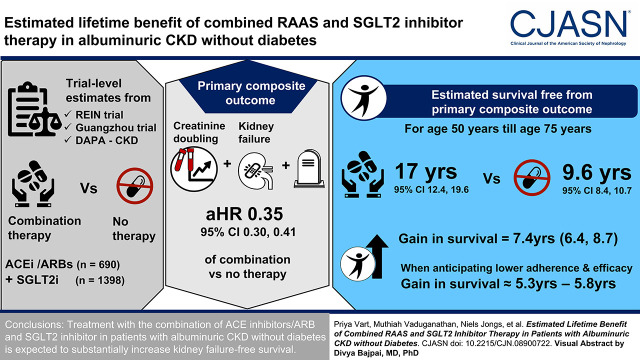
Visual Abstract
Keywords: chronic kidney disease, chronic kidney failure, death, treatment, ACEi/ARB, SGLT2 inhibitor, lifetime benefit, renin-angiotensin system
Abstract
Background and objectives
Despite high rates of complications in patients with CKD without diabetes, the implementation of proven therapies in this group remains low. Expressing the clinical benefit of a therapy in terms of extra years free from the disease or death may facilitate implementation. We estimated lifetime survival free of kidney failure for patients with albuminuric CKD without diabetes treated with the combination therapy of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers and sodium-glucose cotransporter-2 (SGLT2) inhibitors relative to patients not treated.
Design, setting, participants, & measurements
We used trial-level estimates of the effect of treatment with angiotensin-converting enzyme inhibitors/angiotensin receptor blockers (ramipril/benazepril; n=690) and SGLT2 inhibitors (dapagliflozin; n=1398) compared with placebo to derive the effect of combination therapy versus no treatment. Using this effect, we estimated treatment effect of combination therapy to the active treatment group of patients with albuminuric CKD without diabetes participating in the Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease (DAPA-CKD) trial (n=697) and projected eventfree and overall survival for those treated and not treated with combination therapy. We also performed our calculations anticipating lower adherence and less pronounced benefits than were observed in the clinical trials. The primary outcome was a composite of doubling of serum creatinine, kidney failure, or death.
Results
The aggregate estimated hazard ratio comparing combination therapy with angiotensin-converting enzyme inhibitors/angiotensin receptor blockers and SGLT2 inhibitor versus no treatment for the primary end point was 0.35 (95% confidence interval, 0.30 to 0.41). For a 50-year-old patient until the age of 75 years, the estimated survival free from the primary composite end point was 17.0 (95% confidence interval, 12.4 to 19.6) years with the combination therapy and 9.6 years (95% confidence interval, 8.4 to 10.7) with no treatment with any of these agents, corresponding to a gain in eventfree survival of 7.4 (95% confidence interval, 6.4 to 8.7) years. When assuming lower adherence and less pronounced efficacy of combination therapy, the gain in eventfree survival ranged from 5.3 years (95% confidence interval, 4.4 to 6.1) to 5.8 years (95% confidence interval, 4.8 to 6.8).
Conclusions
Treatment with the combination of angiotensin-converting enzyme inhibitors/angiotensin receptor blockers and SGLT2 inhibitor in patients with albuminuric CKD without diabetes is expected to substantially increase kidney failure–free survival.
Clinical Trial registry name and registration number:
Benazepril for Advanced Chronic Renal Insufficiency, NCT00270426, and a Study to Evaluate the Effect of Dapagliflozin on Renal Outcomes and Cardiovascular Mortality in Patients with Chronic Kidney Disease (Dapa-CKD), NCT03036150
Introduction
About half of patients with CKD do not have diabetes but experience high rates of kidney failure and mortality (1). These patients are typically treated with angiotensin-converting enzyme (ACE) inhibitors and/or angiotensin receptor blockers (ARBs), particularly when presenting with albuminuria (2). Recently, the sodium-glucose cotransporter-2 (SGLT2) inhibitor dapagliflozin was shown to reduce the risk of progressive kidney disease by >40% and to significantly reduce the risk of hospitalized heart failure and death from cardiovascular and noncardiovascular causes in patients with CKD and albuminuria, nearly all of whom were already treated with ACE inhibitors/ARBs. Despite these observed benefits, the implementation of proven therapies in routine clinical practice remains low. For instance, studies using real-world data suggest that about 50%–70% of patients with CKD in whom ACE inhibitor/ARB treatment is recommended are not actively treated, particularly patients with nondiabetic CKD (3,4). Consequently, understanding barriers to implementation and development of tools that could increase uptake of medication in these patients are highly desired.
In clinical trials, the benefit of treatment is typically expressed in the form of relative risk reduction, which may be difficult to communicate to patients, clinicians, and policy makers and thereby may contribute to delays in implementation of proven therapies in practice. Because patients with CKD are typically treated for a lifetime and different patients start treatment at different ages, expressing the clinical benefit of a therapy in terms of extra years free from the disease or death may facilitate risk communication in clinical management, increase uptake of these therapies in clinical practice, and inform decision making by policy makers and payers.
Therefore, in this study, we estimated the benefit of pharmacologic treatment with the combination of ACE inhibitor/ARB and SGLT2 inhibitor versus no treatment with any of these agents in patients with albuminuric CKD without diabetes. To this end, we derived aggregate relative risk reduction for the combination therapy from pivotal randomized clinical trials testing individual therapies and used validated actuarial methods (5,6) to project absolute eventfree survival gains for kidney failure and mortality.
Materials and Methods
Overall Study Design
In this study, using overall trial-level estimates from pivotal randomized clinical trials that assessed the efficacy and safety of an ACE inhibitor/ARB and SGLT2 inhibitor in patients with CKD without diabetes, we estimated the cumulative effect of combination therapy with ACE inhibitors/ARBs and SGLT2 inhibitors compared with no treatment. To obtain trial-level estimates, we used data from the Ramipril Efficacy in Nephropathy (REIN) trial (7,8), the Guangzhou trial from Guangzhou, China (9), and the Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease (DAPA-CKD) trial (10). For validation of eventfree survival estimates in a nonclinical trial setting, we modeled data from the Chronic Renal Insufficiency Cohort (CRIC). Participants enrolled in each clinical trial and the CRIC observational study provided written consent. The study protocols of the clinical trials included and the CRIC study were approved by the institutional review board at each participating site.
