Abstract
Evidence showing that neutrophils play a protective role in the host response to infection by different intracellular parasites has been published in the past few years. We assessed this issue with regard to the infection of mice with Mycobacterium tuberculosis. We found a chronic recruitment of neutrophils to the infection foci, namely, to the peritoneal cavity after intraperitoneal infection and to the spleen and liver after intravenous inoculation of the mycobacteria. However, bacilli were never found associated with the recruited neutrophils but rather were found inside macrophages. The intravenous administration of the antineutrophil monoclonal antibody RB6-8C5 during the first week of infection led to selective and severe neutropenia associated with an enhancement of bacillary growth in the target organs of the mice infected by the intravenous route. The neutropenia-associated exacerbation of infection was most important in the liver, where a bacterial load 10-fold higher than that in nonneutropenic mice was found; the exacerbation in the liver occurred both during and after the neutropenic period. Early in infection by M. tuberculosis, neutropenic mice expressed lower levels of mRNAs for gamma interferon and inducible nitric oxide synthase in the liver compared to nondepleted mice. These results point to a protective role of neutrophils in the host defense mechanisms against M. tuberculosis, which occurs early in the infection and is not associated with the phagocytic activity of neutrophils but may be of an immunomodulatory nature.
The neutrophil is a professional phagocyte with a crucial role in the host defenses against infection by extracellular parasites. Recent data from mouse models show that the neutrophil also plays a protective role in infections by intracellular parasites. Results from several groups, including our own, demonstrate that the neutrophil is a key cell in the host defense against primary (4, 16, 21, 40) and secondary (4, 22) infection by Listeria monocytogenes. Likewise, the neutrophil has been found to play a protective role in infections by Candida albicans (30, 41, 42), Salmonella enterica subsp. typhimurium (15), Francisella tularensis (49), Yersinia enterocolitica (15), Chlamydia trachomatis (6), and Toxoplasma gondii (45).
The importance of neutrophils in the host defense against intracellular microbes that cause chronic infections, such as mycobacteria, has previously been dismissed. There are two reasons for this. First, the neutrophil has a short life span, and second, the bacilli grow inside macrophages, thus being sheltered from the phagocytic activity of the neutrophil. However, in support of a role for these cells in chronic infections, we have shown that neutrophils are persistently recruited to the sites of mycobacterial infection (5, 47). This neutrophil response is biphasic, with an early acute peak on the first day of infection followed by a second influx peaking around 8 to 15 days and lasting until the end of the infection. The first peak is nonspecific, while the second is T cell dependent (1, 2). The importance of this neutrophil influx has been addressed by studying the response to mycobacterial strains of differing virulence or by comparing live and dead mycobacteria (47). Importantly, we have shown that the neutrophil response is stronger and more persistent for both virulent and live mycobacteria (47). In addition, the in vitro antimycobacterial activity of peritoneal macrophages was increased when macrophage cultures were supplemented with neutrophil material (47).
Further support for a protective role of neutrophils in mycobacterial infections has been provided by the in vivo depletion of these cells by monoclonal antibody (MAb) RB6-8C5 treatment. In an intravenous (i.v.) model of Mycobacterium avium infection, mice depleted of neutrophils by using RB6-8C5 exhibited increased susceptibility to bacterial growth (3). This increased susceptibility was similar to that of mice which carry the beige mutation (3). Beige mice reconstituted with neutrophils from C57BL/6 mice exhibited increased resistance to M. avium infection (3). More recently, Petrofsky and Bermudez, using the same neutrophil depletion procedure, also showed that neutrophils play a protective role in the early resistance to M. avium infection (38). It is not yet clear what role neutrophils play in the lung, as RB6-8C5 depletion failed to exacerbate growth of bacteria in mice aerogenically infected with M. avium (43).
In the present work, we studied neutrophil recruitment during infection of mice with Mycobacterium tuberculosis and also the effect of in vivo neutrophil depletion on bacterial proliferation. We show that neutrophils, which were found to be present at the foci of M. tuberculosis infection, play an indirect, nonphagocytic role in host protective mechanisms, probably via an effect on innate production of gamma interferon (IFN-γ).
MATERIALS AND METHODS
Mice.
Female BALB/c mice were purchased from Jackson Laboratories (Bar Harbor, Maine) and infected when they were 6 to 8 weeks of age.
Antibodies.
The RB6-8C5 cell line was a kind gift from R. L. Coffman (DNAX Research Institute, Palo Alto, Calif.), and the GL-117 and JES5-2A5 cell lines, secreting β-galactosidase-specific and interleukin-10 (IL-10)-specific MAbs, respectively, were a kind gift from DNAX. These hybridomas were grown in ascites fluid in HSD nude mice purchased from the Gulbenkian Institute (Oeiras, Portugal), and the antibodies were purified by using a protein G-agarose column (Gibco, Paisley, United Kingdom).
Experimental infections.
A virulent laboratory strain of M. tuberculosis (Erdman) was grown from a low-passage seed lot in Proskauer-Beck liquid medium to mid-log phase, aliquoted, and frozen at −70°C.
To quantitatively assess neutrophil influx during the infection by M. tuberculosis, the peritoneal cavity model of infection was used (47). Mice were injected intraperitoneally (i.p.) with 105 CFU of M. tuberculosis or phosphate-buffered saline (PBS), and groups of four or five mice were sacrificed at different time points.
Treated and untreated mice were i.v. infected, via the lateral tail vein, with 105 CFU of strain Erdman. Mycobacterial proliferation was assessed at different time intervals by determining viable counts in liver, spleen, and lung until day 30 of infection. Serial dilutions of whole-organ homogenates were plated on Middlebrook 7H11 agar (Life Technologies, Gaithersburg, Md.), and bacterial colonies were counted after incubation at 37°C for 20 days. The data are expressed as the log10 of the mean number of bacteria recovered per organ (n = 4 animals).
