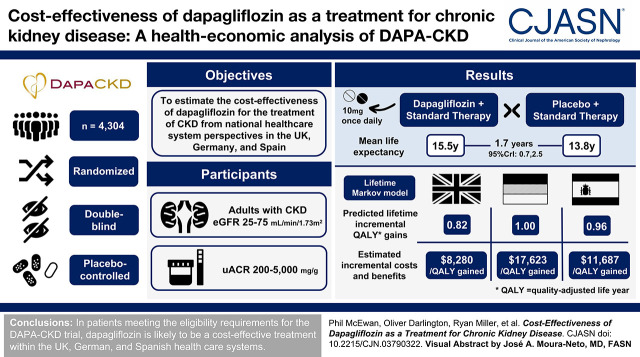
Visual Abstract
Keywords: dapagliflozin, SGLT2 inhibitor, chronic kidney disease, cost-effectiveness
Abstract
Background and objectives
CKD imposes a significant burden on patients and health care providers, particularly upon reaching kidney failure when patients may require KRT. The Dapagliflozin and Prevention of Adverse Outcomes in CKD (DAPA-CKD) trial demonstrated that dapagliflozin, with standard therapy, reduced CKD progression and KRT requirement. The study objective was to estimate the cost-effectiveness of dapagliflozin for the treatment of CKD from payer perspectives in the United Kingdom, Germany, and Spain.
Design, setting, participants, & measurements
We constructed a lifetime Markov model to characterize outcomes in patients with CKD on the basis of the DAPA-CKD trial. Health states were defined by eGFR level and KRT type. Direct health care costs and utility values were sourced from published literature and the DAPA-CKD trial, respectively. Costs and benefits were discounted at 3.5% per annum in the United Kingdom and 3% in Germany and Spain.
Results
In patients eligible for the DAPA-CKD trial, treatment with dapagliflozin was predicted to reduce rates of CKD progression, with patients predicted to spend 1.7 (95% credibility interval, 0.8 to 2.4) more years in the eGFR range 15–89 ml/min per 1.73 m2 versus standard therapy alone (12.1; 95% credibility interval, 8.9 to 14.1 versus 10.4; 95% credibility interval, 7.7 to 12.4 years). Life expectancy (undiscounted) was correspondingly predicted to increase by 1.7 (95% credibility interval, 0.7 to 2.5) years (15.5; 95% credibility interval, 11.1 to 18.2 versus 13.8; 95% credibility interval, 9.9 to 16.5 years). This in addition to reduced incidence of adverse clinical outcomes, including hospitalization for heart failure, resulted in modeled quality-adjusted life year (discounted) gains between 0.82 (95% credibility interval, 0.38 to 1.18) and 1.00 (95% credibility interval, 0.46 to 1.41). These gains translated to incremental cost-effectiveness ratios of $8280, $17,623, and $11,687 in the United Kingdom, Germany, and Spain, respectively, indicating cost-effectiveness at willingness-to-pay thresholds (United Kingdom: $27,510 per quality-adjusted life year; Germany and Spain: $35,503 per quality-adjusted life year).
Conclusions
In patients meeting the eligibility requirements for the DAPA-CKD trial, dapagliflozin is likely to be a cost-effective treatment within the UK, German, and Spanish health care systems.
Clinical Trial registry name and registration number:
Dapagliflozin and Prevention of Adverse Outcomes in CKD (DAPA-CKD), NCT03036150
Introduction
The prevalence of CKD—estimated to be 11%–13% worldwide (1)—continues to increase, driven by both aging populations and the increasing prevalence of type 2 diabetes mellitus (2,3). Patients with CKD are at high risk of progressing to kidney failure and developing cardiovascular complications, including heart failure, myocardial infarction, and stroke (4). Furthermore, the worsening of kidney function over time also leads to anemia, mineral and bone disorders/osteoporosis, and fractures (5,6). Patients with CKD may ultimately require KRT, necessitating a choice between dialysis, kidney transplantation, or conservative care (7). The effect on both individuals and health care systems is substantial, affecting an individual’s life expectancy and health-related quality of life (8) and imposing costs on both health care systems and wider society; patients receiving KRT are estimated to consume between 2% and 4% of national health care expenditure, despite constituting approximately 0.15% of the global population (9).
Current treatment options typically include angiotensin-converting enzyme inhibitors (ACEis) or angiotensin receptor blockers (ARBs), which aim to minimize symptoms and delay disease progression. Nevertheless, many patients will continue to progress toward advanced CKD (9). Other treatments providing additional protective efficacy are therefore needed to slow CKD progression and protect patients against adverse disease-related outcomes.
Dapagliflozin, a sodium-glucose cotransporter-2 inhibitor, was shown to be an efficacious treatment for CKD in the Dapagliflozin and Prevention of Adverse Outcomes in CKD (DAPA-CKD) trial. Eligible patients were those with CKD with and without type 2 diabetes who had received stable treatment with an ACEi or an ARB at the patient maximum tolerated labeled daily dose for at least 4 weeks prior to the study. Patients were randomized to receive either placebo or dapagliflozin 10 mg once daily. Treatment with dapagliflozin led to a statistically significant and clinically meaningful relative risk reduction of 39% (hazard ratio, 0.61; 95% confidence interval, 0.51 to 0.72; P<0.001) for the composite primary end point of a ≥50% sustained decline in eGFR, onset of kidney failure, or incidence of cardiovascular- or kidney-related death. The secondary outcome of death from any cause demonstrated a 31% relative risk reduction (hazard ratio, 0.69; 95% confidence interval, 0.52 to 0.88; P=0.004).
When considering novel therapeutic options, decision makers must evaluate additional expenditures on disease management against long-term clinical benefits achieved through reduced downstream disease and health care burden and improved patient health-related quality of life. Cost-effectiveness analyses represent an important tool to support informed decision making within this context. Consequently, the objective of this study was to estimate the cost-effectiveness of dapagliflozin added to standard therapy compared with standard therapy alone using patient-level data from the DAPA-CKD trial and considered from a multinational European payer perspective.
Materials and Methods
DAPA-CKD was a randomized, double-blind, placebo-controlled, event-driven trial in patients with eGFR ≥25 to ≤75 ml/min per 1.73 m2 and albuminuria. The trial evaluated the efficacy and safety of dapagliflozin 10 mg once daily versus placebo in addition to current standard therapy, defined as stable dosing of an ACEi or ARB. The trial design, baseline characteristics, and results have been previously published (10–12).
Patient Population
The target population for this cost-effectiveness analysis is reflective of the patient population included in the DAPA-CKD trial: adults with CKD (prekidney failure: eGFR ≥25 to ≤75 ml/min per 1.73 m2) and urine albumin-creatinine ratio (UACR) ≥200 to ≤5000 mg/g. The cohort profile is presented in Supplemental Material (Supplemental Table 1).
Economic Model
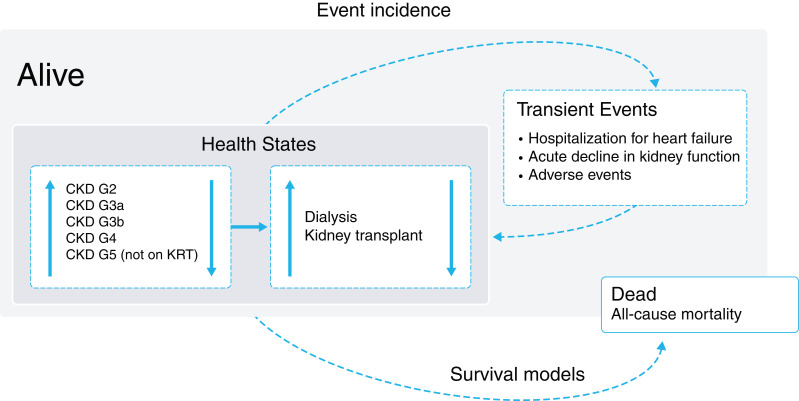
We developed a cohort-level Markov state transition model to assess the cost-effectiveness of dapagliflozin versus placebo in addition to standard therapy (Figure 1). Consistent with a previously published cost-effectiveness evaluation of dapagliflozin in patients with heart failure, we evaluated the cost-effectiveness of dapagliflozin from the perspective of the UK, German, and Spanish health care systems (13). The modeled events were informed by patient-level data from the DAPA-CKD trial, which are more likely to provide robust projections than published cohort data. Differences in country settings are reflected in country-specific costs, utilities, life tables, discount rates, and willingness-to-pay thresholds.