Clinical Trials
The Ramipril Efficacy in Nephropathy Trial.
Between 1994 and 1995, the REIN trial included 352 patients aged between 18 and 70 years who were normotensive or hypertensive, had chronic nephropathy and persistent proteinuria (i.e., urinary protein excretion of ≥1 g/d for at least 3 months without evidence of urinary tract infection or overt heart failure), and had not received ACE inhibitor therapy for at least 2 months. In a stratified randomization procedure on the basis of proteinuria, 177 patients were randomized to ramipril, and 175 were randomized to the placebo group; these patients were followed for a median duration of 2.1 years (7,8).
The Guangzhou Trial.
Between 1999 and 2001, investigators randomized 422 patients with CKD who were 18–70 years of age with serum creatinine concentrations of 1.5–5.0 mg/dl and urinary protein excretion of >0.3 g/d for ≥3 months and who had not received an ACE inhibitor/ARB for at least 6 weeks. After an 8-week run-in period, investigators provided 104 patients with serum creatinine concentrations of 1.5–3.0 mg/dl with 20 mg of benazepril per day, and they randomized 224 patients with serum creatinine concentrations of 3.1–5.0 mg/dl to 20 mg of benazepril per day or placebo. All patients were treated according to local guidelines. Patients were followed for a median duration of 3.0 years (9).
The Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease Trial.
Between 2017 and 2020, the DAPA-CKD trial enrolled 4304 patients aged 18 years or older with CKD and with or without a diagnosis of diabetes, eGFR between 25 and 75 ml/min per 1.73 m2, and a urinary albumin-creatinine ratio (UACR) between 200 and 5000 mg/g. Randomized patients were required to receive an ACE inhibitor or ARB at the maximally tolerated dose as part of standard of care; 97% of participants were receiving ACE inhibitor or ARB at baseline. Eligible patients were randomly assigned to dapagliflozin 10 mg once daily or a placebo. The median duration of follow-up was 2.4 years. For this analysis, we incorporated data from patients without diabetes at baseline (11).
Observational Cohort
The CRIC study is a multicenter, longitudinal, observational cohort study from the United States that enrolled a racially/ethnically diverse sample of persons with CKD (n=3939) (10). Participants were between the ages of 21 and 74 years with a mean eGFR of 43.4 ml/min per 1.73 m2 and a median urinary protein excretion of 0.17 g/d in the original cohort. A total of 2031 (52%) CRIC study participants were not diagnosed with diabetes at baseline. For this analysis, we incorporated data from patients without diabetes and not on ACE inhibitors/ARBs with UACR>300 mg/g. The CRIC study protocol was approved by the institutional review board at each participating site.
Clinical Outcomes
The primary end point for this analysis was a composite of a sustained doubling of serum creatinine, kidney failure (defined as a sustained eGFR ≤15 ml/min per 1.73 m2, initiation of dialysis for at least 30 days, or kidney transplantation), or all-cause mortality. The secondary end point was a composite of sustained doubling of serum creatinine or kidney failure.
Statistical Methods
We estimated the treatment effect with an ACE inhibitor, ramipril or benazepril, from a meta-analysis of the individual patient data from the REIN trial and the Guangzhou trial. We estimated the effect of treatment with an SGLT2 inhibitor, dapagliflozin, from the DAPA-CKD trial. Assuming independent treatment effects of the ACE inhibitors/ARBs and SGLT2 inhibitors, we determined the combined effect of ACE inhibitors/ARBs and SGLT2 inhibitors compared with no treatment using established methods of indirect comparisons (commonly used to assess treatment effects if a placebo was selected in an active-controlled trial) (12,13). We estimated the 95% confidence interval (95% CI) for the combined effect from the square root of the sum of squared standard errors of the individual logarithmic hazard ratios (HRs).
We calculated eventfree survival related to combination therapy with ACE inhibitors/ARBs and SGLT2 inhibitors using data derived from DAPA-CKD participants without diabetes at baseline randomized to dapagliflozin. To estimate eventfree survival when not treated with either ACE inhibitor/ARB or SGLT2 inhibitor, we applied the inverse of the effect of combination therapy to the individual patient data of DAPA-CKD participants without diabetes at baseline. By using the inverse of the combination therapy effect, we were able to estimate eventfree survival for patients (theoretically) not treated with ACE inhibitors/ARBs and SGLT2 inhibitors. Because estimated eventfree survival and overall survival depend on the age at the start of the treatment, we calculated and compared projected eventfree survival estimates for patients of every age between 50 and 65 years until the age of 75 years. To estimate eventfree survival starting at different ages, we used validated age-based methods of calculating nonparametric Kaplan–Meier estimates using age (at baseline and at the time of an event or death) as the time component rather than time from randomization (5). The area under the survival curve reflected projected eventfree survival. Age treatment interactions were not observed in any of the included trials, and consequently, differences in survival curves reflected projected gain in eventfree survival. We applied the inverse of upper and lower bounds of the effect of combination therapy to DAPA-CKD participants randomized to dapagliflozin without diabetes at baseline to estimate uncertainty around these survival gains. Estimates of survival gains were smoothed with a locally weighted scatterplot smoothing procedure (i.e., Lowess smoothing) (14).
