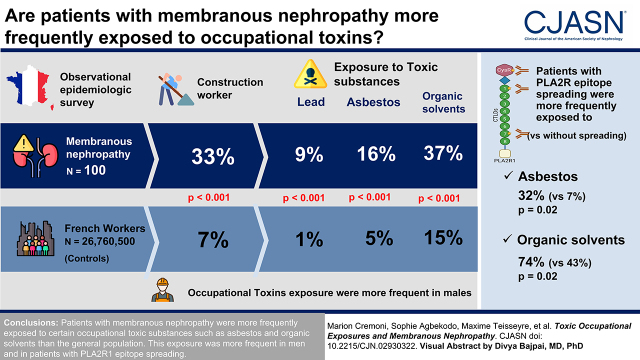
Visual Abstract
Keywords: membranous nephropathy, nephrotic syndrome, occupational exposure
Abstract
Background and objectives
Membranous nephropathy is a rare autoimmune kidney disease whose increasing prevalence in industrialized countries pleads for the involvement of an environmental factor in the development of the disease. In addition, the predominance of men in membranous nephropathy, classically attributed to biologic or genetic differences between men and women, could also be due to different occupational exposures. To support this hypothesis, we sought to describe the toxic occupational exposures of patients with membranous nephropathy.
Design, setting, participants, & measurements
In this observational epidemiologic study, we compared the occupations and toxic occupational exposures of 100 patients with membranous nephropathy with those of the general population, consisting of two cohorts of 26,734,000 and 26,500 French workers. We then compared the characteristics of patients exposed to an occupational toxic substance with those of unexposed patients.
Results
Patients with membranous nephropathy worked more frequently in the construction sector than the general population (33% versus 7%, P<0.001). This difference remained significant by age and sex. They were also more frequently exposed to toxic substances, such as asbestos (16% versus 5%, P<0.001), lead (9% versus 1%, P<0.001), or organic solvents (37% versus 15%, P<0.001), than the general population. The predominance of men in the subgroup of patients occupationally exposed to toxic substances was not observed in unexposed individuals (organic solvents: 80% men versus 41%, P<0.001; asbestos: 90% men versus 55%, P=0.004). In addition, patients with phospholipase A2 receptor 1 (PLA2R1) epitope spreading were more frequently exposed to asbestos and organic solvents than patients without epitope spreading (32% versus 7%, P=0.02 and 74% versus 43%, P=0.02, respectively), with a dose-dependent effect.
Conclusions
Patients with membranous nephropathy were more frequently exposed to certain occupational toxic substances, such as asbestos and organic solvents, than the general population. This occupational exposure was more frequent in men and in patients with PLA2R1 epitope spreading.
Clinical Trial registry name and registration number:
Immunopathological Analysis in a French National Cohort of Membranous Nephropathy (IHMN), NCT04326218.
Podcast
This article contains a podcast at https://www.asn-online.org/media/podcast/CJASN/2022_10_25_CJN02930322.mp3.
Introduction
Membranous nephropathy is one of the most common causes of nephrotic syndrome in nondiabetic White adults (1). This rare autoimmune kidney disease is characterized by subepithelial immune deposits containing IgG and complement fractions with alteration of the glomerular basement membrane structure (2,3). Patients with membranous nephropathy develop autoantibodies against podocyte proteins (4). The most common target antigen is M-type phospholipase A2 receptor 1 (PLA2R1), which is found in 70% of patients with membranous nephropathy (5–7). Of increasing prevalence (8,9), it preferentially affects men between 50 and 60 years old (3). In recent decades, Western countries have been confronted with an increasing incidence of autoimmune diseases. One of the main hypotheses is that this is the result of a complex interaction between genetic predisposition, immune dysregulation, and environmental factors (10). Numerous genome-wide association studies have identified that some single nucleotide polymorphisms within the PLA2R1, HLA-DQA1, and HLA-DRB1 genes are significantly associated with primary membranous nephropathy (11–17). More recently, another genome-wide association study has identified two new risk loci for primary membranous nephropathy encoding transcriptional master regulators of inflammation, NFKB1 and IRF4, suggesting that inflammation may play a part in membranous nephropathy physiopathology (18). Little is known about immune dysregulation in membranous nephropathy, but several studies suggested a disturbed balance between T helper 17 (Th17) and T regulatory cells (19–24). Environmental factors could play a triggering role in immune dysregulation in genetically predisposed individuals, leading to the development of autoimmune diseases (25). Exposure to certain environmental toxic substances, such as mercury (26,27), formaldehyde (28), and air pollution (22,29), has been identified as a potential cause of membranous nephropathy. Occupational exposure to organic solvents is a risk factor for autoimmune diseases (30–32). Although described nearly 50 years ago, exposure to organic solvents remains a controversial cause of GN and/or progression of CKD (33–37). At present, no study has attempted to establish a link between occupational exposure to toxic substances and membranous nephropathy. However, the increasing prevalence and predominance of men in membranous nephropathy could suggest an occupational environment trigger for this autoimmune disease (38). In this study, we sought to describe the occupational exposure of patients with membranous nephropathy to toxic substances in comparison with the general French population to identify potential environmental factors in the onset of the disease.
Materials and Methods
Study Design
First, we conducted an observational cross-sectional epidemiologic survey of occupational exposures of patients with membranous nephropathy followed at Nice University Hospital. We included patients with histologically proven (kidney biopsy–proven) membranous nephropathy. Noninclusion criteria were (1) refusal to participate, (2) language barrier or any other reason that did not allow a standardized collection of data by the questionnaire, and (3) minors or adults under protective supervision for whom informed consent could not be obtained. The data from this epidemiologic survey were then compared with the general French population.
In a second phase, we carried out a comparative observational study of the exposed/unexposed type. The clinical and biologic characteristics of patients with membranous nephropathy exposed to certain occupational toxic substances were compared with unexposed patients with membranous nephropathy.
Data Collection
Exposures.
An occupational health physician collected the epidemiologic data of patients with membranous nephropathy using a heteroquestionnaire (Supplemental Material) (available in French and English) completed during semiguided individual interviews. This data collection was conducted from February to June 2021. The heteroquestionnaire collected details of each patient’s current and past occupational activities: occupation at diagnosis and for how many years; history of occupations before diagnosis and their duration; possible occupational toxic exposures; and nephrotoxic prevention measures implemented by their employer’s occupational health services. Each patient received the same information during the interview, and medical and/or work-related terms were explained. The occupational physician collected exposure data by classifying the level of exposure into three categories: low, moderate, or high exposure. This classification was on the basis of a quantitative estimate following the patient’s detailed description of his or her professional activity. It summarizes a combination of (1) the frequency of exposure during the professional activity, (2) the cumulative length of exposure (in years), (3) the estimated concentration of the toxic substance(s) during the exposure situations, and (4) the adherence or not with the wearing of personal protective equipment (respiratory, skin, and digestive protection). The patients’ jobs and details of their occupational exposure are detailed in Table 1. For example, high exposure to organic solvents could be defined for a mechanic using motor oil >2 h/d indoors without personal protective equipment and for >10 years. The same exposure with personal protective measures in a ventilated area could be considered moderate. In contrast, if the same exposure occurs only occasionally (e.g., once a month), the exposure will be considered low. A second questionnaire focused on nonoccupational toxic exposures to search for possible overexposure outside the workplace (i.e., at home or during leisure activities).
Table 1.
