Abstract
An affinity chromatography technique was utilized to isolate and purify the receptors of Escherichia coli K88ac+ fimbriae from the mucus of the small intestines of newborn piglets. Purified K88ac+ fimbriae were covalently immobilized onto a beaded agarose matrix (Sepharose 4B). The immobilized fimbriae were used for the affinity purification of the K88ac+ receptors. Only two major proteins were tightly and specifically bound to the immobilized fimbriae after the column containing bound receptor was washed exhaustively with a buffer containing a high concentration of salt and a detergent. The receptors were eluted as a single component at a low pH. The isolated proteins were then subjected to enzyme-linked immunosorbent assay, sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and Western blot (immunoblot) analyses. The two proteins were of high purity, were responsible for nearly all of the fimbrial binding capacity of the crude mucus, and had molecular masses of 26 and 41 kDa. The method for isolation of E. coli binding proteins is simple and yields purified intestinal receptors in a single chromatographic run. The intestinal mucus of different piglets has different proportions of the two receptor proteins.
Enterotoxigenic Escherichia coli (ETEC) strains bearing K88 fimbriae can specifically adhere to receptors in the mucus of the small intestines of piglets, causing diarrhea (14, 15). A layer of mucus covers the epithelial cells in the mammalian small intestine, is secreted by specialized goblet cells, and contains receptors that recognize specific adhesion proteins (2, 5, 15, 16). K88 fimbriae are filamentous surface appendages that enable ETEC to bind to the receptor in the mucus layer of the small intestine. This binding prevents the removal of the bacteria by intestinal peristalsis and is a prerequisite for virulence (6, 10, 18, 19, 20). Neonatal piglets are extremely sensitive to infection by ETEC but can be protected by specific anti-K88 egg yolk antibodies (11, 14, 17). Piglets are commonly infected by E. coli strains expressing several serological types of the K88 fimbrial antigens. The K88 fimbrial antigens are classified into three sets designated K88ab, K88ac, and K88ad. Each variant shares a common antigen (a) and expresses one of three variant-specific antigens (b, c, or d, respectively) (6, 7, 9, 12). It is also well documented that genetics play a significant role in the susceptibility of piglets to infectious diarrhea. Piglets are resistant to infection if they are genetically defective in their ability to express K88-specific brush border receptors (9, 18); furthermore, the presence of K88-specific receptors in porcine ileal mucus is age dependent (4, 5). The receptors of porcine small intestine mucus that bind to K88ab fimbriae have been isolated and identified by using gel filtration chromatography and 3H-labeled E. coli (15), while the K88ac fimbrial receptors of porcine intestinal brush border have been identified using by 35SO4-labeled E. coli (3, 6). However, little information is available on the nature of the receptor for K88 in the intestinal mucus of pigs. In the present investigation, an affinity chromatographic technique was developed to isolate and purify the mucus protein targets of K88ac+ fimbriae, to identify the purified receptors by enzyme-linked immunosorbent assay (ELISA) and Western blot (immunoblot) assay, and to show that the proportions of two receptor proteins (41 and 26 kDa) in piglets is variable. The ability to readily purify receptors by using a receptor affinity column greatly facilitated the present studies.
MATERIALS AND METHODS
Chemicals and reagents.
Cyanogen bromide-activated Sepharose 4B, prestained protein molecular weight markers, Coomassie brilliant blue R-250, FAST BCIP/NBT-buffered substrate tablets, Tween 20, alkaline phosphatase substrate, bicinchoninic acid (BCA) protein assay reagent, Bradford reagent, alkaline phosphatase-conjugated sheep anti-mouse immunoglobulin G (IgG), alkaline phosphatase conjugated goat anti-swine IgG, a standard molecular weight reference (Sigma marker), Freund's complete and incomplete adjuvants, sodium dodecyl sulfate (SDS), diethanolamine, swine gamma globulin, and bovine serum albumin (BSA) were from Sigma, St. Louis, Mo. The 96-well ELISA plates (Falcon 3911) were from VWR Canlab, Winnipeg, Manitoba, Canada; monoclonal antibody specific for K88 fimbriae was from Central Veterinary Laboratory, Souris, United Kingdom; rabbit anti-K88ac fimbrial antibodies were from our laboratory; instant skim milk powder (Carnation) was from Nestle, Don Mills, Ontario, Canada; 0.2-μm-pore-size nitrocellulose membrane was from Bio-Rad Laboratories, Richmond, Calif.; 0.45-μm-pore-size membrane filter (MSI) was from Fisher Scientific, Nepean, Ontario, Canada; Trypticase soy broth was from Becton Dickinson, Sparks, Md., and the ETEC K88+ strain was from Animal Health Centre, Veterinary Services Branch, Manitoba Agriculture, Winnipeg, Manitoba, Canada.
Buffers.