Study of neutrophil influx.
The peritoneal leukocyte population in mice injected i.p. with mycobacteria or PBS (control) was analyzed. After peritoneal lavage with 4 ml of PBS, total leukocyte numbers were determined and differential cell counts were performed with cytospin preparations (Shandon cytocentrifuge) stained with the Diffquick stain (Date International, Miami, Fla.). Duplicate cytospin preparations were stained by the Ziehl-Neelsen method for the visualization of M. tuberculosis bacilli.
Neutrophil recruitment to foci of systemic infection was studied in liver and spleen at the time points indicated in Fig. 2 and Table 1. Spleen cell suspensions were treated with a 0.15 M ammonium chloride–0.010 M potassium bicarbonate solution to lyse erythrocytes. The cells were then washed, and total and differential cell numbers were determined as described above for peritoneal leukocytes. Liver tissue was fixed in 10% buffered formalin set in paraffin, sectioned, and stained with hematoxylin and eosin. Neutrophil influx was evaluated by counting the number of neutrophils per microscopic field (with a 100× objective) in at least 50 fields per section. These numbers were transformed to correspond to cell numbers per square millimeter. Duplicate preparations of both spleen cell cytospin preparations and histological liver sections were stained by the Ziehl-Neelsen method for the visualization of M. tuberculosis bacilli.
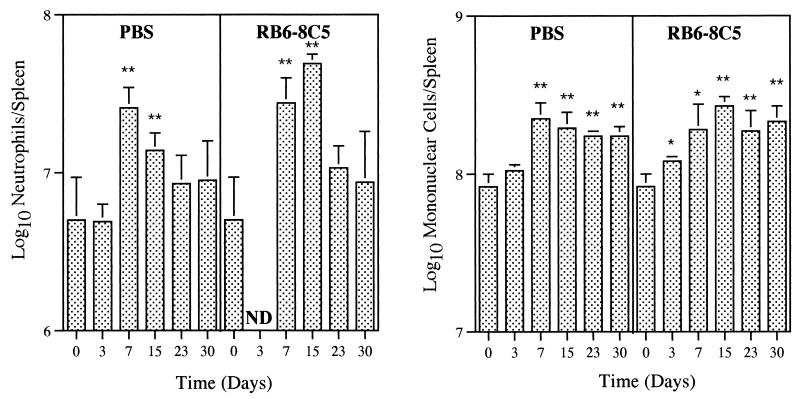
FIG. 2.
Neutrophil and mononuclear cell populations of mouse spleens during systemic infection by M. tuberculosis. Mice were injected i.v. with 105 CFU of M. tuberculosis and treated i.v. with the neutrophil-depleting MAb RB6-8C5 or with PBS (control). The antibody was administrated at 5 h, 2 days, and 4 days of infection. At the indicated time points, groups of four or five mice were sacrificed and spleen cell suspensions were obtained. After erythrocyte lysis, cells were washed and total and differential leukocyte counts were performed. Bars represent means and standard deviations. ND, not detectable. Statistical differences between noninfected and infected mice: ∗, P < 0.05; ∗∗, P < 0.01. Results are from one representative experiment of two independent experiments.
TABLE 1.
Numbers of neutrophils in liver sections of early neutrophil-depleted and nondepleted i.v. infected micea
| Mouse group
|
Neutrophils/mm2 (mean ± SD) on day:
|
|||
|---|---|---|---|---|
| Infection | Treatment | 0 | 3 | 7 |
| None | None | 2.3 ± 0.8 | ||
| M. tuberculosis | PBS | 42.6 ± 10.8 | 72.1 ± 23.6 | |
| M. tuberculosis | RB6-8C5 | 7.4 ± 0.5 | 71.6 ± 22.6 | |
Systemic infection with M. tuberculosis and neutrophil depletion with MAb RB6-8C5 were carried out as described in the legend to Fig. 2. Noninfected and nondepleted mice were used as a control. Statistical differences are significant for neutrophil numbers in infected versus noninfected mice (P < 0.01 for both day 3 and day 7) and for neutrophil numbers in depleted and nondepleted infected mice at day 3 (P < 0.01). Four mice per group were used.
Neutrophil depletion.
In order to assess the role of neutrophils in systemic infection by M. tuberculosis, i.v. infected mice were made neutropenic by treatment with MAb RB6-8C5, as previously described in detail (3, 4). Briefly, mice were injected i.v. in the lateral tail vein with 200 μg of MAb RB6-8C5 or with MAb GL-117 or PBS as controls (in an independent experiment it was found that the effect of the administration of the isotype-matched control MAb GL-117 was not different from that of PBS). Two depletion periods were used in the present study. Early depletion required antibody treatment at 5 h, 2 days, and 4 days of infection. Late depletion required dosing on days 16, 18, and 20 of infection. Previous studies with MAb RB6-8C5, showed severe neutrophil depletion in the peripheral blood, spleen, and peritoneal cavity for 48 h following the i.v. injection of 0.2 mg of the antibody, with the number of neutrophils returning to normal at day 3 after treatment (4). In this model, therefore, neutropenia is maintained until day 6, with recovery at day 7 (3). The numbers of neutrophils in livers and spleens of MAb RB6-8C5-treated mice were determined as described above at the time points indicated in Fig. 2 and Table 1.
By laser-assisted confocal microscopy, we confirmed that MAb RB6-8C5 binds to neutrophils but not to mononuclear cells. This neutrophil-specific binding of RB6-8C5 was observed in both naive and experimentally infected mice (J. Pedrosa et al., unpublished data).
In vivo treatments with rIL-12 or anti-IL-10.
