Visual Abstract
Keywords: chronic kidney disease, end stage kidney disease, dialysis, kidney transplantation, menarche, menopause, menstruation, menstrual disturbances, menstrual irregularities, reproductive life span
Abstract
Background and objectives
Menstrual abnormalities and shortened reproductive lifespan are associated with shorter life expectancy and higher cardiovascular and osteoporosis risk in the general population, although the magnitude of these reproductive factor irregularities in females with CKD is unclear. This systematic review and meta-analysis aimed to summarize the current knowledge regarding menstrual abnormalities and reproductive lifespan among females with CKD.
Design, setting, participants, & measurements
A comprehensive bibliographic search (MEDLINE, Embase, and Cumulative Index to Nursing and Allied Health Literature [CINAHL]) was completed from database inception to February 2022 to identify all original articles reporting on females of reproductive age with nondialysis-dependent/nonkidney transplant CKD, dialysis-dependent CKD, or kidney transplantation and menstruation patterns, age of menarche, and/or menopause. Data extraction and study quality assessment were completed in duplicate. Random effects meta-analyses were used to derive pooled proportions estimates.
Results
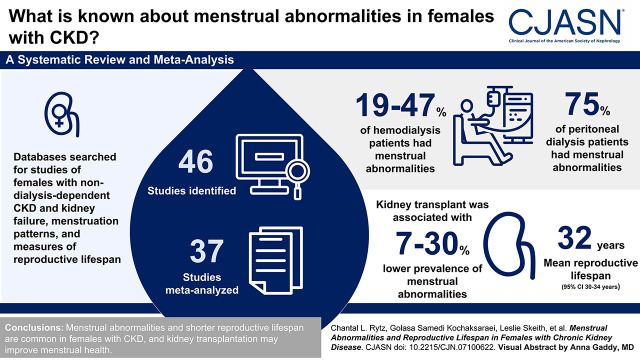
Forty-six studies were identified, and 35 were meta-analyzed, stratified by KRT modality and reported outcome. Menstrual abnormalities were present in 19%–47% of patients on hemodialysis and 75% of patients on peritoneal dialysis. Kidney transplantation was associated with a 7%–30% decrease in menstrual abnormalities. Reproductive lifespan was 32 years (95% confidence interval, 30 to 34 years). Although significant heterogeneity was present, study quality ranged from fair to good, and no evidence of publication bias was noted.
Conclusions
Menstrual abnormalities and shorter reproductive lifespan are common in females with CKD, although kidney transplantation may improve menstrual health.
Introduction
Globally, CKD is more prevalent in women (1), with over 40% affected in the reproductive years (2). Although considerable attention has been placed on improving fertility rates and complications of pregnancy in the CKD population (3), menstrual abnormalities have received notably less consideration despite menarche, menstruation, and menopause being fundamental parts of female reproductive biology. Despite the fact that they are commonly experienced in individuals with CKD (4), studies exploring menstrual abnormalities, shorter reproductive lifespan, and early menopause are often limited by sample size and combine results from populations across CKD stages, including those with kidney transplants (5). Menstrual abnormalities, premature menopause, and shortened reproductive lifespan are associated with lower life expectancy, higher risk of cardiovascular disease (6,7), and osteoporosis (8) in the general population. However, the prevalence of these reproductive factors in the CKD population is unclear. There is increasing recognition that premenopausal-aged women treated with dialysis or kidney transplantation are at high risk of both cardiovascular- and noncardiovascular-related mortality compared with the general population (9) as well as age-matched men treated with dialysis (10). As female reproductive health is associated with important nonreproductive health outcomes, including mortality (7), this underscores the importance of understanding the prevalence of menstrual abnormalities and the reproductive lifespan in the CKD population. Therefore, the objective of this systematic review and meta-analysis was to determine the prevalence of menstrual abnormalities and quantify the reproductive lifespan of females with CKD.
Materials and Methods
Protocol
This systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (11), and the protocol was registered with the Prospective Systematic Review Register.
Information Sources
Published studies were identified through searching electronic databases (MEDLINE, Embase, and Cumulative Index to Nursing and Allied Health Literature [CINAHL]) from inception until February 2022 without language restrictions. Relevant text words and medical subject headings were used to develop a search strategy (Supplemental Table 1) on two broad themes encompassing the population (females with CKD; theme 1) and outcomes (reports on menstrual abnormalities and/or reproductive lifespan; theme 2). The two themes were combined using the Boolean operator “AND,” and the search was limited to original, peer-reviewed human studies. References from selected papers were scanned to ensure completeness. The search strategy was reviewed by research librarians within the University of Calgary. Database-specific subject headings and medical subject heading words were adapted for each database as necessary. Non-English articles were translated using Google Translate.
Study Eligibility
Study Article Identification.
Studies were eligible for inclusion if they (1) reported females with CKD, including those undergoing/who underwent KRT, such as hemodialysis (HD), peritoneal dialysis (PD), or kidney transplantation (if a study included kidney transplantation recipients, menstrual patterns both before and after transplantation were required to be reported for inclusion); (2) reported the menstrual status of females, including the number of individuals with regular menstruation, individuals with irregular menstruation and/or those with menstrual abnormalities, and/or reproductive lifespan and/or reproductive lifespan–associated conditions (e.g., primary ovarian insufficiency); and (3) were an observational study design (i.e., cohort, cross-sectional, or case-control).
Study Selection.
After conducting a calibration exercise, titles and abstracts from the literature search were screened independently and in duplicate by two reviewers (C.L.R. and G.S.K.). The same process was followed for the screening of full-text articles. When data were missing or clarification of published data was needed, the study authors were contacted. Percentage agreement was quantified at each screening stage, and any disagreements in study inclusion were resolved by discussion with a third reviewer (L.S.).
Data Extraction.
Study identifiers (authors and location and year of publication), study design characteristics (sample size and inclusion/exclusion criteria), participant characteristics (age and comorbidities), CKD characteristics (year/age of diagnosis, KRT, and etiology), and menstruation characteristics (hormone therapy use, menstrual abnormalities related to regularity, frequency, duration, amount, age of menarche, and age of menopause) were abstracted using a standardized form. Individuals with primary amenorrhea were not included in counts for amenorrhea. Assessment of study quality was completed using a modified version of the National Institutes of Health Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies and Case-Control Studies (12). Study quality for observational and cross-sectional studies was rated as poor (zero to three of 12 questions), fair (four to seven), or good (eight to 12). Case-control studies were rated as poor (zero to five of 17), fair (six to 11), or good (12 to 17).
Data Synthesis and Analysis.
The proportion of patients with menstrual abnormalities and the mean reproductive lifespan were the primary measures of interest. Denominator sample sizes for menstrual disturbance calculations excluded nonreporting, individuals with primary amenorrhea, postmenopausal individuals, and those with menstrual abnormalities prior to CKD diagnoses. Reporting of both age of menarche and age of menopause was required to calculate the reproductive lifespan. When two values were presented for either age of menarche or menopause (e.g., reporting stratified by KRT modality type), the ages were combined using a sample size weighted mean within each study to produce one overall number. When possible, measures were pooled using DerSimonian Laird random effects models (13). Pooled estimates for the proportions of menstrual abnormalities were assessed separately by KRT. For dialyzed individuals, pooled proportion estimates were further stratified by HD and PD. Pooled mean ages were calculated for reported ages of menarche, menopause, and reproductive lifespan separately. Pooled estimates were used to compare changes in menstrual abnormalities in transplanted females pre- and postkidney transplantation, stratified by menstrual abnormalities type. Heterogeneity was determined using Cochran Q and I2 statistics. Publication bias was determined through visual assessments of funnel plots. A two-sided P=0.05 was considered statistically significant. All analyses were performed using STATA (V16; StataCorp LP, College Station, TX).
