Abstract
Idiopathic nephrotic syndrome often responds to immunosuppressive treatment. Nevertheless, this syndrome—and the drugs used to treat it—remain important causes of patient morbidity. Idiopathic nephrotic syndrome is usually caused by minimal change disease or FSGS, diseases that primarily affect the podocytes. In spite of decades of research, the underlying causes of both diseases remain incompletely understood. There is, however, a large body of observational and experimental data linking the immune system with both minimal change disease and FSGS, including associations with systemic infections and hematologic malignancies. Perhaps most compellingly, many different immunomodulatory drugs are effective for treating idiopathic nephrotic syndrome, including biologic agents that have well-defined immune targets. In fact, the unexpected efficacy of targeted therapeutic agents has provided important new insights into the pathogenesis of these diseases. Given the large number of drugs that are available to deplete or block specific cells and molecules within the immune system, a better understanding of the immunologic causes of idiopathic nephrotic syndrome may lead to better diagnostic and therapeutic approaches.
Keywords: immunology, focal segmental glomerulosclerosis, idiopathic nephrotic syndrome
Introduction
Idiopathic nephrotic syndrome refers to nephrotic syndrome that occurs in the absence of an identifiable systemic cause. Although most pediatric patients with nephrotic syndrome are not biopsied, those who are usually have minimal change disease or FSGS patterns of injury. Both diseases are considered “podocytopathies” (1), and the histology does not usually show the typical hallmarks of an inflammatory disease. The glomeruli are not hypercellular, for example, and deposits of immune complexes and/or complement are generally absent or sparse. Indeed, the only changes apparent in most minimal change disease glomeruli are effacement of the podocyte foot processes (2). As early as the 1970s, however, idiopathic nephrotic syndrome was theorized to have an immune basis (3,4). Shalhoub (4) proposed that “lipoid nephrosis” (now known as minimal change disease) is a T cell–mediated disease. This hypothesis was on the basis of the favorable response to treatment with glucocorticoids, the association of the disease with T cell malignancies and atopy, and the lack of glomerular immune complexes (arguing against it being a B cell–mediated disease) (3,4). Similar associations were observed in FSGS.
Since Shalhoub (4) first published this theory, additional tools for interrogating or modulating the immune system have been utilized to analyze blood, urine, biopsy samples, and the genome of affected patients (5). Perhaps most compellingly, idiopathic nephrotic syndrome frequently responds to treatment with biologic agents that have well-defined immune targets, such as B cell–depleting drugs (6–8). It is also noteworthy that the drugs used to treat idiopathic nephrotic syndrome are also effective in autoimmune kidney diseases, such as membranous nephropathy. This suggests that these glomerular diseases have common mechanisms of injury, even if the histologic findings are different. Nevertheless, the molecular causes of idiopathic nephrotic syndrome remain incompletely understood.
What Is an Immune-Mediated Disease?
The immune system is composed of adaptive immune cells (e.g., T cells and B cells), innate immune cells (e.g., neutrophils and macrophages), and a large number of soluble molecules (cytokines, chemokines, antibodies, and complement proteins). The term “autoimmune” is usually used to describe diseases in which the adaptive immune system inappropriately targets self-tissue. Kidney biopsies are typically immunostained for Ig (IgG, IgM, and IgA) and complement proteins (C1q and C3), and these analyses are well suited to detect immune-complex glomerular diseases. When these immune proteins are detected in idiopathic nephrotic syndrome tissue, however, their significance is not always clear. Subsets of patients have minimal change disease or FSGS patterns by light microscopy, but they also have prominent glomerular deposits of IgM (“IgM nephropathy”) or C1q (“C1q nephropathy”) (9,10). Detection of these proteins does not currently influence the treatment decisions, and outcomes of IgM and C1q nephropathy are similar to those of minimal change disease and FSGS (11).
More broadly, one can define an “immune-mediated” disease as one in which immune cells or molecules are drivers of tissue injury, and standard pathology methods do not detect many of these processes. Inflammation is not usually seen in minimal change disease and FSGS kidneys by light microscopy, for example, yet immunostaining of FSGS biopsies for T cell, B cell, and macrophages markers reveals increased numbers of these cells compared with control samples (12). Immune cells also produce soluble cytokines that can act remotely (13). Levels of these molecules can be measured in serum and urine, but it is difficult to detect their effects on target tissues. Newer “-omics” methods, such as RNA sequencing, can detect the downstream effects of these molecules, and molecular studies of patients with idiopathic nephrotic syndrome have identified transcriptional signatures characteristic of cytokine responses (14,15).
Clinical Associations that Implicate the Immune System in Idiopathic Nephrotic Syndrome
Primary versus Secondary Disease
Secondary causes of minimal change disease and FSGS include malignancies, certain medications, viruses, and “adaptive” causes, such as obesity or low nephron number (16). The distinction between the primary and secondary forms of disease is on the basis of detecting certain histologic features and the absence of an obvious secondary cause (17). It is worth noting, however, that flares of idiopathic nephrotic syndrome are often “triggered” by systemic disease, such as viral infections (Table 1). Similarly, secondary causes of nephrotic syndrome may affect podocytes through the same immune pathways as idiopathic nephrotic syndrome. Lupus podocytopathy, for example, manifests as diffuse podocyte effacement without immune-complex deposition and is usually treated similar to minimal change disease (18). Thus, even though lupus podocytopathy is a secondary cause of nephrotic syndrome, it may be mechanistically closer to minimal change disease than it is to other forms of lupus nephritis.
Table 1.
Examples of genetic and secondary causes of idiopathic nephrotic syndrome with associated pathologic findings
| Nephrotic Syndrome | Examples | Immune Mediated | Biopsy Findings | APOL1 Association |
|---|---|---|---|---|
| Genetic causes | NPHS1, NPHS2, ACTN4, ANLN | No | FSGS, MCD | |
| Secondary | ||||
| Infectious | Viral: HIV, parvovirus B19, EBV, CMV, RSV, influenza, SARS-CoV-2; bacterial: Mycoplasma pneumonia | Likely for CMV and EBV | Collapsing FSGS (HIV, parvovirus B19, EBV, CMV, COVID-19), MCD (examples: RSV, COVID-19) | HIVAN and COVAN |
| Medications | IFN, CNIs, anthracyclines, lithium, heroin, gold, pamidronate, NSAIDs, anabolic steroids, sirolimus; immunizations: influenza, Pfizer-BioNTech COVID-19, Moderna COVID-19 | Likely | FSGS (IFN, CNI), MCD (NSAIDs, vaccines) | Associated with IFN-induced FSGS |
| Malignancy | Hodgkin disease, non-Hodgkin lymphoma, solid organ tumor (renal cell, colon, and ovarian cancer) | Likely | FSGS (NHL, GVHD, renal cell); MCD (Hodgkin lymphoma, ovarian cancer) | |
| Adaptive | Obesity, hypertension, nephropenia (congenital or acquired) | No | FSGS | Associated with FSGS |
| Allergens | Pollen, dander, dust, food allergy | Likely | MCD | |
| Systemic diseases | SLE, GVHD, HLH, IPEX | Likely | MCD (SLE, IPEX), MCD or FSGS with C1q deposits, MCD or FSGS (HLH) | |
MCD, minimal change disease; EBV, Epstein–Barr virus; CMV, cytomegalovirus; RSV, respiratory syncytial virus; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; COVID-19, coronavirus disease 2019; HIVAN, HIV-associated nephropathy; COVAN, COVID-19 associated nephropathy; CNI, calcineurin inhibitor; NSAID, nonsteroidal anti-inflammatory drug; NHL, non-Hodgkin's lymphoma; GVHD, graft versus host disease; HLH, hemophagocytic lymphistiocytosis; IPEX, immune dysregulation, polyendocrinopathy, enteropathy, X linked.
Minimal change disease and FSGS have similar responses to immunosuppressive drugs, raising the question as to whether they have shared pathophysiology. Both conditions are often categorized as “steroid sensitive” or “steroid resistant” (19). Presumably, this clinical response identifies patients whose disease is immune mediated. Several newer techniques have been used to evaluate the different immune signature between those “responders” and “nonresponders.” Flow cytometry has been used to analyze subsets of B cells following treatment of pediatric patients who are steroid sensitive and steroid resistant (20–23). Lymphocyte subsets have also been examined in detail using time-of-flight mass cytometry in patients treated with anti-CD20 antibodies (24). Time-of-flight mass cytometry analysis revealed that anti-CD20 treatment depleted B cells, but frequent relapsers had higher numbers of class-switched memory B cells (including B-regulatory cells) after treatment (24). Recently, urinary single-cell RNA analysis showed higher numbers of urinary immune cells in patients with FSGS (predominantly adults) compared with those with minimal change disease (14). The number of immune cells was also higher in urine from patients with active disease compared with patients in remission. Another single-cell RNA sequencing study of patients with FSGS identified distinct patterns of disease on the basis of the transcriptional patterns in endothelial cells (25). Conversely, whole-exome sequencing of samples from steroid-resistant nephrotic syndrome tends to identify pathologic variants in the genes of podocyte proteins (26), potentially identifying patients in whom the immune system is not the main driver of injury. Future studies will likely provide additional diagnostic methods of distinguishing immune subtypes of these diseases.