Mice were infected i.v. with M. tuberculosis Erdman and treated during the first week of infection with MAb RB6-8C5 or PBS as control. Neutralization of IL-10 was performed by i.p. injection of a single dose of 2 mg of MAb JES5-2A5 at 5 h after infection (36). Recombinant IL-12 (rIL-12) (46) (a kind gift from S. Wolf and J. Sypek, Genetics Institute, Cambridge, Mass.) was reconstituted in PBS and administrated i.p. in two doses of 0.4 μg at 5 h and 2 days after infection.
Semiquantitative reverse transcriptase PCR reaction (RT-PCR).
Liver segments were collected from infected mice, homogenized in Ultraspec (Biotecx Laboratories Inc., Houston, Tex.), rapidly frozen, and stored at −70°C. Total mRNA was extracted and reverse transcribed by using murine Moloney leukemia virus reverse transcriptase (Gibco BRL, Grand Island, N.Y.). Analysis of mRNA-specific cDNA sequences for IFN-γ and inducible nitric oxide synthase (iNOS) was performed by semiquantitative PCR (20). Briefly, cDNA was diluted and amplified by using Taq polymerase (Promega, Madison, Wis.) and specific primers (20, 53). The PCR product was blotted, probed with specific internal probes, and detected by using an enhanced chemiluminescence (ECL) kit (Amersham, Arlington Heights, Ill.). By using a limiting number of cycles for the PCR, a correlation between the amount of signal and the relative amounts of cDNA specific for a particular product is obtained. The amount of readable RNA from each sample was compared by using primers specific for the hypoxanthine phosphoribosyltransferase (HPRT) housekeeping gene. Samples that had similar HPRT signals were compared for the expression of IFN-γ-specific and iNOS-specific signals.
RESULTS
Persistent neutrophil recruitment is a feature of M. tuberculosis infection.
As a first approach to quantitatively assess neutrophil influx during infection by M. tuberculosis, the recruitment of neutrophils to the peritoneal cavity of mice infected i.p. was monitored over time. We used this model since, as previously reported (5, 47), peritoneal leukocyte populations can be easily and accurately studied both qualitatively and quantitatively.
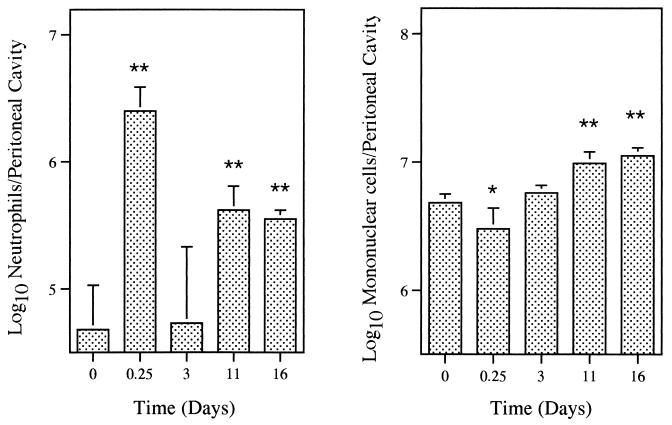
Figure 1 demonstrates that the very low numbers of peritoneal neutrophils seen in noninfected mice increased in the i.p. inoculated animals, with peaks at 6 h and 11 days postinfection. An increase in the number of mononuclear cells in the peritoneal cavity was found after the third day of infection. No significant differences between eosinophil and mast cell populations were seen (not shown). Control mice injected i.p. with PBS did not show a significant influx of neutrophils (not shown).
FIG. 1.
Influx of neutrophils and mononuclear cells to peritoneal cavities of mice infected i.p. with 105 CFU of M. tuberculosis. Peritoneal lavages were performed on groups of four or five mice at different time points, and total and differential leukocyte counts were done. Bars represent means and standard deviations. Statistical differences between noninfected and infected mice: ∗, P < 0.05; ∗∗, P < 0.01. Results are from one representative experiment of two independent experiments.
In order to assess the recruitment of neutrophils to M. tuberculosis infection foci during systemic infection, we studied the leukocyte populations in spleen and liver following infection by the i.v. route. In the spleens of noninfected mice, high numbers of neutrophils were found (Fig. 2). These numbers increased significantly after day 3 in the i.v. inoculated animals (Fig. 2). The histological analysis of liver sections showed that neutrophils were very rare in control mice but were recruited to this organ during the entire course of systemic infection by M. tuberculosis (Table 1).
At the infection foci, M. tuberculosis bacilli were never found associated with neutrophils but rather were found in macrophages.
RB6-8C5 efficiently abrogates neutrophilia at infectious foci during systemic infection by M. tuberculosis.
A severe, transient, and selective neutropenia was obtained by our protocol of administration of MAb RB6-8C5 in mice systemically infected with M. tuberculosis. In fact, during a 6-day period after the beginning of the treatment with MAb RB6-8C5, no neutrophils were found in the spleen by cytological analysis (Fig. 2). A similar neutropenia was found in the liver by histological analysis (Table 1). No significant differences in the numbers of other leukocytes due to the treatment with MAb RB6-8C5 were found in either organ.
The depletion of neutrophils during the first week of infection exacerbates M. tuberculosis proliferation.
