic materials and proteins by CPNCs.
CPNCs have been investigated for the delivery of a broad variety of biomedical cargos, including small molecule drugs (especially chemotherapeutic drugs), genetic materials (i.e., siRNA, RNA-encoded DNAs, and RNPs), proteins, and bioimaging agents. Although localized CPNC-based biomedical systems have been reported, CPNCs are generally designed for biomedical applications via systemic circulation. Structures of CPNCs need to be optimized in order to promote biomedical outcomes. Biomedical functionalization of CPNCs with cell-penetrating peptides and/or targeting moieties is often employed to improve cellular uptake and in vivo bioavailability of CPNC-based biomedical nanosystems. Because CPNCs possess multiple spatial domains (cavities, shells, and shell surface) that have different physicochemical properties, co-delivery of multiple biomedical cargoes by CPNCs is feasible and may lead to synergistic biomedical effects. In particular, theranostic applications of CPNCs via co-delivery of chemotherapeutic drugs and bioimaging contrast agents have been demonstrated.
At the current stage, preparation and functionalization approaches of CPNCs have been well developed, but comprehensive studies of CPNC-based biomedical systems require closely integrated interdisciplinary research efforts by overcoming the collaboration barriers among different disciplines. While the merits of these systems have been broadly revealed through in vitro studies, extensive in vivo studies are needed to verify the remarkable advantages of CPNCs relative to other nanocarriers for specific biomedical delivery applications, in order to pave the way for clinic transformation of CPNC-based therapeutic or diagnostic formations. Because typically CPNCs have more complicated structures and require more challenging preparation than other commonly used nanocarriers, their biomedical applications need to be designed thoughtfully to address the biomedical needs that may not be sufficiently met by using other nanocarriers. Moreover, the development of CPNC-based platforms to apply for emerging biomedical nanotechnologies also represents an important long-term research direction.
ACKNOWLEDGEMENTS
Financial support from the U. S. National Science Foundation (CHE-1412785, and DMR-1609914) and U. S. National Institutes of Health (R21 EB024095-01) is gratefully acknowledged.
Footnotes
CONFLICT OF INTEREST
The authors have declared no conflicts of interest for this article.
Contributor Information
Haotian Sun, Department of Chemical and Biological Engineering, University at Buffalo, the State University of New York, Buffalo, New York 14260, USA.
William Erdman, III, Department of Chemical and Biological Engineering, University at Buffalo, the State University of New York, Buffalo, New York 14260, USA.
Yuan Yuan, Department of Biomedical Engineering, University at Buffalo, the State University of New York, Buffalo, New York 14260, USA.
Mohamed Alaa Mohamed, 1) Department of Chemical and Biological Engineering, University at Buffalo, the State University of New York, Buffalo, New York 14260, USA; 2) Department of Chemistry, Mansoura University, Mansoura 33516, Egypt.
Ruosen Xie, 1) Department of Materials Science and Engineering, University of Wisconsin-Madison, Madison, WI, USA; 2) Wisconsin Institute for Discovery, University of Wisconsin-Madison, Madison, WI, USA.
Yuyuan Wang, 1) Department of Biomedical Engineering, University of Wisconsin-Madison, Madison, WI, USA; 2) Wisconsin Institute for Discovery, University of Wisconsin-Madison, Madison, WI, USA.
Shaoqin Gong, 1) Department of Biomedical Engineering, University of Wisconsin-Madison, Madison, WI, USA; 2) Department of Materials Science and Engineering, University of Wisconsin-Madison, Madison, WI, USA; 3) Wisconsin Institute for Discovery, University of Wisconsin-Madison, Madison, WI, USA.
Chong Cheng, Department of Chemical and Biological Engineering, University at Buffalo, the State University of New York.