Infections and Idiopathic Nephrotic Syndrome
The link between viral infections and idiopathic nephrotic syndrome was part of the original justification of Shalhoub (4) for suspecting a role for T cells. It was observed that measles infections, which dampen T cell immunity, are associated with remission of disease. Viral infections are also common triggers of both FSGS and minimal change disease (27) (Table 1). In HIV-associated nephropathy, which is associated with collapsing FSGS (cFSGS), the virus directly infects podocytes, leading to cytoskeleton damage and upregulation of intracellular mediators that contribute to podocyte dedifferentiation (28,29). Cytomegalovirus, Epstein–Barr virus, and, more recently, severe acute respiratory syndrome coronavirus 2 (coronavirus disease 2019) are also associated with cFSGS (28,30). Similarly, upper respiratory infections with several viruses, including respiratory syncytial virus, influenza, parainfluenza, and coronavirus disease 2019, are associated with relapses of minimal change disease (31,32). Direct viral infection of the kidney is not seen in cytomegalovirus or Epstein–Barr virus, suggesting that these infections cause disease via systemic immune activation by the virus.
Variants in the gene for APOL1 are strongly linked with higher risk of FSGS in patients with HIV-associated nephropathy (28). APOL1 protein is expressed by podocytes, and it is believed that the risk variants exacerbate inflammatory signaling within the podocyte (33). Consistent with this, APOL1 risk variants have been linked with the development of cFSGS following treatment with therapeutic IFN (34). Interestingly, a recent study showed that endothelial APOL1 affects the inflammatory response to sepsis (35), suggesting that this protein also influences inflammatory responses of other cell types, perhaps indirectly affecting podocytes.
Cancer and Idiopathic Nephrotic Syndrome
Hematologic malignancies are associated with idiopathic nephrotic syndrome, most notably Hodgkin lymphoma. In this disease, IL-13 produced by dysregulated Th2 cells is believed to propagate nephrotic syndrome, an effect replicated in rat models (36). Nephrotic syndrome is also associated with other cancer-related conditions, including graft versus host disease after hematopoietic stem cell transplant. In this setting, glomerular disease is believed to be the result of B and T cell activation, which induces local damage by monocytes (37). Case reports also suggest that FSGS and minimal change disease can occur as paraneoplastic syndromes related to renal cell carcinoma or ovarian cancer (38,39). Hemophagocytic lymphohistiocytosis is a rare syndrome of immune overactivation that is associated with infections and malignancies. Hemophagocytic lymphohistiocytosis can cause multiple organ dysfunction via increased production of IFN-γ, IL-1β, and IL-18, and it has been linked with cases of minimal change disease and FSGS (40,41).
Medications and Idiopathic Nephrotic Syndrome
Several classes of medication have been linked to FSGS, including calcineurin inhibitors (CNIs), anthracyclines, and lithium. Some medications, like pamidronate, probably act directly on the podocyte. Others, such as lithium and IFN-α, -β, or -γ, may cause disease through systemic effects on the immune system (42–46). Nonsteroidal anti-inflammatory drugs have been specifically associated with minimal change disease, and this seems to be a variant of acute interstitial nephritis caused by an immune response to the drug (44).
Evidence of Specific Immunologic Factors in Idiopathic Nephrotic Syndrome
T Cells
The initial focus of Shalhoub (4) on a role of T cells in nephrotic syndrome was supported by the improvement of some patients following treatment with steroids or cyclophosphamide and the apparent lack of autoantibodies or immune complexes. Several studies have reported altered numbers or phenotypes of T cell subsets. The relative number of CD4 to CD8 T cells may decrease with disease, although this has not been consistent across all studies (47,48). The Th17 subset of CD4 T cells is increased in some adult patients with idiopathic nephrotic syndrome, whereas the number of T-regulatory cells (Tregs) is decreased compared with controls and with disease recurrence (49–51). Immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome is a rare condition associated with the defective production of Tregs. A patient with immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome who developed minimal change disease was reported, further implicating Tregs in the disease (52).
Many observations indicate that idiopathic nephrotic syndrome is caused by soluble factors in plasma. Idiopathic FSGS rapidly recurs in 40%–60% of transplant recipients (53). In one fascinating report, rapid retransplantation of an affected kidney led to the resolution of FSGS (54). There are also reports of transient nephrotic syndrome in fetuses, presumably due to transplacental passage of causative factors (55,56). Importantly, injection of patient serum into rats can cause proteinuria (57). These observations have prompted researchers to search for a circulating “permeability factor” (58), and much of the focus has been on T cell products. Indeed, supernatant from a T cell hybridoma induced proteinuria when injected into rats, confirming that T cells produce molecules that can affect glomerular permeability (59). Several possible effectors have been identified. IL-13 is produced by Th2 CD4 T cells and has been implicated in the development of minimal change disease (60), although IL-13 levels do not always correlate to disease activity (61). IL-17 is a cytokine produced by the Th17 subset, and levels of IL-17 correlate with glomerulosclerosis in patients with FSGS (62). IL-1 and TNF-α levels are also increased in some patients with idiopathic nephrotic syndrome, providing further evidence of T cell activation (63). A causative role for IL-1 was corroborated by a recent case series, demonstrating the benefit in adults with advanced nephrotic syndrome who were treated with anakinra (an IL-1 receptor antagonist, which blocks the effects of IL-1α and IL-1β) (64). Studies have also shown that FSGS is associated with activation of TNF-α pathways in podocytes (65), although the response to TNF-α blockade is inconsistent (66). In spite of extensive research, however, it is still not known whether specific pathogenic T cell populations or products cause idiopathic nephrotic syndrome.
Role of B Cells
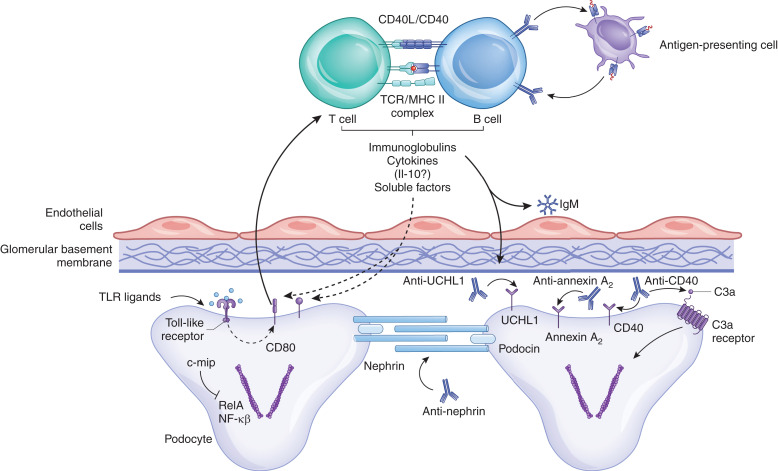
Although the initial focus was on T cells, a growing body of evidence implicates B cells in the pathogenesis of idiopathic nephrotic syndrome. Even though glomerular immune complexes are not prominent, Ig is sometimes seen within the glomeruli by immunofluorescence (67). Furthermore, B cells have other immune functions beyond the production of antibodies (Figure 1). They are antigen-presenting cells that strongly affect the T cell response to antigens. They can also produce a range of pro- and anti-inflammatory cytokines (68). The number of circulating B cells as a percentage of lymphocytes is higher in children with nephrotic syndrome compared with control patients (22). Importantly, this difference is present in samples collected prior to treatment with immunomodulatory drugs. Also of note, B cell numbers are not altered in patients with genetically induced disease, indicating that this is not a nonspecific consequence of nephrotic syndrome. Additionally, a B cell activation marker (sCD23) was found to be higher in patients with relapsed idiopathic nephrotic syndrome than in patients in remission or in healthy controls (69).
Figure 1.
Multiple functions of B cells in the immune response. Autoimmune B cells may be the primary cause of nephrotic syndrome in some patients through the production of antibodies that target podocyte antigens, such as annexin A2, nephrin, and UCHL1. Production of natural antibodies reactive in injury epitopes may also be a secondary factor in disease progression. Complement activation within the glomerulus generates C3a, which ligates the C3a receptor on podocytes and causes morphologic changes. B cells also have other immune functions that may contribute to disease. They are antigen-presenting cells and modulate T cell immunity. They also produce cytokines and other soluble factors that can affect podocytes. c-mip, c-Maf–inducing protein; NF-κβ, nuclear factor-κappa-β; RelA, REL-associated protein; TCR, T-cell receptor; TLR, Toll-like receptor; UCHL1, ubiquitin carboxyl-terminal hydrolase L1.