We performed several additional analyses. First, we estimated gain in eventfree survival for the primary and secondary composite end points in a nonclinical trial setting using data from patients without diabetes at baseline enrolled in the CRIC study. Because a substantial number of patients in the CRIC study were not treated with ACE inhibitors/ARBs and because SGLT2 inhibitors were not used in persons without diabetes, we were able to compare and validate the projected eventfree survival derived from the DAPA-CKD trial when not treated with either ACE inhibitors/ARBs or SGLT2 inhibitors. In the CRIC study analysis, patients were not censored for change in ACEi/ARB use during follow-up because these data were not verified against objective assessment (e.g., pharmacy claim), and the reported ACEi/ARB use was suggested to be stable over time (15). Second, we estimated gain in eventfree survival when comparing treatment with SGLT2 inhibitors with the treatment with ACE inhibitors/ARB inhibitors. For this purpose, we used data from the treatment and placebo arms of the DAPA-CKD trial. Third, we estimated the benefits of combination therapy when assuming scenarios regarding the additivity of the effects of individual treatment (assuming effect additivity of SGLT2 inhibitors of between 120% and 50% of the reported treatment efficacy), adherence to combined medication (assuming medication adherence between 50% and 100% of the observed adherence included clinical trials), change in treatment efficacy over the years after the start of treatment (assuming a yearly change in the efficacy of the combination therapy between a 3% yearly increase and a 10% yearly decline compared with the previous year), and a combination of these mentioned scenarios. Finally, in a sensitivity analysis, we repeated our analyses when directly applying the combination therapy effect to the placebo group of the REIN trial and Guangzhou trial. Because the REIN trial and the Guangzhou trial did not include patients older than 70 years, we estimated eventfree survival between 50 and 70 years.
We conducted all analyses using Stata 17 (StataCorp. 2021 Stata Statistical Software: Release 17; StataCorp LLC, College Station, TX). Two-sided P values of 0.05 were considered statistically significant.
Results
Baseline characteristics of patients in the REIN trial (n=322), the Guangzhou trial (n=328), and the DAPA-CKD trial (n=4304) are shown in Table 1. Mean age ranged from 45 to 62 years, mean eGFR ranged from 20 to 43 ml/min per 1.73 m2, and median UACR ranged from 949 to 1499 mg/g.
Table 1.
Baseline patient characteristics and background medical therapy
| Characteristics | Ramipril Efficacy in Nephropathy, n=322 | Guangzhou, China, n=328 | Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease, n=4304 |
|---|---|---|---|
| Treatment | Ramipril versus placebo | Benazepril versus placebo | Dapagliflozin versus placebo |
| Enrollment period | 1994–1995 | 1999–2001 | 2017–2020 |
| Age, yr | 49±14 | 45±15 | 62±12 |
| Women, N (%) | 73 (23) | 162 (49) | 1425 (33) |
| Race, N (%) | |||
| White | 320 (99) | — | 2290 (53) |
| Black | 2 (0.6) | — | 191 (4) |
| Asian | — | 376 (100) | 1467 (34) |
| Other | — | — | 356 (8.3) |
| Systolic BP, mm Hg | 144±18 | 152±25 | 137±17 |
| eGFR, ml/min per 1.73 m2 | 39±18 | 20±9 | 43±12 |
| Urinary albumin-creatinine ratio, mg/g | 1499 (774, 2506)a | 1484 (835, 2133)a | 949 (477, 1885) |
| Weight, kg | 72±12 | 60±12 | 82±21 |
| Baseline medications, N (%) | |||
| ACEi/ARB | 160 (50) | 216 (66) | 4174 (97) |
| Diuretics | NA | 181 (55) | 1882 (44) |
| β-blockers | NA | 163 (50) | 1680 (39) |
—, no data; ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; NA, not available.
Estimated.
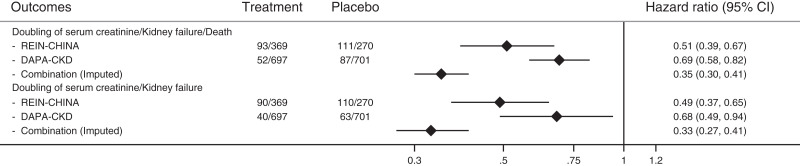
The estimated HRs for the combination of ACE inhibitor/ARB and SGLT2 inhibitor versus no treatment with any of these agents for the primary composite end point of doubling of serum creatinine, kidney failure, or all-cause death was 0.35 (95% CI, 0.30 to 0.41) (Figure 1), and the estimated HR for the doubling of serum creatinine or kidney failure was 0.33 (95% CI, 0.27 to 0.41) (Figure 1).
Figure 1.
Estimated relative treatment effects of combination treatment with the renin-angiotensin-aldosterone system and sodium-glucose cotransporter-2 inhibitors on key outcomes. 95% CI, 95% confidence interval; DAPA-CKD, Dapagliflozin and Prevention of Adverse Outcomes in Chronic Kidney Disease; REIN, Ramipril Efficacy in Nephropathy.
A total of 697 patients without diabetes were randomized to the active treatment arm in the DAPA-CKD trial. The mean age of these participants at baseline was 57±15 years; a total of 215 (31%) were women, 373 (54%) were White, and 268 (38%) were Asian. Mean eGFR was 42±11 ml/min per 1.73 m2, and median UACR was 870 mg/g (25%–75% range, 472–1533) (Supplemental Table 1). During a median follow-up of 2.1 years, a total of 52 (8%) patients experienced the primary composite end point (as defined here; not from the clinical trial itself), and 40 (6%) experienced the secondary composite end point (again, as defined here; event rate, 3.9 events per 100 patient-years; 95% CI, 3.0 to 5.1 and 3.0 events per 100 patient-years; 95% CI, 2.2 to 4.1, respectively).
The estimated difference in absolute risk when treated with the combination of ACE inhibitor/ARB and SGLT2 inhibitor and when not treated with either of these agents was 17%–29% over 3 years for the primary composite end point and 15%–22% over 3 years for the secondary composite end point. The corresponding number needed to treat was four to six to prevent one primary composite end point and five to seven to prevent one secondary composite end point.