REFERENCES
- An P, Ba X, & Lu S (2010). Preparation and characterization of crosslinked poly (methylmethacrylate) heat sensitive color-developing nanocapsules. Polymer bulletin, 64(4), 375–386. 10.1007/s00289-009-0188-y [DOI] [Google Scholar]
- Awino JK, Gudipati S, Hartmann AK, Santiana JJ, Cairns-Gibson DF, Gomez N, & Rouge JL (2017). Nucleic acid nanocapsules for enzyme-triggered drug release. Journal of the American Chemical Society, 139(18), 6278–6281. 10.1021/jacs.6b13087 [DOI] [PubMed] [Google Scholar]
- Bagaria HG, & Wong MS (2011). Polyamine–salt aggregate assembly of capsules as responsive drug delivery vehicles. Journal of Materials Chemistry, 21(26), 9454–9466. 10.1039/c1jm10712g [DOI] [Google Scholar]
- Baier G, Baumann D, Siebert JM, Musyanovych A, Mailänder V, & Landfester K (2012). Suppressing unspecific cell uptake for targeted delivery using hydroxyethyl starch nanocapsules. Biomacromolecules, 13(9), 2704–2715. 10.1021/bm300653v [DOI] [PubMed] [Google Scholar]
- Balan V, Dodi G, Tudorachi N, Ponta O, Simon V, Butnaru M, & Verestiuc L (2015). Doxorubicin-loaded magnetic nanocapsules based on N-palmitoyl chitosan and magnetite: Synthesis and characterization. Chemical Engineering Journal, 279, 188–197. 10.1016/j.cej.2015.04.152 [DOI] [Google Scholar]
- Belbekhouche S, Mansour O, & Carbonnier B (2018). Promising sub-100 nm tailor made hollow chitosan/poly (acrylic acid) nanocapsules for antibiotic therapy. Journal of colloid and interface science, 522, 183–190. 10.1016/j.jcis.2018.03.061 [DOI] [PubMed] [Google Scholar]
- Chen CK, Law WC, Aalinkeel R, Yu Y, Nair B, Wu J, … & Cheng C (2014). Biodegradable cationic polymeric nanocapsules for overcoming multidrug resistance and enabling drug–gene co-delivery to cancer cells. Nanoscale, 6(3), 1567–1572. 10.1039/c3nr04804g [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CK, Wang Q, Jones CH, Yu Y, Zhang H, Law WC, … & Cheng C (2014). Synthesis of pH-responsive chitosan nanocapsules for the controlled delivery of doxorubicin. Langmuir, 30(14), 4111–4119. 10.1021/la4040485 [DOI] [PubMed] [Google Scholar]
- Chen G, Abdeen AA, Wang Y, Shahi PK, Robertson S, Xie R, … & Gong S (2019). A biodegradable nanocapsule delivers a Cas9 ribonucleoprotein complex for in vivo genome editing. Nature nanotechnology, 14(10), 974–980. 10.1038/s41565-019-0539-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Ratnayaka S, Alford A, Kozlovskaya V, Liu F, Xue B, … & Kharlampieva E (2017). Theranostic multilayer capsules for ultrasound imaging and guided drug delivery. ACS Nano, 11(3), 3135–3146. 10.1021/acsnano.7b00151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Peng Z, Zeng Z, She Y, Wei J, & Chen Y (2014). Hairy polymeric nanocapsules with pH-responsive shell and thermoresponsive brushes: Tunable permeability for controlled release of water-soluble drugs. Journal of Polymer Science Part A: Polymer Chemistry, 52(15), 2202–2216. 10.1002/pola.27233 [DOI] [Google Scholar]
- Cheng R, Meng F, Ma S, Xu H, Liu H, Jing X, & Zhong Z (2011). Reduction and temperature dual-responsive crosslinked polymersomes for targeted intracellular protein delivery. Journal of materials chemistry, 21(47), 19013–19020. 10.1039/C1JM13536H [DOI] [Google Scholar]