Results
Study Selection
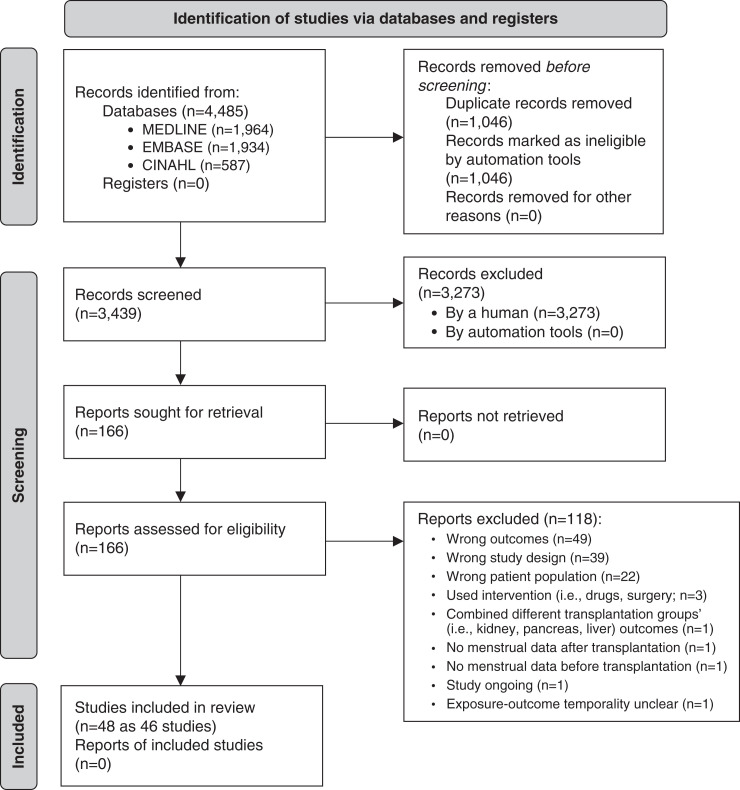
Of 4485 potentially relevant citations identified within the electronic search, 3439 titles were assessed, and 166 full-text publications were retrieved for further review, of which 48 studies met inclusion criteria (Figure 1). Two sets of two studies (set 1 [14,15] and set 2 [16,17]) represented the same study population and were merged to ensure that there was no duplication. Therefore, 46 studies (27 cohort, 17 cross-sectional, and two case-control) were retained representing 5636 individuals. Sixteen articles reported menstrual abnormalities of dialyzed patients, 11 reported menstrual abnormalities of transplanted patients, two studies reported menstrual abnormalities in females treated with dialysis and kidney transplantation, and six studies reported reproductive lifespan. Percentage agreement values between reviewers (C.L.R. and G.S.K.) were 91% and 77% for title and full-text review, respectively.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses flow chart. CINAHL, Cumulative Index to Nursing and Allied Health Literature.
Study Characteristics
Characteristics of included studies are shown in Table 1. The mean age of study cohorts ranged from 15 to 70 years old. Study publication dates ranged from 1975 to 2021, with the majority (n=32) published after 2000. Three articles (18–20) included individuals with nondialysis-dependent CKD (n=1165), and three (5,21,22) included those receiving conservative kidney care (n=92). The majority of individuals were receiving HD (n=3039) or PD (n=229), and 1084 kidney transplant recipients had undergone HD (n=223), PD (n=37), or both (n=8) before transplantation. Thirteen articles did not specify which kind of dialysis, if at all, was undertaken before transplantation (5,22–34).
Table 1.
Study demographics
| Authors (yr) | Study Design | Country | Study Size | Age Range, yr | Mean Age, yr | Ethnicity/Race, % White | Stage of CKD | Months since Diagnosis (SD) | Hemodialysis, n | Peritoneal Dialysis, n | Months on Dialysis (SD) | Kidney Transplantation, n | Months since Kidney Transplantation (SD) | Age at Kidney Transplantation, yr (SD) | Dialysis before Kidney Transplantation | Months on Dialysis Prekidney Transplantation (SD) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ølgaard et al. (1975) (38) | Cross-sectional | Denmark | 20 | 14–58 | HD group: 36; PD group: 40 | NR | 5d | — | 10 | 10 | 19 (range, 0.5–50) | — | — | — | — | — |
| Bierman and Nolan (1977) (36) | RCS | United States | 23 | 13–43 | 24 | NR | 5d | 4.4 | — | — | 9.5 (NR) | 23 | — | 23.7 (NR) | Yes, n=23 HD | — |
| Morley et al. (1979) (24) | RCS | South Africa | 22 | 17–37 | 27 | NR | 5d | — | 17 | — | — | 5 | 24.2 (NR) | — | Unclear | — |
| Lim et al. (1980) (58) | Cross-sectional | United States | 24 | 18–59 | — | NR | 5d | — | 24 | — | Range, 6–48 | — | ||||
| Gómez et al. (1980) (41) | Cross-sectional | Switzerland | 23 | 19–66 | 44 | NR | 5d | — | 23 | — | 16 (2) | — | — | — | — | — |
| Weizman et al. (1983) (51) | Cross-sectional | Israel | 21 | 20–53 | 37 | NR | 5d | — | 21 | — | 21.6 (9.8) | — | — | — | — | — |
| El-Sheikh et al. (1987) (28) | RCS | United Kingdom | 48 | 20–66 | 46 | NR | 5d | — | — | — | — | 48 | — | — | Yes, unclear what type | |
| Schover et al. (1990) (59) | Cross-sectional | United Kingdom | 36 | — | 40 | NR | 5d | — | — | — | — | 36 | 38.4 (NR) | — | Yes; n=31 HD, n=5 PD | HD group: 19.0 (22.2); PD group: 10.0 (5.6) |
| Bianchi et al. (1992) (21) | Cross-sectional | Italy | 36 | — | 47 | NR | Stage 3 (n=13), stage 4 (n=13), stage 5 (n=10) | — | — | — | — | — | — | — | — | — |
| Otieno et al. (1993) (22) | PCS | Kenya | 40 | — | 29 | NR | 5d | — | 13 | 1 | — | — | — | — | — | — |
| Milde et al. (1996) (78) | Cross-sectional | United States | 56 | 25–86 | 53 | 95 | 5d | n=56 <36 (NR) | 41 | 15 | n=38 >36 (NR) | — | — | — | — | — |
| Cochrane and Regan (1997) (5) | PCS | United Kingdom | 100 | 17–70 | 39 | NR | 5d | — | 17 | 9 | — | 44 | — | — | Unclear | — |
| Holley et al. (1997) (42) | RCS | United States | 76 | 15–59 | 42 | 59 | 5d | — | 52 | 24 | 54 (50.4) | — | — | — | — | — |
| Kim et al. (1998) (31) | RCS | South Korea | 72 | 14–54 | 38 | NR | 5d | — | — | — | — | 72 | 40.2 (29.7) | 34.6 (8.8) | Yes, unclear what type | 27.0 (31.9) |
| Kawashima et al. (1998) (55) | RCS | Japan | 25 | 24–48 | 38 | NR | 5d | — | 25 | — | 53.4 (range, 7–144) | — | — | — | — | — |
| Rush et al. (2000) (39) | RCS | United States | 102 | 26–88 | 56 | 15 | 5d | — | 102 | — | 49.2 (4.8) | — | — | — | — | — |
| Weisinger et al. (2000) (47) | Cross-sectional | Venezuela | 74 | — | 37 | NR | 5d | 86.9 (69.4) | 74 | — | 56.9 (43.3) | — | — | — | — | — |
| Jang et al. (2001) (43) | Cross-sectional | Australia | 48 | 20–84 | 56 | NR | 5d | — | 48 | — | 46.8 (range, 1–204) | — | — | — | — | — |
| Shanmugavadivoo and Shaariah (2003) (40) | RCS | Malaysia | 137 | — | — | 0 | 5d | — | 72 | 65 | 33.3 (range, 2–216) | — | — | — | ||
| Kramer et al. (September 2003) (48) | RCS | United States | 41 | — | 37 | 76 | 5d | — | — | — | — | 41 | n=24 >60 (NR); n=17 <60 (NR) | 30.1 (9.6) | Unclear | — |
| Matuszkiewicz-Rowińska et al. (2004) (17) | Cross-sectional | Poland | 75 | — | 35 | NR | 5d | — | 75 | — | 32 (30) | — | — | — | — | — |
| Kramer et al. (March 2003) (37) | Cross-sectional | United States | 238 | 47–89 | 70 | 66 | 5d | — | 238 | — | 38.4 (39.6) | — | — | — | — | — |
| Di Iorio et al. (2004) (57) | Cross-sectional | Italy | 1256 | 25–84 | 63 | NR | 5d | — | 1256 | — | 62.2 (61.4) | — | — | — | — | — |
| Yücel et al. (2004) (53) | RCS | Turkey | 32 | 25–65 | 44 | NR | 5d | — | 32 | — | 86.4 (range, 24–231) | — | — | — | — | |
| Pezeshki et al. (2004) (25) | Case-control | Iran | 50 | — | 28 | NR | 5d | — | — | — | — | 50 | — | — | Unclear | — |
| Guazzelli et al. (2008) (29) | RCS | Brazil | 197 | 28–44 | 36 | NR | 5d | — | — | — | 197 | 108 | 32.7 (8.8) | Yes, unclear what type | — | |
| Song et al. (2008) (69) | Case-control | South Korea | 38 | — | 46 | NR | 5d | — | 38 | — | 98 (range, 12–264) | — | — | — | — | — |
| Filocamo et al. (2009) (54) | PCS | Italy | 39 | — | 36 | NR | 5d | — | — | — | — | 39 | 16 (range, 13–46) | — | Yes, n=39 HD | 18 (range, 12–36) |
| Karayalcin et al. (2010) (30) | RCS | Turkey | 50 | 18–52 | 34 | NR | 5d | — | — | — | — | 50 | 52.8 (50.9) | — | Yes, unclear what type | 23.5 (11.2) |