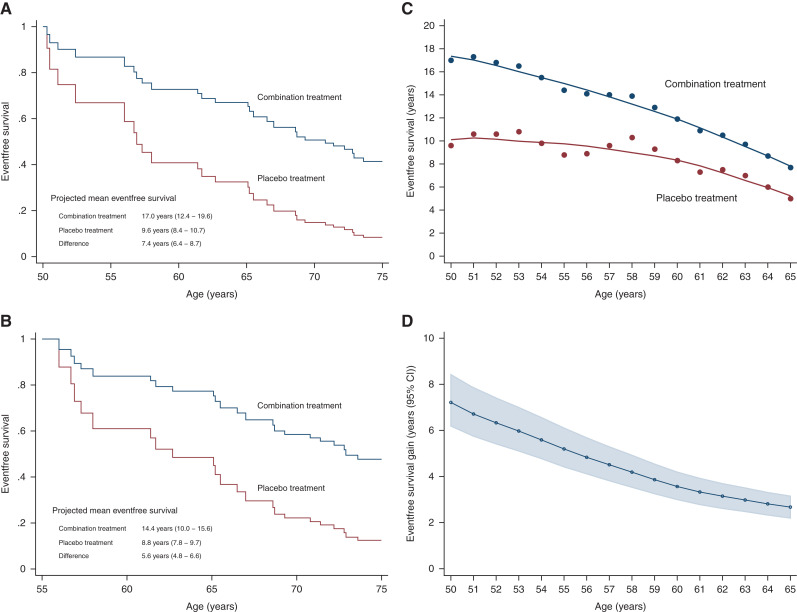
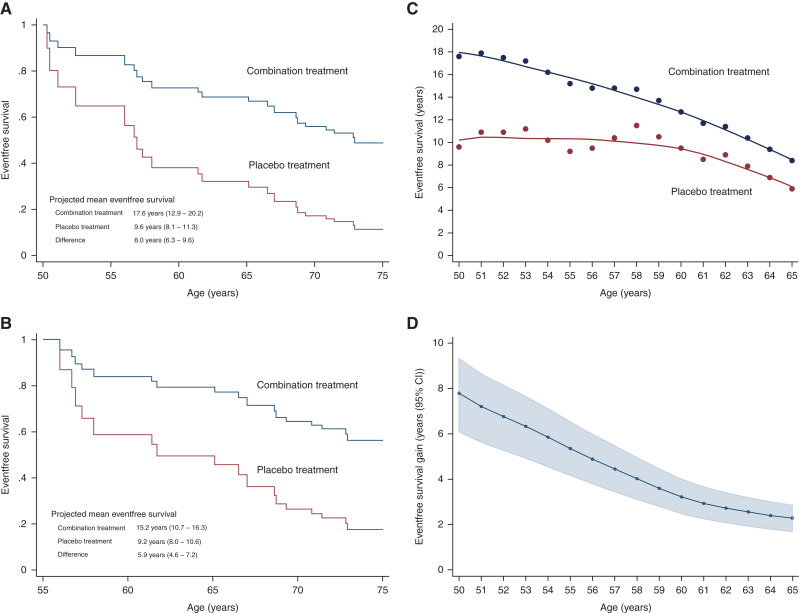
Between the age of 50 and 75 years, the estimated survival free from the primary composite end point was 17.0 years (95% CI, 12.4 to 19.6) with the combination therapy and 9.6 years (95% CI, 8.4 to 10.7) with no treatment (difference, 7.4 years; 95% CI, 6.4 to 8.7) (Figure 2). The corresponding gain in eventfree survival for the secondary composite end point was 8.0 years (95% CI, 6.3 to 9.6) (Figure 3).
Figure 2.
(A and B) Eventfree survival and (C and D) treatment benefits with combination treatment versus placebo treatment for doubling of serum creatinine/kidney failure/death. (A) Kaplan–Meier estimated curves for patients starting at age 50 years. (B) Kaplan–Meier estimated curves for patients starting at age 55 years. (C) Treatment benefits on eventfree survival. (D) Difference in eventfree survival between two treatments.
Figure 3.
(A and B) Eventfree survival and (C and D) treatment benefits with combination treatment versus placebo treatment for doubling of serum creatinine/kidney failure. (A) Kaplan–Meier estimated curves for patients starting at age 50 years. (B) Kaplan–Meier estimated curves for patients starting at age 55 years. (C) Treatment benefits on eventfree survival. (D) Difference in eventfree survival between two treatments.
We estimated absolute eventfree survival gains for every year of age between 50 and 65 years until the age of 75 years (Figures 2 and 3). Regarding primary end point, gains in eventfree survival for the ages of 55, 60, and 65 years were 5.6 years (95% CI, 4.8 to 6.6), 3.6 years (95% CI, 3.0 to 4.2), and 2.8 (95% CI, 2.3 to 3.3), respectively. When investigated for a 70 year old until the age of 75 years, the gain in eventfree survival regarding primary end point was 0.7 years (95% CI, 0.6 to 0.9). For the secondary end point, these numbers for the ages of 55, 60, 65, and 70 years were 5.9 years (95% CI, 4.6 to 7.2), 3.2 years (95% CI, 2.5 to 4.0), 2.4 years (95% CI, 1.8 to 3.0), and 0.6 years (95% CI, 0.5 to 0.8), respectively. As expected, the gain among younger patients was more pronounced, owing to higher rates of nonrenal disease–related deaths in older patients.
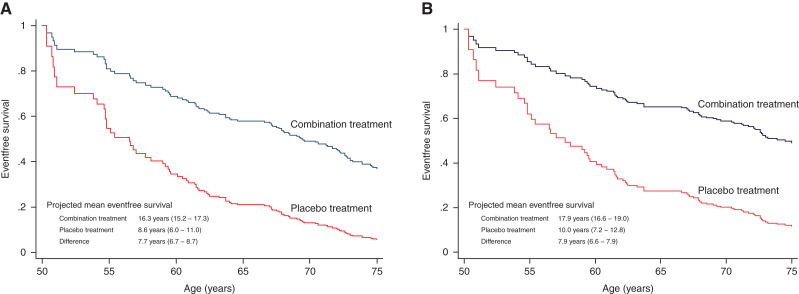
In the first additional analysis, the aggregate treatment effect was applied to the CRIC study participants with CKD without diabetes not using ACE inhibitor/ARB or SGLT2 inhibitor treatment (n=242; mean age, 55±13 years; mean eGFR, 38±14 ml/min per 1.73 m2; median UACR, 730; 25%–75% range, 377–1710). During a median follow-up of 7.7 (interquartile range [IQR], 5.6–8.7) years, 135 primary composite end points (incidence rate, 10.9; IQR, 9.2–12.9 per 100 patient-years) and 107 secondary composite end points (incidence rate, 8.6; IQR, 7.1–10.4 per 100 patient-years) were observed. In CRIC, for the primary composite end point, the projected eventfree survival rate for a participant aged 50 years treated with combination ACE inhibitor/ARB and SGLT2 inhibitor treatment was 16.3 years (95% CI, 15.2 to 17.3), and it was 8.6 years (95% CI, 6.0 to 11.0) when untreated, corresponding to an eventfree survival gain of 7.7 years (95% CI, 6.7 to 8.7) (Figure 4). The corresponding gain in eventfree survival for the secondary composite end point was 7.9 years (95% CI, 6.6 to 9.0) (Figure 4).
