Abstract
The in vivo interactions of platelets with Candida species yeast cells were investigated in a murine model. Mice were injected intravenously via the lateral caudal vein, and blood drawn by periorbital puncture was collected in phosphate-buffered saline–formaldehyde to avoid in vitro platelet activation. The study of the clearance of blastoconidia of Candida albicans and Candida glabrata showed that these cells disappeared quickly from the bloodstream. Microscopic observation of blood samples, stained by Calcofluor white or May Grunwald Giemsa, demonstrated the rapid attachment of platelets to fungal elements of all the Candida spp. tested. The attachment of murine platelets to C. albicans cells, observed by scanning electron microscopy, revealed morphological changes. The platelets lost their discoid shape, generated pseudopodia, and flattened against the yeast cells. The reversibility of platelet binding to C. albicans by chelating agents suggests a cation-dependent link. In contrast, the fixation of C. glabrata and Candida tropicalis was not modified by chelating agents. The mechanisms involved in the in vivo adherence of platelets to Candida cells may therefore differ according to the species of Candida.
Disseminated Candida infections involve the translocation of Candida cells through the blood circulation. In the bloodstream, the fungus may interact with various (soluble) proteins such as fibrinogen (3), fibronectin (16, 28), complement, and other plasma components (8, 9); with the vascular endothelium (27); and with glycoproteins of the subendothelial extracellular matrix (26) such as collagen and laminin (4, 17). It may also interact with blood cells such as neutrophils, which are usually considered to be the primary effective cells against Candida yeast (6). Platelets, known to be critical mediators of homeostasis and blood coagulation, have recently been implicated in the metastatic processes of tumor cells (2, 12, 30) and in the pathogenesis of infectious diseases (34).
Several in vitro studies have suggested that platelets interact with Candida cells. Maish and Calderone demonstrated the adherence of Candida albicans to fibrin-platelet clots (18, 19). The same group showed that cell wall fragments of C. albicans were able to aggregate platelets (29). Previously, we reported that resting platelets adhere to C. albicans germ tubes and that this attachment is associated with morphological changes (24, 25). Klotz et al. studied the adherence of C. albicans to the endothelium and to the subendothelial extracellular matrix and demonstrated that the interaction between yeast and platelets occurred after platelet aggregation (14, 15). However, although these authors detected platelet aggregation with yeast cytosol, they were unable to produce platelet aggregation with viable whole yeast. A recent study by Willcox et al. (30) concludes that C. albicans, unlike all other species, is unable to aggregate platelets. This study also demonstrated the ability of platelets to kill some Candida spp. but not C. albicans. However, Yeaman et al. showed that platelets are activated by Candida species to secrete platelet microbicidal peptides and that these peptides are active against the Candida organisms (32, 33, 35).
In vivo interactions between Candida species and platelets have been only partially studied. Holder and Nathan (11) observed that injection of sonic cell extract of Candida into mice produced platelet aggregation. In a rabbit model, Calderone et al. (5) studied endocarditis elicited by traumatic destruction of aortic valves and demonstrated the close apposition of Candida cells and platelets in the lesion. Demonstration of Candida in blood smears from patients has been reported, and Kates et al. (13) showed platelets in close association with yeast. Platelet anti-Candida activity has been described and has been shown to play a role in the severity of experimental C. albicans endocarditis (33).
Although platelets are considered a factor in the pathogenesis of candidiasis, it is unclear whether these cells promote or limit the progression of the disease. We describe an in vivo procedure for the study of the adherence of mouse platelets to Candida cells. The amount of platelet binding to Candida species was measured by fluorescence microscopy after coloration by Calcofluor white. The results obtained demonstrate an interaction between platelets and all Candida species. In addition, the fixation of platelets to the circulating blastoconidia of C. albicans in vivo was confirmed by scanning electron microscopy (SEM), which showed that the attachment to the platelets was associated with morphological changes.
MATERIALS AND METHODS
Organisms and culture conditions.
C. albicans 1066 (ATCC 66369), originally isolated from a case of septicemia, was used throughout this work. Two clinical isolates of C. albicans and isolates of Candida spp. C. glabrata, C. krusei, C. tropicalis, C. parapsilosis, C. kefyr, and C. guilliermondii were obtained from the Mycological Laboratory of the Faculty of Medicine, Angers, France. All strains were maintained by subculture on Sabouraud dextrose agar slants (Merck, Darmstadt, Germany) at 37°C for 24 h twice a month.
For assay purposes, blastoconidia were inoculated at a concentration of 2 × 106 per ml in medium YNB broth (1.7‰ yeast nitrogen base [Fisher Scientific]) containing 2% glucose and 5‰ ammonium sulfate (YNB-G-SA) for 24 h at 37°C, pH 4.5. In some experiments, germ tubes were obtained by incubation of blastoconidia for 3 h in YNB-G-SA at 37°C and pH 7. Cells were harvested by centrifugation (10 min, 500 × g), washed three times in 0.15 M NaCl, and finally resuspended in this buffer at the required concentration after a hemacytometer count.
