Summary
Objective
Osteoarthritis (OA) is a chronic degenerative disease of the joints characterized by articular cartilage degradation. While clear sex differences exist in human OA development, most pre-clinical research has been conducted solely in male animals, limiting generalizability of findings to both sexes. The objective of this study was to determine if sex impacts the progression and severity of OA in the rat medial meniscal tear (MMT) preclinical model used to surgically induce OA. It was hypothesized that differences would be observed between males and females following MMT surgery.
Design
An MMT model was employed in male and female Lewis rats to induce OA. Animals were euthanized 3 weeks post-surgery and EPIC-μCT was used to quantitatively evaluate articular cartilage structure and composition, osteophyte volumes and subchondral bone structure.
Results
Analysis of medial 1/3 articular cartilage, showed increased cartilage thickness and proteoglycan loss in the MMT of both sexes, when compared to sham. Both male and female MMT groups also saw increased subchondral bone mineral density and larger osteophyte volumes. Significant interactions between sex and OA development were seen in normalized cartilage volume (larger in females), and normalized total osteophyte volumes (larger in males).
Conclusion
This study demonstrates the viability of both sexes in the rat MMT preclinical OA model. Though clear differences exist, this model can be used to model OA development and evaluate sex as a factor in the efficacy of OA therapeutics.
Keywords: Osteoarthritis, MicroCT, Inbred lewis rats, Sex difference
1. Introduction
Osteoarthritis (OA) is the most prevalent chronic condition of synovial joints in humans and remains the leading cause of disability around the world [1]. OA is commonly characterized by degradation of the articular cartilage in the joints with accompanying disease phenotypes of subchondral bone sclerosis, marginal tissue growths on the joint periphery, in the form of osteophytes, and synovial inflammation [2]. The Worldwide Health Organization (WHO) estimates that OA affects 9.6% of men and 18.0% of women over the age of 60 [3]. Women commonly develop more severe disease phenotypes, associated with higher pain levels and more substantial reduction in function and quality of life, when compared to men [4,5]. There are numerous sex differences which can potentially be attributed to this disparity in disease incidence, but this area remains understudied and further investigation is critical in order to fully understand the mechanisms behind this phenomenon.
Two of the most well-established differences that persist in OA between sexes are anatomical make-up and hormone expression. Morphological differences between males and females in articular cartilage may serve as a major contributor to differences seen in OA progression. In humans, men have higher cartilage volume, surface area and thickness and higher bone mineral density relative to women [6,7]. Whole joint biomechanical parameters, particularly in the knee, is also different as women have increased mechanical loading due to differences in lower limb alignment and during activities like running have significantly greater knee loads and contact stress compared to men [8,9]. Men and women demonstrate markedly different expression of hormones (testosterone, estrogen, and leptin among others) and tend to have different physiological responses to the signaling cues generated by these hormones [[10], [11], [12], [13]]. Estrogen has a strong correlation with OA incidence where lower estrogen levels correlates with increased OA incidence [[14], [15], [16]]. This is commonly referred to as ‘menopausal arthritis’ as there is a dramatic increase in the prevalence and severity of OA in women coinciding with the onset of menopause [17]. Furthermore, hormone replacement therapy (HRT) has shown promise in slowing OA progression in animal models of OA as well as in the clinical setting [[14], [15], [16]]. While there is substantial data documenting the differences of OA progression between sexes in humans there remains a disparity of knowledge in the way sex effects OA progression in preclinical animal models. These models create the foundation for clinical research to build upon, are commonly used in mechanistic studies of the disease and are used to develop and test novel therapeutic approaches.
Animal models of OA range broadly from small animals like the mouse destabilization of the medial meniscus (DMM) and rat medial meniscal transection (MMT) to larger animals like goats, guinea pigs and even horses. Though sex is a critical factor in human OA development, most pre-clinical studies do not address sex differences when using animal models to study mechanisms or develop therapies for OA [18]. While sex differences in OA animal models have been addressed to a small extent in certain animal models, there is limited understanding of sex differences in OA development in rat models, even though these models are utilized in a fourth of all pre-clinical OA animal studies [[19], [20], [21], [22], [23], [24]]. Even though certain studies have documented the impact of sex on OA progression in rodent models, mainly mice, weight differences between sexes was not accounted for as a potential confounding variable [[24], [25], [26]]. Furthermore, it is important to note that while these mouse studies reached similar conclusions of increased cartilage degradation in male animals relative to females, these studies reported inconsistent findings for individual cartilage parameters and for osteophyte volumes and subchondral bone metrics [[24], [25], [26]]. The type of analysis conducted in this study has yet to be performed in the rat MMT model which is more often utilized to test novel therapeutic approaches for OA, as opposed to the mouse DMM models used in the previously mentioned studies [[24], [25], [26]].
The objective of the current study was to characterize sex differences in OA development in the common pre-clinical medial meniscus transection (MMT) surgically induced OA model in the Lewis rat. MMT surgery was performed in male and female rats and tibiae were quantitatively evaluated for compositional and structural changes in cartilage, osteophytes, and subchondral bone at the standard 3 week time point using contrast enhanced microCT (μCT) [20]. It was hypothesized that a difference would be seen in the progression of OA between male and female Lewis rats.
2. Methods
2.1. Surgical methods
All animal care and experiments were conducted in accordance with the institutional guidelines of the Atlanta Veteran Affairs Medical Center (VAMC) and experimental procedures were approved by the Atlanta VAMC Institutional Animal Care and Use Committee (IACUC) (Protocol: V002-18).