Figure 4.
In the Chronic Renal Insufficiency Cohort, eventfree survival with combination treatment versus placebo treatment for (A) doubling of serum creatinine/kidney failure/death and (B) doubling of serum creatinine/kidney failure.
When comparing combination therapy versus treatment with ACE inhibitor/ARB only, the gain in eventfree survival for a 50 year old with combination therapy was 2.5 years (95% CI, 1.3 to 3.7) (Supplemental Figure 1) for the primary composite end point and 2.5 years (95% CI, 0.4 to 4.9) (Supplemental Figure 1) for the secondary composite end point. Finally, when assuming the possibility of (1) the effects of ACE inhibitors/ARBs and SGLT2 inhibitors being not fully additive, (2) low adherence to the combination therapy, or (3) decline in the efficacy of the combination therapy over time, the gain in eventfree survival was attenuate, although it remained clinically meaningful (Supplemental Figure 2). Estimates in eventfree survival were 6.8 years (95% CI, 5.7 to 8.0), 5.3 years (95% CI, 4.4 to 6.1), and 5.8 years (95% CI, 4.8 to 6.8) when assuming a subadditive (70%) effect, suboptimal adherence (70%), and a decline in efficacy of combination therapy by 2% per year, respectively (Supplemental Figure 2). If all of the above conditions were met, the estimated gain in eventfree was 3.7 years (95% CI, 3.0 to 4.3) (Supplemental Figure 3). Finally, when directly applying the combination therapy effect to the placebo group of the REIN trial and the Guangzhou trial, the estimated gain in eventfree survival for both of the kidney end points assessed was similar to that obtained from our main analyses (Supplemental Figure 4).
Discussion
In this study, results from clinical trials and observational data show that a patient of age 50 years with albuminuric CKD without diabetes, when treated with a combination of ACE inhibitors/ARBs and SGLT2 inhibitors, may experience about 7 additional years free of kidney failure and death compared with a person not treated with these agents. In a conservative approach assuming that the effect of combination therapy is not completely additive and assuming waning treatment adherence and treatment efficacy over time, there was a considerable gain in eventfree survival. These results highlight the potential and the opportunity to lower the burden of CKD complications by delaying or even preventing kidney failure and premature death if currently available treatments can be appropriately utilized.
About 40%–60% of patients with CKD do not have diabetes (10,16,17). These patients often have other comorbidities and remain at a high risk of kidney failure and death. These patients are typically treated with ACE inhibitors/ARBs when presented with albuminuria (18). Recently, the SGLT2 inhibitor dapagliflozin has been shown to improve prognosis in patients with CKD without diabetes on top of treatment with a maximum-tolerated dose of ACE inhibitor/ARB (11). Recent guidelines already recommend SGLT2 inhibitors and ACE inhibitors/ARBs as first-line treatment in patients with CKD without diabetes (19). Unfortunately, despite clinical recommendations, the uptake of medications remains low (3,17,20), in part due to limited awareness of treatment benefits, suboptimal risk communication between patients and physicians, and costs associated with medication (21). This study provides estimates of treatment benefit expressed in extra years free from the disease or death that are easy to understand for patients, clinicians, and policy makers. This may facilitate risk communication in clinical management, increase uptake of these therapies in clinical practice, and inform decision making by policy makers and payers.
ACE inhibitors/ARBs and SGLT2 inhibitors are believed to exhibit kidney-protective effects via similar and distinct pathways. ACE inhibitors/ARBs mainly inhibit the conversion and binding of angiotensin II to its receptor, and thereby inhibit the intrarenal renin-angiotensin-aldosterone system (RAAS) and reduce glomerular hyperfiltration (22,23). Although it has been suggested that SGLT2 inhibitors inhibit the intrarenal RAAS, clinical studies have shown that they reduce glomerular hyperfiltration through activation of tubule-glomerular feedback secondary to inhibition of the reabsorption of glucose and sodium in proximal tubules of the kidneys (24). Thereby, from a mechanistic perspective, these SGLT2 inhibitors may exhibit an independent and additive effect when combined with ACE inhibitors/ARBs. Importantly, in our study, the aggregate treatment effect was estimated assuming complete additivity of individual treatment effects, and in line, the observed eventfree survival among patients on ACE inhibitors/ARBs and SGLT2 inhibitors (i.e., among patients in the active treatment arm of the DAPA-CKD trial) was essentially similar to the estimated eventfree survival obtained by applying the aggregate treatment effect to those not on either of the agents.