The following buffers or solutions were used: 1 mM HCl; 1 M NaCl; 0.1 M bicarbonate, pH 9.0; 1 M ethanolamine, pH 9.0; binding buffer (3.84 mM NaH2PO4, 6.16 mM Na2HPO4, 0.15 M NaCl, pH 7.2); washing buffer A (56 mM NaH2PO4, 144 mM Na2PO4, 2 M NaCl [pH 7.2] plus 1% Tween 20); washing buffer B (56 mM NaH2PO4, 144 mM Na2PO4, 1 M NaCl, pH 7.2); buffer C (elution buffer) (0.1 M glycine-HCl, pH 2.5); storage buffer (3.84 mM NaH2PO4, 6.16 mM Na2HPO4, 0.15 M NaCl, 0.02% sodium azide, pH 7.2); phosphate-buffered saline (PBS) (0.01 M sodium phosphate, 0.15 M NaCl, pH 7.2); PBST (0.01 M sodium phosphate, 0.15 M NaCl, 0.05% Tween 20); transfer buffer (48 mM Tris base, 39 mM glycine, 20% methanol, 0.037% SDS); and alkaline phosphatase substrate (1 mg/ml in 10 mM diethanolamine and 0.5 mM MgCl2, pH 9.5).
Preparation of purified E. coli K88ac+ fimbriae.
The local strain of hemolytic ETEC K88+ bacteria was identified as being K88ac (11). The K88+ fimbrial antigen was purified by using a modification of the method of Erickson et al. (6). In this procedure, the bacteria were cultured for 48 h at 37°C with shaking (150 rpm) in Trypticase soy broth. The broth cultures were centrifuged at 4°C and 6000 × g for 15 min, and the pellets were washed with PBS. The precipitate was heated to 60°C in a water bath for 30 min to release the fimbriae from the bacteria and, while still hot, was blended for 10 min at high speed in Polytron homogenizer (Kinematika, GMBH, Lucerne, Switzerland). The bacteria were removed by centrifugation (14,000 × g, 4°C, 15 min), and after filtration through a 0.45-μm-pore-size membrane filter, the fimbriae in the supernatant were precipitated by the addition of 2.5% citric acid to a pH of 4.0. The supernatant was kept at 4°C for 2 h to facilitate precipitation of fimbriae, followed by collection of fimbriae by centrifugation (14,000 × g, 4°C, 15 min). The fimbriae were dissolved in 0.1 M PBS, pH 7.2. The pH 4.0 precipitation, washing, and solubilization steps were repeated four times. The final preparation was stored at −20°C in the pH 7.2 PBS at a protein concentration of 1 mg/ml. The concentration of fimbriae was determined by ELISA with the K88-specific monoclonal antibody, and the fimbriae were analyzed for purity by SDS-polyacrylamide gel electrophoresis (SDS-PAGE). The procedures for ELISA and SDS-PAGE are described below. The fimbriae were estimated to be more than 95% pure.
Preparation of polyclonal antibody.
Purified K88ac+ fimbriae (1 mg/ml) were emulsified with an equal volume of Freund's complete adjuvant, and 1 ml was used to subcutaneously inoculate New Zealand White rabbits (approximate 2 to 2.5 kg) at four sites. After 3 weeks, the rabbits were exposed three times biweekly to a second, similar schedule except that the antigen was emulsified with incomplete Freund's adjuvant. The rabbits were bled (8 weeks after the original inoculations), and sera were obtained. They were tested for specificity and titer by ELISA. Antibody specificity was tested against E. coli K88ab, K88ac, K88ad, K99, and 987P. The antibody cross-reacted with K88ab and K88ad but not with K99 and 987P.
Preparations of mucus.
Twenty-two healthy newborn piglets that had not been fed were obtained from the University of Manitoba experimental farm at Glenlea, Manitoba, Canada. They were sacrificed, the small intestines were collected, and the mucus was isolated by using a modification of the procedure of Metcalfe et al. (15). The track of each small intestine for the isolation of mucus was washed with PBS until the buffer was clear. The gut was cut into about 30-cm-long sections and put into binding buffer, and after removal from the buffer, the sections were split along the mesenteric border. The mucus was collected by gently scraping with a glass slide and was transferred into the binding buffer (pH 7.2). All processes were performed in an ice-cold bath. The scraps from each section were pooled, mixed, and centrifuged at 10,000 × g at 4°C for 15 min to remove particulate material. The supernatant containing the crude intestinal mucus was saved. The total protein concentration was determined by using a BCA kit according to the directions of the manufacturer. The final crude protein concentration was adjusted to 2 mg/ml with binding buffer containing 0.05% sodium azide and stored at −20°C. Before use in affinity chromatography, the mucus samples were centrifuged at 10,000 × g at 4°C for 15 min.
Determination of antibody present in crude mucosa preparations.
Crude small intestinal mucus (2 mg of protein per ml) was assayed for the presence of specific anti-K88+ antibody (IgG) by using an ELISA with alkaline phosphatase as the substrate. The procedure was similar to that used by Metcalfe et al. (15). Purified K88+ fimbriae (100 μl; 5 μg/ml) were immobilized on polystyrene microtiter plates for 2 h at 37°C, washed three times with PBST, blocked with 5% skim milk powder for 2 h at 37°C, and washed with PBST. Twofold serial dilutions of each mucus preparation were then added to each well (100 μl), and the plates were incubated for 2 h at 37°C. After washing with PBST, alkaline phosphatase-labeled goat anti-swine IgG (100 μl; 1:3,000) was added, and the plates were incubated for 1 h at 37°C. All plates were then washed with PBST, substrate (100 μl) was added, and the mixture was incubated for 60 min at 37°C. Absorbency was determined at 405 nm in an ELISA plate reader (model 450; Bio-Rad, Hercules, Calif.).