Male (n = 15) Lewis rats, weighing 300–350 g, and female (n = 15) Lewis rats, weighing 275–300 g (strain code: 004; Charles River), were ordered and acclimated to the environment for one-week post arrival to the VAMC. Sample size was calculated via an a priori power analysis to have a minimum n = 12 of total MMT animals to detect a significant difference between male and female OA progression. Exclusion criteria of clearly identifiable damage incurred during dissection and sample preparation in μCT scans led to exclusion of two male MMT animals and one female sham animal from analysis. The MMT pre-clinical model, a model of surgical instability, was conducted to induce OA in rats as previously described [20]. Briefly, isoflurane inhalation was used to anesthetize the animals and SR buprenorphine (ZooPharm, Windsor, CO) was injected subcutaneously at 1 mL/kg. A sterile site was prepared along the medial aspect of the femora-tibial joint, a skin incision was made and then a blunt dissection exposed the medial collateral ligament (MCL), which was then transected. A full thickness cut of the meniscus was made at the narrowest point. The musculature, soft tissue and skin were closed using 4.0 vicryl sutures and staple clips. Sham surgeries were also performed, where the MCL was transected but not the meniscus. Post-surgery, 10 mL of Lactated Ringer's was injected subcutaneously. At 7 days post-surgery, staples were removed from the incision area.
The rats were euthanized via CO2 asphyxiation 3 weeks post-surgery and the hind limbs were collected. At this time point, it has been established that early stage OA has developed in the animals [20]. The samples were stored in 10% neutral buffered formalin for fixation.
2.2. Sample preparation
To prepare the samples for analysis, muscle and connective tissue was removed and the femur was disarticulated from the tibia. The tibiae were removed from formalin and stored in PBS until scanning. Immediately before scanning, the tibiae were immersed in 30% Hexabrix 320 contrast reagent (NDC 67684-5505-5, Guerbet, Villepinte, France) for 30 min at 37 °C [27].
2.3. EPIC-μCT analysis of articular cartilage, osteophytes and subchondral bone
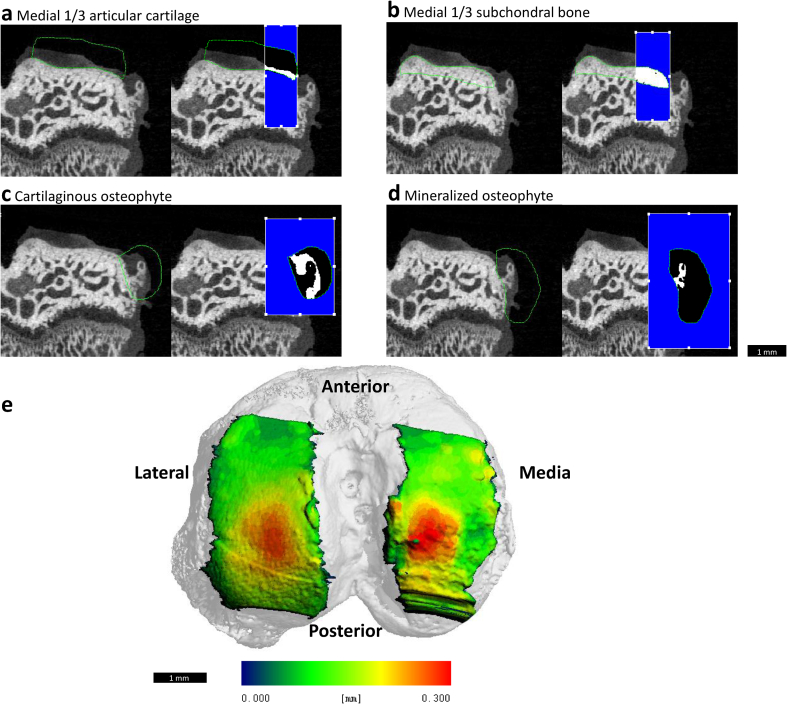
To quantitatively analyze the structure and composition of the articular cartilage and the morphology of osteophytes and the subchondral bone, equilibrium partitioning of an ionic contrast agent based micro-computed tomography (EPIC-μCT) analysis was utilized. The proximal tibiae were scanned with a Scanco μCT 40 (Scanco Medical, Brüttisellen, Switzerland). Scan parameters were: 45 kVp, 177 μA, 200 ms integration time, isotropic 16 μm voxel size, ~27 min scan time [27]. Raw scan data were automatically reconstructed into two-dimensional (2D) grayscale tomograms and orthogonally transposed to produce coronal sections yielding a 3D model reconstruction of the joint (Fig. 1e). Threshold values of 110 and 435 mg hydroxyapatite per cubic centimeter (mg HA/cm3) were used for evaluation of cartilage parameters (articular cartilage and cartilaginous osteophytes) and 435–1000 mg HA/cm3 for bone parameters (subchondral bone, mineralized osteophytes) to isolate the volume of interest (VOI) from surrounding air and tissue. Individual aspects of the joint: articular cartilage, subchondral bone and total osteophyte growth, were manually contoured on 2D coronal sections to isolate the region of interest (Fig. 1a–d). Calibration between microCT intensity and mg hydroxyapatite were calculated in previous studies and applied here [27].
Fig. 1.
Representative EPIC-μCT images depicting contoured volume of interest (VOI) for medial 1/3 articular cartilage and subchondral bone and mineralized and cartilaginous osteophytes. Representative coronal sections show rat medial tibial condyle with contours outlined in green and the medial 1/3 VOI for each respective aspect of the tibia highlighted in white for total medial (a) articular cartilage and (b) subchondral bone. Representative coronal sections show contours outlines in green and analyzed volumes highlighted in white for (c) cartilaginous and (d) mineralized osteophyte in MMT joint. Scale bar is universal for all representative images. (e) Articular cartilage thickness map of rat tibia overlaid on superior aspect of tibia indicating articular cartilage VOI. Heat map correlates with cartilage thickness (blue ~ 0.0 mm; red ~ 0.3 mm).
