Abstract
Objectives
1) Develop unidimensional instruments to measure osteoarthritis (OA) knowledge among people with hip or knee OA, and 2) assess the structural validity, internal consistency, cross-cultural validity/measurement invariance, test-retest reliability, and measurement error of the Hip Osteoarthritis Knowledge Scale (HOAKS) and the Knee Osteoarthritis Knowledge Scale (KOAKS).
Methods
Draft HOAKS and KOAKS were developed and refined following best-practice (COSMIN) guidelines with involvement of consumer research partners. Measurement properties of the HOAKS and KOAKS will be assessed through an online survey. The survey will include the novel HOAKS or KOAKS, the current short form of the Hip or Knee injury and Osteoarthritis Outcome Score (HOOS-12/KOOS-12), and items that gather demographic and OA characteristics and explore self-rated OA knowledge. People will be eligible to participate if aged 18 years and older, can communicate in English, and have either hip or knee OA as diagnosed by a health professional or by meeting diagnostic criteria. We aim to obtain 400 complete HOAKS or KOAKS responses and 100 complete HOAKS or KOAKS retest responses one week after initial completion. Rasch analysis will estimate structural validity, internal consistency and cross-cultural validity/measurement invariance. Assessment will include test-retest reliability (intraclass correlation coefficient) and absolute measurement error (standard error of measurement; smallest detectable change).
Conclusion
This study will produce robust unidimensional instruments to measure hip and knee OA knowledge. We anticipate that the HOAKS and KOAKS scales will be useful in clinical and research settings to identify knowledge gaps or evaluate interventions designed to improve knowledge.
Keywords: Hip osteoarthritis, Knee osteoarthritis, Knowledge, Patient-reported outcome measure, Protocol, Survey
1. Introduction
Hip and knee osteoarthritis (OA) are major contributors to disability, health loss, and healthcare costs [[1], [2], [3]]. Activation to self-manage is central to the healthcare of people with long-term conditions like OA [4,5]. Knowledge and beliefs about OA influence people’s support seeking, physical activity levels, social and leisure participation, emotional well-being, and selection of management options [[6], [7], [8], [9]]. Knowledge and information can assist people to understand their health, improve their management choices, and identify helpful behaviours [4,10,11]. Education is a core component of all international OA management guidelines [[12], [13], [14], [15]].
There are currently no psychometrically robust tools to measure OA knowledge; this hinders the identification of an individual’s knowledge gaps and the evaluation of interventions designed to improve knowledge. The OA Patient Knowledge Questionnaire (PKQ-OA), which focuses on disease and drug knowledge, has previously been described [16]. The internal consistency and test-retest reliability of the PKQ-OA were only tested in a small sample and construct validity was not reported. PKQ-OA limitations include a challenging scoring system (structured as a multi-choice quiz), limited exploration of symptom interpretation or core recommended interventions of exercise and weight management, and not being publicly available.
We aimed to develop novel unidimensional instruments to enable measurement of OA knowledge – the Hip Osteoarthritis Knowledge Scale (HOAKS) and the Knee Osteoarthritis Knowledge Scale (KOAKS). Here we describe the development process and assessment of content validity already undertaken (Part A), and the protocol for the subsequent testing of the structural validity, internal consistency, cross-cultural validity/measurement invariance, test-retest reliability, and measurement error of the HOAKS and KOAKS (Part B).
2. Part A: scale development and content validity
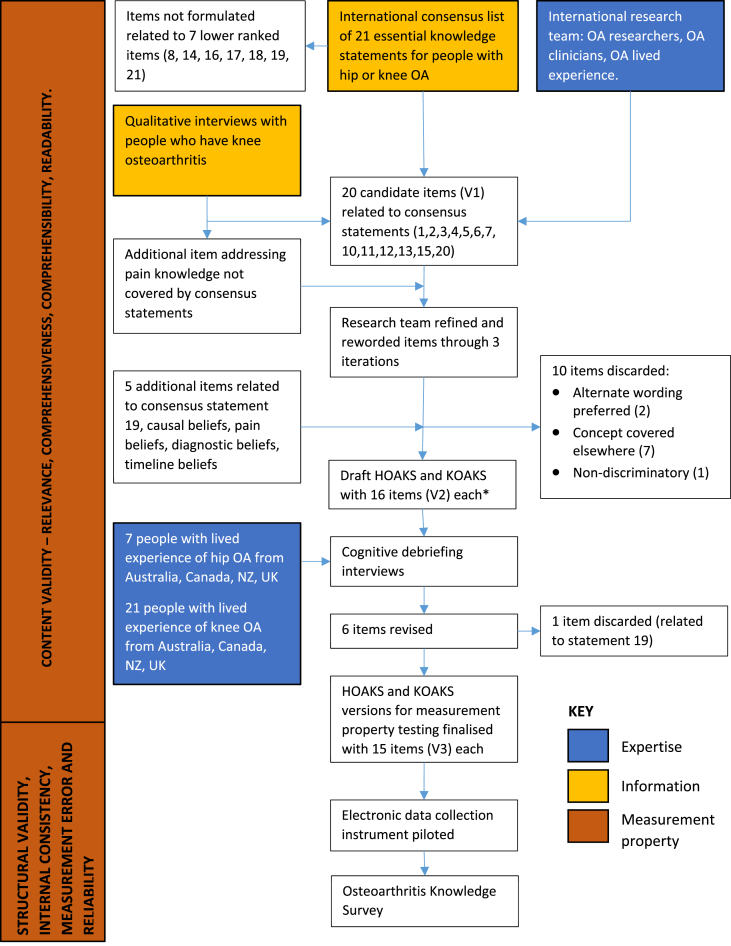
The HOAKS and KOAKS were designed to measure OA knowledge in persons with hip or knee OA of any severity, at any stage in the natural history, and in a variety of settings (from community to tertiary care). They are intended for use in research, self-management programmes, and clinical settings. The development of the knowledge scales followed COnsensus-based Standards for the selection of health Measurement INstruments (COSMIN) recommendations [17] and is presented in Fig. 1.