Preparation of immobilized K88ac+ fimbriae (fimbrial beads).
Two grams of cyanogen bromide-actived Sepharose 4B was swollen in ice-cold distilled water for 2 h and washed twice with 100 ml of ice-cold 1 mM HCl for 30 min. The gel was drained by suction through a glass filtering crucible. It was then washed twice with 100 ml of ice-cold distilled water and twice with ice-cold 0.1 M NaHCO3 (pH 9.0) and partially dried by using the same apparatus. Thirty milligrams of fimbriae in 5 ml of 0.1 M NaHCO3 (pH 9.0) was added to 2 g of the gel at 4°C and left overnight with gentle shaking. The gel was filtered and then washed successively with 100 ml each of 0.1 M NaHCO3 (pH 9.0), 1 M NaCl, and distilled water until the absorbency value was 0.02 optical density (OD) unit at 280 nm. Thirty milliliters of 1 M ethanolamine (pH 9.0) was added to the gel and left at room temperature for 2 h with gentle shaking to block the free amino groups of the gel. The gel was drained by suction, washed successively with 100 ml each of distilled water and 1 M NaCl, and then equilibrated with 50 ml of elution buffer. The gel was finally washed three times with 100 ml of binding buffer and stored at 4°C.
Determination of degree of immobilization of fimbriae on Sepharose 4B.
The soluble fimbrial protein in the mixture was separated from the immobilized fimbriae as described above. The initial and final concentrations of protein in the gel were monitored by using the BCA method (21) as modified by Akins and Tuan (1). BSA was selected as the standard. The final absorbency was measured in a 96-well microplate reader fitted with a 570-nm filter. The amount of immobilized fimbriae was determined by using a competitive assay. In this assay, microplate wells were coated with 0.5 μg of fimbriae in 100 μl of bicarbonate buffer (pH 9.6) at 37°C for 2 h. The plates were washed three times with PBST and subsequently blocked with 5% skim milk powder in PBS for 2 h at 37°C. Following blocking, the plates were washed three times with PBST. A suspension of the gel (100 μl at a fimbria concentration of about 10 μg/100 μl) was added to the first wells in a row, followed by successive double dilutions of the gel in the other wells of each row. The fimbria-free gel and BSA covalently bound to Sepharose 4B were used as controls. Rabbit anti-K88ac+ fimbrial antibody (100 μl; 1:3,000) was added to each well. The mixture was incubated at 37°C for 2 h, followed by three washes with PBST. Alkaline phosphatase-conjugated sheep anti-mouse IgG diluted in PBS (100 μl; 1:3,000) was added to all wells, followed by incubation at 37°C for 1 h and five washes with PBST. Substrate solution (100 μl) was added to each well, the plate was developed for 30 min at 37°C, and absorbencies were determined with an ELISA plate reader fitted with a 405-nm filter.
Elution of receptors from the affinity column.
The gel containing the immobilized fimbriae was suspended in binding buffer, transferred onto a 10-ml column (80 by 15 mm), and equilibrated with 30 ml of the binding buffer. Approximately 60 mg of mucus protein in 30 ml of binding buffer (2 mg of protein per ml) was added to the column at a flow rate of 5 ml/h. After the addition of the mucus solution, the column was washed at a flow rate of 15 ml/h with 30 ml of washing buffer A and then with washing buffer B to remove the detergent (Tween 20). Samples were collected throughout the entire washing procedure, and the OD at 280 nm was monitored. The column was washed with buffer B (30 ml) until the absorbency of the eluate was approximately 0.001 OD unit. The elution buffer (buffer C, 20 ml) was then added to the column at a flow rate of 20 ml/h. The receptor fractions (2 ml of eluate) were collected, and the pH was adjusted immediately to 7.2 with 2 N NaOH. The total protein in each fraction was monitored at 280 nm. The eluted fractions were collected for SDS-PAGE and immunoblotting assay. In another trial, the mucus was applied to the column as described above and the column was washed extensively with the binding buffer (low salt and no detergent), followed by removal of bound proteins with elution buffer C. The eluted fractions (2 ml) were subjected to SDS-PAGE (12%) by the procedure of Laemmli (13). In a third study, 30 ml of BSA (2 mg/ml) was used as a nonspecific binding control, and the first binding and elution procedure was repeated. For the binding study, 100 μl of fimbrial bead samples was collected from each step of the binding and elution process for determination of total bound protein. Finally, after use, the column was washed with storage buffer and stored at 4°C.
Receptor assay.
