Abstract
Objective
To measure the prevalence of dry eye disease (DED) and study the relationship between screen time and dry eye symptoms in the pediatric population during the coronavirus disease 2019 (COVID-19) pandemic using the Ocular Surface Disease Index (OSDI) questionnaire.
Methods
In this descriptive, observational, cross-sectional study, our survey included the pediatric population, ages 1 to 18 years, of both genders, who attended outpatient clinics of two main hospitals in Jeddah, Saudi Arabia. Collected data included age, gender, dry eye symptoms, and common DED risk factors, followed by the Ocular Surface Disease Index (OSDI) questionnaire, which consists of 12 items graded on a five-point scale (0 = never to 4 = all the time).
Results
A total of 329 pediatric participants were included, with more than half of the participants (56.1%) males and 58.5% aged 12-18 years. The most frequently reported symptoms (reported as often or always) were decreased vision (23.0%) and itchy eyes (22.1%). Environmental factors have an effect on developing DED symptoms, as some participants (21.8%) have reported being uncomfortable in windy weather and 15.8% have reported this in places with air conditioners. Based on the OSDI diagnostic criteria, 250 (76.1%) participants had DED. Furthermore, in terms of severity, 44 (13.3%) participants had mild DED, 62 (18.8%) participants had moderate DED, and 145 (43.9%) participants had severe DED. We found that prolonged exposure to mobile screens for two to three hours or four hours or more was associated with a higher DED incidence compared to those exposed for shorter periods. Older age categories were more likely to experience DED (80.8% and 78.2% in age categories 12-18 and 7-12, respectively, versus 57.6% in the youngest age category (p = 0.001)). Additionally, DED was independently associated with participants with a previous history of eyeglasses prescription and those experiencing dry eyes while using electronic devices.
Conclusion
Since many children use electronic devices for education and entertainment, we found that symptoms of DED due to prolonged screen time have increased among the pediatric population during the COVID-19 pandemic. Therefore, awareness efforts should be directed to reduce the rate of controllable risk factors like personal computer use. In addition, educational campaigns are warranted to provide all possible preventive measures against DED, especially to children with uncontrollable risk factors for developing DED.
Keywords: covid 19, osdi questionnaire, screen time, pediatric dry eye, dry eye symptoms
Introduction
Dry eye disease (DED), also known as keratoconjunctivitis sicca (KCS) and keratitis sicca, is a multifactorial, painful inflammatory disorder of the tear film that occurs due to insufficient lubrication by the eyes’ tears [1]. It is attributed to the loss of homeostasis of the tear film, which is found on the outermost corneal surface, leading to various ocular symptoms due to increased evaporation or decreased secretion of the tear film. The disturbance of the tear film homeostasis is thought to be due to eyelid abnormalities, deficits in tear constituents, or inadequate blinking secondary to prolonged time screen [2]. This could also be due to systemic illnesses, allergies, eye surgeries, or autoimmune disorders. Regardless of the cause, DED causes unpleasant symptoms that range from a burning sensation to eye discomfort, pain, photophobia, squint, accommodation spasms, or blurred vision, which eventually affect the quality of life. In addition, DED has been associated with depression, stress, and anxiety, making it a debilitating eye disease [2]. Severe DED increases the risk of developing conjunctivitis, headache, eye infection, corneal abrasion, and ulcers [1-4]. The diagnosis of DED is usually based on subjective symptoms and clinical dry eye examination findings. Signs and symptoms of DED may mimic those of other ocular surface diseases such as infection, allergic conjunctivitis, keratopathy, and episcleritis. The diagnosis of DED is challenging in clinical practice, which leads to a delay in diagnosis until an advanced stage of the disease has ensued.
Additionally, the COVID-19 pandemic has considerably changed people’s lifestyles, especially those of children. That is, governments have been forced to adopt safety precautions to combat the transmission of this novel virus. Due to such preventive measures, schools and academic activities worldwide switched to a virtual learning modality and paperless classrooms. Since this pandemic, more young people have had dry eye and other eye symptoms, mainly due to in-house quarantine, limited outdoor activities, and increased screen time [5]. In other words, prolonged and steady use of visual display terminals (VDTs) or digital screens for professional and recreational purposes has been increasing across all age groups. The use of digital screens is a significant and well-known precipitating factor for DED. This is attributed to the extended use of VDTs with short watching distances, which causes less frequent and incomplete blinking. This, hence, promotes tear film instability by accelerating tear film evaporation [2].