- Davis ME, Chen Z, & Shin DM (2010). Nanoparticle therapeutics: an emerging treatment modality for cancer. Nature Review Drug Discovery, 2008, 7(9), 771–782. 10.1038/nrd2614 [DOI] [PubMed] [Google Scholar]
- Duncan R (2011). Polymer therapeutics as nanomedicines: new perspectives. Current opinion in biotechnology, 22(4), 492–501. 10.1016/j.copbio.2011.05.507 [DOI] [PubMed] [Google Scholar]
- Elsabahy M, & Wooley KL (2012). Design of polymeric nanoparticles for biomedical delivery applications. Chemical Society Reviews, 41(7), 2545–2561. 10.1039/c2cs15327k [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farokhzad OC, & Langer R (2006). Nanomedicine: developing smarter therapeutic and diagnostic modalities. Advanced drug delivery reviews, 58(14), 1456–1459. 10.1016/j.addr.2006.09.011 [DOI] [PubMed] [Google Scholar]
- Freichels H, Wagner M, Okwieka P, Meyer RG, Mailänder V, Landfester K, & Musyanovych A (2013). (Oligo) mannose functionalized hydroxyethyl starch nanocapsules: en route to drug delivery systems with targeting properties. Journal of Materials Chemistry B, 1(34), 4338–4348. 10.1039/C3TB20138D [DOI] [PubMed] [Google Scholar]
- Fu S, Zhang Y, Guan S, Huang Q, Wang R, Tian R, … & Fan X (2018). Reductive-responsive, single-molecular-layer polymer nanocapsules prepared by lateral-functionalized pillar [5] arenes for targeting anticancer drug delivery. ACS applied materials & interfaces, 10(17), 14281–14286. 10.1021/acsami.8b03534 [DOI] [PubMed] [Google Scholar]
- Gu X, Wang J, Wang Y, Wang Y, Gao H, & Wu G (2013). Layer-by-layer assembled polyaspartamide nanocapsules for pH-responsive protein delivery. Colloids and Surfaces B: Biointerfaces, 108, 205–211. 10.1016/j.colsurfb.2013.03.007 [DOI] [PubMed] [Google Scholar]
- Gu Z, Yuan Y, He J, Zhang M, & Ni P (2009). Facile approach for DNA encapsulation in functional polyion complex for triggered intracellular gene delivery: design, synthesis, and mechanism. Langmuir, 25(9), 5199–5208. 10.1021/la804037v [DOI] [PubMed] [Google Scholar]
- Haladjova E, Kyulavska M, Doumanov J, Topouzova-Hristova T, & Petrov P (2017). Polymeric vehicles for transport and delivery of DNA via cationic micelle template method. Colloid and Polymer Science, 295(11), 2197–2205. 10.1007/s00396-017-4193-7 [DOI] [Google Scholar]
- Hoyle CE, Lowe AB, Bowman CN (2010). Thiol-click chemistry: a multifaceted toolbox for small molecule and polymer synthesis. Chemical Society Reviews, 39(4), 1355–1387. 10.1039/B901979K [DOI] [PubMed] [Google Scholar]
- Hu H, Zhang X, Sun J, An L, Du J, Yang H, … & Yang S (2016). Preparation of pH-responsive hollow poly (MAA-co-EGDMA) nanocapsules for drug delivery and ultrasound imaging. RSC Advances, 6(105), 103754–103762. 10.1039/C6RA21411H [DOI] [Google Scholar]
- Huang H, Remsen EE, Kowalewski T, & Wooley KL (1999). Nanocages derived from shell cross-linked micelle templates. Journal of the American Chemical Society, 121(15), 3805–3806. 10.1021/ja983610w [DOI] [Google Scholar]
- Huang Y, Vezeridis AM, Wang J, Wang Z, Thompson M, Mattrey RF, & Gianneschi NC (2016). Polymer-stabilized perfluorobutane nanodroplets for ultrasound imaging agents. Journal of the American Chemical Society, 139(1), 15–18. 10.1021/jacs.6b08800 [DOI] [PubMed] [Google Scholar]