| Arikan et al. (2011) (44) | RCS | Turkey | 30 | 15–45 | 34 | NR | 5d | — | 30 | — | 75.6 (182.2) | — | — | — | — | — |
| Koca et al. (2012) (32) | RCS | Turkey | 117 | — | HD group: 41; PD group: 40; KT group: 37 | NR | 5d | — | 39 | 43 | HD group: 50.4 (39.6); PD group: 56.4 (52.8) | 35 | 36.0 (45.6) | — | Yes, unclear what type | 38.4 (30) |
| van Eps et al. (2012) (79) | PCS | Australia | 7 | — | 41 | NR | 5d | — | 7 | — | — | — | — | — | — | — |
| Yu et al. (2013) (26) | RCS | China | 42 | 25–45 | 29 | NR | 5d | — | — | — | — | 42 | — | — | Yes, unclear what type | 16.0 (range, 1–27 mo) |
| Al-Turki et al. (2014) (35) | Cross-sectional | Saudi Arabia | 40 | 19–90 | 52 | NR | 5d | — | 40 | — | >60 | — | — | — | — | — |
| Cheung et al. (2015) (18) | RCS | United States | 1007 | — | 69 | 50 | Unclear | — | — | — | — | — | — | — | — | — |
| Chakhtoura et al. (2015) (23) | RCS | France | 129 | NR | 5d | — | — | — | — | 102 | — | — | Yes, unclear what type | — | ||
| Teuwafeu et al. (2016) (50) | Cross-sectional | Cameroon | 52 | 18–69 | 38 | NR | 5d | — | 52 | — | Median: 36.0 (IQR, 16–60) | — | — | — | — | — |
| Lin et al. (2016) (34) | Cross-sectional | China | 165 | 14–44 | 35 | NR | 5d | HD group: 57 (21); PD group: 54 (18); KT group: 56 (19) | 86 | 43 | HD group: 52 (8.9); PD group: 48.5 (11.3) | 36 | — | — | Yes, unclear what type | KT group: 49.8 (10.2) |
| Sikora-Grabka et al. (2018) (15) | PCS | Poland | 10 | 31–37 | 34 | NR | 5d | 167 (95% CI, 74 to 258) | — | — | — | — | — | — | Yes, n=8 HD, n=2 PD | Median: 28.6 (95% CI, 11 to 46) |
| Gonçalves et al. (2019) (68) | Cross-sectional | Portugal | 31 | 20–72 | 49 | NR | 5d | 156 (NR) | — | — | — | 31 | Range, 6–60 (NR) | — | Yes, n=105 HD, n=20 PD, n=8 both HD and PD | 52.8 (NR) |
| Yaprak et al. (2019) (52) | RCS | Turkey | 33 | — | 34 | NR | 5d | — | — | — | — | 33 | 29.7 (6.3) | Yes, n=17 HD, n=10 PD | Median: 21.5 (IQR, 6–105) | |
| Fayed et al. (2019) (45) | PCS | Egypt | 60 | 20–35 | 30 | NR | 5d | 79 (42) | 60 | — | 26.9 (8.8) | — | — | — | — | |
| Ramesh et al. (2020) (60) | PCS | Canada | 476 | — | 60 | 75 | 5d | — | 476 | — | — | — | — | — | — | — |
| Serret-Montoya et al. (2020) (20) | PCS | Mexico | 57 | 12–17 | 15 (median) | NR | Stage 4 (n=13), stage 5 (n=44); n=34/44 receiving dialysis | Median: 14 (range, 1–144) | 25 | 19 | Median: 16 (range, 3–60) | — | — | — | — | — |
| Kim et al. (2020) (19) | PCS | United States | 145 | 1–16 | NR | 0 | G2, G3a, G3b, unclear n for each | Median: 168 (IQR, 96–180) | — | — | — | — | — | — | — | — |
| Dines et al. (2021) (27) | Cross-sectional | United States | 190 | 18–44 | NR | 78 | 5d | — | — | — | — | 190 | — | — | Unclear | Unclear |
HD, hemodialysis; PD, peritoneal dialysis; NR, not reported; —, not reported; RCS, retrospective cohort study; PCS, prospective cohort study; KT, kidney transplantation; IQR, interquartile range; 95% CI, 95% confidence interval.
Hormonal Therapy Use
Fourteen studies (18,23,24,27,29,33,35–43) reported hormone therapy/contraceptive use, although numerous (15,34,44–47) considered hormone therapy as exclusion criteria. Eight dialysis-specific articles (24,39–43,48) reporting on 585 individuals reported low use of hormone replacement therapy (n=363), oral contraceptives (n=6), and intrauterine devices (n=2) among study participants. Five transplant-specific articles (23,27,29,33,36) reported 242 and 138 individuals using hormonal contraceptives before and after transplantation, respectively. The most common type was oral contraceptives both before (n=205; 53%) and after (n=124; 34%) transplantation. However, few studies reported on the hormonal composition (e.g., combined or progestogen) of the contraceptive used (23,24,27).
Medication Use
There were 27 studies (14,17,24,26,28–30,33,34,36,38,39,41,43,44,49–56) reporting a combined total of 3534 medications used in 2706 study participants (1.25:1 ratio). The most commonly reported medications included those for anemia treatment (e.g., erythropoietin and ferrous sulfate; n=1612; 46%), immunosuppressant medications (e.g., cyclosporin, tacrolimus, prednisone, mycophenolate mofetil, and mycophenolate sodium; n=1282; 36%), and antihypertensive medications (e.g., propranolol, β-blockers, and calcium channel blockers; n=154; 4%).
Study Outcomes
Menstrual Abnormalities Definitions.
Definitions and descriptions of menstrual conditions are listed in Supplemental Tables 2 and 3 for dialysis and transplantation patients, respectively. Amenorrhea was defined as the absence of menstrual bleeding for 3 (57) to ≥6 months (14,17,23,26,28,33,34,47,55,56), oligomenorrhea was defined as menstruation intervals of >32 days (17) or >35 days apart (34,41,55), polymenorrhea was defined as cycles lasting <28 days (26,54), and irregular menses were defined as excessive (bleeding >5 days), short (<20 days) or long (>45 days) cycles (29), or ranging from occasional spotting to frequent dysfunctional uterine bleeding (58). Several articles failed to provide definitions of oligomenorrhea (22,24,44,50), polymenorrhea (24,50), amenorrhea (22,24,31,35,36,41,44,50,51,58,59), or irregular/sporadic menses (14,15,31,33,35,36,38,40,43,59). Other articles listed definitions of menstrual abnormalities as questions or statements (27,30). One study (20) listed definitions for each menstrual abnormality but did not stratify results by KRT.
Menstrual Abnormalities in Nondialysis-Dependent, Nontransplanted Females.
Only three articles (18–20) included individuals with CKD not treated with dialysis or kidney transplant (n=1165), and three articles (5,21,22) included individuals receiving conservative kidney care (n=92). The ages of menarche and menopause were reported by Kim et al. (19) and Bianchi et al. (21), and only menstrual abnormalities in eight of 13 (61%) individuals who were predialysis were reported by Serret-Montaya et al. (20). Otieno et al. (22) reported menstrual abnormalities in four of 26 (15%) individuals treated with conservative care both before and after treatment initiation. The combined prevalence of menstrual abnormalities among all participants (i.e., conservative treatment, HD, and PD) was reported by Cochrane and Regan (5). Thus, we were unable to report a global assessment of menstrual abnormalities in populations with CKD not treated with dialysis or kidney transplantation.