Autoantibodies in Idiopathic Nephrotic Syndrome.
IgG in the serum of some patients with idiopathic nephrotic syndrome binds to podocytes, and studies have discovered several podocyte proteins that are the targets of autoantibodies. In one study, mass spectrometry identified annexin A2 as a possible autoantigen (70). The authors analyzed samples from 596 incident patients and detected antiannexin A2 antibodies in 18% of the patients (70). They also eluted IgG from three of the kidney biopsies and showed that it bound to annexin A2, confirming that antiannexin A2 antibody was present within the glomeruli. A recent study also found antinephrin IgG in the serum of 29% of patients with idiopathic nephrotic syndrome (71). Antibody levels fell in response to treatment and became undetectable in those patients who had a complete response to treatment. Yet another possible podocyte autoantigen is UCLH1. Anti-UCLH1 antibody titers were higher in pediatric patients with idiopathic nephrotic syndrome compared with those with other diseases, and antibody levels increased during relapse (72). Experiments demonstrated that the anti-UCLH1 antibodies directly altered podocyte morphology in vitro, and they induced proteinuria when injected into mice. Interestingly, anti-UCLH1 levels were higher in pediatric patients with minimal change than they were in adult patients.
In a study of patients with FSGS who underwent kidney transplantation, investigators found that increased levels of anti-CD40 IgG predicted disease recurrence post-transplantation, and they detected CD40 expression on podocytes of the affected kidneys (73). Passive transfer of the antibodies into mice was associated with increased albuminuria. Case reports have also found autoantibodies to other targets in patients with transplants and FSGS, including the angiotensin 2 receptor (74). Thus, although idiopathic nephrotic syndrome is not regarded as an immune-complex disease, a substantial percentage of patients have IgG antibodies reactive with podocyte antigens.
In addition to being a primary cause of injury, antibodies can also bind to antigens that are generated after tissue injury. It has been known for decades that IgM and complement C3 are deposited in the glomeruli of some patients with sclerotic glomeruli (67,75–77). Although it is commonly believed that these proteins are passively trapped in areas of tissue damage, IgM in the glomeruli colocalizes with activated complement fragments in both human (78) and murine (79,80) disease. IgM only activates complement when bound to a cognate ligand, so this indicates that the IgM is bound to a target antigen within the glomerulus. Experiments also showed that natural IgM binds to cardiolipin displayed on injured glomerular endothelial cells (79,80). Detection of glomerular IgM and complement deposits does not consistently associate with a worse prognosis in patients with idiopathic nephrotic syndrome (31,76,81,82). Nevertheless, this may be a mechanism of secondary injury to the glomerulus.
After antibodies bind to glomerular structures, complement activation can mediate glomerular injury. A recent study in mice revealed that injured podocytes downregulate expression of decay accelerating factor (DAF; or CD55), a complement regulatory protein (83). Decreased expression of DAF by the podocyte increases glomerular complement activation and might potentiate antibody-mediated injury. The authors also found that C3a generated during complement activation binds to C3a receptor expressed on podocytes, resulting in cytoskeletal rearrangement in the cells. Examination of samples from patients with FSGS also found decreased DAF expression within the glomeruli and increased glomerular complement activation. Interestingly, a study using a model of diabetic nephropathy also found that C3a generated in the glomerulus alters podocyte morphology and causes proteinuria (84). Thus, C3a may link various different primary glomerular insults with downstream changes in podocytes and proteinuria.
B Cell–Targeted Therapies.
Perhaps the most compelling evidence supporting a role for the immune system in idiopathic nephrotic syndrome comes from studies showing that B cell–depleting drugs are effective in some patients. After an early report of a patient who incidentally entered remission after treatment with rituximab (85), several subsequent randomized trials have supported this finding (7,86,87). Several studies in pediatric patients have shown that disease typically does not recur until after B cells have reconstituted, and recovery of the class-switched memory B cell population may predict relapse (21–23). This raises the possibility that drugs targeting specific subsets of B cells may be effective for treating disease and that B cell markers may be useful for monitoring patients.
In head-to-head trials, rituximab appears more effective at preventing relapses compared with treatment with CNIs (88) or mycophenolate mofetil (86), drugs that affect T cell immunity. Ofamtumumab is a humanized anti-CD20 antibody with higher binding affinity than rituximab. Ofamtumumab and rituximab appeared to be equally effective in a recent trial of pediatric and young adult patients (7). The depletion of B cells by these medications also alters T cell populations, as T and B cell responses are strongly intertwined. Th17 cells decrease after B cell depletion (89), whereas the number of Tregs increases (21,90). Thus, even drugs that specifically target B cells, like rituximab and ofatumumab, may improve nephrotic syndrome through their indirect effects on T cell immunity (91).
Other Soluble Immune Molecules
Other immune-derived molecules may also function as permeability factors. An elegant study identified soluble urokinase plasminogen activator receptor (suPAR) as a possible driver of FSGS (92). However, further studies showed that isolated suPAR does not cause proteinuria, and suPAR levels are also increased in nonproteinuric kidney disease (93,94). Thus, it does not seem to be a specific cause of disease. Similarly, cardiotropin-like cytokine 1 was identified as a possible permeability factor, but this finding has not been confirmed by subsequent studies (95).
Toll-like receptors (TLRs) are innate immune receptors that can detect bacterial and viral constituents. Podocytes express several TLRs, and signaling through these receptors can directly change podocyte morphology and induce expression of chemokines (96–99). TLRs could, therefore, link infection-associated molecules and nephrotic syndrome flares. In addition, TLR signaling increases podocyte expression of CD80, a T cell costimulatory molecule (98,99). TLRs, therefore, could also modulate the interaction between podocytes and T cells. CD80 has been detected in urine and biopsy samples of patients with idiopathic nephrotic syndrome (100,101), raising the possibility that it might be a biomarker of immune activation of podocytes. Furthermore, CTLA4-Ig is a fusion protein that blocks costimulation of T cells by CD80, and it was reported to reduce proteinuria in five patients with FSGS (101). Nevertheless, the detection of CD80 in FSGS biopsies has not been reproduced by other laboratories (102), and treatment with CTLA4-Ig is not effective in all patients (103).
Off-Target Effects of Immunomodulatory Drugs
The efficacy of immunomodulatory drugs in idiopathic nephrotic syndrome is important to our understanding of these diseases. Clinical associations, such as changes to cytokine levels or immune cell numbers in the circulation, might be downstream consequences of disease or its treatments, whereas a clinical response to immunosuppressive drugs provides evidence that the immune system plays a causal role in disease. However, even drugs with specific mechanisms of action can have off-target effects. One important study showed that CNIs can directly stabilize podocytes, independent of their effects on the immune system (104). Another study showed anti-CD20 antibodies bound sphingomyelin phosphodiesterase acid–like 3b on podocytes, stabilizing the cells (105), although no follow-up studies have confirmed this effect. In spite of these potentially beneficial off-target effects, it is striking that so many immunosuppressive drugs with distinct mechanisms of action are effective in idiopathic nephrotic syndrome, including glucocorticoids, CNIs, mycophenolate mofetil, cyclophosphamide, and rituximab/ofatumumab (106). The immunologic effects of these medications and the newer therapeutic options currently being evaluated are summarized in Table 2.
Table 2.