- Iha RK, Wooley KL, Nystrom AM, Burke DJ, Kade MJ, & Hawker CJ (2009). Applications of orthogonal “click” chemistries in the synthesis of functional soft materials. Chemical Reviews, 109(11), 5620–5686. 10.1021/cr900138t [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagielski N, Sharma S, Hombach V, Mailänder V, Rasche V, & Landfester K (2007). Nanocapsules synthesized by miniemulsion technique for application as new contrast agent materials. Macromolecular chemistry and physics, 208(19-20), 2229–2241. 10.1002/macp.200700254 [DOI] [Google Scholar]
- Jaromin A, Zarnowski R, Piętka-Ottlik M, Andes DR, & Gubernator J (2018). Topical delivery of ebselen encapsulated in biopolymeric nanocapsules: drug repurposing enhanced antifungal activity. Nanomedicine, 13(10), 1139–1155. 10.2217/nnm-2017-0337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeyaraman J, Shukla A, & Sivakumar S (2016). Targeted stealth polymer capsules encapsulating Ln3+-doped LaVO4 nanoparticles for bioimaging applications. ACS Biomaterials Science & Engineering, 2(8), 1330–1340. 10.1021/acsbiomaterials.6b00252 [DOI] [PubMed] [Google Scholar]
- Jin L, Zeng X, Liu M, Deng Y, & He N (2014). Current progress in gene delivery technology based on chemical methods and nano-carriers. Theranostics, 4(3), 240. 10.7150/thno.6914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CH, Chen CK, Ravikrishnan A, Rane S, & Pfeifer BA (2013). Overcoming nonviral gene delivery barriers: perspective and future. Molecular pharmaceutics, 10(11), 4082–4098. 10.1021/mp400467x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan M, Ong ZY, Wiradharma N, Attia ABE, & Yang YY (2012). Advanced materials for co-delivery of drugs and genes in cancer therapy. Advanced healthcare materials, 1(4), 373–392. 10.1002/adhm.201200109 [DOI] [PubMed] [Google Scholar]
- Kim E, Kim D, Jung H, Lee J, Paul S, Selvapalam N, … & Kim K (2010). Facile, template - free synthesis of stimuli - responsive polymer nanocapsules for targeted drug delivery. Angewandte Chemie International Edition, 49(26), 4405–4408. 10.1002/anie.201000818 [DOI] [PubMed] [Google Scholar]
- Knop K, Hoogenboom R, Fischer D, & Schubert US (2010). Poly(ethylene glycol) in drug delivery: pros and cons as well as potential alternatives. Angewandte chemie international edition, 49(36), 6288–6308. 10.1002/anie.200902672 [DOI] [PubMed] [Google Scholar]
- Kraft JC, Freeling JP, Wang Z, & Ho RJ (2014). Emerging research and clinical development trends of liposome and lipid nanoparticle drug delivery systems. Journal of pharmaceutical sciences, 103(1), 29–52. 10.1002/jps.23773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon S, Ko H, You DG, Kataoka K, & Park JH (2019). Nanomedicines for reactive oxygen species mediated approach: an emerging paradigm for cancer treatment. Accounts of Chemical Research, 52(7), 1771–1782. 10.1021/acs.accounts.9b00136 [DOI] [PubMed] [Google Scholar]
- Landis RF, Gupta A, Lee YW, Wang LS, Golba B, Couillaud B, … & Rotello VM (2017). Cross-linked polymer-stabilized nanocomposites for the treatment of bacterial biofilms. ACS nano, 11(1), 946–952. 10.1021/acsnano.6b07537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, Bae KH, Kim JS, Nam YS, & Park TG (2011). Intracellular delivery of paclitaxel using oil-free, shell cross-linked HSA–multi-armed PEG nanocapsules. Biomaterials, 32(33), 8635–8644. 10.1016/j.biomaterials.2011.07.063 [DOI] [PubMed] [Google Scholar]