Mice.
Female, outbred Swiss mice, 6 to 8 weeks old, weighing 18 to 20 g (Centre d'Elevage Déprés-France) were maintained under conventional conditions before experimentation. Mice were injected intravenously via the lateral vein, and blood was drawn by periorbital puncture immediately after the mice were killed by cervical dislocation. Blastoconidia of all the Candida spp. studied were suspended in 0.1 ml of 0.15 M NaCl for inoculation.
Clearance of C. albicans and C. glabrata blastoconidia from the bloodstream.
One hundred microliters of C. albicans or C. glabrata blastoconidia suspensions (2 × 107 cells) was injected into the lateral tail veins of mice. One, 3, and 15 min after injection, the mice were bled by periorbital puncture, and 20 μl of the blood was immediately mixed with 250 μl of distilled water. After 5 min the erythrocytes were lysed, and the blastoconidia were labeled by mixing 1 volume of 0.1 mM fluorescent brightener (Calcofluor white [Sigma, St. Louis, Mo.]) in phosphate-buffered saline (PBS; 0.15 M, pH 7.2) with 5 volumes of sample. The enumeration of the blastoconidia was realized by a direct hemacytomer count under fluorescence microscopy using a Nikon incident-light fluorescence microscope with an excited filter with peak transmission at 365 nm and a barrier filter that transmitted light at wavelengths >420 nm.
Interactions of Candida spp. cells with platelets in vivo.
For the adherence assays, the mice were injected intravenously via the lateral tail vein with 5 × 107 to 7 × 107 Candida spp. blastoconidia or C. albicans germ tubes. After 3 min, blood was drawn and 20 μl of blood was immediately mixed in a plastic tube with 1 ml of PBS or PBS containing 3.3 mM formaldehyde to prevent in vitro platelet activation. In experiments to study the role of divalent cations, blood was collected in PBS containing 10 mM EDTA or 10 mM EGTA or 0.13 M sodium citrate. For negative controls, 20 μl of blood from an uninfected mouse was mixed with all the above buffers supplemented with 5 × 105 blastoconidia. The blood samples were incubated in the buffers for 1 to 60 min prior to examination by photonic fluorescence microscopy following yeast labeling with Calcofluor white as described above.
In some experiments, mice were bled 1, 3, and 15 min after inoculation and the blood samples were treated with Calcofluor white or the May Grunwald Giemsa stain (MGG [Ral, Paris, France]) before examination by photonic microscopy.
For the SEM study of platelet attachment to C. albicans ATCC 66369 cells, 5 × 107 blastoconidia were inoculated. After 15 min, 500 to 600 μl of blood was collected in 25 ml of PBS and passed immediately through a filter (10-μm pore size; MSI, Westboro, Mass.) to remove the erythrocytes. Then the filter was washed once again with 25 ml of PBS and set in a sterile tube containing 1 ml of 0.15 M NaCl. Cells were removed by gentle agitation, the filter was discarded, and the preparation containing the holding cells was fixed in 2.5% glutaraldehyde, postfixed in 2% OsO4, dried at the critical point, coated with gold-palladium, and examined with a JEOL JSM 35 microscope as described by Miegeville and Morin (21).
Statistical analyses.
For each experiment, at least three mice were inoculated and each bloodletting was divided into three tubes. Statistical significance was determined by using the paired t test. All comparisons were two sided, and a P value of <0.05 was considered significant.
RESULTS
Clearance of C. albicans ATCC 66369 and C. glabrata blastoconidia from the bloodstream.
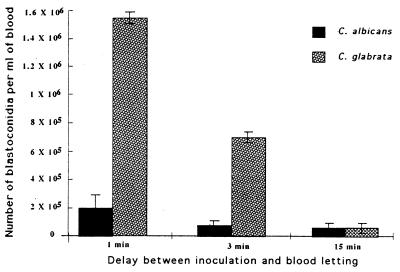
Blastoconidia (2 × 107) were inoculated into the mice by intravenous injection. One, 3, and 15 min after inoculation, blastoconidia were numbered after staining by Calcofluor white. Figure 1 indicates that blastoconidia of C. albicans disappeared more quickly from the bloodstream than did C. glabrata cells. To illustrate, 1 min after inoculation, with the blood volume of mice being approximately 3 ml, the percentages of remaining circulating blastoconidia of C. albicans and C. glabrata were estimated at 3 and 22.5%, respectively, of the blastoconidia initially injected (P < 0.001). Three minutes after inoculation, the decrease of circulating yeast cells became more pronounced, leading to the disappearance of 98 and 89% of the blastoconidia initially injected for C. albicans and C. glabrata, respectively (P <0.01).
FIG. 1.