Details of patients' jobs and occupational exposures
| Business Sectors | Patient Jobs | Type of Product Containing Solvents | Type of Product Containing Lead | Type of Product Containing Asbestos |
|---|---|---|---|---|
| Agricultural workers | Farmers, gardeners | Renovation of an asbestos farm | ||
| Service workers | Office workers, teachers, postmen, cleaners, hairdressers, hospital workers | Bleach, cleaners, disinfectants, household sprays, dyes, ammonia perms, thioglycolic acid, printing ink | ||
| Industry workers | Seamstresses and tailors, technicians in the chemical industry, appliance technicians, gas station attendants | Pharmaceutical products, printing ink, methyl ethyl ketone, glue, resins, degreasers, cleaners, bleach, kerosene, alcohol vapor, nitrogenous solvents, solvent vapor, hydrochloric acid, silver photo developers, fuel, gasoline | Lead nitrate | |
| Construction workers | Electricians, painters, car mechanics, masons, plumbers, carpenters, locksmiths, welders | Form oil, motor oil, oil-based paint, paint thinners, glue, bleach, white spirit, tile cleaning acids, paint stripper, hydrochloric acid, oxidizers, degreasers, acetone, ammonia, corrosive cleaning acids, fuel, petroleum products, brake fluid, varnish, benzine cans, cement, asphalt, tar, glue, shoe polish, paint strippers, dyes | Lead paint, pipes and cables | Renovation of old roofs, mortars on the basis of asbestos plaster, asbestos insulation materials (old buildings), asbestos brake pads and clutches, welding/brazing of asbestos-bearing materials |
The occupations of each patient and the details of their occupational exposure were collected by the occupational medicine department of the Nice University Hospital.
We compared the epidemiologic data of patients with membranous nephropathy with the general population in two national surveys. We analyzed the main sectors of occupational activity and personal toxic exposures. We used two cohorts of French workers as controls. The French National Institute of Statistics and Economic Studies (INSEE) 2018 provides the occupational activity of the French general population by sex and age groups. After excluding unemployed individuals, this information is available for 26,734,000 participants. The 2017 French survey on Medical Surveillance of Employees’ Exposure to Occupational Risks (Surveillance Médicale des Expositions des salariés aux Risques professionnels [SUMER]) provides data on occupational toxic exposure. The statistical department of the French Ministry of Labor conducts this national survey every 7 years among the national working population of employees. In 2017, it included 26,500 participants. To note, in developing the questionnaire for patients with membranous nephropathy, we used similar evaluation criteria to those of the 2017 SUMER survey (i.e., exposition duration, personal and collective protective equipment, and intensity [measured or estimated] of exposure). To be consistent with the 2017 SUMER survey data, we decided to analyze both lifetime exposure of patients with membranous nephropathy data and also over the year 2017.
Membranous Nephropathy.
We collected data concerning the medical history of membranous nephropathy using patients’ records and, whenever necessary, direct patient questioning. The data collected included demographic characteristics, medical history and comorbidities, and clinical presentation of membranous nephropathy, as well as biologic parameters at diagnosis and during follow-up.
Among the 100 patients included, 45 were enrolled during an active phase of the disease (i.e., urine protein-creatinine ratio >3.5 g/g). In these participants, also included in a local cohort (Immunopathological Analysis in a French National Cohort of Membranous Nephropathy [IHMN]), 1 ml of whole blood was collected and stimulated with immune ligands (anti-CD3 as T cells stimulant and R848 as TLR 7/8 agonist) on single lyophilized spheres (LyoSphere; Qiagen) within 8 hours of blood collection. Stimulated blood samples were incubated for 16–24 hours at 37°C and then centrifuged at 2000–3000×g for 15 minutes to harvest the stimulated plasma. Plasma levels of IL-17A were measured using the custom-designed cartridges Ella (ProteinSimple) following the manufacturer’s instructions.
Outcomes
First, we aim to describe the main occupational toxic substances to which patients with membranous nephropathy were exposed and then compare them with those of the general population. Second, using subgroup analyses of patients with membranous nephropathy, we compared the characteristics of patients exposed to an occupational toxic substance with those who were not, including disease progression (epitope spreading and kidney function).
Statistical Analyses
Qualitative variables are presented as numbers and percentages and compared using a Pearson chi-squared test or a Fisher exact test in the case of a small sample. Quantitative variables with a Gaussian distribution (age) are presented as means and SDs and compared by the unpaired t test. Quantitative variables with a non-Gaussian distribution (creatinine, cytokines, and duration of exposure) are presented as medians and interquartile ranges (25th to 75th percentile) and compared with a nonparametric two-tailed test (Mann–Whitney U). The Shapiro–Wilk normality test was used to determine if a variable had Gaussian distribution. We performed a comparison in frequency by sex and age groups to compare the main job categories between patients with membranous nephropathy and the general population of French workers. Frequencies observed in the membranous nephropathy cohort for sex and age groups were used to calculated frequencies of the main job categories for the two cohorts. A multivariable logistic regression model with backward selection (threshold =0.15) was used to investigate independent factors that influence PLA2R1 spreading. Of the 100 patients, ten creatinine values at diagnosis were missing. We ensured that the numbers of missing data were similar between patient groups using a Fisher exact test. The ten missing data were thus simply removed from the analysis. Statistical analyses were performed using GraphPad Prism 8 (GraphPad Software, Inc., San Diego, CA) for unadjusted analysis and SAS Enterprise Guide 7.1 for multivariable analysis. All comparisons were two tailed, and the statistical differences were considered significant when P<0.05.
Results
Cohort Description
We included 100 patients with biopsy-proven membranous nephropathy in this retrospective Nice-based cohort study. The men-women sex ratio was 1.7, and the mean age at diagnosis of membranous nephropathy was 52±15 years. The median follow-up at the time of the study and thus at the time of the questionnaire was 7 (3–13) years. Sixty-two were active or past smokers. Eleven had membranous nephropathy secondary to another disease, such as infection, lupus, or cancer. For 89 patients, the etiologic workup remained negative; 63 had anti-PLA2R1 antibodies, two had antibodies to thrombospondin type 1 domain containing 7A, and 25 had neither anti-PLA2R1 nor antithrombospondin type 1 domain containing 7A antibodies. Among patients with membranous nephropathy mediated by anti-PLA2R1 antibodies, 31 had PLA2R1 epitope spreading beyond the CysR domain. Among the 100 participants, six patients achieved spontaneous complete remission, 70 had persistent nephrotic syndrome and/or required immunosuppressive therapy to achieve remission, and 24 progressed to kidney failure requiring replacement therapy with dialysis and/or kidney transplantation. Patients’ characteristics are detailed in Table 2.
Table 2.
Characteristics of the study population
| Patients' Characteristics | Participants, n=100 |
|---|---|
| Baseline characteristics | |
| Men, n (%) | 63 (63) |
| Sex ratio | 1.7 |
| Age at inclusion, yr | 61 (15) |
| Age at membranous nephropathy diagnosis, yr | 52 (15) |
| 20–39, n (%) | 20 (20) |
| 40–64, n (%) | 60 (60) |
| 65–99, n (%) | 20 (20) |
| Age at diagnosis according to the sex | |
| Women | 52 (18) |
| Men | 51 (13) |
| Job categories, n (%) | |
| Agricultural workers | 6 (6) |
| Industry workers | 14 (14) |
| Service workers | 43 (43) |
| Construction workers | 31 (31) |
| No professional activity | 6 (6) |
| Active or former smoker, n (%) | 62 (62) |
| Membranous nephropathy presentation, n (%) | |
| Secondary membranous nephropathy | 11 (11) |
| Autoimmune disease (e.g., lupus) | 8 (8) |
| Infection | 2 (2) |
| Malignancies | 1 (1) |
| Primary membranous nephropathy | 89 (89) |
| PLA2R1-mediated membranous nephropathy | 63 (63) |
| THSD7A-mediated membranous nephropathy | 2 (2) |
| Double-negative membranous nephropathy | 25 (25) |
| PLA2R1 epitope spreading, n=63 | |
| Yes | 31 (49) |
| No | 30 (48) |
| Missing data | 2 (3) |
| Course of membranous nephropathy | |
| Spontaneous complete remission | 6 (6) |
| Persistent nephrotic syndrome and/or need for immunosuppressive therapy | 70 (70) |
| Kidney failure | 24 (24) |
The numbers (and percentages) of patients are indicated for categorical variables, and mean (SD) are shown for continuous variables. Excluding the two patients for whom we did not have data on their PLA2R1 epitope spreading, there were no missing data. PLA2R1, phospholipase A2 receptor 1; THSD7A, thrombospondin type 1 domain containing 7A.