Figure 1.
Markov model schematic diagram. Patients can move between any eGFR-defined health state prior to kidney failure; once patients suffer kidney failure, they enter irreversibly into KRT-defined health states. Patients can suffer transient events, incurring associated costs and disutilities in the cycle of incidence. Death is an absorbing health state calculated by survival models on the basis of the all-cause mortality end point.
The model included five eGFR-defined states (CKD stages G2–G5 on the basis of laboratory readings from DAPA-CKD) in addition to dialysis and transplant states. As CKD is a chronic and progressive disease associated with high mortality and morbidity, the model uses monthly cycle lengths up to a lifetime horizon, defined as until all patients have died, up to a maximum period of 40 years. The principal model outcome was the incremental cost-effectiveness ratio (ICER), expressed as the cost per quality-adjusted life year (QALY) gained.
Our modeling approach is consistent with other CKD models identified in a recently published systematic review (14). Furthermore, the justification of necessary modeling assumptions and validation results are provided in Supplemental Material.
Disease Progression
Disease progression was captured using transitions between discrete health states, characterized by eGFR-defined CKD stages and KRT modality. Transition matrices were derived for the first 4 months of follow-up and from month 4 onward, reflecting observed patterns in eGFR over time derived from patient-level data in the DAPA-CKD trial (Supplemental Tables 2 and 3). Transition matrices derived for 4 months onward are applied to patients for the duration of the modeled time horizon. To capture the observed benefit of treatment with dapagliflozin on the rate of CKD progression, treatment-specific transition probabilities were estimated, such that the probability of progression was lower in patients treated with dapagliflozin. To validate the approach to deriving the transition matrices, time spent in respective CKD health states was validated against progression in the trial to demonstrate the reliability of the monthly transitions (Supplemental Figure 1). Furthermore, life expectancy and time to kidney failure were externally validated using published data (14).
In the DAPA-CKD trial, there were insufficient data informing transitions after patients initiated KRT. Therefore, patterns of post-KRT resource utilization and outcomes, particularly the probability of receiving a kidney transplant in patients receiving dialysis, the probability of transplant failure, and mortality in patients receiving KRT, were derived from published estimates (14).
To ensure that there was no double counting of mortality, transition probabilities to track disease progression were derived excluding the transition to death. The effect of kidney function on the risk of mortality was captured separately via the multivariable parametric survival model described below.
Mortality and Event Incidence
In addition to CKD progression, the model captured the incidence of events aligned to clinical end points of DAPA-CKD, with patients at risk of hospitalization for heart failure, acute decline in kidney function (predefined end point of doubling of serum creatinine between two visits as a result of acute intermittent events), and all-cause mortality. Although the incidence rates of myocardial infarction and stroke were exploratory end points of DAPA-CKD, dapagliflozin was associated with no significant effect on their incidence, so these events were not considered.
Parametric survival analysis was used to extrapolate patient life expectancy beyond the trial follow-up period following the methods advocated by the National Institute for Health and Care Excellence in the United Kingdom (15). To model recurrent events (hospitalization for heart failure and acute decline in kidney function), generalized estimating equations were used with a Poisson distribution. Parametric and recurring event models were adjusted for treatment received, baseline patient characteristics, and time-updated eGFR levels to replicate escalating risk as patients’ disease progressed. Analysis was conducted from an intention-to-treat perspective. The selection process and full statistical model specifications for parametric and recurrent event models are described in Supplemental Material (Supplemental Figures 2–4, Supplemental Tables 4–6).
Dapagliflozin has a well-established safety profile; nevertheless, adverse events were also included in the model. Patients experienced a constant rate of transient adverse events while on treatment, incurring associated cost- and utility-related effects in the cycle of incidence only. Modeled adverse events were volume depletion, major hypoglycemic events, fractures, diabetic ketoacidosis, and amputation.
The annual frequencies of clinical and adverse events are presented in Supplemental Material (Supplemental Table 7).
Resource Use and Costs
Our analyses considered direct health care costs only from payer perspectives in the United Kingdom, Germany, and Spain. These were attributed to patients on the basis of eGFR and clinical outcomes. Annual costs were stratified by eGFR-defined CKD stage and KRT modality and were obtained from the published literature. Incidence of hospitalization for heart failure, acute decline in kidney function, and adverse events incurred an event-specific cost applied only in the cycle of incidence, with inputs sourced from published literature. Costs taken from sources published in prior years were inflated to 2021 prices utilizing country-specific medical care inflation indices and converted to US dollars (16).
Dapagliflozin treatment costs were applied in addition to the cost of standard therapy while patients remained on dapagliflozin. Upon discontinuation of dapagliflozin, only the costs of standard therapy were applied. Patient time on treatment was a function of a constant annual probability of discontinuation derived from DAPA-CKD (6% of patients discontinuing per annum). Costs were discounted at an annual rate of 3.5% in the United Kingdom and 3% in Germany and Spain, according to local guidelines (15,17,18). Country-specific cost inputs are reported in Table 1.
Table 1.
Cost inputs for the United Kingdom, Germany, and Spain
| Parameter | United Kingdom, $ | Germany, $ | Spain, $ |
|---|---|---|---|
| Treatment (per annum) | |||
| Dapagliflozin | 665 (27) | 898a | 477a |
| Standard therapy | 67 (27) | 39b | 55a |
| Additional monitoring costs (dapagliflozin, first year only) | 202 (29) | 100 (30) | 99 (30) |
| CKD management (per annum) | |||
| CKD G2, 60–89 ml/min per 1.73 m2 | 1689 (31) | 10,207c | 5484 (32) |
| CKD G3a, 45–59 ml/min per 1.73 m2 | 1689 (31) | 10,207 (33) | 5768 (32) |
| CKD G3b, 30–44 ml/min per 1.73 m2 | 1689 (31) | 10,207 (33) | 5768 (34) |
| CKD G4, 15–29 ml/min per 1.73 m2 | 5913 (31) | 12,406 (33) | 9548 (34) |
| CKD G5, not on KRT; <15 ml/min per 1.73 m2 | 20,731 (31) | 56,405 (33) | 16,184 (34) |
| Dialysis | 47,565 (7) | 73,896 (35) | 58,062 (34) |
| Transplant | 28,199 (36) | 146,899 (37) | 26,989 (38) |
| Events | |||
| Hospitalization for heart failure | 7255 (39) | 6576 (40) | 4000 (38) |
| Acute decline in kidney function | 187 (29) | 100 (30) | 99 (30) |
| Volume depletion | 46 (41) | 36 (30) | 3193 (38) |
| Major hypoglycemic event | 514 (42) | 470 (42) | 845 (43) |
| Diabetic ketoacidosis | 3147 (44) | 2911 (44) | 4689 (38) |
| Fracture | 3535 (29) | 8559 (45) | 5173 (46) |
| Amputation | 19,197 (47) | 16,913 (48) | 10,025 (38) |
All prices are inflated to 2021 prices and converted to US dollars using Organisation for Economic Cooperation and Development conversion rates. SEM ranges of 10% are sampled using a γ-distribution in the probabilistic sensitivity analysis.
Internal information from AstraZeneca.
CompuGroup Medical. Lauer-Taxe. 2022. https://www.cgm.com/deu_de/produkte/apotheke/lauer-taxe.html
Assumed to be the same as CKD 3a.