Comparison between levels of C. albicans ATCC 66369 and C. glabrata blastoconidium clearance from the bloodstream after intravenous injection of 2 × 107 cells into the lateral tail veins of mice. Cells recovered by periorbital puncture were counted 1, 3, and 15 min after the inoculation. Data are means ± standard deviations of three mice, with triplicate samples for each.
However, for a longer period (15 min), the observed difference between the concentrations of blastoconidia in blood for C. albicans and C. glabrata was not significant (P > 0.1).
As it was difficult to bleed several mice precisely 1 min after injection and as the number of blastoconidia per milliliter of blood was very low 15 min after injection, 3 min was chosen as the time to bleed mice after injection for further studies.
Choice of the blood buffer for the studies of interaction between platelets and blastoconidia.
As the time between the taking and the observation of blood may vary, the composition of the buffer and the time of incubation of blood in this buffer, ensuring no modification of the in vivo interaction of platelets with yeast, were determined for C. albicans ATCC 66369 and C. glabrata (Table 1).
TABLE 1.
Effect of incubation time and buffer composition for blood collection on C. albicans ATCC 66369 and C. glabrata platelet fixationa
| Candida spp. | Blood collection buffer | % of yeast cells bound to platelets in blood from:
|
|||||
|---|---|---|---|---|---|---|---|
| Inoculated mice at:
|
Uninfected miceb at:
|
||||||
| <1 min | 5 min | 30 min | <1 min | 5 min | 30 min | ||
| C. albicans | PBS | 92.0 ± 0.6 | 90.0 ± 0.4 | 88.0 ± 0.4 | 3.0 ± 0.7 | 5.0 ± 0.8 | 47.4 ± 0.8 |
| PBS-For | 96.2 ± 0.3 | 95.0 ± 0.2 | 87.0 ± 1.8 | 1.2 ± 0.3 | 1.8 ± 0.8 | 0.8 ± 0.2 | |
| C. glabrata | PBS | 98.0 ± 0.3 | 98.2 ± 0.6 | 93.0 ± 0.4 | 0.2 ± 0.1 | 0.5 ± 0.1 | 66.2 ± 0.9 |
| PBS-For | 98.7 ± 0.8 | 97.5 ± 0.6 | 95.8 ± 0.4 | 0.2 ± 0.1 | 0.4 ± 0.2 | 0.2 ± 0.1 | |
Mice were inoculated as described in Materials and Methods, and blood recovered by periorbital puncture was incubated for 1, 5, and 15 min in 0.15 M PBS (pH 7.2) or PBS–3.3 mM formaldehyde (PBS-For) prior to examination. Data are means ± standard deviations of three observations of six independent experiments.
Negative controls were performed by incubating blood from uninfected mice under the same conditions as those for blood from infected mice and with the same buffers supplemented with Candida cells.
Blood samples recovered 3 min after inoculation showed a high percentage of yeast cells associated with platelets whatever the time of incubation, the buffer composition (PBS or PBS-formaldehyde), and the Candida species tested (87 to 93%; P > 0.1) (Table 2).
TABLE 2.
Interaction between Candida spp. and plateletsa
| Candida spp. | % of yeast cells bound to platelets in blood from:
|
|
|---|---|---|
| Inoculated mice | Uninfected mice | |
| C. albicans | 95.0 ± 0.2 | 1.8 ± 0.8 |
| C. albicans isolate 1 | 90.2 ± 1.1 | 1.2 ± 0.3 |
| C. albicans isolate 2 | 92.6 ± 0.8 | 1.7 ± 0.4 |
| C. glabrata | 99.8 ± 0.2 | 2.5 ± 0.9 |
| C. guilliermondii | 98.6 ± 1.3 | 0 |
| C. tropicalis | 98.7 ± 0.4 | 0 |
| C. kefyr | 96.4 ± 0.1 | 0 |
| C. parapsilosis | 99.9 ± 0.1 | 0 |
Mice were inoculated as described in Materials and Methods, and blood recovered by periorbital puncture and diluted in PBS–3.3 mM formaldehyde. Negative controls were performed as described for Table 1. Data are means ± standard deviations of three observations of six independent experiments.
When the blood from an uninfected mouse was collected in PBS supplemented with blastoconidia, immediate or delayed (5 min) observations showed a low percentage of yeast bound to platelets: 3 to 5% for C. albicans and 0.2 to 0.5% for C. glabrata. However, after 30 min of incubation the percentage of yeast associated with platelets was considerably higher: 47.4% for C. albicans and 66.2% for C. glabrata. In contrast, when the blood was diluted in PBS-formaldehyde, no modification of the initial binding occurred during the 30-min incubation period. These results were proof of in vitro fixation of platelets to blastoconidia when blood was collected in PBS. To avoid this, PBS–3.3 mM formaldehyde was chosen as the blood buffer and the time of incubation was 1 to 30 min for further studies.
Interaction between C. albicans and platelets: results of photonic microscopy.