Fig. 1.
Development of the Hip Osteoarthritis Knowledge Scale (HOAKS) and Knee Osteoarthritis Knowledge Scale (KOAKS). ∗ The HOAKS and KOAKS have identical wording except for references to ‘hip’ or ‘knee’.
The HOAKS and KOAKS were initially developed by a group of twelve OA experts from Australia, Canada, New Zealand, and the United Kingdom. This core group included OA researchers and clinicians with backgrounds in chiropractic, health coaching, physiotherapy, psychology and psychometrics, and a person with lived experience of OA. Expertise and diversity characteristics of this group are presented in Table 1.
Table 1.
Characteristics of the core group who developed the osteoarthritis knowledge scales.
| Characteristic | Core group representation |
|---|---|
| Gender | Male 6 (BD, JHA, AMB, SF, CK, DOB) |
| Female 6 (KB, MB, JC, SD, RSH, JLW) | |
| Country | Australia 4 (KB,AMB,SF,RSH) |
| Canada 1 (JW) | |
| New Zealand 6 (BD,JHA,MB,JC,CK,DOB) | |
| United Kingdom 1 (SD) | |
| Discipline | Chiropractic 1 (SF) |
| Consumer research partner 1 (JC) | |
| Health coaching (MB) | |
| Physiotherapy 8 (BD,JHA,KB,AMB,SD,RSH,DOB,JW) | |
| Psychology 2 (SD,CK) | |
| Career | Clinician researcher 2 (BD,AMB) |
| Researcher 9 (JHA,KB,MB,SD,SF,RSH,CK, DOB,JLW) | |
| Consultant 1 (JC) | |
| Authored hip and/or knee OA paper in peer reviewed journal | Yes 10 (BD,JHA,KB,AMB, MB,SD,SF,RSH,DOB,JLW) |
| No 2 (CK,JC) | |
| Presented about hip and/or knee OA at international conference | Yes 6 (JHA,KB,AMB,SF,RSH,JLW) |
| No 6 (BD,MB,JC,SD,CK,DOB) | |
| Held nationally competitive grant funding as the lead Chief Investigator for a clinical research grant investigating hip and/or knee OA | Yes 6 (BD,JHA,KB,AMB,RSH,JW) |
| No 6 (MB,JC,SD,SF,CK,DOB) | |
| Developed or tested measurement instrument | Yes (BD,JHA,AMB,KB,SD,SF,RSH,CK,DOB,JLW) |
| No (MB,JC) |
OA, osteoarthritis.
International consensus of essential knowledge for people with hip or knee OA formed the conceptual framework for content inclusion [18]. The international consensus emerged from a Delphi process involving 51 OA experts (clinicians and researchers) from 13 countries and nine persons with lived experience of OA from Australia [18]. The consensus statements were reviewed in relation to findings from recent qualitative research [8,9].
Twenty candidate items (V1) for inclusion in the instruments were drafted to reflect the content of consensus statements (the development of items is presented in Appendix A) [18]. Some statements were challenging due to their compound nature with multiple concepts that were unable to be reflected through a single item; these required multiple candidate items that related to each concept. Other statements were challenging because they included technical terms or complex words (such as ‘cartilage’, ‘ligaments’, ‘individualised’, ‘mechanical blocking’) that may be unfamiliar to people with OA. Candidate items were not developed in relation to seven lower ranked consensus statements due to difficulties developing these into readable items or consensus that these were unlikely to be discriminatory. An additional item was drafted to reflect pain knowledge (not represented in the consensus statements) that qualitative research indicated was important for informing approaches to OA self-management [8]. Candidate items were then discussed and refined by the core group through multiple iterations and consensus to develop V2 items. As part of this process, additional items were suggested to reflect knowledge about causation, pain, diagnosis, and disease course that had emerged as important in qualitative research [8,9], and one consensus statement initially excluded. Ten items were discarded when alternate wording was preferred, the item was expected to be non-discriminatory, or the content was adequately covered by other items. V2 items were used to create draft instruments. Items were initially drafted in relation to knee OA for use in the KOAKS. The HOAKS items were created by replacing the word ‘knee’ with the word ‘hip’.
To facilitate navigation and completion, the scale is presented in three sections containing conceptually related information: statements about hip/knee joint OA; statements about what you should do if you have hip/knee OA; and statements about treatment for hip/knee OA [19]. Each item presents a statement with a five-point Likert scale scored from 1 to 5. Respondents are asked to rate each statement as False (1), Possibly False (2), Unsure (3), Possibly True (4), or True (5) [20]. Each scale point is labelled with a descriptive title, rather than numeric value, to increase reliability and reduce positivity bias [19]. In contrast to dichotomous true-false responses, Likert scales allow gradation of knowledge and may enable the tool to be more discriminative or indicate areas of uncertainty in which OA messaging could be more clear [20]. Recent psychometric analysis has demonstrated that response patterns from this answer format can be adequately analysed by Rasch analysis [21]. Items were all worded positively to reduce cognitive load but scored in both forward (True end of response choices indicating better knowledge), and reverse directions (False end of response choices indicating better knowledge).
Readability was assessed through the Readability Test Tool (https://www.webfx.com/tools/read-able/). We aimed for items to be understandable by those with a reading age of 12 years; however, this was not able to be achieved with all items. Terms for which the research team was not able to come up with acceptable alternatives (including ‘exercise’, ‘ability’, and ‘medications’) resulted in higher reading age requirements for three items included in the final version (10, 12, 14).