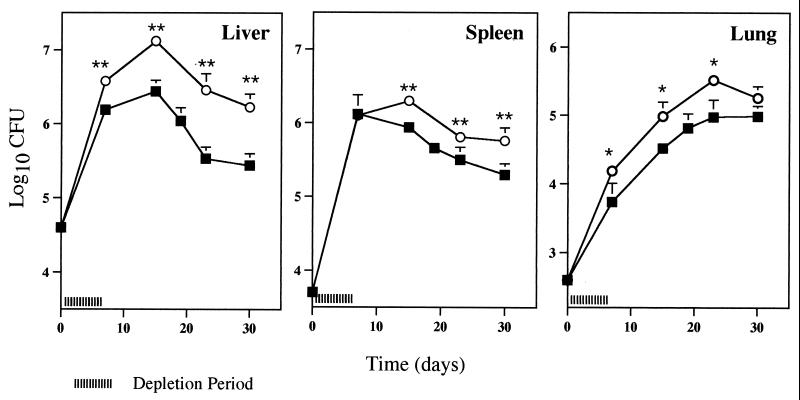
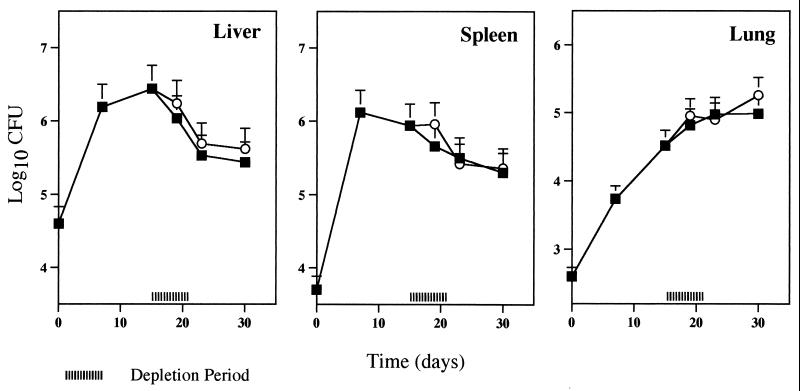
Neutrophil depletion was carried out early in infection by the administration of three doses of the antibody at 5 h, 2 days, and 4 days after the inoculation of mycobacteria. The effect of this early neutrophil depletion on bacterial numbers was different in each organ. In the lung, a significant exacerbation of mycobacterial proliferation was found at the end of the effective period of depletion, i.e., 7 days (Fig. 3). In the spleen, bacterial numbers were higher in MAb-treated animals than in control mice from day 15 onwards. Finally, in the liver, the exacerbation of infection induced by the treatment with MAb RB6-8C5 occurred both during the neutropenic period and after recovery from the neutropenia. In this organ, at day 15 of infection the bacterial loads in neutropenic mice were 10-fold higher than those in the nondepleted animals. Differences in the mycobacterial numbers in the latter two organs persisted throughout the remaining course of the infection.
FIG. 3.
Effect of early neutrophil depletion on susceptibility of BALB/c mice to M. tuberculosis infection. Mice were injected i.v. with 105 CFU of M. tuberculosis and treated with MAb RB6-8C5 (circles) or PBS as a control (squares) as described for Fig. 2. Groups of mice were sacrificed at different time points, and numbers of viable bacteria were determined by plating serial dilutions of organ homogenates on Middlebrook 7H11 medium. The results represent the geometric means and standard deviations of the CFU of four animals. Statistical differences between mice treated with MAb RB6-8C5 and mice treated with PBS: ∗, P < 0.05; ∗∗, P < 0.01. Results are from one representative experiment of three independent experiments.
In order to address the role of the antibody treatment in early bacterial growth, two independent experiments were performed wherein bacterial numbers in the liver, spleen, and lung were determined at day 3 of infection. Table 2 shows that no exacerbation of bacterial growth occurred at this early time point.
TABLE 2.
Effect of early neutrophil depletion of BALB/c mice on bacterial loads at day 3 of infection with M. tuberculosisa
| Treatment | Log10 CFU (mean ± SD) in:
|
||
|---|---|---|---|
| Liver | Spleen | Lung | |
| Control | 5.56 ± 0.17 | 4.89 ± 0.16 | 3.43 ± 0.08 |
| RB6-8C5 | 5.43 ± 0.13 (P = 0.29) | 4.86 ± 0.17 (P = 0.85) | 3.58 ± 0.15 (P = 0.14) |
Early neutrophil depletion causes decreased expression of mRNAs for IFN-γ and iNOS in vivo.
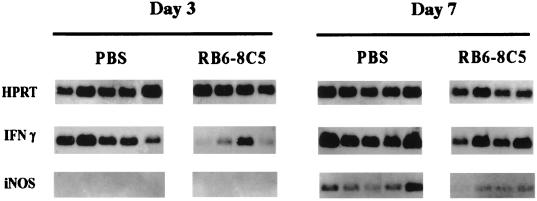
In order to assess the mechanism(s) through which neutrophils participate in the host defense against systemic infection by M. tuberculosis, we analyzed the expression of mRNAs for IFN-γ and iNOS in the livers of neutropenic and nondepleted mice infected with the bacilli, using RT-PCR.
We found that neutropenic mice expressed lower levels of mRNA for IFN-γ early in infection compared to nondepleted mice. This decreased level of mRNA for IFN-γ was found during the neutropenic period, at day 3 of infection (Fig. 4). At this time point, no message for iNOS was found in either group of mice. Later in infection (day 7), we found a lower level of message for iNOS in the livers of mice that had been previously treated with the antineutrophil MAb RB6-8C5 than in the controls (Fig. 4).
FIG. 4.
Semiquantitative RT-PCR analysis of in vivo expression of IFN-γ, iNOS, and HPRT in mouse livers at 3 and 7 days after systemic infection with 105 CFU of M. tuberculosis. Liver fragments were collected from control mice (mice injected with PBS) and from mice made neutropenic by treatment with MAb RB6-8C5 as described for Fig. 2. Each lane represents data from one animal after scanning of the Southern blots. Results are from one representative experiment of two independent experiments.
IL-10 neutralization or IL-12 administration partially abrogates the exacerbation of infection induced by early neutrophil depletion.
The lower levels of mRNA expression for IFN-γ found in infected neutropenic mice compared to infected nondepleted mice could be the result of either a decreased production of IL-12 or an increase in the levels of IL-10, or both. In order to test this hypothesis we assessed the effect of the neutralization of IL-10 as well as the effect of the administration of IL-12 in neutropenic mice and in nondepleted mice.