Although this relationship in adults has been well-studied [6], few studies have reported the prevalence of dry eye disease among children during the COVID-19 pandemic using different assessment tools [6-7]. A cross-sectional study performed at pediatric ophthalmology clinics at two institutes in Egypt studied the effect of the COVID-19 pandemic on screen time and its relationship with DED symptoms in a pediatric population using the Standard Patient Evaluation of Eye Dryness (SPEED) questionnaire [6]. They concluded that the increased screen time in children during the COVID-19 pandemic might contribute to the symptoms of DED. Therefore, this study aims to measure the prevalence of dry eye disease in the pediatric population during the COVID-19 pandemic and other attributing factors using the Ocular Surface Disease Index (OSDI) questionnaire.
Materials and methods
A descriptive observational cross-sectional study was conducted using a self-administrated questionnaire targeting all pediatric populations of both genders, aged 10-18 years, attending outpatient pediatric ophthalmology clinics at King Fahd Armed Forces Hospital and King Abdulaziz Medical City, Jeddah. The data were collected from October 2021 to January 2022. The estimated sample size was calculated using Roasoft, Inc (Seattle, WA). The required sample size of 329 children was deemed sufficient to provide a one-sided 95% confidence level, with an estimated 50% response distribution and an error margin of ±5%.
A questionnaire translated into Arabic was given to the children or their parents. Informed consent was obtained from the legal guardian of each child before the survey, but after elucidating the confidentiality measures and the study's objectives. No inducements were provided. Ethical approval was obtained from the ethics review committee of the Armed Forces Scientific Research Center, Saudi Arabia. Confidentiality was maintained thoroughly, as no personal information was obtained and all questionnaires were kept anonymous. A nonprobability convenience sampling technique was used, as any child aged 1 to 18 years who attended the pediatric outpatient clinics was included in the study.
OSDI questionnaire and score calculation
The OSDI is a questionnaire specific to DED that measures the severity of DED-related symptoms. The questionnaire consists of 12 items graded on a five-point scale (0 = Never to 4 = all the time). The questionnaire was divided into three sections: vision-related functions, ocular symptomatology, and environmental triggers [8]. Scores of each section were computed, and a final raw score was obtained by summing up the section scores. The raw final score ranged between 0 and 48. A final percentage score was calculated using the formula: (raw score*25)/the number of questions answered [9]. Participants with normal eyes scored an OSDI score of 0-12 while those with mild, moderate, and severe DED scored 13-22, 23- 32, and > 33, respectively.
Statistical analysis
The Statistical Package for the Social Sciences (IBM Corp. Released 2019. IBM SPSS Statistics for Windows, Version 26.0. Armonk, NY: IBM Corp) was used for statistical analysis. The reliability of the overall OSDI questionnaire items and the OSDI subscales was assessed statistically using Cronbach's alpha test. Descriptive statistics were adopted to express categorical (frequencies and percentages) and numerical data (medians and interquartile ranges [IQRs]). The relationship between DED and demographic, clinical, and lifestyle characteristics was investigated using a chi-squared test. The significant variables from the univariate analysis were subsequently used in a logistic regression model, using the enter method, to assess the risk factors for DED. The dependent variable was DED status (no versus yes), and the significantly associated variables were used as independent variables. Results were expressed as odds ratios (ORs) and their respective 95% confidence intervals (95% CIs). A p-value of < 0.05 was used to indicate statistical significance.
Results
Participants’ demographics, characteristics, and clinical history
A total of 336 responses were initially received; however, seven were omitted due to missing data. Therefore, 329 pediatric patients were included in the current study. More than half of the participants were males (56.1%) and aged 12-18 (58.5%). A history of chronic diseases was found in 8.8% of all participants, and 7.9% have used medications daily. In addition, approximately one-quarter (23.0%) of the respondents had allergic conditions (asthma, allergic rhinitis, allergic eczema, food allergy, or allergic conjunctivitis), and 15.2% received anti-allergic medications. Regarding the ophthalmologic history, 8.8% of the participants had received a medical/surgical intervention for eye disease, and 3.9% had undergone eye surgery in the past six months. More details about the clinical history of patients are listed in Table 1.