2.1. Patient and public involvement
A person with lived experience of knee OA (JC), who had been involved in our previous research, joined the research team as a consumer research partner. As part of the research team they contributed to scale development and conducted cognitive debrief interviews. JC was paid for their time.
The draft instruments (with V2 items) were further developed with the assistance of an additional 28 research partners with lived experience of OA from Australia (n = 12), Canada (n = 3), New Zealand (n = 7), and the United Kingdom (n = 5) (see Acknowledgements). Research partners, who were not paid for their time, were identified through databases of previous research participants (Australia and NZ), the Arthritis Research Canada Arthritis Patient Advisory Board, and the Patient for Research Panel at the Exeter (UK) Knee Reconstruction Unit. They ranged in age from 44 to 80 years old, were predominantly women (72%), were predominantly Caucasian (Māori and Samoan people participated in NZ), and had experienced joint pain for 3–40 years. Fourteen research partners had received joint replacements. Research partners participated in cognitive debriefing interviews to assess scale relevance, comprehensiveness, and comprehensibility. Research partners were presented with the scale in the presence of a member of the research team (either face-to-face or by telephone/videoconference) and were asked to think aloud as they completed the questionnaire and talked about how they understood each item. They were then asked about ease of completion, suggestions for improvement, items that were repetitious or unimportant, and any important areas of arthritis knowledge that had not been included. A member of the research team (BM [Australia], JW [Canada], JC or MB [New Zealand], SD [United Kingdom]) manually recorded comments provided by these research partners. The feedback was then collated, tabulated, and discussed by the research team to enable a final consensus to be achieved (V3 items). Six items were revised based on the cognitive debriefing interviews. One item related to arthroscopy was discarded because those without first-hand experience of the procedure usually did not know what the statement was referring to and those who had first-hand experience based their answer on their individual response to the intervention (or that of someone they knew). For these reasons, the research team concluded that the item could not be meaningfully answered by much of the target population. Some additional areas of OA knowledge were suggested by a small number of research partners (such as the impact of weather or the benefits of specific injections, medications, or supplements), but the majority of people did not suggest additional areas to include.
3. Part B: protocol for assessing the measurement properties of the HOAKS and KOAKS
3.1. Aim
We aim is to test the structural validity, internal consistency, cross-cultural validity/measurement invariance, test-retest reliability, and measurement error of HOAKS and KOAKS.
3.2. Participants
People will be eligible to participate in the OA Knowledge Survey if they are 18 years of age or older, can communicate (read and write) in the English language, and have a diagnosis of hip or knee OA made either by a health professional or by meeting UK National Institute for Health and Care Excellence (NICE) hip or knee diagnostic criteria (assessed by questionnaire; see Table 2) [15,22,23]. Although people need to be over 45 years of age to fulfil NICE criteria, those aged between 18 and 44 years who have been diagnosed with hip or knee OA by a health professional will be eligible to participate. Allowing inclusion of people diagnosed by both mechanisms will expand the pool of eligible people. There are no exclusion criteria. People will be able to participate in the OA Knowledge Study if their hip or knee joint has been replaced.
Table 2.
NICE OA diagnostic criteria [19] (as used in this study).
| NICE hip OA diagnostic criteria: | NICE knee OA diagnostic criteria: |
|
|
NICE, United Kingdom National Institute for Health and Care Excellence; OA, osteoarthritis.
3.3. Recruitment
Potential participants will be invited to access the webpage describing the project (www.otago.ac.nz/oaks) through social media, arthritis consumer advocacy groups (such as Arthritis New Zealand, Arthritis Australia, Arthritis Research Canada, Versus Arthritis), and invitations sent to existing OA consumer databases for which appropriate consent and approvals exist. Health professionals will be asked to post recruitment posters in their clinics.
All respondents will be screened for hip and knee OA by asking (for each hip or knee joint in which they report pain) whether they a) have been diagnosed with OA by a health professional or b) meet NICE diagnostic criteria. Those who report more than one joint with a diagnosis of OA or meeting NICE diagnostic criteria will be asked which is most troublesome; they will be asked to complete the survey about this joint only. Pain, function, and quality of life items will adapt to refer specifically to this most troublesome joint.
No compensation or remuneration will be provided for taking part. All participants completing the survey who choose to provide their email address will receive an email to thank them for their time. Participants will be able to indicate that they wish to receive a copy of the study results by email.
3.3.1. Sample size
Consistent with COSMIN recommendations, we aim to obtain at least 400 complete HOAKS/KOAKS responses and at least 100 complete HOAKS/KOAKS retest responses [17]. This sample size will be adequate for Rasch analysis and enable us to state with 99% confidence that item calibration is within +0.50 logits [24]. All participants will be invited to the retest phase to provide the most precise estimate of test-retest performance available from this study. A sample size of n = 100 will provide a 95% confidence interval on the ICC between ± 0.07 (when ICC = 0.8) to 0.1 (when ICC = 0.7) [25]; a larger achieved sample size will return a narrower confidence interval.
3.4. Measures
The survey instrument will include the novel HOAKS or KOAKS, the existing short form of the Hip or Knee injury and Osteoarthritis Outcome Score (HOOS-12 or KOOS-12), demographic and OA disease characteristics items, and a single item exploring self-rated OA knowledge.
3.4.1. Hip or Knee Osteoarthritis Knowledge Scale (HOAKS or KOAKS)
The HOAKS and KOAKS use the same items with the only difference being the replacement of the word ‘hip’ with ‘knee’. The HOAKS and KOAKS currently each have 15 items that all relate to the construct of OA knowledge and are conceived as unidimensional scales (Appendix A). Seven items are scored in the forward direction and eight items are scored in the reverse direction. Total scores for the scale range from 15 to 75 and higher scores indicate greater knowledge about OA.