Current and future treatment options for idiopathic nephrotic syndrome and their mechanisms of action on the immune system
| Medication Class | Medication Name | Site(s) of Action | Mechanism of Action | Indication | Population |
|---|---|---|---|---|---|
| Glucocorticoids | Prednisone; methylprednisolone | Glucocorticoid receptor in T and B cells as well as inflammatory cells; may also directly act on podocytes, parietal epithelial cells | Global reduction in cytokines, chemokines, inflammatory cells; stabilization of cytoskeleton and increased podocyte survival | MCD, FSGS | Pediatric, adult |
| Alkylating agents | Cyclophosphamide | T cells | Decreases Tregs and secretion of IFN-γ and IL-12; increases IL-4, IL-10 secretion | Frequently relapsing/steroid-dependent MCD, frequently relapsing FSGS | Pediatric, adult |
| Purine analogs | Mycophenolate mofetil | T cells, B cells | Interrupts purine synthesis in activated T and B cells, causing lymphocyte depletion | Frequently relapsing/steroid-dependent MCD; steroid-sparing idiopathic NS | Pediatric, adult |
| Calcineurin inhibitors | Tacrolimus; cyclosporin | T cells; podocyte cytoskeleton | Binds and inhibits calcineurin, leading to decreased T cell activation; stabilizes podocyte cytoskeleton | Steroid-sparing idiopathic NS; frequently relapsing/steroid-dependent MCD, steroid-resistant FSGS | Pediatric, adult |
| mAbs | Rituximab | B cells; podocyte basement membrane | Binds to CD20 on B cells; depletes B cells, reducing autoantibody formation; indirectly affects T cells; may stabilize podocytes via binding of SMPDL-3b | Frequently relapsing/steroid-dependent MCD; initial treatment in MCD; recurrent FSGS | Pediatric, adult |
| mAbs | Ofatumumab | B cells | Binds to CD20 on B cells; depletes B cells, reducing autoantibody formation; indirectly affects T cells | Steroid-dependent NS | Pediatric |
| mAbs | Belimumab | B cell–activating factor | Binds to B cell–activating factor, preventing B cell survival and differentiation into plasma cells | Frequently relapsing NS | Pediatric |
| mAbs | Obinutuzumab and daratumumab | Obinutuzumab: B cells; daratumumab: plasma cells | Obinutuzumab: binds to CD20 on naïve and memory B cells; daratumumab: binds to CD38 on plasma cells | Steroid-dependent NS | Pediatric |
| mAbs | Bleselumab | B cells | Binds to CD40, preventing CD40–CD154 interactions between B cells, T cells, and antigen-presenting cells | Recurrent FSGS | Adult |
| mAbs | Adalimumab | Immune system; locally at the level of kidney parenchyma | Inhibits TNF-α, which may reduce kidney fibrosis, glomerular permeability | Treatment-resistant MCD; FSGS | Adult |
| Melanocortin peptide | ACTH | Immune system; adrenal cortex; podocyte | Steroidal effects via the MCR2 receptor at the adrenal gland; nonsteroidal effects via MCR1 affecting the immune system and locally at the podocyte | Steroid-dependent/steroid-resistant FSGS; recurrent FSGS | Pediatric, adult |
| Leukotriene receptor antagonist | Montelukast | Th2 cells | Suppresses Th2 cytokine production (IL-4, IL-13), reducing podocyte permeability and cytoskeletal changes in vitro | Undergoing evaluation for use in frequently relapsing NS | Pediatric |
| IL receptor blocker | Anakinra (case series) | IL-1 receptor | Targets and inactivates IL-1R1, preventing IL-1β and IL-1R1 signaling | Treatment-resistant/ frequently relapsing FSGS and MCD | Pediatric, adult |
| Cytokines | Proleukin | IL-2 | Increases Tregs, which secrete CTLA4 to bind CD80 and prevents activation of T cells | Undergoing evaluation for use in treatment-resistant NS | Pediatric |
| Chemokine receptor inhibitor | DMX-200/repargeranium currently in clinical trials, ClinicalTrials.gov identifier NCT05183646 | CCR2 receptor | Antagonizes chemokine receptors expressed in podocytes, reducing proteinuria | FSGS | Adult |
| Chemokine receptor inhibitor | Propergeranium ClinicalTrials.gov identifier NCT03649152 | CCR2 receptor | Antagonizes chemokine receptors expressed in podocytes, reducing proteinuria | FSGS | Adult |
Recurrent FSGS indicates FSGS that has recurred following kidney transplant. MCD, minimal change disease; Treg, T-regulatory cell; NS, nephrotic syndrome; SMPDL-3b, sphingomyelin phosphodiesterase acid–like 3b; ACTH, adrenocorticotrophic hormone; MCR2, melanocortin receptor 2; MCR1, melanocortin receptor 1; Th2, T helper 2; CTLA4, cytotoxic T-lymphocyte associated protein 4; CCR2, CC chemokine receptor 2.
Overall, there is overwhelming evidence that idiopathic nephrotic syndrome is immune mediated, at least in some patients. Several new treatments have become available, most notably B cell–targeted drugs. Unfortunately, none of the available drugs lead to remission in all patients, and we do not have accurate methods for predicting which patients will respond to treatment. Patients with identical clinical and biopsy findings can have widely different responses to treatment. Consequently, some patients with idiopathic nephrotic syndrome are exposed to an array of potentially harmful medications without deriving any clinical benefit. It is important, therefore, that we identify “druggable” targets in nephrotic syndrome and equally important to develop reliable biomarkers for patients in whom the target is activated. New methods of detecting particular immune signatures in affected patients are needed. Once accomplished, classifying patients as having primary or secondary disease will become less important than stratifying patients as “likely to respond” or “unlikely to respond” to the available treatments.
Disclosures
J.M. Thurman receives royalties from Alexion Pharmaceuticals, Inc. and is a consultant for Q32 Bio, Inc., a company developing complement inhibitors. He holds stock in, receives research funding from, and will receive royalty income from Q32 Bio, Inc. The remaining author has nothing to disclose.
Funding
None.
Acknowledgments
R.E. Campbell and J.M. Thurman acknowledge and credit BioRender.com for the assistance in creating Figure 1.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Author Contributions
R.E. Campbell and J.M. Thurman conceptualized the study, wrote the original draft, and reviewed and edited the manuscript.
References
- 1.Barisoni L, Schnaper HW, Kopp JB: A proposed taxonomy for the podocytopathies: A reassessment of the primary nephrotic diseases. Clin J Am Soc Nephrol 2: 529–542, 2007. 10.2215/CJN.04121206 [DOI] [PubMed] [Google Scholar]
- 2.Kopp JB, Anders HJ, Susztak K, Podestà MA, Remuzzi G, Hildebrandt F, Romagnani P: Podocytopathies. Nat Rev Dis Primers 6: 68, 2020. 10.1038/s41572-020-0196-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meadow SR, Sarsfield JK, Scott DG, Rajah SM: Steroid-responsive nephrotic syndrome and allergy: Immunological studies. Arch Dis Child 56: 517–524, 1981. 10.1136/adc.56.7.517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shalhoub RJ: Pathogenesis of lipoid nephrosis: A disorder of T-cell function. Lancet 2: 556–560, 1974. 10.1016/s0140-6736(74)91880-7 [DOI] [PubMed] [Google Scholar]