Table 1. Characteristics and clinical history of participants (n=329).
| Parameter | Category | Frequency | Percent |
| Gender | Male | 185 | 56.1 |
| Female | 144 | 43.6 | |
| Age | 1-6 y | 59 | 17.9 |
| 7-12 y | 78 | 23.6 | |
| 12-18 y | 192 | 58.5 | |
| Chronic diseases | Yes | 29 | 8.8 |
| Use of daily medications | Yes | 26 | 7.9 |
| Medical/surgical eye treatment | Yes | 29 | 8.8 |
| Eye surgery during the last 6 months | Yes | 13 | 3.9 |
| Current conjunctivitis | Yes | 20 | 6.1 |
| Diagnosed with any eye disease | Yes | 55 | 16.7 |
| Prescribed eyeglasses? | Yes | 102 | 30.9 |
| Dry eye while using electronic | Yes | 75 | 22.8 |
| Allergies | Yes | 76 | 23.0 |
| Use of any anti-allergic medication | Yes | 50 | 15.2 |
Dry eye symptoms and vision-related function
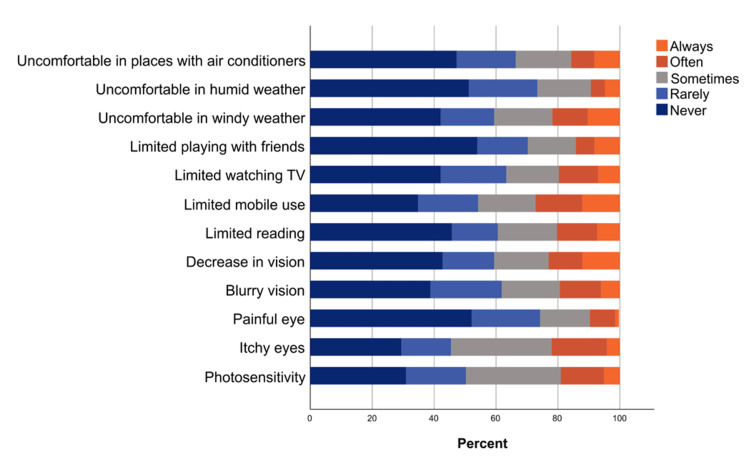
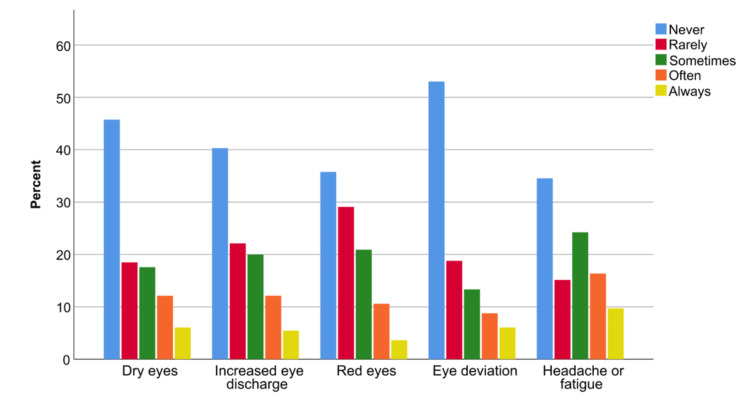
The detailed responses of participants to the different items of the OSDI questionnaire are depicted in Figure 1. The most frequently reported symptoms (reported as often or always) were decreased vision (23.0%) and itchy eyes (22.1%). Moreover, dry eye symptoms have been reported to cause discomfort to some participants during mobile use (27.3%) and reading (20.3%). Regarding environmental factors, participants felt uncomfortable in windy weather (21.8%) and places with air conditioners (15.8%). There were other reported symptoms, although they are not included in the OSDI. The most-reported ones (reported as often or always) were headache/fatigue (26.1%), dry eyes (18.2%), and increased eye discharge (17.6%, Figure 2).
Figure 1. The percentages of participants' responses to different items of the OSDI questionnaire.