- Lee K, Bae KH, Lee Y, Lee SH, Ahn CH, & Park TG (2010). Pluronic/polyethylenimine shell crosslinked nanocapsules with embedded magnetite nanocrystals for magnetically triggered delivery of siRNA. Macromolecular bioscience, 10(3), 239–245. 10.1002/mabi.200900291 [DOI] [PubMed] [Google Scholar]
- Lertsutthiwong P, Rojsitthisak P, & Nimmannit U (2009). Preparation of turmeric oil-loaded chitosan-alginate biopolymeric nanocapsules. Materials Science and Engineering: C, 29(3), 856–860. 10.1016/j.msec.2008.08.004 [DOI] [Google Scholar]
- Li A, Ma H, Feng S, & Liu J (2016). A copolymer capsule with a magnetic core for hydrophilic or hydrophobic drug delivery via thermo-responsive stimuli or carrier biodegradation. RSC Advances, 6(39), 33138–33147. 10.1039/c5ra27839b [DOI] [Google Scholar]
- Li J, Zhang L, Liu Y, Wen J, Wu D, Xu D, … & Wang H (2016). An intracellular protein delivery platform based on glutathione-responsive protein nanocapsules. Chemical Communications, 52(93), 13608–13611. 10.1039/c6cc05099a [DOI] [PubMed] [Google Scholar]
- Liang S, Liu Y, Jin X, Liu G, Wen J, Zhang L, … & Lu Y (2016) Phosphorylcholine polymer nanocapsules prolong the circulation time and reduce the immunogenicity of therapeutic proteins. Nano Research, 9(4), 1022–1031. 10.1007/s12274-016-0991-3 [DOI] [Google Scholar]
- Lin G, Hu R, Law WC, Chen CK, Wang Y, Li Chin H, … & Yong K-T (2013). Biodegradable nanocapsules as siRNA carriers for mutant K-Ras gene silencing of human pancreatic carcinoma cells. Small, 9(16), 2757–2763. 10.1002/smll.201201716 [DOI] [PubMed] [Google Scholar]
- Liong M, Lu J, Kovochich M, Xia T, Ruehm SG, Nel AE, … & Zink JI (2008). Multifunctional inorganic nanoparticles for imaging, targeting, and drug delivery. ACS nano, 2(5), 889–896. 10.1021/nn800072t [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma WF, Wu KY, Tang J, Li D, Wei C, Guo J, … & Wang CC (2012). Magnetic drug carrier with a smart pH-responsive polymer network shell for controlled delivery of doxorubicin. Journal of Materials Chemistry, 22(30), 15206–15214. 10.1039/C2JM31721D [DOI] [Google Scholar]
- Meier W (2000). Polymer nanocapsules. Chemical Society Reviews, 29(5), 295–303. 10.1039/A809106D [DOI] [Google Scholar]
- Mora-Huertas CE, Fessi H, & Elaissari A (2010). Polymer-based nanocapsules for drug delivery. International journal of pharmaceutics, 385(1–2), 113–142. 10.1016/j.ijpharm.2009.10.018 [DOI] [PubMed] [Google Scholar]
- Mura S, Nicolas J, & Couvreur P (2013). Stimuli-responsive nanocarriers for drug delivery. Nature materials, 12(11), 991–1003. 10.1038/nmat3776 [DOI] [PubMed] [Google Scholar]
- Nguyen HTP, Munnier E, Soucé M, Perse X, David S, Bonnier F, … & Chourpa I (2015). Novel alginate-based nanocarriers as a strategy to include high concentrations of hydrophobic compounds in hydrogels for topical application. Nanotechnology, 26(25), 255101. 10.1088/0957-4484/26/25/255101 [DOI] [PubMed] [Google Scholar]
- Odian G Principles of Polymerization, 4th Ed., John Wiley & Sons, 2004. [Google Scholar]
- Pejawar-Gaddy S, Kovacs JM, Barouch DH, Chen B, & Irvine DJ (2014). Design of lipid nanocapsule delivery vehicles for multivalent display of recombinant Env trimers in HIV vaccination. Bioconjugate chemistry, 25(8), 1470–1478. 10.1021/bc5002246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petros RA, & DeSimone JM (2010). Strategies in the design of nanoparticles for therapeutic applications. Nature reviews Drug discovery, 9(8), 615. 10.1038/nrd2591 [DOI] [PubMed] [Google Scholar]