Occupational Exposures of Patients with Membranous Nephropathy
Main Job Categories.
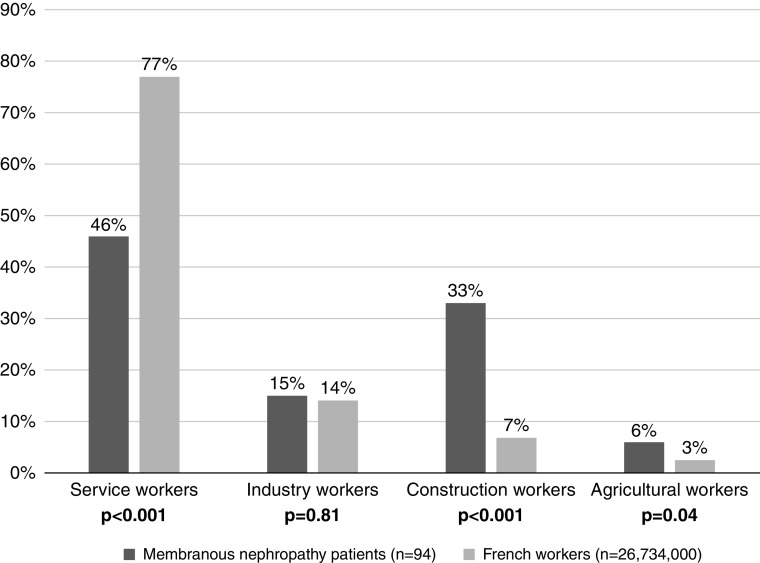
The main job categories of the 94 patients with membranous nephropathy who had a professional activity during their lifetime were compared with those of the 26,734,000 French workers in 2018 (INSEE data) and are represented in Figure 1. Patients with membranous nephropathy worked significantly more in the construction (33% versus 7%, P<0.001) and agricultural sectors (6% versus 3%, P=0.04) and less in the service sector (46% versus 77%, P<0.001) than the general population of French workers. A detailed comparison of demographic and socioprofessional characteristics between patients with membranous nephropathy and French workers is provided in Supplemental Table 1. We also performed a comparison of the main job categories between French workers and the subgroup of the 83 patients with primary membranous nephropathy who had a professional activity (i.e., excluding patients with secondary membranous nephropathy), and we found the same results (Supplemental Figure 1). Because patients with membranous nephropathy and the general population of French workers had noncomparable demographic characteristics (sex and age), we performed a comparison in frequency (sex and age groups) of the main job categories; patients with membranous nephropathy over 30 years old worked significantly more in the construction sector than the general population of French workers (Table 3). In this cohort, considering service workers under 50 as the reference population, we estimated that the risk of developing membranous nephropathy is eight times higher in construction workers, regardless of age and sex (Supplemental Table 2).
Figure 1.
Comparison of the main job categories between patients with membranous nephropathy and the general population of French workers. The data source for French workers is the French National Institute of Statistics and Economic Studies 2018 and covers 26,734,000 individuals. Individuals who have never worked were not included in the analysis. Patients with membranous nephropathy worked significantly more in the construction (P<0.001) and agricultural sectors (P=0.04) and less in the service sector (P<0.001) than the general population of French workers. Statistical significance was determined by a chi-squared test or a Fisher exact test according to patient sample size. There were no missing data.
Table 3.
Comparison in frequency (sex and age) of the main job categories between patients with membranous nephropathy and the general population of French workers
| Job Categories | French Workers, 100% (n=26,734,000) | Patients with Membranous Nephropathy, 100% (n=94) | P Value |
|---|---|---|---|
| Men (63% a ), % | |||
| Age 15–29 (10%a) | |||
| Service workers | 5 (1,296,599) | 3 (3) | ns |
| Industry workers | 0.9 (235,259) | 1 (1) | ns |
| Construction workers | 0.4 (117,630) | 2 (2) | ns |
| Agricultural workers | 0.2 (42,774) | (0) | ns |
| Age 30–49 (34%a) | |||
| Service workers | 16 (4,408,437) | 10 (9) | ns |
| Industry workers | 3 (802,020) | 3 (3) | ns |
| Construction workers | 2 (401,010) | 7 (7) | 0.01b |
| Agricultural workers | 0.5 (141,690) | 1 (1) | ns |
| Age ≥50 (56%a) | |||
| Service workers | 27 (7,260,954) | 16 (15) | 0.01b |
| Industry workers | 5 (1,320,660) | 5 (5) | ns |
| Construction workers | 2 (660,330) | 12 (11) | 0.02b |
| Agricultural workers | 0.9 (235,259) | 2 (2) | ns |
| Women (37% a ), % | |||
| Age 15–29 (10%a) | |||
| Service workers | 3 (761,919) | 2 (2) | ns |
| Industry workers | 0.5 (139,017) | 1 (1) | ns |
| Construction workers | 0.3 (69,508) | 1 (1) | ns |
| Agricultural workers | 0.1 (24,061) | (0) | ns |
| Age 30–49 (34%a) | |||
| Service workers | 10 (2,590,525) | 5 (5) | ns |
| Industry workers | 3 (470,518) | 2 (2) | ns |
| Construction workers | 0.9 (235,259) | 4 (4) | 0.01b |
| Agricultural workers | 0.3 (80,202) | 1 (1) | ns |
| Age ≥50 (56%a) | |||
| Service workers | 16 (4,264,073) | 10 (9) | ns |
| Industry workers | 3 (775,286) | 3 (3) | ns |
| Construction workers | 1 (387,643) | 6 (6) | 0.01b |
| Agricultural workers | 0.5 (136,343) | 1 (1) | ns |
Main job categories between patients with membranous nephropathy and the general population of French workers are compared using a Fisher exact test. Differences between groups were considered to be statistically significant when P values were <0.05. ns, not significant.
The frequency observed in the membranous nephropathy cohort was used to calculate the frequency (by sex and age groups) of the main job categories for both cohorts.
Significant associations.
Occupational Toxic Agents.
Compared with the general population of French workers (2017 SUMER survey), patients with membranous nephropathy were more frequently exposed to three toxic substances during their professional activity: lead (14% lifetime prevalence and 9% 2017 prevalence for patients with membranous nephropathy versus 1% prevalence for the general population; P<0.001 and P<0.001, respectively), asbestos (21% lifetime prevalence and 16% 2017 prevalence for patients with membranous nephropathy versus 5% prevalence for the general population; P<0.001 and P<0.001, respectively), and organic solvents (57% lifetime prevalence and 37% 2017 prevalence for patients with membranous nephropathy versus 15% prevalence for the general population; P<0.001 and P<0.001, respectively) (Figure 2). It should be noted that environmental and domestic nonoccupational toxic exposures identified through a second questionnaire were negligible (data not shown).
Figure 2.