OSDI: Ocular Surface Disease Index
Figure 2. The percentages of participants’ responses to selected eye symptoms.
Results of the OSDI scale and subscales
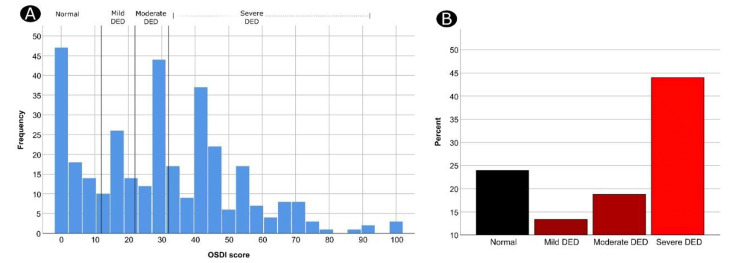
In general, the three OSDI subscales showed robust to reliable statistical reliability (with a Cronbach’s alpha between 0.805 and 0.862) [10]. Additionally, all OSDI questionnaire items were reliable (Cronbach’s alpha = 0.907). The median (IQR) overall OSDI score for all the participants was 29.2 (12.5-44.3) (Table 2). Based on the OSDI diagnostic criteria, 79 (23.9%) participants had no DED, whereas 250 (76.1%) patients had DED. Furthermore, 44 (13.3%) participants had mild DED, 62 (18.8%) had moderate DED, and 145 (43.9%) had severe DED (Figure 3).
Table 2. Descriptive statistics of the OSDI scale and subscales.
OSDI: Ocular Surface Disease Index
| Parameter | Number of Items | Minimum - Maximum | Cronbach's Alpha | Median (IQR) |
| Eye symptoms | 5 | 0-20 | 0.805 | 6.0 (2.8-9.0) |
| Vision-related function | 4 | 0-16 | 0.862 | 4.0 (0.0-8.0) |
| Environmental triggers | 3 | 0-12 | 0.814 | 3.0 (0.0-6.0) |
| Raw OSDI | 12 | 0-48 | 0.907 | 14.0 (6.0-21.3) |
| Percent OSDI | 12 | 0-100 | 0.907 | 29.2 (12.5-44.3) |
Figure 3. Frequency distribution of OSDI scores among the participants (A) and the different OSDI categories of DED (B).
OSDI: Ocular Surface Disease Index; DED: dry eye disease
Factors associated with DED
Results of the univariate analysis showed that increased age was associated with DED (80.8% and 78.2% in age categories 12-18 and 7-12, respectively, versus 57.6% in the youngest age category (p = 0.001). Furthermore, compared to their counterparts, DED was significantly higher among participants with a positive history of using daily medications (92.3% versus 74.6%, p = 0.042), medical/surgical eye treatment (96.6% versus 74.1%, p = 0.007), conjunctivitis (95% versus 74.8%, p = 0.041), an eye disease (92.7% versus 72.7%, p = 0.002), allergy (90.8% versus 71.7%, p = 0.001), and dry eye while using electronic devices (97.3% versus 69.7%, p < 0.0001). Moreover, DED was found significantly high in participants who use eyeglasses (94.1% versus 68%, p < 0.0001), those who cannot close their eyelids (100% versus 75.1%, p = 0.039), and those currently using anti-allergic medications (90% versus 73.6%, p = 0.012) or eye drops for conjunctivitis (100% versus 72.6%, p < 0.0001, Table 3).
Table 3. Demographic and clinical factors associated with dry eye disease according to OSDI score.
