Abstract
Reactive oxygen species (ROS) and nitric oxide (NO) have been implicated in chondrocyte senescence and cartilage aging, pathogenesis of osteoarthritis (OA), and rheumatoid arthritis. Naturally occurring polyphenolic compounds (PPCs), such as curcumin (turmeric), resveratrol (grape), and epigallocatechin-3-gallate (EGCG) (green tea), have been known for their anti-inflammatory and chondroprotective effects. However, the potential protective effects of these PPCs against oxidative stress in chondrocytes are unclear. To investigate this, bovine articular chondrocytes and human osteoarthritic chondrocytes were pre-treated with PPCs at varying concentrations, and then exposed to hydrogen peroxide (H2O2) as an ROS inducer or S-nitroso-N-acetylpenicillamine (SNAP) as a NO donor. Alternatively, chondrocytes were co-treated with polyphenols and H2O2. Intracellular ROS/NO were measured using a fluorescent dye technique (H2DCF-DA for ROS; DAF-FM for NO). Our findings showed that PPC pre-/co-treatment inhibited both H2O2-induced ROS and SNAP-induced NO at different concentrations in both bovine chondrocytes and human osteoarthritic chondrocytes. Curcumin also increased glutathione peroxidase activity in the presence of H2O2 in bovine chondrocytes. Taken together, these findings indicate that PPCs are capable of suppressing oxidative stress- induced responses in chondrocytes, which may have potential therapeutic value for OA clinical application.
Keywords: Chondrocytes, Polyphenols, Reactive oxygen species, Oxidative stress
Abbreviations: EGCG, epigallocatechin-3-gallate; NAC, N-acetyl-l-cysteine; DMSO, dimethyl sulfoxide; H2O2, hydrogen peroxide; DMEM, Dulbecco's Modified Eagle's Medium; FBS, fetal bovine serum; PBS, phosphate-buffered saline; H2DCF-DA, 2′,7′-dichlorodihydrofluorescein diacetate; DAF-FM, 4-amino-5-methylamino-2′,7′-difluorofluorescein; EDTA, ethylenediaminetetraacetic acid; l-NAME, Nω-nitro-l-arginine methyl ester hydrochloride; SNAP, S-nitroso-N-acetylpenicillamine; GPx, glutathione peroxidase; ROS, reactive oxygen species; NO, nitric oxide; DMOADs, disease modifying osteoarthritis drugs
1. Introduction
Osteoarthritis (OA) is a progressive disease caused by destruction of articular cartilage and proliferative remodeling of subchondral bone. OA is the most common form of arthritis, affecting an estimated 12% of adults of ages 25–74 in the U.S [1]. The clinical management of OA continues to be a challenge. The conventional treatment for OA is the administration of analgesic nonsteroidal anti-inflammatory drugs, which have well known, severe side effects and are only temporarily and marginally effective for reducing symptoms, such as pain and inflammation, or for maintaining joint mobility, and/or limiting the loss of function. Therefore, novel, safe, and more efficacious agents are needed for OA treatment. The search for candidate disease modifying osteoarthritis drugs (DMOADs) is one of the major goals of current OA research.
The balance between reactive oxygen species (ROS) production and antioxidant defense determines the extent of cellular oxidative stress. It is widely accepted, but not conclusively proven, that the level of oxidative stress increases with aging and leads to the damage of cellular macromolecules such as DNA. In cartilage, the extracellular matrix (ECM) proteins are also likely to be key targets of ROS [2,3]. Chondrocytes are capable of producing hydroxyl radical [4], hydrogen peroxide [5], and superoxide species [6]. While there is growing support that ROS may contribute to vital cell signaling mechanisms [[7], [8], [9]], a large body of evidence has demonstrated the detrimental effects of these highly reactive and labile molecules in cartilage. For example, ROS lead to inhibition of proteoglycan synthesis [10,11] and have been implicated in the degeneration of the ECM through lipid peroxidation and formation of malondialdehyde and hydroxynonenal adducts [2]. There is accumulating evidence to suggest that excessive levels of ROS are cytotoxic to chondrocytes [[12], [13], [14]]. To prevent an accumulation of ROS-mediated damages, chondrocytes possess a well-coordinated enzymatic antioxidant system formed principally by catalase, glutathione peroxidase (GPx), and superoxide dismutase (SOD) [15]. In addition, nitric oxide (NO) and its redox derivatives appear to have a number of different functions in both normal and pathophysiological joint conditions [16]. NO is produced in osteoarthritic cartilage. Inducible nitric oxide synthase (iNOS) enzyme is also upregulated in osteoarthritic chondrocytes, resulting in an excess of NO and perpetuating the release of inflammatory cytokines and other catabolic processes. NO inhibits both proteoglycan and collagen synthesis, activates matrix metalloproteinases (MMPs), mediates chondrocyte apoptosis, and promotes chondrocyte inflammatory responses [16]. All of these activities contribute to the catabolic consequences of NO in cartilage.
Natural plant products have been used throughout human history for various purposes. Experimental studies on animals as well as cultured human cell lines suggest the potential of the natural products, polyphenolic compounds (PPCs, see Fig. 1) in the treatment of a number of diseases, such as cardiovascular diseases, cancers, neurodegenerative diseases, diabetes, osteoporosis, and OA [17]. PPCs exhibit a range of biological activities, including anti-inflammatory, anti-carcinogenic, and estrogenic activities as well as cardiovascular protection, free-radical scavenging, inhibition/induction of apoptosis, and inhibition of platelet aggregation [[17], [18], [19], [52], [53]]. Thus, PPCs, which are mostly derived from edible plants or plant products, most likely act on pathways common to a variety of cellular/biochemical activities. PPCs show many beneficial effects; however, over-consumption may cause adverse effects [19]. More experimental and clinical trials with PPCs, either alone or in combination with existing pharmacotherapeutics, are clearly needed to fully evaluate their potential. Their safety, combined with low cost and generic systemic beneficial effects, make nutraceutical PPCs attractive and promising agents to be explored for prevention and treatment of diseases.
Fig. 1.
Chemical structures of EGCG, curcumin and resveratrol.
In the context of cartilage degeneration, PPCs have been shown to exhibit potent inflammation modulatory activities. For example, curcumin (turmeric) (1) inhibits the action of inflammatory and catabolic mediators, such as PGE2, COX-2 and, interleukin (IL)-6 in human chondrocytes [18], and (2) promotes chondrogenesis of mesenchymal stem cells by suppression of nuclear factor (NF)-κB [20]. Also, curcumin-phosphatidylcholine complex (Meriva®) decreased joint pain and improved joint function in OA patients [21]. A recent study showed that short-term supplementation with curcuminoids attenuated systemic oxidative stress in patients with osteoarthritis [22]. Another PPC, resveratrol (grape) also displays anti-inflammatory and chondroprotective activities [23]. Intra-articular resveratrol treatment reduced cartilage loss in a rabbit model [24]. EGCG (green tea) showed multiple anti-arthritic effects, such as reducing levels of MMPs [25], COX-2 [26], nitric oxide [26] and prostaglandin E2 [26], and suppressing mitogen activated protein (MAP) kinases [27], activator protein (AP)-1 [27], expression of growth factors [28], angiogenic factors [28], and cytokines and chemokines [28] in IL-1β-stimulated human osteoarthritic chondrocytes. Recent publications showed that pomegranate juice (containing punicalagin and ellagic acid) reduced MMP-13 and increased glutathione peroxidase (GPx) in patients with knee OA [29]. Other studies have shown reduced ROS and NO production and pro-inflammatory cytokines by ginsenoside Rb1 in young rat chondrocytes [30], and the synergistic effect of resveratrol and curcumin on inhibiting IL-1β induced inflammation and apoptosis [31].