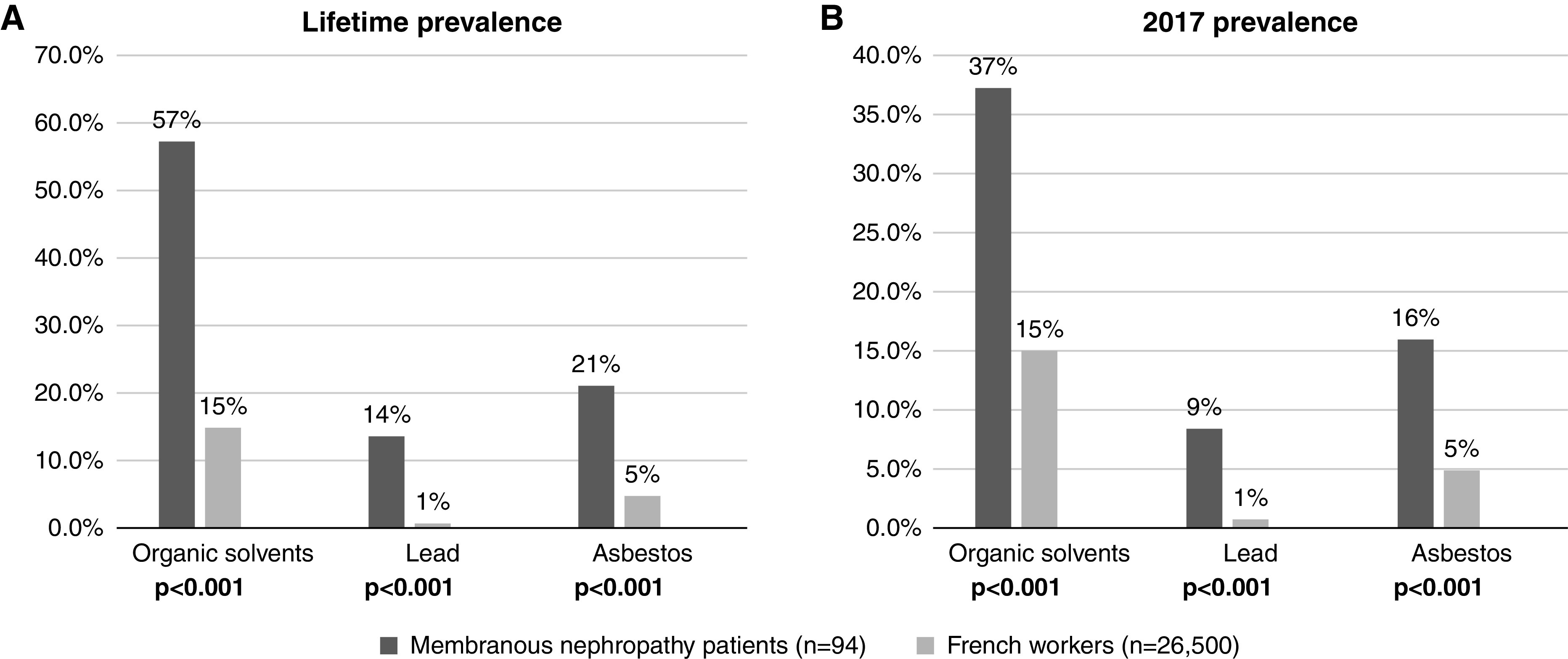
Comparison of (A) lifetime and (B) 2017 prevalence of occupational exposure between patients with membranous nephropathy and the general population of French workers. The data source for French workers is the 2017 French Medical Surveillance of Employees’ Exposure to Occupational Risks survey, which is a national survey of occupational exposures developed by the French Ministry of Labor that reported the proportion of workers exposed to toxic agents among the employed population between April 2016 and September 2017. Patients with membranous nephropathy were more frequently exposed to lead, asbestos, and organic solvents during their professional activity than the general population of French workers. Statistical significance was determined by a chi-squared test or a Fisher exact test according to patient sample size. There were no missing data.
Relationship between Membranous Nephropathy Characteristics and Occupational Exposure to Toxic Substances
Demographic Characteristics.
As shown in Figure 3, patients exposed to occupational toxic substances showed a predominance of men for two toxic agents: organic solvents (80% men in exposed patients versus 41% men in unexposed patients, P<0.001) and asbestos (90% men in exposed patients versus 55% men in unexposed patients, P=0.004). Interestingly, the sex ratio of unexposed patients with membranous nephropathy was balanced. For the three toxic substances studied, the age at diagnosis was not different between exposed and unexposed patients. Moreover, no difference in smoking habits was found between patients exposed to asbestos or lead and nonexposed patients (P=0.21 and P=0.36, respectively). In contrast, patients exposed to organic solvents were more likely to be active or past smokers than unexposed patients (P=0.03).
Figure 3.
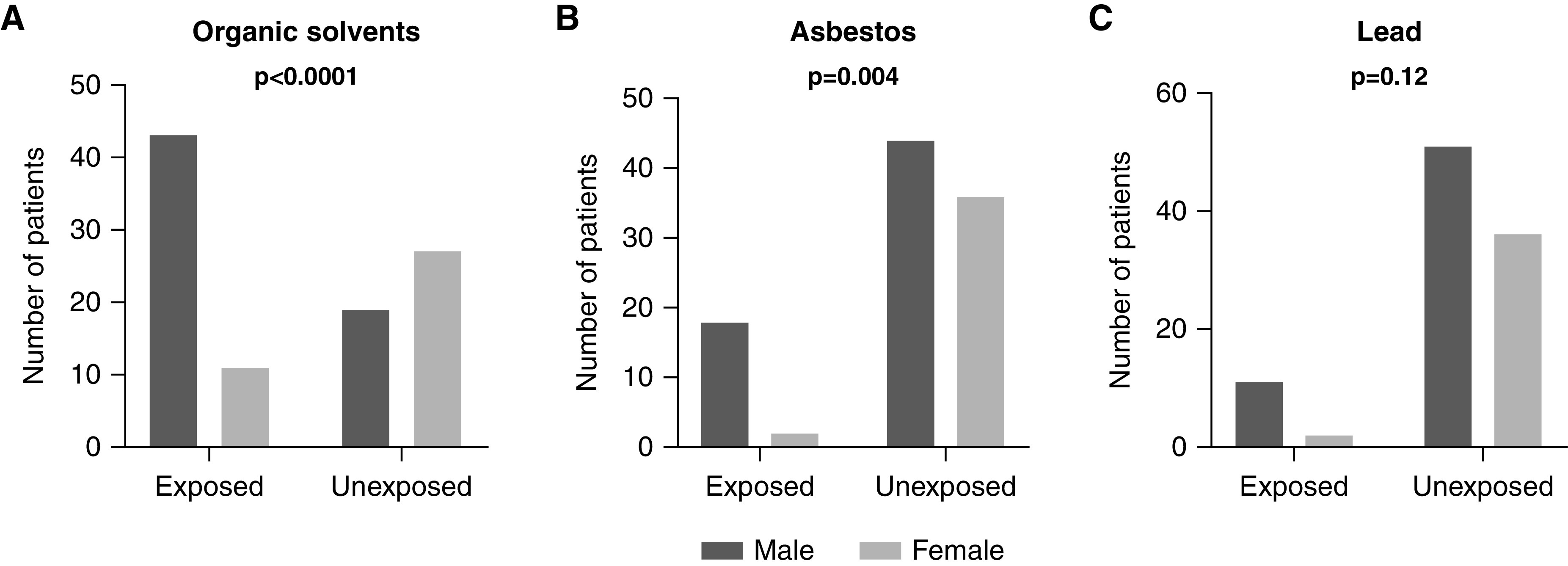
Sex ratio by toxic occupational exposure to (A) organic solvents, (B) asbestos, and (C) lead. Patients with membranous nephropathy exposed to occupational toxic substances seemed to be more often men than women: organic solvents (80% men in exposed patients versus 41% men in unexposed patients, P<0.001), asbestos (90% men in exposed patients versus 55% men in unexposed patients, P=0.004), and lead (85% men in exposed patients versus 59% men in unexposed patients, P=0.12). Statistical significance was determined by a chi-squared test or a Fisher exact test according to patient sample size. There were no missing data.
Epitope Spreading.