3.4.2. 12-Item short form of the Hip or Knee injury and Osteoarthritis Outcome Score (HOOS-12/KOOS-12)
The HOOS-12 and KOOS-12 are psychometrically robust 12-item instruments that have three domains (4 items each) exploring hip/knee-related pain, function, and quality of life [26]. These instruments were selected because they are relatively brief, are appropriate across the disease course, and provide valid domain scores as well as a total summary score.
3.4.3. Self-rated knowledge about osteoarthritis
Respondents will be asked to self-rate their OA knowledge using an item from the Brief Illness Perception Questionnaire (B-IPQ) [27]; ‘How well do you feel you understand knee/hip osteoarthritis?’ with response options from 0 (don’t understand at all) to 10 (understand very clearly). This item was selected as it came from a validated instrument previously used in the target population [28].
3.4.4. Demographic and osteoarthritis disease characteristics data
Respondents will also be asked to provide demographic and OA disease characteristics data to enable description of the sample, exploration of differential item functioning (DIF), and sensitivity analyses. Data collected and response categories are presented in Table 3.
Table 3.
Demographic and osteoarthritis disease characteristics items to be collected and response categories.
| Item | Response categories |
|---|---|
| Birth year | Year (1900–2002) |
| Gender | Male |
| Female | |
| Gender diverse | |
| Prefer not to answer | |
| Ethnicity | Free text (no response option framework appropriate for all countries) |
| Country of residence | All countries |
| Socioeconomic circumstance [41] | 1 Not at all difficult |
| 2 | |
| 3 | |
| 4 | |
| 5 Extremely difficult | |
| Rurality | Urban |
| Rural 1 (25–60 min travel to urban centre of 30,000 people or more) | |
| Rural 2 (60–90 min travel to urban centre of 30,000 people or more) | |
| Rural 3 (>90 min travel to urban centre of 30,000 people or more) | |
| Highest level of education | Some secondary education (high school) |
| Completed secondary education (graduated high school) | |
| Trade/technical/vocational training | |
| Some undergraduate education (college or university) | |
| Completed undergraduate education (college or university) | |
| Some postgraduate education | |
| Completed postgraduate education (masters or doctorate) | |
| Other (please specify) | |
| Occupation | Manager |
| Professional | |
| Technician or Trades Worker | |
| Community or Personal Service Worker | |
| Clerical or Administrative Worker | |
| Sales Worker | |
| Machinery Operator or Driver | |
| Labourer | |
| Homeworker | |
| Unemployed looking for work | |
| Unemployed not looking for work | |
| Student | |
| Retired | |
| Unable to work due to health reasons | |
| Other (please specify) | |
| Pain duration | Less than one year |
| One to two years | |
| Two to five years | |
| Five to ten years | |
| Ten to fifteen years | |
| Fifteen to twenty years | |
| More than twenty years | |
| Diagnosis of OA by health professional (multiple options may be selected) | Nil |
| Left hip | |
| Right hip | |
| Left knee | |
| Right knee | |
| Joint replacement and year (multiple options may be selected) | Nil |
| Left hip | |
| Right hip | |
| Left knee | |
| Right knee | |
| Where received OA information (multiple options may be selected) | No information received |
| GP or family doctor | |
| Surgeon | |
| Another doctor (such as sports doctor or rheumatologist) | |
| Nurse | |
| Physiotherapist or physical therapist | |
| Osteopath | |
| Chiropractor | |
| OA rehabilitation programme | |
| Arthritis educator | |
| Arthritis support group | |
| Other people with OA | |
| Family or friends | |
| Internet/website | |
| Television | |
| Information booklets | |
| Other (please specify) |
3.5. Patient and public involvement
A person with lived experience of knee OA (JC), who was also involved in scale development, contributed to survey development and project design, and will continue to contribute to data interpretation, reporting, and communication to consumer groups. JC was paid for their time.
3.6. Data collection
Data collection will be via a secure Qualtrics online survey that can be completed with using a range of electronic devices (e.g. PC, laptop, tablet, mobile phone). The survey settings will ensure that only one survey can be submitted from a unique device.
Data collection commenced October 21, 2020. The survey will remain open until the recruitment target has been met. Participants will be asked for consent to be sent an invitation to complete the HOAKS or KOAKS a second time, at one week after initial completion to enable assessment of test-retest reliability. Those who are sent this retest invitation will have up to one week to complete this second survey. One week (minimum response interval) has been deemed sufficient to assume that participants will not be able to recall their first set of responses and two weeks (maximum response interval) is given as the upper limit so that respondents’ clinical status will be stable between the test and retest data collection (given OA is a long-term condition) [17]. Beyond the eligibility criteria there are no further quotas or restrictions on participation by country or other demographic groupings.
3.7. Data management
Quantitative electronic data will be stored on a secure Qualtrics™ data centre server (located in Sydney, Australia) during the data collection, accessible only to the lead author (BD).
3.7.1. Privacy and confidentiality
Each participant will be automatically allocated a unique ID by Qualtrics. Some identifying information will be collected to enable the delivery of retest surveys, results summaries, and requests to participate in further research (for those who consent to these actions). Following completion of data collection, identifying details (name and email address) will be removed from the analysis file prior to sharing amongst the research team. Following completion of research team analyses, anonymised data will be added to a publicly accessible registry.
3.8. Data analysis
3.8.1. Demographic and OA disease characteristics
Demographic data will be analysed descriptively. Data for each demographic characteristic will be presented separately for HOAKS and KOAKS respondents. Response data will be presented as: the number who commenced screening, the number who were screened as eligible (for each survey or both), the number included in HOAKS/KOAKS (test and retest) and HOOS-12/KOOS-12 analyses.