- Radhakrishnan K, Thomas MB, Pulakkat S, Gnanadhas DP, Chakravortty D, & Raichur AM (2015). Stimuli-responsive protamine-based biodegradable nanocapsules for enhanced bioavailability and intracellular delivery of anticancer agents. Journal of Nanoparticle Research, 17(8), 341. 10.1007/s11051-015-3145-8 [DOI] [Google Scholar]
- Rai M, Yadav A, & Gade A (2009). Silver nanoparticles as a new generation of antimicrobials. Biotechnology advances, 27(1), 76–83. 10.1016/j.biotechadv.2008.09.002 [DOI] [PubMed] [Google Scholar]
- Razzaque S, Cheng Y, Hussain I, & Tan B (2020) Synthesis of surface functionalized hollow microporous organic capsules for doxorubicin delivery to cancer cells. Polymer Chemistry, 11(12), 2110–2118. 10.1039/C9PY01772K [DOI] [Google Scholar]
- Santiana JJ, Gudipati S, Hartmann AK, & Rouge JL (2019). Hybrid Nucleic Acid Nanocapsules for Targeted, Enzyme-Specific Drug Delivery and Intracellular Gene Regulation. In Targeted Nanosystems for Therapeutic Applications: New Concepts, Dynamic Properties, Efficiency, and Toxicity (pp. 59–77). American Chemical Society. 10.1021/bk-2019-1309.ch003 [DOI] [Google Scholar]
- Santiana JJ, Sui B, Gomez N, & Rouge JL (2017). Programmable peptide-cross-linked nucleic acid nanocapsules as a modular platform for enzyme specific cargo release. Bioconjugate chemistry, 28(12), 2910–2914. 10.1021/acs.bioconjchem.7b00629 [DOI] [PubMed] [Google Scholar]
- Shung KK, Smith M, & Tsui BM (2012). Principles of medical imaging. Academic Press. [Google Scholar]
- Siebert JM, Baier G, Musyanovych A, & Landfester K (2012). Towards copper-free nanocapsules obtained by orthogonal interfacial “click” polymerization in miniemulsion. Chemical Communications, 48(44), 5470–5472. 10.1039/C2CC30253E [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, & Jemal A (2019). Cancer statistics, 2019. CA: a cancer journal for clinicians, 69(1), 7–34. 10.3322/caac.21551 [DOI] [PubMed] [Google Scholar]
- Sun H, Chen C, Cui H, & Cheng C (2016). Crosslinked polymer nanocapsules. Polymer International, 65(4), 351–361. 10.1002/pi.5077 [DOI] [Google Scholar]
- Sun H, Yan L, Carter KA, Zhang J, Caserto JS, Lovell JF, … & Cheng C (2019). Zwitterionic Cross-Linked Biodegradable Nanocapsules for Cancer Imaging. Langmuir, 35(5), 1440–1449. 10.1021/acs.langmuir.8b01633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H, Yarovoy I, Capeling M, & Cheng C (2017). Polymers in the co-delivery of siRNA and anticancer drugs for the treatment of drug-resistant cancers. Topics in Current Chemistry, 375, 24. 10.1007/s41061-017-0113-z [DOI] [PubMed] [Google Scholar]
- Taheri S, Baier G, Majewski P, Barton M, Förch R, Landfester K, & Vasilev K (2014). Synthesis and antibacterial properties of a hybrid of silver–potato starch nanocapsules by miniemulsion/polyaddition polymerization. Journal of Materials Chemistry B, 2(13), 1838–1845. 10.1039/C3TB21690J [DOI] [PubMed] [Google Scholar]
- Taylor-Pashow KM, Della Rocca J, Huxford RC, & Lin W (2010). Hybrid nanomaterials for biomedical applications. Chemical Communications, 46(32), 5832–5849. 10.1039/C002073G [DOI] [PubMed] [Google Scholar]