Because epitope spreading is a poor prognostic factor in PLA2R1-associated membranous nephropathy (39,40), we investigated whether certain factors influence PLA2R1 epitope spreading, such as occupational exposure to toxic substances. After unadjusted analysis, two variables appeared to be significantly associated with epitope spreading: asbestos exposure (ten of 31 spreaders [32%] versus two of 30 nonspreaders [7%], P=0.02) and organic solvent exposure (23 of 31 spreaders [74%] versus 13 of 30 nonspreaders [43%], P=0.02). The association between PLA2R1 epitope spreading and asbestos exposure was confirmed by multivariable analysis (odds ratio, 6.67; 95% confidence interval, 1.32 to 33.69; P=0.02). In addition, patients exposed to organic solvents and asbestos with moderate or high intensity were more likely to have PLA2R1 epitope spreading than patients with no or low exposure (P=0.04 and P=0.04, respectively). The data are summarized in Table 4.
Table 4.
Comparison of baseline characteristics and occupational exposure between patients with membranous nephropathy and phospholipase A2 receptor 1 epitope spreading and those with membranous nephropathy without phospholipase A2 receptor 1 epitope spreading
| Patients' Characteristics and Exposure | Spreading, n=31 | No Spreading, n=30 | Unadjusted P Value |
|---|---|---|---|
| Baseline characteristics | |||
| Men | 25 (81%) | 17 (57%) | 0.06 |
| Sex ratio men-women | 4.2 | 1.3 | |
| Age at diagnosis, yr | 52 (16) | 55 (15) | 0.52 |
| Job categories | |||
| Construction workers | 14 (45%) | 7 (23%) | 0.11 |
| Other sector of activity | 17 (55%) | 23 (77%) | |
| Smoking | 19 (61%) | 20 (67%) | 0.79 |
| Occupational exposure | |||
| Asbestos | 10 (32%) | 2 (7%) | 0.02a |
| Lead | 4 (13%) | 2 (7%) | 0.67 |
| Organic solvents | 23 (74%) | 13 (43%) | 0.02a |
| Level of exposure | |||
| Asbestos | 0.04a | ||
| High/moderate exposure | 9 (29%) | 2 (7%) | |
| Low/no exposure | 22 (71%) | 28 (93%) | |
| Lead | 0.19 | ||
| High/moderate exposure | 4 (13%) | 1 (3%) | |
| Low/no exposure | 27 (87%) | 29 (97%) | |
| Organic solvents | 0.04a | ||
| High/moderate exposure | 20 (65%) | 11 (37%) | |
| Low/no exposure | 11 (36%) | 19 (66%) | |
Categorial (number and percentage) and continuous (mean and SD) variables are shown for patients with membranous nephropathy with or without epitope spreading. Baseline characteristics and occupational exposures were compared using a Fisher exact test and unpaired t test for categorial and continuous variables, respectively. All variables with P<0.15 in unadjusted analysis were used for multivariable logistic regression (i.e., men, construction worker, exposure to asbestos, and exposure to organic solvents). After backward selection, only asbestos was retained in the multivariable model (P=0.02). The proportion of patients with or without PLA2R1 epitope spreading is then shown for each exposure level and for each toxic agent in number and percentage and compared using a Fisher exact test. Differences between groups were considered to be statistically significant when P values were <0.05. There were no missing data.
Significant associations.
Kidney Prognosis.
We found no significant difference in the kidney function at diagnosis according to the presence or absence of exposure during occupational activity. Creatinine at diagnosis in patients exposed to organic solvents compared with nonexposed patients was 1.09 (0.97–1.68) mg/dL and 1.01 (0.77–1.68) mg/dL, respectively (P=0.30). Creatinine at diagnosis in patients exposed to lead compared with nonexposed patients was 1.11 (1.02–1.60) mg/dL and 1.03 (0.78–1.69) mg/dL, respectively (P=0.38). Creatinine at diagnosis in patients exposed to asbestos compared with nonexposed patients was 1.39 (1.01–1.82) μmol/L and 1.02 (0.78–1.67) mg/dL, respectively (P=0.09). To note, ten creatinine values at diagnosis were missing, but the proportions of missing values between groups were not different. We did not find any effect of toxic occupational exposures on the progression of membranous nephropathy to kidney failure (Supplemental Table 3).
T Helper 17 Cytokine Profile.
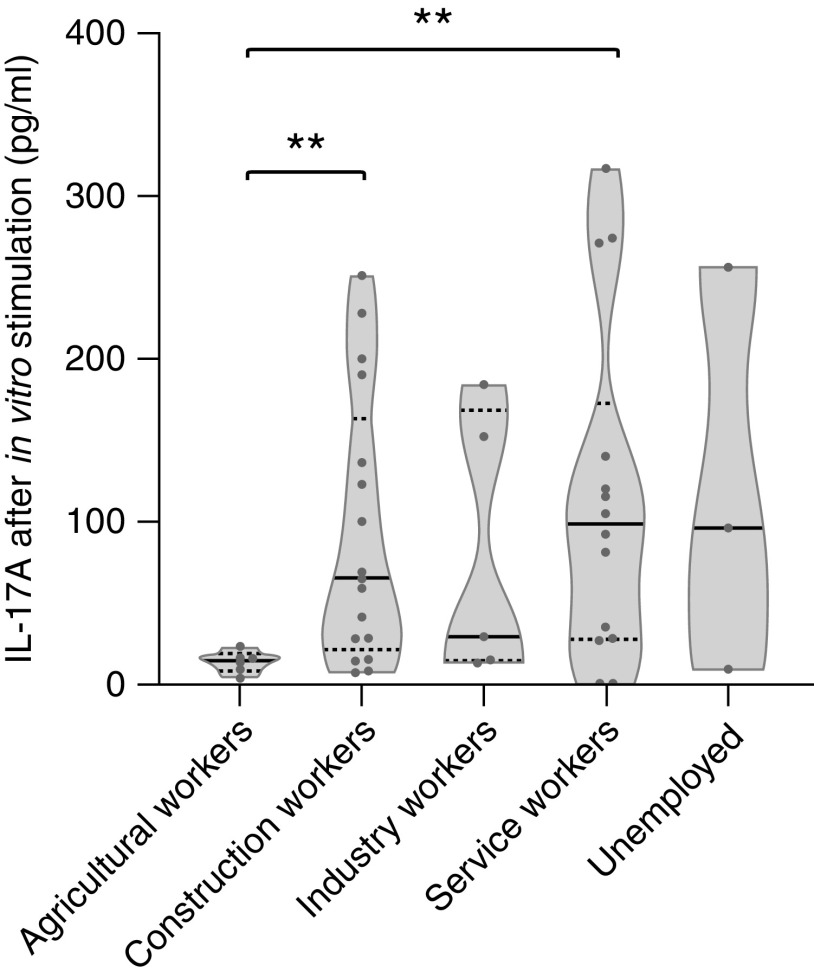
We previously described an association between exposure to fine particles PM2.5 and cytokines produced after in vitro nonspecific stimulation of the immune cells of patients with membranous nephropathy (22). Patients exposed to high levels of PM2.5 exhibited a higher level of cytokine from the Th17 pathway (22). Cytokine assays were performed for 45 patients in the cohort and analyzed according to job categories. Agricultural workers had significantly lower IL-17A levels than construction and service workers (14.5 [7.8–18.5] pg/ml versus 65.0 [21.5–163.0] pg/ml, P=0.01 and 98.5 [27.8–172.8] pg/ml, P=0.01, respectively). Results are shown in Figure 4.
Figure 4.
IL-17A levels secreted by immune cells after in vitro stimulation. Immune cells were stimulated at 37°C for 24 hours in whole blood with anti-CD3 agonist mAbs and a Toll-like receptor 7/8 agonist. IL-17A levels were measured in cellular supernatants. Statistical significance of the differences between job categories was assessed using the Mann–Whitney U nonparametric test. **P=0.01.