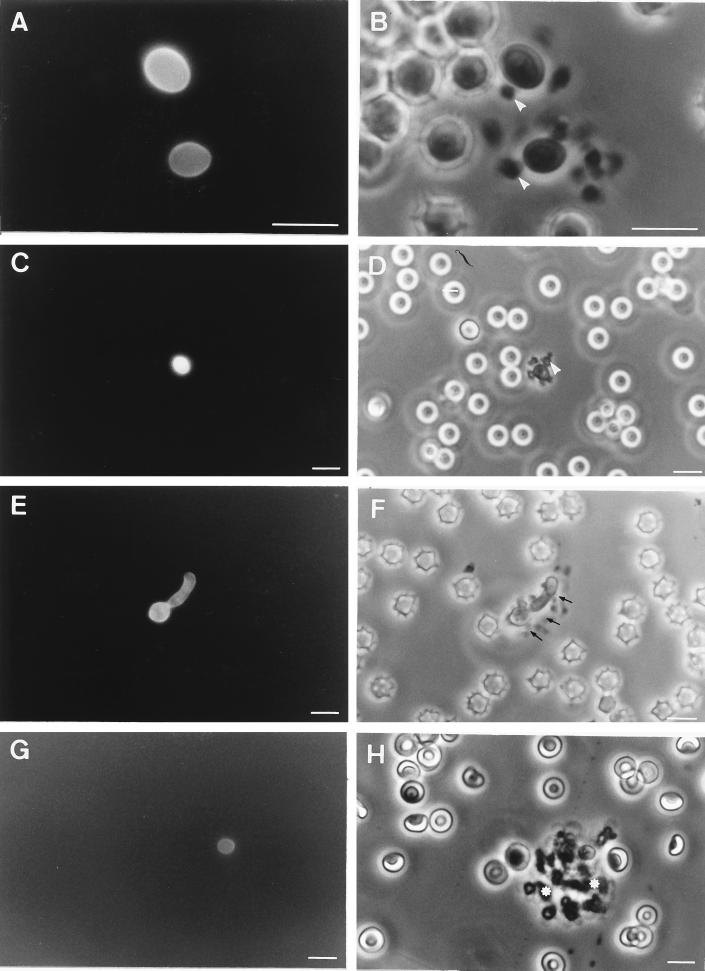
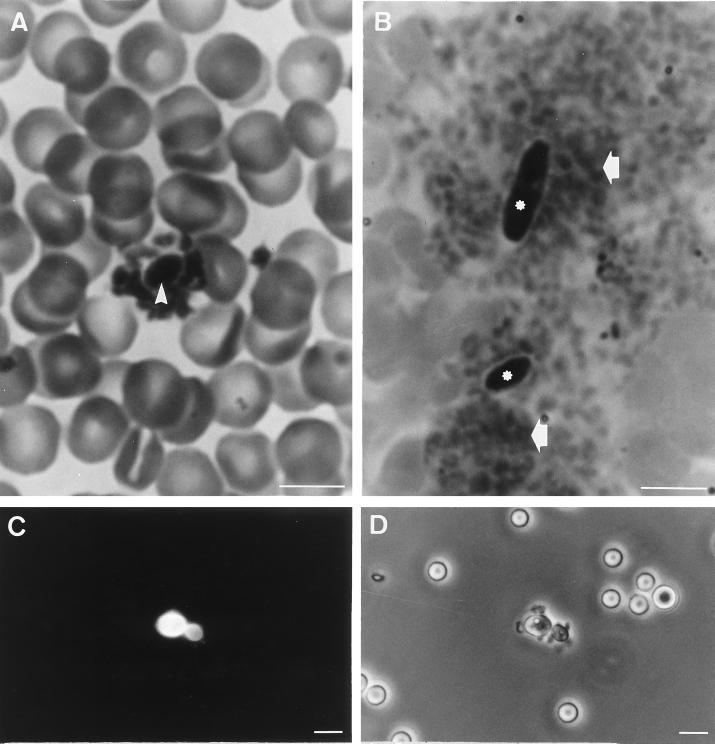
When blood from mice inoculated with blastoconidia or germ tubes of C. albicans ATCC 66369 was observed in the presence of Calcofluor white with a UV microscope, it was very easy to locate yeast cells (Fig. 2A, C, E, and G). Platelets rapidly attached to germ tubes (Fig. 2F) or blastoconidia (Fig. 2B, D, and H): 1 to 3 min after inoculation yeast cells fixed one or two platelets or formed microaggregates (Fig. 2A to F) with platelets. Fifteen to 30 min after inoculation, only a few blastoconidia (Fig. 2G and H) or germ tubes (data not shown) were observed, usually associated with large aggregates of platelets. Quantitative studies showed that whatever the strain of C. albicans used (ATCC 66369 or clinical isolates), the proportion of blastoconidia bound to platelets was high (90 to 95%) (Table 2). The attachment of platelets to fungal elements was confirmed when blood smears stained by MGG were examined by photonic microscopy (Fig. 3A and B). Platelets were seen in close association with blastoconidia (Fig. 3A) or germ tubes (data not shown). However the distribution of the yeast on the blood smear was heterogeneous: free yeast cells or yeast cells with a crown of platelets were observed in the center of the smear, whereas yeast cells associated with large aggregates of platelets were observed at the front edge and sides of the smears.