Coronal sections for articular cartilage were evaluated across the full length of the tibial condyle and analyzed specifically in the medial 1/3 region of the medial tibial condyle as this region has been shown to demonstrate the most damage in the MMT-induced OA model [28]. The Scanco μCT 40 generated values for articular cartilage volume, thickness and attenuation. Cartilage attenuation is inversely related to sulfated glycosaminoglycan (sGAG) content [27]. Cartilage degradation leads to lower levels of sGAG in the cartilage leading to higher concentrations of Hexabrix 320 contrast reagent in the extra cellular matrix of the cartilage. Higher Hexabrix corresponds with lower sGAG and higher attenuation levels [27]. Osteophyte volumes were evaluated using coronal sections generated by the scanning of the tibiae [29,30]. Coronal sections for the subchondral bone were evaluated across the full length of the tibial condyle and the medial 1/3 values were generated for subchondral bone volume, thickness, attenuation and porosity.
2.4. Surface roughness analysis of articular cartilage
To quantify surface roughness, coronal sections of scanned tibiae were exported from the Scanco μCT 40 as 2D TIFF images and imported into MATLAB R2016a (MathWorks, Natick, MA). A custom code created a 3D surface with these images by scanning section images sequentially and fitting the 3D surface along a previously generated 3D polynomial surface: fourth order along the ventral-dorsal axis and second order along the medial-lateral axis [29]. The surface roughness was quantified as the root mean square of differences between the 3D surface created with the exported TIFF images and the polynomial fitted surface. Surface roughness was calculated across the medial 1/3 region of the tibial plateau.
2.5. Histology
Representative tibiae were selected after being scanned and decalcified with formic/citrate decalcifying solution (Newcomer Supply 10429C, Middleton, WI) for 7 days. Samples were processed, paraffin-embedded, sectioned into 5 μm-thick slices and stained with hematoxylin and eosin (H&E; Fisherbrand™ 517-28-2, Waltham, MA) or safranin O and fast green (Saf-O; Electron Microscopy Sciences® 20800, Hatfield, PA), following manufacturer protocols.
2.6. Statistical analysis
All figures are presented as the calculated mean±standard deviation (SD). Significance was determined using two-way analysis of variance (ANOVA) test between groups with post-hoc Tukey Honest analysis for articular cartilage and subchondral bone parameters. Significance for osteophyte data sets was determined using two-way ANOVA with a non-parametric post-hoc Bonferroni analysis, as the osteophyte parameters did not follow a normal distribution. For all statistical analyses, significance was set at p < 0.05. Data were analyzed and significance was determined using GraphPad Prism software version 6.0 (GraphPad Software Inc., La Jolla, CA).
3. Results
3.1. Qualitative analysis of MMT induced OA
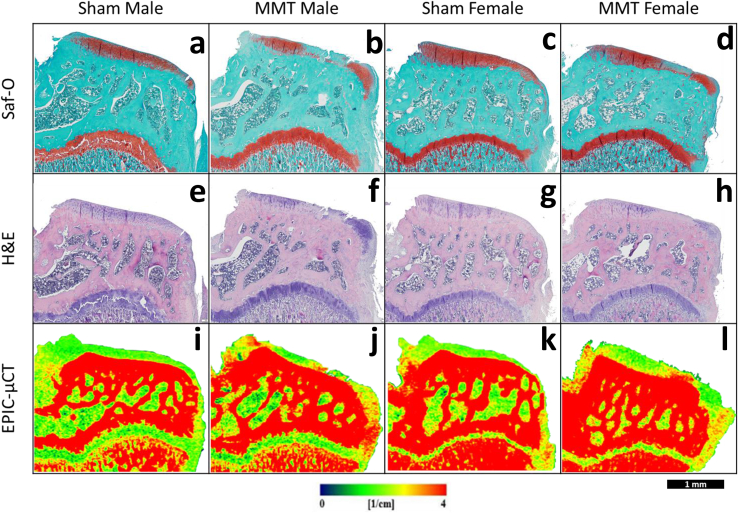
Histological staining of tibial sections by H&E and Saf-O was used to qualitatively assess the extent of damage caused by the MMT model. Samples from the sham groups for both males and females depicted intact cartilage with smooth superficial surfaces and relatively uniform proteoglycan content across the medial tibial condyle. Osteophytes were not apparent on the marginal edges of the joint for either of the sham groups. MMT samples from both sexes depicted large amounts of proteoglycan loss, apparent in qualitatively less Saf-O (red) staining, especially in the medial 1/3 region of the medial tibial condyle. Both Saf-O and H&E staining showed fibrillations and lesions on the cartilage surface in both males and females with MMT. MMT samples in both sexes also showed the presence of osteophytes along the medial edge of the tibiae, although no differences could be noted between their cartilaginous and mineralized portions (Fig. 2). EPIC-μCT images depicted similar qualitative observations in cartilage composition and structure and osteophyte formation as seen in the histological analyses (Fig. 2).
Fig. 2.
Histological and EPIC-μCT Representative coronal sections of rat medial tibial condyle for sham and MMT joints for both male and female animals. (a–d) Saf-O and (e–h) H&E stained rat medial tibia indicate the presence of fibrillations on the articular cartilage surface, osteophyte formation, proteoglycan loss (as depicted by absence of red coloration in (a–d) Saf-O stained samples) and loss in cell viability in articular cartilage layer (as depicted by absence of hematoxylin (violet coloration) in (e–h) H&E stained samples) for MMT (b, f) male and (d, h) female animals. Sham (a, e) male and (c, g) females animals show no damage. EPIC-uCT representative images show the presence of osteophytes, fibrillations and cartilage degradation (more red is inversely proportional to proteoglycan content) in (j, l) MMT samples and no damage in (i, k) sham samples. All images are oriented with medial aspect of the tibia on the right. Scale bar (bottom right corner) is universal for images.