Strengths of this study include the use of individual patient data from key clinical trials that allowed harmonization of end points and therefore a robust estimation of the combination therapy effect. Additionally, the availability of data from the observational cohort allowed validation of the results in a “real-life” setting. There are also several limitations. First, for the combination therapy, there was no direct information available on the effect additivity, constancy, and adherence over a lifetime. Regarding effect additivity, other than the known mechanism of action of ACE inhibitors/ARBs and SGLT2 inhibitors, the possibility of other unknown overlapping pathways cannot be completely ruled out. Similarly, it is unclear whether combination therapy will continue to exhibit the same effect throughout life. Although treatment with ACE inhibitors/ARBs and SGLT2 inhibitors has been shown to exhibit consistent benefit over a long time (25), persistence in the use of RAAS inhibition in clinical practice can be variable and in some settings quite poor, often due to concerns regarding the use of RAAS inhibitors in advanced CKD and episodes of hyperkalemia or AKI (26). Thereby, the benefit experienced by patients may be lower than estimated. However, it should be noted that in a detailed assessment of various possibilities of effect additivity, constancy, and treatment adherence, results showed considerable clinical benefit with the combination therapy. Second, the study investigated the benefits of combination therapy regarding kidney outcomes and mortality and did not investigate associated costs. SGLT2 inhibitors were recently added to the World Health Organization’s Essential Medicines List and are becoming available in generic form in many global markets, which is expected to improve their affordability and accessibility. This study also did not investigate the adverse effects of combination therapy. However, combination therapy is reported to have a similar safety profile as ACE inhibitors/ARBs alone, which has been widely studied and demonstrated to be safe. In this study, the effect of SGLT2 inhibitors was on the basis of dapagliflozin alone. Results of other SGLT2 inhibitors (e.g., empagliflozin) are expected in the near future, and at present, it is unclear whether efficacy of other SGLT2 inhibitors is comparable with dapagliflozin and whether this effect is similar among patients with and without diabetes. Finally, individuals examined in this study were assumed to be representative of patients with albuminuric CKD without diabetes in routine clinical practice.
In conclusion, combination disease-modifying treatment with ACE inhibitors/ARBs and SGLT2 inhibitors in patients with albuminuric CKD without diabetes but with proteinuria may substantially increase the number of years free from kidney failure and mortality. Treatment benefit with the combination therapy remains considerable even in the presence of lower effect additivity, treatment adherence, and decline in treatment efficacy over time.
Disclosures
G.M. Chertow reports consultancy agreements with Akebia, Ardelyx, AstraZeneca, Cricket, DiaMedica, Gilead, Miromatrix, Reata, Sanifit, Unicycive, and Vertex; ownership interest in Ardelyx, CloudCath, Durect, DxNow, Eliaz Therapeutics, Outset, Physiowave, PuraCath, and Renibus; research funding from Amgen, the National Institute of Allergy and Infectious Diseases, and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK); stock options in Ardelyx, CloudCath, Durect, and Miromatrix; serving on advisory boards for Ardelyx, Baxter, CloudCath, Cricket, DiaMedica, Durect, Miromatrix, and Reata Pharmaceuticals; serving on steering committees for Akebia, AstraZeneca, Gilead, Sanifit, and Vertex; serving as Coeditor of Brenner & Rector’s The Kidney (Elsevier) and on the Satellite Healthcare Board of Directors; and providing Data and Safety Monitoring Board (DSMB) service for Angion, Bayer, Gilead, Mineralys, NIDDK, Palladio, and ReCor. H.J.L. Heerspink reports ongoing consultancy agreements with AstraZeneca, Bayer, Boehringer Ingelheim, CSL Behring, Chinook, Dimerix, Eli Lilly, Gilead, GoldFinch, Janssen, Merck, Novo Nordisk, and Travere Pharmaceuticals; research funding from AstraZeneca, Janssen research support (grant funding directed to the employer), and Novo Nordisk; lecture fees from AstraZeneca; speakers bureau for AstraZeneca; and funding/honoraria and consulting fees for steering committee membership and/or advisory board participation from Abbvie, AstraZeneca, Bayer, Boehringer Ingelheim, Chinook, CSL Pharma, Dimerix, Gliead, GoldFinch, Janssen, Fresenius, Merck, Mitsubishi Tanabe, MundiPharma, Novo Nordisk, and Travere Pharmaceuticals. F.F. Hou is a member of the DAPA-CKD study executive committee and is a study investigator. She reports personal fees from AbbVie, consultancy agreements with AstraZeneca, and honoraria from AstraZeneca. F. McCausland reports consultancy agreements with GlaxoSmithKline; research grants from Advanced Instruments, Fifth Eye, the National Institutes of Health (NIDDK), and Satellite Healthcare; and research funding paid to the institution from Advanced Medical and Fifth Eye. G. Remuzzi reports employment with the Mario Negri Institute for Pharmacological Research; speaker honorarium/travel reimbursements from Akebia Therapeutics, Alexion Pharmaceuticals Inc., Alnylam, BioCryst Pharmaceuticals, Janssen Pharmaceutical, Novartis, and Silence Therapeutics (no personal remuneration is accepted, and compensations are paid to his institution for research and educational activities); honoraria from Akebia Therapeutics, Alexion Pharmaceuticals Inc., Alnylam, Catalyst Biosciences, Janssen Pharmaceutical, Novartis, and Silence Therapeutics; and serving as a member of numerous editorial boards of scientific medical journals. M. Vaduganathan reports consultancy agreements with American Regent, Amgen, AstraZeneca, Baxter Healthcare, Bayer AG, Boehringer Ingelheim, Cytokinetics, Lexicon Pharmaceuticals, Novartis, Pharmacosmos, Relypsa, Roche Diagnostics, Sanofi, and Tricog Health; research funding from American Regent, Amgen, AstraZeneca, Bayer AG, Boehringer Ingelheim, Galmed, Impulse Dynamics, Novartis, Occlutech, and Roche Diagnostics; speakers bureau for AstraZeneca, Novartis, and Roche Diagnostics; receiving research grant support or serving on advisory boards for American Regent, Amgen, AstraZeneca, Baxter Healthcare, Bayer AG, Boehringer Ingelheim, Cytokinetics, Lexicon Pharmaceuticals, Relypsa, and Roche Diagnostics; speaker engagements with Novartis and Roche Diagnostics; and participating on clinical end point committees for studies sponsored by Galmed and Novartis. P. Vart is an editor for Clinical Kidney Journal. D.C. Wheeler reports consultancy agreements with Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Gilead, GlaxoSmithKline, Janssen, Merck Sharp and Dohme, Mundipharma, Napp, Tricida, Vifor Fesenius, and Zydus; honoraria from Amgen, Astellas, AstraZeneca, Bayer, Boehringer Ingelheim, Gilead, GlaxoSmithKline, Janssen, Merck Sharp and Dohme, Mundipharma, Napp, Reata, Pharmacosmos, Tricida, Vifor Fresenius, and Zidus; an advisory or leadership role for AstraZeneca; speakers bureau for Amgen, Astellas, AstraZeneca, Janssen, Merck Sharp and Dohme, Mundipharma, Napp, and Vifor Fresenius; and serving as an Honorary Professorial Fellow for the George Institute for Global Health. The remaining author has nothing to disclose.
