Abstract
Introduction:
Hyalocytes are sentinel macrophages residing within the posterior vitreous cortex anterior to the retinal inner limiting membrane (ILM). Following anomalous PVD and vitreoschisis, hyalocytes contribute to paucicellular (vitreo-macular traction syndrome, macular holes) and hypercellular (macular pucker, proliferative vitreo-retinopathy, proliferative diabetic vitreo-retinopathy) diseases.
Areas covered:
Studies of human tissues employing dark-field, phase, and electron microscopy; immunohistochemistry; and in vivo imaging of human hyalocytes.
Expert opinion:
Hyalocytes are important in early pathophysiology, stimulating cell migration and proliferation, as well as subsequent membrane contraction and vitreo-retinal traction. Targeting hyalocytes early could mitigate advanced disease. Ultimately, eliminating the role of vitreous and hyalocytes may prevent proliferative vitreo-retinal diseases entirely.
Keywords: Vitreous, hyalocytes, anomalous PVD, vitreoschisis, macular pucker, proliferative diabetic vitreo-retinopathy, proliferative vitreo-retinopathy
1. Introduction
Hyalocytes play an important role in proliferative vitreo-retinal diseases that feature prominent hypercellular membranes such as macular pucker (MPK), proliferative diabetic vitreo-retinopathy (PDVR), and post-retinal detachment proliferative vitreo-retinopathy (PVR). There are also paucicellular vitreo-maculopathies such as macular holes (MH) and vitreo-macular traction syndrome (VMTS) where hyalocytes might play a role. The fundamental pathogenetic conditions common to all these conditions are anomalous posterior vitreous detachment (APVD) and vitreoschisis (VS).
The first article in this series of expert reviews on hyalocytes focused on the origin and turnover of hyalocytes, cell morphology, and imaging[1], while the second article exploits technical advances in molecular biology to elucidate the functions of hyalocytes during development and in adult homeostasis as well as in inflammatory and neurodegenerative disorders[2]. The current article, which is the third in this 3-part series, will summarize the pathogenesis and progression of these conditions emphasizing the critical contribution(s) of hyalocytes. Improved understanding of the role of hyalocytes in pathophysiology may lead to targeted therapies directed at these cells, which may be particularly potent given the role of hyalocytes in the early stages of proliferative vitreo-retinal diseases. Moreover, based upon our current understanding of vitreous anatomy, the pathologic phenomena of anomalous PVD and vitreoschisis, as well as the role of hyalocytes, preventative strategies have already arisen and are being implemented, most notably in the prevention of PVR. Indeed, eliminating the roles of vitreous and hyalocytes in PVR serves as a paradigm for how to one day induce an innocuous posterior vitreous detachment and substantially mitigate, if not entirely prevent proliferative vitreo-retinal diseases.
2. Anomalous posterior vitreous detachment (APVD) and vitreoschisis (VS)
In youths, vitreous is a solid clear gel composed of water (98%) and structural macromolecules (collagen and hyaluronan), as well as critical extracellular matrix constituents[3–5]. Aging, myopia, and diabetes are associated with fibrous liquefaction and degeneration of the vitreous body, destabilizing the entire vitreous body. When there is concurrent weakening of vitreo-retinal adhesion, dehiscence at the vitreo-retinal interface and collapse of the vitreous body result in an innocuous posterior vitreous detachment (PVD)[6,7]. However, if there is excess fibrous liquefaction/degeneration internally and/or insufficient weakening of vitreo-retinal adhesion, an anomalous PVD can occur[8–10]. There are various consequences of anomalous PVD, which differ based upon the topographic location of vitreo-retinal separation and whether or not the outer vitreous shell, called the posterior vitreous cortex, remains intact (Fig 1).
Figure 1: Anomalous PVD.
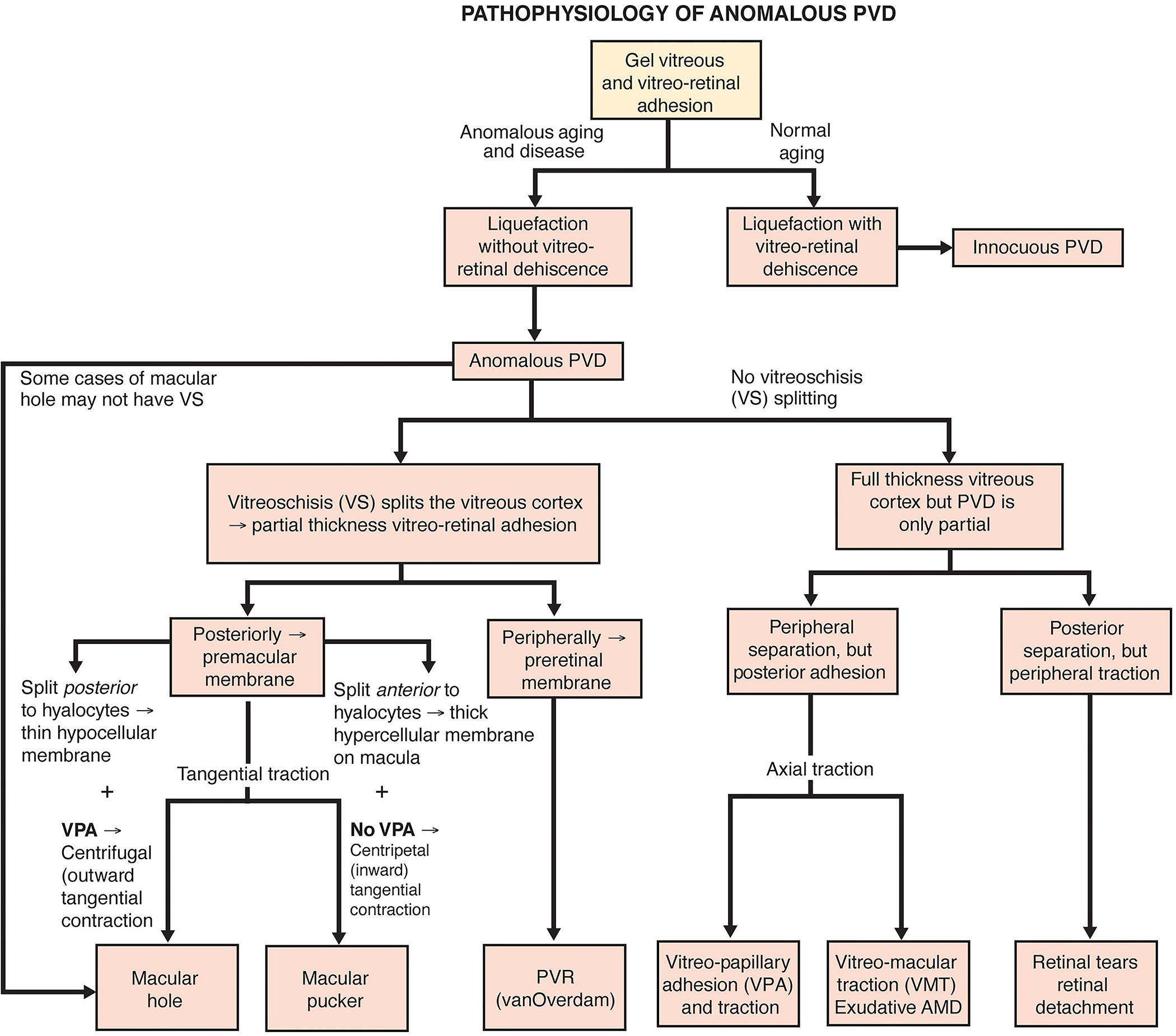
When gel liquefaction and weakening of vitreo-retinal adhesion occur concurrently, the vitreous body separates away from the retina without sequelae. If the gel liquefies without concurrent dehiscence at the vitreo-retinal interface, there can be various untoward consequences, depending upon where vitreous is most adherent. If separation of vitreous from retina is full-thickness but topographically incomplete, there can be different forms of partial PVD (right side of diagram). Posterior separation with persistent peripheral vitreo-retinal attachment can induce retinal breaks and detachments. Peripheral vitreo-retinal separation with persistent full-thickness attachment of vitreous to the retina posteriorly can induce traction upon the macula (VMT), known as the vitreo-macular traction syndrome. Persistent full-thickness adherence to the macula is associated with (and may promote) exudative age-related macular degeneration (AMD). Persistent attachment to the optic disc can induce vitreo-papillopathies and also contribute to neovascularization and vitreous hemorrhage in ischemic retinopathies, as well as some cases of full-thickness macular hole.
If, during PVD, the posterior vitreous cortex splits (vitreoschisis), there can be different effects depending upon the level (plane) of the split. Vitreoschisis more anteriorly leaves a relatively thick, cellular membrane adherent to the macula with embedded hyalocytes which recruit circulatiung monocytes and retinal glial cells. If there is also separation from the optic disc (found in 90% of macular pucker cases), inward (centripetal) contraction of this premacular membrane induces macular pucker. In the periphery, vitreoschisis in eyes with retinal detachment can result in vitreous cortex remnants (VCRs, as named by van Overdam[56,57]) with hyalocytes that promote proliferative vitreo-retinopathy (PVR) and recurrent retinal detachment[111]. If the vitreoschisis split occurs more posteriorly, the remaining premacular membrane is relatively thin and hypocellular. Persistent vitreopapillary adhesion (VPA, present in 87.5% or more of cases of macular holes) influences the vector of force in the tangential plane, resulting in outward (centrifugal) tangential traction (especially nasally), opening a central dehiscnece which is recognized clinically as a macular hole. Reproduced with permission from [10], © 2014 Springer Science+Business Media New York.
Splitting between the lamellae of the posterior vitreous cortex, known as vitreoschisis[10,11] (Fig 2), plays a critical role in the pathophysiology of hypercellular proliferative vitreo-retinopathies[12,13]. This is because persistent attachment of the outer lamellae of the posterior vitreous cortex to the retina can contain many hyalocytes. These cells are no longer modulated, regulated, or tempered by overlying vitreous (which is known to possess anti-migratory and anti-proliferative properties[4,5]) since it is displaced anteriorly following vitreous separation.
Figure 2: Vitreoschisis.
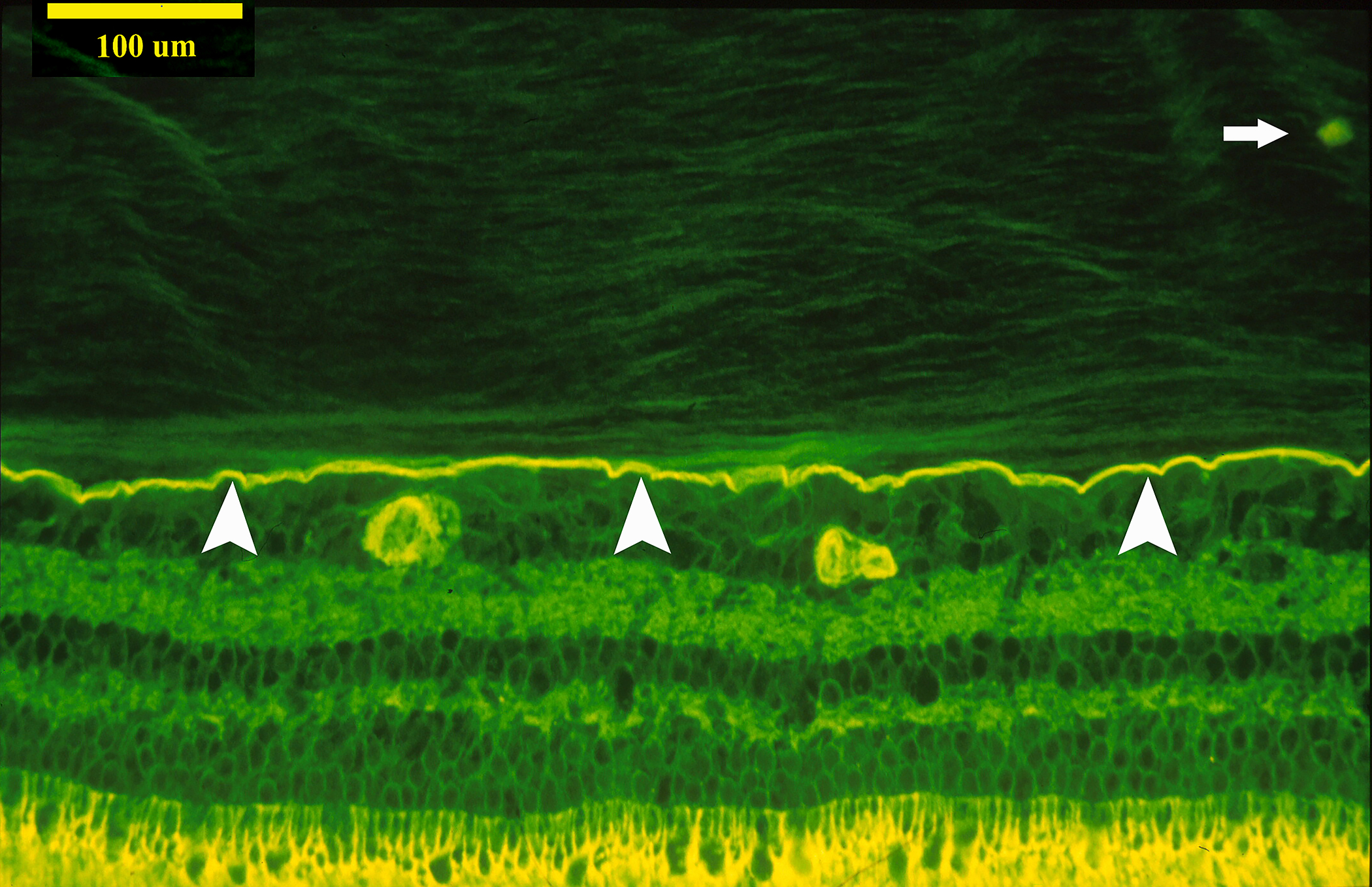
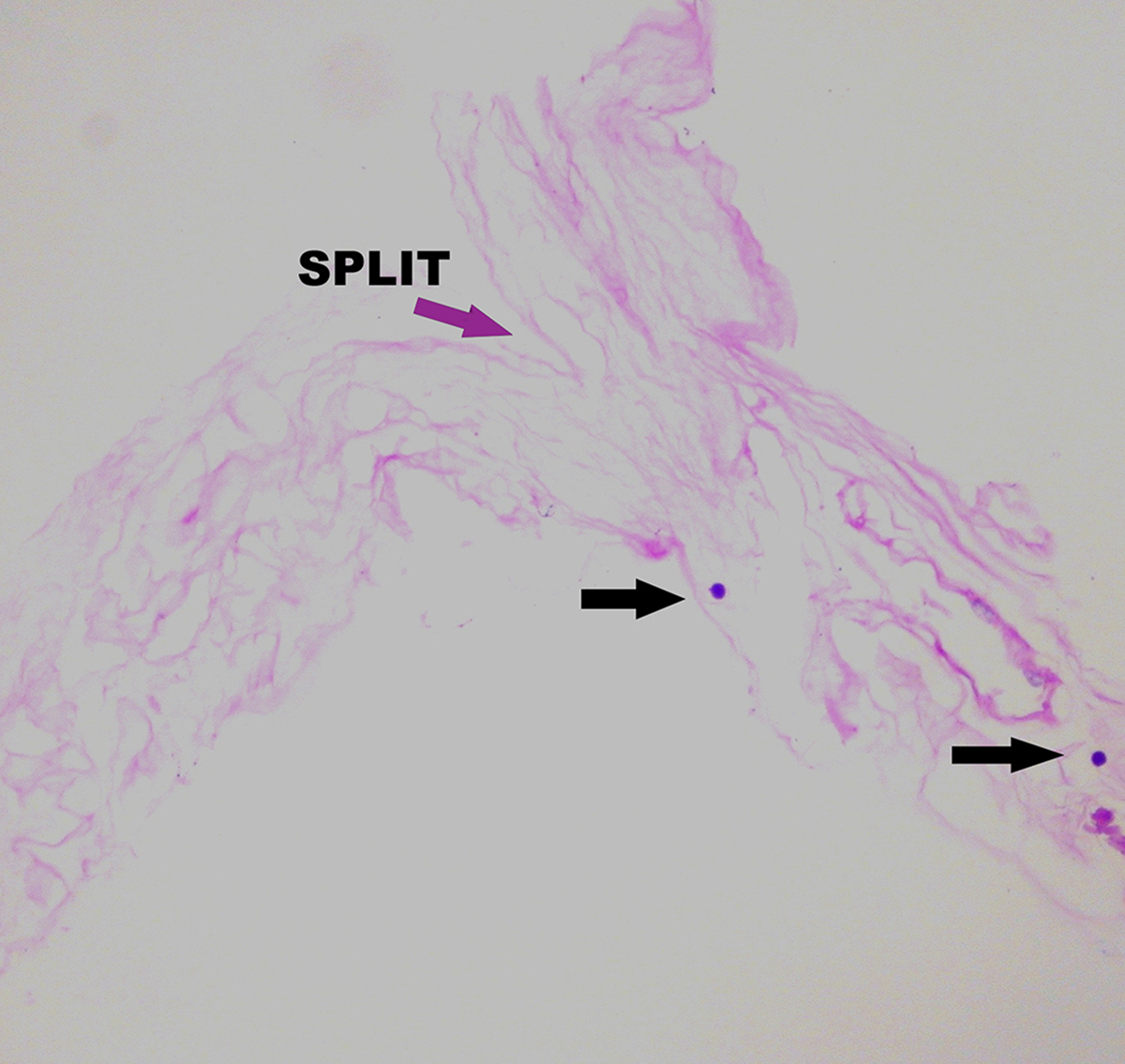
Left: The lamellar structure of the mammalian posterior vitreous cortex is demonstrated in an adult monkey eye stained with fluorescein-conjugated ABA lectin stain. The retina (below) and vitreous (above) are separated by the ILM (white arrowheads), above which is the posterior vitreous cortex whose lamellar structure is clearly evident. These potential cleavage planes can split apart during anomalous PVD or during vitrectomy surgery with membrane peeling, in each instance leaving a layer of vitreous attached to the macula. A hyalocyte (white arrow) is embedded in the posterior vitreous cortex (bar = 100 μM) Image courtesy of G Hageman. Middle: Spectral domain OCT/SLO imaging of macular pucker demonstrating splitting of the posterior vitreous cortex (left side of scan), known as “vitreoschisis”. Reproduced with permission from [13], © 2011 BMJ Publishing Group Ltd. Right: The eye shown in the middle panel underwent vitrectomy surgery with membrane peeling and the specimen was examined histologically. The area designated as “split’ is the site where the posterior vitreous cortex splits into the vitreoschisis cavity seen on OCT (middle panel). The arrows indicate hyalocytes embedded in the posterior vitreous cortex (Periodic Acid Schiff stain, magnification = 225x). Image courtesy of N Rao, Doheny Eye Institute.