FIG. 2.
Immunofluorescence (A, C, E, and G) and phase-contrast (B, D, F, and H) photographs of platelet adherence to C. albicans ATCC 66369 blastoconidia (A to D, G, and H) and germ tubes (E and F). Blood samples were recovered 3 (A to F) or 15 min (G and H) after inoculation of the mice, and yeast cells were visualized by Calcofluor white. Note that platelets (arrowheads) adhered to blastoconidia. Note the aggregates of platelets (small arrows) in close association with germ tubes or with blastoconidia (∗). Bars, 10 μm.
FIG. 3.
In vivo studies of the interactions between C. albicans ATCC 66369 (A) and C. krusei (B) and platelets. Blood smears were stained with MGG. Immunofluorescence (C) and phase-contrast (D) photographs of platelet adherence to C. parapsilosis blastoconidia are shown. Note blastoconidia of C. albicans (arrowhead) with a crown of platelets. Note blastoconidia of C. krusei (∗) associated with big aggregates of platelets (arrows). Bars, 10 μm.
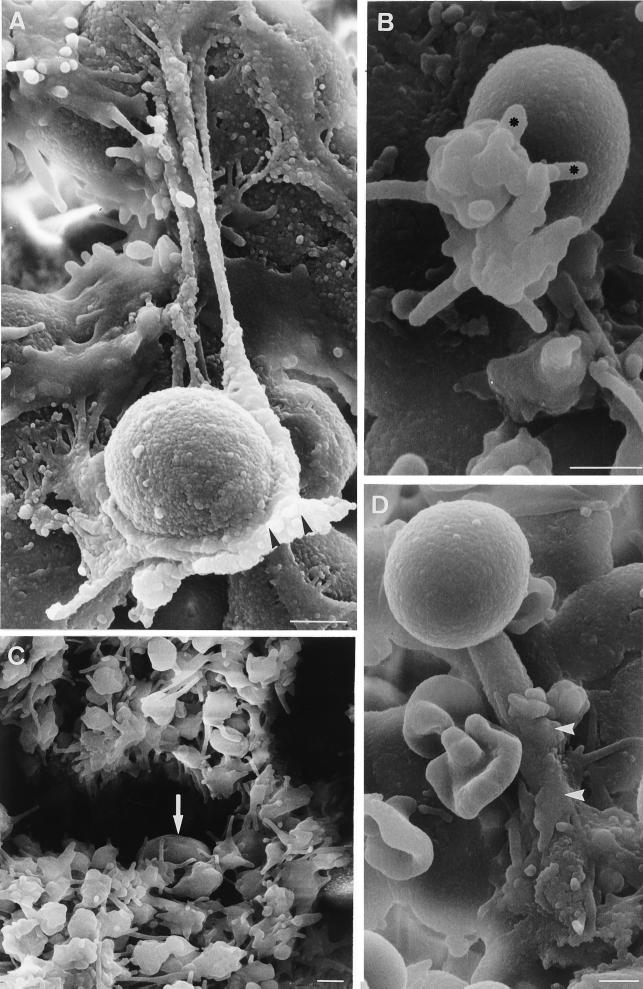
Interactions between C. albicans and platelets: results of SEM.
The binding of platelets to blastoconidia or germ tubes of C. albicans ATCC 66369 was observed 15 min after inoculation. The results of SEM (Fig. 4) show yeast cells in aggregates of platelets (Fig. 4C) and platelets attached to fungal elements (Fig. 4A, B, and D). After fixation on the surface on the fungus, the platelets lose their discoid shape, producing spikes or pseudopodia (Fig. 4A and B). Sometimes the platelets flatten and spread over the blastoconidia (Fig. 4A) or germ tubes (Fig. 4D).
FIG. 4.
SEM visualization of platelet adherence to C. albicans ATCC 66369. Adherence assays were performed, and platelets that adhered to the fungus were examined by SEM as described in Materials and Methods. Binding of platelets to blastoconidia (A, B, and C) or germ tubes (D) of C. albicans was observed 15 min after inoculation. Note that platelets with pseudopods (∗) adhered to blastoconidia (arrow) and that some platelets partially spread on germ tubes (arrowheads). Note the blastoconidium (arrow) associated with a big aggregate of platelets. Bars, 1 μm.
Interactions between Candida spp. and platelets.