Health-Related Quality of Life
Utility estimates were derived from a pooled analysis of individual patient data (EQ-5D-5L questionnaires) from the DAPA-CKD trial (Table 2). Utility decrements were applied upon the incidence of hospitalization for heart failure and adverse events in the cycle of incidence only, with no chronic utility decrement. Benefits were discounted at an annual rate of 3.5% in the United Kingdom and 3% in Germany and Spain (15,17,18). Further technical details are provided in Supplemental Material.
Table 2.
Utility inputs for the United Kingdom, Germany, and Spain
| Parameter | Utility or Utility Decrementa | Source | ||
|---|---|---|---|---|
| United Kingdom | Germany | Spain | ||
| Health state | ||||
| CKD G2, 60–89 ml/min per 1.73 m2 | 0.765 (0.005) | 0.873 (0.005) | 0.829 (0.005) | DAPA-CKD (49) |
| CKD G3a, 45–59 ml/min per 1.73 m2 | 0.765 (0.005) | 0.882 (0.002) | 0.837 (0.003) | DAPA-CKD (49) |
| CKD G3b, 30–44 ml/min per 1.73 m2 | 0.765 (0.005) | 0.882 (0.002) | 0.837 (0.003) | DAPA-CKD (49) |
| CKD G4, 15–29 ml/min per 1.73 m2 | 0.757 (0.006) | 0.874 (0.003) | 0.825 (0.033) | DAPA-CKD (49) |
| CKD G5, not on KRT; <15 ml/min per 1.73 m2 | 0.727 (0.010) | 0.852 (0.009) | 0.792 (0.009) | DAPA-CKD (49) |
| Dialysis | 0.679 (0.014) | 0.814 (0.013) | 0.759 (0.013) | DAPA-CKD (49) |
| Transplant | 0.710 (0.070) | 0.710 (0.070) | 0.710 (0.070) | Lee et al. (50) |
| Event | ||||
| Hospitalization for heart failure | −0.087 (0.037) | −0.063 (0.034) | −0.068 (0.034) | DAPA-CKD (49) |
| Volume depletion | −0.05 (0.010) | −0.05 (0.010) | −0.05 (0.010) | McEwan et al. (51) |
| Major hypoglycemic events | −0.01 (0.001) | −0.01 (0.001) | −0.01 (0.001) | Beaudet et al. (52) and Currie et al. (53) |
| Diabetic ketoacidosis | −0.01 (0.010) | −0.01 (0.010) | −0.01 (0.010) | Peasgood et al. (54) |
| Fracture | −0.094 (0.032) | −0.072 (0.031) | −0.067 (0.031) | DAPA-CKD (49) |
| Amputation | −0.256 (0.050) | −0.266 (0.049) | −0.257 (0.049) | DAPA-CKD (49) |
DAPA-CKD, Dapagliflozin and Prevention of Adverse Outcomes in CKD.
Stated values are expressed as mean (SEM), with SEMs sampled using a β-distribution in the probabilistic sensitivity analysis.
Subgroup Analyses
Subgroup analysis was conducted to demonstrate that dapagliflozin would be a cost-effective therapy across all subgroups. The analysis considered patients with and without type 2 diabetes, patients with an eGFR ≥45 versus <45 ml/min per 1.73 m2, patients aged ≥65 versus <65 years, and patients with a UACR=30–300 versus >300 mg/g at baseline.
Scenario Analyses
The DAPA-CKD trial had a median follow-up period of 2.4 years, whereas our model predicts outcomes over a lifetime horizon. To ensure that outcomes are not sensitive to the selected time horizon, we evaluated dapagliflozin over varying time horizons.
There is a paucity of alternative evidence regarding long-term treatment discontinuation. Therefore, two alternative scenario analyses were conducted where patients were assumed to discontinue dapagliflozin treatment at 3 years or linearly taper discontinuation to 0% after 4 years.
Sensitivity Analyses
One-way sensitivity analyses were conducted to assess the effect of varying key model inputs on the base case outcomes. Probabilistic sensitivity analysis was conducted to assess uncertainty in all parameters of the model. Random sampling of parameters (1000 replicates) was conducted using standard distributions to generate 95% credibility intervals (95% CrIs).
Results
Patients treated with dapagliflozin were predicted to experience health benefits from improved life expectancy and reduced rates of CKD progression and hospitalization for heart failure. In all considered countries, patients treated with dapagliflozin in addition to standard therapy had a mean life expectancy (undiscounted) of approximately 15.5 (95% CrI, 11.1 to 18.2) years, a 1.7-year increase compared with patients treated with placebo (13.8; 95% CrI, 9.9 to 16.5) in addition to standard therapy (Table 3).
Table 3.
Base case clinical outcomes
| Outcome | Dapagliflozin plus Standard Therapy | Standard Therapy | Incremental |
|---|---|---|---|
| Survival | |||
| 1-yr survival, % | 99 | 98 | 0.6 |
| 5-yr survival, % | 90 | 85 | 4 |
| 10-yr survival, % | 71 | 63 | 8 |
| Total LYs gained, undiscounted (95% CrI) | 15.5 (11.2 to 18.2) | 13.8 (9.9 to 16.5) | 1.7 (0.7 to 2.5) |
| Mean time in each CKD stage (eGFR range, ml/min per 1.73 m2), yr (95% CrI) | |||
| CKD G2 (60–89) | 0.9 (0.6 to 1.0) | 0.7 (0.6 to 0.9) | 0.1 (0.0 to 0.2) |
| CKD G3 (30–59) | 7.1 (5.2 to 8.2) | 5.8 (4.4 to 7.0) | 1.3 (0.7 to 3.6) |
| CKD G4 (15–29) | 4.1 (2.8 to 5.0) | 3.9 (2.7 to 4.9) | 0.3 (–0.1 to 0.6) |
| CKD G5 (not on KRT, <15) | 0.5 (0.3 to 0.7) | 0.5 (0.3 to 0.7) | 0.0 (–0.1 to 0.1) |
| KRT | 2.9 (1.9 to 3.6) | 2.8 (1.9 to 3.6) | 0.0 (–0.4 to 0.5) |
| Event incidence, per 1000 patients | |||
| Hospitalization for heart failure | 81 | 100 | −19 |
| Acute decline in kidney function | 363 | 390 | −28 |
| Adverse eventsa | 936 | 746 | 190 |
The outcomes presented here are taken from the UK analysis; there is minimal variation in the values for Germany and Spain corresponding to country-specific life tables. Incremental values may not correspond exactly to the treatment arms due to rounding. LY, life year; 95% CrI, 95% credibility interval.
Adverse events include volume depletion, hypoglycemic events, fractures, diabetic ketoacidosis, and amputation.
Despite significantly slower progression to kidney failure in patients treated with dapagliflozin, the overall time spent with kidney failure was approximately equivalent between arms as a result of improved patient life expectancy in those treated with dapagliflozin (2.9 years for patients treated with dapagliflozin versus 2.8 years for those treated with placebo). However, patients treated with dapagliflozin spent a smaller proportion of their lives with kidney failure (19% versus 21%), spending 1.7 (95% CrI, 1.5 to 1.6) more years in the eGFR range from 15 to 89 ml/min per 1.73 m2 (CKD stages G2–G4) versus patients treated with placebo (12.1; 95% CrI, 8.9 to 14.1 versus 10.4; 95% CrI, 7.7 to 12.4 years). Treatment with dapagliflozin was also expected to result in 19 fewer hospitalizations for heart failure and 28 fewer episodes of acute decline in kidney function per 1000 treated patients over a lifetime horizon.
Treatment with dapagliflozin led to predicted lifetime incremental QALY gains of 0.82 (95% CrI, 0.34 to 1.17), 1.00 (95% CrI, 0.43 to 1.40), and 0.96 (95% CrI, 0.43 to 1.40) in the UK, Germany, and Spain, respectively, with differences in benefits between countries resulting from the application of local discounting rates, utility tariffs, and life tables (Table 4).
Table 4.