3.2. EPIC-μCT quantitative analysis of medial 1/3 articular cartilage
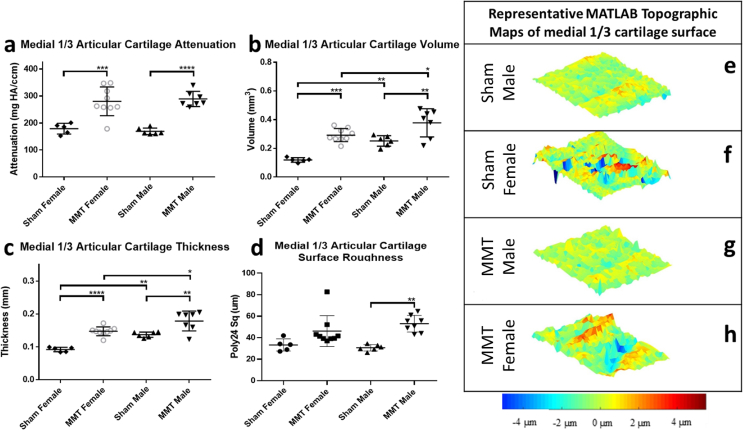
To quantitatively analyze OA progression in male and female rats in the MMT model, the medial 1/3 region of the articular cartilage of the medial tibial condyle was evaluated using EPIC-μCT. Cartilage damage has been shown to initiate in this region of the tibial condyle and is detectable by EPIC-μCT at the 3 week post-surgery time point in the MMT model (28). Induction of the MMT was shown to be a significant factor among both sexes resulting in increased cartilage proteoglycan loss (attenuation), surface roughness volume and thickness (Fig. 3a–c). However, sex was only a significant factor for extrinsic properties of the cartilage such as volume and thickness, with the male animals showing greater values than females (Fig. 3b and c). To account for the inherent size differences between male and female animals, cartilage volume and thickness values were weight normalized to permit direct comparison of the magnitude changes between sham and MMT animals between the two sexes (Supp. Fig. 1). Upon weight normalization, there was significant interaction between OA progression and sex and post-hoc analysis demonstrated higher magnitudes of cartilage volume and thickness in MMT females, relative to MMT males highlighting the presence of a potential sex difference.
Fig. 3.
EPIC-μCT quantitative analysis of medial 1/3 articular cartilage in sham and MMT joints from both sexes. (a) Medial 1/3 articular cartilage attenuation increased in MMT joints compared to sham joints for both male and female animals with no significant differences between male and female animals. Medial 1/3 articular cartilage (b) volume and (c) thickness increased in MMT joints compared to sham joints for both male and female animals with males showing larger values than females when comparing MMT males to MMT females and sham males to sham females for both parameters. Medial 1/3 articular cartilage surface roughness significantly increased quantitatively in only MMT male animals compared to male sham animals. (e–h) MATLAB generated representative topographic maps of medial 1/3 cartilage surface depict the deviation of a corresponding sample's cartilage surface renderings from a standardized 3D polynomial surface. Topographic maps show qualitatively increased surface roughness in MMT (f) male and (h) female animals compared to (e, g) shams. Data shown as mean±SD. n = 5 for sham females, n = 9 for MMT females, n = 6 for sham males and n = 7 for MMT males. ∗p < 0.05; ∗∗p ≤ 0.01; ∗∗∗p ≤ 0.001; ∗∗∗∗p ≤ 0.0001.
MATLAB analysis of cartilage surface roughness showed a significant increase in roughness for male animals but not female animals in the MMT group compared to the sham group (Fig. 3d). Qualitative analysis of the cartilage surface roughness, in the form of topographic maps, for all study groups were completed by subtracting individual 3D polynomial surfaces from the corresponding cartilage surface renderings yielded (Fig. 3 e-h).
3.3. EPIC-μCT quantitative analysis of osteophytes
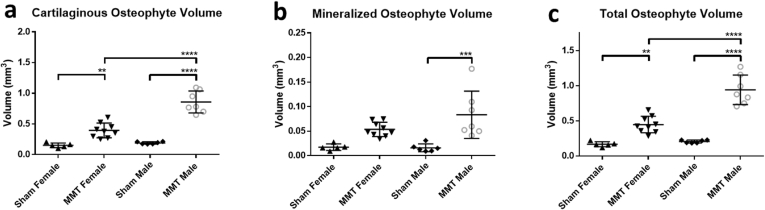
EPIC-μCT was used to quantitatively asses the volumes of osteophytes, defined as thickenings and partial mineralization's of the tissue at the marginal edge of the medial tibial plateau [31]. Cartilage volumes were subdivided into mineralized and cartilaginous components. Total osteophyte volumes were calculated as a sum of the component volumes. MMT induction was shown to be a significant factor in increasing cartilaginous, mineralized and total osteophyte volumes in both sexes. A significant interaction between sex and OA was observed for cartilaginous and total osteophyte volumes with males showing significantly larger volumes. However, sex was not observed to have an impact on mineralized osteophyte volumes. (Fig. 4a–c). To again account for the inherent size differences between male and female animals all osteophyte data sets were normalized between the sexes by dividing the respective volume measurements by the mass of the animals (Supp. Fig. 2). The persistence of a significant interaction between sex and OA induction in normalized data with males presenting larger osteophyte growths indicate another differences in OA progression seen between sexes in this model.