The fimbrial beads (100 μl) were analyzed for protein at various stages of purification of the receptor. The samples were mixed with 5 ml of Bradford reagent, the absorbencies were measured at 595 nm, and the amount of protein bound to the beads was quantified by using pure swine globulin as a reference standard. The protein concentrations of the mucus samples (before and after affinity isolation) and of the isolated fractions were adjusted with PBS to 0.1 mg/ml. The concentration of the receptor was determined by using the ELISA. In this assay, the wells of a 96-well microplate were coated by adding 100 μl of each sample to the first well of each row, followed by double dilution in the other wells of the other rows with 0.1 M bicarbonate buffer, pH 9.6. The plates were incubated at 37°C for 2 h, washed three times with PBST, and blocked with 5% dry skim milk powder for 2 h at 37°C. After three rinses with PBST, 100 μl of E. coli K88ac fimbriae (20 μg/ml) was added to each well. The plate was then incubated at 37°C for 2 h. After three washes with PBST, 100 μl of anti-K88 monoclonal antibody (1:3,000) was added to each well, and the mixture was incubated at 37°C for 2 h. After three washes, 100 μl of alkaline phosphatase-conjugated sheep anti-mouse IgG (1:3,000) was added to each well and incubated at 37°C for 1 h. The plate was washed five times with PBST, 100 μl of substrate solution was added to each well, and the plate was incubated at 37°C until a strong color developed (about 45 min). The OD was determined at 405 nm with a plate reader.
Analysis of receptors by SDS-PAGE and immunoblotting.
The crude mucus sample (10 μl) and 30 μl of each fraction for protein staining were mixed with 30 μl of SDS sample buffer. The samples subjected to SDS-PAGE (12%) by the procedure of Laemmli (13). The gel was stained with 0.5% Coomassie blue at room temperature for 30 min. The protein bands were visualized by destaining with 20% methanol and 5% acetic acid. The molecular masses were determined by using a standard molecular mass reference (Sigma marker). The same samples were separated by SDS-PAGE for the immunoblotting assay. Following electrophoresis, the gel was equilibrated for 30 min in transfer buffer and electroblotted onto nitrocellulose membranes by using a power supply (model 200/2.0; Bio-Rad) set at 15 V for 1 h. The membrane was then rinsed three times with PBS, 5% skim milk diluted with PBS was added, and the membrane was incubated at 37°C for 2 h with agitation. The blot was washed twice for 5 min each in PBST. K88 fimbriae (0.1 mg/ml) were added to the blot and incubated at 37°C for 2 h. After five washes, rabbit anti-K88ac+ fimbrial antibody (1:1,000) was added to the blot, followed by incubation at 37°C for 2 h with agitation. After three washes with PBST for 5 min, alkaline phosphatase-labeled secondary antibody (1:1,000) was added to the blot, followed by incubation at 37°C for 2 h with agitation. The blot was washed with four changes of PBST for 5 min each and then placed in the Sigma FAST BCIP/NBT substrate solution with agitation. The blot was transferred into water when the color of the bands was of sufficient intensity.
RESULTS
Immobilization of fimbriae.
The K88+ fimbriae readily reacted with the cyanogen bromide-activated Sepharose 4B (fimbrial beads). The concentration of fimbrial protein in the eluate when 5 ml of a 6-mg/ml solution was added to 2 g of the active Sepharose decreased from 6.0 to 3.5 mg/ml in 5 ml of solution. This indicated that 12.5 mg of the 30 mg of K88+ fimbriae was covalently bound to 2 g of the Sepharose beads. Similar yields of bound fimbriae were obtained following the direct assay of the modified Sepharose for protein after washing.
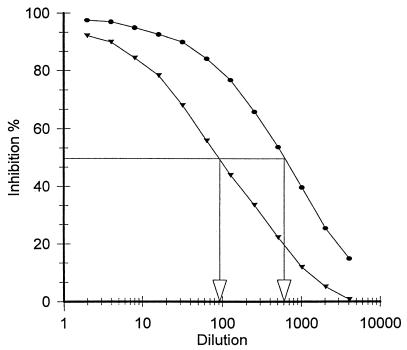
The ability of the fimbria-bound beads to react with rabbit anti-K88ac+ fimbrial antibody was assessed by using the competitive ELISA (Fig. 1). The results demonstrated that the binding of antiserum to K88+ fimbriae was inhibited in the presence of both free fimbriae and the fimbrial beads but not in the presence of BSA-treated beads. The free fimbriae, however, were an approximately sevenfold-better competitor for the antibody than the same concentration of fimbriae bound to Sepharose (referred to as fimbrial beads). This may be attributed to a partial inactivation of the fimbriae during the binding process and to the inability of all of the bound fimbriae to react with the antibody.
FIG. 1.
Competitive binding of immobilized fimbriae to their antibody as determined by ELISA. Fimbriae that were applied to the surface of the titer plate wells competed with suspended fimbrial beads for rabbit anti-K88ac fimbrial antibodies. Values show percent inhibition obtained with fimbriae covalently bound to Sepharose 4B (▾) and fimbriae prior to being bound to Sepharose 4B (●). BSA was not bound to the Sepharose 4B-fimbrial column (data not shown). The initial concentration of the competitor was 10 μg/100 μl. See Materials and Methods for further details.
Affinity purification of the fimbrial binding protein from mucus.