Base case discounted health economic results
| Outcome | Dapagliflozin plus Standard Therapy | Standard Therapy | Incremental |
|---|---|---|---|
| United Kingdom | |||
| Total costs (95% CrI), $ | 109,596 (77,765 to 133,287) | 102,774 (74,017 to 126,749) | 6822 (–3293 to 17,138) |
| Drug acquisition | 6034 | 700 | 5334 |
| CKD management (not on KRT) | 36,815 | 34,920 | 1895 |
| KRT | 63,357 | 63,826 | –469 |
| Adverse events, hospitalization for heart failure, and acute decline in kidney function | 3391 | 3328 | 63 |
| Total QALYs gained (95% CrI) | 8.68 (6.79 to 9.72) | 7.86 (6.21 to 9.00) | 0.82 (0.34 to 1.17) |
| ICER, $/QALY | — | — | 8280 |
| Germany | |||
| Total costs (95% CrI), $ | 254,579 (186,892 to 304,520) | 236,908 (174,288 to 286,922) | 17,671 (–3328 to 35,900) |
| Drug acquisition | 7428 | 417 | 7011 |
| CKD management (not on KRT) | 128,095 | 117,133 | 10,962 |
| KRT | 114,735 | 115,391 | –656 |
| Adverse events, hospitalization for heart failure, and acute decline in kidney function | 4321 | 3967 | 354 |
| Total QALYs gained (95% CrI) | 10.32 (7.96 to 11.49) | 9.32 (7.28 to 10.65) | 1.00 (0.43 to 1.40) |
| ICER, $/QALY | — | — | 17,623 |
| Spain | |||
| Total costs (95% CrI), $ | 164,048 (118,905 to 202,510) | 152,862 (112,237 to 191,511) | 11,186 (–2903 to 24,614) |
| Drug acquisition | 4447 | 596 | 3851 |
| CKD management (not on KRT) | 74,305 | 67,320 | 6985 |
| KRT | 81,490 | 81,660 | –170 |
| Adverse events, hospitalization for heart failure, and acute decline in kidney function | 3807 | 3286 | 521 |
| Total QALYs gained (95% CrI) | 9.79 (7.56 to 11.20) | 8.83 (6.89 to 10.28) | 0.96 (0.43 to 1.41) |
| ICER, $/QALY | — | — | 11,687 |
Incremental values may not correspond exactly to the treatment arms due to rounding. 95% CrI, 95% credibility interval; QALY, quality-adjusted life year; ICER, incremental cost-effectiveness ratio; —, not applicable.
Treatment with dapagliflozin in addition to standard therapy was associated with increased total costs versus placebo with standard therapy in the United Kingdom ($6822; 95% CrI, −$3293 to $17,138), Germany ($17,671; 95% CrI, −$3328 to $35,900), and Spain ($11,168; 95% CrI, −$2903 to $24,614). This was driven by a combination of increased drug acquisition cost and CKD management costs as a result of increased life expectancy; however, reduced rates of CKD progression and kidney failure, among other clinical events, provided important cost offsets.
Estimated incremental costs and benefits translated to ICERs of $8280/QALY gained, $17,623/QALY gained, and $11,687/QALY gained in UK, German, and Spanish settings, respectively (Table 4; outcomes are provided in native currencies in Supplemental Table 8). The cumulative projected total costs and QALYs accrued over the modeled lifetime horizons in the United Kingdom, Germany, and Spain are presented in Supplemental Figure 5. Differences in cost-effectiveness between countries were driven by differences in drug acquisition costs, the cost of CKD management, and complications in each country. The countries with higher costs associated with background management of CKD had greater total incremental costs due to increased life expectancy, whereas those with larger relative costs relating to CKD progression demonstrated greater cost offsets associated with delayed CKD progression.
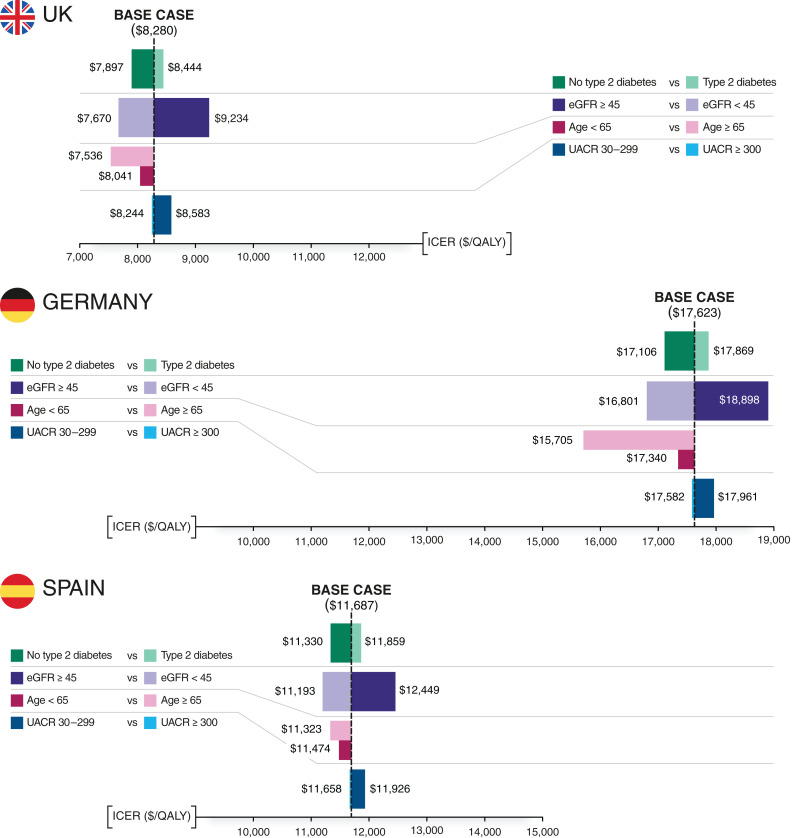
Subgroup Analyses
Model results were consistent across settings and patient subgroups, with all estimated ICERs falling well below established willingness-to-pay thresholds (Figure 2). Subgroups with the largest effect on cost-effectiveness varied by country, driven by differences in the relative cost of background CKD management. For example, the estimated ICER ($15,705/QALY) for patients in Germany aged 65 years or older was notably lower than the base case ICER ($17,623/QALY), owing to the higher costs attributed to CKD management in the German setting in combination with the reduced life expectancy of this subgroup.
Figure 2.
Subgroup analysis: change relative to the base case incremental cost-effectiveness ratio (ICER; discounted) in patient subgroups dichotomously stratified by baseline type 2 diabetes status, eGFR level, or urine albumin-creatinine ratio (UACR). The degree to which the ICER of the subgroup of interest increases or decreases relative to the ICER relating to the base case population receiving dapagliflozin with standard therapy versus standard therapy alone. QALY, quality-adjusted life year.
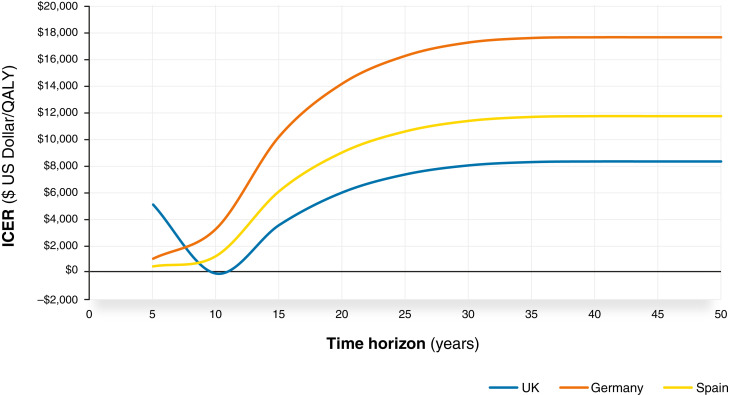
Scenario Analyses
Figure 3 presents the continuous effect of alternative time horizons. For analyses that ran over a period of 10 years or fewer, dapagliflozin was generally expected to be dominant versus placebo (cost saving with increased health benefit). ICERs increased gradually up to a lifetime horizon, remaining cost effective throughout and demonstrating that the conclusions are independent of the horizon selected, with life expectancy estimates comparable with real-world estimates (19).
Figure 3.