The interactions between mouse platelets and several common species of Candida were studied in vivo by photonic microscopy. The results show that the platelets are able to bind to blastoconidia of all strains (Table 2). As we observed for C. albicans in blood smears or after staining with Calcofluor white, the platelets were seen in close association with circulating blastoconidia. To illustrate, Fig. 3C and D show about eight platelets attached to a blastoconidium of C. parapsilosis and its bud after staining with Calcofluor white and Fig. 3B shows two blastoconidia of C. krusei associated with large aggregates of platelets at the edge of a blood smear.
Role of divalent cations in the interaction between platelet and yeast.
When blood from mice inoculated with C. albicans ATCC 66369 was collected in PBS-EDTA or in PBS-sodium citrate, 30 min after incubation in these buffers, there was a small but significant decline (P < 0.02) in the percentages of blastoconidia bound to the platelets (PBS-EDTA, 64.5% ± 3.1%; PBS-citrate, 59.2% ± 2.7%) compared to the percentages observed after 5 min of incubation (PBS-EDTA, 92% ± 3.2%; PBS-citrate, 85.8% ± 3.1%). After 30 min of incubation in PBS-EGTA the percentage of blastoconidia bound to platelets was greatly depressed (20.2% ± 1.3%; P < 0.001). Similarly, the percentages of C. albicans bound to platelets decreased sharply after 60 min (PBS-EDTA, 44.3% ± 3.5%; P < 0.001; PBS-citrate, 42.3% ± 1.9%; P < 0.001).
For C. glabrata and C. tropicalis, whatever the buffer used or the time of incubation, no statistically significant differences in the reduction of the proportion of yeast cells binding to platelets were observed. To illustrate, the percentages of C. tropicalis bound to platelets after incubation with EDTA were 96% ± 0.8% after 5 min and 90.8% ± 1.7% after 60 min.
DISCUSSION
In the bloodstream, platelets circulate in the form of disk-shaped cell fragments that do not normally interact with the vascular endothelial lining. When the endothelial lining is damaged or the blood vessel is injured, platelets adhere to subendothelial components such as collagen and microfibrils. This adherence elicits the secondary activation of the platelets for the generation of a hemostatic plug. In vitro, the addition of collagen or ADP to blood or platelet-rich plasma (PRP) induces the activation and aggregation of platelets. To aggregate to each other, the platelets in the bloodstream or in PRP must be activated and express fibrinogen receptors.
Apart from the fact that platelets have a vital function in hemostats, they may have a role in the pathogenesis of tumoral metastases (20) and in the dissemination of bacteria (7, 10) or parasites (1, 23, 36). There is circumstantial evidence that platelets may interact with Candida (15, 24, 25, 29). Although numerous studies have investigated the ability of yeast cells and yeast cell components to aggregate platelets in PRP (22, 29, 30), little is known concerning the adherence of platelets to yeast cells, which could be a critical initial event in inducing the activation and aggregation of platelets.
In the present study, we have used a murine model to study the abilities of different species of Candida to bind platelets in vivo. The data presented here indicate that after injection of blastoconidia into mice the rate of clearance of C. albicans from the bloodstream was high, leading to the disappearance of 97% of the inoculated cells within the first minute of incubation. In contrast, the rate of clearance observed with C. glabrata was eightfold lower.
Microscopic observation of blood samples stained by Calcofluor white or MGG showed rapid binding of platelets to blastoconidia of all Candida spp. The attachment of murine platelets onto C. albicans cells observed by SEM was associated with morphological changes. Platelets lost their discoid shape, generated spikes or pseudopodia, and flattened against the yeast cells. Some fungal elements were trapped in platelet aggregates. These findings suggest an activation of the platelets. Our observations support earlier results obtained by an in vitro procedure for the study of the interaction between washed resting human platelets and C. albicans germ tubes (24, 25). These results have been confirmed by Yeaman et al. in a flow cytometric analysis of C. albicans adherence to platelets in vitro, which indicated that these organisms bind directly to platelets in the absence of plasma (31). Our present results may be compared to those reported by Klotz et al., who showed that blastoconidia of C. albicans bound firmly to the surfaces of platelets aggregated by ADP whereas inactivated platelets did not adhere to yeast cells (15).
The reversibility of platelet binding to C. albicans blastoconidia by EDTA, EGTA, and sodium citrate suggests a cation-dependent link. In contrast these chelating agents did not modify the attachment of platelets to blastoconidia of other Candida species, suggesting that the mechanisms involved in the in vivo interaction of platelets with Candida cells may differ according to the species of Candida.
There is some discrepancy between our results and those of Klotz et al., who concluded that whole C. albicans cells are unable to aggregate platelets in vitro (15).
Other species of Candida have been shown to be able to cause platelet aggregation. It has been suggested that C. albicans does not aggregate platelets and is not killed by antimicrobial proteins released by activated platelets, implying a lack of recognition that may in fact contribute to the survival of this species (30). However, the presence of sodium citrate in the PRP might explain the nonadherence of platelets to C. albicans.