Some studies have also shown conflicting results for the effect of curcumin and resveratrol on articular chondrocytes, depending on the model system used [18,30,32]. Therefore, in this study, we aimed to investigate the effect of multiple PPCs on chondrocyte oxidative stress, by using cells from healthy adult bovine chondrocytes, as well as chondrocytes from adult patients with OA as models of cartilage oxidative stress injury initiation and progression, respectively. In this investigation, we report that PPC treatment is capable of suppressing oxidative stress-induced responses in both human osteoarthritic and bovine healthy chondrocytes, which may have potential therapeutic value for clinical applications of PPC for OA treatment.
2. Materials and methods
2.1. Chemicals
Epigallocathechin-3-gallate (EGCG), resveratrol and curcumin were purchased from LKT laboratories, Inc (St. Paul, MN) (Fig. 1). Hydrogen peroxide (H2O2) was obtained from Sigma-Aldrich (St. Louis, MO). Dulbecco's Modified Eagle's Medium (DMEM), F12 medium, antibiotic-antimycotic (Anti-anti), ethylenediaminetetraacetic acid (EDTA), trypsin-EDTA (Invitrogen), penicillin-streptomycin (Pen Strep), fetal bovine serum (FBS), phosphate-buffered saline (PBS), 2′,7′-dichlorodihydrofluorescein diacetate (H2DCF-DA), 4-amino-5-methylamino-2′,7′-difluorofluorescein diacetate (DAF-FM diacetate), Amplex Red Catalase activity assay kit, Nω-nitro-l-arginine methyl ester hydrochloride (l-NAME) as a NO inhibitor, and S-nitroso-N-acetylpenicillamine (SNAP) were purchased from Invitrogen (Carlsbad, CA). Collagenase type 2 was purchased from Worthington Biochemical Corporation (Lakewood, NJ). Bradford protein assay reagent was purchased from BioRad (Hercules, CA). Stock solutions of 100 mM EGCG, 100 mM curcumin, 1 M NAC, 1 M l-NAME were prepared in DMSO and stored at −20 °C until use. 10 mM H2DCF-DA and 5 mM DCF-DA-diacetate were prepared as stock solutions in DMSO, stored at −20 °C, and diluted in PBS to 20 μM and 10 μ M, respectively, prior to use.
2.2. Cell culture
Samples of human osteoarthritic articular cartilage (3 Female patients, age 54, 54, 55 (average age: 54) were obtained as total joint arthroplasty surgical waste according to approved IRB protocol (University of Pittsburgh, PA, USA and University of Washington, Seattle, WA, USA). Joint surface regions without macroscopic signs of degeneration but adjacent to lesion sites were individually harvested, to represent cartilage tissues exposed to an inflammatory environment and likely primed for degeneration. Chondrocytes were enzymatically isolated from the articular cartilage using a standard protocol in the laboratory [33,34].
Bovine articular cartilage was harvested from the patello-femoral groove of the hind-leg stifle of 2–3 year old cows within 24 h of slaughter (JW Trueth and Sons, Baltimore, MD) [35]. Bovine chondrocytes were isolated enzymatically using a standard protocol [33]. Both human and bovine chondrocytes were used at relatively low passage number (Passage 2) in order to maintain the chondrogenic phenotype while using a sufficient number of chondrocytes for our experiments. Both human and bovine chondrocytes at passage 1 were seeded into 150 cm2 tissue culture flasks at a density of ~40,000 cells/cm2 in DMEM/F12 with 10% FBS and antibiotic-antimycotic. After culturing at 37 °C for 2 days when ~80% confluence was reached, cells were detached with 0.25% trypsin-EDTA and further cultured at a density of ~40,000 cells/cm2 in DMEM/F12 with 10% FBS for 1 day, and then serum starved, in DMEM/F12 with 1% FBS overnight, followed by treatments with PPC and either H2O2 or SNAP treatment.
2.3. Detection of reactive oxygen species (ROS) and NO production in chondrocytes
Production of cellular ROS was evaluated by analyzing changes in fluorescence intensity resulting from the oxidation of the intracellular fluoroprobe H2DCF-DA [36]. H2DCF-DA enters cells passively and is de-acetylated by cytoplasmic esterase to the non-fluorescent dichloro-dihydrofluorescein (DCFH). DCFH reacts with ROS to form dichlorofluorescein (DCF), a fluorescent product. Briefly, for ROS detection, bovine chondrocytes and human osteoarthritic chondrocytes (1 × 104 cells/well) were plated into 96-well plates. After 24 h, cells were pre-labeled with 10 μM H2DCF-DA for 15 min, and then co-treated with 100 μM H2O2 and with or without EGCG (10–50 μM), curcumin (10–50 μM), or resveratrol (10–50 μM), for 30 min.
Production of cellular NO was evaluated by analyzing changes in fluorescence intensity resulting from the oxidation of the intracellular fluoroprobe DCF-DA-diacetate [36]. DCF-DA-diacetate enters cells passively and is de-acetylated by cytoplasmic esterase to the weakly fluorescent DCF-DA. DCF-DA reacts with NO to form benzotriazole derivative, a fluorescent product. For NO detection, bovine chondrocytes and human osteoarthritic chondrocytes (1 × 104 cells/well) were plated into 96-well plates. After 24 h, cells were pre-labeled with 10 μM DCF-FM for 15 min, and then co-treated with 500 μM SNAP (Sigma) and with or without EGCG (10–50 μM), curcumin (10–50 μM), or resveratrol (10–50 μM), for 30 min. Fluorescence signals were recorded at excitation/emission of 485 nm/528 nm (BioTek, Winooski, VT).