ICER (US dollars per QALY) evolution over time in the United Kingdom, Germany, and Spain. Negative ICERs represent scenarios where dapagliflozin is considered the dominant treatment option (i.e., a lower cost and higher QALYs compared with placebo).
When patients were assumed to discontinue dapagliflozin treatment after 3 years, dapagliflozin remains highly cost effective over all country settings (Supplemental Table 9). Patients receive an additional treatment benefit for 3 years, but they are not subject to lifetime costs of dapagliflozin, resulting in much lower incremental costs than in the base case analysis.
When the discontinuation rate for dapagliflozin tapers linearly to 0% over a 4-year period, ICERs across country settings were slightly increased due to the rise in treatment costs in the dapagliflozin arm as patients remain on dapagliflozin for longer. When compared with the base case, estimated life years and QALYs are increased because patients receive the benefits of dapagliflozin longer.
Sensitivity Analyses
Deterministic sensitivity analyses showed that the results were robust to the choice of input parameters (Supplemental Table 10).
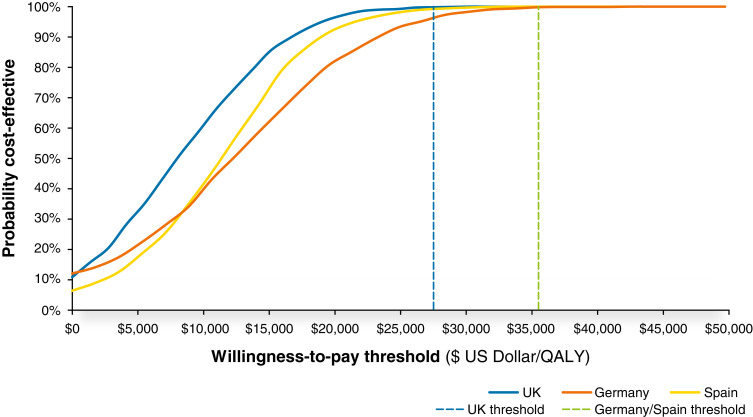
Results of the probabilistic sensitivity analysis were consistent with the deterministic analysis, indicating that model results are robust to joint uncertainty in the input parameters. Under probabilistic analysis, 99.8% of simulations were cost effective at a threshold of $27,510/QALY (£20,000/QALY) gained in the United Kingdom, with 99% and 100% being cost effective at a threshold of $35,503/QALY (€30,000/QALY) gained in Germany and Spain, respectively (Figure 4).
Figure 4.
Cost-effectiveness acceptability curves for the United Kingdom, Germany, and Spain. The probability of dapagliflozin with standard therapy being cost effective at a given willingness-to-pay threshold versus standard therapy alone. The established willingness-to-pay threshold for each country is indicated by a vertical line from the y axis.
Discussion
In this study, we developed a model driven by patient-level data from the pivotal DAPA-CKD trial to evaluate the potential long-term clinical and economic effects of dapagliflozin in the treatment of CKD. Our analysis indicates that dapagliflozin is expected to be a cost-effective treatment versus placebo when used in addition to standard therapy for a population reflective of the DAPA-CKD trial. Furthermore, the outcomes of this analysis align with recent cost-effectiveness analyses of dapagliflozin in other populations, including in high-risk patients with type 2 diabetes (20) and nondiabetic kidney disease (21), demonstrating the importance of slowing eGFR and UACR decline progression across interconnected disease populations. Empagliflozin has also demonstrated cost-effectiveness in an analysis of patient-level data from the BI 10773 (Empagliflozin) Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients (EMPA-REG OUTCOME) trial conducted in patients with diabetic kidney disease (22). Hence, treatment of CKD with dapagliflozin represents a good use of health care resources if adopted into clinical practice in Germany, Spain, and the United Kingdom, in line with recent published analyses in Thailand and the United States (21,23).
Results were consistent across UK, German, and Spanish health care settings and important patient subgroups, and they were primarily driven by attenuating disease progression, thereby delaying initiation of dialysis and kidney transplantation and reducing rates of hospitalization for heart failure and death. An estimated average increase in life expectancy with dapagliflozin of 1.7 years compares favorably with other treatments for CKD, such as ARBs, which have been estimated to extend life expectancy by 0.2 years in patients with CKD (24).
Delayed CKD progression to kidney failure and reduced incidence of hospitalization for heart failure provided important cost offsets to the drug acquisition cost of dapagliflozin. However, in German and Spanish settings, incremental CKD management costs exceeded incremental treatment costs due to the predicted gain in life expectancy and comparatively low treatment acquisition costs.
Although cost-effectiveness analysis is an effective method of establishing whether the incremental cost of new technology is justified by associated health gains and cost offsets, this approach does not consider the full value of a new treatment to a health care system. This is particularly relevant in patients with CKD given the resource requirements of kidney failure and provision of KRT, which impose a burden on health care systems beyond the consideration of the annual costs associated with their management due to capacity restraints. Therefore, the overall benefit of the addition of dapagliflozin to standard therapy may be underestimated in this analysis, as it does not capture the potential effect of this new therapy on health care service delivery. Furthermore, sodium-glucose cotransporter-2 inhibitors are associated with benefits not captured in this analysis, such as weight loss, improved glycemic control, decreased BP, and increased hemoglobin levels (25,26).
This study, as with any cost-effectiveness analysis, has several limitations. First, DAPA-CKD had a median follow-up of 2.4 years, and extrapolation of data beyond this time is subject to uncertainty. However, sensitivity analyses demonstrated that model results were robust to the choice of input parameters and the uncertainty associated with those parameters. Additionally, although the trial was stopped prematurely, potentially reducing the statistical power of some outcomes, the treatment effect was considered to have strong validity with minimal effect on extrapolations (10). Second, we assumed that heterogeneity between patients was adequately reflected by discrete eGFR-defined health states, and although outcomes may differ within individual health states, clinical guidelines for the management of CKD are aligned to eGFR-defined CKD stage classifications, consistent with those implemented within the model. Third, this analysis represents patients eligible for the DAPA-CKD trial, and therefore, it is not completely representative of the general CKD population. Therefore, modeled results on the basis of extrapolations of the DAPA-CKD trial are applicable only to patients reflective of the trial’s inclusion criteria. Finally, we were unable to use DAPA-CKD trial data to model outcomes in patients who started dialysis or underwent transplantation, for whom we did not have sufficient follow-up. Therefore, post-KRT outcomes were sourced from a published systematic literature review of CKD modeling.
In conclusion, our results show that dapagliflozin, added to standard therapy, is a cost-effective treatment option for CKD well below established willingness-to-pay thresholds in the United Kingdom, Germany, and Spain.