- 5.Gadegbeku CA, Gipson DS, Holzman LB, Ojo AO, Song PX, Barisoni L, Sampson MG, Kopp JB, Lemley KV, Nelson PJ, Lienczewski CC, Adler SG, Appel GB, Cattran DC, Choi MJ, Contreras G, Dell KM, Fervenza FC, Gibson KL, Greenbaum LA, Hernandez JD, Hewitt SM, Hingorani SR, Hladunewich M, Hogan MC, Hogan SL, Kaskel FJ, Lieske JC, Meyers KE, Nachman PH, Nast CC, Neu AM, Reich HN, Sedor JR, Sethna CB, Trachtman H, Tuttle KR, Zhdanova O, Zilleruelo GE, Kretzler M: Design of the Nephrotic Syndrome Study Network (NEPTUNE) to evaluate primary glomerular nephropathy by a multidisciplinary approach. Kidney Int 83: 749–756, 2013. 10.1038/ki.2012.428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ravani P, Bonanni A, Rossi R, Caridi G, Ghiggeri GM: Anti-CD20 antibodies for idiopathic nephrotic syndrome in children. Clin J Am Soc Nephrol 11: 710–720, 2016. 10.2215/CJN.08500815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ravani P, Colucci M, Bruschi M, Vivarelli M, Cioni M, DiDonato A, Cravedi P, Lugani F, Antonini F, Prunotto M, Emma F, Angeletti A, Ghiggeri GM: Human or chimeric monoclonal anti-CD20 antibodies for children with nephrotic syndrome: A superiority randomized trial. J Am Soc Nephrol 32: 2652–2663, 2021. 10.1681/ASN.2021040561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ruggenenti P, Ruggiero B, Cravedi P, Vivarelli M, Massella L, Marasà M, Chianca A, Rubis N, Ene-Iordache B, Rudnicki M, Pollastro RM, Capasso G, Pisani A, Pennesi M, Emma F, Remuzzi G; Rituximab in Nephrotic Syndrome of Steroid-Dependent or Frequently Relapsing Minimal Change Disease Or Focal Segmental Glomerulosclerosis (NEMO) Study Group : Rituximab in steroid-dependent or frequently relapsing idiopathic nephrotic syndrome. J Am Soc Nephrol 25: 850–863, 2014. 10.1681/ASN.2013030251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vizjak A, Ferluga D, Rozic M, Hvala A, Lindic J, Levart TK, Jurcić V, Jennette JC: Pathology, clinical presentations, and outcomes of C1q nephropathy. J Am Soc Nephrol 19: 2237–2244, 2008. 10.1681/ASN.2007080929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connor TM, Aiello V, Griffith M, Cairns T, Roufosse CA, Cook HT, Pusey CD: The natural history of immunoglobulin M nephropathy in adults. Nephrol Dial Transplant 32: 823–829, 2017. 10.1093/ndt/gfw063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devasahayam J, Erode-Singaravelu G, Bhat Z, Oliver T, Chandran A, Zeng X, Dakshinesh P, Pillai U: C1q nephropathy: The unique underrecognized pathological entity. Anal Cell Pathol (Amst) 2015: 490413, 2015. 10.1155/2015/490413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benz K, Büttner M, Dittrich K, Campean V, Dötsch J, Amann K: Characterisation of renal immune cell infiltrates in children with nephrotic syndrome. Pediatr Nephrol 25: 1291–1298, 2010. 10.1007/s00467-010-1507-0 [DOI] [PubMed] [Google Scholar]
- 13.Yatim KM, Lakkis FG: A brief journey through the immune system. Clin J Am Soc Nephrol 10: 1274–1281, 2015. 10.2215/CJN.10031014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Latt KZ, Heymann J, Jessee JH, Rosenberg AZ, Berthier CC, Arazi A, Eddy S, Yoshida T, Zhao Y, Chen V, Nelson GW, Cam M, Kumar P, Mehta M, Kelly MC, Kretzler M, Ray PE, Moxey-Mims M, Gorman GH, Lechner BL, Regunathan-Shenk R, Raj DS, Susztak K, Winkler CA, Kopp JB; Nephrotic Syndrome Study Network (NEPTUNE); Accelerating Medicines Partnership in Rheumatoid Arthritis and Systemic Lupus Erythematosus (AMP RA/SLE) Consortium : Urine single-cell RNA sequencing in focal segmental glomerulosclerosis reveals inflammatory signatures. Kidney Int Rep 7: 289–304, 2021. 10.1016/j.ekir.2021.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chung CF, Kitzler T, Kachurina N, Pessina K, Babayeva S, Bitzan M, Kaskel F, Colmegna I, Alachkar N, Goodyer P, Cybulsky AV, Torban E: Intrinsic tumor necrosis factor-α pathway is activated in a subset of patients with focal segmental glomerulosclerosis. PLoS One 14: e0216426, 2019. 10.1371/journal.pone.0216426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rosenberg AZ, Kopp JB: Focal segmental glomerulosclerosis. Clin J Am Soc Nephrol 12: 502–517, 2017. 10.2215/CJN.05960616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Vriese AS, Sethi S, Nath KA, Glassock RJ, Fervenza FC: Differentiating primary, genetic, and secondary FSGS in adults: A clinicopathologic approach. J Am Soc Nephrol 29: 759–774, 2018. 10.1681/ASN.2017090958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu L, Murray B, Tomaszewski JE: Lupus podocytopathy superimposed on diabetic glomerulosclerosis: A case report. Medicine (Baltimore) 100: e27077, 2021. 10.1097/MD.0000000000027077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.International Study of Kidney Disease in Children : The primary nephrotic syndrome in children. Identification of patients with minimal change nephrotic syndrome from initial response to prednisone. A report of the International Study of Kidney Disease in Children. J Pediatr 98: 561–564, 1981. 10.1016/s0022-3476(81)80760-3 [DOI] [PubMed] [Google Scholar]
- 20.Ling C, Wang X, Chen Z, Fan J, Meng Q, Zhou N, Sun Q, Hua L, Gui J, Liu X: Altered B-lymphocyte homeostasis in idiopathic nephrotic syndrome. Front Pediatr 7: 377, 2019. 10.3389/fped.2019.00377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhatia D, Sinha A, Hari P, Sopory S, Saini S, Puraswani M, Saini H, Mitra DK, Bagga A: Rituximab modulates T- and B-lymphocyte subsets and urinary CD80 excretion in patients with steroid-dependent nephrotic syndrome. Pediatr Res 84: 520–526, 2018. 10.1038/s41390-018-0088-7 [DOI] [PubMed] [Google Scholar]
- 22.Colucci M, Carsetti R, Cascioli S, Serafinelli J, Emma F, Vivarelli M: B cell phenotype in pediatric idiopathic nephrotic syndrome. Pediatr Nephrol 34: 177–181, 2019. 10.1007/s00467-018-4095-z [DOI] [PubMed] [Google Scholar]
- 23.Colucci M, Carsetti R, Cascioli S, Casiraghi F, Perna A, Ravà L, Ruggiero B, Emma F, Vivarelli M: B cell reconstitution after rituximab treatment in idiopathic nephrotic syndrome. J Am Soc Nephrol 27: 1811–1822, 2016. 10.1681/ASN.2015050523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fribourg M, Cioni M, Ghiggeri G, Cantarelli C, Leventhal JS, Budge K, Bin S, Riella LV, Colucci M, Vivarelli M, Angeletti A, Perin L, Cravedi P: CyTOF-enabled analysis identifies class-switched B cells as the main lymphocyte subset associated with disease relapse in children with idiopathic nephrotic syndrome. Front Immunol 12: 726428, 2021. 10.3389/fimmu.2021.726428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menon R, Otto EA, Hoover P, Eddy S, Mariani L, Godfrey B, Berthier CC, Eichinger F, Subramanian L, Harder J, Ju W, Nair V, Larkina M, Naik AS, Luo J, Jain S, Sealfon R, Troyanskaya O, Hacohen N, Hodgin JB, Kretzler M, Kpmp; Nephrotic Syndrome Study Network (NEPTUNE) : Single cell transcriptomics identifies focal segmental glomerulosclerosis remission endothelial biomarker. JCI Insight 5: 133267, 2020. 10.1172/jci.insight.133267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Warejko JK, Tan W, Daga A, Schapiro D, Lawson JA, Shril S, Lovric S, Ashraf S, Rao J, Hermle T, Jobst-Schwan T, Widmeier E, Majmundar AJ, Schneider R, Gee HY, Schmidt JM, Vivante A, van der Ven AT, Ityel H, Chen J, Sadowski CE, Kohl S, Pabst WL, Nakayama M, Somers MJG, Rodig NM, Daouk G, Baum M, Stein DR, Ferguson MA, Traum AZ, Soliman NA, Kari JA, El Desoky S, Fathy H, Zenker M, Bakkaloglu SA, Müller D, Noyan A, Ozaltin F, Cadnapaphornchai MA, Hashmi S, Hopcian J, Kopp JB, Benador N, Bockenhauer D, Bogdanovic R, Stajić N, Chernin G, Ettenger R, Fehrenbach H, Kemper M, Munarriz RL, Podracka L, Büscher R, Serdaroglu E, Tasic V, Mane S, Lifton RP, Braun DA, Hildebrandt F: Whole exome sequencing of patients with steroid-resistant nephrotic syndrome. Clin J Am Soc Nephrol 13: 53–62, 2018. 10.2215/CJN.04120417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang H, Wang Z, Dong L, Guo Y, Wu J, Zhai S: New insight into the pathogenesis of minimal change nephrotic syndrome: Role of the persistence of respiratory tract virus in immune disorders. Autoimmun Rev 15: 632–637, 2016. 10.1016/j.autrev.2016.02.007 [DOI] [PubMed] [Google Scholar]