2.4. Catalase and GPx assays
Chondrocytes (4 × 105/well) were seeded on 6-well plates. After 24 h, cells were co-incubated with 100 μM H2O2 and curcumin for 30 min. Cells were lysed with 0.5% Triton X-100 solution in PBS for 20 min at room temperature (for catalase assay) or with GPx assay buffer (for GPx assay), and the supernatant after centrifugation at 10,000g × for 10 min at 4 °C was used for enzyme assay. (1) Catalase -The Amplex Red Catalase Assay Kit (Invitrogen, Carlsbad, CA) was used according to the manufacturer's protocol [36]. In brief, reaction mixtures containing 50 μl of 100 μM Amplex Red containing 0.4 U/mL HRP, 25 μL of 40 μM H2O2, 0.2 units/mL horseradish peroxidase, and 25 μL of cell lysate were incubated at 37 °C for 30 min. Catalase activity was determined spectrophotometrically based on A570 and normalized with respect to protein concentration of each sample. (2) GPX – The GPx assay Kit (BioVision, Milpitas, CA) was used according to the manufacturer's protocol [36], and enzyme activity was determined spectrophotometrically based on A340 and normalized with respect to protein concentration of each sample.
2.5. Statistical analysis
Significant differences were assessed with two-tailed Student's t-test for two-group comparisons, and denoted as p < 0.05 (∗) and p < 0.0 1 (∗∗).
3. Results
3.1. PPCs suppress inducible ROS/NO levels in bovine chondrocytes
A number of studies have demonstrated that oxidative damage due to over-production of NO and other ROS may be involved in the pathogenesis of OA [[37], [38], [39], [40]]. Our initial experiment thus aimed to explore whether PPC treatment of chondrocytes protected them against insults of ROS and NO. To investigate this hypothesis, we first used chondrocytes isolated from healthy bovine articular cartilage and challenged them with H2O2 as an ROS inducer or SNAP as an NO donor to simulate the conditions of elevated cellular ROS/RNS, with or without PPC treatment.
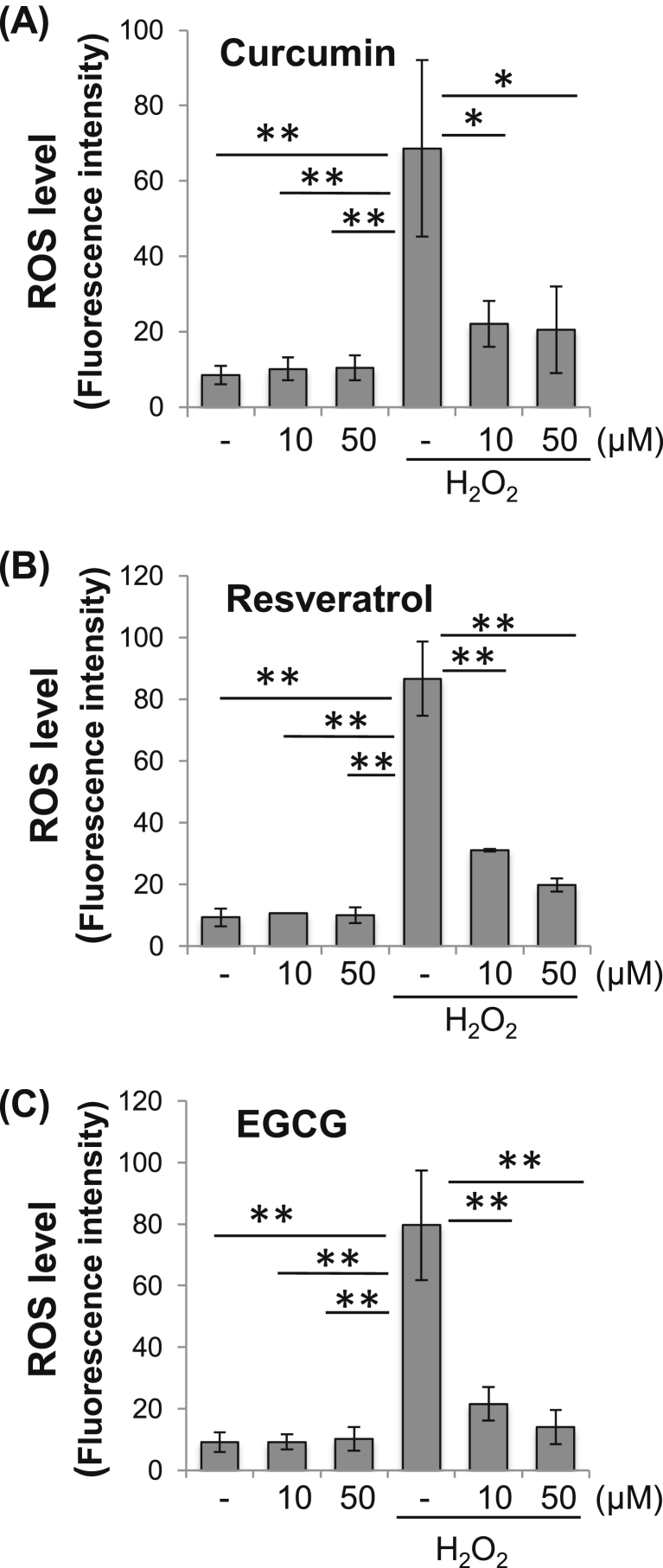
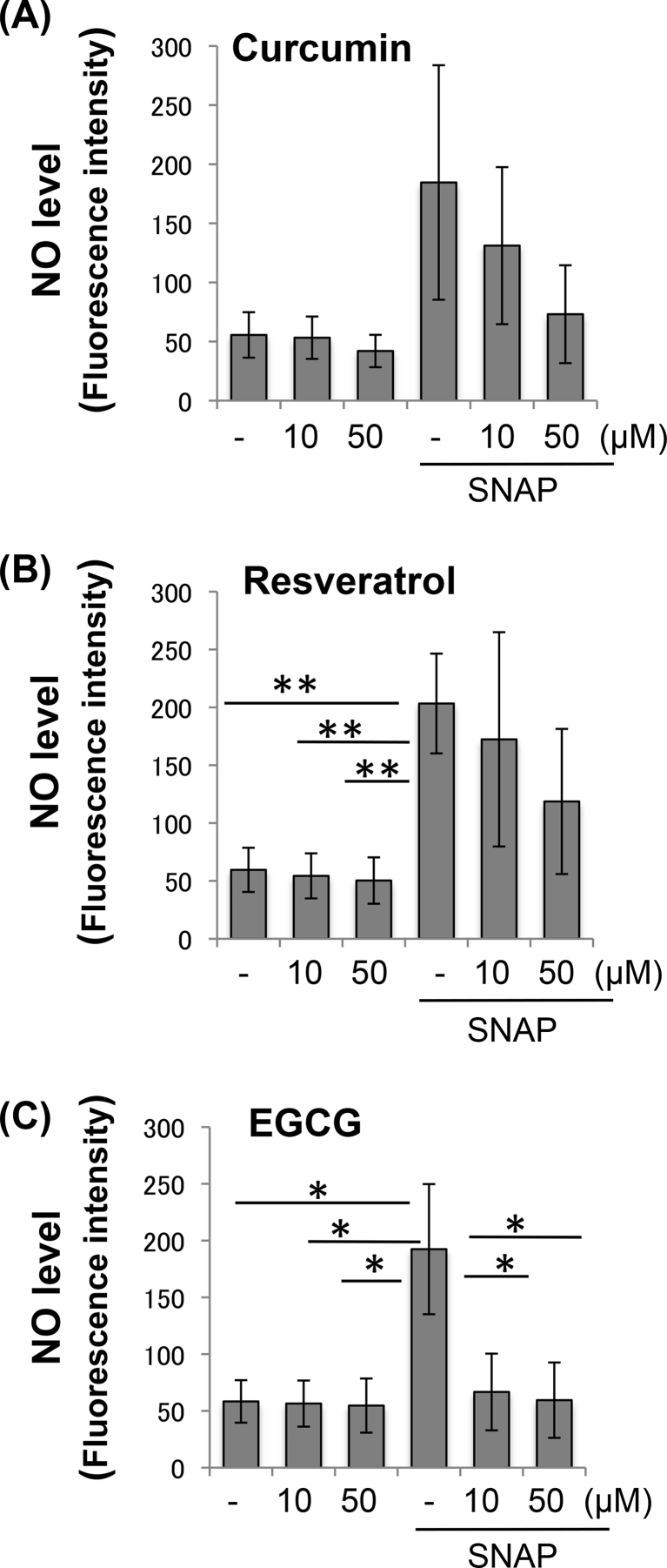
To investigate whether EGCG, curcumin and resveratrol could inhibit intracellular ROS production, bovine articular chondrocytes were co-treated with the PPCs at varying concentrations, and H2O2. Our results indicated that treatment with any of the three PPCs rapidly and substantially reduced H2O2-induced ROS productions (Fig. 2). We previously showed that PPCs did not directly affect H2O2 in the absence of cells [36]. We next investigated whether these three PPCs could inhibit cellular NO accumulation. Bovine chondrocytes were co-treated with PPCs at varying concentrations, and then exposed to SNAP as an NO donor. As shown in Fig. 3, EGCG treatment resulted in a reduction in SNAP-induced NO levels, while no statistical significant difference was seen upon treatment with the other two PPCs.