This alteration of normal vitreo-retinal homeostasis begins a cascade of events initiated by hyalocyte recruitment of cells from the circulation (monocytes) as well as cells from the local environment, such as retinal glial cells, retinal pigment epithelial cells (when there is a retinal break), and vascular endothelial cells (in the case of neovascularization). The following will explore how anomalous PVD and vitreoschisis influence the particular features of hyalocyte engagement in the pathophysiology of specific proliferative vitreo-retinal disorders.
3. Hypercellular tractional vitreo-retinopathies
Hyalocytes are an important cell type in hypercellular, tractional premacular membranes, contributing mightily to pathogenesis in macular pucker (MPK), proliferative vitreo-retinopathy (PVR), and proliferative diabetic vitreo-retinopathy (PDVR). In contrast, non-tractional premacular proliferation consists mostly of glial cells[5,14].
3.1. Macular pucker
First described in 1865 by Iwanoff[15], macular pucker (MPK) is defined as a premacular, avascular, fibrocellular membrane with folds and striae in the underlying inner retina, accompanied by disturbed cytoarchitecture in the outer retina[16,17]. The term “epiretinal membrane (ERM)” is not as accurate as the term “premacular membrane (PMM)”, which is the tissue that causes macular pucker, the term that correctly refers to the effects of this membrane on the macula. Although the term “idiopathic” is common in the literature, it is no longer appropriate because MPK is now known to be caused by vitreous pathology. The prevalence of MPK is between 6% and 11.8%[18–20]. Over the age of 70 years the prevalence is as high as 15.1%[20], with bilateral involvement between 19.5%[18] and 31%[19] of cases. Chinese may have a higher prevalence than Caucasians, African Americans, or Hispanics[21]. The most common patient complaints are metamorphopsia and blurring, although micropsia and monocular diplopia have also been reported[22].
Early theories on the pathogenesis of MPK focused on the retina and largely ignored the role of vitreous[23]. The two contemporary theories on etiology are breaks in the inner limiting membrane (ILM) with consequent glial cell migration, and anomalous posterior vitreous detachment (PVD) (Fig 1)[8] with vitreoschisis[13]. (Fig 2). The latter theory highlights the role of hyalocytes. Common to both theories is the role of PVD, which is found in 80–95% of cases[24–29]. Admittedly, the methods used to diagnose PVD in many of these studies may be suspect, making it possible that many cases diagnosed with total PVD are incomplete[13]. However, this prevalence is significantly higher than the 53% prevalence of PVD in the general population over age 50[30,31]. Furthermore, it is probable that PVD in cases of MPK is anomalous with vitreoschisis.
The retinal break/glial cell theory postulates that microbreaks in the ILM resulting from PVD create pores through which glial cells migrate to proliferate along the retinal surface. This theory is supported by the fact that the cells in MPK membranes resemble glial cells in both morphology[32] and immunofluorescence[33]. Evidence against this theory is that retinal breaks have not been documented on histologic examination. The hyalocyte theory more directly implicates vitreous, as it involves anomalous PVD[8] with vitreoschisis[11,12] (Fig 2). Spectral domain OCT found vitreoschisis in 42% of MPK eyes[13], although the true prevalence is likely higher as future studies employing swept source OCT will likely demonstrate. Indeed, at surgery, 80% of MPK eyes have evidence of vitreoschisis[34]. The level (plane) through which vitreoschisis occurs influences pathology[13]. If vitreoschisis splits the posterior vitreous cortex farther anteriorly, more hyalocytes will remain adherent to the macula in the premacular membrane and begin the process of MPK by recruiting monocytes from the circulation and glial cells from the retina. This could be the pathophysiology underlying lamellar hole associated preretinal proliferation (LHEP)[35]. Further, it has been shown that hyalocytes embedded within this vitreous layer are found within the premacular membranes excised from patients with MPK (Fig 3)[3,23,36]. Lastly, hyalocytes transdifferentiate into myofibroblasts[37,38] causing tangential membrane contraction exerting inward (centripetal) tangential force on the retina. It is this action that causes the irregular (corrugated) retinal contour typical of MPK.
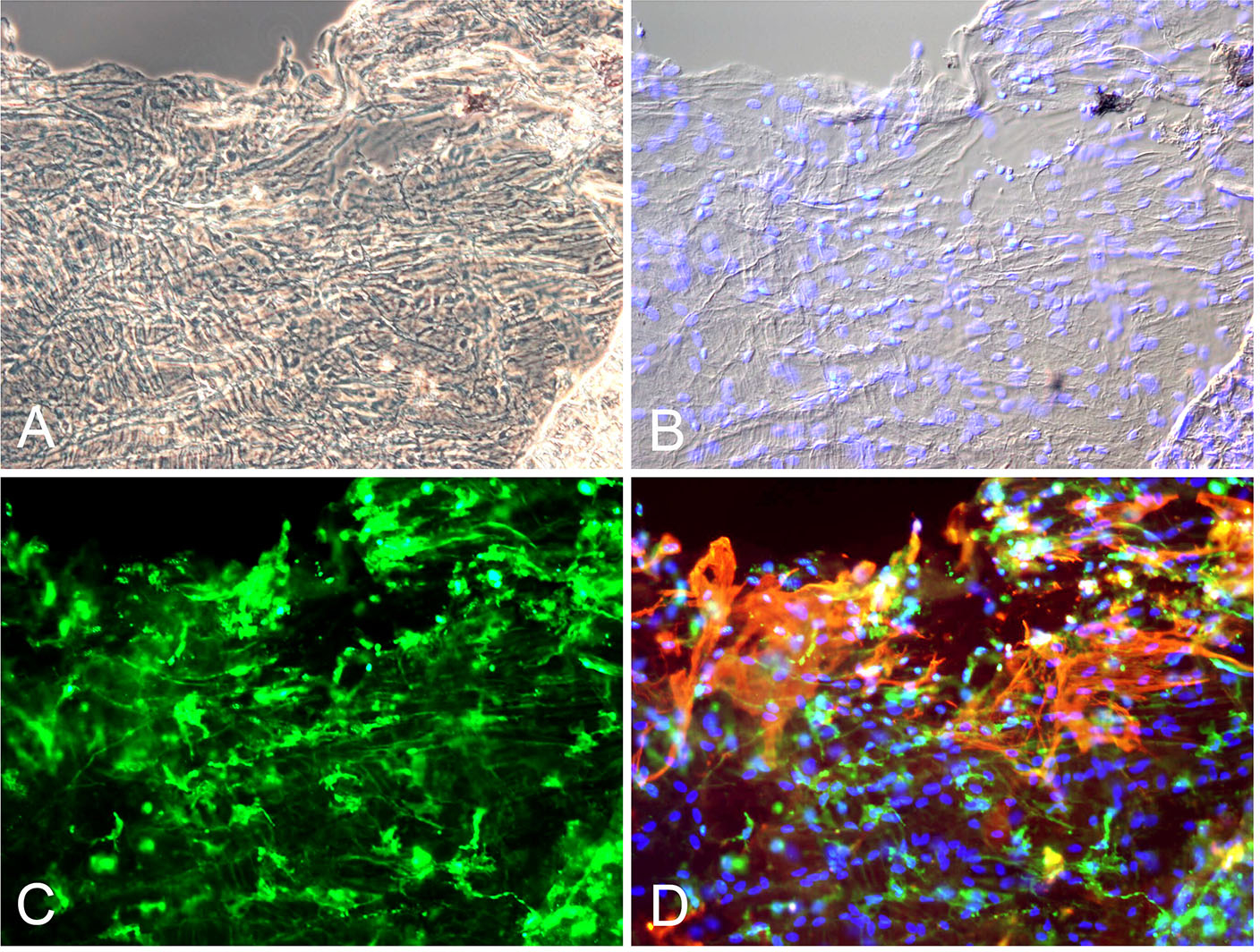
Figure 3: Transmission electron microscopy of hyalocytes in macular pucker.
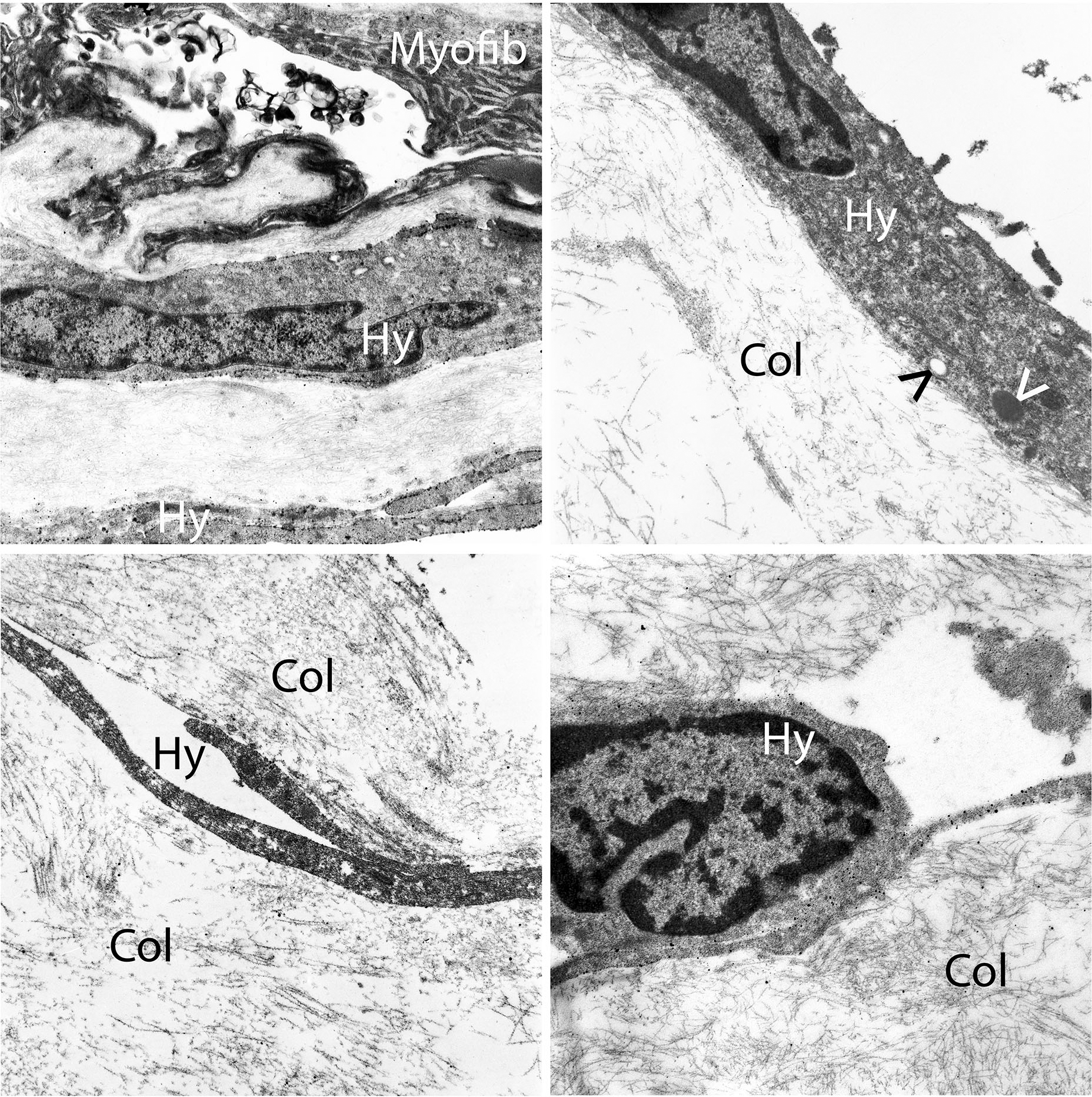
Multilayers of vitreous collagen (col) with hyalocytes (Hy) and myofibroblasts (My) are evident in this fibrocellular membrane. Hyalocytes possess an oval nucleus with marginal chromatin, vacuoles, dense granules, and thin cytoplasmic protrusions. Myofibroblasts are distinguished by cytoplasmic aggregates of actin microfilaments forming stress bundles. Image courtesy of author R Schumann.
Vogt and colleagues[36] found that the cellular composition of premacular membranes in MPK included glial cells, myofibroblasts, and fibroblasts, implicating the possibility of transdifferentiation of hyalocytes into myofibroblasts[36]. Indeed, Kohno and colleagues[39] found that exposing cultured hyalocytes to TGF-β2 induced contraction, and cells were positive for αSMA and negative for GFAP, indicating a conversion into myofibroblasts; astrocytes did not exhibit such changes. Additionally, immunofluorescence of surgically removed premacular membranes showed GFAP stained cells in non-contracted areas, and αSMA positive cells in areas of contraction. The investigators hypothesized that TGF-β2 induces hyalocyte transdifferentiation into myofibroblasts[39]. TGF-β2 also induces hyalocytes to secrete connective tissue growth factor (CTGF), which was found in higher concentrations in vitreous from eyes with proliferative vitreo-retinal disorders[40]. Platelet derived growth factor (PDGF) has also been implicated as working with TGF-β2 to phosphorylate myosin light chains (important in contraction of both muscle fibers and non-muscle cells) via Rho-Kinase[41]. Thus, Rho Kinase inhibitors could alter and possibly prevent hyalocyte-mediated membrane contraction in MPK[37]. This concept is supported by the findings of Vogt et al. who studied premacular membranes excised from humans with MPK, finding that hyalocytes, macroglia, and microglia comprised the main cell composition with trans-differentiation of cells, suggesting therapeutic value in anti-fibrosis treatment strategies (see below)[36]. Currently, however, vitrectomy with membrane peeling is the cure[42]. Pre-operative vision depends upon whether there are multiple centers of retinal contraction[26] (Fig 4), the presence of macular edema[43], alterations in the photoreceptor layer[44–46], and integrity of the inner segment/outer segment junction[44,45,47,48].
Figure 4: Coronal plane (en face) imaging of macular pucker.