Discussion
Membranous nephropathy is a rare autoimmune kidney disease. Its increasing prevalence in industrialized countries (25,29) pleads for the involvement of environmental factors acting as an adjuvant at the origin of an immune dysregulation that could favor the onset of the disease. In addition, the predominance of men (38) in membranous nephropathy, classically attributed to biologic or genetic differences between men and women, could also be due to different occupational exposures. Here, we highlighted that patients with membranous nephropathy work more frequently in the construction sector with exposure to occupational toxic substances, such as asbestos, lead, and organic solvents. The association between organic solvents and GN had already been suggested (36,37) but remains controversial (35). We here show for the first time an association between membranous nephropathy and exposure to lead and asbestos. We suggest that the well-known predominance of men may be due to a subgroup of patients exposed to occupational toxic substances. Toxic exposures outside the workplace were negligible. These observations need to be confirmed in a large prospective cohort of patients with membranous nephropathy. This is one of the objectives of an ongoing prospective national French research program called the IHMN study. We will assess whether an exposure to environmental toxic substances alters membranous nephropathy prognosis.
We found an association between exposure to organic solvents or asbestos and PLA2R1 epitope spreading, with a dose-dependent effect. PLA2R1 epitope spreading is a biomarker of poor prognosis and of nonresponse to rituximab therapy (39,40). However, in contrast to the Jacob et al. (36) study, exposure to occupational toxic agents had no effect on the progression of membranous nephropathy to kidney failure in our study, possibly due to the improved management of this disease since the discovery of anti-PLA2R1 antibodies (4,5) and the use of effective immunosuppressive therapies, such as rituximab (41–43). We investigated whether other confounding factors might be involved in the pathogenesis of the membranous nephropathy, and we found that patients exposed to organic solvents were also more often active or past smokers than nonexposed patients. Patients with membranous nephropathy exposed to other toxic substances and with PLA2R1 epitope spreading were not more likely to be smokers. Therefore, smoking is unlikely to be directly involved in the pathogenesis of the disease.
In this study, patients working in the agricultural sector secreted less IL-17A after in vitro stimulation of immune cells than patients working in other sectors. In a previous study, our team showed that increased IL-17A levels were associated with a higher risk of thrombosis and relapse (22), which could not be confirmed here, likely due to a small sample size. These increased IL-17A levels were more likely in patients living in urban areas exposed to high levels of fine particles PM2.5 (22). An environmental factor may induce an immune dysregulation with an imbalance of the Th17-T regulatory ratio, causing a more inflammatory membranous nephropathy that would increase the risk of thrombosis and relapse. Several environmental factors, such as exposure to organic solvents or exposure to PM2.5, can be suspected. This immune dysregulation toward the Th17 pathway induced by such environmental factors must be confirmed in vitro on cell culture, as already shown in other pathologies, such as hypersensitivity dermatitis with organic solvents (44).
Nevertheless, our study has several limitations. First, it is a retrospective study with a long follow-up period, which may induce a bias in the patients' memory of their previous occupations and the toxic substances to which they may have been exposed. However, only prolonged exposures over several years seem relevant, and it is unlikely that patients will forget an occupation that they have performed for years. Moreover, as membranous nephropathy is a rare disease, it seems difficult to design a prospective exposed/unexposed study. Second, although the sample size is reasonable for a rare disease, it is a single-center study, and the limited size of the patient cohort induces an imbalance with general population cohorts. It would be interesting to conduct the same study in other geographic areas and/or a retrospective analytic study of matched case-control type to confirm these results. Third, general population data were extracted from publicly available but incomplete databases. It would have been interesting to have continuous age rather than age ranges, as well as health data. Fourth, although the occupational health physicians who conducted the questionnaires were experienced, it cannot be excluded that their questions may have guided the patients' responses. Fifth, the duration of patient follow-up was variable, leading to differences in management (pre- and postrituximab eras) and delays that do not allow conclusions to be drawn regarding the subsequent occurrence of kidney failure in newly diagnosed patients. Finally, we used backward selection in the multivariable model, although this may increase the type 1 error. Most of these limitations will be controlled in the national French prospective IHMN study previously described.
Although various studies report the existence of a dose-response relationship between exposure to organic solvents and the occurrence of GN (33,34,36,45–47) as well as the beneficial effect obtained by avoiding this exposure (46), professional practices do not seem to have been modified, and the nephrotoxicity of this exposure is still not recognized by the French health authorities. This lack of recognition as an occupational disease could be partly explained by the fact that no direct correlation has yet been established in the absence of in vitro studies on cell cultures or in vivo studies in animals.
In conclusion, patients with membranous nephropathy are more frequently exposed to certain occupational toxic substances (organic solvents, lead, and asbestos) than the general population. This predominance of men in exposures, particularly high levels of asbestos exposure, may favor PLA2R1 epitope spreading. These results justify adherence to the use of personal protective equipment and increased surveillance of proteinuria in exposed workers. The occurrence of membranous nephropathy in the context of high occupational exposure to organic solvents or asbestos, without any other obvious etiology, seems to justify recognition of an occupational disease.
Disclosures
S. Benzaken reports an advisory or leadership role on the BMS Advisory Committee and other interests or relationships with the French Health Authority and the Regional Health Agency: Public Health National Committee. S. Benzaken’s spouse reports employment with the Imaging Center: La Grande Bleue, Nice, France. V. Esnault reports consultancy agreements with AstraZeneca, Bayer, BMS-Pfizer, Boehringer-Ingelheim, and Novartis; research funding from AstraZeneca, BMS-Pfizer, Boehringer-Ingelheim, Fresenius, Hemotech, and Novartis; honoraria from Amgen, Bayer, BMS-Pfizer, Boehringer-Ingelheim, Fresenius, Lilly, and Novartis; and advisory or leadership roles for AstraZeneca, Bayer, and Boehringer-Ingelheim. B. Seitz-Polski reports consultancy agreements with Novartis and patents with Euroimmun and is the coinventor on the patents “Methods and kits for monitoring membranous nephropathy” and “Prognosis and monitoring of membranous nephropathy based on the analysis of PLA2R1 epitope profile and spreading.” All remaining authors have nothing to disclose.
Funding
This work was supported by GIRCI Méditerranée Grant 18-GIRCI-03 and grants from ORKID (grant AAP-ORKID-2019).
Supplementary Material
Acknowledgments
We thank all of the patients involved in this study.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Author Contributions
B. Seitz-Polski conceptualized the study; S. Agbekodo and M. Cremoni were responsible for data curation; B. Seitz-Polski was responsible for investigation; S. Agbekodo and M. Cremoni were responsible for formal analysis; M. Cremoni, B. Seitz-Polski, and K. Zorzi were responsible for methodology; M. Teisseyre was responsible for validation; S. Benzaken, J.-H. Planchard, and M. Teisseyre were responsible for visualization; S. Benzaken and B. Seitz-Polski were responsible for funding acquisition; B. Seitz-Polski provided supervision; M. Cremoni wrote the original draft; and S. Agbekodo, V. Brglez, V. Esnault, and B. Seitz-Polski reviewed and edited the manuscript.
Data Sharing Statement
Data supporting the conclusions of this study are available upon request to M. Cremoni and B. Seitz-Polski.
Supplemental Material
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.02930322/-/DCSupplemental.
Supplemental Material. Questionnaire.
Supplemental Table 1. Comparison of demographic and socioprofessional characteristics between patients with membranous nephropathy and the general population of French workers.
Supplemental Table 2. Estimated risk of membranous nephropathy for each occupation compared with the referent occupation (service sector) adjusted for age and sex.
Supplemental Table 3. Comparison of baseline characteristics and occupational exposure between patients with membranous nephropathy and kidney failure and those with membranous nephropathy without kidney failure.
Supplemental Figure 1. Comparison of the main job categories between patients with primary membranous nephropathy and the general population of French workers.