Fig. 2.
PPCs suppress inducible ROS levels in bovine chondrocytes. Bovine chondrocytes pre-labeled with 10 μM H2DCF-DA were exposed to 100 μM H2O2 for 30 min, with or without PPC co-treatment at various concentrations: (A) curcumin, (B) resveratrol, and (C) EGCG. Fluorescence signals corresponding to ROS levels were recorded at excitation/emission of 485 nm/528 nm. Results are expressed as mean ± SD (N = 3). Difference relative to untreated controls: ∗, p < 0.05; ∗∗, p < 0.01.
Fig. 3.
PPCs suppress inducible NO levels in bovine chondrocytes. Bovine chondrocytes pre--labeled with 10 μM DCF-FM were exposed to 500 μM SNAP (Sigma) for 30 min, with or without PPC co-treatment at various concentrations: (A) curcumin, (B) resveratrol, and (C) EGCG. Fluorescence signals corresponding to NO levels were recorded at excitation/emission of 485 nm/528 nm. Results are expressed as mean ± SD (N = 3). Difference relative to untreated controls: ∗, p < 0.05; ∗∗, p < 0.01.
It should be noted that we had initially tested a number of treatment periods for both co-treatment with PPC and H2O2 (Supplemental Figure 1) and pre-treatment with PPC, followed by treatment with H2O2 (Supplemental Fig. 2). With co-treatment, the PPC effect was attenuated with longer incubation times (Supplemental Figure 1). We also observed that longer PPC pre-incubation time (>3 h) resulted in increased ROS production in the absence of H2O2; in particular, EGCG, when administered alone, showed significant pro-oxidative effects (3.6-fold versus untreated control), while curcumin (1.4-fold) and resveratrol (1.3-fold) showed lower effects (Supplemental Fig. 2). On the other hand, a 3-h pre-treatment with PPCs followed by treatment with H2O2 did result in a reduction of H2O2 induced ROS at 30 min, but the effect was lost after 24 h (Supplemental Fig. 2).
Because of the possible pro-oxidant effect of longer time exposure to PPCs, a short interval of simultaneous PPC co-treatment with H2O2 or SNAP was therefore used for all subsequent experiments. It is noteworthy that, among the PPCs tested for a longer time (23 h) treatment, curcumin appeared to have an overall more robust effect on reducing H2O2–induced ROS (Supplemental Figure 1), which could translate into a more sustained in vivo antioxidant effect in OA. We therefore next aimed to investigate the mechanism of action of curcumin on cellular ROS and NO metabolism.
3.2. Curcumin increases GPx activity in the presence of H2O2
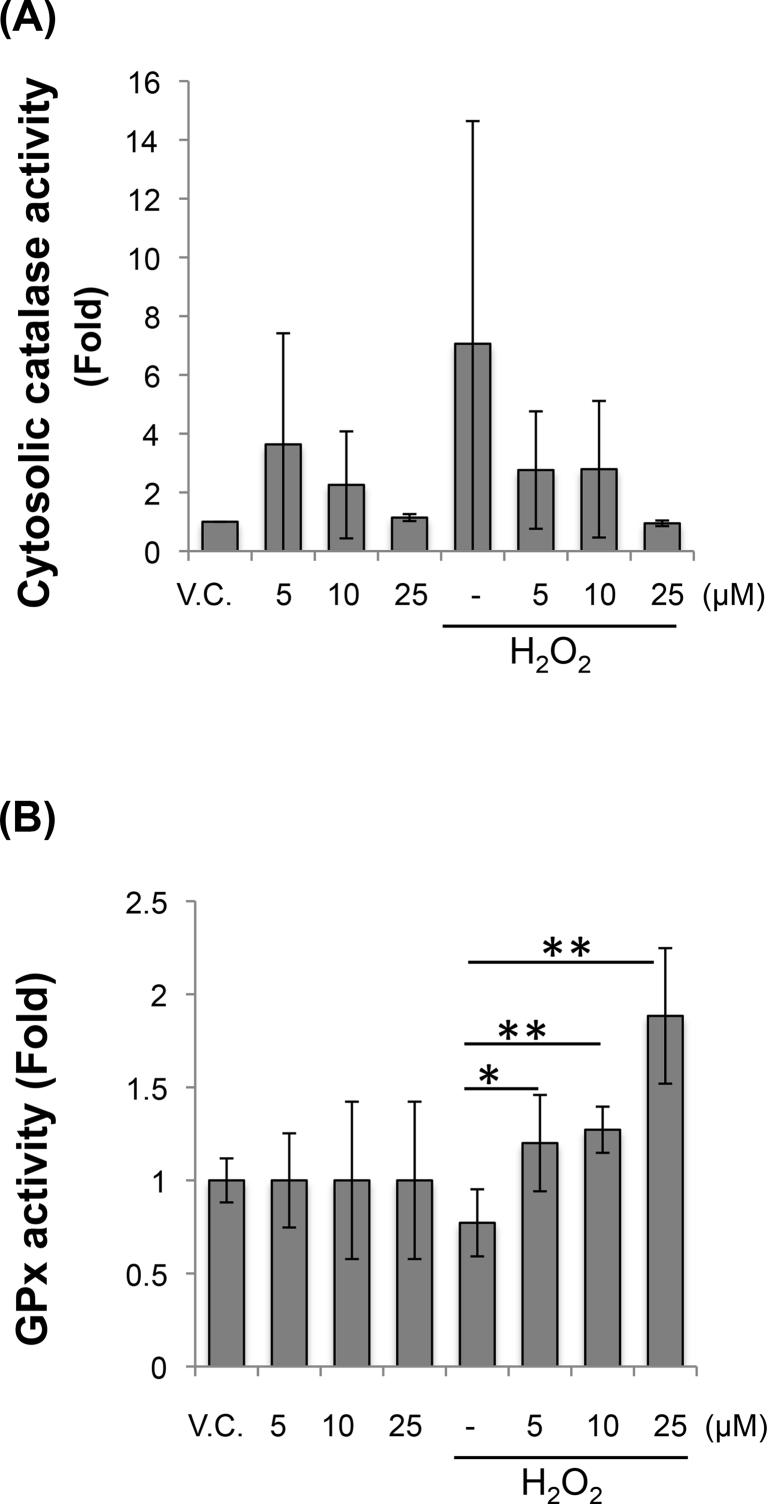
Alterations in intracellular ROS levels could also result from changes in cellular antioxidant defense systems. Specifically, the antioxidant enzymes, SOD, catalase and GPx, play significant roles in protecting cells against radicals-mediated damage. We therefore exposed bovine chondrocytes to 100 μM H2O2 for 30 min, with or without co-treatment with curcumin, and measured the activity of the anti-oxidant enzymes, catalase and GPx, which have been shown to be responsive to ROS [36].