- Tian H, Du J, Wen J, Liu Y, Montgomery SR, Scott TP, … & Lu Y (2016). Growth-factor nanocapsules that enable tunable controlled release for bone regeneration. ACS Nano, 10(8), 7362–7369. 10.1021/acsnano.5b07950 [DOI] [PubMed] [Google Scholar]
- Tian K, Zeng J, Zhao X, Liu L, Jia X, & Liu P (2015). Synthesis of multi-functional nanocapsules via interfacial AGET ATRP in miniemulsion for tumor micro-environment responsive drug delivery. Colloids and Surfaces B: Biointerfaces, 134, 188–195. 10.1016/j.colsurfb.2015.06.057 [DOI] [PubMed] [Google Scholar]
- Tong R, & Cheng J (2007). Anticancer polymeric nanomedicines. Journal of Macromolecular Science, Part C: Polymer Reviews, 47(3), 345–381. 10.1080/15583720701455079 [DOI] [Google Scholar]
- Utama RH, Jiang Y, Zetterlund PB, & Stenzel MH (2015). Biocompatible glycopolymer nanocapsules via inverse miniemulsion periphery RAFT polymerization for the delivery of gemcitabine. Biomacromolecules, 16(7), 2144–2156. 10.1021/acs.biomac.5b00545 [DOI] [PubMed] [Google Scholar]
- Wang Y, Ma B, Abdeen AA, Chen C, Xie R, Saha K, & Gong S (2018). Versatile Redox-Responsive Polyplexes for the Delivery of Plasmid DNA, Messenger RNA, and CRISPR-Cas9 Genome-Editing Machinery. ACS Applied Materials & Interfaces, 10(38), 31915–31927. 10.1021/acsami.8b09642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Ye M, Xie R, & Gong S (2019). Enhancing the In Vitro and In Vivo Stabilities of Polymeric Nucleic Acid Delivery Nanosystems. Bioconjugate Chemistry, 30(2), 325–337. 10.1002/adma.201807557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells PN (2006). Ultrasound imaging. Physics in Medicine & Biology, 51(13), R83. 10.1088/0031-9155/51/13/R06 [DOI] [PubMed] [Google Scholar]
- Wu D, Qin M, Xu D, Wang L, Liu C, Ren J, … & Lu Y (2019). A bioinspired platform for effective delivery of protein therapeutics to the central nervous system. Advanced Materials, 31(18), 1807557. 10.1002/adma.201807557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Meng F, & Zhong Z (2009). Reversibly crosslinked temperature-responsive nano-sized polymersomes: synthesis and triggered drug release. Journal of materials chemistry, 19(24), 4183–4190. 10.1039/B901141B [DOI] [Google Scholar]
- Yan M, Du J, Gu Z, Liang M, Hu Y, Zhang W, … & Lu Y (2010). A novel intracellular protein delivery platform based on single-protein nanocapsules. Nature nanotechnology, 5(1), 48–53. 10.1038/nnano.2009.341 [DOI] [PubMed] [Google Scholar]
- Yan M, Liang M, Wen J, Liu Y, Lu Y, & Chen IS (2012). Single siRNA nanocapsules for enhanced RNAi delivery. Journal of the American Chemical Society, 134(33), 13542–13545. 10.1021/ja304649a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan M, Wen J, Liang M, Lu Y, Kamata M, & Chen IS (2015). Modulation of gene expression by polymer nanocapsule delivery of DNA cassettes encoding small RNAs. PloS one, 10(6), e0127986. 10.1371/journal.pone.0127986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang WJ, Zhao T, Zhou P, Chen S, Gao Y, Liang L, … & Wang L (2017). “Click” functionalization of dual stimuli-responsive polymer nanocapsules for drug delivery systems. Polymer Chemistry, 8(20), 3056–3065. 10.1039/C7PY00161D [DOI] [Google Scholar]