3.8.2. Missing data
Missing data will be handled by conducting complete case analysis. The survey software will request that any unanswered items are completed to minimise missing data, but responses will not be forced to reduce the risk of early survey termination. The number included in each analysis will be reported. Patterns of missing data will be analysed. The intended psychometric analyses (Rasch analysis) do not require imputation of missing values.
3.8.3. Scale scores
Descriptive statistics will be calculated for HOAKS and KOAKS item scores and total scale scores (reversed item scores [items 1,2, 4, 5, 6, 10, 11, 15] will be corrected prior to total score calculation). A minimum of 12 item responses will be required to calculate a total score: those with at least 12 items (but less than 15) will have their total score derived by person-mean substitution, substituting values for these missing items with their mean score for completed items [29].
Descriptive statistics will be calculated for HOOS-12/KOOS-12 domains (hip/knee-related pain, function, and quality of life) and the total scale scores for these instruments. A minimum of 3 item responses will be required in each subscale (and to calculate a total score): those with at least 3 items (but less than 4) will have their subscale score derived by person mean substitution, in line with the HOOS-12 instructions [26].
3.8.4. Measurement properties
Measurement properties that will be analysed are structural validity, internal consistency, cross-cultural validity/measurement invariance, reliability, and measurement error (Table 4) [17]. Content validity has been achieved through the development process, following best practice [17].
Table 4.
Measurement properties for patient-reported outcomes from the COSMIN checklist [17] to be analysed in this study and analytic technique.
| Measurement property | Analysed | Analytic technique |
|---|---|---|
| Content Validity | NA (criterion met through previous work [described in Part A]) | |
| Structural validity | Yes | Rasch analysis |
| Internal consistency | Yes | Rasch analysis - person separation index |
| Cross-cultural validity/measurement invariance | Yes | Rasch – differential item functioning |
| Measurement error | Yes | Standard error of measurement and smallest detectable change |
| Test-retest reliability | Yes | Intraclass correlation coefficient |
| Criterion validity | No (no gold standard for knowledge exists) | |
| Hypothesis testing for construct validity | No (no appropriate comparator for knowledge exists) | |
| Responsiveness | No (this study will not assess knowledge change over time) |
HOAKS and KOAKS data will be pooled for analyses as: 1) these scales were developed from the same set of knowledge statements; and 2) it is intended that the same items will be included in each scale (excepting the use of ‘hip’ or ‘knee’) for ease of application. HOAKS and KOAKS data will be analysed separately if the participant groups completing each scale are significantly different in terms of age, gender, duration of symptoms, HOOS-12/KOOS-12 score, joint replacement status, or if differential item functioning analysis indicates a significant effect for joint. As each respondent will only be providing data for one joint, all observations will be independent.
Prior to any statistical analyses, the data will be screened for response sets as well as floor and ceiling effects. Psychometric analyses will be conducted with Partial-Credit Rasch analysis using RUMM2030 software [30]. While Rasch analysis will provide the main approach for psychometric analyses, prior auxiliary analyses such as exploratory or confirmatory factor analysis may be conducted if required [31].
3.8.4.1. Rasch analysis
Rasch analysis will be conducted in an iterative fashion to assess structural validity, internal consistency and cross-cultural validity/measurement invariance [32]. A satisfactory fit to the Rasch model will be indicated by a non-significant (p > .05, Bonferroni adjusted) chi-square fit statistic for item-trait interaction. The acceptable range for fit residuals of individual items is considered to be −2.50 to 2.50 [30].
In order to retain maximum usability of the scale in subsequent administration and scoring, items with severely disordered thresholds (where response categories of an item are not predicting respondents’ latent traits in an orderly fashion) will be deleted rather than re-scored. While this increases the likelihood that items will be deleted, this will be offset by the use of subtests to address local dependency between items [33]. Item deletion will also be informed by item locations such that items will be preferably retained if they represent a unique level of item difficulty or may be more likely deleted if another item already covers a very similar level of difficulty. At each iterative model fit, the residuals correlation matrix will be inspected for evidence of local dependency: a residual correlation between a pair of items will be indicated when any correlation coefficient in this matrix exceeds 0.20 of the mean of all correlation coefficients [34].
The subtest approach will allow exploration of the dimensionality of the scale. Rasch analysis distinguishes between local trait dependency due to underlying dimensionality and local response dependency, which occurs when the response to one item is influenced by the response to another item, such as a method effect [33]. The presence of each type of dependency will be explored by entering locally dependent items into so-called subtests. If the resulting model is unidimensional, it can be concluded that local response dependency has been successfully resolved [35], and that there is no evidence of local trait dependency.
At each iterative step, dimensionality will be tested using the method proposed by Smith [36]. Internal consistency reliability will be estimated in terms of person separation index, which is interpreted as equivalent to Cronbach’s alpha [37]. Differential item functioning analyses will investigate the extent to which items or subtests perform similarly by completion of HOAKS or KOAKS versions of the scale or by the personal factors: gender (male, female, gender diverse); age (<50 years, 50–70 years, >70 years); country (provided sufficient responses are obtained from a range of countries); pain duration (<2 years, 2–5 years, 6–10 years, > 10 years); formal OA diagnosis (yes, no); and joint replacement status (yes, no). Lastly, once a suitable model has been found, algorithms will be generated that allow transformation of ordinal-level scores of the scale to interval-level scores, which serves to increase precision of the scale and render it suitable for parametric statistical analyses [37].
3.8.5. Test-retest reliability
For the HOAKS/KOAKS, the intraclass correlation coefficient (ICC) will be calculated with 95% confidence interval using a two-way random effects model (ICC2,1 with absolute agreement) [17]. An ICC will be considered acceptable if the lower bound of the 95% confidence interval is 0.75 or higher [38]. We will use a Bland-Altman plot to report limits of agreement [39].