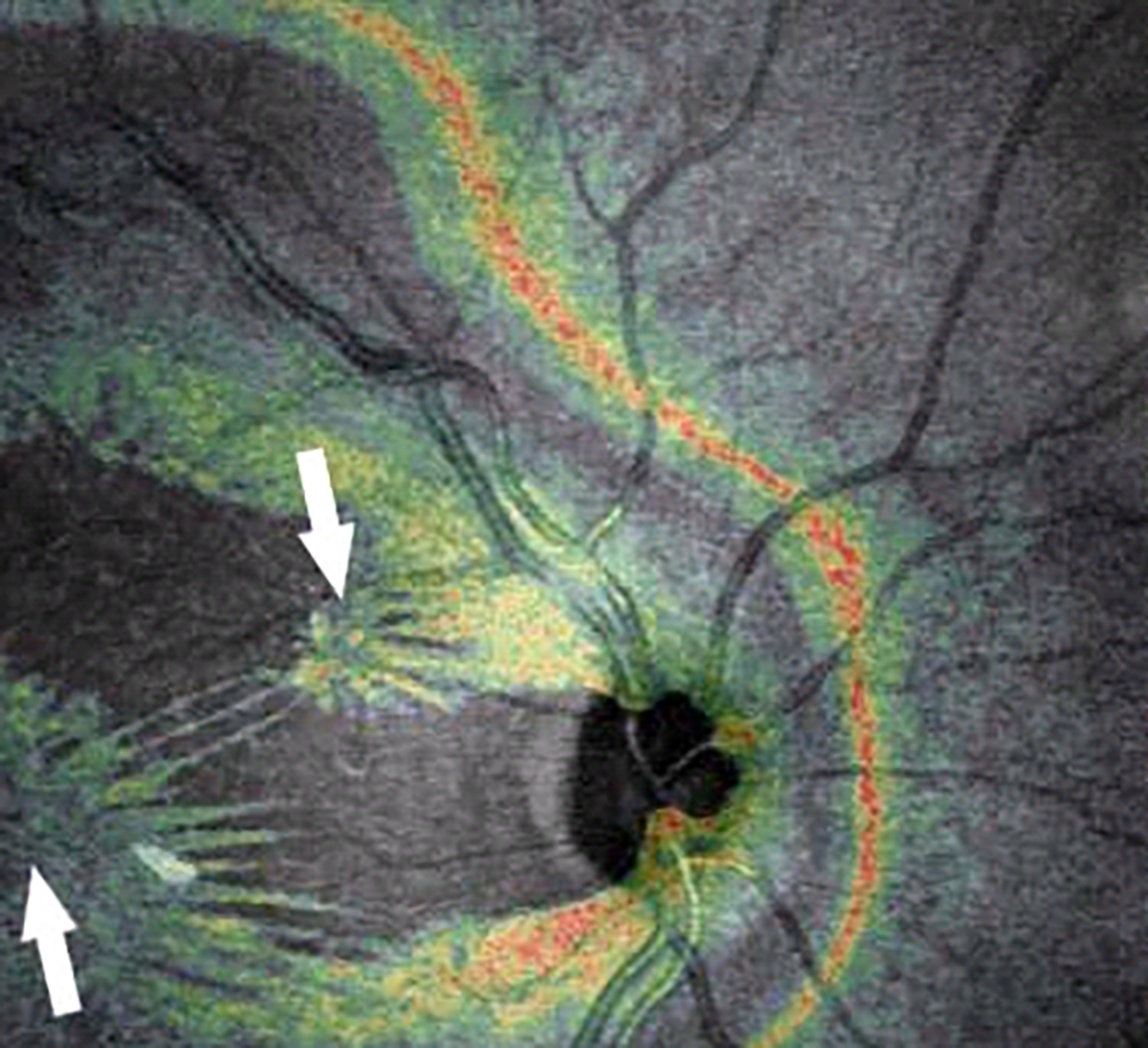
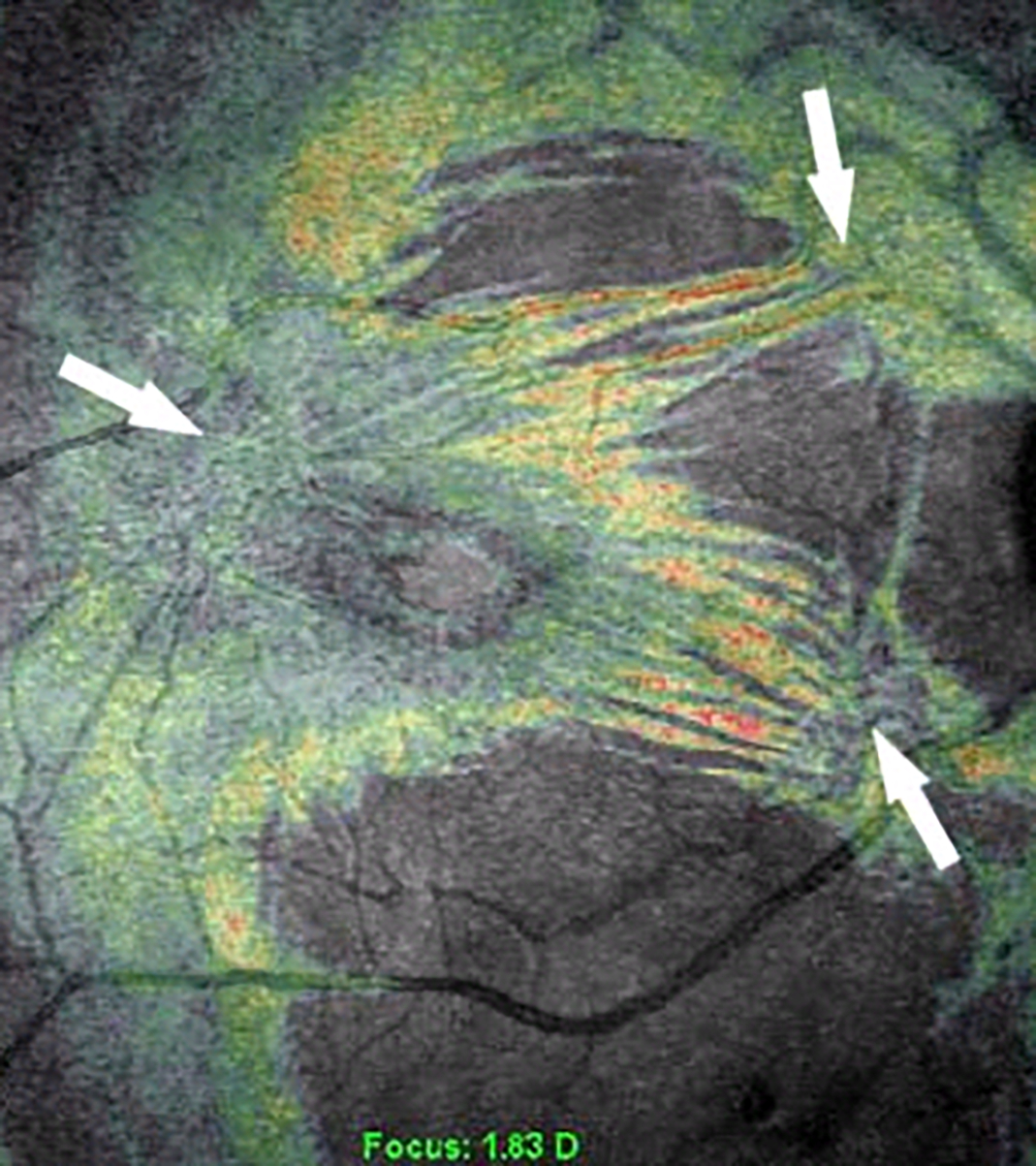
Combined OCT-SLO imaging with superimposition of coronal plane OCT (color) onto SLO (grayscale) images demonstrate macular pucker with multiple centers of retinal contraction (arrows): bi-centric (left), compared to three centers of retinal contraction (right). Eyes with 3 or 4 centers of retinal contraction were found to have a higher prevalence of intraretinal cysts and a thicker macula than eyes with 1 or 2 centers of retinal contraction. Reproduced with permission from [26], © 2008 The Ophthalmic Communications Society, Inc.
3.2. Proliferative vitreo-retinopathy
Proliferative vitreo-retinopathy (PVR) is a fibroproliferative disorder characterized by the formation of preretinal contractile, fibrocellular membranes which can lead to retinal break re-opening/formation and recurrent retinal detachment. Subretinal membranes can also develop, as well as intraretinal gliosis, although the cell types in these membranes may differ, and rather than induce tangential traction, these membranes induce retinal stiffness and shortening. PVR can occur after primary rhegmatogenous retinal detachment (RRD), surgical intervention, or trauma. The development of PVR in the peripheral fundus is the primary cause for surgical failure after RRD repair, which occurs in 10–15% of cases[49]. Similar pathogenic mechanisms result in proliferative membranes on the posterior pole causing MPK[50–52]. Although PVR and MPK have similarities in cell distribution and immunoreactivity, there are differences in cell composition[53], with hyalocytes predominating in premacular membranes causing MPK in one study[14]. Another study using imaging mass cytometry showed that surgically excised preretinal PVR membranes consist of numerous IBA1-positive myeloid cells (hyalocytes and/or microglia) that simultaneously co-express α-SMA, strongly suggesting transdifferentiation of myeloid cells into myofibroblasts as a common pathophysiological feature during PVR formation[54]. All studies emphasize the importance of transdifferentiation by hyalocytes into myofibroblasts with strong contractile properties.
PVR is classically characterized in three overlapping stages: inflammation, cell proliferation, and extracellular matrix (ECM) remodeling. PVR is associated with breakdown of the blood-retinal barrier which facilitates the entry of inflammatory growth factors and cytokines. Inflammation is known to play a crucial role in fibrosis, and multiple profibrotic and inflammatory mediators have been identified in vitreous[14]. The early stages are associated with significant hypercellularity in part due the aforementioned anterior level of vitreoschisis splitting the vitreous cortex. The major cell types are myeloid cells, most likely hyalocytes but also retinal microglia, retinal pigment epithelial (RPE) cells, and retinal astroglia. Hyalocytes play an important role in the initiation and development of PVR, as they can modulate immune and inflammatory processes, initiate intraretinal gliosis, and transdifferentiate into myofibroblasts resulting in tractional fibrocellular membranes[14,37]. Other myeloid cells such as retinal microglia could play an important role later in the pathophysiology of PVR. The transition into a final fibrotic stage is associated with membrane maturation (fibroblast activity?) and tangential contraction.
Several studies and many years of surgical experience have emphasized the essential role of vitreous in the development of PVR. The posterior vitreous cortex (PVC) has been shown to provide a scaffold for fibrocellular proliferation and is an active player in the ECM remodeling. The PVC contains sufficient profibrotic and inflammatory mediators, as well as cells (hyalocytes) to induce ECM contraction[37]. Further, the lamellar structure of the PVC (Fig 2, left) predisposes to vitreoschisis (VS), an important initiating event in the pathogenesis of PVR and other vitreo-retinopathies[8,9], since after anomalous PVD the outermost lamellae of the PVC can remain attached to the retinal surface posterior to the vitreous base[8,9,11]. These VS-induced vitreous cortex remnants (VCR) are attached to the peripheral (and macular) retinal surface acting as a scaffold for PVR to develop. Hyalocytes are likely to be present in VCR if VS splits the PVC anteriorly. These cells are critical since hyalocytes recruit circulating monocytes, secrete profibrotic cytokines inducing the synthesis of ECM, and promote myofibroblast differentiation which, in turn, also induces synthesis and contraction of the ECM[37,55].
Indeed, histopathologic analyses of PVR membranes suggest that PVR develops in the presence of VCR, as both native collagen and newly formed ECM have been detected[53]. In addition, different areas within PVR membranes have different cell and extracellular matrix characteristics, which could represent different stages of PVR formation. These paucicellular, collagen-rich areas with hyalocytes (Fig 5) most likely represent vitreous cortex remnants (VCR) that resulted from vitreoschisis (VS; Fig 2)[56]. A prevalence of VCR on the peripheral retinal surface of around 35% has been reported in patients undergoing vitrectomy for RRD[57,58]. Furthermore, VCR on the peripheral retina has been linked to the development of PVR after surgery for RRD[57–60]. Cases of primary RRD with VCR had an incidence of re-detachment of 12%, compared to 2% in cases that did not have VS & VCR[57]. Interestingly, VCR on the macula has been reported in 15–41% of patients undergoing surgery for a primary RRD, and may be associated with the development of premacular membrane proliferation and contraction causing macular pucker[61] (see above), especially in diabetes and highly myopic eyes[13,34,50,52,53]. Additional factors that influence growth and development of these pathologic membranes are cytokines translated by TGFB1and FGF2 genes which are activated by macrophages and mTOR stimulation by cytokine secretion predominantly in the early stages of PVR[62].
Figure 5: Proliferative vitreo-retinopathy (PVR).
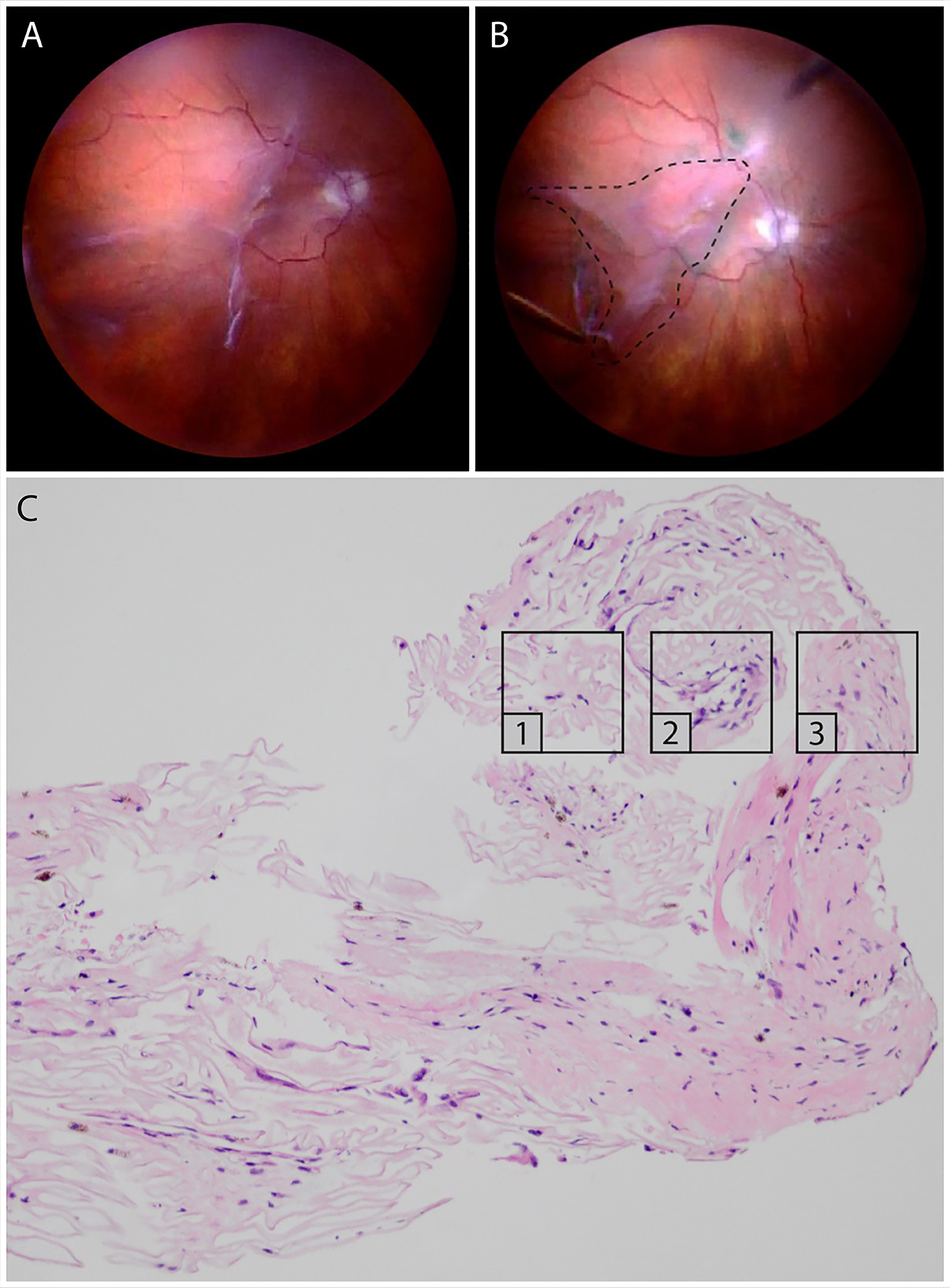
Intraoperative imaging before (A) and after (B) peeling of a PVR membrane, extending from the superior arcade to the inferotemporal periphery. The dashed line indicates the area from where the membrane was excised. (C) Light microscopy of the PVR membrane stained with hematoxylin and eosin. Different continuous membrane areas can be distinguished, representing different stages of PVR: paucicellular, lamellar collagen-rich areas with hyalocytes, suggestive for VCR (1); areas with increased cellular infiltration by RPE and glial cells (2); more fibrotic areas with low cellularity and myofibroblasts (3). Images courtesy of author K van Overdam.
3.3. Proliferative diabetic vitreo-retinopathy
Diabetic retinopathy is the leading cause of severe vision loss in working-age adults[63] and, given the increasing prevalence of diabetes, represents a significant medical and socio-economic challenge. The advanced stage of the disease, proliferative diabetic vitreo-retinopathy (PDVR), is characterized by poorly perfused retina causing a hypoxia-induced release of pro-angiogenic growth factors eventually leading to the formation of retinal neovascularization (RNV) and/or neovascularization of the optic disc[64]. Vascular endothelial growth factor (VEGF) is a pro-angiogenic factor inhibited effectively via intravitreal injections of inhibitors in routine clinical practice for the treatment of diabetic macular edema and PDVR[65]. However, the involvement of other mechanisms in the pathophysiology of PDVR is likely, since some patients have only a moderate or poor response to anti-VEGF therapy[66], resulting in contractile vascular membranes at the vitreo-retinal and vitreo-papillary interface[67]. It appears that vitreous is critical in this process, hence the term PDVR[68]. In similar fashion to PVR (see above), vitreous plays an important role in advanced DR, as evidenced by the facts that florid neovascularization is muted in an eye with posterior vitreous detachment (PVD)[69] and that neovascularization generally does not recur following vitrectomy[70]. Indeed, it has been suggested that the mechanism by which panretinal laser photocoagulation works to mitigate neovascularization is by inducing PVD[71,72]. Within the scaffold of collagen fibrils in the posterior vitreous cortex reside hyalocytes, which appear to play as important a role in PDVR, just as they do in other proliferative vitreo-retinopathies[41]. The following presents experimental as well as clinical evidence of the role of hyalocytes in PDVR.
3.3.1. Experimental evidence of hyalocyte involvement in PDVR
In the “oxygen-induced-retinopathy” mouse model, myeloid cells, (retinal microglia and vitreous hyalocytes) accumulate at sites of retinal ischemia and neovascularization[73] and may influence the formation of pathological ocular neovascularization[74]. Similarly, studies in the Ins2Akita mouse model revealed an increased number of myeloid cells, alterations in their network organization, and evidence of cell shape changes regarded as classical signs of activation. In older diabetic mice with myeloid cells deficient of Cx3cr1, these changes were exacerbated and an accumulation of Iba-1+ hyalocytes was observed[75]. Furthermore, in vitro experiments on cultured bovine hyalocytes suggest that hyalocytes are involved in the formation of fibrous contractile membranes in vitreo-retinal disease[76], probably in a TGF-β1-dependent manner[41], and that VEGF expression by hyalocytes is enhanced under hypoxic conditions[77]. Human studies have further demonstrated that hypoxia in the diabetic vitreous stimulates the expression of angiogenic and inflammatory cytokines[78].