Disclosures
K. Bergenheim reports employment with and ownership interest in AstraZeneca. A. Briggs is a director and shareholder of Avalon Health Economics; reports consultancy agreements with Abbott Vascular, Amgen, AstraZeneca, Bayer, Daichii Sankyo, Eisai, GSK, Janssen, Merck & Co., Novartis, Roche, Sword Health, and Takeda; and reports ownership interest in Avalon Health Economics and Occam Research. O. Darlington reports employment with Health Economics & Outcomes Research Ltd., Cardiff, United Kingdom, which received fees from AstraZeneca in relation to this study. J.J. Garcia Sanchez reports employment with, ownership interest in, and research funding from AstraZeneca. H.J.L. Heerspink reports ongoing consultancy agreements with AbbVie, AstraZeneca, Bayer, Boehringer Ingelheim, Chinook, CSL Behring, Dimerix, Fresenius, Gilead, Goldfinch, Janssen, Merck, Mitsubishi Tanabe, Mundi Pharma, Novo Nordisk, and Travere Pharmaceuticals; research funding from AbbVie, AstraZeneca, Boehringer Ingelheim, Janssen research support (grant funding directed to employer), and Novo Nordisk; and speakers bureau for AstraZeneca. P. McEwan reports employment with Health Economics and Outcomes Research Ltd., Cardiff, United Kingdom, which received fees from AstraZeneca in relation to this study; consultancy agreements with Akebia Therapeutics Inc., AstraZeneca (Global), AstraZeneca Malaysia, Bayer Aktiengesellschaft, Genmab, Gilead Sciences, Medtelligence, Moderna, Novo Nordisk A/S, Novo Nordisk Ltd., RTI Health Solutions, SOBI Ltd., the Swedish Institute for Health Economics, Think Research, Third Bridge, Vifor Fresenius Medical Care Renal Pharma, Vifor (International) Ltd., and Wickenstones; research funding from Sanofi; honoraria from Akebia Therapeutics Inc., AstraZeneca (Global), AstraZeneca Malaysia, Bayer Aktiengesellschaft, Genmab, Gilead Sciences, Medtelligence, Moderna, Novo Nordisk A/S, Novo Nordisk Ltd., RTI Health Solutions, SOBI Ltd., the Swedish Institute for Health Economics, Think Research, Third Bridge, Vifor Fresenius Medical Care Renal Pharma, Vifor (International) Ltd., and Wickenstones; and serves as a National Institute for Health and Care Excellence board member. J.J.V. McMurray reports personal lecture fees from Abbott Diabetes Care, Alkem Metabolics, Eris Lifesciences, Hikma, Lupin, Medscape/Heart.Org, ProAdWise Communications, Radcliffe Cardiology, Servier, Sun Pharmaceutical Industries Inc., and The Corpus; other interests and relationships as the director of Global Clinical Trial Partners Ltd; nonfinancial support and other from AstraZeneca during the conduct of the study; nonfinancial support and other from Abbvie, Amgen, Cardiorentis, Oxford University/Bayer, and Theracos outside the submitted work; and other from Bayer, Bristol-Myers Squibb, DalCor, GlaxoSmithKline, Kidney Research UK, Merck, Novartis, Pfizer, and Vifor Fresenius. J.J.V. McMurray's employer, Glasgow University, has been paid for his participation in advisory boards organized by AstraZeneca and Novartis. Payments have been made to Glasgow University for J.J.V. McMurray's work on clinical trials, consulting, and other activities from Abbvie, Alnylam, Amgen, AstraZeneca, Bayer, BMS, Boehringer Ingelheim, Cardurion, Cytokinetics, DalCor, GSK, Ionis, KBP Biosciences, Novartis, Pfizer, and Theracos. R. Miller reports employment with Health Economics & Outcomes Research Ltd., Cardiff, United Kingdom, which received fees from AstraZeneca in relation to this study. D.C. Wheeler reports consultancy agreements with Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Gilead, GlaxoSmithKline, Janssen, Merck Sharp and Dohme, Mundipharma, Napp, Tricida, Vifor Fresenius, and Zydus; honoraria from Amgen, Astellas, AstraZeneca, Bayer, Boehringer Ingelhiem, GlaxoSmithKline, Janssen, Medscape, Merck Sharp and Dohme, Mundipharma, Napp, Pharmacosmos, Reata, Takeda, and Vifor Fresenius; an advisory or leadership role for AstraZeneca; speakers bureau for Amgen, Astellas, AstraZeneca, Janssen, Merck Sharp and Dohme, Mundipharma, Napp, and Vifor Fresenius; and other interests and relationships as an Honorary Professorial Fellow of the George Institute for Global Health.
Funding
This work was supported by AstraZeneca, which provided support for the analysis and medical writing for this study.
Supplementary Material
Acknowledgments
Medical writing and editorial support were provided by Peter Gabb of Health Economics and Outcomes Research Ltd.
The funding agreement with AstraZeneca ensured the authors’ independence in designing the study, interpreting the data, and preparing the manuscript for publication.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “A Blueprint for Assessing Affordability of SGLT2 Inhibitors in the United States: The Cost-Effectiveness of Dapagliflozin in Three European Countries,” on pages 1707–1709.
Author Contributions
K. Bergenheim, A. Briggs, O. Darlington, J.J. Garcia Sanchez, H.J.L. Heerspink, P. McEwan, J.J.V. McMurray, and D.C. Wheeler conceptualized the study; O. Darlington and R. Miller were responsible for investigation; R. Miller was responsible for formal analysis; A. Briggs, O. Darlington, J.J. Garcia Sanchez, P. McEwan, and R. Miller were responsible for methodology; J.J. Garcia Sanchez was responsible for project administration; J.J. Garcia Sanchez was responsible for resources; A. Briggs, H.J.L Heerspink, J.J.V. McMurray, and D.C. Wheeler were responsible for validation; J.J. Garcia Sanchez was responsible for funding acquisition; P. McEwan provided supervision; O. Darlington and P. McEwan wrote the original draft; and A. Briggs, K. Bergenheim, J.J. Garcia Sanchez, H.J.L. Heerspink, J.J.V. McMurray, and D.C. Wheeler reviewed and edited the manuscript.
Data Sharing Statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data sharing policy, which is described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.03790322/-/DCSupplemental.
Supplemental Material. Supplemental text.
Supplemental Table 1. Patient profile.
Supplemental Table 2. CKD transition matrix—dapagliflozin and standard therapy.
Supplemental Table 3. CKD transition matrix—placebo and standard therapy.
Supplemental Table 4. Parameterizations of adjusted all-cause mortality parametric (Gompertz) survival equations.
Supplemental Table 5. Adjusted generalized estimating equations predicting hospitalization for heart failure events.
Supplemental Table 6. Adjusted generalized estimating equations predicting acute decline in kidney function events.
Supplemental Table 7. Annual probability of clinical and adverse events.
Supplemental Table 8. Base case discounted health economic results (native currency).
Supplemental Table 9. The effect of alternative treatment discontinuation assumptions.
Supplemental Table 10. Deterministic sensitivity analysis.
Supplemental Figure 1. Stacked area plot of predicted (dashed lines) and observed (solid lines) patient distribution in CKD stages in the model and DAPA-CKD trial, respectively.
Supplemental Figure 2. Observed (solid lines) and predicted (dotted lines) incidence of all-cause mortality.
Supplemental Figure 3. Extrapolated mortality rate of patients treated with dapagliflozin and standard therapy versus placebo and standard therapy over a lifetime horizon using a Gompertz survival equation.
Supplemental Figure 4. Observed (rhombuses) and predicted (circles) incidence of acute decline in kidney function and hospitalization for heart failure across subgroups.
Supplemental Figure 5. Cumulative accrual of QALYs (left panels) and total costs (right panels) in patients treated with dapagliflozin and standard therapy (blue) and standard therapy only (grey) in the United Kingdom (top panel), Germany (middle panel), and Spain settings (bottom panel).