3.8.6. Measurement error
From the ICC calculated above, we will derive the standard error of measurement (SEM) and smallest detectable change (SDC) for the HOAKS/KOAKS [38].
3.8.7. Sensitivity analyses
Sensitivity analyses will explore whether mean total HOAKS/KOAKS scale scores or subgroup analyses were influenced by specific groups of respondents, including:
-
•
People diagnosed with OA by NICE criteria rather than by health professionals
-
•
People who have had any hip or knee joint replacement
-
•
People who have had a joint replacement of the joint about which they complete the survey.
3.9. Ethics
This Osteoarthritis Knowledge Survey (D20/301) was approved by the Department of Primary Health Care and General Practice, University of Otago, Wellington. A participant information sheet describing the study will be presented on the opening page of the survey. Participants indicate their consent to participate by advancing to the next page of the survey.
4. Discussion
The initial development of the HOAKS and KOAKS followed best practice guidelines for patient-reported outcome measurement instruments [17]. The construct of OA knowledge amongst people with hip or knee OA is clearly described. The conceptual framework was informed by international consensus statements of important knowledge [18]; the framework was expanded with items developed through additional qualitative research and to include domains of knowledge central to OA self-management, such as pain [8,9].
The structural validity of the draft scales will now be tested and developed using Rasch analysis to ensure the scales are unidimensional and can be analysed as an interval scale using parametric tests. Key measurement properties of internal consistency, cross-cultural validity/measurement invariance, measurement error and test-retest reliability will be reported to inform future users of the scales.
4.1. Strengths and limitations
The consensus list of essential OA knowledge statements that informed the theoretical framework for the HOAKS and KOAKS contained more statements pertaining to exercise and physical activity than any other construct. Although a number of these statements were consolidated when developing the HOAKS/KOAKS, the instruments remain biased toward physical activity and rehabilitative exercise (despite also exploring knowledge related to body weight, medication and surgery). Given engagement in physical activity and rehabilitative exercise is the health behaviour that is most amenable to change in the majority of people with OA, exploring this knowledge in more depth appears appropriate. Further item reduction may occur as a result of Rasch analysis.
Diverse groups of people who have OA and professionals from research and clinical backgrounds were included at all stages of this initial development (conceptual framework development, item development, item refinement) [40]. The consumer research partners who participated in scale development were from four different countries and had a broad range of symptom duration. A limitation of this group is that most participants were Caucasian and no data were collected about their socioeconomic or health literacy status.
Respondents will only be able to complete the survey that assesses scale measurement properties electronically due to resource constraints; this will exclude those who do not have access to an internet-connected device. The recruitment strategy will use social media, arthritis advocacy networks, researcher databases, and clinicians in order to maximise the diversity of the sample. This recruitment strategy may result in differential levels of response from populations with hip or knee OA or from participants from particular geographical regions. Analyses will be adjusted as described to account for differential response.
Assessment of construct validity is prevented in this study by the lack of an existing gold standard for OA knowledge against which we can confidently assess the HOAKS and KOAKS. Future studies could compare HOAKS/KOAKS scores amongst those who have and have not completed evidence-based OA education.
4.2. Future use
We anticipate that the HOAKS and KOAKS scales will be useful in clinical and research settings to identify knowledge gaps or evaluate interventions designed to improve knowledge. These scales will be amenable to electronic or paper completion and will be translated into non-English languages.
Declaration of competing interest
All authors attest that there are no conflicts of interest.
Acknowledgements
The core research team gratefully acknowledge the contribution of people who have lived experience of OA to the development of this scale:
New Zealand: Blair Duckett, Annette Jensen, Irene Tawera, Moana Aiatu, Murray Leckie, Vivienne Grant.
Australia: Jacqueline de Ferranti, Rosalie Fields, Shirley Kenny, Liz Seaward, Brenda McGuire, Chrisy Dennis, June Bates, Roger Lloyd, Joan Barker, Gary Cantwell, Keri Jones, Angela Wray.
Canada: Members of the Arthritis Research Canada Arthritis Patient Advisory Board - Trish Silvester-Lee, Shannon McQuitty, Isla Steele.
United Kingdom: Patient for Research Panel at the Exeter Knee Reconstruction Unit including Dr David Hillebrandt and Graham Wills.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ocarto.2021.100160.
Contributor Information
Ben Darlow, Email: ben.darlow@otago.ac.nz.
Haxby Abbott, Email: haxby.abbott@otago.ac.nz.
Kim Bennell, Email: k.bennell@unimelb.edu.au.
Andrew M. Briggs, Email: a.briggs@curtin.edu.au.
Melanie Brown, Email: melanie.brown@otago.ac.nz.
Jane Clark, Email: jclark_73@yahoo.com.au.
Sarah Dean, Email: S.Dean@exeter.ac.uk.
Simon French, Email: simon.french@mq.edu.au.
Rana S. Hinman, Email: ranash@unimelb.edu.au.
Chris Krägeloh, Email: chris.krageloh@aut.ac.nz.
Ben Metcalf, Email: b.metcalf@unimelb.edu.au.
Daniel O’Brien, Email: daniel.obrien@aut.ac.nz.
James Stanley, Email: james.stanley@otago.ac.nz.
Jackie L. Whittaker, Email: jackie.whittaker@ubc.ca.