- 28.Muehlig AK, Gies S, Huber TB, Braun F: Collapsing focal segmental glomerulosclerosis in viral infections. Front Immunol 12: 800074, 2022. 10.3389/fimmu.2021.800074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dettmar AK, Oh J: Infection-related focal segmental glomerulosclerosis in children. BioMed Res Int 2016: 7351964, 2016. 10.1155/2016/7351964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.May RM, Cassol C, Hannoudi A, Larsen CP, Lerma EV, Haun RS, Braga JR, Hassen SI, Wilson J, VanBeek C, Vankalakunti M, Barnum L, Walker PD, Bourne TD, Messias NC, Ambruzs JM, Boils CL, Sharma SS, Cossey LN, Baxi PV, Palmer M, Zuckerman JE, Walavalkar V, Urisman A, Gallan AJ, Al-Rabadi LF, Rodby R, Luyckx V, Espino G, Santhana-Krishnan S, Alper B, Lam SG, Hannoudi GN, Matthew D, Belz M, Singer G, Kunaparaju S, Price D, Chawla S, Rondla C, Abdalla MA, Britton ML, Paul S, Ranjit U, Bichu P, Williamson SR, Sharma Y, Gaspert A, Grosse P, Meyer I, Vasudev B, El Kassem M, Velez JCQ, Caza TN: A multi-center retrospective cohort study defines the spectrum of kidney pathology in Coronavirus 2019 Disease (COVID-19). Kidney Int 100: 1303–1315, 2021. 10.1016/j.kint.2021.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang YM, Gu QH, Huang J, Qu Z, Wang X, Meng LQ, Wang F, Liu G, Cui Z, Zhao MH: Clinical significance of IgM and C3 glomerular deposition in primary focal segmental glomerulosclerosis. Clin J Am Soc Nephrol 11: 1582–1589, 2016. 10.2215/CJN.01190216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Enya T, Morimoto Y, Oshima R, Miyazaki K, Miyazawa T, Okada M, Sugimoto K: Nephrotic syndrome relapse in a boy with COVID-19. CEN Case Rep 10: 431–434, 2021. 10.1007/s13730-021-00587-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wakashin H, Heymann J, Roshanravan H, Daneshpajouhnejad P, Rosenberg A, Shin MK, Hoek M, Kopp JB: APOL1 renal risk variants exacerbate podocyte injury by increasing inflammatory stress. BMC Nephrol 21: 371, 2020. 10.1186/s12882-020-01995-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nichols B, Jog P, Lee JH, Blackler D, Wilmot M, D’Agati V, Markowitz G, Kopp JB, Alper SL, Pollak MR, Friedman DJ: Innate immunity pathways regulate the nephropathy gene apolipoprotein L1. Kidney Int 87: 332–342, 2015. 10.1038/ki.2014.270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu J, Ma Z, Raman A, Beckerman P, Dhillon P, Mukhi D, Palmer M, Chen HC, Cohen CR, Dunn T, Reilly J, Meyer N, Shashaty M, Arany Z, Haskó G, Laudanski K, Hung A, Susztak K: APOL1 risk variants in individuals of African genetic ancestry drive endothelial cell defects that exacerbate sepsis. Immunity 54: 2632–2649.e6, 2021. 10.1016/j.immuni.2021.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morel A, Meuleman MS, Moktefi A, Audard V: Renal diseases associated with hematologic malignancies and thymoma in the absence of renal monoclonal immunoglobulin deposits. Diagnostics (Basel) 11: 710, 2021. 10.3390/diagnostics11040710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brukamp K, Doyle AM, Bloom RD, Bunin N, Tomaszewski JE, Cizman B: Nephrotic syndrome after hematopoietic cell transplantation: Do glomerular lesions represent renal graft-versus-host disease? Clin J Am Soc Nephrol 1: 685–694, 2006. 10.2215/CJN.00380705 [DOI] [PubMed] [Google Scholar]
- 38.Dabrowski D, Ozluk E, Barbeito S, Wei EX: Focal segmental glomerulosclerosis preceding type 2 papillary renal cell carcinoma. Case Rep Pathol 2020: 8811905, 2020. 10.1155/2020/8811905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.González-Fontal GR, Restrepo JG, Henao-Martínez AF: Minimal-change disease as a paraneoplastic syndrome in a patient with ovarian carcinoma. NDT Plus 4: 427–429, 2011. 10.1093/ndtplus/sfr106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pathak LK, Dabbas W: Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis syndrome and collapsing focal segmental glomerulosclerosis. Proc Bayl Univ Med Cent 33: 606–607, 2020. 10.1080/08998280.2020.1769797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gebregeorgis W, Patel I, Thakur M, Bhutani D, Woldie I: Collapsing glomerulopathy associated with hemophagocytic syndrome in a patient with NK/T cell lymphoma. Clin Nephrol Case Stud 4: 11–17, 2016. 10.5414/CNCS108586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Markowitz GS, Appel GB, Fine PL, Fenves AZ, Loon NR, Jagannath S, Kuhn JA, Dratch AD, D’Agati VD: Collapsing focal segmental glomerulosclerosis following treatment with high-dose pamidronate. J Am Soc Nephrol 12: 1164–1172, 2001. 10.1681/ASN.V1261164 [DOI] [PubMed] [Google Scholar]
- 43.Davis J, Desmond M, Berk M: Lithium and nephrotoxicity: Unravelling the complex pathophysiological threads of the lightest metal. Nephrology (Carlton) 23: 897–903, 2018. 10.1111/nep.13263 [DOI] [PubMed] [Google Scholar]
- 44.Bakhriansyah M, Souverein PC, van den Hoogen MWF, de Boer A, Klungel OH: Risk of nephrotic syndrome for non-steroidal anti-inflammatory drug users. Clin J Am Soc Nephrol 14: 1355–1362, 2019. 10.2215/CJN.14331218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Markowitz GS, Bomback AS, Perazella MA: Drug-induced glomerular disease: Direct cellular injury. Clin J Am Soc Nephrol 10: 1291–1299, 2015. 10.2215/CJN.00860115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Markowitz GS, Nasr SH, Stokes MB, D’Agati VD: Treatment with IFN-alpha, -beta, or -gamma is associated with collapsing focal segmental glomerulosclerosis. Clin J Am Soc Nephrol 5: 607–615, 2010. 10.2215/CJN.07311009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kemper MJ, Zepf K, Klaassen I, Link A, Muller-Wiefel DE: Changes of lymphocyte populations in pediatric steroid-sensitive nephrotic syndrome are more pronounced in remission than in relapse. Am J Nephrol 25: 132–137, 2005. 10.1159/000085357 [DOI] [PubMed] [Google Scholar]
- 48.Lapillonne H, Leclerc A, Ulinski T, Balu L, Garnier A, Dereuddre-Bosquet N, Watier H, Schlageter MH, Deschênes G: Stem cell mobilization in idiopathic steroid-sensitive nephrotic syndrome. Pediatr Nephrol 23: 1251–1256, 2008. 10.1007/s00467-008-0793-2 [DOI] [PubMed] [Google Scholar]
- 49.Ye Q, Zhou C, Li S, Wang J, Liu F, Liu Z, Mao J, Fu H: The immune cell landscape of peripheral blood mononuclear cells from PNS patients. Sci Rep 11: 13083, 2021. 10.1038/s41598-021-92573-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stachowski J, Barth C, Michałkiewicz J, Krynicki T, Jarmoliński T, Runowski D, Lewandowska-Stachowiak M, Zaniew M, Warzywoda A, Bortkiewicz E, Dobosz M, Maciejewski J, Baldamus CA: Th1/Th2 balance and CD45-positive T cell subsets in primary nephrotic syndrome. Pediatr Nephrol 14: 779–785, 2000. 10.1007/PL00013437 [DOI] [PubMed] [Google Scholar]
- 51.Liu LL, Qin Y, Cai JF, Wang HY, Tao JL, Li H, Chen LM, Li MX, Li XM, Li XW: Th17/Treg imbalance in adult patients with minimal change nephrotic syndrome. Clin Immunol 139: 314–320, 2011. 10.1016/j.clim.2011.02.018 [DOI] [PubMed] [Google Scholar]
- 52.Hashimura Y, Nozu K, Kanegane H, Miyawaki T, Hayakawa A, Yoshikawa N, Nakanishi K, Takemoto M, Iijima K, Matsuo M: Minimal change nephrotic syndrome associated with immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome. Pediatr Nephrol 24: 1181–1186, 2009. 10.1007/s00467-009-1119-8 [DOI] [PubMed] [Google Scholar]
- 53.Kienzl-Wagner K, Waldegger S, Schneeberger S: Disease recurrence: The sword of Damocles in kidney transplantation for primary focal segmental glomerulosclerosis. Front Immunol 10: 1669, 2019. 10.3389/fimmu.2019.01669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gallon L, Leventhal J, Skaro A, Kanwar Y, Alvarado A: Resolution of recurrent focal segmental glomerulosclerosis after retransplantation. N Engl J Med 366: 1648–1649, 2012. 10.1056/NEJMc1202500 [DOI] [PubMed] [Google Scholar]
- 55.Shuster S, Ankawi G, Licht C, Reiser J, Wang X, Wei C, Chitayat D, Hladunewich M: Fetal renal echogenicity associated with maternal focal segmental glomerulosclerosis: The effect of transplacental transmission of permeability factor suPAR. J Clin Med 7: 324, 2018. 10.3390/jcm7100324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kemper MJ, Wolf G, Müller-Wiefel DE: Transmission of glomerular permeability factor from a mother to her child. N Engl J Med 344: 386–387, 2001. 10.1056/NEJM200102013440517 [DOI] [PubMed] [Google Scholar]