Menstrual Abnormalities in Dialyzed Females.
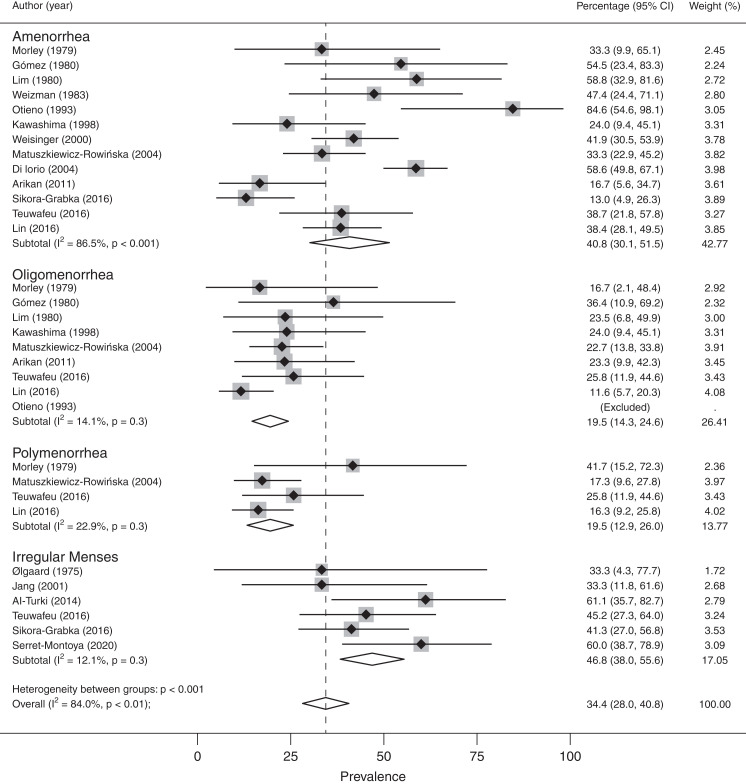
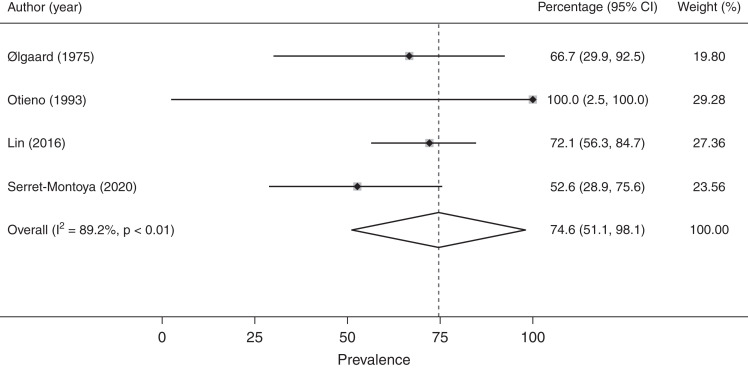
Data on menstrual abnormalities in patients on dialysis were collected and summarized in Figures 2 and 3, Supplemental Figure 1, and Supplemental Table 2. The overall rate of menstrual abnormalities in patients on dialysis from 18 studies was 63% (95% confidence interval [95% CI], 51% to 75%) (Supplemental Figure 1); however, significant heterogeneity was noted (I2: 95%; P<0.001). Stratification by menstrual disturbance type within the HD group (Figure 2) showed proportions of irregular menses (47%; 95% CI, 38% to 56%; I2: 12%; n=6 studies), polymenorrhea (19%; 95% CI, 13% to 26%; I2: 23%; n=4), amenorrhea (41%; 95% CI, 30% to 52%; I2: 87%; n=13), and oligomenorrhea (19%; 95% CI, 14% to 25%; I2: 14%; n=9). The proportion of menstrual abnormalities in females undergoing PD (Figure 3) was 75% (95% CI, 51% to 98%; n=4) with notable heterogeneity (I2: 89%; P<0.001). There were too few studies including PD populations to stratify by menstrual disturbance type.
Figure 2.
Prevalence of menstrual abnormalities stratified by menstrual disturbance type in females undergoing hemodialysis. 95% CI, 95% confidence interval.
Figure 3.
Prevalence of menstrual abnormalities in females undergoing peritoneal dialysis.
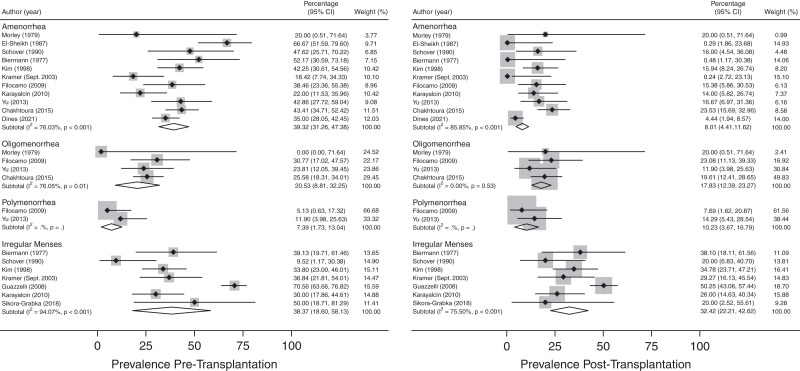
Menstrual Abnormalities in Transplanted Females.
Menstrual abnormalities in kidney transplant recipients were reported in 13 studies (15,23,24,26–31,33,36,54,59), and both pre- and post-transplantation estimates were collected from studies and are summarized in Figure 4 and Supplemental Table 3. The overall prevalence of amenorrhea decreased post-transplantation (8.01; 95% CI, 4.41 to 11.62) as compared with pretransplantation (39.32; 95% CI, 31.26 to 47.38). No significant differences were observed in the prevalence of oligomenorrhea (pretransplant: 20.53; 95% CI, 8.81 to 32.25; post-transplant: 17.83; 95% CI, 12.39 to 23.27), polymenorrhea (pretransplant: 7.39; 95% CI, 1.73 to 13.04; post-transplant: 10.23; 95% CI, 3.67 to 16.79), or irregular menses (pretransplant: 38.37; 95% CI, 18.60 to 58.13; post-transplant: 32.42; 95% CI, 22.21 to 42.62) with kidney transplantation. High levels of between-study heterogeneity were noted across most subgroups (P=0.05).
Figure 4.
Prevalence of menstrual abnormalities stratified by menstrual disturbance type in females pre- and post-transplantation.
Menarche, Menopause, and Reproductive Lifespan.
The age of menarche was reported in 1116 individuals in 12 articles (19,20,23,27,29,31,32,36,40,42,45,53); it ranged from 11.9 to 15.6 years and did not appear to differ among nondialyzed (13.73; 95% CI, 13.36 to 14.10; n=1 study), dialyzed (12.90; 95% CI, 12.48 to 13.32; n=6), and transplanted females (13.68; 95% CI, 12.96 to 14.40; n=6) (Supplemental Figure 2). The age of menopause was reported in 1587 individuals in 14 articles (18,21,23,27,28,31,33,39,40,42,43,50,53,60); it ranged from 36 to 64 years and did not appear to differ among nondialyzed (47.62; 95% CI, 46.66 to 48.57; n=2), dialyzed (46.99; 95% CI, 44.83 to 49.14; n=8), and transplanted females (46.29; 95% CI, 43.19 to 49.40; n=3) (Supplemental Figure 3). Only two articles (39,40) reported when menopause was reached with respect to dialysis initiation, where the majority reached menopause before (n=97; 78%) as compared with after (n=28; 22%) dialysis initiation. Similarly, only three articles (23,28,31) reported that all individuals (n=107; 100%) reached menopause after kidney transplantation. Of the 12 and 14 articles reporting age of menarche and age of menopause, respectively, only six articles (23,27,31,40,42,53) reported both the age of menarche and the age of menopause and were thus used to calculate reproductive lifespan (Supplemental Figure 4). Reproductive lifespan length was found to be 32 (95% CI, 30 to 34) years, although there was significant heterogeneity (I2: 92%; P<0.001). Primary ovarian insufficiency was reported in 12 individuals from two studies (5,23), at least five of whom were transplant recipients (the remaining seven were from one study [5] reporting dialyzed and transplantation recipients combined). Early menopause (<45 years old, n=264 of 1007 [26%]; 45–50 years old, n=227 of 1007 [22%]) was reported in women with CKD from one study (18); however, the type of KRT undergone, if any, was not reported and was therefore not included in analyses.