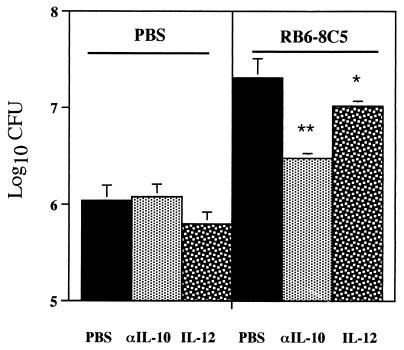
While the administration of rIL-12 did not affect mycobacterial numbers in nondepleted mice, it clearly reduced the exacerbation of infection in the livers of mice treated with the antineutrophil antibody (Fig. 5). Treatment with anti-IL-10 MAb also resulted in reduced exacerbation of infection due to neutrophil depletion (Fig. 5).
FIG. 5.
Effects of IL-10 neutralization or IL-12 administration on host susceptibility to M. tuberculosis infection for normal and neutropenic mice. Mice were injected i.v. with 105 CFU of M. tuberculosis and treated during the first week of infection with MAb RB6-8C5 (right panel) or PBS (left panel) as described for Fig. 2. Recombinant IL-12 was administrated i.p. at 5 h and 2 days after infection, and neutralization of IL-10 was done by i.p. injection, at 5 h after infection, of 2 mg of MAb JES5-2A5 (αIL-10); as a control for these treatments, mice were injected i.p. with PBS. Groups of mice were sacrificed at 14 days of infection, and numbers of viable bacteria were assessed. The results represent the geometric means and standard deviations of the CFU of four animals. Statistical differences between mice treated i.p. with PBS and mice treated i.p. with IL-12 or with MAb JES5-2A5: ∗, P < 0.05; ∗∗, P < 0.01. Results are from one representative experiment of two independent experiments.
Depletion of neutrophils during the third week of infection does not affect the proliferation of M. tuberculosis.
If the increased susceptibility seen during MAb RB6-8C5 treatment is related to decreased innate responses, we would expect late depletion to have very little effect on bacterial growth. We therefore depleted previously infected mice and compared the effects of such treatment with a control infection. As shown in Fig. 6, this depletion of neutrophils late in infection had no effect on the mycobacterial loads in the three target organs studied.
FIG. 6.
Effect of late neutrophil depletion on the susceptibility of BALB/c mice to M. tuberculosis infection. The procedure and symbols are as for Fig. 3, except that MAb RB6-8C5 was injected at days 16, 18, and 20 after infection. Results are from one representative experiment of two independent experiments. Four mice were used per time point.
DISCUSSION
As already reported for other mycobacteria (5, 47), we show here that infection with M. tuberculosis led to the persistent recruitment of neutrophils to the sites of infection, namely, to the peritoneal cavity after i.p. infection and to the liver and spleen after i.v. infection. Importantly, the protective role of this neutrophilic response was demonstrated by the increased susceptibility of neutropenic mice to M. tuberculosis.
Clearly, the depletion of neutrophils during the first week of infection allowed increased growth of M. tuberculosis in liver, spleen, and lung; this increase was most pronounced in the liver. At present we have no explanation for the importance of neutrophils in protective responses in the liver. Interestingly, however, other related reports, on studies using a variety of infectious agents, have also noted increased susceptibility in the livers of neutrophil-depleted mice (4, 6, 15, 21).
We do not feel that the protective function of the neutrophils is as a direct killer cell in the host resistance against M. tuberculosis. In support of this hypothesis, we did not find M. tuberculosis bacteria associated with neutrophils in either the peritoneal or splenic cytospin preparations or the histological sections of the liver; rather, the bacteria were clearly associated with or within macrophages. This observation indicates that there is little opportunity for direct phagocytosis and intracellular killing of M. tuberculosis by neutrophils in vivo. Other studies have addressed the role of neutrophils in direct killing of M. tuberculosis. Brown (10) and Jones et al. (31) have demonstrated that human neutrophils are capable of controlling the growth of M. tuberculosis in cultures of human neutrophils, but their assays did not distinguish between intra- and extracellular killing. In contrast, Denis reported that human neutrophils were unable to kill M. tuberculosis even after activation with cytokines (24).
There are several plausible mechanisms that could mediate this indirect protective role of neutrophils against infection by intracellular parasites. These include immunomodulation through production of cytokines and chemotactic factors (8, 9, 25, 37–39, 41, 42, 50), secretion of granule components that can activate infected macrophages (26, 34, 35), and transfer of neutrophilic antimicrobial materials to the macrophage (29, 33, 34, 47).
Although we cannot exclude the other mechanisms mentioned above, we favor an immunomodulatory activity of the neutrophil, particularly in the liver. In fact, the increase in bacterial proliferation observed in the livers of the neutrophil-depleted mice occurred not only during the period of neutropenia but also later when neutrophils were again present in the host. The possibility of an immunomodulatory role for neutrophils in the protective mechanisms against M. tuberculosis is supported by the fact that mouse neutrophils are known to secrete several cytokines, such as IL-1α/β, IL-6, IL-10, IL-12, and tumor necrosis factor alpha (9, 11–13, 38, 41, 42).
Several of these cytokines are known to modulate the expression of IFN-γ, which is a key cytokine in the host defense mechanisms against M. tuberculosis in the mouse (18, 27). A second major component of the protective response is the macrophage product nitric oxide. This molecule is required to create a toxic environment within the infected macrophage and is dependent on the enzyme iNOS for its production. In turn, the expression of iNOS is highly dependent upon IFN-γ expression (27). Interestingly, we observed that the neutrophil-depleted mice expressed less mRNA for IFN-γ early during depletion (day 3) and that the loss of this IFN-γ resulted in reduced expression of iNOS mRNA later (day 7). These observations clearly suggest that the presence of neutrophils in the infection foci is important for the early production of IFN-γ and that the decreased production of IFN-γ in neutropenic mice affects the nitric oxide-mediated antimycobacterial activity of infected macrophages.