3.3.2. Human studies
Development and progression of PDVR relate to the formation of preretinal fibrocellular membranes[79–83]. Pathologic fibrocellular changes at the vitreo-macular interface are present in all diabetic eyes irrespective of the stage of DR[82,84]. Clinico-pathologic studies found multi-layered tractional membranes situated on the vitread side of the ILM with cells embedded in masses of native vitreous cortex collagen[79,80,82,84]. According to Gandorfer and colleagues, two types of membranes exist: membranes with cellular components directly adjacent to the ILM, and membranes with vitreous collagen strands interposed between ILM and cells[80]. As demonstrated in Figure 6, the cellular composition typically consists of myeloid cells, most likely hyalocytes, and myofibroblasts. Glial cells were also present, but without predominance in eyes with PDVR. Hyalocytes in these pathologic eyes can be directly situated on the retinal surface (first type alluded to above), or within the vitreous collagen fibrils that are anterior to the ILM. Studies have found these cells to be immunopositive for CD45, CD64 and IBA1[38,85,86]. Due to their role in extracellular matrix synthesis and regulation of intraocular inflammation, hyalocytes can change their phenotype and cell function. Similar to PVR, hyalocytes are able to transdifferentiate into myofibroblast-like cells. Although myofibroblasts can transdifferentiate from numerous cell types, in diabetic eyes they are believed to predominantly originate from hyalocytes.
Figure 6: Histopathology of proliferative diabetic vitreo-retinopathy (PDVR).
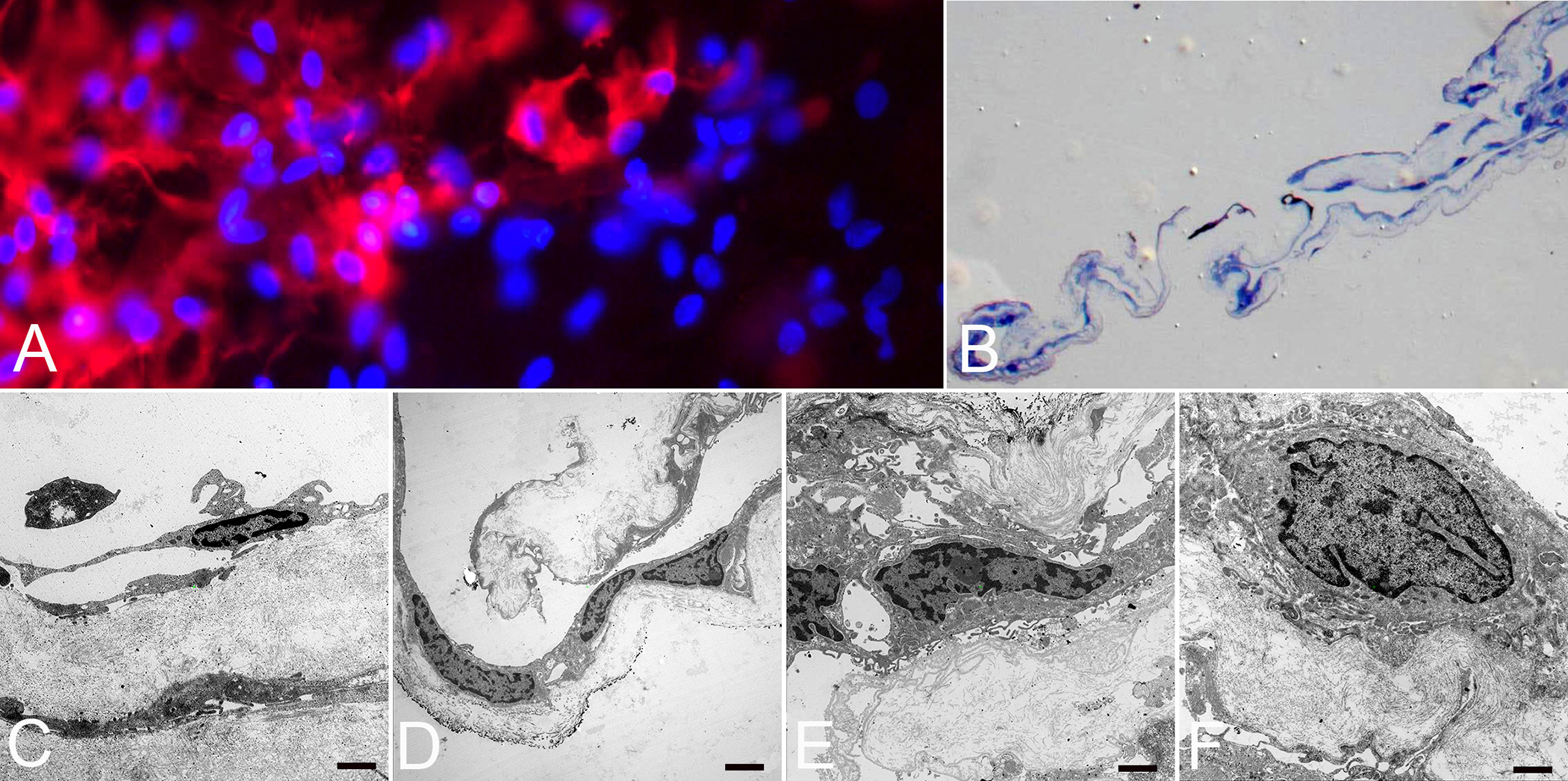
Immunofluorescence microscopy (A), light (B) and transmission electron microscopy (C-F) of a fibrocellular, premacular membrane surgically removed from an eye with PDVR. A) Flat-mounted membrane with anti-CD45 (red) positive staining merged with cell nuclei staining (blue) indicating the presence of hyalocytes (original magnification x400). B) Semithin section of membrane demonstrated folded fibrocellular composition with thick collagen strands (original magnification x100). C) Ultrastructural analysis revealed hyalocytes with elongated cell bodies and thin cell processes in abundance of native vitreous collagen (original magnification x7000, bar = 1000 nm). D) Hyalocytes and myofibroblasts situated on layers of vitreous collagen strands (original magnification x3000, bar = 2000 nm). E) Myofibroblasts surrounded by newly formed collagen (original magnification x7000, bar = 1000 nm). F) Fibroblast with large cell nucleus and newly formed collagen (original magnification x7000, bar = 1000 nm). Images courtesy of author R Schumann.
Myofibroblasts are the contractile components of fibrocellular membranes. Their contractile properties and their ability to produce newly formed collagen have been demonstrated in numerous studies of the diabetic vitreo-retinal interface[68,87]. In particular, ultrastructural analyses of the co-localization α-SMA filaments and collagen type I and III proved the presence of hyalocytes in fibrocellular membranes of diabetic eyes[84]. Hyalocytes and myofibroblasts are usually embedded in thick layers of native vitreous collagen. In addition, newly-formed collagen and fibrous long-spacing collagen were seen in DR representing a remodelling process of vitreous cortex collagen[79,84,88]. Pathologic changes at the vitreo-macular interface were present in all eyes irrespective of the presence of tractional fibrocellular membranes.
A recent study by Boneva and colleagues showed that retinal neovascular complexes contained α-SMA and IBA-1 positive myeloid cells, which are most likely hyalocytes given their proximity to preretinal PDVR membranes[89]. Their studies found an abundance of α-SMA -positive myofibroblasts, HLA-DR (human leukocyte antigen – DR isotype)-positive antigen-presenting immune cells, PECAM-1 (platelet endothelial cell adhesion molecule)-positive endothelial cells, and CD8a (cluster of differentiation 8a)-positive cytotoxic lymphocytes in RNV tissue, compared to a fainter staining for these markers in premacular membranes excised from eyes with MPK (Fig. 7). Further in vitro analyses once again identified that myeloid cells, most probably hyalocytes, had the potential for myofibroblastic transdifferentiation[89]. Finally, TGF-β was significantly upregulated in human RNV when compared to control tissue[89]. This cytokine has been shown to induce α-SMA (a classic myofibroblast marker) expression in cultured myeloid cells[90] and stimulate the contraction of hyalocytes in vitro[91]. In summary, these data suggest that TGF-β-mediated myofibroblastic transdifferentiation of hyalocytes is a key event in the course of contractile fibrovascular membranes formation in advanced PDVR.
Figure 7: Imaging mass cytometry of preretinal diabetic retinal neovascularization membranes compared to premacular membranes in macular pucker (non-diabetic).
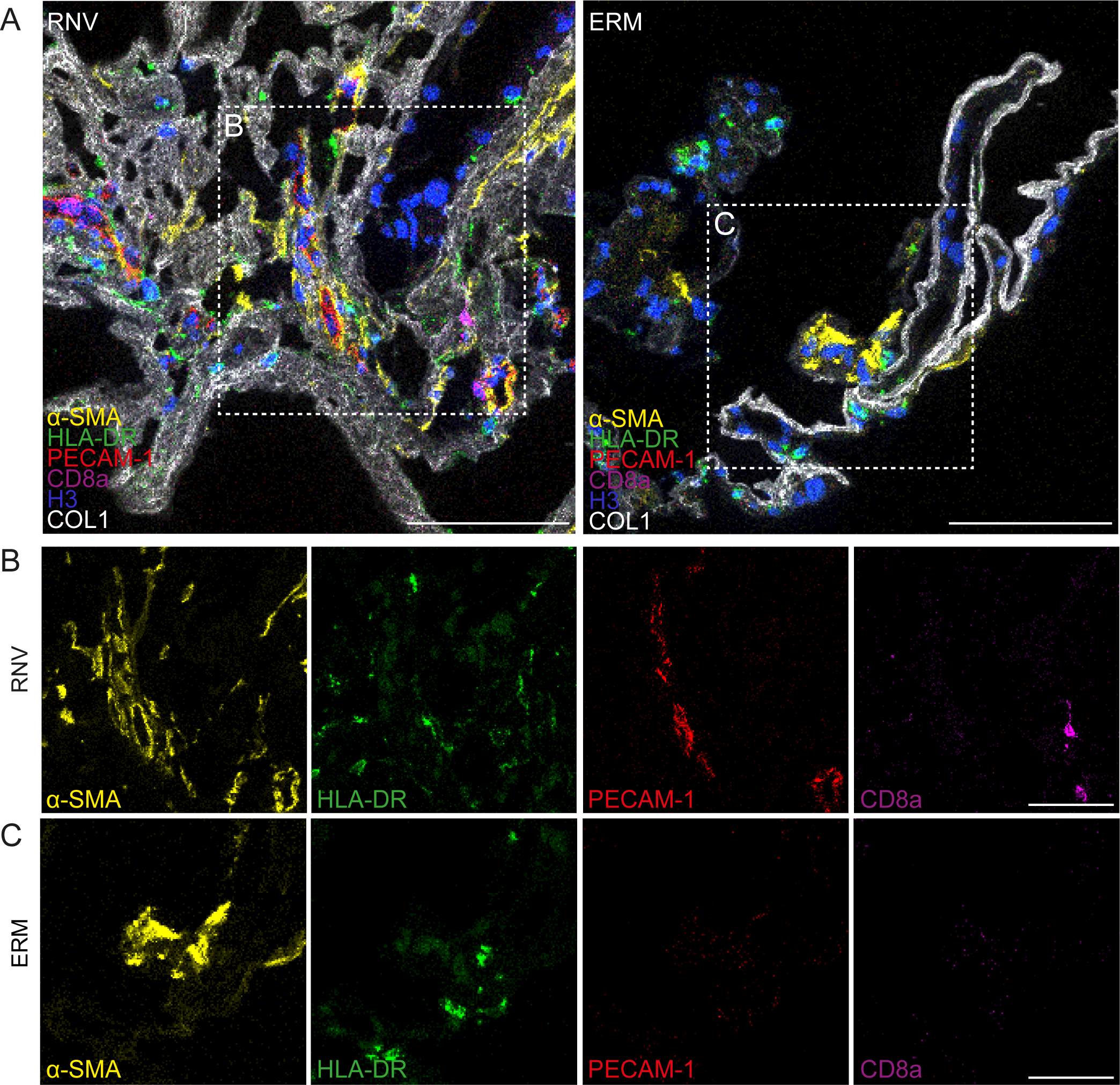
Imaging mass cytometry of human retinal neovascularization (“RNV”) and macular pucker (“ERM”) tissue samples from humans. Multiplexed stainings for α-SMA (α-smooth muscle actin, yellow), HLA-DR (human leukocyte antigen – DR isotype, green), PECAM-1 (platelet endothelial cell adhesion molecule, red), CD8a (cluster of differentiation 8a, magenta), Histone H3 (blue) and COL1 (collagen type I, white) are presented. Higher magnification of the sections within the dashed white squares are shown in the panels in B and C, respectively. Scale bars correspond to 100 μm (A) and 50 μm (B and C). Reproduced from [89], licensed under CC-BY4.0 (http://creativecommons.org/licenses/by/4.0/)
3.3.3. In vivo hyalocyte imaging in PDVR
Hyalocyte clustering and altered cell morphology have been detected in patients with PDVR using clinical OCT[92,93]. Clusters of hyalocytes with plumper appearance in a patient with PDVR are shown in Fig. 8 and Movie 1. In this patient, hyalocyte proliferation around the sites of neovascularization was observed (Fig. 8). High resolution Adaptive Optics Scanning Light Ophthalmoscopy (AOSLO) imaging of hyalocyte morphology and movement dynamics in the living human eye without exogenous labeling has provided better understanding of the roles these cells play in diabetic retinopathy[94]. Fig. 9 shows a comparison of hyalocytes imaged in a healthy control and a patient with PDVR. In the healthy control, hyalocytes appear more ramified with multiple fine and long processes, while these cells look less ramified and more amoeboid shaped in the diabetic patient. A better understanding of these changes in cell morphology and behavior may provide more insight into their roles in disease progression and response to therapy by pharmacologic and non-pharmacologic means.
Figure 8: Imaging human hyalocytes in vivo.
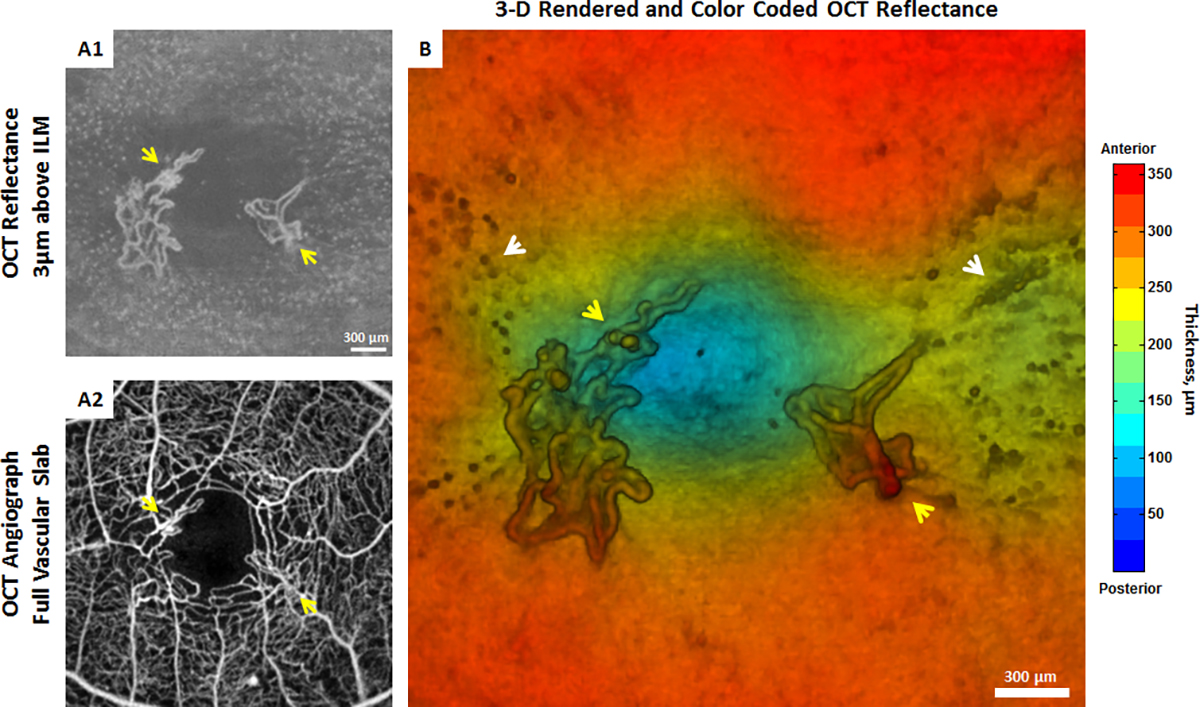
In vivo imaging of hyalocytes in a 61-year-old patient with proliferative diabetic vitreo-retinopathy using clinical OCT. A1) 3μm OCT reflectance slab located above the ILM centered at the fovea. Clustering of hyalocytes near the sites of neovascularization is observed. A2) Corresponding OCTA image shows the foveal full vascular layer. B) 3-D rendered and color-coded OCT reflectance image. Yellow arrows indicate neovascularization near the margin of the foveal avascular zone. White arrows indicate clustering of hyalocytes near neovascularization. (See also Movie 1 for a 3-D rendered and color-coded OCT reflectance movie). Images courtesy of authors TYP Chui and RB Rosen.