The cytosolic levels of catalase activity were not significantly altered by H2O2, and therefore the effect of curcumin on H2O2-induced chondrocyte oxidative stress could not be evaluated (Fig. 4A) [41]. We noticed a similar lack of effect of H2O2 on GPx activity; however, upon co-treatment with curcumin and H2O2, an increased in GPx activity was observed compared to treatment with H2O2 alone (Fig. 4B).
Fig. 4.
Curcumineffects onROS-induced enzyme changes in bovine chondrocytes. Bovine chondrocytes co-treated with 100 μM H2O2 and curcumin for 30 min were lysed and assayed for (A) catalase and (B) GPx activities. Results are expressed as mean ± SD (N = 3). Difference relative to untreated controls (VC, vehicle control): ∗, p < 0.05; ∗∗, p < 0.01.
3.3. Curcumin suppresses ROS/NO production in human osteoarthritic chondrocytes
We next investigated whether PPC treatment was also effective in human cells, particularly chondrocytes derived from osteoarthritic articular cartilage. Human osteoarthritic chondrocytes were treated with H2O2 and SNAP to simulate the oxidative stress environment observed during OA progression. As shown in Fig. 5, treatment with 100 μM H2O2 or 500 μM SNAP significantly induced ROS and NO, respectively, in human osteoarthritic chondrocytes. Co-treatment with curcumin effectively reduced the elevation of ROS and NO levels in osteoarthritic chondrocytes (Fig. 5).
Fig. 5.
Curcumin suppresses inducible ROS/NO levels in human osteoarthritic chondrocytes. (A) ROS. Human osteoarthritic chondrocytes pre-labeled with 10 μM H2DCF-DA were exposed to 100 μM H2O2 for 30 min, with or without curcumin co-treatment for 30 min. (B) NO. Human osteoarthritic chondrocytes pre-labeled with 10 μM DAF-FM were co-exposed to 500 μM SNAP for 30 min, with or without co-treatment with curcumin. Fluorescence signals were recorded at excitation/emission of 485 nm/528 nm. Results are expressed as mean ± SD (N = 3). Difference relative to untreated controls: ∗, p < 0.05; ∗∗, p < 0.01.
4. Discussion
In this investigation, we have induced elevated ROS/NO levels in cultured chondrocytes to mimic the oxidative stress conditions during OA pathogenesis. Our findings showed that treatment of bovine chondrocytes with the three PPCs inhibited H2O2-induced-intracellular ROS. EGCG was the only treatment that showed a reduction in NO in bovine chondrocytes exposed to SNAP. Curcumin also blocked H2O2/SNAP induced-intracellular ROS/NO in human osteoarthritic chondrocytes. In addition, curcumin increased GPx activity in the presence of H2O2; however, catalase activity was not significantly changed either by H2O2 or curcumin treatment. This observation could be because our catalase assay was not sufficiently sensitive to detect the changes at the H2O2 concentrations used. While both higher doses and longer treatment with H2O2 could be employed to elicit measurable effects on antioxidant enzyme activities in cultured cells [42], we intentionally kept H2O2 concentration at the low end of the spectrum to minimize non-specific cytotoxicity [43]. On the other hand, PPCs showed rapid inhibition of elevated ROS/NO, being effective when co-introduced with ROS/NO inducing agents for 30 min (Fig. 2, Fig. 3) or even shorter time (data not shown).
From our pilot study (Supplemental Figs. 1 and 2), pre-incubation with PPCs before exposure to H2O2 did not provide additional beneficial effects compared to co-treatment, and the longer H2O2 exposure minimized the PPC effect, with the exception of curcumin, which appeared to maintain the ROS reduction effect even at 24 h. This feature suggests that PPCs may have a more beneficial effect when provided in an acute process, such as injury to cartilage in post-traumatic OA, either locally or by intra-articular injection, to reduce the local, injury-induced ROS production. Animal studies have shown a beneficial effect of intra-articular injection of PPC (EGCG and tannic acid) in a model of collagen induced arthritis [44], and a reduction in disease progression by curcumin when applied topically in a model of post-traumatic OA [45].
In our previous report, we observed that treatment with PPCs showed similar rapid action in human bone marrow-derived mesenchymal stem cells [36]. The PPCs used here are known as typical amphipathic drugs that are able to bind to cell membranes [46,47]. Previous studies have shown rapid incorporation of PPCs into cells and binding of PPCs to cell membrane with concomitant anti-oxidative effects [46,48,49]. It is thus likely that the effects of PPCs on chondrocytes are mediated by PPC first binding to and diffusing across the cell membrane, followed by entry into the cytoplasm, to result in effective reduction of ROS/NO in the cytosol as well as the nucleus.