References
- 1.Hill NR, Fatoba ST, Oke JL, Hirst JA, O’Callaghan CA, Lasserson DS, Hobbs FDR: Global prevalence of chronic kidney disease: A systematic review and meta-analysis. PLoS One 11: e0158765, 2016. 10.1371/journal.pone.0158765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Midtvedt K, Heldal K: Chronic kidney disease and the aging population. Transplantation 97: e64, 2014. 10.1097/tp.0000000000000172 [DOI] [PubMed] [Google Scholar]
- 3.Thomas MC, Cooper ME, Zimmet P: Changing epidemiology of type 2 diabetes mellitus and associated chronic kidney disease. Nat Rev Nephrol 12: 73–81, 2016. 10.1038/nrneph.2015.173 [DOI] [PubMed] [Google Scholar]
- 4.Jankowski J, Floege J, Fliser D, Böhm M, Marx N: Cardiovascular disease in chronic kidney disease: Pathophysiological insights and therapeutic options. Circulation 143: 1157–1172, 2021. 10.1161/circulationaha.120.050686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palaka E, Grandy S, van Haalen H, McEwan P, Darlington O: The impact of CKD anaemia on patients: Incidence, risk factors, and clinical outcomes—A systematic literature review. Int J Nephrol 2020: 7692376, 2020. 10.1155/2020/7692376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inker LA, Grams ME, Levey AS, Coresh J, Cirillo M, Collins JF, Gansevoort RT, Gutierrez OM, Hamano T, Heine GH, Ishikawa S, Jee SH, Kronenberg F, Landray MJ, Miura K, Nadkarni GN, Peralta CA, Rothenbacher D, Schaeffner E, Sedaghat S, Shlipak MG, Zhang L, van Zuilen AD, Hallan SI, Kovesdy CP, Woodward M, Levin A; CKD Prognosis Consortium : Relationship of estimated GFR and albuminuria to concurrent laboratory abnormalities: An individual participant data meta-analysis in a global consortium. Am J Kidney Dis 73: 206–217, 2019. 10.1053/j.ajkd.2018.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Institute for Health and Care Excellence : Renal Replacement Therapy and Conservative Management. NICE guideline [NG107], 2018. Available at: https://www.nice.org.uk/guidance/ng107. Accessed October 30, 2020 [PubMed]
- 8.Pagels AA, Söderkvist BK, Medin C, Hylander B, Heiwe S: Health-related quality of life in different stages of chronic kidney disease and at initiation of dialysis treatment. Health Qual Life Outcomes 10: 71, 2012. 10.1186/1477-7525-10-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vanholder R, Annemans L, Brown E, Gansevoort R, Gout-Zwart JJ, Lameire N, Morton RL, Oberbauer R, Postma MJ, Tonelli M, Biesen WV, Zoccali C; European Kidney Health Alliance : Reducing the costs of chronic kidney disease while delivering quality health care: A call to action. Nat Rev Nephrol 13: 393–409, 2017. 10.1038/nrneph.2017.63 [DOI] [PubMed] [Google Scholar]
- 10.Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou F-F, Mann JFE, McMurray JJV, Lindberg M, Rossing P, Sjöström CD, Toto RD, Langkilde A-M, Wheeler DC; DAPA-CKD Trial Committees and Investigators : Dapagliflozin in patients with chronic kidney disease. N Engl J Med 383: 1436–1446, 2020. 10.1056/NEJMoa2024816 [DOI] [PubMed] [Google Scholar]
- 11.Wheeler DC, Stefansson BV, Batiushin M, Bilchenko O, Cherney DZI, Chertow GM, Douthat W, Dwyer JP, Escudero E, Pecoits-Filho R, Furuland H, Górriz JL, Greene T, Haller H, Hou FF, Kang S-W, Isidto R, Khullar D, Mark PB, McMurray JJV, Kashihara N, Nowicki M, Persson F, Correa-Rotter R, Rossing P, Toto RD, Umanath K, Van Bui P, Wittmann I, Lindberg M, Sjöström CD, Langkilde AM, Heerspink HJL: The Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease (DAPA-CKD) trial: Baseline characteristics. Nephrol Dial Transplant 35: 1700–1711, 2020. 10.1093/ndt/gfaa234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heerspink HJL, Stefansson BV, Chertow GM, Correa-Rotter R, Greene T, Hou F-F, Lindberg M, McMurray J, Rossing P, Toto R, Langkilde AM, Wheeler DC; DAPA-CKD Investigators : Rationale and protocol of the Dapagliflozin And Prevention of Adverse outcomes in Chronic Kidney Disease (DAPA-CKD) randomized controlled trial. Nephrol Dial Transplant 35: 274–282, 2020. 10.1093/ndt/gfz290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McEwan P, Darlington O, McMurray JJV, Jhund PS, Docherty KF, Böhm M, Petrie MC, Bergenheim K, Qin L: Cost-effectiveness of dapagliflozin as a treatment for heart failure with reduced ejection fraction: A multinational health-economic analysis of DAPA-HF. Eur J Heart Fail 22: 2147–2156, 2020. 10.1002/ejhf.1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sugrue DM, Ward T, Rai S, McEwan P, van Haalen HGM: Economic modelling of chronic kidney disease: A systematic literature review to inform conceptual model design. PharmacoEconomics 37: 1451–1468, 2019. 10.1007/s40273-019-00835-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Institute for Health and Care Excellence : Guide to the Methods of Technology Appraisal, 2013. Available at: https://www.nice.org.uk/process/pmg9/resources/guide-to-the-methods-of-technology-appraisal-2013-pdf-2007975843781. Accessed October 28, 2020 [PubMed]
- 16.Organisation for Economic Co-operation and Development (OECD) : Exchange Rates, 2022. Available at: https://data.oecd.org/conversion/exchange-rates.htm. Accessed May 25, 2022
- 17.Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen : General Methods for the Assessment of the Relation of Benefits to Costs. Available at: https://www.iqwig.de/download/General_Methods_for_the_Assessment_of_the_Relation_of_Benefits_to_Costs.pdf. Accessed October 30, 2020
- 18.López-Bastida J, Oliva J, Antoñanzas F, García-Altés A, Gisbert R, Mar J, Puig-Junoy J: Spanish recommendations on economic evaluation of health technologies. Eur J Health Econ 11: 513–520, 2010. 10.1007/s10198-010-0244-4 [DOI] [PubMed] [Google Scholar]
- 19.Turin TC, Tonelli M, Manns BJ, Ravani P, Ahmed SB, Hemmelgarn BR: Chronic kidney disease and life expectancy. Nephrol Dial Transplant 27: 3182–3186, 2012. 10.1093/ndt/gfs052 [DOI] [PubMed] [Google Scholar]
- 20.McEwan P, Morgan AR, Boyce R, Bergenheim K, Gause-Nilsson IAM, Bhatt DL, Leiter LA, Johansson PA, Mosenzon O, Cahn A, Wilding JPH: The cost-effectiveness of dapagliflozin in treating high-risk patients with type 2 diabetes mellitus: An economic evaluation using data from the DECLARE-TIMI 58 trial. Diabetes Obes Metab 23: 1020–1029, 2021. 10.1111/dom.14308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tisdale RL, Cusick MM, Aluri KZ, Handley TJ, Joyner AKC, Salomon JA, Chertow GM, Goldhaber-Fiebert JD, Owens DK: Cost-effectiveness of dapagliflozin for non-diabetic chronic kidney disease. J Gen Intern Med 37: 3380–3387, 2022. 10.1007/s11606-021-07311-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reifsnider OS, Kansal AR, Wanner C, Pfarr E, Koitka-Weber A, Brand SB, Stargardter M, Wang C, Kuti E, Ustyugova A: Cost-effectiveness of empagliflozin in patients with diabetic kidney disease in the United States: Findings based on the EMPA-REG OUTCOME trial. Am J Kidney Dis 79: 796–806, 2022. 10.1053/j.ajkd.2021.09.014 [DOI] [PubMed] [Google Scholar]
- 23.Vareesangthip K, Deerochanawong C, Thongsuk D, Pojchaijongdee N, Permsuwan U: Cost-utility analysis of dapagliflozin as an add-on to standard of care for patients with chronic kidney disease in Thailand. Adv Ther 39: 1279–1292, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Palmer AJ, Annemans L, Roze S, Lamotte M, Rodby RA, Bilous RW: An economic evaluation of the Irbesartan in Diabetic Nephropathy Trial (IDNT) in a UK setting. J Hum Hypertens 18: 733–738, 2004. 10.1038/sj.jhh.1001729 [DOI] [PubMed] [Google Scholar]
