Abstract
Objective
Determine the feasibility of a 6-month exercise and weight management intervention for people with hip osteoarthritis (OA).
Design
18 participants with clinical and radiographic hip OA with a body mass index ≥28 kg/m2 and <41 kg/m2 participated. Six consultations with a physiotherapist and six consultations with a dietitian via videoconferencing over six months to deliver, and support, an exercise program and a ketogenic very low-calorie diet with meal replacements. Recruitment rate and retention rate, adherence, adverse events and intervention acceptability were assessed. Overall hip pain, physical function and body weight were assessed via numeric rating scale (NRS, 0–10), Western Ontario and McMaster Universities Osteoarthritis Index physical function subscale (WOMAC, 0–68) and home-scales respectively, at baseline, 3 and 6 months.
Results
Eighteen (11% of 157 people screened) participants were enrolled and 16 (89%) completed 6-month assessments. Participants reported acceptable adherence to the intervention. Most (88%) participants were “extremely satisfied” with the intervention. Ten minor adverse events were exercise related. Overall hip pain reduced by −1.9 units (95%CI -2.8 to −0.9) at 3 months and by −3.3 (−4.3 to −2.2) at 6 months. Physical function improved by −8.5 units (95%CI -13.2 to −3.6) and −14.2 (−18.1 to −7.5) at 3 and 6 months respectively. Body weight reduced by 9.8% [95%CI -12% to −8%] and 11.3% [-13.6% to −9%] at 3 and 6 months respectively.
Conclusions
The feasibility of a large clinical trial evaluating this exercise and weight management intervention is supported.
Keywords: Hip osteoarthritis, Physiotherapy, Physical therapy, Dietetics, Exercise, Weight loss, Obesity, Pain, Function, Feasibility
Trial registration Australian New Zealand Clinical Trials Registry (ACTRN12619001045101);
1. Introduction
Hip osteoarthritis (OA) is a chronic joint disease that is often painful and disabling. The prevalence of symptomatic hip OA among older adults is 6.2% [1] and up to 85% of those with hip OA are overweight or have obesity [2]. There is no cure for OA and rates of joint replacement surgery for hip OA are exploding, with a life-time risk of hip replacement for hip OA as high as 1 in 7 for women and 1 in 10 for men [3]. Treatments that effectively relieve symptoms and delay the need for joint replacement are critical.
Exercise is strongly recommended in clinical guidelines to manage hip OA symptoms [[4], [5], [6], [7], [8]] based on evidence from several clinical trials. Exercise has small to moderate beneficial effects on pain and physical function in people with hip OA [[9], [10], [11]], with the majority of trials evaluating strengthening exercise. Although there is some evidence supporting the use of exercise to delay hip joint replacement in people with hip OA [12], exercise alone may be insufficient to reduce symptoms adequately and delay the need for joint replacement in those who are overweight or have obesity.
Clinical guidelines for hip OA management provide conflicting recommendations about weight loss. Recent clinical guidelines from the Osteoarthritis Research Society International do not recommend weight loss for hip OA, due to the absence of clinical trial evidence about the effectiveness of weight loss in people with hip OA [5]. In contrast, other clinical guidelines do recommend weight loss for hip OA [4,[6], [7], [8]] based on indirect evidence from studies in knee OA, advising reductions in body weight by 5% for improvement in clinical and mechanistic outcomes [8]. One uncontrolled study in people with hip OA reported a 5% reduction in body weight using a combination of exercise and weight loss that was associated with potentially clinical meaningful improvements in hip OA symptoms [13]. Although adherence to consultations with clinicians supervising the intervention was excellent (≥82%), safety of this face-to-face delivered intervention was not reported.