- 57.Zimmerman SW: Increased urinary protein excretion in the rat produced by serum from a patient with recurrent focal glomerular sclerosis after renal transplantation. Clin Nephrol 22: 32–38, 1984 [PubMed] [Google Scholar]
- 58.Savin VJ, Sharma R, Sharma M, McCarthy ET, Swan SK, Ellis E, Lovell H, Warady B, Gunwar S, Chonko AM, Artero M, Vincenti F: Circulating factor associated with increased glomerular permeability to albumin in recurrent focal segmental glomerulosclerosis. N Engl J Med 334: 878–883, 1996 [DOI] [PubMed] [Google Scholar]
- 59.Koyama A, Fujisaki M, Kobayashi M, Igarashi M, Narita M: A glomerular permeability factor produced by human T cell hybridomas. Kidney Int 40: 453–460, 1991 [DOI] [PubMed] [Google Scholar]
- 60.Yap HK, Cheung W, Murugasu B, Sim SK, Seah CC, Jordan SC: Th1 and Th2 cytokine mRNA profiles in childhood nephrotic syndrome: Evidence for increased IL-13 mRNA expression in relapse. J Am Soc Nephrol 10: 529–537, 1999. 10.1681/ASN.V103529 [DOI] [PubMed] [Google Scholar]
- 61.Savin VJ, Sharma M, Zhou J, Genochi D, Sharma R, Srivastava T, Ilahe A, Budhiraja P, Gupta A, McCarthy ET: Multiple targets for novel therapy of FSGS associated with circulating permeability factor. BioMed Res Int 2017: 6232616, 2017. 10.1155/2017/6232616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhai S, Sun B, Zhang Y, Zhao L, Zhang L: IL-17 aggravates renal injury by promoting podocyte injury in children with primary nephrotic syndrome. Exp Ther Med 20: 409–417, 2020. 10.3892/etm.2020.8698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lama G, Luongo I, Tirino G, Borriello A, Carangio C, Salsano ME: T-lymphocyte populations and cytokines in childhood nephrotic syndrome. Am J Kidney Dis 39: 958–965, 2002. 10.1053/ajkd.2002.32769 [DOI] [PubMed] [Google Scholar]
- 64.Angeletti A, Magnasco A, AntonellaTrivelli, Degl’Innocenti LM, Piaggio G, Lugani F, Caridi G, Verrina E, Cravedi P, Ghiggeri GM: Refractory minimal change disease and focal segmental glomerular sclerosis treated with anakinra. Kidney Int Rep 7: 121–124, 2021. 10.1016/j.ekir.2021.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Otalora L, Chavez E, Watford D, Tueros L, Correa M, Nair V, Ruiz P, Wahl P, Eddy S, Martini S, Kretzler M, Burke GW 3rd, Fornoni A, Merscher S: Identification of glomerular and podocyte-specific genes and pathways activated by sera of patients with focal segmental glomerulosclerosis. PLoS One 14: e0222948, 2019. 10.1371/journal.pone.0222948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Joy MS, Gipson DS, Powell L, MacHardy J, Jennette JC, Vento S, Pan C, Savin V, Eddy A, Fogo AB, Kopp JB, Cattran D, Trachtman H: Phase 1 trial of adalimumab in focal segmental glomerulosclerosis (FSGS). II. Report of the FONT (Novel Therapies for Resistant FSGS) study group. Am J Kidney Dis 55: 50–60, 2010. 10.1053/j.ajkd.2009.08.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Desilva F: Minimal change nephrotic syndrome - Focal sclerosis complex. In: Renal Pathology with Clinical and Functional Correlations, edited by Tisher CC, Brenner BM, Philadelphia, Lippincott Williams & Wilkins, 1994, 347–350 [Google Scholar]
- 68.Hoffman W, Lakkis FG, Chalasani G: B cells, antibodies, and more. Clin J Am Soc Nephrol 11: 137–154, 2016. 10.2215/CJN.09430915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kemper MJ, Meyer-Jark T, Lilova M, Müller-Wiefel DE: Combined T- and B-cell activation in childhood steroid-sensitive nephrotic syndrome. Clin Nephrol 60: 242–247, 2003 [DOI] [PubMed] [Google Scholar]
- 70.Ye Q, Zhang Y, Zhuang J, Bi Y, Xu H, Shen Q, Liu J, Fu H, Wang J, Feng C, Tang X, Liu F, Gu W, Zhao F, Zhang J, Qin Y, Shang S, Shen H, Chen X, Shen H, Liu A, Xia Y, Lu Z, Shu Q, Mao J: The important roles and molecular mechanisms of annexin A2 autoantibody in children with nephrotic syndrome. Ann Transl Med 9: 1452, 2021. 10.21037/atm-21-3988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Watts AJB, Keller KH, Lerner G, Rosales I, Collins AB, Sekulic M, Waikar SS, Chandraker A, Riella LV, Alexander MP, Troost JP, Chen J, Fermin D, Yee JL, Sampson MG, Beck LH Jr., Henderson JM, Greka A, Rennke HG, Weins A: Discovery of autoantibodies targeting nephrin in minimal change disease supports a novel autoimmune etiology. J Am Soc Nephrol 33: 238–252, 2022. 10.1681/ASN.2021060794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jamin A, Berthelot L, Couderc A, Chemouny JM, Boedec E, Dehoux L, Abbad L, Dossier C, Daugas E, Monteiro RC, Deschênes G: Autoantibodies against podocytic UCHL1 are associated with idiopathic nephrotic syndrome relapses and induce proteinuria in mice. J Autoimmun 89: 149–161, 2018. 10.1016/j.jaut.2017.12.014 [DOI] [PubMed] [Google Scholar]
- 73.Delville M, Sigdel TK, Wei C, Li J, Hsieh SC, Fornoni A, Burke GW, Bruneval P, Naesens M, Jackson A, Alachkar N, Canaud G, Legendre C, Anglicheau D, Reiser J, Sarwal MM: A circulating antibody panel for pretransplant prediction of FSGS recurrence after kidney transplantation. Sci Transl Med 6: 256ra136, 2014. 10.1126/scitranslmed.3008538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alachkar N, Gupta G, Montgomery RA: Angiotensin antibodies and focal segmental glomerulosclerosis. N Engl J Med 368: 971–973, 2013. 10.1056/NEJMc1207233 [DOI] [PubMed] [Google Scholar]
- 75.Ainsworth SK, Hirsch HZ, Brackett NC Jr, Brissie RM, Williams AV Jr, Hennigar GR: Diabetic glomerulonephropathy: Histopathologic, immunofluorescent, and ultrastructural studies of 16 cases. Hum Pathol 13: 470–478, 1982 [DOI] [PubMed] [Google Scholar]
- 76.Zeis PM, Kavazarakis E, Nakopoulou L, Moustaki M, Messaritaki A, Zeis MP, Nicolaidou P: Glomerulopathy with mesangial IgM deposits: Long-term follow up of 64 children. Pediatr Int 43: 287–292, 2001 [DOI] [PubMed] [Google Scholar]
- 77.Habib R, Girardin E, Gagnadoux MF, Hinglais N, Levy M, Broyer M: Immunopathological findings in idiopathic nephrosis: Clinical significance of glomerular “immune deposits.” Pediatr Nephrol 2: 402–408, 1988 [DOI] [PubMed] [Google Scholar]
- 78.Trachtman H, Laskowski J, Lee C, Renner B, Feemster A, Parikh S, Panzer SE, Zhong W, Cravedi P, Cantarelli C, Kulik L, You Z, Satchell S, Rovin B, Liu F, Kalled SL, Holers VM, Jalal D, Thurman JM: Natural antibody and complement activation characterize patients with idiopathic nephrotic syndrome. Am J Physiol Renal Physiol 321: F505–F516, 2021. 10.1152/ajprenal.00041.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Strassheim D, Renner B, Panzer S, Fuquay R, Kulik L, Ljubanović D, Holers VM, Thurman JM: IgM contributes to glomerular injury in FSGS. J Am Soc Nephrol 24: 393–406, 2013. 10.1681/ASN.2012020187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Panzer SE, Laskowski J, Renner B, Kulik L, Ljubanovic D, Huber KM, Zhong W, Pickering MC, Holers VM, Thurman JM: IgM exacerbates glomerular disease progression in complement-induced glomerulopathy. Kidney Int 88: 528–537, 2015. 10.1038/ki.2015.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mirioglu S, Caliskan Y, Ozluk Y, Dirim AB, Istemihan Z, Akyildiz A, Yazici H, Turkmen A, Kilicaslan I, Sever MS: Co-deposition of IgM and C3 may indicate unfavorable renal outcomes in adult patients with primary focal segmental glomerulosclerosis. Kidney Blood Press Res 44: 961–972, 2019. 10.1159/000501827 [DOI] [PubMed] [Google Scholar]