Figure 9: Human hyalocytes in vivo.
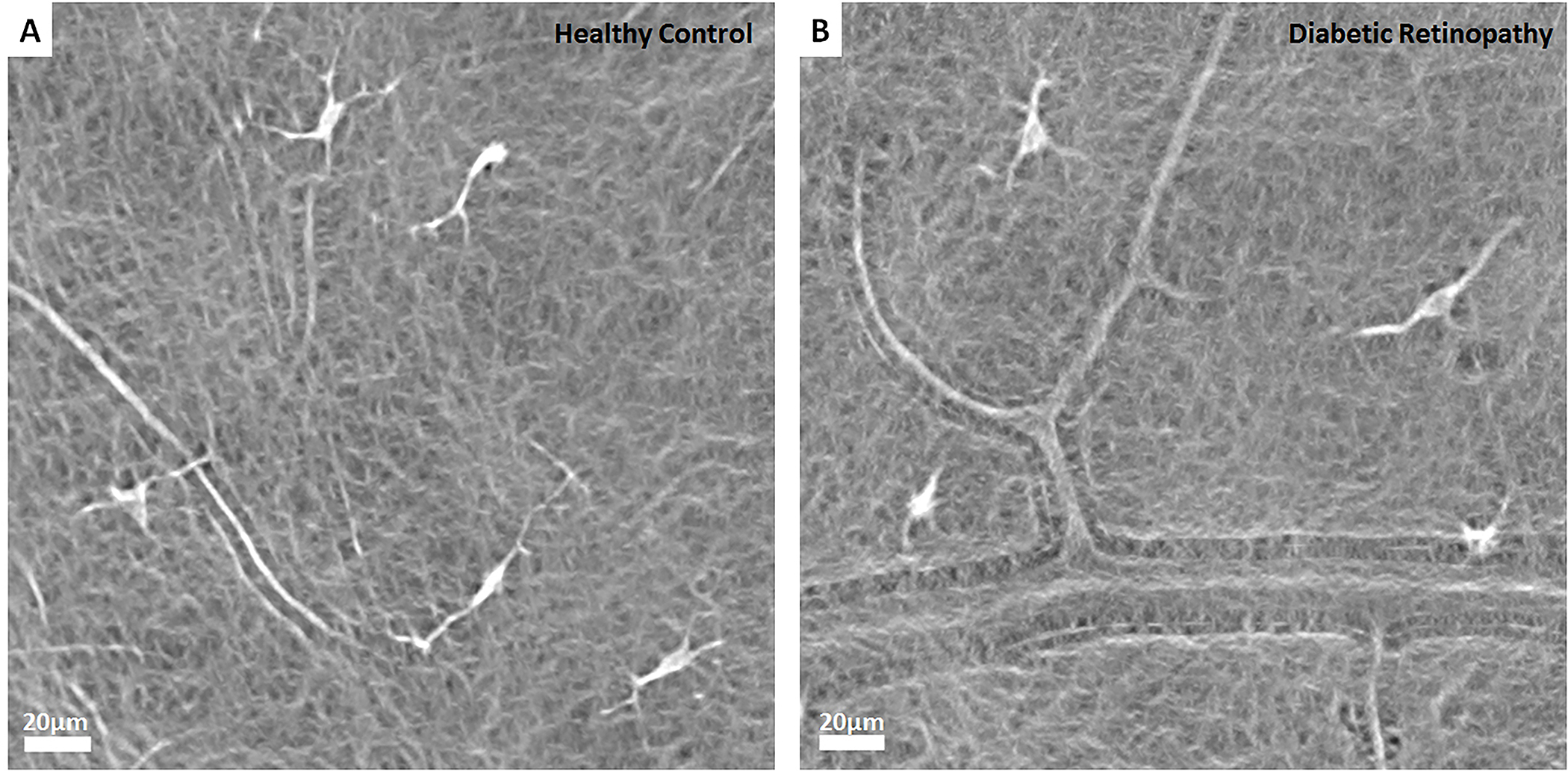
Comparison of hyalocytes imaged using non-confocal quadrant detection Adaptive Optics Scanning Light Ophthalmoscopy in a 32-year-old healthy control (A) and a 26-year-old patient with proliferative diabetic vitreo-retinopathy (B). Hyalocytes in the healthy control appear more ramified with multiple fine and long processes. In contrast, hyalocytes in the diabetic patient look relatively less ramified (top two cells) and more amoeboid shaped (bottom two cells). Images courtesy of authors TYP Chui and RB Rosen.
4. Pauci-cellular tractional vitreo-maculopathies
Following anomalous PVD, persistent full-thickness vitreo-macular adhesion, vitreoschisis, and fibrocellular membranes cause a variety of tractional vitreo-maculopathies. In contrast to the foregoing which described pathologies arising from pathologic hypercellular membranes, there are paucicellular vitreo-retinopathies that result in a heterogeneous group of macular pathologies. These include vitreo-macular traction syndrome, macular holes, and myopic foveoschisis[8,11,12,95,96].
In vitreo-macular traction (VMTS), the aforementioned age-related fibrous liquefaction/degeneration of the vitreous body with persistent adhesion of the posterior vitreous cortex to the ILM of the retina does not induce vitreoschisis, but instead results in full-thickness posterior vitreous cortex traction upon the ILM in an axial direction (Fig 10). In VMTS the vitreo-retinal adhesion site co-localizes with cell cluster formation on the ILM[38,85,97]. The presence of cell clusters thus represents the initial stage of pathologic membrane formation, even when paucicellular. Indeed, the degree (size and strength) of persistent vitreo-retinal adhesion at the ILM seems to affect the diversity of vitreo-macular traction pathologies[11,96].
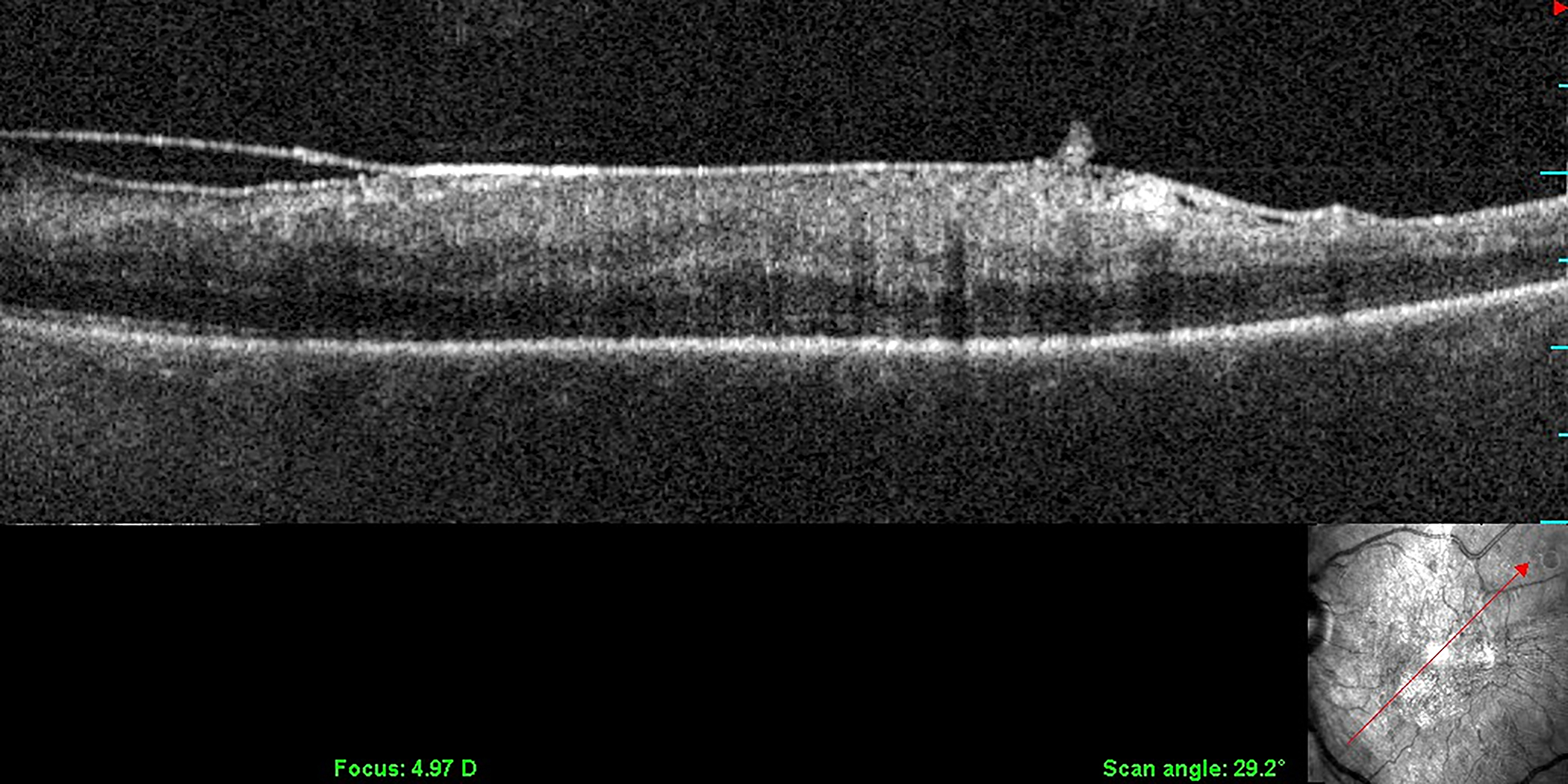
Figure 10: Vitreo-macular traction syndrome.
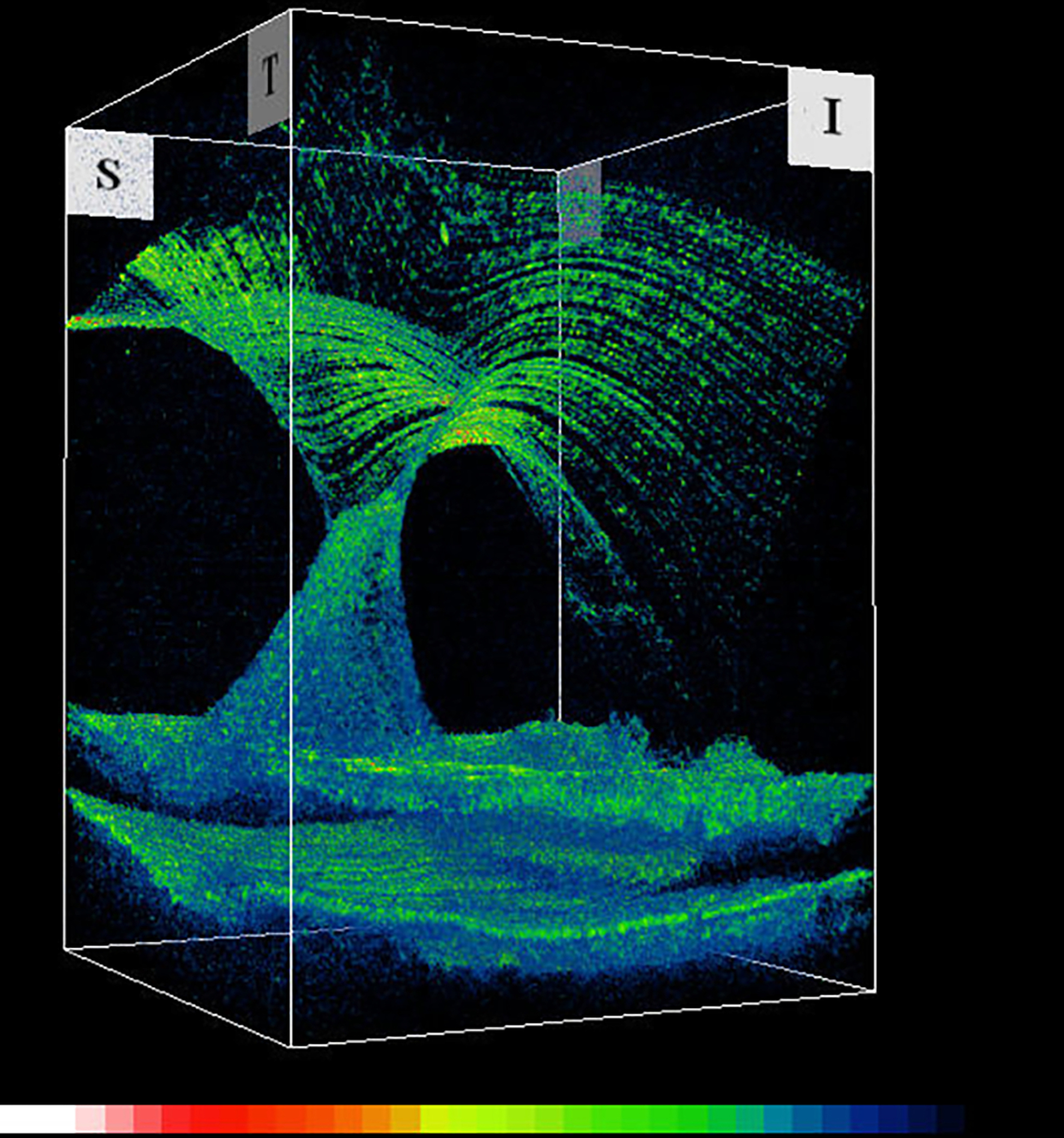
Anomalous PVD with persistent full-thickness attachment to the fovea can induce significant axial traction. There is relatively less cellular involvement in this process compared to macular pucker and PVR. Left: 3D-OCT. Image courtesy of author Michael Engelbert. Right: Combined OCT (color) – SLO (scanning laser ophthalmoscopy, in greyscale). Reproduced with permission from [23], © 2014 Springer Science+Business Media New York.
Hyalocyte activation is implicated in the development of vitreo-macular traction[12,25,98], even when paucicellular[14]. Although visualization of premacular vitreous cells in situ is now possible using B-scan spectral-domain OCT[92], this cannot provide conclusive cell identification, given that the morphology and behavior of both microglia and hyalocytes are similar because of origin and function (see article 1 in this series of expert review on hyalocytes[1]). Previous correlative light and electron microscopy studies of premacular cells on the ILM presented evidence for the presence of hyalocytes at the vitreo-macular interface[98], revealing ultrastructural features of hyalocytes in the majority of CD45-positive cells. These CD45-positive hyalocytes possessed an oval nucleus with marginal chromatin, vacuoles, dense granules, and thin cytoplasmic protrusions (Fig 3). A fluorescein-tagged immunonanogold particle was used as a secondary antibody which was visualized under both the fluorescence and transmission electron microscopes. Moreover, various immuno-histologic analyses of surgically-excised ILM specimens removed from VMTS and macular holes revealed hyalocytes with positive immunoreactivity for CD45 or CD64 (Fig 11)[38,85]. These premacular hyalocytes had similar ultrastructural features as vitreous-derived hyalocytes in guinea pig, chicken, and rodent eyes[99–103]. (Fig 12 E & F) In traumatic macular holes, hyalocytes were found on the vitread side of the ILM[104].
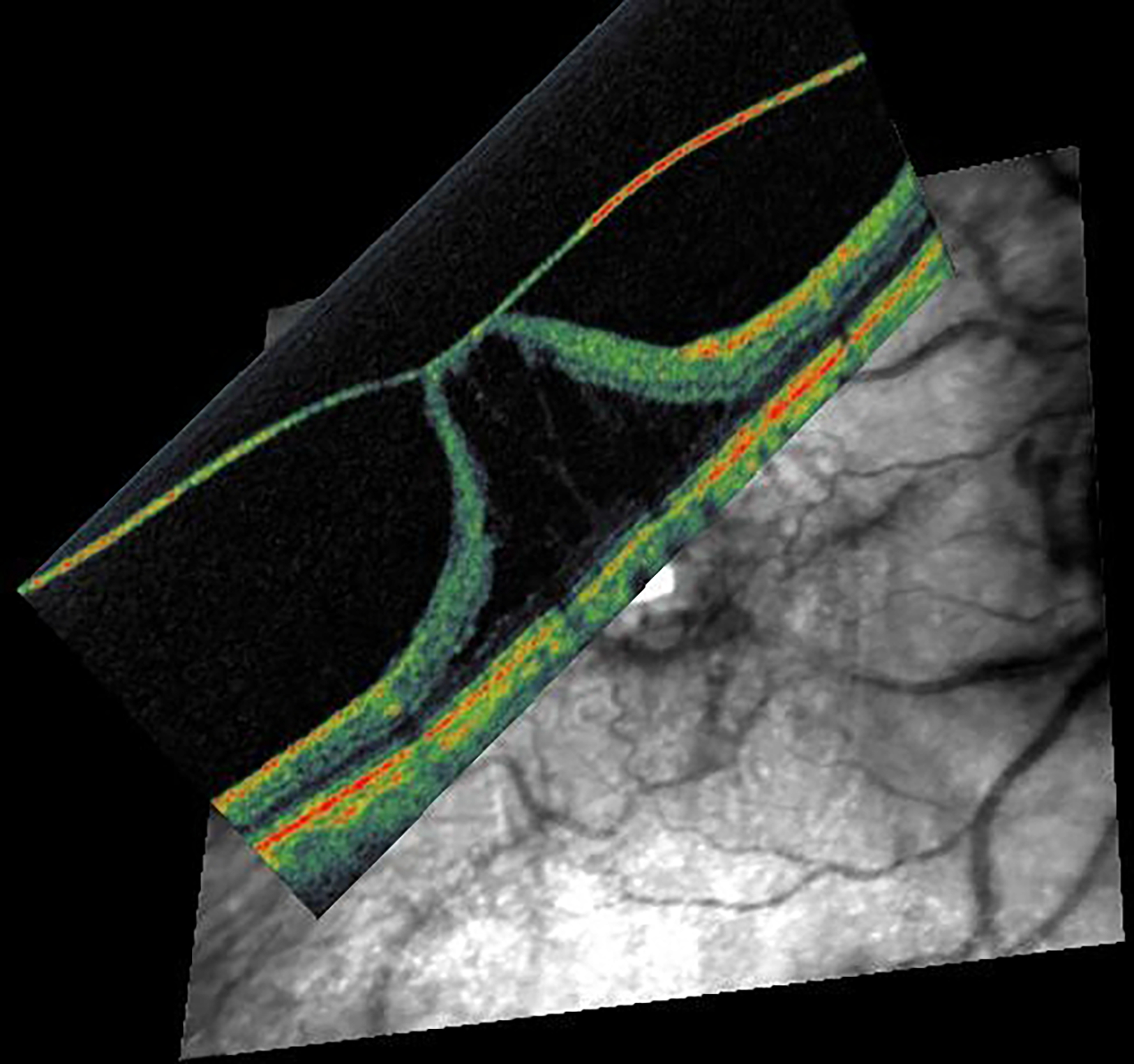
Figure 11: Immunofluorescence microscopy of ILM surgical specimen removed in vitreo-macular traction syndrome.