Contributions
All authors have made substantial contributions to the conception and design of the study, or acquisition of data, or analysis and interpretation of data. Jane Clark contributed her lived experience of OA throughout the project. Blair Duckett, Annette Jensen, Irene Tawera, Moana Aiatu, Murray Leckie, Vivienne Grant, Jacqueline de Ferranti, Rosalie Fields, Shirley Kenny, Liz Seaward, Brenda McGuire, Chrisy Dennis, June Bates, Roger Lloyd, Joan Barker, Gary Cantwell, Keri Jones, Angela Wray, Trish Silvester-Lee, Shannon McQuitty, Isla Steele, and members of Patient for Research Panel at the Exeter Knee Reconstruction Unit including Dr David Hillebrandt and Graham Wills contributed to HOAKS/KOAKS item development. All authors contributed to drafting the article or revising it critically for important intellectual content and approved the submitted version. A/Prof Ben Darlow (ben.darlow@otago.ac.nz) takes responsibility for the integrity of the work as a whole, from inception to finished article.
Funding
The study has not received any external funding. Sarah Dean’s time is partly supported by the National Institute for Health Research Applied Research Collaboration South West Peninsula. The views expressed in this publication are those of the author(s) and not necessarily those of the National Institute for Health Research or the Department of Health and Social Care, UK. Rana S. Hinman is supported by a National Health and Medical Research Council Senior Research Fellowship (#1154217). Kim Bennell is supported by a National Health and Medical Research Council Investigator Grant (#1174431). Funding received from a Health Research Council Grant (HRC19/675) led to support for Jane Clark’s time by the University of Otago. No funders had any role in study design, the collection, analysis or interpretation of data, the writing of the manuscript, or the decision to submit the manuscript for publication.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Vos T., Flaxman A.D., Naghavi M., Lozano R., Michaud C., Ezzati M., et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dieleman J.L., Cao J., Chapin A., Chen C., Li Z., Liu A., et al. US health care spending by payer and health condition. 1996-2016. JAMA. 2020;323:863–884. doi: 10.1001/jama.2020.0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vos T., Lim S.S., Abbafati C., Abbas K.M., Abbasi M., Abbasifard M., et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Small N., Bower P., Chew-Graham C.A., Whalley D., Protheroe J. Patient empowerment in long-term conditions: development and preliminary testing of a new measure. BMC Health Serv. Res. 2013;13:263. doi: 10.1186/1472-6963-13-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ministry of Health . second ed. 2016. Self-management Support for People with Long-Term Conditions. Wellington, NZ. [Google Scholar]
- 6.Holden D.M., Nicholls M.E., Young M.J., Hay P.E., Foster P.N. The role of exercise for knee pain: what do older adults in the community think? Arthritis Care Res. 2012;64:1554–1564. doi: 10.1002/acr.21700. [DOI] [PubMed] [Google Scholar]
- 7.Wallis J.A., Taylor N.F., Bunzli S., Shields N. Experience of living with knee osteoarthritis: a systematic review of qualitative studies. BMJ Open. 2019;9 doi: 10.1136/bmjopen-2019-030060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darlow B., Brown M., Thompson B., Hudson B., Grainger R., McKinlay E., et al. Living with osteoarthritis is a balancing act: an exploration of patients’ beliefs about knee pain. BMC Rheumatol. 2018;2:15. doi: 10.1186/s41927-018-0023-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bunzli S., O’Brien P., Ayton D., Dowsey M., Gunn J., Choong P., et al. Misconceptions and the acceptance of evidence-based nonsurgical interventions for knee osteoarthritis. A qualitative study. Clin. Orthop. Relat. Res. 2019;477:1975–1983. doi: 10.1097/corr.0000000000000784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahola A.J., Groop P.-H. Barriers to self-management of diabetes. Diabet. Med. 2013;30:413–420. doi: 10.1111/dme.12105. [DOI] [PubMed] [Google Scholar]
- 11.McCorkle R., Ercolano E., Lazenby M., Schulman-Green D., Schilling L.S., Lorig K., et al. Self-management: enabling and empowering patients living with cancer as a chronic illness. CA A Cancer J. Clin. 2011;61:50–62. doi: 10.3322/caac.20093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bannuru R.R., Osani M.C., Vaysbrot E.E., Arden N.K., Bennell K., Bierma-Zeinstra S.M.A., et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 2019;27:1578–1589. doi: 10.1016/j.joca.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 13.Kolasinski S.L., Neogi T., Hochberg M.C., Oatis C., Guyatt G., Block J., et al. American college of rheumatology/arthritis foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Rheum. 2019;72(2020):220–233. doi: 10.1002/art.41142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandes L., Hagen K.B., Bijlsma J.W., Andreassen O., Christensen P., Conaghan P.G., et al. EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis. Ann. Rheum. Dis. 2013;72:1125–1135. doi: 10.1136/annrheumdis-2012-202745. [DOI] [PubMed] [Google Scholar]
- 15.National Clinical Guideline Centre (UK) National Institute for Health and Care Excellence; London: 2014. Osteoarthritis: Care and Management in Adults (CG177) UK. [PubMed] [Google Scholar]
- 16.Hill J., Bird H. Patient knowledge and misconceptions of osteoarthritis assessed by a validated self-completed knowledge questionnaire (PKQ-OA) Rheumatology. 2006;46:796–800. doi: 10.1093/rheumatology/kel407. [DOI] [PubMed] [Google Scholar]
- 17.Mokkink L.B., Prinsen C.A., Patrick D.L., Alonso J., Bouter L.M., De Vet H., et al. COSMIN; Amsterdam, NL: 2019. COSMIN Study Design Checklist for Patient-Reported Outcome Measurement Instruments. [Google Scholar]