In conclusion, attempts to define the role of platelets in disseminated candidiasis have produced conflicting results. Further in vivo studies using variant strains should be carried out to determine whether the adherence of platelets to blastoconidia of C. albicans or other Candida species and the subsequent activation and aggregation of platelets are injurious or beneficial to the host.
ACKNOWLEDGMENTS
This work was supported in part by grants from the Programme de Recherche Fondamentale en Microbiologie et Maladies Infectieuses et Parasitaires, Ministère de l'Education Nationale, de la Recherche et de la Technologie, Réseau Infections fongiques (RIF).
We thank Paulette Avranche for her technical assistance.
REFERENCES
- 1.Auriault J P, Capron A, Vorn H, Viens H. A new function for platelets: IgE-dependent killing of schistosomes. Nature. 1983;303:810–812. doi: 10.1038/303810a0. [DOI] [PubMed] [Google Scholar]
- 2.Biggerstaff J P, Seth N B, Meyer T V, Amirkhosravi A, Francis J L. Fibrin monomer increases platelet adherence to tumor cells in a flowing system: a possible role in metastasis? Thromb Res. 1998;92(Suppl. 2):S53–S58. doi: 10.1016/s0049-3848(98)00161-3. [DOI] [PubMed] [Google Scholar]
- 3.Bouali A, Robert R, Tronchin G, Senet J M. Binding of human fibrinogen to Candida albicans in vitro: a preliminary study. J Med Vet Mycol. 1986;24:345–348. doi: 10.1080/02681218680000511. [DOI] [PubMed] [Google Scholar]
- 4.Bouchara J P, Tronchin G, Annaix V, Robert R, Senet J M. Laminin receptors on Candida albicans germ tubes. Infect Immun. 1990;58:48–54. doi: 10.1128/iai.58.1.48-54.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calderone R, Cihlar R L, Lee D D, Hoberg K, Scheld W M. Yeast adhesion in the pathogenesis of endocarditis due to Candida albicans: studies with adherence-negative mutants. J Infect Dis. 1985;152:710–715. doi: 10.1093/infdis/152.4.710. [DOI] [PubMed] [Google Scholar]
- 6.Calderone R, Diamond R, Senet J M, Warmington J, Filler S, Edwards J E. Host cell-fungal cell interactions. J Med Vet Mycol. 1994;32:151–168. doi: 10.1080/02681219480000801. [DOI] [PubMed] [Google Scholar]
- 7.Clawson C C, White J G. Platelet interaction with bacteria. I. Reaction phases and effects of inhibitors. Am J Pathol. 1971;65:367–380. [PMC free article] [PubMed] [Google Scholar]
- 8.Edwards J E, Gaither T A, O'Shea J J, Rotrosen D, Lawley T J, Wright S A, Frank M M, Green I. Expression of specific binding sites on Candida with functional and antigenic characteristics of human complement receptors. J Immunol. 1986;137:3577–3583. [PubMed] [Google Scholar]
- 9.Gilmore B J, Restinas E M, Lorentz J S, Hostetter M K. An iC3b receptor on Candida albicans: structure, function, and correlates for pathogenicity. J Infect Dis. 1988;157:38–46. doi: 10.1093/infdis/157.1.38. [DOI] [PubMed] [Google Scholar]
- 10.Henderson W R J. The role of the platelet in the pathogenesis of infectious diseases. Curr Opin Infect Dis. 1992;5:375–380. [Google Scholar]
- 11.Holder I A, Nathan P. Effect in mice of viable Candida albicans and a cell-free sonic extract on circulating platelets. Infect Immun. 1973;7:468–472. doi: 10.1128/iai.7.3.468-472.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Honn K V, Tang D G, Crissman J D. Platelets and cancer metastasis: a causal relationship? Cancer Metastasis Rev. 1992;11:325–351. doi: 10.1007/BF01307186. [DOI] [PubMed] [Google Scholar]
- 13.Kates M M, Phair J B, Yungbluth M, Weil S C. Demonstration of Candida in blood smears. Lab Med. 1988;19:25. [Google Scholar]
- 14.Klotz S. Adherence of Candida albicans to components of the subendothelial extracellular matrix. FEMS Microbiol Lett. 1990;68:249–254. doi: 10.1016/s0378-1097(05)80049-7. [DOI] [PubMed] [Google Scholar]
- 15.Klotz S, Harrison J, Misra R. Aggregated platelets enhance adherence of Candida yeasts to endothelium. J Infect Dis. 1989;160:669–677. doi: 10.1093/infdis/160.4.669. [DOI] [PubMed] [Google Scholar]
- 16.Klotz S, Hein R C, Smith R L, Rouse J B. The fibronectin adhesin of Candida albicans. Infect Immun. 1994;62:4679–4681. doi: 10.1128/iai.62.10.4679-4681.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klotz S, Smith R L. Gelatin fragments block adherence of Candida albicans to extracellular matrix proteins. Microbiology. 1995;141:2681–2684. doi: 10.1099/13500872-141-10-2681. [DOI] [PubMed] [Google Scholar]