- 82.Juozapaite S, Cerkauskiene R, Laurinavicius A, Jankauskiene A: The impact of IgM deposits on the outcome of nephrotic syndrome in children. BMC Nephrol 18: 260, 2017. 10.1186/s12882-017-0674-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Angeletti A, Cantarelli C, Petrosyan A, Andrighetto S, Budge K, D’Agati VD, Hartzell S, Malvi D, Donadei C, Thurman JM, Galešić-Ljubanović D, He JC, Xiao W, Campbell KN, Wong J, Fischman C, Manrique J, Zaza G, Fiaccadori E, La Manna G, Fribourg M, Leventhal J, Da Sacco S, Perin L, Heeger PS, Cravedi P: Loss of decay-accelerating factor triggers podocyte injury and glomerulosclerosis. J Exp Med 217: e20191699, 2020. 10.1084/jem.20191699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Morigi M, Perico L, Corna D, Locatelli M, Cassis P, Carminati CE, Bolognini S, Zoja C, Remuzzi G, Benigni A, Buelli S: C3a receptor blockade protects podocytes from injury in diabetic nephropathy. JCI Insight 5: 131849, 2020. 10.1172/jci.insight.131849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Benz K, Dötsch J, Rascher W, Stachel D: Change of the course of steroid-dependent nephrotic syndrome after rituximab therapy. Pediatr Nephrol 19: 794–797, 2004. 10.1007/s00467-004-1434-z [DOI] [PubMed] [Google Scholar]
- 86.Ravani P, Lugani F, Drovandi S, Caridi G, Angeletti A, Ghiggeri GM: Rituximab vs low-dose mycophenolate mofetil in recurrence of steroid-dependent nephrotic syndrome in children and young adults: A randomized clinical trial. JAMA Pediatr 175: 631–632, 2021. 10.1001/jamapediatrics.2020.6150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Iijima K, Sako M, Nozu K, Mori R, Tuchida N, Kamei K, Miura K, Aya K, Nakanishi K, Ohtomo Y, Takahashi S, Tanaka R, Kaito H, Nakamura H, Ishikura K, Ito S, Ohashi Y; Rituximab for Childhood-onset Refractory Nephrotic Syndrome (RCRNS) Study Group : Rituximab for childhood-onset, complicated, frequently relapsing nephrotic syndrome or steroid-dependent nephrotic syndrome: A multicentre, double-blind, randomised, placebo-controlled trial. Lancet 384: 1273–1281, 2014. 10.1016/S0140-6736(14)60541-9 [DOI] [PubMed] [Google Scholar]
- 88.Basu B, Sander A, Roy B, Preussler S, Barua S, Mahapatra TKS, Schaefer F: Efficacy of rituximab vs tacrolimus in pediatric corticosteroid-dependent nephrotic syndrome: A randomized clinical trial. JAMA Pediatr 172: 757–764, 2018. 10.1001/jamapediatrics.2018.1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.van de Veerdonk FL, Lauwerys B, Marijnissen RJ, Timmermans K, Di Padova F, Koenders MI, Gutierrez-Roelens I, Durez P, Netea MG, van der Meer JW, van den Berg WB, Joosten LA: The anti-CD20 antibody rituximab reduces the Th17 cell response. Arthritis Rheum 63: 1507–1516, 2011. 10.1002/art.30314 [DOI] [PubMed] [Google Scholar]
- 90.Ravani P, Ponticelli A, Siciliano C, Fornoni A, Magnasco A, Sica F, Bodria M, Caridi G, Wei C, Belingheri M, Ghio L, Merscher-Gomez S, Edefonti A, Pasini A, Montini G, Murtas C, Wang X, Muruve D, Vaglio A, Martorana D, Pani A, Scolari F, Reiser J, Ghiggeri GM: Rituximab is a safe and effective long-term treatment for children with steroid and calcineurin inhibitor-dependent idiopathic nephrotic syndrome. Kidney Int 84: 1025–1033, 2013. 10.1038/ki.2013.211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chan CY, Liu ID, Resontoc LP, Ng KH, Chan YH, Lau PY, Than M, Jordan SC, Lam KP, Yeo WS, Yap HK: T lymphocyte activation markers as predictors of responsiveness to rituximab among patients with FSGS. Clin J Am Soc Nephrol 11: 1360–1368, 2016. 10.2215/CJN.11941115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wei C, El Hindi S, Li J, Fornoni A, Goes N, Sageshima J, Maiguel D, Karumanchi SA, Yap HK, Saleem M, Zhang Q, Nikolic B, Chaudhuri A, Daftarian P, Salido E, Torres A, Salifu M, Sarwal MM, Schaefer F, Morath C, Schwenger V, Zeier M, Gupta V, Roth D, Rastaldi MP, Burke G, Ruiz P, Reiser J: Circulating urokinase receptor as a cause of focal segmental glomerulosclerosis. Nat Med 17: 952–960, 2011. 10.1038/nm.2411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hayek SS, Sever S, Ko YA, Trachtman H, Awad M, Wadhwani S, Altintas MM, Wei C, Hotton AL, French AL, Sperling LS, Lerakis S, Quyyumi AA, Reiser J: Soluble urokinase receptor and chronic kidney disease. N Engl J Med 373: 1916–1925, 2015. 10.1056/NEJMoa1506362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wei C, Sigdel TK, Sarwal MM, Reiser J: Circulating CD40 autoantibody and suPAR synergy drives glomerular injury. Ann Transl Med 3: 300, 2015. 10.3978/j.issn.2305-5839.2015.11.08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McCarthy ET, Sharma M, Savin VJ: Circulating permeability factors in idiopathic nephrotic syndrome and focal segmental glomerulosclerosis. Clin J Am Soc Nephrol 5: 2115–2121, 2010. 10.2215/CJN.03800609 [DOI] [PubMed] [Google Scholar]
- 96.Banas MC, Banas B, Hudkins KL, Wietecha TA, Iyoda M, Bock E, Hauser P, Pippin JW, Shankland SJ, Smith KD, Stoelcker B, Liu G, Gröne HJ, Krämer BK, Alpers CE: TLR4 links podocytes with the innate immune system to mediate glomerular injury. J Am Soc Nephrol 19: 704–713, 2008. 10.1681/ASN.2007040395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shimada M, Ishimoto T, Lee PY, Lanaspa MA, Rivard CJ, Roncal-Jimenez CA, Wymer DT, Yamabe H, Mathieson PW, Saleem MA, Garin EH, Johnson RJ: Toll-like receptor 3 ligands induce CD80 expression in human podocytes via an NF-κB-dependent pathway. Nephrol Dial Transplant 27: 81–89, 2012. 10.1093/ndt/gfr271 [DOI] [PubMed] [Google Scholar]
- 98.Ishimoto T, Shimada M, Gabriela G, Kosugi T, Sato W, Lee PY, Lanaspa MA, Rivard C, Maruyama S, Garin EH, Johnson RJ: Toll-like receptor 3 ligand, polyIC, induces proteinuria and glomerular CD80, and increases urinary CD80 in mice. Nephrol Dial Transplant 28: 1439–1446, 2013. 10.1093/ndt/gfs543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Reiser J, von Gersdorff G, Loos M, Oh J, Asanuma K, Giardino L, Rastaldi MP, Calvaresi N, Watanabe H, Schwarz K, Faul C, Kretzler M, Davidson A, Sugimoto H, Kalluri R, Sharpe AH, Kreidberg JA, Mundel P: Induction of B7-1 in podocytes is associated with nephrotic syndrome. J Clin Invest 113: 1390–1397, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Garin EH, Mu W, Arthur JM, Rivard CJ, Araya CE, Shimada M, Johnson RJ: Urinary CD80 is elevated in minimal change disease but not in focal segmental glomerulosclerosis. Kidney Int 78: 296–302, 2010. 10.1038/ki.2010.143 [DOI] [PubMed] [Google Scholar]
- 101.Yu CC, Fornoni A, Weins A, Hakroush S, Maiguel D, Sageshima J, Chen L, Ciancio G, Faridi MH, Behr D, Campbell KN, Chang JM, Chen HC, Oh J, Faul C, Arnaout MA, Fiorina P, Gupta V, Greka A, Burke GW 3rd, Mundel P: Abatacept in B7-1-positive proteinuric kidney disease. N Engl J Med 369: 2416–2423, 2013. 10.1056/NEJMoa1304572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Novelli R, Gagliardini E, Ruggiero B, Benigni A, Remuzzi G: Any value of podocyte B7-1 as a biomarker in human MCD and FSGS? Am J Physiol Renal Physiol 310: F335–F341, 2016. 10.1152/ajprenal.00510.2015 [DOI] [PubMed] [Google Scholar]
- 103.Hansrivijit P, Puthenpura MM, Ghahramani N: Efficacy of abatacept treatment for focal segmental glomerulosclerosis and minimal change disease: A systematic review of case reports, case series, and observational studies. Clin Nephrol 94: 117–126, 2020. 10.5414/CN110134 [DOI] [PubMed] [Google Scholar]
- 104.Faul C, Donnelly M, Merscher-Gomez S, Chang YH, Franz S, Delfgaauw J, Chang JM, Choi HY, Campbell KN, Kim K, Reiser J, Mundel P: The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat Med 14: 931–938, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fornoni A, Sageshima J, Wei C, Merscher-Gomez S, Aguillon-Prada R, Jauregui AN, Li J, Mattiazzi A, Ciancio G, Chen L, Zilleruelo G, Abitbol C, Chandar J, Seeherunvong W, Ricordi C, Ikehata M, Rastaldi MP, Reiser J, Burke GW 3rd: Rituximab targets podocytes in recurrent focal segmental glomerulosclerosis. Sci Transl Med 3: 85ra46, 2011. 10.1126/scitranslmed.3002231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tan L, Li S, Yang H, Zou Q, Wan J, Li Q: Efficacy and acceptability of immunosuppressive agents for pediatric frequently-relapsing and steroid-dependent nephrotic syndrome: A network meta-analysis of randomized controlled trials. Medicine (Baltimore) 98: e15927, 2019. 10.1097/MD.0000000000015927 [DOI] [PMC free article] [PubMed] [Google Scholar]