A number of PPCs are well known for their antioxidant and anti-inflammatory activities, and are consumed as micronutrients in the human diet, with an average consumption of 1 g/day [44,50,51]. PPCs taken orally are extensively metabolized in the intestinal and hepatic systems, with the resultant metabolites present in the plasma exhibiting different biological activities [44,51]. PPCs have been reported to exhibit a range of biological activities, including anti-inflammatory, anti-carcinogenic, and estrogenic activities, as well as cardiovascular protection, free-radical scavenging, inhibition/induction of apoptosis, and inhibition of platelet aggregation [31,52,53]. However, their therapeutic value has been limited because of their light sensitivity, low water solubility, and poor oral bioavailability [54]. Interestingly, a recent study showed that curcumin encapsulated in nanoparticle-sized liposomes for improved drug bioavailability may slow osteoarthritis progression [54]. Also, Theracumin® (Theravalues, Tokyo, Japan), a surface-controlled water-dispersible form of curcumin, showed effectiveness in a double-blind study in osteoarthritis [55]. The bioavailability of Theracumin® in humans, estimated as the area under the blood concentration–time curve after administration, was 27-fold higher than that of curcumin powder, suggesting that this formulation may represent a major breakthrough in the clinical use of this natural PPC product.
Surprisingly, we also observed pro-oxidant effects for all three PPCs after 3 h of incubation, when introduced alone to the cells, without addition of H2O2 (Supplemental Fig. 2). It has been shown previously that PPCs can be oxidized in culture medium, thus interfering with assessment of their antioxidative capabilities [56], and while addition of 10% FBS and pyruvate to medium should ameliorate this effect, our serum starving conditions might have contributed to this increase in oxidation. However, when H2O2 was added with the PPC, this pro-oxidant effect was lost, and the introduction of PPC actually decreased the ROS increase induced by H2O2. This feature might also explain why co-incubation of cells with PPC and H2O2 can reduce H2O2-induced ROS levels even after 24 h, while pre-incubation of cells with PPC for 3 h before addition of H2O2 fails to have the same beneficial effect observed at 24 h.
The pro-oxidant effect of PPC observed in vitro could have been only an artifact of culture conditions, as some animal studies mentioned above [[24], [25], [26], [27], [28]] have reported beneficial effect of PPC treatments. However, the effect of PPCs on ROS should be carefully monitored in models of OA to avoid increasing even slightly the oxidative stress, especially if applied prophylactically when oxidative stress has not been induced yet.
In this study we have shown the beneficial effect of three selected PPCs, that actually exhibited slight differences in the reduction of ROS and NO radicals in chondrocytes. For example, while all three PPCs were capable of reducing H2O2–induced ROS, only EGCG reduced NO production induced by SNAP in healthy bovine chondrocytes, and curcumin was capable of decreasing NO in human osteoarthritic chondrocytes. The beneficial effect of PPCs in reducing ROS was observed in both normal bovine chondrocytes treated with H2O2 as a model of acute injury and immediate treatment, and in human osteoarthritic chondrocytes, as a model of reducing degenerative disease progression. In addition, we have shown possible pro-oxidant effects of the PPC when added in the absence of oxidative stress, and while this could be a culture artifact, additional studies should be done to investigate if prophylactic use of PPCs might increase oxidative stress in healthy cartilage in vivo.
Taken together, the findings reported here suggest that selected PPCs are capable of suppressing oxidative stress-induced responses in chondrocytes. While further studies are needed to exclude potential adverse effects and to address the optimal timing and duration of their action, PPC treatments have promising potential therapeutic value as DMOADs for OA clinical applications.
Author contribution
HY: design, experimentation, and data analysis; VU: design, experimentation, and data analysis; RT: supervision and guidance of experimentation. All authors drafted, revised, and approved the manuscript.
Role of funding sources
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Declaration of Competing Interest
The authors have no conflict of interest to declare.
Acknowledgement
This study was supported in part by the Commonwealth of Pennsylvania Department of Health (SAP4100062224, SAP4100050913) and Arthritis Foundation Postdoctoral Fellowship (VU).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ocarto.2020.100064.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Cisternas M.G., Murphy L., Sacks J.J., Solomon D.H., Pasta D.J., Helmick C.G. Alternative methods for defining osteoarthritis and the impact on estimating prevalence in a us population-based survey. Arthritis Care Res. 2016;68:574–580. doi: 10.1002/acr.22721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tiku M.L., Shah R., Allison G.T. Evidence linking chondrocyte lipid peroxidation to cartilage matrix protein degradation. Possible role in cartilage aging and the pathogenesis of osteoarthritis. J. Biol. Chem. 2000;275:20069–20076. doi: 10.1074/jbc.M907604199. [DOI] [PubMed] [Google Scholar]
- 3.Eble J.A., de Rezende F.F. Redox-relevant aspects of the extracellular matrix and its cellular contacts via integrins. Antioxidants Redox Signal. 2014;20:1977–1993. doi: 10.1089/ars.2013.5294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tiku M.L., Yan Y.P., Chen K.Y. Hydroxyl radical formation in chondrocytes and cartilage as detected by electron paramagnetic resonance spectroscopy using spin trapping reagents. Free Radic. Res. 1998;29:177–187. doi: 10.1080/10715769800300211. [DOI] [PubMed] [Google Scholar]
- 5.Rathakrishnan C., Tiku K., Raghavan A., Tiku M.L. Release of oxygen radicals by articular chondrocytes: a study of luminol-dependent chemiluminescence and hydrogen peroxide secretion. J. Bone Miner. Res. 1992;7:1139–1148. doi: 10.1002/jbmr.5650071005. [DOI] [PubMed] [Google Scholar]
- 6.Rathakrishnan C., Tiku M.L. Lucigenin-dependent chemiluminescence in articular chondrocytes. Free Radical Biol. Med. 1993;15:143–149. doi: 10.1016/0891-5849(93)90053-w. [DOI] [PubMed] [Google Scholar]
- 7.Esplugues J.V. No as a signalling molecule in the nervous system. Br. J. Pharmacol. 2002;135:1079–1095. doi: 10.1038/sj.bjp.0704569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guzik T.J., Korbut R., Adamek-Guzik T. Nitric oxide and superoxide in inflammation and immune regulation. J. Physiol. Pharmacol. 2003;54:469–487. [PubMed] [Google Scholar]