DED: dry eye disease; OSDI: Ocular Surface Disease Index; **means P < .01; * means .01 < P < .05
| Variable | Category | Normal (0-12 points) N (%) | Symptomatic DED (≥13 points) N (%) | P |
| Age (yrs) | 1-6 y | 25 (42.4) | 34 (57.6) | 0.001** |
| 7-12 y | 17 (21.8) | 61 (78.2) | ||
| 12-18 y | 37 (19.2) | 156 (80.8) | ||
| Gender | Male | 50 (27.0) | 135 (73.0) | 0.147 |
| Female | 29 (20.1) | 115 (79.9) | ||
| Chronic diseases | No | 75 (24.9) | 226 (75.1) | 0.180 |
| Yes | 4 (13.8) | 25 (86.2) | ||
| Use of daily medications | No | 77 (25.4) | 226 (74.6) | 0.042* |
| Yes | 2 (7.7) | 24 (92.3) | ||
| Medical/surgical eye treatment | No | 78 (25.9) | 223 (74.1) | 0.007** |
| Yes | 1 (3.4) | 28 (96.6) | ||
| Eye surgery during the last 6 months | No | 78 (24.6) | 239 (75.4) | 0.161 |
| Yes | 1 (7.7) | 12 (92.3) | ||
| Conjunctivitis | No | 78 (25.2) | 232 (74.8) | 0.041* |
| Yes | 1 (5.0) | 19 (95.0) | ||
| Diagnosed with any eye diseases | No | 75 (27.3) | 200 (72.7) | 0.002** |
| Yes | 4 (7.3) | 51 (92.7) | ||
| Prescribed eyeglasses | No | 73 (32.0) | 155 (68.0) | < 0.0001** |
| Yes | 6 (5.9) | 96 (94.1) | ||
| Cannot close the whole lid | No | 79 (24.9) | 238 (75.1) | 0.039* |
| Yes | 0 (0.0) | 13 (100.0) | ||
| Dry eye while using electronic devices | No | 77 (30.3) | 177 (69.7) | < 0.0001** |
| Yes | 2 (2.7) | 73 (97.3) | ||
| Presence of Allergic conditions | No | 72 (28.3) | 182 (71.7) | 0.001** |
| Yes | 7 (9.2) | 69 (90.8) | ||
| Use of any anti-allergic medication | No | 74 (26.4) | 206 (73.6) | 0.012* |
| Yes | 5 (10.0) | 45 (90.0) | ||
| Used drops for conjunctivitis | No | 79 (27.4) | 209 (72.6) | < 0.0001** |
| Yes | 0 (0.0) | 41 (100.0) |
Regarding the effect of lifestyle factors and screen time on DED, we found that prolonged exposure to mobile screens for two to three hours in 78.1% or four hours or more in 81.8% was associated with more DED incidence compared to those who were exposed for shorter periods (62.3% and 72.9% for those exposed for one to two hours and zero to one hour, respectively, p = 0.024). Although there was also a significant difference (p = 0.007, Table 4) in exposure to TV screens, with higher proportions of participants with DED who watched TV for one to two hours (84.8%) compared to those who watched TV for zero to one hour (77.7%), two to three hours (60.3%), or four or more hours (77.7%). We cannot conclude that watching TV is a risk factor for DED, as the minimum watching TV hours are almost equal to the maximum watching hours in terms of DED incidence.
Table 4. Lifestyle-related factors associated with DED according to OSDI score.
DED: dry eye disease; OSDI: Ocular Surface Disease Index; **means P < .01; * means .01 < P < .05
| Variable | Category | Normal (0-12 points) N (%) | Symptomatic DED (≥13 points) N (%) | p |
| Time spent using the mobile | 0-1h | 13 (27.1) | 35 (72.9) | 0.024* |
| 1-2h | 23 (37.7) | 38 (62.3) | ||
| 2-3h | 16 (21.9) | 57 (78.1) | ||
| 4h and more | 27 (18.2) | 121 (81.8) | ||
| Time spent using electronic devices | 0-1h | 45 (29.8) | 106 (70.2) | 0.137 |
| 1-2h | 13 (21.3) | 48 (78.7) | ||
| 2-3h | 10 (18.2) | 45 (81.8) | ||
| 4h and more | 11 (17.5) | 52 (82.5) | ||
| Time spent watching TV | 0-1h | 31 (22.3) | 108 (77.7) | 0.007** |
| 1-2h | 12 (15.2) | 67 (84.8) | ||
| 2-3h | 25 (39.7) | 38 (60.3) | ||