Fig. 4.
EPIC-μCT quantitative analysis of osteophyte formation on medial tibia of sham and MMT joints from both sexes. (a) Cartilaginous and (c) total osteophyte volumes were significantly increased in MMT joints compared to shams for both sexes with MMT male volumes significantly higher than MMT females. (b) Mineralized osteophyte volumes only saw a significant increase in MMT male animals compare to sham males. Data shown as mean±SD. n = 5 for sham females, n = 9 for MMT females, n = 6 for sham males and n = 7 for MMT males. ∗p < 0.05; ∗∗p ≤ 0.01; ∗∗∗p ≤ 0.001; ∗∗∗∗p ≤ 0.0001.
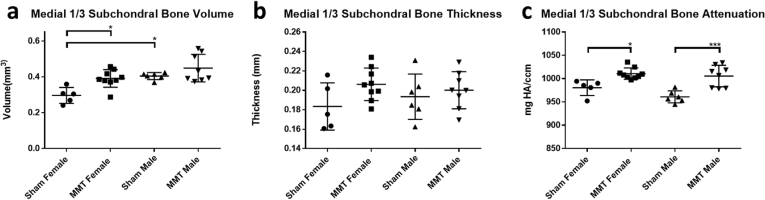
3.4. EPIC-μCT quantitative analysis of subchondral bone
EPIC-μCT was used to quantify the effects of MMT surgery on changes to the subchondral bone in the medial 1/3 region of the subchondral bone. Analysis of subchondral bone revealed the impact of MMT induction as a significant factor in increasing bone volumes and density (Fig. 5a, c). However, for thickness of the subchondral bone, there were no detectable differences between sham and MMT for either male or female animals (Fig. 5b). To account for the inherent size differences between male and female animals, as was done with articular cartilage and osteophyte analyses, subchondral bone volume was normalized by mass to permit direct comparison of the relative magnitude changes between sham and MMT animals between the sexes (Supp. Fig. 3). Normalized data showed sex as a significant factor, but it was female animals that demonstrated the larger increase in subchondral bone volumes due to OA development. However, no significant interaction between sex and OA induction was observed for any of the parameters measured in subchondral bone.
Fig. 5.
EPIC-μCT quantitative analysis of medial 1/3 subchondral bone in sham and MMT joints from both sexes. (a) Medial 1/3 subchondral bone volumes significantly increased in only MMT female animals compared to sham female animals and sham male animals showed significantly higher volumes than sham female animals. (b) Medial 1/3 subchondral bone thickness saw no significant increases in MMT joints compared to sham joints for both sexes and no significant differences were detected between male and female animals. (c) Medial 1/3 subchondral bone attenuation (density) increased in MMT joints compared to sham joints for both male and female animals with no significant differences between male and female animals. Data shown as mean±SD. n = 5 for sham females, n = 9 for MMT females, n = 6 for sham males and n = 7 for MMT males. ∗p < 0.05; ∗∗p ≤ 0.01; ∗∗∗p ≤ 0.001; ∗∗∗∗p ≤ 0.0001.
4. Discussion
Pre-clinical animal models of OA are commonly used to elucidate the underlying mechanisms of the disease and to assess the efficacy of therapeutics being developed to treat OA. Accounting for how critical factors influence changes over the course of disease progression permits more comprehensive conclusions to be drawn therefore allowing for more effective translation to be made towards the clinic. Sex of the individual may directly influence the efficacy of treatment and progression of disease but remains largely understudied. The objective of the current study was to characterize the effect of sex differences in OA progression in the MMT pre-clinical Lewis rat model.
The results of the current study showed that male rats had increased cartilage volume and thickness relative to females; these pre-clinical structural findings are consistent with previously published data from humans [6,32]. Animals, in the current study, were not weight or age matched due to the generally lower weight of female animals at a given age; animal weight varied significantly between the sexes with males having a mean weight of 325 g and females having a mean weight of 211 g. While this is a potential limitation of the study, regardless of the differences in cartilage morphology and body mass, both males and females developed OA after MMT surgery. Both sexes showed significant increases in cartilage attenuation (indicative of proteoglycan loss) volume and thickness (due to an influx of water following proteoglycan loss) in the MMT groups compared to their respective sham groups. These changes in joint morphology are indicative of successful MMT induction of OA.
The Lewis rat MMT model is commonly used for new therapeutic interventions and the presence of sex differences in this model for OA has not been previously investigated. Though other studies have characterized sex differences in other rodent models of OA these studies have primarily evaluated difference using semi-quantitative histopathology [24,26,33]. Histopathological analysis quantifies OA progression with different levels of severity, but it is difficult to normalize for differences in size and weight between sexes [24,33]. In our study, we utilized contrast enhanced μCT to quantitatively analyze structural and compositional features of OA pathogenesis which also allowed for the size differences between the animals to be used to normalize the data. Previous studies have suggested that male mice exhibit a higher degree of cartilage damage due to OA induction [24,26,33]. We observed similar increases in magnitudes of normalized articular cartilage volume in both male and female MMT cartilage volumes when compared to the corresponding shams. Conversely, normalized data indicated the presence of a significant interaction between sex and OA in regard to larger cartilage volume and thickness in females suggesting that OA can induce larger morphological changes in female animals for parameters when the size of the animal is considered as a factor. Compositional changes in the cartilage were also addressed, as sex was observed to not be a significant factor in proteoglycan loss due to OA, with both male and female animals showing statistically equivalent increases with OA induction. Overall, we saw significant structural and compositional impacts on the articular cartilage of the knee in both sexes of this preclinical OA model.