Study Quality and Risk of Bias.
Study quality is described in Supplemental Table 4. All studies were considered of either fair (n=32) or good (n=14) quality. The most common study quality factors reported included temporality of exposure and outcome measures and clearly stated research question/objective and population. Study quality factors that were least reported included sample size justifications and measurement of confounding variables. There was no evidence of publication bias through visual assessment of funnel plots (Supplemental Figure 5).
Discussion
This systematic review and meta-analysis represents the first comprehensive review, to our knowledge, summarizing menstrual abnormalities and reproductive lifespan among females with nondialysis-dependent CKD without kidney transplantation, women with dialysis-dependent CKD, and women with kidney transplantation. The findings from this systematic review and meta-analysis are three-fold. (1) The prevalence of menstrual abnormalities is high (63% overall) in dialyzed females, specifically irregular menses (47%) and amenorrhea (41%). (2) Kidney transplantation is associated with a reduction in menstrual abnormalities, particularly amenorrhea (approximately 30% reduction), although between-study heterogeneity was high. (3) The length of reproductive lifespan in females with CKD is 32 years. Thus, these results have important implications for the gynecologic and kidney care for this patient population (3).
Menstrual abnormalities are common among the general population of females (61), ranging from 6% to 40%, and they vary by disturbance type, socioeconomic position, and lifestyle factors (62). Menstrual abnormalities have significant public health ramifications (63), including missing work or school, which exacerbates sex inequity on a global level. The high prevalence of menstrual disorders in those with CKD is a concern and underscores reproductive health as an important aspect in the care of this population. However, nearly two thirds of nephrologists report not feeling confident managing (64) menstrual abnormalities in females with CKD, and almost two thirds of patients reported being unaware that menstruation may change with kidney transplantation (31).
Individuals with kidney failure treated with HD have abnormal hypothalamic-pituitary-ovarian axis function, with a reduction in the pulsatile release of gonadotropin-releasing hormone, resulting in lowered levels of follicle-stimulating hormone and luteinizing hormone (58) and no concurrent change in estradiol levels (60). However, the CKD stage at which disruption of the hypothalamic-pituitary gonadal axis occurs is unknown. Similarly, higher levels of prolactin may contribute to anovulation through negative feedback inhibition on the hypothalamic-pituitary-ovarian axis (65). Additionally, autoimmune disorders are more commonly a cause of CKD in premenopausal females (66), and thus, menstrual abnormalities may also be attributable to gonadotoxic medications (i.e., cyclophosphamide) in the CKD population (67) or the disease process itself. However, studies on menstrual health in the setting of CKD have previously been limited by small sample sizes, considerable exclusion criteria, and lack of stratification by CKD stage as well as biased survey techniques. For example, numerous studies considered inclusion of only married females (26,51,68,69) or interviewed individuals other than the patient (e.g., spouse or child) on menstrual pattern history (40). Thus, the current understanding of menstrual health in the CKD population warrants additional exploration.
KRT modalities, such as dialysis and transplantation, may have differing effects on regulating menstrual cycles. It has been noted that both HD and PD rarely improve the hypothalamic-pituitary-ovarian axis, sustaining abnormal hormonal milieus (17), although menstrual abnormalities can improve marginally. In contrast, successful kidney transplantation may improve hypothalamic-pituitary-ovarian axis irregularities (70), causing gradual restoration of luteinizing hormone, prolactin, and estradiol levels similar to those of healthy controls (71), although estradiol increases may be caused by immunosuppressive agents that interfere with estrogen receptor binding (72). However, irrespective of KRT modality, menstrual abnormalities are not completely alleviated. As noted in this systematic review, it is unclear why menstrual abnormalities other than amenorrhea are not improved following kidney transplantation. We speculate that nonsignificant differences may be due to between-study heterogeneity, differences in study cohorts, and a wide-spanning range of publication dates, as well as potential differences in the definitions, descriptions, and collection of menstrual abnormalities in each study population. Similarly, confounding factors, such as medication use, may have affected results. Taken together, these factors underscore the implications for fertility and overall health in females with CKD (3).
Although our findings indicate that the pooled average age of menarche of 13 years is similar to the general population, the pooled average age of menopause of 47 years and the reproductive lifespan of 32 years are considerably shorter (73). Reproductive lifespan has recently emerged as an important sex-specific factor that is inversely proportional to kidney and cardiovascular risk (6,74). Whether these associations represent a causal relationship or simply reflect a shared pathophysiology with cardiovascular disease in individuals with CKD is unclear. Similarly, earlier onset of menopause has been associated with lower life expectancy, greater risk for osteoporosis and cardiovascular disease, and lower risk of breast and gynecologic cancers in the general population (75). However, it has not been investigated if these same associations apply to the population with CKD, and therefore, they represent an unmet need in nephrology research and care.
This study has strengths and limitations. First, there was a lack of clarity and unanimity across studies for definitions of the types of menstrual abnormalities that may affect the synthesis of results. To the best of our abilities, definitions presented by authors were used verbatim to summarize menstrual abnormalities. Second, there was significant heterogeneity noted in results, which may be due to differing diagnostic criteria, definitions, and sampling processes; as such, our results are to be interpreted with caution. Further, because of the limited reporting, we were unable to adjust analyses by hormonal contraceptive use, which is known to induce amenorrhea and/or regulate menstrual cycles, although contraceptive use is low in this population (76,77). Similarly, we were unable to adjust analyses by immunosuppressive use or rates of fertility, which can also contribute to menstrual abnormalities (3). However, this review highlights the high prevalence of menstrual abnormalities and shorter reproductive lifespan in the CKD population and underscores the urgency of addressing this important component of overall health.
Menstrual disorders are common in females with CKD and are more than a reproductive health issue (63), with implications for quality of life and socioeconomic status (63). These sex-specific factors of women are associated with cardiovascular (6,7) and bone health (8) as well as malignancy risk (9) in the general population; whether these same associations exist in the CKD population is unknown. This lack of data precludes guidance in this key aspect of care of female patients across the stages of CKD and represents an important knowledge gap in nephrology.
Disclosures
S.B. Ahmed reports serving as an Advisory Board Member of the Canadian Institutes of Health Research Institute (CIHR) of Gender and Health (volunteer position), as a Canadian Medical Association Journal Governance Council Member (elected position), and as the President-Elect of the Organization for the Study of Sex Differences (elected volunteer position) and reports research funding from the Canadian Institutes of Health Research. M. Robert reports research funding from Can Health West Network, a CIHR Operation Grant, Cumming Medical Research Fund (CMRF), and a Women's Health Clinical Mentorship Grant; serving as the Medical Director of the Calgary Chronic Pain Program; and serving as a cochair of the Alberta Pain Strategy Outcomes Subcommittee (not paid). L. Skeith reports research funding from CSL Behring and honoraria from Leo Pharma and Sanofi. All remaining authors have nothing to disclose.
Funding
None.
Supplementary Material
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
See related editorial, “Reproductive Health in Women with Kidney Disease,” on pages 1716–1718.
Author Contributions
S.B. Ahmed, G.S. Kochaksaraei, C.L. Rytz, and L. Skeith conceptualized the study; S.M. Dumanski, G.S. Kochaksaraei, and C.L. Rytz were responsible for data curation; G.S. Kochaksaraei and C.L. Rytz were responsible for investigation; P.E. Ronksley and C.L. Rytz were responsible for formal analysis; M. Robert, P.E. Ronksley, C.L. Rytz, and L. Skeith were responsible for methodology; P.E. Ronksley and C.L. Rytz were responsible for software; S.B. Ahmed, P.E. Ronksley, and C.L. Rytz were responsible for validation; P.E. Ronksley and C.L. Rytz were responsible for visualization; S.B. Ahmed, S.M. Dumanski, P.E. Ronksley, and L. Skeith provided supervision; C.L. Rytz wrote the original draft; and S.B. Ahmed, S.M. Dumanski, G.S. Kochaksaraei, M. Robert, P.E. Ronksley, C.L. Rytz, and L. Skeith reviewed and edited the manuscript.