Funding
H.J.L. Heerspink is supported by Netherlands Organisation for Scientific Research Vidi grant 917.15.306 and by Innovative Medicines Initiative 2 Joint Undertaking grant 115974. This joint undertaking receives support from the European Union’s Horizon 2020 Research and Innovation Programme and European Federation of Pharmaceutical Industries and Associations (EFPIA). This work is supported by British Heart Foundation Centre of Research Excellence grant RE/18/6/34217. AstraZeneca supported the analysis of the DAPA-CKD trial. Funding support was provided by AstraZeneca for journal publication charges.
Supplementary Material
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Toward Guideline-Directed Medical Therapy in Nephrology: Lifetime Benefit of RAAS and SGLT2 Inhibition in Nondiabetic Kidney Disease,” on pages 1710–1712.
Author Contributions
H.J.L. Heerspink and P. Vart conceptualized the study; G.M. Chertow, H.J.L. Heerspink, F.F. Hou, N. Jongs, F. McCausland, G. Remuzzi, M. Vaduganathan, P. Vart, and D.C. Wheeler were responsible for investigation; H.J.L. Heerspink, N. Jongs, and P. Vart were responsible for formal analysis; H.J.L. Heerspink, M. Vaduganathan, and P. Vart were responsible for methodology; H.J.L. Heerspink, F.F. Hou, and G. Remuzzi were responsible for resources; H.J.L. Heerspink provided supervision; P. Vart wrote the original draft; and G.M. Chertow, H.J.L. Heerspink, F.F. Hou, N. Jongs, F. McCausland, G. Remuzzi, M. Vaduganathan, P. Vart, and D.C. Wheeler reviewed and edited the manuscript.
Data Sharing Statement
Due to agreement under patient privacy, data can only be shared on reasonable request.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.08900722/-/DCSupplemental.
Supplemental Figure 1. Eventfree survival from combination treatment compared with RAAS inhibitor treatment for the primary composite outcome (i.e., doubling of serum creatinine/ESKD/death; A) and the secondary composite outcome (i.e., doubling of serum creatinine/ESKD; B).
Supplemental Figure 2. Projected eventfree survival gain for the primary composite outcome by possible effect additivity levels of combination treatment (A), possible yearly change in efficacy (B), and adherence (C) of combination treatment.
Supplemental Figure 3. Projected eventfree survival gain for primary composite outcome by possible levels of efficacy additivity and decline in efficacy assuming 70% of the total adherence observed in included trials.
Supplemental Figure 4. Eventfree survival with combination treatment versus no treatment with either of the agents for the primary and secondary composite outcomes when directly applying combination treatment effect to the placebo group of the REIN trial and the trial from Guangzhou, China (A and B, respectively), and corresponding results in the DAPA-CKD trial (C and D, respectively).
Supplemental Table 1. Baseline characteristics of patients with diabetes by background medical therapy in DAPA-CKD.
References
- 1.Fox CS, Matsushita K, Woodward M, Bilo HJ, Chalmers J, Heerspink HJ, Lee BJ, Perkins RM, Rossing P, Sairenchi T, Tonelli M, Vassalotti JA, Yamagishi K, Coresh J, de Jong PE, Wen CP, Nelson RG; Chronic Kidney Disease Prognosis Consortium : Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: A meta-analysis. Lancet 380: 1662–1673, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.MacKinnon M, Shurraw S, Akbari A, Knoll GA, Jaffey J, Clark HD: Combination therapy with an angiotensin receptor blocker and an ACE inhibitor in proteinuric renal disease: A systematic review of the efficacy and safety data. Am J Kidney Dis 48: 8–20, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Tuttle KR, Alicic RZ, Duru OK, Jones CR, Daratha KB, Nicholas SB, McPherson SM, Neumiller JJ, Bell DS, Mangione CM, Norris KC: Clinical characteristics of and risk factors for chronic kidney disease among adults and children: An analysis of the CURE-CKD Registry. JAMA Netw Open 2: e1918169, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qiao Y, Shin JI, Chen TK, Sang Y, Coresh J, Vassalotti JA, Chang AR, Grams ME: Association of albuminuria levels with the prescription of renin-angiotensin system blockade. Hypertension 76: 1762–1768, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Claggett B, Packer M, McMurray JJ, Swedberg K, Rouleau J, Zile MR, Jhund P, Lefkowitz M, Shi V, Solomon SD; PARADIGM-HF Investigators : Estimating the long-term treatment benefits of sacubitril-valsartan. N Engl J Med 373: 2289–2290, 2015 [DOI] [PubMed] [Google Scholar]
- 6.Vaduganathan M, Claggett BL, Jhund PS, Cunningham JW, Pedro Ferreira J, Zannad F, Packer M, Fonarow GC, McMurray JJV, Solomon SD: Estimating lifetime benefits of comprehensive disease-modifying pharmacological therapies in patients with heart failure with reduced ejection fraction: A comparative analysis of three randomised controlled trials. Lancet 396: 121–128, 2020 [DOI] [PubMed] [Google Scholar]
- 7.The GISEN Group (Gruppo Italiano di Studi Epidemiologici in Nefrologia) : Randomised placebo-controlled trial of effect of ramipril on decline in glomerular filtration rate and risk of terminal renal failure in proteinuric, non-diabetic nephropathy. Lancet 349: 1857–1863, 1997 [PubMed] [Google Scholar]