- 18.Maish P A, Calderone R A. Adherence of Candida albicans to a fibrin-platelet matrix formed in vitro. Infect Immun. 1980;27:650–656. doi: 10.1128/iai.27.2.650-656.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maish P A, Calderone R A. Role of the surface mannan in the adherence of Candida albicans to fibrin-platelet clots formed in vitro. Infect Immun. 1981;32:92–97. doi: 10.1128/iai.32.1.92-97.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehta P. Potential role of platelets in the pathogenesis of tumor metastasis. Blood. 1984;1:55–63. [PubMed] [Google Scholar]
- 21.Miegville M, Morin O. Observation de differentes souches de levures et de leurs protoplastes en microscopie électronique à balayage. C R Acad Sci Paris. 1976;283D:417. [PubMed] [Google Scholar]
- 22.Nosal R, Menyhardtova Z. The effect of glycoprotein from Candida albicans on functions of rat platelets. Toxicon. 1976;14:313–318. doi: 10.1016/0041-0101(76)90028-3. [DOI] [PubMed] [Google Scholar]
- 23.Peyron F, Polack B, Lamotte D, Kolodie L, Ambroise-Thomas P. Plasmodium falciparum growth inhibition by human platelets in vitro. Parasitology. 1989;99:317–322. doi: 10.1017/s0031182000059011. [DOI] [PubMed] [Google Scholar]
- 24.Robert R, Mahaza C, Miegeville M, Ponton J, Marot-Leblond A, Senet J M. Binding of resting platelets to Candida albicans germ tubes. Infect Immun. 1996;64:3752–3757. doi: 10.1128/iai.64.9.3752-3757.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robert R, Senet J M, Mahaza C, Annaix V, et al. Molecular basis of the interactions between Candida albicans, fibrinogen and platelets. J Mycol Med. 1992;2:19–25. [Google Scholar]
- 26.Rotrosen D, Calderone R A, Edwards J E. Adherence of Candida species to host tissues and plastic surfaces. Rev Infect Dis. 1986;8:73–85. doi: 10.1093/clinids/8.1.73. [DOI] [PubMed] [Google Scholar]
- 27.Scheld W M, Sande M A. Endocarditis and intravascular infections. In: Mandell G L, Bennett J E, Dolin R, editors. Principles and practice of infectious diseases. 4th ed. Vol. 1. New York, N.Y: Churchill Livingstone; 1995. pp. 740–783. [Google Scholar]
- 28.Skerl K G, Calderone R A, Segal E, Sreevalsan T, Scheld W M. In vitro binding of Candida albicans yeast cells to human fibronectin. Can J Microbiol. 1984;30:221–227. doi: 10.1139/m84-033. [DOI] [PubMed] [Google Scholar]
- 29.Skerl K G, Calderone R A, Sreevalsan T. Platelet interaction with Candida albicans. Infect Immun. 1981;34:938–943. doi: 10.1128/iai.34.3.938-943.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Willcox M D P, Webb B C, Thakur A, Harty D W S. Interactions between Candida species and platelets. J Med Microbiol. 1998;47:103–110. doi: 10.1099/00222615-47-2-103. [DOI] [PubMed] [Google Scholar]
- 31.Yeaman M R, Sullam P M, Dazin P F, Ghannoum M A, Edwards J E, Bayer A S. Fluconazole and platelet microbicidal protein inhibit Candida adherence to platelets in vitro. Antimicrob Agents Chemother. 1994;38:1460–1465. doi: 10.1128/aac.38.7.1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yeaman M R, Ibrahim A S, Edwards J E, Bayer A S, Ghannoum M A. Thrombin induced rabbit platelet microbicidal protein is fungicidal in vitro. Antimicrob Agents Chemother. 1993;37:46–553. doi: 10.1128/aac.37.3.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yeaman M R, Soldan S S, Ghannoum M A, Edwards J E, Filler S G, Bayer A S. Resistance to platelet microbicidal protein results in increased severity of experimental Candida albicans endocarditis. Infect Immun. 1996;64:1379–1384. doi: 10.1128/iai.64.4.1379-1384.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yeaman M R. The role of platelets in antimicrobial host defense. Clin Infect Dis. 1997;25:951–970. doi: 10.1086/516120. [DOI] [PubMed] [Google Scholar]
- 35.Yeaman M R, Tang Y Q, Shen A J, Bayer A S, Selsted M E. Purification and in vitro activities of rabbit platelet microbicidal proteins. Infect Immun. 1997;65:1023–1031. doi: 10.1128/iai.65.3.1023-1031.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yong E C, Chi E Y, Fritsche T R, Henderson W R. Human platelet-mediated cytotoxicity against Toxoplasma gondii: role of thromboxane A2. J Exp Med. 1991;173:65–78. doi: 10.1084/jem.173.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]