Data Sharing Statement
All data used in this study are available in this article.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.07100622/-/DCSupplemental.
Supplemental Figure 1. Prevalence of menstrual disturbances in dialyzed females.
Supplemental Figure 2. Pooled age of menarche stratified by KRT type in females with CKD.
Supplemental Figure 3. Pooled age of menopause stratified by KRT type in females with CKD.
Supplemental Figure 4. Pooled reproductive lifespan in females with CKD.
Supplemental Figure 5. Funnel plot for assessment of publication bias of pooled risk difference of menstrual disturbance in females with CKD pre- and postkidney transplantation.
Supplemental Table 1. MEDLINE search strategy.
Supplemental Table 2. Menstrual disturbances in dialyzed females living with CKD.
Supplemental Table 3. Menstrual disturbances in transplanted females living with CKD.
Supplemental Table 4. Study quality assessment.
References
- 1.Carrero JJ, Hecking M, Chesnaye NC, Jager KJ: Sex and gender disparities in the epidemiology and outcomes of chronic kidney disease. Nat Rev Nephrol 14: 151–164, 2018. 10.1038/nrneph.2017.181 [DOI] [PubMed] [Google Scholar]
- 2.Hill NR, Fatoba ST, Oke JL, Hirst JA, O’Callaghan CA, Lasserson DS, Hobbs FDR: Global prevalence of chronic kidney disease: A systematic review and meta-analysis. PLoS One 11: 0158765, 2016. 10.1371/journal.pone.0158765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dumanski SM, Ahmed SB: Fertility and reproductive care in chronic kidney disease. J Nephrol 32: 39–50, 2019. 10.1007/s40620-018-00569-9 [DOI] [PubMed] [Google Scholar]
- 4.Chang DH, Dumanski SM, Ahmed SB: Female reproductive and gynecologic considerations in chronic kidney disease: Adolescence and young adulthood. Kidney Int Rep 7: 152–164, 2021. 10.1016/j.ekir.2021.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cochrane R, Regan L: Undetected gynaecological disorders in women with renal disease. Hum Reprod 12: 667–670, 1997 [DOI] [PubMed] [Google Scholar]
- 6.Ley SH, Li Y, Tobias DK, Manson JE, Rosner B, Hu FB, Rexrode KM: Duration of reproductive lifespan, age at menarche, and age at menopause are associated with risk of cardiovascular disease in women. J Am Heart Assoc 6: 006713, 2017. 10.1161/JAHA.117.006713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y-X, Arvizu M, Rich-Edwards JW, Stuart JJ, Manson JE, Missmer SA, Pan A, Chavarro JE: Menstrual cycle regularity and length across the reproductive lifespan and risk of premature mortality: Prospective cohort study. BMJ 371: m3464, 2020. 10.1136/bmj.m3464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grainge MJ, Coupland CAC, Cliffe SJ, Chilvers CED, Hosking DJ: Reproductive, menstrual and menopausal factors: Which are associated with bone mineral density in early postmenopausal women? Osteoporos Int 12: 777–787, 2001. 10.1007/s001980170055 [DOI] [PubMed] [Google Scholar]
- 9.De La Mata NL, Rosales B, MacLeod G, Kelly PJ, Masson P, Morton RL, Wyburn K, Webster AC: Sex differences in mortality among binational cohort of people with chronic kidney disease: Population based data linkage study. BMJ 375: 068247, 2021. 10.1136/BMJ-2021-068247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carrero JJ, de Jager DJ, Verduijn M, Ravani P, De Meester J, Heaf JG, Finne P, Hoitsma AJ, Pascual J, Jarraya F, Reisaeter AV, Collart F, Dekker FW, Jager KJ: Cardiovascular and noncardiovascular mortality among men and women starting dialysis. Clin J Am Soc Nephrol 6: 1722–1730, 2011. 10.2215/CJN.11331210 [DOI] [PubMed] [Google Scholar]
- 11.Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, McKenzie JE: PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 372: n160, 2021. 10.1136/bmj.n160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National Institutes of Health : Study Quality Assessment Tools. US National Heart, Lung, and Blood Institute, 2014. Available at: www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. Accessed November 7, 2022
- 13.DerSimonian R, Kacker R: Random-effects model for meta-analysis of clinical trials: An update. Contemp Clin Trials 28: 105–114, 2007. 10.1016/j.cct.2006.04.004 [DOI] [PubMed] [Google Scholar]
- 14.Sikora-Grabka E, Adamczak M, Kuczera P, Szotowska M, Madej P, Wiecek A: Serum anti-Müllerian hormone concentration in young women with chronic kidney disease on hemodialysis, and after successful kidney transplantation. Kidney Blood Press Res 41: 552–560, 2016. 10.1159/000443458 [DOI] [PubMed] [Google Scholar]
- 15.Sikora-Grabka E, Adamczak M, Kuczera P, Wiecek A: Serum sex hormones concentrations in young women in the early period after successful kidney transplantation. Endokrynol Pol 69: 150–155, 2018. 10.5603/EP.2018.0019 [DOI] [PubMed] [Google Scholar]
- 16.Matuszkiewicz-Rowińska J, Skórzewska K, Radowicki S, Niemczyk S, Przedlacki J, Sokalski A, Wardyn K, Puka J, Świtalski M, Grochowski J, Ostrowski K: [Menstrual disturbances and alternations in hypophyseal gonadal axis in end-stage premenopausal women undergoing hemodialysis: A multi-center study]. Pol Arch Med Wewn 109: 609–615, 2003 [PubMed] [Google Scholar]
- 17.Matuszkiewicz-Rowińska J, Skórzewska K, Radowicki S, Niemczyk S, Sokalski A, Przedlacki J, Puka J, Świtalski M, Wardyn K, Grochowski J, Ostrowski K: Endometrial morphology and pituitary-gonadal axis dysfunction in women of reproductive age undergoing chronic haemodialysis--A multicentre study. Nephrol Dial Transplant 19: 2074–2077, 2004. 10.1093/ndt/gfh279 [DOI] [PubMed] [Google Scholar]
- 18.Cheung KL, Stefanick ML, Allison MA, LeBlanc ES, Vitolins MZ, Shara N, Chertow GM, Winkelmayer WC, Kurella Tamura M: Menopausal symptoms in women with chronic kidney disease. Menopause 22: 1006–1011, 2015. 10.1097/GME.0000000000000416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim HS, Ng DK, Matheson MB, Atkinson MA, Warady BA, Furth SL, Ruebner RL: Delayed menarche in girls with chronic kidney disease and the association with short stature. Pediatr Nephrol 35: 1471–1475, 2020. 10.1007/s00467-020-04559-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Serret-Montaya J, Zurita-Cruz JN, Villasís-Keever MA, Aguilar-Kitsu A, Del Carmen Zepeda-Martinez C, Cruz-Anleu I, Hernández-Hernández BC, Alonso-Flores SR, Manuel-Apolinar L, Damasio-Santana L, Hernandez-Cabezza A, Romo-Vázquez JC: Hyperprolactinemia as a prognostic factor for menstrual disorders in female adolescents with advanced chronic kidney disease. Pediatr Nephrol 35: 1041–1049, 2020. 10.1007/s00467-020-04494-7 [DOI] [PubMed] [Google Scholar]
- 21.Bianchi ML, Colantonio G, Montesano A, Trevisan C, Ortolani S, Rossi R, Buccianti G: Bone mass status in different degrees of chronic renal failure. Bone 13: 225–228, 1992 [DOI] [PubMed] [Google Scholar]
- 22.Otieno MRB, McLigeyo SO, Kigondu CS, Rogo KO: Menstrual disorders in patients with chronic renal failure. East Afr Med J 70: 6–9, 1993 [PubMed] [Google Scholar]