- 9.Li H., Forstermann U. Nitric oxide in the pathogenesis of vascular disease. J. Pathol. 2000;190:244–254. doi: 10.1002/(SICI)1096-9896(200002)190:3<244::AID-PATH575>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 10.Bates E.J., Lowther D.A., Johnson C.C. Hyaluronic acid synthesis in articular cartilage: an inhibition by hydrogen peroxide. Biochem. Biophys. Res. Commun. 1985;132:714–720. doi: 10.1016/0006-291x(85)91191-x. [DOI] [PubMed] [Google Scholar]
- 11.Panasyuk A., Frati E., Ribault D., Mitrovic D. Effect of reactive oxygen species on the biosynthesis and structure of newly synthesized proteoglycans. Free Radical Biol. Med. 1994;16:157–167. doi: 10.1016/0891-5849(94)90139-2. [DOI] [PubMed] [Google Scholar]
- 12.Collins J.A., Wood S.T., Bolduc J.A., Nurmalasari N.P.D., Chubinskaya S., Poole L.B., et al. Differential peroxiredoxin hyperoxidation regulates map kinase signaling in human articular chondrocytes. Free Radical Biol. Med. 2019;134:139–152. doi: 10.1016/j.freeradbiomed.2019.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Loeser R.F. The role of aging in the development of osteoarthritis. Trans. Am. Clin. Climatol. Assoc. 2017;128:44–54. [PMC free article] [PubMed] [Google Scholar]
- 14.Bolduc J.A., Collins J.A., Loeser R.F. Reactive oxygen species, aging and articular cartilage homeostasis. Free Radical Biol. Med. 2019;132:73–82. doi: 10.1016/j.freeradbiomed.2018.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernandez-Moreno M., Soto-Hermida A., Pertega S., Oreiro N., Fernandez-Lopez C., Rego-Perez I., et al. Mitochondrial DNA (mtdna) haplogroups and serum levels of anti-oxidant enzymes in patients with osteoarthritis. BMC Muscoskel. Disord. 2011;12:264. doi: 10.1186/1471-2474-12-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abramson S.B. Osteoarthritis and nitric oxide. Osteoarthritis Cartilage. 2008;16(Suppl 2):S15–S20. doi: 10.1016/S1063-4584(08)60008-4. [DOI] [PubMed] [Google Scholar]
- 17.Scalbert A., Manach C., Morand C., Remesy C., Jimenez L. Dietary polyphenols and the prevention of diseases. Crit. Rev. Food Sci. Nutr. 2005;45:287–306. doi: 10.1080/1040869059096. [DOI] [PubMed] [Google Scholar]
- 18.Henrotin Y., Clutterbuck A.L., Allaway D., Lodwig E.M., Harris P., Mathy-Hartert M., et al. Biological actions of curcumin on articular chondrocytes. Osteoarthritis Cartilage. 2010;18:141–149. doi: 10.1016/j.joca.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Cory H., Passarelli S., Szeto J., Tamez M., Mattei J. The role of polyphenols in human health and food systems: a mini-review. Front. Nutr. 2018;5:87. doi: 10.3389/fnut.2018.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buhrmann C., Mobasheri A., Matis U., Shakibaei M. Curcumin mediated suppression of nuclear factor-kappab promotes chondrogenic differentiation of mesenchymal stem cells in a high-density co-culture microenvironment. Arthritis Res. Ther. 2010;12:R127. doi: 10.1186/ar3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belcaro G., Cesarone M.R., Dugall M., Pellegrini L., Ledda A., Grossi M.G., et al. Product-evaluation registry of meriva(r), a curcumin-phosphatidylcholine complex, for the complementary management of osteoarthritis. Panminerva Med. 2010;52:55–62. [PubMed] [Google Scholar]
- 22.Panahi Y., Azizi T., Moghadam M.R., Amin G., Parvin S., Sahebkar A. Oral health status among iranian veterans exposed to sulfur mustard: a case-control study. J. Clin. Expt. Dent. 2015;7:e192–196. doi: 10.4317/jced.52112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Csaki C., Keshishzadeh N., Fischer K., Shakibaei M. Regulation of inflammation signalling by resveratrol in human chondrocytes in vitro. Biochem. Pharmacol. 2008;75:677–687. doi: 10.1016/j.bcp.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 24.Elmali N., Esenkaya I., Harma A., Ertem K., Turkoz Y., Mizrak B. Effect of resveratrol in experimental osteoarthritis in rabbits. Inflamm. Res. 2005;54:158–162. doi: 10.1007/s00011-004-1341-6. [DOI] [PubMed] [Google Scholar]
- 25.Rasheed Z., Anbazhagan A.N., Akhtar N., Ramamurthy S., Voss F.R., Haqqi T.M. Green tea polyphenol epigallocatechin-3-gallate inhibits advanced glycation end product-induced expression of tumor necrosis factor-alpha and matrix metalloproteinase-13 in human chondrocytes. Arthritis Res. Ther. 2009;11:R71. doi: 10.1186/ar2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ahmed S., Rahman A., Hasnain A., Lalonde M., Goldberg V.M., Haqqi T.M. Green tea polyphenol epigallocatechin-3-gallate inhibits the il-1 beta-induced activity and expression of cyclooxygenase-2 and nitric oxide synthase-2 in human chondrocytes. Free Radical Biol. Med. 2002;33:1097–1105. doi: 10.1016/s0891-5849(02)01004-3. https://doi.org/S0891584902010043. [DOI] [PubMed] [Google Scholar]
- 27.Singh R., Ahmed S., Malemud C.J., Goldberg V.M., Haqqi T.M. Epigallocatechin-3-gallate selectively inhibits interleukin-1beta-induced activation of mitogen activated protein kinase subgroup c-jun n-terminal kinase in human osteoarthritis chondrocytes. J. Orthop. Res. 2003;21:102–109. doi: 10.1016/S0736-0266(02)00089-X. [DOI] [PubMed] [Google Scholar]
- 28.Akhtar N., Haqqi T.M. Epigallocatechin-3-gallate suppresses the global interleukin-1beta-induced inflammatory response in human chondrocytes. Arthritis Res. Ther. 2011;13:R93. doi: 10.1186/ar3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghoochani N., Karandish M., Mowla K., Haghighizadeh M.H., Jalali M.T. The effect of pomegranate juice on clinical signs, matrix metalloproteinases and antioxidant status in patients with knee osteoarthritis. J. Sci. Food Agric. 2016;96:4377–4381. doi: 10.1002/jsfa.7647. [DOI] [PubMed] [Google Scholar]
- 30.Kim H.J., Braun H.J., Dragoo J.L. The effect of resveratrol on normal and osteoarthritic chondrocyte metabolism. Bone Joint Res. 2014;3:51–59. doi: 10.1302/2046-3758.33.20002263/3/51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Csaki C., Mobasheri A., Shakibaei M. Synergistic chondroprotective effects of curcumin and resveratrol in human articular chondrocytes: inhibition of il-1beta-induced nf-kappab-mediated inflammation and apoptosis. Arthritis Res. Ther. 2009;11:R165. doi: 10.1186/ar2850ar2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toegel S., Wu S.Q., Piana C., Unger F.M., Wirth M., Goldring M.B., et al. Comparison between chondroprotective effects of glucosamine, curcumin, and diacerein in il-1beta-stimulated c-28/i2 chondrocytes. Osteoarthritis Cartilage. 2008;16:1205–1212. doi: 10.1016/j.joca.2008.01.013S1063-4584(08)00026-5. [DOI] [PubMed] [Google Scholar]