References
- 1.Lassalle M, Ayav C, Frimat L, Jacquelinet C, Couchoud C; Au Nom du Registre REIN : The essential of 2012 results from the French Renal Epidemiology and Information Network (REIN) ESRD registry. Nephrol Ther 11: 78–87, 2015 [DOI] [PubMed] [Google Scholar]
- 2.Beck LH Jr., Salant DJ: Membranous nephropathy: From models to man. J Clin Invest 124: 2307–2314, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Couser WG: Primary membranous nephropathy. Clin J Am Soc Nephrol 12: 983–997, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cravedi P, Jarque M, Angeletti A, Favà À, Cantarelli C, Bestard O: Immune-monitoring disease activity in primary membranous nephropathy. Front Med (Lausanne) 6: 241, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beck LH Jr., Bonegio RGB, Lambeau G, Beck DM, Powell DW, Cummins TD, Klein JB, Salant DJ: M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med 361: 11–21, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tomas NM, Beck LH Jr., Meyer-Schwesinger C, Seitz-Polski B, Ma H, Zahner G, Dolla G, Hoxha E, Helmchen U, Dabert-Gay AS, Debayle D, Merchant M, Klein J, Salant DJ, Stahl RAK, Lambeau G: Thrombospondin type-1 domain-containing 7A in idiopathic membranous nephropathy. N Engl J Med 371: 2277–2287, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tomas NM, Hoxha E, Reinicke AT, Fester L, Helmchen U, Gerth J, Bachmann F, Budde K, Koch-Nolte F, Zahner G, Rune G, Lambeau G, Meyer-Schwesinger C, Stahl RA: Autoantibodies against thrombospondin type 1 domain-containing 7A induce membranous nephropathy. J Clin Invest 126: 2519–2532, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hurtado A, Johnson RJ: Hygiene hypothesis and prevalence of glomerulonephritis. Kidney Int Suppl 68: S62–S67, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Swaminathan S, Leung N, Lager DJ, Melton LJ 3rd, Bergstralh EJ, Rohlinger A, Fervenza FC: Changing incidence of glomerular disease in Olmsted County, Minnesota: A 30-year renal biopsy study. Clin J Am Soc Nephrol 1: 483–487, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Versini M, Jeandel P-Y, Bashi T, Bizzaro G, Blank M, Shoenfeld Y: Unraveling the Hygiene Hypothesis of helminthes and autoimmunity: Origins, pathophysiology, and clinical applications. BMC Med 13: 81, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stanescu HC, Arcos-Burgos M, Medlar A, Bockenhauer D, Kottgen A, Dragomirescu L, Voinescu C, Patel N, Pearce K, Hubank M, Stephens HA, Laundy V, Padmanabhan S, Zawadzka A, Hofstra JM, Coenen MJ, den Heijer M, Kiemeney LA, Bacq-Daian D, Stengel B, Powis SH, Brenchley P, Feehally J, Rees AJ, Debiec H, Wetzels JF, Ronco P, Mathieson PW, Kleta R: Risk HLA-DQA1 and PLA(2)R1 alleles in idiopathic membranous nephropathy. N Engl J Med 364: 616–626, 2011 [DOI] [PubMed] [Google Scholar]
- 12.Coenen MJH, Hofstra JM, Debiec H, Stanescu HC, Medlar AJ, Stengel B, Boland-Augé A, Groothuismink JM, Bockenhauer D, Powis SH, Mathieson PW, Brenchley PE, Kleta R, Wetzels JF, Ronco P: Phospholipase A2 receptor (PLA2R1) sequence variants in idiopathic membranous nephropathy. J Am Soc Nephrol 24: 677–683, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lv J, Hou W, Zhou X, Liu G, Zhou F, Zhao N, Hou P, Zhao M, Zhang H: Interaction between PLA2R1 and HLA-DQA1 variants associates with anti-PLA2R antibodies and membranous nephropathy. J Am Soc Nephrol 24: 1323–1329, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bullich G, Ballarín J, Oliver A, Ayasreh N, Silva I, Santín S, Díaz-Encarnación MM, Torra R, Ars E: HLA-DQA1 and PLA2R1 polymorphisms and risk of idiopathic membranous nephropathy. Clin J Am Soc Nephrol 9: 335–343, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cui Z, Xie LJ, Chen FJ, Pei ZY, Zhang LJ, Qu Z, Huang J, Gu QH, Zhang YM, Wang X, Wang F, Meng LQ, Liu G, Zhou XJ, Zhu L, Lv JC, Liu F, Zhang H, Liao YH, Lai LH, Ronco P, Zhao MH: MHC class II risk alleles and amino acid residues in idiopathic membranous nephropathy. J Am Soc Nephrol 28: 1651–1664, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Le W-B, Shi J-S, Zhang T, Liu L, Qin HZ, Liang S, Zhang YW, Zheng CX, Jiang S, Qin WS, Zhang HT, Liu ZH: HLA-DRB1*15:01 and HLA-DRB3*02:02 in PLA2R-related membranous nephropathy. J Am Soc Nephrol 28: 1642–1650, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaga H, Komatsuda A, Omokawa A, Okuyama S, Mori K, Wakui H, Takahashi N: Analysis of PLA2R1 and HLA-DQA1 sequence variants in Japanese patients with idiopathic and secondary membranous nephropathy. Clin Exp Nephrol 22: 275–282, 2018 [DOI] [PubMed] [Google Scholar]
- 18.Xie J, Liu L, Mladkova N, Li Y, Ren H, Wang W, Cui Z, Lin L, Hu X, Yu X, Xu J, Liu G, Caliskan Y, Sidore C, Balderes O, Rosen RJ, Bodria M, Zanoni F, Zhang JY, Krithivasan P, Mehl K, Marasa M, Khan A, Ozay F, Canetta PA, Bomback AS, Appel GB, Sanna-Cherchi S, Sampson MG, Mariani LH, Perkowska-Ptasinska A, Durlik M, Mucha K, Moszczuk B, Foroncewicz B, Pączek L, Habura I, Ars E, Ballarin J, Mani LY, Vogt B, Ozturk S, Yildiz A, Seyahi N, Arikan H, Koc M, Basturk T, Karahan G, Akgul SU, Sever MS, Zhang D, Santoro D, Bonomini M, Londrino F, Gesualdo L, Reiterova J, Tesar V, Izzi C, Savoldi S, Spotti D, Marcantoni C, Messa P, Galliani M, Roccatello D, Granata S, Zaza G, Lugani F, Ghiggeri G, Pisani I, Allegri L, Sprangers B, Park JH, Cho B, Kim YS, Kim DK, Suzuki H, Amoroso A, Cattran DC, Fervenza FC, Pani A, Hamilton P, Harris S, Gupta S, Cheshire C, Dufek S, Issler N, Pepper RJ, Connolly J, Powis S, Bockenhauer D, Stanescu HC, Ashman N, Loos RJF, Kenny EE, Wuttke M, Eckardt KU, Köttgen A, Hofstra JM, Coenen MJH, Kiemeney LA, Akilesh S, Kretzler M, Beck LH, Stengel B, Debiec H, Ronco P, Wetzels JFM, Zoledziewska M, Cucca F, Ionita-Laza I, Lee H, Hoxha E, Stahl RAK, Brenchley P, Scolari F, Zhao MH, Gharavi AG, Kleta R, Chen N, Kiryluk K: The genetic architecture of membranous nephropathy and its potential to improve non-invasive diagnosis. Nat Commun 11: 1600, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roccatello D, Sciascia S, Di Simone D, Solfietti L, Naretto C, Fenoglio R, Baldovino S, Menegatti E: New insights into immune mechanisms underlying response to Rituximab in patients with membranous nephropathy: A prospective study and a review of the literature. Autoimmun Rev 15: 529–538, 2016 [DOI] [PubMed] [Google Scholar]
- 20.Rosenzwajg M, Languille E, Debiec H, Hygino J, Dahan K, Simon T, Klatzmann D, Ronco P: B- and T-cell subpopulations in patients with severe idiopathic membranous nephropathy may predict an early response to rituximab. Kidney Int 92: 227–237, 2017 [DOI] [PubMed] [Google Scholar]