- 23.Chakhtoura Z, Meunier M, Caby J, Mercadal L, Arzouk N, Barrou B, Touraine P: Gynecologic follow up of 129 women on dialysis and after kidney transplantation: A retrospective cohort study. Eur J Obstet Gynecol Reprod Biol 187: 1–5, 2015. 10.1016/j.ejogrb.2015.01.004 [DOI] [PubMed] [Google Scholar]
- 24.Morley JE, Distiller LA, Epstein S, Katz M, Gold C, Sagel J, Kaye G, Pokroy M, Kalk J: Menstrual disturbances in chronic renal failure. Horm Metab Res 11: 68–72, 1979 [DOI] [PubMed] [Google Scholar]
- 25.Pezeshki M, Taherian AA, Gharavy M, Ledger WL: Menstrual characteristics and pregnancy in women after renal transplantation. Int J Gynaecol Obstet 85: 119–125, 2004 [DOI] [PubMed] [Google Scholar]
- 26.Yu L, Xia R, Zhou M: [Sexual function in premenopausal women before and after renal transplantation]. Nan Fang Yi Ke Da Xue Bao 33: 910–912, 917, 2013. 10.3969/j.issn.1673-4254.2013.06.27 [DOI] [PubMed] [Google Scholar]
- 27.Dines VA, Garovic VD, Parashuram S, Cosio FG, Kattah AG: Pregnancy, contraception, and menopause in advanced chronic kidney disease and kidney transplant. Womens Health Rep (New Rochelle) 2: 488–496, 2021. 10.1089/whr.2021.0053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El-Sheikh MM, Lamki H, Mcgeown MG: Routine gynaecological screening of patients with kidney transplants. J Obstet Gynaecol (Lahore) 8: 170–172, 1987. 10.3109/01443618709008791 [DOI] [Google Scholar]
- 29.Guazzelli CAFF, Torloni MR, Sanches TF, Barbieri M, Pestana JOMA: Contraceptive counseling and use among 197 female kidney transplant recipients. Transplantation 86: 669–672, 2008. 10.1097/TP.0b013e3181817e7d [DOI] [PubMed] [Google Scholar]
- 30.Karayalcin R, Genc V, Oztuna D, Huseynova N, Ersoz S: Gynecologic symptoms and sexual function in female kidney allograft recipients. Transplant Proc 42: 2551–2555, 2010. 10.1016/j.transproceed.2010.05.147 [DOI] [PubMed] [Google Scholar]
- 31.Kim JH, Chun CJ, Kang CM, Kwak JY: Kidney transplantation and menstrual changes. Transplant Proc 30: 3057–3059, 1998. 10.1016/S0041-1345(98)00928-2 [DOI] [PubMed] [Google Scholar]
- 32.Koca TG, Koca N, Ersoy A: The comparison of the relationship between sociocultural-economic features and sexual dysfunction frequency in sexually active premenopausal female patients on renal replacement therapy. J Sex Med 9: 3171–3179, 2012. 10.1111/j.1743-6109.2012.02952.x [DOI] [PubMed] [Google Scholar]
- 33.Kramer HM, Tolkoff-Rubin NE, Williams WW, Cosimi AB, Pascual MA: Reproductive and contraceptive characteristics of premenopausal kidney transplant recipients. Prog Transplant 13: 193–196, 2003. 10.7182/prtr.13.3.q0743uh352168x80 [DOI] [PubMed] [Google Scholar]
- 34.Lin C-T, Liu X-N, Xu H-L, Sui H-Y: Menstrual disturbances in premenopausal women with end-stage renal disease: A cross-sectional study. Med Princ Pract 25: 260–265, 2016. 10.1159/000444879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Al-Turki HA, Al-Hwiesh AK, Al-Muhanna FA, Taha IS, AlAwdahs N: Reproductive and gynaecological issues in Saudi women with end stage renal disease. J Pak Med Assoc 64: 337–338, 2014 [PubMed] [Google Scholar]
- 36.Bierman M, Nolan GH: Menstrual function and renal transplantation. Obstet Gynecol 49: 186–189, 1977 [PubMed] [Google Scholar]
- 37.Kramer HM, Curhan GC, Singh A; Hemodialysis and Estrogen Levels in Postmenopausal Patients Study Group : Permanent cessation of menses and postmenopausal hormone use in dialysis-dependent women: The HELP study. Am J Kidney Dis 41: 643–650, 2003 [DOI] [PubMed] [Google Scholar]
- 38.Ølgaard K, Hagen C, McNeilly AS: Pituitary hormones in women with chronic renal failure: The effect of chronic intermittent haemo- and peritoneal dialysis. Acta Endocrinol (Copenh) 80: 237–246, 1975. 10.1016/B978-0-7216-9540-2.50231-0 [DOI] [PubMed] [Google Scholar]
- 39.Rush H, Neugarten J, Coco M: Women’s health issues in a dialysis population. Clin Nephrol 54: 455–462, 2000 [PubMed] [Google Scholar]
- 40.Shanmugavadivoo K, Shaariah W: Health issues in dialysis-dependent female patients. Perit Dial Int 23[Suppl 2]: S192–S195, 2003. 10.1177/089686080302302s40 [DOI] [PubMed] [Google Scholar]
- 41.Gómez F, de la Cueva R, Wauters JP, Lemarchand-Béraud T: Endocrine abnormalities in patients undergoing long-term hemodialysis. The role of prolactin. Am J Med 68: 522–530, 1980 [DOI] [PubMed] [Google Scholar]
- 42.Holley JL, Schmidt RJ, Bender FH, Dumler F, Schiff M: Gynecologic and reproductive issues in women on dialysis. Am J Kidney Dis 29: 685–690, 1997. 10.1016/S0272-6386(97)90120-7 [DOI] [PubMed] [Google Scholar]
- 43.Jang C, Bell RJ, White VS, Lee PS, Dwyer KM, Kerr PG, Davis SR: Women’s health issues in haemodialysis patients. Med J Aust 175: 298–301, 2001 [DOI] [PubMed] [Google Scholar]
- 44.Arikan DC, Bozkurt S, Arikan I, Turgut E: Hormone profiles and their relation with menstrual cycles in patients undergoing hemodialysis. Turk Jinekoloji ve Obstet Dern Derg 8: 32–39, 2011. 10.5505/tjod.2011.80378 [DOI] [Google Scholar]
- 45.Fayed A, Soliman A, Naguib M, Soliman M, Salaheldin M: Ovarian reserve in an Egyptian cohort with end-stage kidney disease on hemodialysis and after successful kidney transplantation: A prospective study. Int Urol Nephrol 51: 737–743, 2019. 10.1007/s11255-019-02089-2 [DOI] [PubMed] [Google Scholar]
- 46.Skórzewska K, Radowicki S, Matuszkiewicz-Rowinska J, Szlendak-Sauer K: Morphological changes in endometrium of hemodialyzed women of reproductive age. Gynecol Endocrinol 23: 523–526, 2007. 10.1080/09513590701557523 [DOI] [PubMed] [Google Scholar]
- 47.Weisinger JR, Gonzalez L, Alvarez H, Hernandez E, Carlini RG, Capriles F, Cerviño M, Martinis R, Paz-Martínez V, Bellorín-Font E: Role of persistent amenorrhea in bone mineral metabolism of young hemodialyzed women. Kidney Int 58: 331–335, 2000. 10.1046/j.1523-1755.2000.00170.x [DOI] [PubMed] [Google Scholar]
- 48.Kramer HM, Curhan G, Singh A; HELP Study Group : Hemodialysis and estrogen levels in postmenopausal (HELP) patients: The multicenter HELP study. Am J Kidney Dis 41: 1240–1246, 2003 [DOI] [PubMed] [Google Scholar]
- 49.Lim VS, Auletta F, Kathpalia S, Frohman LA: Gonadal function in women with chronic renal failure: A study of the hypothalamo-pituitary-ovarian axis. Proc Clin Dial Transplant Forum 7: 39–47, 1977 [PubMed] [Google Scholar]
- 50.Teuwafeu D, Ashuntantang G, Essi MJ, Kaze F, Maimouna M, Balepna JY, Gobina R, Kengne AP, Ndjitoyap EC: Sexual function and correlates in women undergoing maintenance hemodialysis in Cameroon: A multi-centric study. Open Urol Nephrol J 9: 51–59, 2016. 10.2174/1874303x01609010051 [DOI] [Google Scholar]
- 51.Weizman R, Weizman A, Levi J, Gura V, Zevin D, Maoz B, Wijsenbeek H, Ben David M: Sexual dysfunction associated with hyperprolactinemia in males and females undergoing hemodialysis. Psychosom Med 45: 259–269, 1983. 10.1097/00006842-198306000-00008 [DOI] [PubMed] [Google Scholar]
- 52.Yaprak M, Doğru V, Sanhal CY, Avanaz A, Erman M: Fertility outcome after renal transplantation: A single-center experience. Transplant Proc 51: 1108–1111, 2019. 10.1016/j.transproceed.2019.01.111 [DOI] [PubMed] [Google Scholar]