- 8.Ruggenenti P, Perna A, Gherardi G, Garini G, Zoccali C, Salvadori M, Scolari F, Schena FP, Remuzzi G: Renoprotective properties of ACE-inhibition in non-diabetic nephropathies with non-nephrotic proteinuria. Lancet 354: 359–364, 1999 [DOI] [PubMed] [Google Scholar]
- 9.Hou FF, Zhang X, Zhang GH, Xie D, Chen PY, Zhang WR, Jiang JP, Liang M, Wang GB, Liu ZR, Geng RW: Efficacy and safety of benazepril for advanced chronic renal insufficiency. N Engl J Med 354: 131–140, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Lash JP, Go AS, Appel LJ, He J, Ojo A, Rahman M, Townsend RR, Xie D, Cifelli D, Cohan J, Fink JC, Fischer MJ, Gadegbeku C, Hamm LL, Kusek JW, Landis JR, Narva A, Robinson N, Teal V, Feldman HI; Chronic Renal Insufficiency Cohort (CRIC) Study Group : Chronic Renal Insufficiency Cohort (CRIC) Study: Baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol 4: 1302–1311, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heerspink HJL, Stefánsson BV, Correa-Rotter R, Chertow GM, Greene T, Hou FF, Mann JFE, McMurray JJV, Lindberg M, Rossing P, Sjöström CD, Toto RD, Langkilde AM, Wheeler DC; DAPA-CKD Trial Committees and Investigators : Dapagliflozin in patients with chronic kidney disease. N Engl J Med 383: 1436–1446, 2020 [DOI] [PubMed] [Google Scholar]
- 12.Durrleman S, Chaikin P: The use of putative placebo in active control trials: Two applications in a regulatory setting. Stat Med 22: 941–952, 2003 [DOI] [PubMed] [Google Scholar]
- 13.Fisher LD, Gent M, Büller HR: Active-control trials: How would a new agent compare with placebo? A method illustrated with clopidogrel, aspirin, and placebo. Am Heart J 141: 26–32, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Cleveland WS: Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc 74: 829–836, 1979 [Google Scholar]
- 15.Arora N, Katz R, Bansal N: ACE inhibitor/angiotensin receptor blocker use patterns in advanced CKD and risk of kidney failure and death. Kidney Med 2: 248–257, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar V, Yadav AK, Sethi J, Ghosh A, Sahay M, Prasad N, Varughese S, Parameswaran S, Gopalakrishnan N, Kaur P, Modi GK, Kamboj K, Kundu M, Sood V, Inamdar N, Jaryal A, Vikrant S, Nayak S, Singh S, Gang S, Baid-Agrawal S, Jha V: The Indian Chronic Kidney Disease (ICKD) study: Baseline characteristics. Clin Kidney J 15: 60–69, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kotsis F, Schultheiss UT, Wuttke M, Schlosser P, Mielke J, Becker MS, Oefner PJ, Karoly ED, Mohney RP, Eckardt KU, Sekula P, Köttgen A; GCKD Investigators : Self-reported medication use and urinary drug metabolites in the German Chronic Kidney Disease (GCKD) study. J Am Soc Nephrol 32: 2315–2329, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members : Evaluation and management of chronic kidney disease: Synopsis of the Kidney Disease: Improving Global Outcomes 2012 clinical practice guideline. Ann Intern Med 158: 825–830, 2013 [DOI] [PubMed] [Google Scholar]
- 19.Kidney Disease Improving Global Outcomes : KDIGO 2022 clinical practice guideline for diabetes management in chronic kidney disease, 2022. Available at: https://kdigo.org/wp-content/uploads/2022/03/KDIGO-2022-Diabetes-Management-GL_Public-Review-draft_1Mar2022.pdf. Accessed July 12, 2022
- 20.Pecoits-Filho R, Fliser D, Tu C, Zee J, Bieber B, Wong MMY, Port F, Combe C, Lopes AA, Reichel H, Narita I, Stengel B, Robinson BM, Massy Z; CKDopps Investigators : Prescription of renin-angiotensin-aldosterone system inhibitors (RAASi) and its determinants in patients with advanced CKD under nephrologist care. J Clin Hypertens (Greenwich) 21: 991–1001, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Godman B, Bucsics A, Vella Bonanno P, Oortwijn W, Rothe CC, Ferrario A, Bosselli S, Hill A, Martin AP, Simoens S, Kurdi A, Gad M, Gulbinovič J, Timoney A, Bochenek T, Salem A, Hoxha I, Sauermann R, Massele A, Guerra AA Jr, Petrova G, Mitkova Z, Achniotou G, Laius O, Sermet C, Selke G, Kourafalos V, Yfantopoulos J, Magnusson E, Joppi R, Oluka M, Kwon HY, Jakupi A, Kalemeera F, Fadare JO, Melien O, Pomorski M, Wladysiuk M, Marković-Peković V, Mardare I, Meshkov D, Novakovic T, Fürst J, Tomek D, Zara C, Diogene E, Meyer JC, Malmström R, Wettermark B, Matsebula Z, Campbell S, Haycox A: Barriers for access to new medicines: Searching for the balance between rising costs and limited budgets. Front Public Health 6: 328, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Givertz MM: Manipulation of the renin-angiotensin system. Circulation 104: E14–E18, 2001. 10.1161/hc3001.094733 [DOI] [PubMed] [Google Scholar]
- 23.Sen T, Heerspink HJL: A kidney perspective on the mechanism of action of sodium glucose co-transporter 2 inhibitors. Cell Metab 33: 732–739, 2021 [DOI] [PubMed] [Google Scholar]
- 24.Williams GH: Aldosterone: The missing cardiorenal link. Am J Nephrol 50: 329–332, 2019 [DOI] [PubMed] [Google Scholar]
- 25.Gislason GH, Rasmussen JN, Abildstrøm SZ, Gadsbøll N, Buch P, Friberg J, Rasmussen S, Køber L, Stender S, Madsen M, Torp-Pedersen C: Long-term compliance with beta-blockers, angiotensin-converting enzyme inhibitors, and statins after acute myocardial infarction. Eur Heart J 27: 1153–1158, 2006 [DOI] [PubMed] [Google Scholar]
- 26.McCoy IE, Han J, Montez-Rath ME, Chertow GM: Barriers to ACEI/ARB use in proteinuric chronic kidney disease: An observational study. Mayo Clin Proc 96: 2114–2122, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.