(A, B) Interference microscopy shows a complex cellular membrane with numerous cell nuclei (blue). (C, D) Positive immunostaining for anti-CD64 (green) and alpha-smooth muscle actin (red) indicates presence of hyalocytes and large myofibroblasts with intracytoplasmatic stress bundles. (Magnification x200). Images courtesy of author R Schumann.
Figure 12: Cells of the vitreo-macular interface.
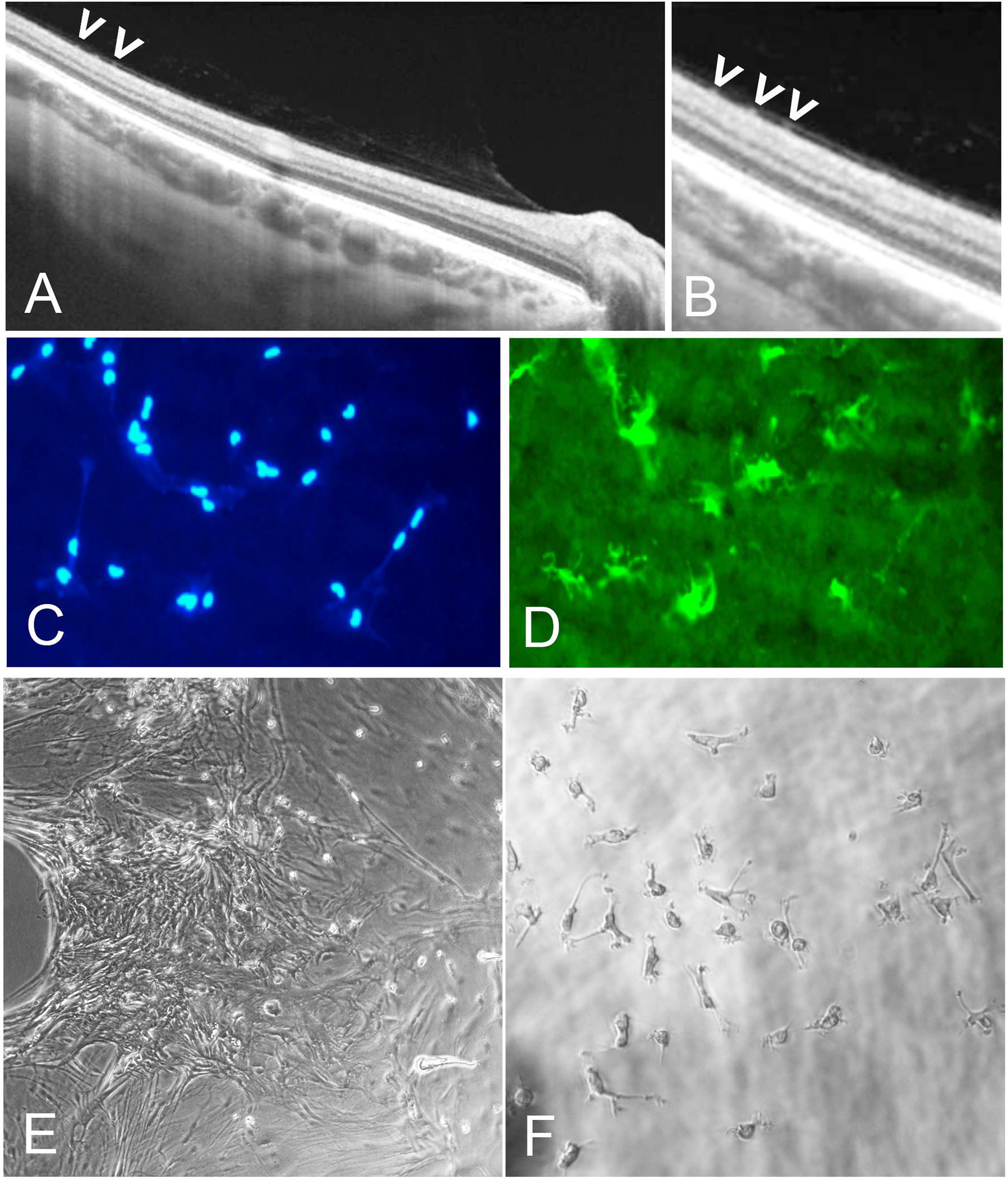
(A) Spectral-domain OCT of vitreoschisis with dots (presumed cells) on retinal surface. (B) Higher magnification of image A indicates premacular cells (arrowheads). (C, D) Fluorescence microscopy with cell nuclei staining (blue) of IBA1-positive (green) premacular cells of flat mounted inner limiting membrane (ILM) specimen removed during macular surgery for macular hole. (E, F) Interference microscopy of ILM with premacular cells in tissue culture demonstrates presence of small dot-like premacular cells that represent macrophage-like phenotype and behavior, consistent with their identity as hyalocytes. Images courtesy of author R Schumann.
Transdifferentiation of hyalocytes into myofibroblasts appears to occur early in various vitreo-retinal disorders, including paucicellular tractional vitreo-maculopathies such as macular holes[85]. Myofibroblasts are a subset of fibroblasts distinguished by cytoplasmic aggregates of actin microfilaments forming stress bundles. Similar to smooth muscle cells, they are characterized by α-smooth muscle actin (α-SMA) immunoreactivity. When cultured on collagen lattice in vitro, human myofibroblasts were shown to generate more potent traction forces than smooth muscle cells[37,105]. In VMTS, myofibroblasts were reported to predominate the cellular composition of fibrocellular membranes[96]. Further, hyalocytes are not only believed to undergo phenotypic changes developing contractile properties, but are also capable of collagen production within the premacular cell population[98], first described by Newsome in 1976[106] and more recently reported in a porcine hyalocyte cell line in vitro[107]. Most hyalocytes found in pathologic membranes are situated on a collagen fibril network identified as native vitreous collagen. In contrast, myofibroblast-like cells were demonstrated in masses mostly embedded in layers of newly formed collagen.
5. Therapeutic and preventative considerations
As the foregoing illustrates, there has been a growing understanding of the important roles various cells play in different proliferative vitreo-retinopathies, both hypercellular and paucicellular. Yet, there have been no specific targeted therapeutic strategies for preventing conditions such as PVR and recurrent retinal detachment, or the development of macular pucker, VMTS, and macular holes following anomalous PVD, other than attempting to prevent PVR by infusing non-specific anti-proliferative drugs[108,109]. A recent surgical approach, however, employed aggressive chromodissection[110] to remove all potentially pathologic tissues from the retinal surface[56,57]. The rationale was to excise the peripheral vitreous that remained attached to the ILM as a result of vitreoschisis, and in so doing remove any vitreous cortex remnants (VCR) with hyalocytes to eliminate their stimulatory role. Furthermore, this removed the scaffold for monocyte (from the circulation) as well as glial cell (from the retina) migration, and active cell proliferation, as well as pathologic extracellular membrane synthesis and contraction. This approach has been shown to improve surgical outcomes and reduce PVR-induced recurrent retinal detachment and premacular proliferation with macular pucker[52,57,59,60]. van Overdam and colleagues therefore recommend that when performing vitrectomy for primary and recurrent retinal detachment, it is important to completely remove all vitreous via repeated, targeted use of triamcinolone acetonide for visualization and chromodissection[110] of vitreous and vitreous cortex remnants, employing vitreous shaving with indentation at the vitreous base, and detection and removal of VCR over the macula and peripheral retinal surface[37,57,111] (Fig 13).
Figure 13: Chromodissection of preretinal membranes in rhegmatogenous retinal detachment.
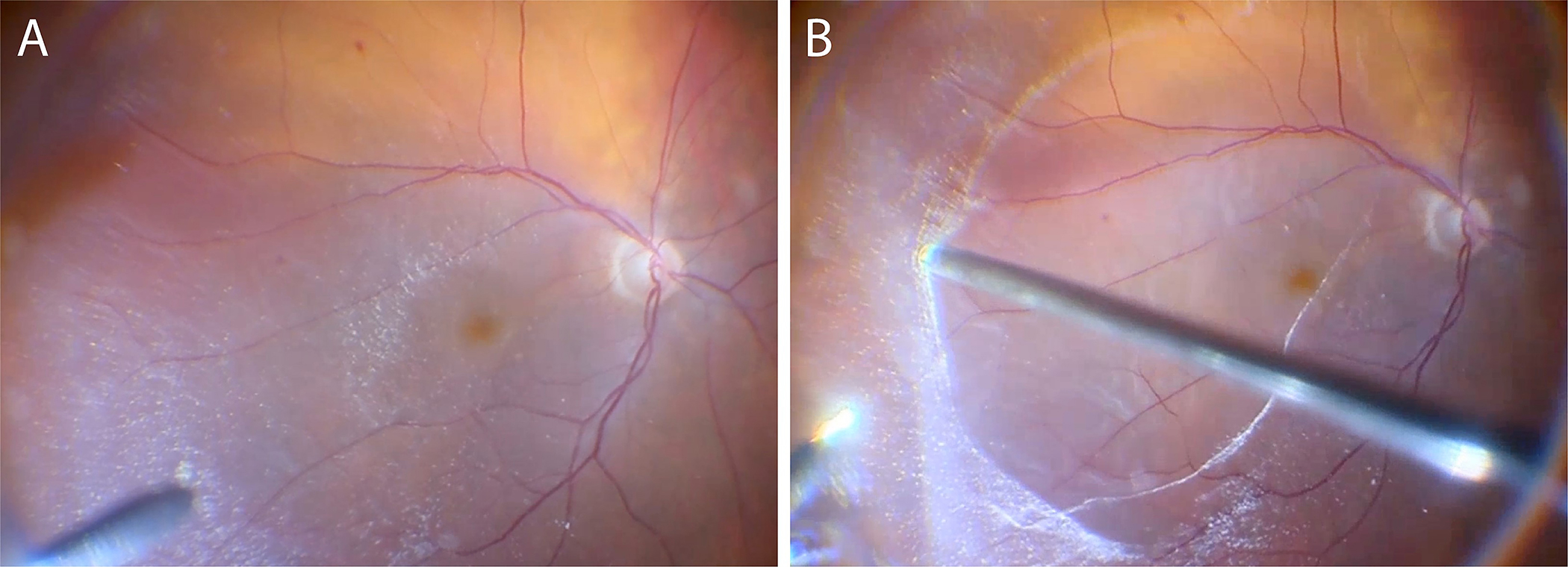
Intraoperative imaging during vitrectomy for primary rhegmatogenous retinal detachment with presumed pre-operative PVD. After core vitrectomy and repeated, targeted use of triamcinolone acetonide for vitreous and VCR visualization, VCR membranes were detected (A) and removed (B) from the macula and peripheral retinal surface. Images courtesy of author K van Overdam, and P van Etten.
The effects of intravitreally applied pharmacologic agents on retinal and choroidal endothelial cells have been studied extensively[112–117]. However, little is known about their impact on hyalocytes, which reside in the primary injection site of these drugs, the vitreous body. Hyalocytes have been found to suppress the effects of bevacizumab and dexamethasone in a co-culture with stimulated human retinal endothelial cells (HRECs) in vitro, suggesting a relevant expression of vascular endothelial growth factor (VEGF) and inflammatory cytokines by hyalocytes[118]. The converse may also true, since bevacizumab has been shown to reduce the intravitreal levels of VEGF and diverse inflammatory cytokines in the vitreous fluid of proliferative diabetic retinopathy patients[119], which is of interest as most of these molecules are expressed strongly by hyalocytes[120]. In vitro experiments on cultured bovine hyalocytes have demonstrated an inhibition of VEGF production in hyalocytes by dexamethasone via an attenuation of hypoxia-inducible factor 1-alpha (HIF1α) protein levels, which points to a possible involvement of hyalocytes in various vitreo-retinal diseases[77]. Electron microscopy studies have demonstrated the presence of hyalocytes on the retinal ILM of macular hole patients associated with unsuccessful ocriplasmin treatment[121], implying the accumulation of preretinal cells on the ILM as a possible obstacle for pharmacologic vitreolysis[122–124]. In order to further study the effects of intravitreal agents applied in the clinical routine on hyalocytes and identify novel therapeutic options with a specific effect on hyalocytes in neovascular and proliferative vitreo-retinal disease, state-of-the-art computational methods[54,125] should be utilized in the future.
Given the important role of vitreous and hyalocytes in various proliferative vitreo-retinopathies, it is logical to seek even better ways to eliminate their untoward effects. Prophylactic vitrectomy is an untenable approach, owing to the required magnitude, cost, and attendant risks. However, the induction of innocuous PVD by pharmacologic means may be a very effective way to prevent anomalous PVD, vitreoschisis, and various vitreo-retinopathies (see above). Indeed, pharmacologic vitreolysis[122,123] has been shown to be somewhat effective[124] and relatively safe[126,127]. Future studies should seek to identify individuals at high risk of the conditions described above and develop pharmacologic means to mitigate these risks.
6. Conclusions
Hyalocytes play an important role in several proliferative vitreo-retinal pathologies, both hypercellular ones, such as macular pucker, proliferative vitreo-retinopathy, and proliferative diabetic vitreo-retinopathy, as well as paucicellular conditions such as vitreo-macular traction syndrome and macular holes. These can all significantly impact vision and ocular health. Owing to their residence in the preretinal posterior vitreous cortex, hyalocytes are early responders that stimulate progressive disease by eliciting the migration and participating in the proliferation and transdifferentiation of various cell types. Lastly, hyalocytes can promote the contractile effects that impact the retina both posteriorly and peripherally. Advances in visualizing hyalocytes in diseased human eyes should lead to a better understanding of their role in pathogenesis and earlier detection of their contribution to pathophysiology. Finally, enhanced appreciation of the role(s) of hyalocytes in health and disease will lead to the development of ways to not only treat proliferative vitreo-retinal disorders, but also prevent vitreo-retinal pathology and promote ocular health.
7. Expert opinion
Owing to a paucity of basic science information and clinical understanding of the roles of vitreous in health and disease, this enigmatic yet exquisite structure is often overlooked as an important participant in vitreo-retinal diseases. This is particularly true concerning current concepts of the role(s) of hyalocytes in proliferative disorders at the vitreo-retinal interface. That hyalocytes reside within the dense collagen matrix of the posterior vitreous cortex (see the first article in this series[1]) makes a compelling argument for their participation in proliferative vitreo-retinal diseases. Further, hyalocytes have been identified in all pathologic membranes of humans with proliferative vitreo-retinal diseases. What remains to be better elucidated, however, is the mechanism by which hyalocytes influence the pathophysiology of these diseases.
Posterior vitreous detachment (PVD) is a common occurrence in older individuals, usually featuring innocuous separation of the posterior vitreous cortex from the inner limiting membrane of the retina. This typically results in complete separation without any remnants of vitreous adherent to the retina. However, if liquefaction of the vitreous body is not coupled with dehiscence of vitreo-retinal adhesion, anomalous PVD can occur with a variety of consequences. If vitreoschisis (splitting between the lamellae of the posterior vitreous cortex) occurs between anterior lamellae of the posterior vitreous cortex, hyalocytes often remain attached to the retina. This promotes hypercellular proliferative membranes such as those seen in macular pucker, proliferative vitreo-retinopathy following retinal detachment, and proliferative diabetic vitreo-retinopathy. If the vitreoschisis split occurs between more posterior lamellae, fewer hyalocytes remain adherent to the retina and hypocellular membranes develop, such as those seen in vitreo-macular traction syndrome and macular holes. The cellularity of these pathologic membranes is further influenced by the capacity of hyalocytes to stimulate cell migration from the circulation (monocytes) and retina (glial cells). Lastly, hyalocytes induce membrane contraction with untoward effects on the retina that profoundly impact vision. Studies have shown that myofibroblasts are important in this regard. While there is much evidence to suggest that hyalocytes transdifferentiate into myofibroblasts, much of the evidence is circumstantial representing a weakness in this hypothetical mechanism of disease pathophysiology. In macular pucker, where there is a very high prevalence of vitreous separation from the optic disc, the vector of tangential forces is inward (centripetal) resulting in puckering of the underlying retina. In macular holes, where there is a high prevalence of vitreo-papillary adhesion, the vector of tangential forces is outward (centrifugal) causing dehiscence in the central macula.