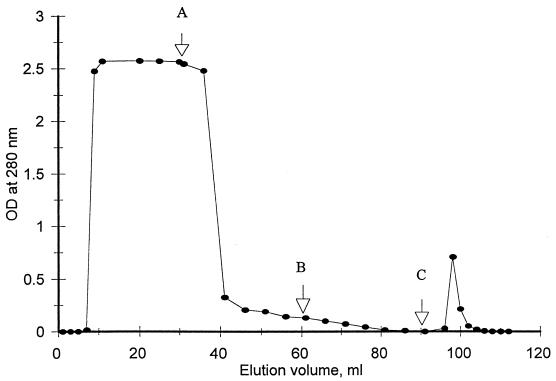
All samples of mucus were first monitored for the presence of specific antibodies to K88+ fimbriae by ELISA, as these antibodies would interfere with subsequent assays for fimbriae. This could have occurred if the piglets inadvertently had received colostrum containing the K88 antibodies. Samples that did not contain the specific antibody were used for the subsequent studies. Initial studies demonstrated that 55 mg of the 60 mg of protein in 30 ml in the mucus fraction was eluted from the column following extensive washing with binding buffer and that the additional 5 mg was eluted from the column with the low-pH elution buffer (buffer C). In another study, 60 mg of the mucus fraction in 30 ml was applied to the column in the binding buffer, and the column was exhaustively washed with elution buffer A (high detergent and salt concentrations) followed by buffer B (high salt). The low-pH elution buffer (buffer C) was used to elute the fimbrial binding protein (Fig. 2, small peak), which was estimated from the absorbency at 280 nm to contain 0.6 mg of protein. Nonspecific binding was tested by using BSA (30 ml; 2 mg/ml) in the binding buffer. The nonspecific binding of BSA on the gel could be dissociated when the gel was washed with buffers A and B, with essentially no proteins being eluted with the elution buffer.
FIG. 2.
Affinity purification of fimbrial binding receptor from intestinal mucus by using a column containing fimbriae covalently bound to Sepharose 4B-fimbrial beads. A mucus extract (30 ml containing 60 mg of protein) was applied to the column, and the column was washed successively with 30 ml each of buffers A, B, and C (arrows). The absorbency of the eluate at 280 nm was approximately 0.001 OD unit when buffer C (the receptor elution buffer) was applied. Application of 30 ml of BSA in buffer A followed by washing and elution with buffers A, B, and C yielded a pattern similar to that obtained with mucus, except no BSA was eluted with buffer C. The total protein in the peak eluted after 20 ml was approximately 600 μg. See Materials and Methods for further details.
Degree of purification of fimbrial binding proteins.
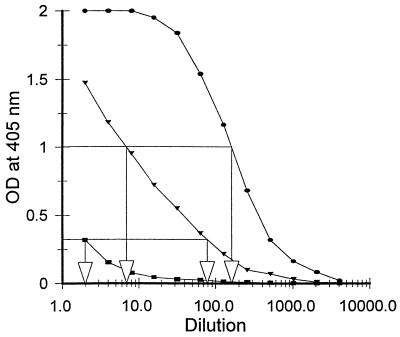
The ELISA indicated that the concentration of the fimbrial binding protein in the purified mucus was increased approximately 24-fold compared to that in the original mucus; that is, the dilution required to give an OD of 1.0 at 405 nm was approximately 7-fold for the crude mucus and 170-fold for the purified mucus, a difference of 24-fold (Fig. 3). In addition, the concentration of the fimbrial binding protein in the residual mucus fraction after affinity purification was reduced approximately 35-fold (i.e., the dilution of fimbriae required to give an OD at 405 nm of 0.4 was 2, while that of the original mucus was 70) (Fig. 3). These results demonstrate that there was considerable purification of the fimbrial binding protein (the receptor) following affinity chromatography, that very little (approximate 3%) was left in the residual fraction, and that most of the receptor binding activity (greater than 97%) was recovered from the affinity column. These results are supported by those obtained in Fig. 2. The purification of the affinity binding protein was 100-fold if it is assumed that 0.6 mg of the receptor was eluted from a mixture containing 60 mg of total protein and that the recovery was 97%.
FIG. 3.
ELISA curves obtained with different dilutions of crude mucus (▾), mucus residue after removal of receptors following affinity isolation (■), and purified receptor (●). The relative receptor concentration was quantitated on the basis of its ability to react with K88ac fimbriae, as discussed in Materials and Methods and Results. See Materials and Methods for further details.
Electrophoresis and immunoblotting assay.
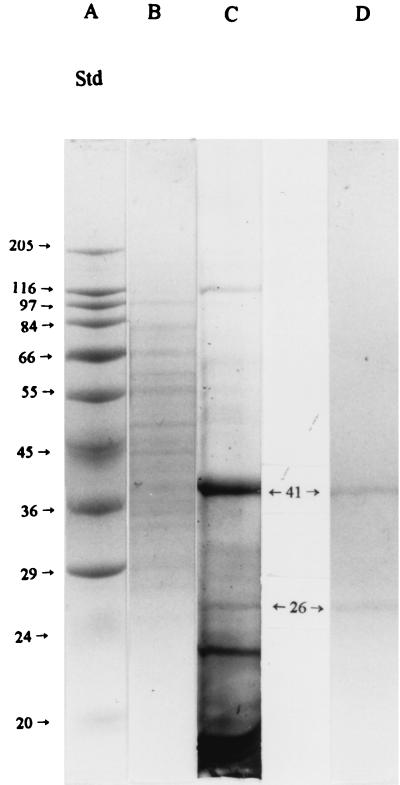
SDS-PAGE (Fig. 4) indicated that there were approximately 19 proteins present in the crude mucus extract (lane B) and that there were only 5 major protein bands when the mucus proteins that had been bound to the affinity column were first washed with a buffer having low ionic strength and no detergent (binding buffer) prior to being eluted with buffer C, the receptor elution buffer (lane C). In addition, the final eluate contained numerous minor bands. However, washing of the bound mucus proteins from the affinity column with both buffers A (containing additional salt and detergent) and B (containing additional salt) followed by elution of the proteins with a low-pH buffer (buffer C) yielded a preparation that had only two major bands with molecular masses of 26 and 41 kDa (lane D). Samples for lanes B and C were from the same preparation; that for lane D was from a different preparation, as the original preparation was lost. Typically, the intensities of the 26- and 41-kDa bands in lanes C and D are similar to each other. However, their relative concentrations can vary considerably among samples from different pigs as discussed below.