| 4h and more | 11 (22.4) | 38 (77.6) | ||
| Time spent on reading | 0-1h | 52 (26.9) | 141 (73.1) | 0.260 |
| 1-2h | 12 (15.8) | 64 (84.2) | ||
| 2-3h | 7 (21.9) | 25 (78.1) | ||
| 4h and more | 8 (27.6) | 21 (72.4) | ||
| Time doing outdoor activities | 0-1h | 27 (22.5) | 93 (77.5) | 0.336 |
| 1-2h | 16 (20.8) | 61 (79.2) | ||
| 2-3h | 19 (22.9) | 64 (77.1) | ||
| 4h and more | 17 (34.0) | 33 (66.0) | ||
| Sleep time | 1-5h | 8 (17.0) | 39 (83.0) | 0.331 |
| 6-8h | 45 (26.9) | 122 (73.1) | ||
| 8h and more | 26 (22.4) | 90 (77.6) |
Risk factors for DED
The outcome of the regression analysis showed that the participants in the older age categories were more likely to experience DED (OR = 2.79, 95%CI, 1.09 to 7.12, p = 0.032 for the 7-12 age category and OR = 3.20, 95%CI, 1.37 to 7.48, p = 0.007 for the 12-18 age category). Additionally, DED was independently associated with participants with a previous history of eyeglasses prescription (OR = 4.41, 95%CI, 1.32 to 14.72, p = 0.016) and those experiencing dry eye while using electronic devices (OR = 6.74, 95%CI, 1.43 to 31.70, p = 0.016). However, participants who watched TV for two to three hours were less likely to experience DED symptoms (OR = 0.45, 95%CI, 0.21 to 0.97, p = 0.043, Table 5).
Table 5. Risk factors for dry eye disease among the participants.
OR: odds ratio; CI: confidence interval. **means P < .01; * means .01 < P < .05
| Parameter | Category | OR (95%CI) | P |
| Age | 1-6 y | Reference | |
| 7-12 y | 2.79 (1.09-7.12) | 0.032* | |
| 12-18 y | 3.20 (1.37-7.48) | 0.007** | |
| Use of daily medications | No | Reference | 0.990 |
| Yes | 1.01 (0.14-7.53) | ||
| Medical/surgical eye treatment | No | Reference | 0.361 |
| Yes | 2.95 (0.29-29.90) | ||
| Conjunctivitis | No | Reference | 0.734 |
| Yes | 1.49 (0.15-15.08) | ||
| Diagnosed with any eye diseases | No | Reference | 0.653 |
| Yes | 1.44 (0.30-6.94) | ||
| Prescribed eyeglasses | No | Reference | 0.016* |
| Yes | 4.41 (1.32-14.72) | ||
| Dry eye while using electronic | No | Reference | 0.016* |
| Yes | 6.74 (1.43-31.70) | ||
| Presence of allergies | No | Reference | 0.109 |
| Yes | 3.65 (0.75-17.76) | ||
| Use of any anti-allergic medication | No | Reference | 0.481 |
| Yes | 0.50 (0.07-3.42) | ||
| Time spent using the mobile | 0-1h | Reference | |
| 1-2h | 0.50 (0.18-1.39) | 0.187 | |
| 2-3h | 1.02 (0.36-2.85) | 0.975 | |
| 4h and more | 0.82 (0.30-2.21) | 0.691 | |
| Time spent watching TV | 0-1h | Reference | |
| 1-2h | 1.99 (0.88-4.48) | 0.096 | |
| 2-3h | 0.45 (0.21-0.97) | 0.043* | |
| 4h and more | 0.54 (0.20-1.43) | 0.214 |
Discussion
The prevalence of DED in children has been increasing in the last few years with the popularity of electronic device use. The global prevalence of DED, according to the Dry Eye Workshop (DEWS) II in 2017, ranged from 20% to 50% [11]. DED causes variable symptoms that may not correlate with the severity of the disruption in the ocular surface. The disease is progressive and may lead to permanent ocular surface damage if left untreated [12]. According to the Tear Film Ocular Surface Society (TFOS) Dry Eye Workshop (DEWS) II criteria, DED has been categorized as either aqueous deficient or evaporative dry [13]. Clinically, patients often present with symptoms of both types of the disease.