Method of delivering health care services is an increasingly important consideration to ensure accessibility and scalability of care, particularly in light of the COVID-19 pandemic. Evidence supports the use of telerehabilitation by health professionals including physiotherapists and dietitians to reduce knee pain [14] and body weight [15], respectively. Telerehabilitation is acceptable to people with OA [[16], [17], [18]], physiotherapists [16,19], and is endorsed by national physiotherapy [20] and dietitian professional organisations [21]. However, to our knowledge no previous studies have evaluated a remotely-delivered exercise and weight management program by physiotherapists and dietitians in hip OA.
The objectives of this pilot study were to assess the feasibility of delivering an online 6-month exercise and weight management intervention to people with hip OA and overweight or obesity to determine: i) participant recruitment rates and retention; ii) adherence; iii) safety; iv) acceptability of the intervention, particularly in relation to the weight management component, and; v) changes in hip OA symptoms, body weight and body composition.
2. Methods
2.1. Study design and setting
This study was designed as a pilot study. Reporting followed items of the CONSORT [22] that are applicable to a non-randomised design. This study was prospectively registered in the Australian New Zealand Clinical Trials Registry (ACTRN12619001045101) and conducted at the University of Melbourne.
2.2. Participants
Participants were recruited from the community in Melbourne, Australia between August 2019 and October 2019 via social media (Facebook). Only social media was used to recruit participants due to its reach, efficiency and cost-effectiveness. Eligibility criteria are listed in Table 1.
Table 1.
Eligibility criteria.
| Inclusion criteria | Exclusion criteria |
|---|---|
| i) 50 years or older | i) unstable weight (>2 kg self-reported change over the previous 3 months); |
| ii) hip OA fulfilling the American College of Rheumatology classification criteria of pain and radiographic changes [59]; | ii) on waiting list for or planning spinal or lower limb surgery in next 12 months |
| iii) pain in the groin or hip region on most days of the past month for >3 months | iii) previous arthroplasty on affected hip |
| iv) average hip pain intensity during walking in the previous week ≥4/10 on an 11-point numerical rating scale (NRS) (no pain = 0; worst pain possible = 10), | iv) hip surgery within the past 6 months; |
| v) body mass index (BMI) between 28 kg/m2 and 41 kg/m2 | v) self-reported inflammatory arthritis (e.g. rheumatoid arthritis); |
| vi) unable to give informed consent to participate fully in the intervention and assessment procedures; | |
| vii) inability to weigh themselves; | |
| viii) any neurological condition affecting lower limbs; | |
| ix) inability to safely participate in moderate-intensity exercise as determined by the Exercise and Sports Science Australia [60] pre-exercise screening form, and; | |
| x) unable to undertake a ketogenic very low calorie diet without close supervision of a medical practitioner, including self-reported: a) diagnosis of Type 1 diabetes; b) Type 2 diabetes requiring insulin or other medication apart from metformin; c) warfarin use; d) stroke or cardiac event in previous 6 months; e) unstable cardiovascular condition; f) fluid intake restriction, or; g) severe chronic kidney disease, defined as estimated glomerular filtration rate of <30 mL/min/1.73m2. |
2.3. Procedures
Volunteers were screened via an online survey followed by telephone screening to confirm eligibility. Potentially eligible participants underwent a standard antero-posterior supine pelvic view x-ray. Participants who had a hip x-ray in the prior 12-months and could provide the images to the research team for screening were not required to undergo new x-rays due to ethical concerns of additional radiation exposure. For participants with bilateral symptoms, the most symptomatic eligible hip was regarded as the study hip. Participants were advised to continue with usual medication during the study. Participant-reported data were collected via REDCap online questionnaire and stored on a password-protected server. Body composition data was collected via dual-energy x-ray absorptiometry at the Be Active Sleep Eat Facility, Monash University, Melbourne. Ethical approval was obtained from the Human Research Ethics Committee at the University of Melbourne (#1954589) and all participants provided written informed consent.