The cellular source for the early IFN-γ is unlikely to be conventional T cells, since the effect occurs earlier than antigen-specific T cells emerge (17). There has been a recent spate of publications implicating many types of cells of the innate immune system in the production of IFN-γ. Natural killer (NK) cells produce IFN-γ in response to IL-12 and tumor necrosis factor alpha (28). In addition, NK cells which express an αβ T-cell receptor (NK T cells) have been shown to release several cytokines and chemokines very rapidly upon activation and have been suggested to play a role in initiating and/or modulating the acquired immune response (7). At this time we have no data supporting the role of any particular cell type in the neutrophil-dependent early expression of IFN-γ. We suggest, however, that the presence of neutrophils is required for IFN-γ production by cells of the innate immune system early after infection. Consistent with this interpretation, no effect on bacterial proliferation was seen as a consequence of a later neutrophil depletion induced during the third week of infection, when protective T cells have already been induced.
IL-10 and IL-12 are cytokines that are induced by bacterial infection and which also modulate IFN-γ expression (51, 52). IL-12 acts directly on cells to both induce and expand their ability to make IFN-γ. In contrast, IL-10 acts on IL-12-producing cells to inhibit production of this cytokine and therefore indirectly limit IFN-γ production (23, 32). The protective role of IL-12 in infections by intracellular parasites, including M. tuberculosis and M. avium, have been well described (14, 19, 20, 44, 48). Moreover, in experimental models of murine candidiasis and toxoplasmosis, one source of protective IL-12 was the neutrophil population (9, 42). In addition, it was recently reported that neutrophils isolated from mice early after infection with M. avium produce increased amounts of IL-12 compared to neutrophils from uninfected mice (38). We therefore hypothesize that in normal mice exposed to M. tuberculosis, neutrophils produce excess IL-12 which limits the effect of any IL-10 expressed. This excess IL-12 then tips the balance in favor of early IFN-γ production. In the neutrophil-depleted mice there is insufficient IL-12, and therefore IL-10 dominates, resulting in reduced early IFN-γ. In support of this hypothesis, we report here that both the addition of rIL-12 and the inhibition of IL-10 resulted in a reduced exacerbatory effect of the antineutrophil treatment.
In summary, our results show that neutrophils play a protective role in a murine model of systemic infection by M. tuberculosis. In particular, these cells are important in limiting bacterial growth in the liver. The protective nature of the neutrophil was in evidence during the early period of the infection and was mediated via a nonphagocytic, possibly immunomodulatory, mechanism which affected IFN-γ production.
ACKNOWLEDGMENTS
This work was supported by grants from NIH (contract AI40488) and JNICT (Lisbon, Portugal) (contract PECS/P/SAU/60/95).
We thank R. L. Coffman and the DNAX Research Institute for providing hybridoma cell lines and S. Wolf and J. Sypek (Genetics Institute) for providing rIL-12.
REFERENCES
- 1.Appelberg R. Interferon-gamma (IFN-γ) and macrophage inflammatory proteins (MIP)-1 and -2 are involved in the regulation of the T cell-dependent chronic peritoneal neutrophilia of mice infected with mycobacteria. Clin Exp Immunol. 1992;89:269–273. doi: 10.1111/j.1365-2249.1992.tb06943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Appelberg R. T cell regulation of the chronic peritoneal neutrophilia during mycobacterial infections. Clin Exp Immunol. 1992;89:120–125. doi: 10.1111/j.1365-2249.1992.tb06889.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Appelberg R, Castro A G, Gomes S, Pedrosa J, Silva M T. Susceptibility of beige mice to Mycobacterium avium: role of neutrophils. Infect Immun. 1995;63:3381–3387. doi: 10.1128/iai.63.9.3381-3387.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Appelberg R, Castro A G, Silva M T. Neutrophils as effector cells of T-cell-mediated, acquired immunity in murine listeriosis. Immunology. 1994;83:302–307. [PMC free article] [PubMed] [Google Scholar]
- 5.Appelberg R, Pedrosa J M, Silva M T. Host and bacterial factors control the Mycobacterium avium-induced chronic peritoneal granulocytosis in mice. Clin Exp Immunol. 1991;83:231–236. doi: 10.1111/j.1365-2249.1991.tb05620.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barteneva N, Theodor I, Peterson E M, de la Maza L M. Role of neutrophils in controlling early stages of a Chlamydia trachomatis infection. Infect Immun. 1996;64:4830–4833. doi: 10.1128/iai.64.11.4830-4833.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bendelac A, Rivera M N, Park S H, Roark J H. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu Rev Immunol. 1997;15:535–562. doi: 10.1146/annurev.immunol.15.1.535. [DOI] [PubMed] [Google Scholar]
- 8.Bliss S K, Marshall A J, Zhang Y, Denkers E Y. Human polymorphonuclear leukocytes produce IL-12, TNF-alpha, and the chemokines macrophage-inflammatory protein-1 alpha and -1 beta in response to Toxoplasma gondii antigens. J Immunol. 1999;162:7369–7375. [PubMed] [Google Scholar]