During the COVID-19 lockdown in several countries, all academic activities of schools and universities were made online, which led to prolonged eye exposure to screens and electronic devices [14]. The latest recommendations from the AAP advise caregivers to limit screen time in children aged two to five years old to one hour or less each day [15]. However, the Royal College of Paediatrics and Child Health (RCPCH) in their recent 2019 guidance recommended that the amount of time spent on devices should be tailored to each child [16]. The OSDI is a clinical instrument that allows physicians to measure DED-related symptoms' severity and understand their impact on visual function and daily life with a sensitivity of 60% and a specificity of 79%. In comparison to the short form 12-Health Survey (SF-12) and McMonnies Questionnaire (MQ), the OSDI has a higher sensitivity and specificity [9]. The current study aimed to measure the prevalence of dry eye disease and its impact on visual function in the pediatric population during the COVID-19 pandemic using a web and hardcopy-based OSDI questionnaire.
In our study, 329 children participated in filling out the OSDI form. Based on the OSDI diagnostic criteria, 250 (76.1%) participants had symptomatic DED (OSDI score above 22), whereas 79 (23.9%) participants had no DED. We also found that 145 (43.9%) children reported severe DED symptoms. These findings are comparable to the D. García-Ayuso et al. study who assessed the prevalence of dry eye symptoms among university students during COVID-19 using OSDI. In their research, the prevalence of symptomatic DED (OSDI score above 22) among 676 participants was 51.8% [7].
Furthermore, Neti et al., who evaluated the impact of COVID‐19 health measures on dry eye symptoms, found that young to middle-aged participants reported worsening dry eye symptoms during the lockdown. They proposed that the lifestyles of the elderly were not significantly altered as much as younger individuals [16]. Their study found two independent risk factors for DED increased VDT usage and female gender. These findings were consistent with another study by D. García-Ayuso et al., which found that the prevalence of DED was higher in females [7]. In contrast, our current study found no statistically significant difference between both genders. However, we found that children who used smartphones for four hours or more had a statistically significant increase in DED incidence. In addition, we found that children with a previous history of eyeglasses prescription had a higher incidence of dry eye symptoms. In previous studies, increased screen time has been associated with a higher incidence of dry eye symptoms in the pediatric population [4]. The Osaka study by Uchino and colleagues demonstrated that patients with definite DED who had prolonged exposure to VDT had lower mucin secreted by goblet cells in the conjunctiva [17].
Strengths and limitations
This is the first study to assess the prevalence of dry eye disease among the pediatric Saudi population in the COVID-19 era. In addition, our findings may provide helpful information to the health authorities regarding the impact of implementing restrictions on educational institutions on children and adolescents. Also, the large sample size allows our findings to be generalized to other populations. One of the limitations of this study is that the influence of environmental factors on the development of dry eye symptoms was not considered. Furthermore, the reported symptoms of DED in the survey may be caused by other ocular surface diseases. Self-reporting, as in any questionnaire, can have recall bias. When concluding the findings, it is essential to consider that some children often have difficulty expressing dry eye symptoms.
Conclusions
This study highlights the importance of recognizing DED in its early stages, as severe forms can become chronic and more challenging to treat. Since many children use electronic devices for education and entertainment, we found that symptoms of DED due to prolonged screen time have increased among the pediatric population during the COVID-19 pandemic. Therefore, awareness efforts should be directed to reduce the rate of controllable risk factors like personal computer or digital device use. In addition, educational campaigns are warranted to provide all possible preventive measures against DED, especially to children with uncontrollable risk factors for developing DED.
Acknowledgments
We would like to acknowledge Dr. Waleed Khayyat at King Khaled Eye Specialist Hospital for his valuable contribution and advice regarding the data analysis interpretations.
The content published in Cureus is the result of clinical experience and/or research by independent individuals or organizations. Cureus is not responsible for the scientific accuracy or reliability of data or conclusions published herein. All content published within Cureus is intended only for educational, research and reference purposes. Additionally, articles published within Cureus should not be deemed a suitable substitute for the advice of a qualified health care professional. Do not disregard or avoid professional medical advice due to content published within Cureus.
The authors have declared that no competing interests exist.
Human Ethics
Consent was obtained or waived by all participants in this study. King Fahad Armed Forces Hospital - Jeddah, Research and Ethics Committee issued approval REC 454. We are pleased to inform you that the above research proposal was approved today through an expedited pathway of the Research and Ethics Committee. The committee is hereby giving you the authority to commence with your research study
Animal Ethics
Animal subjects: All authors have confirmed that this study did not involve animal subjects or tissue.