- Yang P, Li D, Jin S, Ding J, Guo J, Shi W, & Wang C (2014). Stimuli-responsive biodegradable poly(methacrylic acid) based nanocapsules for ultrasound traced and triggered drug delivery system. Biomaterials, 35(6), 2079–2088. 10.1016/j.biomaterials.2013.11.057 [DOI] [PubMed] [Google Scholar]
- Yang X, Grailer JJ, Rowland IJ, Javadi A, Hurley SA, Matson VZ, … & Gong S (2010). Multifunctional stable and pH-responsive polymer vesicles formed by heterofunctional triblock copolymer for targeted anticancer drug delivery and ultrasensitive MR imaging. ACS nano, 4(11), 6805–6817. 10.1021/nn101670k [DOI] [PubMed] [Google Scholar]
- Yang X, Grailer JJ, Rowland IJ, Javadi A, Hurley SA, Steeber DA, & Gong S (2010). Multifunctional SPIO/DOX-loaded wormlike polymer vesicles for cancer therapy and MR imaging. Biomaterials, 31(34), 9065–9073. 10.1016/j.biomaterials.2010.08.039 [DOI] [PubMed] [Google Scholar]
- Yassin MA, Appelhans D, Mendes RG, Rümmeli MH, & Voit B (2012). pH-Dependent Release of Doxorubicin from Fast Photo-Cross-Linkable Polymersomes Based on Benzophenone Units. Chemistry–A European Journal, 18(39), 12227–12231. 10.1002/chem.201201312 [DOI] [PubMed] [Google Scholar]
- Yi Q, Ma J, Kang K, & Gu Z (2018). Bioreducible nanocapsules for folic acid-assisted targeting and effective tumor-specific chemotherapy. International journal of nanomedicine, 13, 653–667. 10.2147/IJN.S149458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin H, Kanasty RL, Eltoukhy AA, Vegas AJ, Dorkin JR, & Anderson DG (2014). Non-viral vectors for gene-based therapy. Nature Reviews Genetics, 15(8), 541. 10.1038/nrg3763 [DOI] [PubMed] [Google Scholar]
- Yin Y, Xu S, Chang D, Zheng H, Li J, Liu X, … & Xiong F (2010). One-pot synthesis of biopolymeric hollow nanospheres by photocrosslinking. Chemical Communications, 46(43), 8222–8224. 10.1039/c0CC03129a [DOI] [PubMed] [Google Scholar]
- Yoshinaga N, Ishii T, Naito M, Endo T, Uchida S, Cabral H, … & Kataoka K (2017) Polyplex Micelles with Phenylboronate/Gluconamide Cross-Linking in the Core Exerting Promoted Gene Transfection through Spatiotemporal Responsivity to Intracellular pH and ATP Concentration. Journal of the American Chemical Society, 139(51), 18567–18575. 10.1021/jacs.7b08816 [DOI] [PubMed] [Google Scholar]
- Zhang J, Li Y, Wang J, Qi S, Song X, Tao C, … & Chen J (2017). Dual redox-responsive PEG–PPS–cRGD self-crosslinked nanocapsules for targeted chemotherapy of squamous cell carcinoma. RSC Advances, 7(84), 53552–53562. 10.1039/C7RA10499E [DOI] [Google Scholar]
- Zhang P, Sun F, Tsao C, Liu S, Jain P, Sinclair A, … & Jiang S (2015). Zwitterionic gel encapsulation promotes protein stability, enhances pharmacokinetics, and reduces immunogenicity. Proceedings of the National Academy of Sciences, 112(39), 12046–12051. 10.1073/pnas.1512465112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Biswas A, Hu B, Joo KI, Wang P, Gu Z, & Tang Y (2011). Redox-responsive nanocapsules for intracellular protein delivery. Biomaterials, 32(22), 5223–5230. 10.1016/j.biomaterials.2011.03.060 [DOI] [PubMed] [Google Scholar]
- Zou J, Hew CC, Themistou E, Li Y, Chen C-K, Alexandridis P, & Cheng C (2011). Clicking well-defined biodegradable nanoparticles and nanocapsules by UV-Induced thiol-ene cross-linking in transparent miniemulsions. Advanced Materials, 23(37), 4274–4277. 10.1002/adma.201101646 [DOI] [PubMed] [Google Scholar]