- 9.Bliss S K, Zhang Y, Denkers E Y. Murine neutrophil stimulation by Toxoplasma gondii antigen drives high level production of IFN-γ-independent IL-12. J Immunol. 1999;163:2081–2088. [PubMed] [Google Scholar]
- 10.Brown A E. Capacity of human neutrophils to kill Mycobacterium tuberculosis. J Infect Dis. 1987;156:985–989. doi: 10.1093/infdis/156.6.985. [DOI] [PubMed] [Google Scholar]
- 11.Cassatella M A. Cytokine production by neutrophils in vivo. In: Cassatella M A, editor. Cytokine produced by polymorphonuclear neutrophils: molecular and biological aspects. R. G. Austin, Tex: Landes Company; 1996. pp. 163–180. [Google Scholar]
- 12.Cassatella M A. The production of cytokines by polymorphonuclear neutrophils. Immunol Today. 1995;16:21–26. doi: 10.1016/0167-5699(95)80066-2. [DOI] [PubMed] [Google Scholar]
- 13.Cassatella M A, Meda L, Gasparini S, D'Andrea A, Ma X, Trinchieri G. Interleukin-12 production by human polymorphonuclear leukocytes. Eur J Immunol. 1996;25:1–5. doi: 10.1002/eji.1830250102. [DOI] [PubMed] [Google Scholar]
- 14.Castro A G, Silva R A, Appelberg R. Endogenously produced IL-12 is required for the induction of protective T cells during Mycobacterium avium infections in mice. J Immunol. 1995;155:2013–2019. [PubMed] [Google Scholar]
- 15.Conlan J W. Critical roles of neutrophils in host defense against experimental systemic infections of mice by Listeria monocytogenes, Salmonella typhimurium, and Yersinia enterocolitica. Infect Immun. 1997;65:630–635. doi: 10.1128/iai.65.2.630-635.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conlan J W, North R J. Neutrophils are essential for early anti-Listeria defense in the liver, but not in the spleen or peritoneal cavity, as revealed by a granulocyte-depleting monoclonal antibody. J Exp Med. 1994;179:259–268. doi: 10.1084/jem.179.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cooper A M, Callahan J E, Keen M, Belisle J T, Orme I M. Expression of memory immunity in the lung following re-exposure to Mycobacterium tuberculosis. Tuber Lung Dis. 1997;78:67–73. doi: 10.1016/s0962-8479(97)90017-4. [DOI] [PubMed] [Google Scholar]
- 18.Cooper A M, Dalton D K, Stewart T A, Griffin J P, Russell D G, Orme I M. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J Exp Med. 1993;178:2243–2247. doi: 10.1084/jem.178.6.2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cooper A M, Magram J, Ferrante J, Orme I M. Interleukin 12 (IL-12) is crucial to the development of protective immunity in mice intravenously infected with Mycobacterium tuberculosis. J Exp Med. 1997;186:39–45. doi: 10.1084/jem.186.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cooper A M, Roberts A D, Rhoades E R, Calahan J E, Getzy D M, Orme I M. The role of interleukin-12 in acquired immunity to Mycobacterium tuberculosis infection. Immunology. 1995;84:423–432. [PMC free article] [PubMed] [Google Scholar]
- 21.Czuprynski C J, Brown J F, Maroushek N, Wagner R D, Steinberg H. Administration of anti-granulocyte mAb RB6-8C5 impairs the resistance of mice to Listeria monocytogenes infection. J Immunol. 1994;156:1836–1846. [PubMed] [Google Scholar]
- 22.Czuprynski C J, Brown J F, Wagner R D, Steinberg H. Administration of antigranulocyte monoclonal antibody RB6-8C5 prevents expression of acquired resistance to Listeria monocytogenes infection in previously immunized mice. Infect Immun. 1994;62:5161–5163. doi: 10.1128/iai.62.11.5161-5163.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.D'Andrea A, Aste-Amezaga M, Valiante N M, Ma X, Kubin M, Trinchieri G. Interleukin 10 (IL-10) inhibits human lymphocyte interferon gamma-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. J Exp Med. 1993;178:1041–1048. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Denis M. Human neutrophils, activated with cytokines or not, do not kill virulent Mycobacterium tuberculosis. J Infect Dis. 1991;163:919–920. doi: 10.1093/infdis/163.4.919. [DOI] [PubMed] [Google Scholar]
- 25.Doherty D E, Downey G P, Worthen G S, Haslett C, Henson P M. Monocyte retention and migration in pulmonary inflammation. Requirement for neutrophils. Lab Invest. 1988;59:200–213. [PubMed] [Google Scholar]
- 26.El-Hag A, Clark R A. Immunosuppression by activated human neutrophils. Dependence on the myeloperoxidase system. J Immunol. 1987;139:2406–2413. [PubMed] [Google Scholar]
- 27.Flynn J L, Chan J, Triebold K J, Dalton D K, Stewart T A, Bloom B R. An essential role for interferon gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gazzinelli R T, Hieny S, Wynn T A, Wolf S, Sher A. Interleukin 12 is required for the T-lymphocyte-independent induction of interferon gamma by an intracellular parasite and induces resistance in T-cell-deficient hosts. Proc Natl Acad Sci USA. 1993;90:6115–6119. doi: 10.1073/pnas.90.13.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heifets L, Imai K, Goren M B. Expression of peroxidase-dependent iodonation by macrophages ingesting neutrophil debris. J Reticuloendothel Soc. 1980;28:391–404. [PubMed] [Google Scholar]
- 30.Jensen J, Warner T, Balish E. The role of phagocytic cells in resistance to disseminated candidiasis in granulocytopenic mice. J Infect Dis. 1994;170:900–905. doi: 10.1093/infdis/170.4.900. [DOI] [PubMed] [Google Scholar]