References
- 1.COVID-19 and dry eye. Koh S, Rhee MK. Eye Contact Lens. 2021;47:317–322. doi: 10.1097/ICL.0000000000000797. [DOI] [PubMed] [Google Scholar]
- 2.TFOS DEWS II pathophysiology report. Bron AJ, de Paiva CS, Chauhan SK, et al. Ocul Surf. 2017;15:438–510. doi: 10.1016/j.jtos.2019.08.007. [DOI] [PubMed] [Google Scholar]
- 3.Ocular surface health in Shanghai University students: a cross-sectional study. Li S, He J, Chen Q, Zhu J, Zou H, Xu X. BMC Ophthalmol. 2018;18:245. doi: 10.1186/s12886-018-0825-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Relationship between screen time and dry eye symptoms in pediatric population during the COVID-19 pandemic. Elhusseiny AM, Eleiwa TK, Yacoub MS, et al. Ocul Surf. 2021;22:117–119. doi: 10.1016/j.jtos.2021.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Impact of the COVID-19 pandemic on eye strain and dry eye symptoms. Saldanha IJ, Petris R, Makara M, Channa P, Akpek EK. Ocul Surf. 2021;22:38–46. doi: 10.1016/j.jtos.2021.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.New approaches for diagnosis of dry eye disease. Elhusseiny A. Int J Ophthalmol. 2019;12:1618–1628. doi: 10.18240/ijo.2019.10.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Assessment of dry eye symptoms among university students during the COVID-19 pandemic. García-Ayuso D, Di Pierdomenico J, Moya-Rodríguez E, Valiente-Soriano FJ, Galindo-Romero C, Sobrado-Calvo P. Clin Exp Optom. 2022;105:507–513. doi: 10.1080/08164622.2021.1945411. [DOI] [PubMed] [Google Scholar]
- 8.A review of quality of life measures in dry eye questionnaires. Grubbs J, Tolleson-Rinehart S, Huynh K, Davis R. Cornea. 2014;33:215–218. doi: 10.1097/ICO.0000000000000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reliability and validity of the Ocular Surface Disease Index. Schiffman RM, Christianson MD, Jacobsen G, Hirsch JD, Reis BL. Arch Ophthalmol. 2000;118:615–621. doi: 10.1001/archopht.118.5.615. [DOI] [PubMed] [Google Scholar]
- 10.The use of Cronbach’s alpha when developing and reporting research instruments in science education. Taber K. Res Sci Educ. 2017;48:1273–1296. [Google Scholar]
- 11.TFOS DEWS II epidemiology report. Stapleton F, Alves M, Bunya VY, et al. Ocul Surf. 2017;15:334–365. doi: 10.1016/j.jtos.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 12.A narrative review of current understanding and classification of dry eye disease with new insights on the impact of dry eye during the COVID-19 pandemic. Barabino S. Ophthalmol Ther. 2021;10:495–507. doi: 10.1007/s40123-021-00373-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.TFOS DEWS II definition and classification report. Craig JP, Nichols KK, Akpek EK, et al. Ocul Surf. 2017;15:276–283. doi: 10.1016/j.jtos.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 14.Provocation of dry eye disease symptoms during COVID-19 lockdown. Neti N, Prabhasawat P, Chirapapaisan C, Ngowyutagon P. Sci Rep. 2021;11:24434. doi: 10.1038/s41598-021-03887-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Digital screen time limits and young children's psychological well‐being: evidence from a population‐based study. Przybylski AK, Weinstein N. Child Dev. 2019;90:0–65. doi: 10.1111/cdev.13007. [DOI] [PubMed] [Google Scholar]
- 16.Screen time in children and adolescents: is there evidence to guide parents and policy? Ashton JJ, Beattie RM. Lancet Child Adolesc. 2019;3:292–294. doi: 10.1016/S2352-4642(19)30062-8. [DOI] [PubMed] [Google Scholar]
- 17.Alteration of tear mucin 5AC in office workers using visual display terminals: the Osaka Study. Uchino Y, Uchino M, Yokoi N, et al. JAMA Ophthalmol. 2014;132:985–992. doi: 10.1001/jamaophthalmol.2014.1008. [DOI] [PubMed] [Google Scholar]