- 53.Yücel AE, Kart-Köseoglu H, Isiklar I, Kuruinci E, Özdemir FN, Arslan H: Bone mineral density in patients on maintenance hemodialysis and effect of chronic hepatitis C virus infection. Ren Fail 26: 159–164, 2004. 10.1081/JDI-120038501 [DOI] [PubMed] [Google Scholar]
- 54.Filocamo MT, Zanazzi M, Li Marzi V, Lombardi G, Del Popolo G, Mancini G, Salvadori M, Nicita G: Sexual dysfunction in women during dialysis and after renal transplantation. J Sex Med 6: 3125–3131, 2009. 10.1111/j.1743-6109.2009.01400.x [DOI] [PubMed] [Google Scholar]
- 55.Kawashima R, Douchi T, Oki T, Yoshinaga M, Nagata Y: Menstrual disorders in patients undergoing chronic hemodialysis. J Obstet Gynaecol Res 24: 367–373, 1998. 10.1111/j.1447-0756.1998.tb00110.x [DOI] [PubMed] [Google Scholar]
- 56.Kim JM, Song RK, Kim MJ, Lee DY, Jang HR, Kwon CHD, Huh WS, Kim GS, Kim SJ, Choi DS, Joh JW, Lee SK, Oh HY: Hormonal differences between female kidney transplant recipients and healthy women with the same gynecologic conditions. Transplant Proc 44: 740–743, 2012. 10.1016/j.transproceed.2011.12.072 [DOI] [PubMed] [Google Scholar]
- 57.Di Iorio BR, Stellato D, De Santo NG, Cirillo M; Campanian Dialysis Registry Research Group : Association of gender and age with erythropoietin resistance in hemodialysis patients: Role of menstrual status. Blood Purif 22: 423–427, 2004. 10.1159/000080234 [DOI] [PubMed] [Google Scholar]
- 58.Lim VS, Henriquez C, Sievertsen G, Frohman LA: Ovarian function in chronic renal failure: Evidence suggesting hypothalamic anovulation. Ann Intern Med 93: 21–27, 1980. 10.7326/0003-4819-93-1-21 [DOI] [PubMed] [Google Scholar]
- 59.Schover LR, Novick AC, Steinmuller DR, Goormastic M: Sexuality, fertility, and renal transplantation: A survey of survivors. J Sex Marital Ther 16: 3–13, 1990. 10.1080/00926239008405961 [DOI] [PubMed] [Google Scholar]
- 60.Ramesh S, James MT, Holroyd-Leduc JM, Wilton SB, Seely EW, Hemmelgarn BR, Tonelli M, Wheeler DC, Ahmed SB: Estradiol and mortality in women with end-stage kidney disease. Nephrol Dial Transplant 35: 1965–1972, 2020. 10.1093/ndt/gfaa126 [DOI] [PubMed] [Google Scholar]
- 61.Omani Samani R, Almasi Hashiani A, Razavi M, Vesali S, Rezaeinejad M, Maroufizadeh S, Sepidarkish M: The prevalence of menstrual disorders in Iran: A systematic review and meta-analysis. Int J Reprod Biomed (Yazd) 16: 665–678, 2018 [PMC free article] [PubMed] [Google Scholar]
- 62.Bae J, Park S, Kwon JW: Factors associated with menstrual cycle irregularity and menopause. BMC Womens Health 18: 36, 2018. 10.1186/s12905-018-0528-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Babbar K, Martin J, Ruiz J, Parray AA, Sommer M: Menstrual health is a public health and human rights issue. Lancet Public Health 7: 10–11, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ramesh S, James MT, Holroyd-Leduc JM, Wilton SB, Seely EW, Wheeler DC, Ahmed SB: Sex hormone status in women with chronic kidney disease: Survey of nephrologists’ and renal allied health care providers’ perceptions. Can J Kidney Health Dis 4: 2054358117734534, 2017. 10.1177/2054358117734534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Holley JL: The hypothalamic-pituitary axis in men and women with chronic kidney disease. Adv Chronic Kidney Dis 11: 337–341, 2004. 10.1053/j.ackd.2004.07.004 [DOI] [PubMed] [Google Scholar]
- 66.Mauvais-Jarvis F, Bairey Merz N, Barnes PJ, Brinton RD, Carrero JJ, DeMeo DL, De Vries GJ, Epperson CN, Govindan R, Klein SL, Lonardo A, Maki PM, McCullough LD, Regitz-Zagrosek V, Regensteiner JG, Rubin JB, Sandberg K, Suzuki A: Sex and gender: Modifiers of health, disease, and medicine. Lancet 396: 565–582, 2020. 10.1016/S0140-6736(20)31561-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Harward LE, Mitchell K, Pieper C, Copland S, Criscione-Schreiber LG, Clowse MEB: The impact of cyclophosphamide on menstruation and pregnancy in women with rheumatologic disease. Lupus 22: 81–86, 2013. 10.1177/0961203312468624 [DOI] [PubMed] [Google Scholar]
- 68.Gonçalves PRC, Loureiro LM, Fernandes MID: Sexual function of kidney transplant recipients. Rev Enferm Ref 2019: 47–56, 2019. 10.12707/RIV19009 [DOI] [Google Scholar]
- 69.Song YS, Yang HJ, Song ES, Han DC, Moon C, Ku JH: Sexual function and quality of life in Korean women with chronic renal failure on hemodialysis: Case-control study. Urology 71: 243–246, 2008. 10.1016/j.urology.2007.10.020 [DOI] [PubMed] [Google Scholar]
- 70.Holley JL, Schmidt RJ: Changes in fertility and hormone replacement therapy in kidney disease. Adv Chronic Kidney Dis 20: 240–245, 2013. 10.1053/j.ackd.2013.01.003 [DOI] [PubMed] [Google Scholar]
- 71.Wang GC, Zheng JH, Xu LG, Min ZL, Zhu YH, Qi J, Duan QL: Measurements of serum pituitary-gonadal hormones and investigation of sexual and reproductive functions in kidney transplant recipients. Int J Nephrol 2010: 612126, 2010. 10.4061/2010/612126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rao BR: Isolation and characterization of an estrogen binding protein which may integrate the plethora of estrogenic actions in non-reproductive organs. J Steroid Biochem Mol Biol 65: 3–41, 1998. 10.1016/S0960-0760(98)00019-3 [DOI] [PubMed] [Google Scholar]
- 73.Appiah D, Nwabuo CC, Ebong IA, Wellons MF, Winters SJ: Trends in age at natural menopause and reproductive lifespan among US women, 1959-2018. JAMA 325: 1328–1330, 2021. 10.1001/JAMA.2021.0278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mishra SR, Chung HF, Waller M, Dobson AJ, Greenwood DC, Cade JE, Giles GG, Bruinsma F, Simonsen MK, Hardy R, Kuh D, Gold EB, Crawford SL, Derby CA, Matthews KA, Demakakos P, Lee JS, Mizunuma H, Hayashi K, Sievert LL, Brown DE, Sandin S, Weiderpass E, Mishra GD: Association between reproductive lifespan and incident nonfatal cardiovascular disease: A pooled analysis of individual patient data from 12 studies. JAMA Cardiol 5: 1410–1418, 2020. 10.1001/jamacardio.2020.4105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vellanki K, Hou S: Menopause in CKD. Am J Kidney Dis 71: 710–719, 2018. 10.1053/j.ajkd.2017.12.019 [DOI] [PubMed] [Google Scholar]
- 76.Shah S, Christianson AL, Bumb S, Verma P: Contraceptive use among women with kidney transplants in the United States. J Nephrol 35: 629–638, 2022. 10.1007/s40620-021-01181-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shah S, Christianson AL, Thakar CV, Kramer S, Meganathan K, Leonard AC: Contraceptive use among women with end-stage kidney disease on dialysis in the United States. Kidney Med 2: 707–715, 2020. 10.1016/j.xkme.2020.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Milde FK, Hart LK, Fearing MO. Sexuality and fertility concerns of dialysis patients. ANNA J. 23: 307–313, 1996 [PubMed] [Google Scholar]
- 79.Van Eps C, Hawley C, Jeffries J, Johnson DW, Campbell S, Isbel N, Mudge DW, and Prins J: Changes in serum prolactin, sex hormones and thyroid function with alternate nightly nocturnal home haemodialysis. Nephrology 17: 42–47 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.