FIG. 4.
Typical SDS-PAGE profile of proteins in crude mucus extract (lane B), proteins eluted from the affinity column with buffer C (pH 2.5 glycine buffer) after washing with binding buffer (low concentration of salt and no detergent) (lane C), and proteins eluted from affinity column with buffer C after washing with washing buffers A (pH 7.2 buffer plus 2 M NaCl plus 1% Tween 20) and B (pH 7.2 buffer plus 1 M NaCl) (lane D). Lane A shows the elution profile of the standard (Std) molecular mass markers, with the corresponding molecular masses (kilodaltons) indicated to the left. There were approximately 19 bands in lane B, 19 in lane C (five of which [17, 21, 26, 41, and 116 kDa] were more predominant than others), and two in lane D (26 and 41 kDa). The concentrations of protein in the samples applied to lanes B, C, and D were 2,000, 2,000, and 300 μg/ml, with the volume being 10 μl. Samples for lanes B and C were from the some preparation, and those for lane D were from a different preparation. See Materials and Methods for further details.
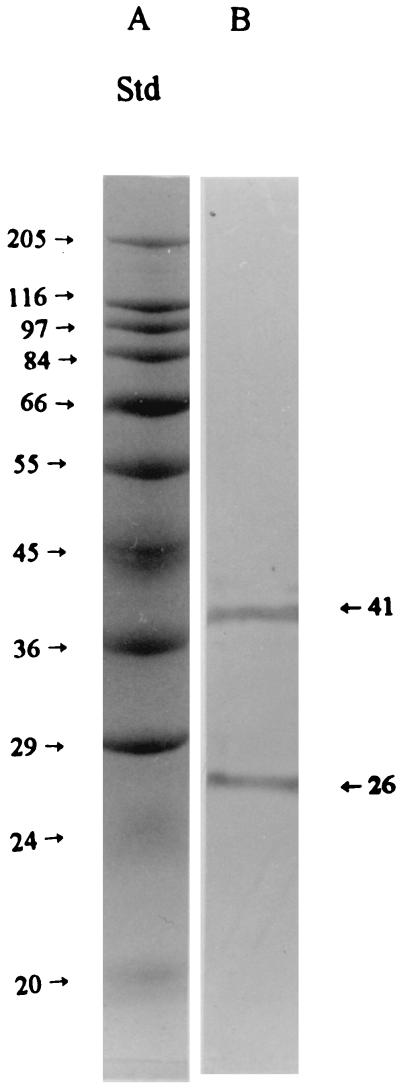
The immunoblotting assay with the same samples as used in Fig. 4 (lanes C and D) produced positive immunoblot reactions (Fig. 5, lane B). Immunoblotting of the crude mucus did not yield a clear pattern due to the low concentration of receptors (data not shown). These results demonstrated that the proteins in Fig. 4, lane D, and those in the immunoblot (Fig. 5, lane B), had identical molecular masses (26 and 41 kDa), suggesting that these proteins were the target receptors for K88ac+ fimbriae and that they had been purified to a relatively high level.
FIG. 5.
Typical immunoblotting of fimbrial binding proteins from the intestinal mucus of pigs. The mucus proteins were bound to the affinity column, and the column was successively washed with buffers A and B. The bound receptors were then eluted from the column with elution buffer (buffer C), and the purified receptor proteins (10 μl; 300 μg/ml) were applied to the electrophoresis gel in binding buffer (lane B). The proteins were separated by SDS-PAGE and electroblotted onto nitrocellulose membranes, and the receptors for fimbriae were stained by procedures described in Materials and Methods. Molecular mass standards (Std) are shown in lane A, with the corresponding molecular masses (kilodaltons) indicated to the left. The immunostaining pattern of the fimbrial binding receptors was the same when the receptors were bound to the column in binding buffer and directly eluted with buffer C (data not shown).
In another study, mucus samples were collected from 22 newborn piglets. Each sample was successively purified by using the affinity column, and the protein bands of each purified fraction were visualized by SDS-PAGE. The 26- and 41-kDa major protein bands appeared in all mucus samples but were in different proportions in some samples. In 32% (7 of 22) of the purified mucus samples, approximately 80% of the protein was associated with the 41-kDa band and only 20% was associated with the 26-kDa band. In contrast, 23% (5 of 22) of the purified mucus samples had a much higher concentration of the 26-kDa band (80%) than of the 41-kDa band (20%). The two receptor proteins appeared in the same proportion in 36.4% (8 of 22) of the samples. In 9% (2 of 22) of the samples, almost all protein was associated with the 41-kDa band and very little was associated with the 26 kDa band.