- 18.French S.D., Bennell K.L., Nicolson P.J., Hodges P.W., Dobson F.L., Hinman R.S. What do people with knee or hip osteoarthritis need to know? An international consensus list of essential statements for osteoarthritis. Arthritis Care Res. 2015;67:809–816. doi: 10.1002/acr.22518. [DOI] [PubMed] [Google Scholar]
- 19.Groves R.M., Fowler F.J., Couper M.P., Lepkowski J.M., Singer E., Tourangeau R. second ed. Wiley; Chichester: 2009. Survey Methodology. [Google Scholar]
- 20.Darlow B., Perry M., Mathieson F., Stanley J., Melloh M., Marsh R., et al. The development and exploratory analysis of the back pain attitudes questionnaire (Back-PAQ) BMJ Open. 2014;4 doi: 10.1136/bmjopen-2014-005251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krägeloh C., Medvedev O.N., Dean S., Stanley J., Dowell A., Darlow B. Rasch analysis of the back pain attitudes questionnaire (Back-PAQ) Disabil. Rehabil. 2020:1–8. doi: 10.1080/09638288.2020.1861484. [DOI] [PubMed] [Google Scholar]
- 22.Young J.J., Skou S.T., Koes B.W., Grønne D.T., Roos E.M. Comparison of clinical criteria for hip osteoarthritis in primary care: a cross sectional study from good life with osteoarthritis in Denmark (GLA:D) Osteoarthritis Cartilage. 2020;28:S406. doi: 10.1016/j.joca.2020.02.635. [DOI] [PubMed] [Google Scholar]
- 23.Skou S.T., Koes B.W., Grønne D.T., Young J., Roos E.M. Comparison of three sets of clinical classification criteria for knee osteoarthritis: a cross-sectional study of 13,459 patients treated in primary care. Osteoarthritis Cartilage. 2020;28:167–172. doi: 10.1016/j.joca.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Linacre J. Sample size and item calibration stability. Rasch Measurement Transactions. 1994;7:328. [Google Scholar]
- 25.Bonett D.G. Sample size requirements for estimating intraclass correlations with desired precision. Stat. Med. 2002;21:1331–1335. doi: 10.1002/sim.1108. [DOI] [PubMed] [Google Scholar]
- 26.Gandek B., Roos E., Franklin P.D., Ware J.E., Jr. A 12-item short form of the Knee injury and Osteoarthritis Outcome Score (KOOS-12): tests of reliability, validity and responsiveness. Osteoarthritis Cartilage. 2019;27:762–770. doi: 10.1016/j.joca.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 27.Broadbent E., Petrie K.J., Main J., Weinman J. The brief illness perception questionnaire. J. Psychosom. Res. 2006;60:631–637. doi: 10.1016/j.jpsychores.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 28.Hunter D.J., Hinman R.S., Bowden J.L., Egerton T., Briggs A.M., Bunker S.J., et al. Effectiveness of a new model of primary care management on knee pain and function in patients with knee osteoarthritis: protocol for THE PARTNER STUDY. BMC Muscoskel. Disord. 2018;19:132. doi: 10.1186/s12891-018-2048-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Downey R.G., King C.V. Missing data in Likert ratings: a comparison of replacement methods. J. Gen. Psychol. 1998;125:175–191. doi: 10.1080/00221309809595542. [DOI] [PubMed] [Google Scholar]
- 30.Andrich D., Sheridan B., Luo G. RUMM Laboratory; Perth, Australia: 2009. RUMM 2030. [Google Scholar]
- 31.Kim E., Krägeloh C.U., Medvedev O.N., Duncan L.G., Singh N.N. Interpersonal mindfulness in parenting scale: testing the psychometric properties of a Korean version. Mindfulness. 2019;10:516–528. doi: 10.1007/s12671-018-0993-1. [DOI] [Google Scholar]
- 32.Siegert R.J., Tennant A., Turner-Stokes L. Rasch analysis of the Beck Depression Inventory-II in a neurological rehabilitation sample. Disabil. Rehabil. 2010;32:8–17. doi: 10.3109/09638280902971398. [DOI] [PubMed] [Google Scholar]
- 33.Nilsson Å.L., Tennant A. Past and present issues in Rasch analysis: the functional independence measure (FIMTM) revisited. J. Rehabil. Med. 2011;43:884–892. doi: 10.2340/16501977-0871. [DOI] [PubMed] [Google Scholar]
- 34.Christensen K.B., Makransky G., Horton M. Critical values for Yen’s Q 3: identification of local dependence in the Rasch model using residual correlations. Appl. Psychol. Meas. 2017;41:178–194. doi: 10.1177/0146621616677520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Medvedev O.N., Titkova E.A., Siegert R.J., Hwang Y.-S., Krägeloh C.U. Evaluating short versions of the five facet mindfulness questionnaire using Rasch analysis. Mindfulness. 2018;9:1411–1422. [Google Scholar]
- 36.Smith E.V., Jr. Detecting and evaluating the impact of multidimensionality using item fit statistics and principal component analysis of residuals. J. Appl. Meas. 2002;3:205–231. [PubMed] [Google Scholar]
- 37.Tennant A., Conaghan P.G. The Rasch measurement model in rheumatology: what is it and why use it? When should it be applied, and what should one look for in a Rasch paper? Arthritis Care Res. 2007;57:1358–1362. doi: 10.1002/art.23108. [DOI] [PubMed] [Google Scholar]
- 38.Weir J.P. Quantifying test-retest reliability using the intraclass correlation coefficient and the SEM. J. Strength Condit Res. 2005;19:231–240. doi: 10.1519/15184.1. [DOI] [PubMed] [Google Scholar]
- 39.Martin Bland J., Altman D. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;327:307–310. doi: 10.1016/S0140-6736(86)90837-8. [DOI] [PubMed] [Google Scholar]
- 40.Brett J., Staniszewska S., Mockford C., Herron-Marx S., Hughes J., Tysall C., et al. Mapping the impact of patient and public involvement on health and social care research: a systematic review. Health Expect. 2014;17:637–650. doi: 10.1111/j.1369-7625.2012.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.International Network on Financial Education . In: Publishing O., editor. OECD; Geneva: 2012. Supplementary questions: optional survey questions for the OECD INFE financial literacy core questionnaire. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.