The formation of osteophytes in the medial aspect of the tibia is also an indicator of OA development and a key feature that is replicated in the pre-clinical rat MMT model. Though current understanding of osteophyte formation is limited, generally, osteophytes are defined as marginal tissue growths that form on the medial aspects of the tibial condyle and consist of cartilaginous and mineralized parts [34]. Increased body mass, which leads to increased loads on the knees, has been shown previously to increase osteophyte formation in the knee joint [35,36]. Quantitative analysis in the current study showed a significant increase in total osteophyte volume in both male and female MMT animals; male animals showed larger total osteophyte growth compared to females while MMT females did not show any significant increase in mineralized osteophyte volumes compared to shams. While these findings of larger osteophyte formations in male animals align with findings of a previous mouse study [25] other studies in mice have also indicated no differences in osteophyte development between sexes [26]. It is important to note that the latter study used OARSI scoring for osteophyte assessment while our study and previous studies indicating increased osteophyte development in male animals used μCT [25]. However, the effect of the confounding variable of weight and associated mechanical load changes on osteophyte formation was not addressed nor was the distinction between the different types of osteophyte growths [25,26]. Here we took into account the impact of weight on total osteophyte volumes, while also making a distinction between the key components of the growths in their cartilaginous and mineralized portions. When weight was used to normalize for osteophyte volumes in the current study, both sexes demonstrated significant increases in all osteophyte formations. However, male animals still yielded significantly larger total osteophyte volumes than female animals as demonstrated by a significant interaction between sex and OA factors in normalized and unnormalized data. The differences in osteophyte development may be attributed to the difference in weight between the two sexes, consistent with the general understanding of osteophyte formation. Furthermore, the increased joint size and size of the tibial condyle in male animals may potentially provide additional surface area from which the osteophytes could form [37]. While these differences are important to consider, our data shows that both sexes in this rat model developed osteophytes with mineralized and cartilaginous portions. Mineralized osteophyte formations are of particular importance in the clinic as X-rays are often unable to differentiate between cartilage and soft tissue since they are similar in composition and look identical when examined via X-ray [38].
The subchondral bone is another critical tissue and component of joint health that can be impacted during OA development and progression. As the disease progresses the subchondral bone will often undergo both hardening and thickening (sclerosis) [39]. In our current study, we assessed subchondral bone changes by analyzing subchondral bone morphometry and composition using μCT. μCT data showed that both male and female animals had an increase in bone mineral density after MMT surgery, suggesting the induction of sclerosis in this underlying bone for these animals. However, the absence of any significant interaction between OA and sex suggests no sex difference in regard to subchondral bone changes due to OA. While increased bone mineral density was observed in the current study, previous studies involving the male MMT models have demonstrated that significant morphological changes in the subchondral bone tend to emerge at later time points [40]. The MMT model used for OA induction yielded expected subchondral bone morphologies at this time point, and both males and females developed these changes in the subchondral bone. Later timepoints potentially would show more pronounced changes in the subchondral bone.
This study uniquely demonstrated that OA in the MMT model can be induced in both sexes and that overall, the development of OA in both sexes of this preclinical model was largely similar with certain aspects varying between male and female animals. Analyzing individual aspects of joint health allowed for comprehensive quantitative analysis of disease development in three dimensions and showed changes in articular cartilage, osteophytes and subchondral bone in both sexes. The analyses conducted showed intrinsic factors associated with OA development which are compositional in nature and independent of weight, such as attenuation and bone density, did not present any significant interaction between sex and OA factors; however, structural parameters associated with OA development, such as mineralized osteophyte and medial 1/3 subchondral bone and articular cartilage volumes presented significant interaction between sex and MMT. Additionally, hormonal and certain morphological differences could also be included in assessment of disease progression as these factors could also potentially contribute to the results observed in this study. These differences due to sex may have an impact on the effect of therapeutics which highlights the importance of including sex as a variable when developing new therapeutic approaches. Due to the utility of both sexes of the MMT model in replicating preclinical OA and assessing the efficacy of novel OA therapeutics, this study demonstrates that it is important to use both sexes in pre-clinical studies to ensure that therapies provide efficacy in treating OA in both sexes.
Authorship
This manuscript has been read and approved by all named authors. KAP, JMM and NJW contributed to the conception of this project’s study design. All names authors played a roll in any surgical procedures done for this study. MicroCT analysis was completed by KAP, JMM and JMF. Data analysis, figure assembly and manuscript drafting were done by KAP and JMM. All authors assisted with final manuscript editing and perpetration. NJW is the principle investigator and overlooked the project from conception to completion while also the obtain funding needed to carry out this project.
Declaration of Competing Interest
No conflict of interest exists for any of the authors.
Acknowledgement of other contributors
The authors would like to thank Mila Friedman for all the histological analysis done in this study. Additionally, we would like to thank Fabrice Bernard and Emily Devereaux for assistance with the surgical procedures conducted in this study and Colleen Oliver for her support with the live animal aspects of this study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ocarto.2020.100066.
Declaration of funding
Funding for this study was provided by VA (SPiRE) Grant I21RX002372-01A1 from the United States (U.S.) Department of Veterans Affairs Rehabilitation Research and Development Service.