DISCUSSION
A simple and rapid procedure for the purification of the fimbrial binding receptor in E. coli K88 was developed. The procedure involves the isolation and purification of fimbriae from E. coli K88, the covalent binding of the fimbriae to a solid matrix such as Sepharose, and the use of the Sepharose-fimbrial matrix for the affinity purification of the corresponding intestinal receptor. The results demonstrated that in the presence of a buffer having a low ionic strength, many mucus matrix proteins can bind nonspecifically to the Sepharose-fimbrial column. However, nearly all of the proteins that interact nonspecifically with the column matrix can be eluted by using a buffer having a high ionic strength and a high concentration of detergent. Presumably, such treatments greatly reduce the strength of the hydrophobic and hydrophilic interactions between the mucus proteins and the column matrix, and as a result macromolecules not specifically bound to the column are eluted while the receptors are not dissociated from the column. They can, however, be displaced from a column at a low pH in a manner similar to that of the antigen in an antibody-antigen reaction (8). This procedure yielded, in a single chromatographic run, a mixture of two highly purified receptors with molecular masses of 26 and 41 kDa. These two receptors, as indicated by the ELISA, accounted for nearly all of the fimbrial binding capacity of the crude mucus. The affinity column did not resolve the two receptors, however, as they were eluted as a single peak. However, this separation was achieved by using SDS-PAGE but presumably could also be accomplished by using gel filtration, as the receptors had different molecular masses, or by modification of the conditions for elution from the affinity column. Although the immunoblotting assay was able to detected the two receptors in the affinity-purified preparation, it did not detect them in the original mucus extract because of their low concentration. The column could also be readily used along with molecular sieving techniques to purify active digests of the receptor and could be used in competition structure-activity analysis in an attempt to identify its active site. These studies are currently being conducted.
The porcine small intestine mucus receptor for K88ab fimbrial adhesion has been partially purified by using gel filtration chromatography followed by SDS-PAGE (15). Although Metcalfe and coworkers (15) were able to only partially purify the K88ac receptors from the crude porcine mucus preparation, their results suggested that the K88-specific receptor was a 40- to 42-kDa glycoprotein. Their method, however, would not have identified the presence of a 26-kDa receptor, as the indicator molecules, the fimbriae, would have masked the presence of this receptor since it also has a molecular mass of 27 kDa (12). The results from the present study are in partial agreement with those of Metcalfe et al. (15), as one of the two major protein receptors had a molecular mass of 41 kDa. However, our studies also demonstrated that there was a second K88ac receptor present and that it had a molecular mass of approximately 26 kDa. Studies with individual pigs also demonstrated that they contained different proportions of the two receptors. A considerable number of the pigs (36%) had equal proportions of the two receptors, while 41% had mostly the 41-kDa receptor and the balance of the pigs (23%) had mostly the 26-kDa receptor. It is not known, however, if the 26-kDa receptor is a different receptor than the 41-kDa receptor or if it is a breakdown product of the larger receptor.
In contrast to the results obtained in the present study and that of Metcalfe et al. (15), Billey et al. (3) reported that the intestinal brush border membranes contain 210- and 240-kDa glycoprotein receptors. They suggested that there were three different K88 adhesion receptors (receptors bcd, bc, and d) in pigs to account for the five different phenotypes of pigs. The present studies did not compare the specificities of the mucus receptors and the intestinal epithelial receptors, nor did they determine the specificities of the 26- and 41-kDa receptors for the different types of fimbriae (K88ab, K88ac, and K88ad).
In conclusion, this research confirmed the presence of the 41-kDa receptor in the mucus and provided evidence for a second form of this receptor, a receptor having a molecular mass of 26 kDa. Also, a simple, rapid, and highly specific method was developed for the purification of the mucus receptors; such a technique has not been previously utilized, as far as we are aware, for the isolation of E. coli receptors. This simple procedure may also be highly effective for the purification of other bacterial receptors, including the K88 receptors present in intestinal epithelial cells. The ability to readily obtain high-purity receptors should greatly facilitate future studies on the purification of the receptor's active site following its partial hydrolysis. In addition, the fimbrial column as described here was used to prepare monospecific polyclonal antibodies (data not shown).
ACKNOWLEDGMENTS
This research was supported by The Natural Science and Engineering Research Council of Canada (NSERC); Manitoba Pork Producers Est, Agri-Food Research and Development Initiative, Winnipeg, Manitoba, Canada; the University of Manitoba, Winnipeg, Manitoba, Canada; and a fellowship to Lin Fang from the Shanghai Laboratory Animal Centre, Academia Sinica.