- 21.Li H, Wu H, Guo Q, Yu H, Xu Y, Yu J, Wang Z, Yi H: Myeloid-derived suppressor cells promote the progression of primary membranous nephropathy by enhancing Th17 response. Front Immunol 11: 1777, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cremoni M, Brglez V, Perez S, Decoupigny F, Zorzi K, Andreani M, Gérard A, Boyer-Suavet S, Ruetsch C, Benzaken S, Esnault V, Seitz-Polski B: Th17-immune response in patients with membranous nephropathy is associated with thrombosis and relapses. Front Immunol 11: 574997, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Motavalli R, Etemadi J, Soltani-Zangbar MS, Ardalan MR, Kahroba H, Roshangar L, Nouri M, Aghebati-Maleki L, Khiavi FM, Abediazar S, Mehdizadeh A, Hojjat-Farsangi M, Mahmoodpoor A, Kafil HS, Zolfaghari M, Ahmadian Heris J, Yousefi M: Altered Th17/Treg ratio as a possible mechanism in pathogenesis of idiopathic membranous nephropathy. Cytokine 141: 155452, 2021 [DOI] [PubMed] [Google Scholar]
- 24.Zhao Q, Dai H, Liu X, Jiang H, Liu W, Feng Z, Zhang N, Gao Y, Dong Z, Zhou X, Du J, Zhang N, Rui H, Yuan L, Liu B: Helper T cells in idiopathic membranous nephropathy. Front Immunol 12: 665629, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu X, Nie S, Ding H, Hou FF: Environmental pollution and kidney diseases. Nat Rev Nephrol 14: 313–324, 2018 [DOI] [PubMed] [Google Scholar]
- 26.Li S-J, Zhang S-H, Chen H-P, Zeng CH, Zheng CX, Li LS, Liu ZH: Mercury-induced membranous nephropathy: Clinical and pathological features. Clin J Am Soc Nephrol 5: 439–444, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chakera A, Lasserson D, Beck LH, Roberts ISD, Winearls CG: Membranous nephropathy after use of UK-manufactured skin creams containing mercury. QJM 104: 893–896, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Breysse P, Couser WG, Alpers CE, Nelson K, Gaur L, Johnson RJ: Membranous nephropathy and formaldehyde exposure. Ann Intern Med 120: 396–397, 1994 [DOI] [PubMed] [Google Scholar]
- 29.Xu X, Wang G, Chen N, Lu T, Nie S, Xu G, Zhang P, Luo Y, Wang Y, Wang X, Schwartz J, Geng J, Hou FF: Long-term exposure to air pollution and increased risk of membranous nephropathy in China. J Am Soc Nephrol 27: 3739–3746, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barragán-Martínez C, Speck-Hernández CA, Montoya-Ortiz G, Mantilla RD, Anaya J-M, Rojas-Villarraga A: Organic solvents as risk factor for autoimmune diseases: A systematic review and meta-analysis. PLoS One 7: e51506, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marie I, Gehanno J-F, Bubenheim M, Duval-Modeste AB, Joly P, Dominique S, Bravard P, Noël D, Cailleux AF, Weber J, Lagoutte P, Benichou J, Levesque H: Prospective study to evaluate the association between systemic sclerosis and occupational exposure and review of the literature. Autoimmun Rev 13: 151–156, 2014 [DOI] [PubMed] [Google Scholar]
- 32.Ouchene L, Muntyanu A, Lavoué J, Baron M, Litvinov IV, Netchiporouk E: Toward understanding of environmental risk factors in systemic sclerosis. J Cutan Med Surg 25: 188–204, 2021 [DOI] [PubMed] [Google Scholar]
- 33.Beirne GJ, Brennan JT: Glomerulonephritis associated with hydrocarbon solvents: Mediated by antiglomerular basement membrane antibody. Arch Environ Health 25: 365–369, 1972 [DOI] [PubMed] [Google Scholar]
- 34.Zimmerman SW, Groehler K, Beirne GJ: Hydrocarbon exposure and chronic glomerulonephritis. Lancet 2: 199–201, 1975 [DOI] [PubMed] [Google Scholar]
- 35.Fored CM, Nise G, Ejerblad E, Fryzek JP, Lindblad P, McLaughlin JK, Elinder CG, Nyrén O: Absence of association between organic solvent exposure and risk of chronic renal failure: A nationwide population-based case-control study. J Am Soc Nephrol 15: 180–186, 2004 [DOI] [PubMed] [Google Scholar]
- 36.Jacob S, Héry M, Protois J-C, Rossert J, Stengel B: Effect of organic solvent exposure on chronic kidney disease progression: The GN-PROGRESS cohort study. J Am Soc Nephrol 18: 274–281, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jacob S, Héry M, Protois J-C, Rossert J, Stengel B: New insight into solvent-related end-stage renal disease: Occupations, products and types of solvents at risk. Occup Environ Med 64: 843–848, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pollard KM: Gender differences in autoimmunity associated with exposure to environmental factors. J Autoimmun 38: J177–J186, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seitz-Polski B, Dolla G, Payré C, Girard CA, Polidori J, Zorzi K, Birgy-Barelli E, Jullien P, Courivaud C, Krummel T, Benzaken S, Bernard G, Burtey S, Mariat C, Esnault VL, Lambeau G: Epitope spreading of autoantibody response to PLA2R associates with poor prognosis in membranous nephropathy. J Am Soc Nephrol 27: 1517–1533, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Salant DJ: Does epitope spreading influence responsiveness to rituximab in PLA2R-associated membranous nephropathy? Clin J Am Soc Nephrol 14: 1122–1124, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dahan K, Debiec H, Plaisier E, Cachanado M, Rousseau A, Wakselman L, Michel PA, Mihout F, Dussol B, Matignon M, Mousson C, Simon T, Ronco P; GEMRITUX Study Group : Rituximab for severe membranous nephropathy: A 6-month trial with extended follow-up. J Am Soc Nephrol 28: 348–358, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seitz-Polski B, Dahan K, Debiec H, Rousseau A, Andreani M, Zaghrini C, Ticchioni M, Rosenthal A, Benzaken S, Bernard G, Lambeau G, Ronco P, Esnault VLM: High-dose rituximab and early remission in PLA2R1-related membranous nephropathy. Clin J Am Soc Nephrol 14: 1173–1182, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Trivin-Avillach C, Beck LH: Management of membranous nephropathy after MENTOR. Clin J Am Soc Nephrol 15: 415–417, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jia Q, Zang D, Yi J, Dong H, Niu Y, Zhai Q, Teng Y, Bin P, Zhou W, Huang X, Li H, Zheng Y, Dai Y: Cytokine expression in trichloroethylene-induced hypersensitivity dermatitis: An in vivo and in vitro study. Toxicol Lett 215: 31–39, 2012 [DOI] [PubMed] [Google Scholar]
- 45.von Schéele C, Althoff P, Kempi V, Schelin U: Nephrotic syndrome due to subacute glomerulonephritis—Association with hydrocarbon exposure? Acta Med Scand 200: 427–429, 1976 [DOI] [PubMed] [Google Scholar]
- 46.Ravnskov U, Forsberg B: Improvement of glomerulonephritis after discontinuation of solvent exposure. Lancet 1: 1194, 1979 [DOI] [PubMed] [Google Scholar]
- 47.Bell GM, Gordon AC, Lee P, Doig A, MacDonald MK, Thomson D, Anderton JL, Robson JS: Proliferative glomerulonephritis and exposure to organic solvents. Nephron 40: 161–165, 1985 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.