Role of the funding source
Study sponcsors had no role outside of funding this publication.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Woolf A.D., Pfleger B. Burden of major musculoskeletal conditions. Bull. World Health Organ. 2003;81(9):646–656. [PMC free article] [PubMed] [Google Scholar]
- 2.Loeser R.F., Goldring S.R., Scanzello C.R., Goldring M.B. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012 Jun;64(6):1697–1707. doi: 10.1002/art.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anonymous . Who. World Health Organization; 2016. WHO | Chronic Rheumatic Conditions [Internet]https://www.who.int/chp/topics/rheumatic/en/ [cited 2018 Dec 20]. p. 1. Available from: [Google Scholar]
- 4.Srikanth V.K., Fryer J.L., Zhai G., Winzenberg T.M., Hosmer D., Jones G. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthritis Cartilage. 2005 Sep 1;13(9):769–781. doi: 10.1016/j.joca.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 5.Zhang W., Nuki G., Moskowitz R.W., Abramson S., Altman R.D., Arden N.K., et al. OARSI recommendations for the management of hip and knee osteoarthritis. Part III: changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis Cartilage. 2010;18(4):476–499. doi: 10.1016/j.joca.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 6.Faber S.C., Eckstein F., Lukasz S., Mühlbauer R., Hohe J., Englmeier K.H., et al. Gender differences in knee joint cartilage thickness, volume and articular surface areas: assessment with quantitative three-dimensional MR imaging. Skeletal Radiol. 2001;30(3):144–150. doi: 10.1007/s002560000320. [DOI] [PubMed] [Google Scholar]
- 7.Lee J.Y., Harvey W.F., Price L.L., Paulus J.K., Dawson-Hughes B., McAlindon T.E. Relationship of bone mineral density to progression of knee osteoarthritis. Arthritis Rheum. 2013 Jun 1;65(6):1541–1546. doi: 10.1002/art.37926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ro D., Han H., Kim B., Lee S., Lee M. Gender difference in knee joint loading: cross-sectional study in normal geriatric population. Osteoarthritis Cartilage. 2016 Apr 1;24:S122. [Google Scholar]
- 9.Sinclair J., Selfe J. Sex differences in knee loading in recreational runners. J. Biomech. 2015 Jul 16;48(10):2171–2175. doi: 10.1016/j.jbiomech.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 10.Kinney R.C., Schwartz Z., Week K., Lotz M.K., Boyan B.D. Human articular chondrocytes exhibit sexual dimorphism in their responses to 17β-estradiol. Osteoarthritis Cartilage. 2005 Apr 1;13(4):330–337. doi: 10.1016/j.joca.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 11.Richette P., Corvol M., Bardin T. Estrogens, cartilage, and osteoarthritis. Jt. Bone Spine. 2003 Aug 1;70(4):257–262. doi: 10.1016/s1297-319x(03)00067-8. [DOI] [PubMed] [Google Scholar]
- 12.Hussain S.M., Cicuttini F.M., Giles G.G., Graves S.E., Wang Y. Relationship between circulating sex steroid hormone concentrations and incidence of total knee and hip arthroplasty due to osteoarthritis in men. Osteoarthritis Cartilage. 2016;24(8):1408–1412. doi: 10.1016/j.joca.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 13.Teichtahl A.J., Wluka A.E., Proietto J., Cicuttini F.M. Obesity and the female sex, risk factors for knee osteoarthritis that may be attributable to systemic or local leptin biosynthesis and its cellular effects. Med. Hypotheses. 2005 Jan 1;65(2):312–315. doi: 10.1016/j.mehy.2005.02.026. [DOI] [PubMed] [Google Scholar]
- 14.Oliveria S.A., Felson D.T., Reed J.I., Cirillo P.A., Walker A.M. Incidence of symptomatic hand, hip, and knee osteoarthritis among patients in a health maintenance organization. Arthritis Rheum. 1995 Aug 1;38(8):1134–1141. doi: 10.1002/art.1780380817. [DOI] [PubMed] [Google Scholar]
- 15.Gao W., Zeng C., Cai D., Liu B., Li Y., Wen X., et al. Serum concentrations of selected endogenous estrogen and estrogen metabolites in pre- and postmenopausal Chinese women with osteoarthritis. J. Endocrinol. Invest. 2010 Oct 27;33(9):644–649. doi: 10.1007/BF03346664. [DOI] [PubMed] [Google Scholar]
- 16.Cirillo D.J., Wallace R.B., Wu L., Yood R.A. Effect of hormone therapy on risk of hip and knee joint replacement in the women's health initiative. Arthritis Rheum. 2006 Oct 1;54(10):3194–3204. doi: 10.1002/art.22138. [DOI] [PubMed] [Google Scholar]
- 17.Wluka A.E., Cicuttini F.M., Spector T.D. Menopause, oestrogens and arthritis. Maturitas. 2000 Jun 30;35(3):183–199. doi: 10.1016/s0378-5122(00)00118-3. [DOI] [PubMed] [Google Scholar]
- 18.Malfait A.M., Little C.B. On the predictive utility of animal models of osteoarthritis. Arthritis Res. Ther. 2015 Dec 14;17(1):225. doi: 10.1186/s13075-015-0747-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carlson C.S., Loeser R.F., Purser C.B., Gardin J.F., Jerome C.P. Osteoarthritis in cynomolgus macaques III: effects of age, gender, and subchondral bone thickness on the severity of disease. J. Bone Miner. Res. 2009 Dec 3;11(9):1209–1217. doi: 10.1002/jbmr.5650110904. [DOI] [PubMed] [Google Scholar]
- 20.Bendele A.M. Animal models of osteoarthritis. J. Musculoskelet. Neuronal Interact. 2001;1 [PubMed] [Google Scholar]