- 25.Oshima M, Neuen BL, Jardine MJ, Bakris G, Edwards R, Levin A, Mahaffey KW, Neal B, Pollock C, Rosenthal N, Wada T, Wheeler DC, Perkovic V, Heerspink HJL: Effects of canagliflozin on anaemia in patients with type 2 diabetes and chronic kidney disease: A post-hoc analysis from the CREDENCE trial. Lancet Diabetes Endocrinol 8: 903–914, 2020. 10.1016/S2213-8587(20)30300-4 [DOI] [PubMed] [Google Scholar]
- 26.Pinto LC, Rados DV, Remonti LR, Kramer CK, Leitao CB, Gross JL: Efficacy of SGLT2 inhibitors in glycemic control, weight loss and blood pressure reduction: A systematic review and meta-analysis. Diabetol Metab Syndr 7[Suppl 1]: A58, 2015. 10.1186/1758-5996-7-S1-A58 [DOI] [Google Scholar]
- 27.Haymarket Media Group : Database of Prescription and Generic Drugs, Clinical Guidelines. Monthly Index of Medical Specialities. Available at: https://www.mims.co.uk/. Accessed October 30, 2020
- 28.Deutsches Institut fur Medizinische Dokumentation und Information : Index. Available at: https://portal.dimdi.de/festbetragsrecherche/browseIndex.xhtml. Accessed October 28, 2020
- 29.Department of Health : NHS reference costs 2019 to 2020. Available at: https://www.england.nhs.uk/national-cost-collection/. Accessed October 30, 2020
- 30.World Health Organization : CHOosing Interventions that are Cost Effective (WHO-CHOICE). Available at: https://www.who.int/teams/health-systems-governance-and-financing/economic-analysis/costing-and-technical-efficiency/quantities-and-unit-prices-(cost-inputs). Accessed October 28, 2020 [DOI] [PMC free article] [PubMed]
- 31.Kent S, Schlackow I, Lozano-Kühne J, Reith C, Emberson J, Haynes R, Gray A, Cass A, Baigent C, Landray MJ, Herrington W, Mihaylova B; SHARP Collaborative Group : What is the impact of chronic kidney disease stage and cardiovascular disease on the annual cost of hospital care in moderate-to-severe kidney disease? BMC Nephrol 16: 65, 2015. 10.1186/s12882-015-0054-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Darbà J, Marsà A: Chronic kidney disease in Spain: Analysis of patient characteristics, incidence and direct medical costs (2011-2017). J Med Econ 23: 1623–1629, 2020. 10.1080/13696998.2020.1830782 [DOI] [PubMed] [Google Scholar]
- 33.Gandjour A, Armsen W, Wehmeyer W, Multmeier J, Tschulena U: Costs of patients with chronic kidney disease in Germany. PLoS One 15: e0231375, 2020. 10.1371/journal.pone.0231375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lorenzo-Sellares V, Pedrosa MI, Santana-Expósito B, García-González Z, Barroso-Montesinos M: Análisis de costes y perfil sociocultural del enfermo renal: Impacto de la modalidad de tratamiento. Nefrología (Madrid) 34: 458–468, 2014 [DOI] [PubMed] [Google Scholar]
- 35.Icks A, Haastert B, Gandjour A, Chernyak N, Rathmann W, Giani G, Rump L-C, Trapp R, Koch M: Costs of dialysis—A regional population-based analysis. Nephrol Dial Transplant 25: 1647–1652, 2010. 10.1093/ndt/gfp672 [DOI] [PubMed] [Google Scholar]
- 36.National Health Service Blood and Transplant : Cost-effectiveness of transplantation, 2009. Available at: https://nhsbtmediaservices.blob.core.windows.net/organ-donation-assets/pdfs/Organ_Donation_Registry_Fact_Sheet_7_21337.pdf. Accessed October 30, 2020
- 37.Kleophas W, Reichel H: International study of health care organization and financing: Development of renal replacement therapy in Germany. Int J Health Care Finance Econ 7: 185–200, 2007. 10.1007/s10754-007-9020-0 [DOI] [PubMed] [Google Scholar]
- 38.Región de Murcia Consejería de Salud : El Conjunto Mínimo Básico de Datos. Informe regional CMBD 2015 Urología. Available at: https://www.murciasalud.es/pagina.php?id=154065. Accessed October 30, 2020
- 39.Kent S, Briggs A, Eckermann S, Berry C: Are value of information methods ready for prime time? An application to alternative treatment strategies for NSTEMI patients. Int J Technol Assess Health Care 29: 435–442, 2013. 10.1017/s0266462313000433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gesundheitsberichterstattung des Bundes : Das Informationssystem der Gesundheitsberichterstattung des Bundes. Available at: http://www.gbe-bund.de/oowa921-install/servlet/oowa/aw92/WS0100/_XWD_FORMPROC. Accessed October 28, 2020
- 41.Personal Social Services Research Unit : Unit Costs of Health and Social Care, 2021. Available at: https://www.pssru.ac.uk/project-pages/unit-costs/unit-costs-2020/. Accessed June 28, 2021
- 42.Hammer M, Lammert M, Mejías SM, Kern W, Frier BM: Costs of managing severe hypoglycaemia in three European countries. J Med Econ 12: 281–290, 2009. 10.3111/13696990903336597 [DOI] [PubMed] [Google Scholar]
- 43.Parekh W, Hoskins N, Baker-Knight J, Ramirez de Arellano A, Mezquita Raya P: The economic burden of insulin-related hypoglycemia in Spain. Diabetes Ther 8: 899–913, 2017. 10.1007/s13300-017-0285-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dhatariya KK, Skedgel C, Fordham R: The cost of treating diabetic ketoacidosis in the UK: A national survey of hospital resource use. Diabet Med 34: 1361–1366, 2017. 10.1111/dme.13427 [DOI] [PubMed] [Google Scholar]
- 45.Bleibler F, König H-H: Cost-effectiveness of intravenous 5 mg zoledronic acid to prevent subsequent clinical fractures in postmenopausal women after hip fracture: A model-based analysis. Hamburg Center for Health Economics Research Paper No. 2016/10, Hamburg, Germany, University of Hamburg, Hamburg Center for Health Economics, 2016
- 46.Oblikue Consulting : eSalud - Información económica del sector sanitario. Available at: http://esalud.oblikue.com/. Accessed October 28, 2020
- 47.Alva ML, Gray A, Mihaylova B, Leal J, Holman RR: The impact of diabetes-related complications on healthcare costs: New results from the UKPDS (UKPDS 84). Diabet Med 32: 459–466, 2015. 10.1111/dme.12647 [DOI] [PubMed] [Google Scholar]
- 48.Kähm K, Laxy M, Schneider U, Rogowski WH, Lhachimi SK, Holle R: Health care costs associated with incident complications in patients with type 2 diabetes in Germany. Diabetes Care 41: 971–978, 2018. 10.2337/dc17-1763 [DOI] [PubMed] [Google Scholar]
- 49.AstraZeneca : A Study to Evaluate the Effect of Dapagliflozin on Renal Outcomes and Cardiovascular Mortality in Patients with Chronic Kidney Disease (Dapa-CKD), 2021. Available at: https://clinicaltrials.gov/ct2/show/NCT03036150. Accessed June 4, 2020
- 50.Lee AJ, Morgan CL, Conway P, Currie CJ: Characterisation and comparison of health-related quality of life for patients with renal failure. Curr Med Res Opin 21: 1777–1783, 2005. 10.1185/030079905x65277 [DOI] [PubMed] [Google Scholar]
- 51.McEwan P, Darlington O, McMurray JJV, Jhund PS, Docherty KF, Böhm M, Petrie MC, Bergenheim K, Qin L: Cost-effectiveness of dapagliflozin as a treatment for heart failure with reduced ejection fraction: A multinational health-economic analysis of DAPA-HF. Eur J Heart Fail 22: 2147–2156, 2020. 10.1002/ejhf.1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Beaudet A, Clegg J, Thuresson PO, Lloyd A, McEwan P: Review of utility values for economic modeling in type 2 diabetes. Value Health 17: 462–470, 2014. 10.1016/j.jval.2014.03.003 [DOI] [PubMed] [Google Scholar]
- 53.Currie CJ, Morgan CL, Poole CD, Sharplin P, Lammert M, McEwan P: Multivariate models of health-related utility and the fear of hypoglycaemia in people with diabetes. Curr Med Res Opin 22: 1523–1534, 2006. 10.1185/030079906X115757 [DOI] [PubMed] [Google Scholar]
- 54.Peasgood T, Brennan A, Mansell P, Elliott J, Basarir H, Kruger J: The impact of diabetes-related complications on preference-based measures of health-related quality of life in adults with type I diabetes. Med Decis Making 36: 1020–1033, 2016. 10.1177/0272989x16658660 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.