- 31.Jones G S, Amirault H J, Andersen B R. Killing of Mycobacterium tuberculosis by neutrophils: a nonoxidative process. J Infect Dis. 1990;162:700–704. doi: 10.1093/infdis/162.3.700. [DOI] [PubMed] [Google Scholar]
- 32.Koch F, Stanzl U, Jennewein P, Janke K, Heufler C, Kampgen E, Romani N, Schuler G. High level IL-12 production by murine dendritic cells: upregulation via MHC class II and CD40 molecules and downregulation by IL-4 and IL-10. J Exp Med. 1996;184:741–746. doi: 10.1084/jem.184.2.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leung K P, Goren M B. Uptake and utilization of human polymorphonuclear leucocyte granule myeloperoxidase. Cell Tissue Res. 1989;257:653–656. doi: 10.1007/BF00221477. [DOI] [PubMed] [Google Scholar]
- 34.Lima M F, Kierszenbaum F. Lactoferrin effects on phagocytic cells function. I. Increased uptake and killing of an intracellular parasite by murine macrophages and human monocytes. J Immunol. 1985;134:4176–4183. [PubMed] [Google Scholar]
- 35.Lincoln J A, Lefkowitz D L, Castro A, Lefkowitz S S, Moguilevsky N, Bollen A. Exogenous myeloperoxidase enhances bacterial phagocytosis and intracellular killing by macrophages. Infect Immun. 1995;63:3042–3047. doi: 10.1128/iai.63.8.3042-3047.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marchant A, Bruyns C, Vandenabeele P, Ducarme M, Gerard C, Delvaux A, De Groote D, Abramowicz D, Velu T, Goldman M. Interleukin-10 controls interferon-gamma and tumor necrosis factor production during experimental endotoxemia. Eur J Immunol. 1994;24:1167–1171. doi: 10.1002/eji.1830240524. [DOI] [PubMed] [Google Scholar]
- 37.Pereira H A, Shafer W M, Pohl J, Martin L E, Spitznagel J K. CAP37, a human neutrophil-derived chemotactic factor with monocyte specific activity. J Clin Invest. 1990;85:1468–1476. doi: 10.1172/JCI114593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Petrofsky M, Bermudez L E. Neutrophils from Mycobacterium avium-infected mice produce TNF-α, IL-12, and IL-1β and have a putative role in early host response. Clin Immunol. 1999;91:354–358. doi: 10.1006/clim.1999.4709. [DOI] [PubMed] [Google Scholar]
- 39.Riedel D D, Kaufmann S H. Chemokine secretion by human polymorphonuclear granulocytes after stimulation with Mycobacterium tuberculosis and lipoarabinomannan. Infect Immun. 1997;65:4620–4623. doi: 10.1128/iai.65.11.4620-4623.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rogers H W, Unanue E R. Neutrophils are involved in acute, nonspecific resistance to Listeria monocytogenes in mice. Infect Immun. 1993;61:5090–5096. doi: 10.1128/iai.61.12.5090-5096.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Romani L, Mencacci A, Cenci E, Sero G D, Bistoni F, Puccetti P. An immunoregulatory role for neutrophils in CD4+ T helper subset selection in mice with candidiasis. J Immunol. 1997;158:2356–2362. [PubMed] [Google Scholar]
- 42.Romani L, Mencacci A, Cenci E, Spaccapelo R, Del Sero G, Nicoletti I, Trinchieri G, Bistoni F, Puccetti P. Neutrophil production of IL-12 and IL-10 in candidiasis and efficacy of IL-12 therapy in neutropenic mice. J Immunol. 1997;158:5349–5356. [PubMed] [Google Scholar]
- 43.Saunders B M, Cheers C. Intranasal infection of beige mice with Mycobacterium avium complex: role of neutrophils and natural killer cells. Infect Immun. 1996;64:4236–4241. doi: 10.1128/iai.64.10.4236-4241.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saunders B M, Zhan Y, Cheers C. Endogenous interleukin-12 is involved in resistance of mice to Mycobacterium avium complex infection. Infect Immun. 1995;63:4011–4015. doi: 10.1128/iai.63.10.4011-4015.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sayles P C, Johnson L L. Exacerbation of toxoplasmosis in neutrophil-depleted mice. Nat Immun. 1996;15:249–258. [PubMed] [Google Scholar]
- 46.Schoenhaut D S, Chua A O, Wolitzsky A G, Quinn P M, Dwyer C M, McComas W, Familletti P C, Gately M K, Gubler U. Cloning and expression of murine IL-12. J Immunol. 1992;148:3433–3440. [PubMed] [Google Scholar]
- 47.Silva M T, Silva M N T, Appelberg R. Neutrophil-macrophage cooperation in the host defence against mycobacterial infections. Microb Pathog. 1989;6:369–380. doi: 10.1016/0882-4010(89)90079-x. [DOI] [PubMed] [Google Scholar]
- 48.Silva R A, Pais T, Appelberg R. Evaluation of IL-12 in immunotherapy and vaccine design in experimental Mycobacterium avium infections. J Immunol. 1998;161:5578–5585. [PubMed] [Google Scholar]
- 49.Sjostedt A, Conlan J W, North R J. Neutrophils are critical for host defense against primary infection with the facultative intracellular bacterium Francisella tularensis in mice and participate in defense against reinfection. Infect Immun. 1994;62:2779–2783. doi: 10.1128/iai.62.7.2779-2783.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Territo M C, Ganz T, Selsted M E, Lehrer R. Monocyte-chemotactic activity of defensins from human neutrophils. J Clin Invest. 1989;84:2017–2020. doi: 10.1172/JCI114394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 52.Trinchieri G, Gerosa F. Immunoregulation by interleukin-12. J Leukoc Biol. 1996;59:505–511. doi: 10.1002/jlb.59.4.505. [DOI] [PubMed] [Google Scholar]
- 53.Wynn T A, Eltoum I, Cheever A W, Lewis F A, Gause W C, Sher A. Analysis of cytokine mRNA expression during primary granuloma formation induced by eggs of Schistosoma mansoni. J Immunol. 1993;151:1430–1440. [PubMed] [Google Scholar]