REFERENCES
- 1.Akins R E, Tuan R S. Ultra-fast protein determinations using microwave enhancement. Mol Biotechnol. 1990;4:17–24. doi: 10.1007/BF02907468. [DOI] [PubMed] [Google Scholar]
- 2.Allen A. The structure and function of gastrointestinal mucus. In: Boedecker E C, editor. Attachment of organisms to the gut mucusa. Vol. 2. Boca Raton, Fla: CRC Press, Inc.; 1984. pp. 3–11. [Google Scholar]
- 3.Billey L O, Erickson A K, Francis D H. Multiple receptors on porcine intestinal epithelial cells for three variants of Escherichia coli K88 fimbrial adhesion. Vet Microbiol. 1998;59:203–212. doi: 10.1016/s0378-1135(97)00193-4. [DOI] [PubMed] [Google Scholar]
- 4.Blomberg L, Conway P L. An in vitro study of ileal colonization resistance to Escherichia coli strain Bd 1107/75 08(K88) in relation to indigenous squamous gastric colonization in piglets of varying ages. Microb Ecol Health Dis. 1989;2:285–291. [Google Scholar]
- 5.Conway P L, Welin A, Cohen P S. Presence of K88-specific receptors in porcine ileal mucus is age dependent. Infect Immun. 1990;58:3178–3182. doi: 10.1128/iai.58.10.3178-3182.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Erickson A K, Willgohs J A, McFarland S Y, Benfield D A, Francis D H. Identification of two porcine brush border glycoproteins that bind the K88ac adhesion of Escherichia coli and correlation of these glycoproteins with adhesive phenotype. Infect Immun. 1992;60:983–988. doi: 10.1128/iai.60.3.983-988.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guinee P A M, Jansen W H. Behavior of Escherichia coli K antigens K88ab, K88ac, and K88ad in immunoelectrophoresis, double diffusion, and hemagglutination. Infect Immun. 1979;23:700–705. doi: 10.1128/iai.23.3.700-705.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1988. [Google Scholar]
- 9.Hu Z L, Hasler-Rapacz J, Huang S C, Rapacz J. Studies in swine on inheritance and variaton in expression of small intestinal receptors mediating adhesion of the K88 enteropathogenic Escherichia coli variants. J Hered. 1993;84:157–165. doi: 10.1093/oxfordjournals.jhered.a111309. [DOI] [PubMed] [Google Scholar]
- 10.Jones G W, Rutter J M. Role of K88 antigen in the pathogenesis of neonatal diarrhea caused by Escherichia coli in piglets. Infect Immun. 1972;6:918–927. doi: 10.1128/iai.6.6.918-927.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim J W, Jin L Z, Cho S H, Marquardt R R, Frohlich A A, Baidoo S K. Use of chicken egg-yolk antibodies against K88+ fimbrial antigen for quantitative analysis of enterotoxigenic Escherichia coli (ETEC) K88+ by a sandwich ELISA. J Sci Food Agric. 1999;79:1513–1518. [Google Scholar]
- 12.Klemm P. Fimbrial adhesion of Escherichia coli. Rev Infect Dis. 1985;73:321–340. doi: 10.1093/clinids/7.3.321. [DOI] [PubMed] [Google Scholar]
- 13.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 14.Marquardt R R, Jin L Z, Kim J W, Fang L, Frohlich A A, Baidoo S K. Passive protective effect of egg-yolk antibodies against enterotoxigenic Escherichia coli K88+ infection in neonatal and early-weaned piglets. FEMS Immun Med Microbiol. 1999;23:283–288. doi: 10.1111/j.1574-695X.1999.tb01249.x. [DOI] [PubMed] [Google Scholar]
- 15.Metcalfe J E, Krogfelt K A, Krivan H C, Cohen P S, Laux D C. Characterization and identification of a porcine small intestine mucus receptor for the K88ab fimbrial adhesion. Infect Immun. 1991;59:91–96. doi: 10.1128/iai.59.1.91-96.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neutra M R. The mechanism of intestinal mucus secretion. In: Boedecker E C, editor. Attachment of organisms to the gut mucosa. Vol. 2. Boca Raton, Fla: CRC Press Inc.; 1984. pp. 33–41. [Google Scholar]
- 17.Rutter J M, Jones G W, Brown G T H, Burrows M R, Luther P D. Antibacterial activity in colostrum and milk associated with protection against enteric disease caused by K88-positive Escherichia coli. Infect Immun. 1976;13:667–676. doi: 10.1128/iai.13.3.667-676.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sellwood R. The K88 adherence system in swine. In: Boedecker E C, editor. Attachment of organisms to the gut mucosa. Vol. 2. Boca Raton, Fla: CRC Press, Inc.; 1984. pp. 21–29. [Google Scholar]
- 19.Smith H W, Huggins M B. The influence of plasmid determined and other characteristics of enteropathogenic Escherichia coli on their ability to proliferate in the alimentary tracts of piglets. J Med Microbiol. 1978;11:471–492. doi: 10.1099/00222615-11-4-471. [DOI] [PubMed] [Google Scholar]
- 20.Smith H W, Lingood M A. Observations on the pathogenic properties of the K88, Hyl, and Ent plasmids of Escherichia coli with particular reference to porcine diarrhoea. J Med Microbiol. 1971;4:467–485. doi: 10.1099/00222615-4-4-467. [DOI] [PubMed] [Google Scholar]
- 21.Smith P K, Krohn R I. Measurement of protein using bicinchonic acid. Anal Biochem. 1975;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]