A better understanding of the role of hyalocytes in the early stages of proliferative disorders at the vitreo-retinal interface could result in new treatment strategies to prevent progression to more advanced stages of disease. In that context, the prevention of anomalous PVD and vitreoschisis could mitigate the role of hyalocytes in proliferative vitreo-retinopathies. Should anomalous PVD induce vitreoschisis, however, then treatment strategies to mitigate the effects of hyalocytes could prevent progression to more advanced stages of disease, especially in PVR following retinal detachment, and in proliferative diabetic vitreo-retinopathy. Alternatively, the induction of innocuous (total) PVD early in the natural history of disease could prevent hyalocyte-mediated proliferative vitreo-retinal disorders entirely. This is already being done in rhegmatogenous retinal detachment where aggressive surgical removal of peripheral vitreous and hyalocytes has had very beneficial preventative effects. Pharmacologic vitreolysis might provide similar beneficial effects without surgical intervention, representing a worthy path of future research and development.
Supplementary Material
Movie 1: 3-D rendered and color-coded OCT reflectance in a 61-year-old patient with proliferative diabetic vitreo-retinopathy using clinical OCT. 3-D rendered OCT reflectance volume is generated using MIPAV (Medical Image Processing, Analysis, and Visualization, version 10.0.0; US National Institutes of Health, Bethesda, Maryland, USA). See also Figure 8. Images courtesy of authors TYP Chui and RB Rosen.
Article highlights.
Following anomalous PVD and vitreoschisis, hyalocytes often remain attached to the retina.
Hyalocytes stimulate migration of monocytes (circulation) and glial cells (retina).
Hyalocytes promote cell proliferation causing macular pucker, proliferative vitreo-retinopathy, and proliferative diabetic vitreo-retinopathy.
Hyalocytes induce membrane contraction impacting the retina and vision.
A better understanding of hyalocytes in early vitreo-retinal proliferation could result in new treatment strategies to prevent progression to advanced stages.
Preventing anomalous PVD and vitreoschisis could mitigate the role of hyalocytes in proliferative diseases.
Prophylactic induction of innocuous PVD could prevent hyalocyte-mediated proliferative vitreo-retinopathies.
Funding
This paper was funded by the VMR Research Foundation (J Sebag), Research to Prevent Blindness (TYP Chui and RB Rosen), the Marrus Family Foundation (TYP Chui and RB Rosen), the New York Eye and Ear Infirmary Foundation (TYP Chui and RB Rosen) and the National Institutes of Health (R01EY027301, R01HL159116; TYP Chui and RB Rosen).
Abbreviations
- PVD
Posterior vitreous detachment
- PDVR
proliferative diabetic vitreo-retinopathy
- ILM
inner limiting membrane
- MPK
macular pucker
- PVR
proliferative vitreo-retinopathy
- MH
macular holes
- VMTS
vitreo-macular traction syndrome
- APVD
anomalous posterior vitreous detachment
- VS
vitreoschisis
- PMM
premacular membrane
- OCT
Optical Coherence Tomography
- LHEP
lamellar hole associated preretinal proliferation
- GFAP
Glial Fibrillary Acidic Protein
- CTGF
connective tissue growth factor
- PDGF
platelet derived growth factor
- RRD
rhegmatogenous retinal detachment
- ECM
extracellular matrix
- RPE
retinal pigment epithelium
- PVC
posterior vitreous cortex
- VCR
vitreous cortex remnants
- VS
vitreoschisis
- NVD
neovascularization of the disc
- RNV
retinal neovascularization
- VEGF
vascular endothelial growth factor
- DR
diabetic retinopathy
- OIR
oxygen-induced-retinopathy
- VMI
vitreo-macular interface
- HLA-DR
human leukocyte antigen – DR isotype
- PECAM-1
platelet endothelial cell adhesion molecule
- CD8a
cluster of differentiation 8a
- AOSLO
Adaptive Optics Scanning Light Ophthalmoscopy
- AMD
age-related macular degeneration
- VMT
vitreomacular traction
- VPA
vitreopapillary adhesion
- SLO
Scanning Laser Ophthalmoscopy
- ABA
Applied Behavior Analysis
- Col
vitreous collagen
- Hy
hyalocytes
- My
myofibroblasts
- ERM
macular pucker
- COL1
collagen type I
- OCTA
Optical Coherence Tomography Angiography
- MIPAV
Medical Image Processing, Analysis, and Visualization
- HREC
human retinal endothelial cell
- HIF1α
hypoxia-inducible factor 1-alpha
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
Papers of special note have been highlighted as either of interest (*) or of considerable interest (**) to readers.
- [1].Wieghofer P, Engelbert M, Chui TY et al. Hyalocyte origin, structure, and imaging. Exp. Rev. Opthalmol. [In Press] (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Boneva S, Wolf J, Wieghofer P et al. Hyalocyte functions and immunology. Exp. Rev. Opthalmol. [In Press] (2022). [Google Scholar]
- [3].Sebag J. Vitreous & Vitreo-Retinal Interface. In: Schachat AP, editor. Ryan’s Retina. 6th ed. Elsevier; 2018. p. 544–581. [Google Scholar]
- [4].Sebag J. Vitreous and Vision Degrading Myodesopsia. Progress in Retinal and Eye Research. 2020;79:100847. [DOI] [PubMed] [Google Scholar]
- [5].Chew L, Sebag J. Vitreous. Adler’s Physiology of the Eye. 12th ed. Philadelphia: Elsevier; 2022. p. (in press). [Google Scholar]
- [6].Sebag J Classifying posterior vitreous detachment: A new way to look at the invisible. British Journal of Ophthalmology. 1997;81:521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Sebag J Posterior Vitreous Detachment. Ophthalmology. 2018;125:1384–1385. [DOI] [PubMed] [Google Scholar]
- [8]. Sebag J Anomalous posterior vitreous detachment: A unifying concept in vitreo-retinal disease. Graefe’s Archive for Clinical and Experimental Ophthalmology. 2004;242:690–698. **This seminal article lays the foundations for the mechanisms underlying proliferative vitreo-retinopathies and the role of hyalocytes.
- [9].Sebag J Vitreous Anatomy, Aging, and Anomalous Posterior Vitreous Detachment. In: Besharshe J, Dana R, Dartt D, editors. Encyclopedia of the Eye. Vol. 4. Oxford: Elsevier; 2010. p. 307–315. [Google Scholar]
- [10].Sebag J, Niemeyer M, Koss M. Anomalous PVD & Vitreoschisis. In: Sebag J, editor. Vitreous: In Health & Disease. New York: Springer; 2014. p. 241–265. [Google Scholar]
- [11].Sebag J. Vitreoschisis. Graefe’s Archive for Clinical and Experimental Ophthalmology. 2008;246:329–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sebag J, Gupta P, Rosen RR, et al. Macular holes and macular pucker: The role of vitreoschisis as imaged by optical coherence tomography/scanning laser ophthalmoscopy. Transactions of the American Ophthalmological Society. 2007;105:121–129. [PMC free article] [PubMed] [Google Scholar]
- [13].Gupta P, Yee KMP, Garcia P, et al. Vitreoschisis in macular diseases. British Journal of Ophthalmology. 2011;95:376–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Schumann RG, Hagenau F, Guenther SR, et al. Premacular cell proliferation profiles in tangential traction vitreo-maculopathies suggest a key role for hyalocytes. Ophthalmologica. 2019;242:106–112. * This landmark study provided histopathologic evidence for previous theories of the role of hyalocytes in vitreo-maculopathies from traction.
- [15].Iwanoff A Beiträge zur normalen und pathologischen Anatomie des Auges. Graefes Arch Clin Exp Ophthalmol. 1865;11:135–170. [Google Scholar]
- [16].Foos R Vitreoretinal juncture; epiretinal membranes and vitreous. Invest Ophthalmol Vis Sci. 1977;16:416–422. [PubMed] [Google Scholar]
- [17].Hiscott P, Grierson I, McLeod D. Natural history of fibrocellular epiretinal membranes: a quantitative, autoradiographic, and immunohistochemical study. Br J Ophthalmol. 1985;69:810–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Klein R, Klein B, Wang Q, et al. The epidemiology of epiretinal membranes. Trans Am Ophthalmol Soc. 1994;92:403. [PMC free article] [PubMed] [Google Scholar]
- [19].Mitchell P, Smith W, Chey T, et al. Prevalence and associations of epiretinal membranes. The Blue Mountains Eye Study, Australia. Ophthalmology. 1997;104:1033–1040. [DOI] [PubMed] [Google Scholar]
- [20].McCarty D, Mukesh B, Chikani V, et al. Prevalence and associations of epiretinal membranes in the visual impairment project. Am J Ophthalmol. 2005;140:288–294. [DOI] [PubMed] [Google Scholar]
- [21].Ng C, Cheung N, Wang J, et al. Prevalence and risk factors for epiretinal membranes in a multi-ethnic United States population. Ophthalmology. 2011;118:694–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Johnson T, Johnson M. Epiretinal Membrane. In: Yanoff M, Duker J, editors. Ophthalmology. St. Louis: Mosby; 2004. p. 686–687. [Google Scholar]
- [23].Tozer K, Sebag J. Vitreous in the pathobiology of macular pucker. In: Sebag J, editor. Vitreous - in Health & Disease. New York: Springer; 2014. p. 311–328. [Google Scholar]
- [24].Appiah A, Hirose T, Kado M. A review of 324 cases of idiopathic premacular gliosis. Am J Ophthalmol. 1988;106:533–535. [DOI] [PubMed] [Google Scholar]
- [25].Johnson M Posterior vitreous detachment: evolution and complications of its early stages. Am J Ophthalmol. 2010;149:371–382. [DOI] [PubMed] [Google Scholar]
- [26].Gupta P, Sadun A, Sebag J. Multifocal retinal contraction in macular pucker analyzed by combined optical coherence tomography/scanning laser ophthalmoscopy. Retina. 2008;28:447–452. [DOI] [PubMed] [Google Scholar]
- [27].Kishi S, Shimizu K. Oval defect in detached posterior hyaloid membrane in idiopathic preretinal macular fibrosis. Am J Ophthalmol. 1994;118:451–456. [DOI] [PubMed] [Google Scholar]
- [28].Sidd R, Fine S, Owens S, et al. Idiopathic preretinal gliosis. Am J Ophthalmol. 1982;94:44–48. [DOI] [PubMed] [Google Scholar]
- [29].Wise G Clinical features of idiopathic preretinal macular fibrosis. Schoenberg Lecture. Am J Ophthalmol. 1975;79:349–7. [DOI] [PubMed] [Google Scholar]
- [30].Foos R, Wheeler N. Vitreoretinal juncture. Synchysis senilis and posterior vitreous detachment. Ophthalmology. 1982;89:1502–1512. [DOI] [PubMed] [Google Scholar]
- [31].Lindner B Acute posterior vitreous detachment and its retinal complications. Acta Ophthalmol. 1966;87:1. [Google Scholar]
- [32].Foos R Vitreoretinal juncture—simple epiretinal membranes. Graefes Arch Clin Exp Ophthalmol. 1974;189:231–250. [DOI] [PubMed] [Google Scholar]
- [33].Snead D, James S, Snead M. Pathological changes in the vitreoretinal junction 1: epiretinal membrane formation. Eye. 2008;22:1310–1317. [DOI] [PubMed] [Google Scholar]
- [34].Yamashita T, Uemura A, Sakamoto T. Intraoperative characteristics of the posterior vitreous cortex in patients with epiretinal membrane. Graefes Arch Clin Exp Ophthalmol. 2008;246:333–337. [DOI] [PubMed] [Google Scholar]
- [35].Pang C, Maberley D, Freund K, et al. Lamellar hole-associated epiretinal proliferation: A clinicopathologic correlation. Retina. 2016;36:1408–1412. [DOI] [PubMed] [Google Scholar]
- [36].Vogt D, Vielmuth F, Wertheimer C, et al. Premacular membranes in tissue culture. Graefe’s Archive for Clinical and Experimental Ophthalmology. 2018;256:1589–1597. [DOI] [PubMed] [Google Scholar]
- [37]. Sakamoto T, Ishibashi T. Hyalocytes: Essential cells of the vitreous in vitreoretinal pathophysiology? Retina. 2011;31:222–228. * This in-depth publication on the role of hyalocytes in vitreo-retinal physiology and pathology lays the groundwork for future investigations.
- [38].Zhao F, Gandorfer A, Haritoglou C, et al. Epiretinal cell proliferation in macular pucker and vitreomacular traction syndrome: Analysis of flat-mounted internal limiting membrane specimens. Retina. 2013;33:77–88. [DOI] [PubMed] [Google Scholar]
- [39].Kohno R, Hata Y, Kawahara S, et al. Possible contribution of hyalocytes to idiopathic epiretinal membrane formation and its contraction. Br J Ophthalmol. 2009;93:1020–1026. [DOI] [PubMed] [Google Scholar]
- [40].Kita T, Hata Y, Kano K, et al. Transforming growth factor-β2 and connective tissue growth factor in proliferative vitreoretinal diseases - possible involvement of hyalocytes and therapeutic potential of Rho Kinase Inhibitor. Diabetes. 2007;56:231–238. [DOI] [PubMed] [Google Scholar]
- [41].Hirayama K, Hata Y, Noda Y, et al. The involvement of the rho-kinase pathway and its regulation in cytokine-induced collagen gel contraction by hyalocytes. Invest Ophthalmol Vis Sci. 2004;45:3896–3903. [DOI] [PubMed] [Google Scholar]
- [42].Nguyen J, Yee K, Sadun A, et al. Quantifying visual dysfunction and the response to surgery in macular pucker. Ophthalmology. 2016;123:1500–1510. [DOI] [PubMed] [Google Scholar]
- [43].Jivrajka R, Kim J, Fink W, et al. Quantitative analysis of central visual field defects in macular edema using three-dimensional computer-automated threshold Amsler grid testing. Graefe’s Arch Clin Exp Ophthalmol. 2009;247:165–170. [DOI] [PubMed] [Google Scholar]
- [44].Okamoto F, Sugiura Y, Okamoto Y, et al. Associations between Metamorphopsia and Foveal Microstructure in Patients with Epiretinal Membrane. Invest Ophthalmol Vis Sci. 2012;53:6770–6775. [DOI] [PubMed] [Google Scholar]
- [45].Oster S, Mojana F, Brar M, et al. Disruption of the photoreceptor inner segment/outer segment layer on spectral domain-optical coherence tomography is a predictor of poor visual acuity in patients with epiretinal membranes. Retina. 2010;30:713–718. [DOI] [PubMed] [Google Scholar]
- [46].Michalewski J, Michalewska Z, Cisiecki S, et al. Morphologically functional correlations of macular pathology connected with epiretinal membrane formation in spectral optical coherence tomography (SOCT). Graefes Arch Clin Exp Ophthalmol. 2007;245:1623–1631. [DOI] [PubMed] [Google Scholar]
- [47].Niwa T, Terasaki H, Kondo M, et al. Function and morphology of macula before and after removal of idiopathic epiretinal membrane. Invest Ophthalmol Vis Sci. 2003;44:1652–1656. [DOI] [PubMed] [Google Scholar]
- [48].Suh M, Seo J, Park K, et al. Associations between macular findings by optical coherence tomography and visual outcomes after epiretinal membrane removal. Am J Ophthalmol. 2009;147:473–480. [DOI] [PubMed] [Google Scholar]
- [49].Enders P, Schick T, Schaub F, et al. Risk of multiple recurring retinal detachment after primary rhegmatogenous retinal detachment repair. Retina. 2017;37:930–935. [DOI] [PubMed] [Google Scholar]
- [50].Sebag J, Green W. Vitreous and the vitreo-retinal interface. In: Ryan S, editor. Retina. 5th ed. Philadelphia: Saunders; 2012. p. 482–516. [Google Scholar]
- [51].Coffee R, Jiang L, Rahman S. Proliferative vitreoretinopathy: advances in surgical management. Int Ophthalmol Clin. 2014;54:91–109. [DOI] [PubMed] [Google Scholar]
- [52].Kato Y, Inoue M, Hirakata A. Effect of Foveal Vitreous Cortex Removal to Prevent Epiretinal Membrane after Vitrectomy for Rhegmatogenous Retinal Detachment. Ophthalmology Retina. 2021;5:420–428. [DOI] [PubMed] [Google Scholar]
- [53].Guenther S, Schumann R, Hagenau F, et al. Comparison of surgically excised premacular membranes in eyes with macular pucker and proliferative vitreoretinopathy. Curr Eye Res. 2019;44:341–349. [DOI] [PubMed] [Google Scholar]
- [54]. Laich Y, Wolf J, Hajdú R, et al. Single-cell protein and transcriptional characterization of epiretinal membranes from patients with proliferative vitreoretinopathy. Invest Ophthalmol Vis Sci. 2022; 63(5):17. *This most recent publication from the world’s leaders in hyalocyte biology will likely be oft-cited and held in high esteem.
- [55].Song Y, Liao M, Zhao X, et al. Vitreous M2 Macrophage-Derived Microparticles Promote RPE Cell Proliferation and Migration in Traumatic Proliferative Vitreoretinopathy. Invest Ophthalmol Vis Sci. 2021;62:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].van Overdam K, Busch E, Verdijk R, et al. The role of vitreous cortex remnants in proliferative vitreoretinopathy formation demonstrated by histopathology: a case report. Am J Ophthalmol Case Rep. 2021;24:101219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57]. van Overdam K Vitreoschisis-induced vitreous cortex remnants: missing link in proliferative vitreoretinopathy. Acta Ophthalmologica. 2020;98:e261–e262. * This outstanding publication applied scientifc considerations to clincial care and significantly improved the lot of retinal detachment patients world-wide.