- 33.Wang D., Taboas J.M., Tuan R.S. Pthrp overexpression partially inhibits a mechanical strain-induced arthritic phenotype in chondrocytes. Osteoarthritis Cartilage. 2011;19:213–221. doi: 10.1016/j.joca.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang Y., Lin H., Shen H., Wang B., Lei G., Tuan R.S. Mesenchymal stem cell-derived extracellular matrix enhances chondrogenic phenotype of and cartilage formation by encapsulated chondrocytes in vitro and in vivo. Acta Biomater. 2018;69:71–82. doi: 10.1016/j.actbio.2017.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alexander P.G., Yingjie S., Taboas J.M., Chen F.H., Melvin G.M., Manner P.A., et al. Development of a spring-loaded impact device to deliver injurious mechanical impacts to the articular cartialge surface. Cartilage. 2013;4:52–62. doi: 10.1177/1947603512455195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yagi H., Tan J., Tuan R.S. Polyphenols suppress hydrogen peroxide-induced oxidative stress in human bone-marrow derived mesenchymal stem cells. J. Cell. Biochem. 2013;114:1163–1173. doi: 10.1002/jcb.24459. [DOI] [PubMed] [Google Scholar]
- 37.Del Carlo M., Jr., Loeser R.F. Nitric oxide-mediated chondrocyte cell death requires the generation of additional reactive oxygen species. Arthritis Rheum. 2002;46:394–403. doi: 10.1002/art.10056. [DOI] [PubMed] [Google Scholar]
- 38.Pelletier J.P., Jovanovic D.V., Lascau-Coman V., Fernandes J.C., Manning P.T., Connor J.R., et al. Selective inhibition of inducible nitric oxide synthase reduces progression of experimental osteoarthritis in vivo: possible link with the reduction in chondrocyte apoptosis and caspase 3 level. Arthritis Rheum. 2000;43:1290–1299. doi: 10.1002/1529-0131(200006)43:6<1290::AID-ANR11>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 39.Stadler J., Stefanovic-Racic M., Billiar T.R., Curran R.D., McIntyre L.A., Georgescu H.I., et al. Articular chondrocytes synthesize nitric oxide in response to cytokines and lipopolysaccharide. J. Immunol. 1991;147:3915–3920. [PubMed] [Google Scholar]
- 40.Studer R., Jaffurs D., Stefanovic-Racic M., Robbins P.D., Evans C.H. Nitric oxide in osteoarthritis. Osteoarthritis Cartilage. 1999;7:377–379. doi: 10.1053/joca.1998.0216. [DOI] [PubMed] [Google Scholar]
- 41.Yagi H., Ulici V., Tuan R.S. Polyphenols suppress oxidative stress in bovine articular chondrocytes. Faseb. J. 2012;26 doi: 10.1016/j.ocarto.2020.100064. https://www.fasebj.org/doi/abs/10.1096/fasebj.26.1_supplement.823.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miguel F., Augusto A.C., Gurgueira S.A. Effect of acute vs chronic h2o2-induced oxidative stress on antioxidant enzyme activities. Free Radic. Res. 2009;43:340–347. doi: 10.1080/10715760902751894908658737. [DOI] [PubMed] [Google Scholar]
- 43.Hutadilok N., Smith M.M., Ghosh P. Effects of hydrogen peroxide on the metabolism of human rheumatoid and osteoarthritic synovial fibroblasts in vitro. Ann. Rheum. Dis. 1991;50:219–226. doi: 10.1136/ard.50.4.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Natarajan V., Madhan B., Tiku M.L. Intra-articular injections of polyphenols protect articular cartilage from inflammation-induced degradation: suggesting a potential role in cartilage therapeutics. PloS One. 2015;10 doi: 10.1371/journal.pone.0127165PONE-D-14-37669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Z., Leong D.J., Xu L., He Z., Wang A., Navati M., et al. Curcumin slows osteoarthritis progression and relieves osteoarthritis-associated pain symptoms in a post-traumatic osteoarthritis mouse model. Arthritis Res. Ther. 2016;18:128. doi: 10.1186/s13075-016-1025-y. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 46.Jaruga E., Sokal A., Chrul S., Bartosz G. Apoptosis-independent alterations in membrane dynamics induced by curcumin. Exp. Cell Res. 1998;245:303–312. doi: 10.1006/excr.1998.4225. [DOI] [PubMed] [Google Scholar]
- 47.Khajavi M., Shiga K., Wiszniewski W., He F., Shaw C.A., Yan J., et al. Oral curcumin mitigates the clinical and neuropathologic phenotype of the trembler-j mouse: a potential therapy for inherited neuropathy. Am. J. Hum. Genet. 2007;81:438–453. doi: 10.1086/519926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yano S., Fujimura Y., Umeda D., Miyase T., Yamada K., Tachibana H. Relationship between the biological activities of methylated derivatives of (-)-epigallocatechin-3-o-gallate (egcg) and their cell surface binding activities. J. Agric. Food Chem. 2007;55:7144–7148. doi: 10.1021/jf071176o. [DOI] [PubMed] [Google Scholar]
- 49.Zenda N., Okubo S., Hu Z.Q., Hara Y., Shimamura T. Erythrocyte-dependent mitogenic activity of epigallocatechin gallate on mouse splenic b cells. Int. J. Immunopharm. 1997;19:399–403. doi: 10.1016/s0192-0561(97)00074-x. [DOI] [PubMed] [Google Scholar]
- 50.Pandey K.B., Rizvi S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009;2:270–278. doi: 10.4161/oxim.2.5.94989498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Manach C., Scalbert A., Morand C., Remesy C., Jimenez L. Polyphenols: food sources and bioavailability. Am. J. Clin. Nutr. 2004;79:727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 52.Faggio C., Sureda A., Morabito S., Sanches-Silva A., Mocan A., Nabavi S.F., et al. Flavonoids and platelet aggregation: a brief review. Eur. J. Pharmacol. 2017;807:91–101. doi: 10.1016/j.ejphar.2017.04.009. [DOI] [PubMed] [Google Scholar]
- 53.Xia N., Daiber A., Forstermann U., Li H. Antioxidant effects of resveratrol in the cardiovascular system. Br. J. Pharmacol. 2017;174:1633–1646. doi: 10.1111/bph.13492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yeh C.C., Su Y.H., Lin Y.J., Chen P.J., Shi C.S., Chen C.N., et al. Evaluation of the protective effects of curcuminoid (curcumin and bisdemethoxycurcumin)-loaded liposomes against bone turnover in a cell-based model of osteoarthritis. Drug Des. Dev. Ther. 2015;9:2285–2300. doi: 10.2147/DDDT.S78277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nakagawa Y., Mukai S., Yamada S., Matsuoka M., Tarumi E., Hashimoto T., et al. Short-term effects of highly-bioavailable curcumin for treating knee osteoarthritis: a randomized, double-blind, placebo-controlled prospective study. J. Orthop. Sci. 2014;19:933–939. doi: 10.1007/s00776-014-0633-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chang Y.J., Chang Y.C., Liu R.H., Chen C.W., Lee I., Yang N.C. Resveratrol can be stable in a medium containing fetal bovine serum with pyruvate but shortens the lifespan of human fibroblastic hs68 cells. Oxid. Med. Cell. Longev. 2018;(2018):2371734. doi: 10.1155/2018/2371734. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.