- 21.Pritzker K.P.H. Animal models for osteoarthritis: processes, problems and prospects. Ann. Rheum. Dis. 1994 Jun 1;53(6):406–420. doi: 10.1136/ard.53.6.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCoy A.M. Animal models of osteoarthritis: comparisons and key considerations. Vet. Pathol. 2015 Sep 10;52(5):803–818. doi: 10.1177/0300985815588611. [DOI] [PubMed] [Google Scholar]
- 23.Kuyinu E.L., Narayanan G., Nair L.S., Laurencin C.T. Animal models of osteoarthritis: classification, update, and measurement of outcomes. J. Orthop. Surg. Res. 2016 Dec 2;11(1):19. doi: 10.1186/s13018-016-0346-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ma H.L., Blanchet T.J., Peluso D., Hopkins B., Morris E.A., Glasson S.S. Osteoarthritis severity is sex dependent in a surgical mouse model. Osteoarthritis Cartilage. 2007 Jun 1;15(6):695–700. doi: 10.1016/j.joca.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 25.Huang H., Skelly J.D., Ayers D.C., Song J. Age-dependent changes in the articular cartilage and subchondral bone of C57BL/6 mice after surgical destabilization of medial meniscus. Sci. Rep. 2017 Sep 9;7(1):42294. doi: 10.1038/srep42294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Temp J., Labuz D., Negrete R., Sunkara V., Machelska H. Pain and knee damage in male and female mice in the medial meniscal transection-induced osteoarthritis. Osteoarthritis Cartilage. 2019 Dec 10;28(4):475–485. doi: 10.1016/j.joca.2019.11.003. [DOI] [PubMed] [Google Scholar]
- 27.Palmer A.W., Guldberg R.E., Levenston M.E. Analysis of cartilage matrix fixed charge density and three-dimensional morphology via contrast-enhanced microcomputed tomography. Proc. Natl. Acad. Sci. U. S. A. 2006 Dec 19;103(51):19255–19260. doi: 10.1073/pnas.0606406103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thote T., Lin A.S.P., Raji Y., Moran S., Stevens H.Y., Hart M., et al. Localized 3D analysis of cartilage composition and morphology in small animal models of joint degeneration. Osteoarthritis Cartilage. 2013 Aug;21(8):1132–1141. doi: 10.1016/j.joca.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 29.Reece D.S., Thote T., Lin A.S.P., Willett N.J., Guldberg R.E. Contrast enhanced μCT imaging of early articular changes in a pre-clinical model of osteoarthritis. Osteoarthritis Cartilage. 2018 Jan;26(1):118–127. doi: 10.1016/j.joca.2017.10.017. [DOI] [PubMed] [Google Scholar]
- 30.Willett N.J., Thote T., Lin A.S.P., Moran S., Raji Y., Sridaran S., et al. Intra-articular injection of micronized dehydrated human amnion/chorion membrane attenuates osteoarthritis development. Arthritis Res. Ther. 2014 Feb 6;16(1) doi: 10.1186/ar4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Kraan P.M., van den Berg W.B. Osteophytes: relevance and biology. Osteoarthritis Cartilage. 2007 Mar 1;15(3):237–244. doi: 10.1016/j.joca.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 32.Cicuttini F., Forbes A., Morris K., Darling S., Bailey M., Stuckey S. Gender differences in knee cartilage volume as measured by magnetic resonance imaging. Osteoarthritis Cartilage. 1999;7(3):265–271. doi: 10.1053/joca.1998.0200. [DOI] [PubMed] [Google Scholar]
- 33.van Osch G.J.V.M., van der Kraan P.M., Vitters E.L., Blankevoort L., van den Berg W.B. Induction of osteoarthritis by intra-articular injection of collagenase in mice. Strain and sex related differences. Osteoarthritis Cartilage. 1993 Jul 1;1(3):171–177. doi: 10.1016/s1063-4584(05)80088-3. [DOI] [PubMed] [Google Scholar]
- 34.Van Den Berg W.B. Osteophyte formation in osteoarthritis. Osteoarthritis Cartilage. 1999;7(3):333. doi: 10.1053/joca.1998.0186. [DOI] [PubMed] [Google Scholar]
- 35.Al-Rawahi M., Luo J., Pollintine P., Dolan P., Adams M.A. Mechanical function of vertebral body osteophytes, as revealed by experiments on cadaveric spines. Spine. 2011 May 1;36(10):770–777. doi: 10.1097/BRS.0b013e3181df1a70. (Phila Pa 1976) [DOI] [PubMed] [Google Scholar]
- 36.Hsia A.W., Collette N.M., Christiansen B.A. Osteophyte Formation and the effect of mechanical loading: comparisons to fracture healing. Osteoarthritis Cartilage. 2017 Apr 1;25:S305–S306. [Google Scholar]
- 37.Kim B.T., Mosekilde L., Duan Y., Zhang X.Z., Tornvig L., Thomsen J.S., et al. The structural and hormonal basis of sex differences in peak appendicular bone strength in rats. J. Bone Miner. Res. 2003;18(1):150–155. doi: 10.1359/jbmr.2003.18.1.150. [DOI] [PubMed] [Google Scholar]
- 38.Li J., Zhong Z., Lidtke R., Kuettner K.E., Peterfy C., Aliyeva E., et al. Radiography of soft tissue of the foot and ankle with diffraction enhanced imaging. J. Am. Podiatr. Med. Assoc. 2004 May;94(3):315–322. doi: 10.7547/0940315. [DOI] [PubMed] [Google Scholar]
- 39.Castañeda S., Roman-Blas J.A., Largo R., Herrero-Beaumont G. Subchondral bone as a key target for osteoarthritis treatment. Biochem. Pharmacol. 2012 Feb 1;83(3):315–323. doi: 10.1016/j.bcp.2011.09.018. [DOI] [PubMed] [Google Scholar]
- 40.Yu D.G., Nie S.B., Liu F.X., Wu C.L., Tian B., Wang W.G., et al. Dynamic alterations in microarchitecture, mineralization and mechanical property of subchondral bone in rat medial meniscal tear model of osteoarthritis. Chin. Med. J. 2015 Nov 5;128(21):2879–2886. doi: 10.4103/0366-6999.168045. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.