- [58].Assi A, Khoueir Z. Prevalance of vitreous cortex remnants in eyes with primary rhegmatogenous retinal detachment undergoing vitrectomy. Retina. 2021;41:1403–1406. [DOI] [PubMed] [Google Scholar]
- [59].Sartini F, Menchini M, Loiudice P, et al. Surgical technique for removing vitreous cortex remnants using a diamond-dusted membrane scraper. Acta Ophthalmol. 2021;doi: 10.1111/aos.14933. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- [60].Rizzo S, de Angelis L, Barca F, et al. Vitreoschisis and retinal detachment: New insight in proliferative vitreoretinopathy. Eur J Ophthalmol. 2021; [DOI] [PubMed] [Google Scholar]
- [61].Gui W, Sebag J. Re: Ishida et al. : Risk Factors, Onset, and Progression of Epiretinal Membrane after 25-Gauge Pars Plana Vitrectomy for Rhegmatogenous Retinal Detachment. Ophthalmol Retina. 2020;4:e10–e11. [DOI] [PubMed] [Google Scholar]
- [62].Stepp MA, Menko AS. Immune responses to injury and their links to eye disease. Translational Research. 2021;236:52–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Cheung N, Mitchell P, Wong T. Diabetic retinopathy. Lancet. 2010;376:124–136. [DOI] [PubMed] [Google Scholar]
- [64].Lange C, Bainbridge J. Oxygen Sensing in Retinal Health and Disease. Ophthalmologica. 2012;227:115–131. [DOI] [PubMed] [Google Scholar]
- [65].El Rami H, Barham R, Sun J, et al. Evidence-Based Treatment of Diabetic Retinopathy. Seminars in Ophthalmology. 2017;32:67–74. [DOI] [PubMed] [Google Scholar]
- [66].Duh EJ, Sun JK, Stitt AW. Diabetic retinopathy: Current understanding, mechanisms, and treatment strategies. JCI Insight. 2017;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Agarwal D, Gelman R, Prospero Ponce C, et al. The Vitreomacular Interface in Diabetic Retinopathy. Journal of Ophthalmology. 2015;2015:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Kroll P, Rodrigues E, Meyer C. Proliferative diabetic vitreo-retinopathy. In: Sebag J, editor. Vitreous - in Health & Disease. New York: Springer; 1984. p. 421–436. [Google Scholar]
- [69].Akiba J, Arzabe C, Trempe C. Posterior Vitreous Detachment and Neovascularization in Diabetic Retinopathy. Ophthalmology. 1990;97:889–891. [DOI] [PubMed] [Google Scholar]
- [70].Blankenship G, Machemer R. Long-term diabetic vitrectomy results. Report of 10 year follow-up. Ophthalmology. 1985;92:503–506. [DOI] [PubMed] [Google Scholar]
- [71].Sebag J, Buzney S, Belyea D, et al. Posterior vitreous detachment following panretinal laser photocoagulation. Graefe’s Arch Clin Exp Ophthalmol. 1990;228:5–8. [DOI] [PubMed] [Google Scholar]
- [72].Sebag J, Nguyen-Cuu J. The effects of vitreous on proliferative diabetic retinopathy and the response to panretinal photocoagulation. Graefe’s Arch Clin Exp Ophthalmol. 2017;255:421–422. [DOI] [PubMed] [Google Scholar]
- [73].Boeck M, Thien A, Wolf J, et al. Temporospatial distribution and transcriptional profile of retinal microglia in the oxygen-induced retinopathy mouse model. Glia. 2020;68:1859–1873. [DOI] [PubMed] [Google Scholar]
- [74].Wieghofer P, Hagemeyer N, Sankowski R, et al. Mapping the origin and fate of myeloid cells in distinct compartments of the eye by single-cell profiling. EMBO J. 2021;40:e105123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Kezic J, Chen X, Rakoczy E, et al. The Effects of Age and Cx 3 cr1 Deficiency on Retinal Microglia in the Ins2 Akita Diabetic Mouse. Invest Ophthalmol Vis Sci. 2013;54:854–863. [DOI] [PubMed] [Google Scholar]
- [76].Noda Y, Hata Y, Hisatomi T, et al. Functional Properties of Hyalocytes under PDGF-Rich Conditions. Invest Ophthalmol Vis Sci. 2004;45:2107–2114. [DOI] [PubMed] [Google Scholar]
- [77].Hata Y, Sassa Y, Kita T, et al. Vascular endothelial growth factor expression by hyalocytes and its regulation by glucocorticoid. British Journal of Ophthalmology. 2008;92:1540–1544. [DOI] [PubMed] [Google Scholar]
- [78].Lange C, Stavrakas P, Luhmann U, et al. Intraocular Oxygen Distribution in Advanced Proliferative Diabetic Retinopathy. American Journal of Ophthalmology. 2011;152:406–412.e3. [DOI] [PubMed] [Google Scholar]
- [79].Gandorfer A, Rohleder M, Grosselfinger S, et al. Epiretinal pathology of diffuse diabetic macular edema associated with vitreomacular traction. Am J Ophthalmol. 2005;139:638–652. [DOI] [PubMed] [Google Scholar]
- [80].Gandorfer A Diffuse diabetic macular edema: pathology and implications for surgery. Dev Ophthalmol. 2007;38:88–95. [DOI] [PubMed] [Google Scholar]
- [81].Loukovaara S, Nurkkala H, Tamene F, et al. Quantitative Proteomics Analysis of Vitreous Humor from Diabetic Retinopathy Patients. J Proteome Res. 2015;14:5131–5143. [DOI] [PubMed] [Google Scholar]
- [82].Matsunaga N, Ozeki H, Hirabayashi Y, et al. Histopathologic evaluation of the internal limiting membrane surgically excised from eyes with diabetic maculopathy. Retina. 2005;25:311–316. [DOI] [PubMed] [Google Scholar]
- [83].Ophir A, Martinez M. Epiretinal membranes and incomplete posterior vitreous detachment in diabetic macular edema, detected by spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52:6414–6420. [DOI] [PubMed] [Google Scholar]
- [84].Hagenau F, Vogt D, Ziada J, et al. Vitrectomy for diabetic macular edema: Optical coherence tomography criteria and pathology of the vitreomacular interface. Am J Ophthalmol. 2019;200:34–46. [DOI] [PubMed] [Google Scholar]
- [85].Schumann R, Eibl K, Zhao F, et al. Immunocytochemical and ultrastructural evidence of glial cells and hyalocytes in internal limiting membrane specimens of idiopathic macular holes. Invest Ophthalmol Vis Sci. 2011;52:7822–7834. [DOI] [PubMed] [Google Scholar]
- [86].Ziada J, Hagenau F, Compera D, et al. Vitrectomy for intermediate age-related macular degeneration associated with tangential vitreomacular traction: a clinicopathologic correlation. Retina. 2018;38:531–540. [DOI] [PubMed] [Google Scholar]
- [87].Schumann R, Hagenau F, Guenther S, et al. Premacular cell proliferation profiles in tangential traction vitreo-maculopathies suggest a key role for hyalocytes. Ophthalmologica. 2019;242:106–112. [DOI] [PubMed] [Google Scholar]
- [88].Tamura K, Yokoyama T, Ebihara N MA. Histopathologic analysis of the internal limiting membranesurgically peeled from eyes with diffuse diabetic macular edema. Jpn J Ophthalmol. 2012;56:280–287. [DOI] [PubMed] [Google Scholar]
- [89]. Boneva SK, Wolf J, Hajdú RI, et al. In-Depth Molecular Characterization of Neovascular Membranes Suggests a Role for Hyalocyte-to-Myofibroblast Transdifferentiation in Proliferative Diabetic Retinopathy. Frontiers in Immunology. 2021;12:1–15. * This study is an excellent example of the value of applying modern scientific analyses to surgical specimens and improve our understanding of human disease, specifically the role of hyalocytes in neovascular diseases of the eye.
- [90].Little K, Ma J, Yang N, et al. Myofibroblasts in macular fibrosis secondary to neovascular age-related macular degeneration - the potential sources and molecular cues for their recruitment and activation. EBioMedicine. 2018;38:283–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Kita T, Hata Y, Arita R, et al. Role of TGF-β in proliferative vitreoretinal diseases and ROCK as a therapeutic target. Proceedings of the National Academy of Sciences. 2008;105:17504–17509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Castanos M, Zhou D, Lindermann A, et al. Imaging of macrophage-like cells of living human retina using clincial OCT. Invest Ophthalmol Vis Sci. 2020;61:48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Ong JX, Nesper PL, Fawzi AA, et al. Macrophage-like cell density is increased in proliferative diabetic retinopathy characterized by optical coherence tomography angiography. Investigative Ophthalmology and Visual Science. 2021;32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Migacz J V, Otero-Marquez O, Zhou R, et al. Imaging of vitreous cortex hyalocyte dynamics using non-confocal quadrant-detection adaptive optics scanning light ophthalmoscopy in human subjects. Biomedical Optics Express. 2022;13:1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Johnson M Perifoveal vitreous detachment and its macular complications. Trans Am Ophthalmol Soc. 2005;103:537–567. [PMC free article] [PubMed] [Google Scholar]
- [96].Gandorfer A, Rohleder M, Kampik A. Epiretinal pathology of vitreomacular traction syndrome. Br J Ophthalmol. 2002;86:902–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Gandorfer A, Scheler R, Schumann R, et al. Interference microscopy delineates cellular proliferations on flat mounted internal limiting membrane specimens. Br J Ophthalmol. 2009;93:120–122. [DOI] [PubMed] [Google Scholar]
- [98].Schumann R, Gandorfer A, Ziada J, et al. Hyalocytes in idiopathic epiretinale membranes : a correlative light and electron microscopic study. Graefes Arch Clin Exp Ophthalmol. 2014;252:1887–1894. [DOI] [PubMed] [Google Scholar]
- [99].Ogawa K Scanning electron microscopic study of hyalocytes in the guinea pig eye. Arch Histol Cytol. 2002;65:263–268. [DOI] [PubMed] [Google Scholar]
- [100].Saga T, Tagawa Y, Takeuchi T, et al. Electron microscopic study of cells in vitreous of guinea pig. Jpn J Ophthalmol. 1984;28:239–247. [PubMed] [Google Scholar]
- [101].Uerara M, Imagawa T, Kitagawa H. Morphological studies of the hyalocytes in the chicken eye: scanning electron microscopy and inflammatory response after the intravitreous injection of carbon particles. J Anat. 1996;188:661–669. [PMC free article] [PubMed] [Google Scholar]
- [102].Salu P, Claeskens W, de Wilde A, et al. Light and electron microscopic studies of the rat hyalocyte after perfusion fixation. Ophthalmic Res. 1985;17:125–130. [DOI] [PubMed] [Google Scholar]
- [103].Qiao H, Hisatomi T, Sonoda K-H, et al. The characterisation of hyalocytes: the origin, phenotype and turnover. Br J Ophthalmol. 2005;89:513–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Guenther SR, Schumann RG, Zaytseva Y, et al. Cell composition at the vitreomacular interface in traumatic macular holes. Graefe’s Archive for Clinical and Experimental Ophthalmology. 2021; [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Kita T, Sakamoto T, Ishibashi T. Hyalocytes: essential vitreous cells in vitreoretinal health and disease. In: Sebag J, editor. Vitreous in Health and Disease. New York, NY: Springer New York; 2014. p. 151–164. [Google Scholar]
- [106].Newsome D, Linsenmayer T, Trelstad R. Vitreous body collagen. Evidence for a dual origin from the neural retina and hyalocytes. J Cell Biol. 1976;71:59–6734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Nishitsuka K, Kashiwagi Y, Tojo N, et al. Hyaluronan production regulation from porcine hyalocyte cell line by cytokines. Exp Eye Res. 2007;85:539–545. [DOI] [PubMed] [Google Scholar]
- [108].Asaria R, Kon C, Bunce C, et al. Adjuvant 5-fluorouracil and heparin prevents proliferative vitreoretinopathy : Results from a randomized, double-blind, controlled clinical trial. Ophthalmology. 2001;108:1179–1183. [DOI] [PubMed] [Google Scholar]
- [109].Sebag J Shaken not stirred (Guest Editorial). Ophthalmology. 2001;108:1177–1178. [DOI] [PubMed] [Google Scholar]
- [110].Haritoglou C, Sebag J. Indications and considerations for chromodissection. Retin Physician. 2014;11:34–39. [Google Scholar]
- [111].van Overdam KA, van den Bosch TPP, van Etten PG, et al. Novel insights into the pathophysiology of proliferative vitreoretinopathy: The role of vitreoschisis-induced vitreous cortex remnants. Acta Ophthalmologica. 2022;1–11. [DOI] [PubMed] [Google Scholar]
- [112].Deissler HL, Deissler H, Lang GE. Actions of bevacizumab and ranibizumab on microvascular retinal endothelial cells: Similarities and differences. British Journal of Ophthalmology. 2012;96:1023–1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Rusovici R, Patel CJ, Chalam KV. Bevacizumab inhibits proliferation of choroidal endothelial cells by regulation of the cell cycle. Clinical Ophthalmology. 2013;7:321–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Deissler H, Deissler H, Lang S, et al. VEGF-induced effects on proliferation, migration and tight junctions are restored by ranibizumab (Lucentis) in microvascular retinal endothelial cells. British Journal of Ophthalmology. 2008;92:839–843. [DOI] [PubMed] [Google Scholar]
- [115].Stewart EA, Samaranayake GJ, Browning AC, et al. Comparison of choroidal and retinal endothelial cells: Characteristics and response to VEGF isoforms and anti-VEGF treatments. Experimental Eye Research. 2011;93:761–766. [DOI] [PubMed] [Google Scholar]
- [116].Deissler HL, Lang GK, Lang GE. Capacity of aflibercept to counteract VEGF-stimulated abnormal behavior of retinal microvascular endothelial cells. Experimental Eye Research. 2014;122:20–31. [DOI] [PubMed] [Google Scholar]
- [117].Sacks D, Baxter B, Campbell BCV, et al. Multisociety Consensus Quality Improvement Revised Consensus Statement for Endovascular Therapy of Acute Ischemic Stroke. International Journal of Stroke. 2018;13:612–632. [DOI] [PubMed] [Google Scholar]
- [118].Tojo N, Kashiwagi Y, Yamamoto S, et al. The in vitro response of human retinal endothelial cells to cytokines and other chemically active agents is altered by coculture with vitreous-derived hyalocytes. Acta Ophthalmologica. 2010;88:66–72. [DOI] [PubMed] [Google Scholar]
- [119].Suzuki Y, Suzuki K, Yokoi Y, et al. Effects of intravitreal injection of bevacizumab on inflammatory cytokines in the vitreous with proliferative diabetic retinopathy. Retina. 2014;34:165–171. [DOI] [PubMed] [Google Scholar]
- [120].Boneva SK, Wolf J, Rosmus DD, et al. Transcriptional Profiling Uncovers Human Hyalocytes as a Unique Innate Immune Cell Population. Frontiers in Immunology. 2020;11:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Schumann RG, Wolf A, Mayer WJ, et al. Pathology of Internal Limiting Membrane Specimens Following Intravitreal Injection of Ocriplasmin. American Journal of Ophthalmology. 2015;160:767–778. [DOI] [PubMed] [Google Scholar]
- [122].Sebag J Pharmacologic vitreolysis. Retina. 1998;18:1–3. [DOI] [PubMed] [Google Scholar]
- [123].Sebag J Is pharmacologic vitreolysis brewing? Retina. 2002;22:1–3. [DOI] [PubMed] [Google Scholar]
- [124].Sebag J Pharmacologic vitreolysis - Premise and promise of the first decade. Retina. 2009;29:871–874. [DOI] [PubMed] [Google Scholar]
- [125].Boneva S, Wolf J, Hajdú R, et al. In-Depth Molecular Characterization of Neovascular Membranes Suggests a Role for Hyalocyte-to-Myofibroblast Transdifferentiation in Proliferative Diabetic Retinopathy. Front Immunol. 2021;12:757607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Khoshnevis M, Sebag J. Pharmacologic vitreolysis with ocriplasmin: Rationale for use and therapeutic potential in vitreo-retinal disorders. BioDrugs. 2015;29:103–112. [DOI] [PubMed] [Google Scholar]
- [127].Sebag J Pharmacologic vitreolysis. In: Sebag J, editor. Vitreous - in Health & Disease. New York: Springer; 2014. p. 799–816. [Google Scholar]
- [128].Sebag J Pathophysiology of the aging vitreous. In: Girach A, de Smet MD, editors. Diseases of the Vitreo-Macular Interface. Berlin, Heidelberg: Springer Berlin Heidelberg; 2014. p. 29–42. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movie 1: 3-D rendered and color-coded OCT reflectance in a 61-year-old patient with proliferative diabetic vitreo-retinopathy using clinical OCT. 3-D rendered OCT reflectance volume is generated using MIPAV (Medical Image Processing, Analysis, and Visualization, version 10.0.0; US National Institutes of Health, Bethesda, Maryland, USA). See also Figure 8. Images courtesy of authors TYP Chui and RB Rosen.