2.4. Intervention
The intervention was modelled on a knee OA exercise and weight management intervention [23]. The development of the intervention, including stakeholder involvement, has been described in detail elsewhere [23]. Summary of services/resources provided to participants is detailed in online supplementary Appendix 1. Informed by the Behaviour Change Wheel [24], clinical consultations were based on our previous and ongoing research into exercise [14,[25], [26], [27], [28]] and weight management [23] for hip and knee OA. Behaviour change techniques applicable for exercise [29,30] and weight management [23,30] are embedded into the physiotherapy and dietitian consultations and resources (online supplementary Appendix 2). Our intervention is described using the TIDierR (Template for Intervention Description and Replication) [31] checklist to ensure we provide sufficient detail for replication.
2.5. Exercise program
The Exercise program consisted of educational information and six individual consultations with a physiotherapist over six months via videoconferencing (Zoom Video Communications Inc., California USA). The physiotherapist consultations included education about OA and self-management, as well as an individualised, structured, progressive strengthening exercise program to be performed at home. Educational resources (online supplementary Appendix 1) were provided in hardcopy via post. Participants were also posted four exercise resistance bands (yellow, green, red, blue) and one adjustable (0.5–5.0 kg) ankle-cuff weight for strengthening exercises.
Prior to their first consultation with the physiotherapist and dietitian, participants completed a pre-consultation survey asking about their main problems and goals, previous weight management, a brief history of their hip symptoms, and other health problems. The initial physiotherapy consultation was approximately 45 min long, with follow-up consultations approximately 30 min. Consultations were recommended to occur in weeks 1, 3, 7, 11, 16 and 21, but the timing was flexible and was negotiated between each participant and their physiotherapist. Together with participants, the physiotherapist developed an individualised management plan to include the following components: i) a structured and progressive muscle strengthening exercise program; ii) information about some of the common barriers to exercise and ways to overcome them, and; iii) advice about self-monitoring and managing flare-ups.
The strengthening exercise program was comprised of exercises used in previous trials by the Centre [27] (see online supplementary Appendix 3). The physiotherapists prescribed 4–6 exercises, including a minimum of one from each of hip extensor, hip abductor and hip flexor strengthening and functional exercises. The exact number of exercises, as well as sets/repetitions, were negotiated between the physiotherapist and participant. Intensity was determined using the Borg CR10 Rating of Perceived Exertion [32] where it should feel “hard” to “very hard” (5–7) to perform a full set of each exercise. The study logbooks were used as a motivational tool for participants and for their physiotherapist to assess their progress through the exercise. Participants recorded their program plan in an exercise logbook and were provided with a booklet demonstrating the starting position and instructions for each exercise. Participants were encouraged to complete exercises three times per week. At subsequent consultations, the physiotherapist reviewed participant progress, adjusted the strengthening program as needed (including removing/adding exercises and changing the dosage) and corrected exercise performance where required. Participants were asked to record their exercise completion in their logbook between consultations. Physiotherapists also recommended that participants complete regular arm strengthening exercises (online supplementary Appendix 3), in addition to their leg strengthening program, to reduce the risk of loss of muscle mass during their weight management program.
During each consultation, physiotherapists provided education and advice, prescribed exercises with progressions, discussed modifications/flare-ups and adverse events, as applicable. The physiotherapist checked the participant's logbook to assist with adherence to the exercise program.
2.6. Weight management program
In conjunction with the Exercise program, participants received six individual videoconferencing consultations with a dietitian. Dietitians individualised a weight management program involving a ketogenic very low-calorie diet (VLCD) [33] for each participant. A VLCD has been shown to lead to greater weight loss in the short-term when compared to a low-fat diet [34,35] and the production of ketones by the liver during fatty acid oxidation while fasting or on a diet that restricts carbohydrates, can reduce appetite [33]. Furthermore, participants with obesity are more likely to achieve a weight loss target using a ketogenic VLCD than a standard hypocaloric diet, and the rate of subsequent weight regain is not significantly different between the two dietary approaches [34]. Although at least 5% weight loss is recommended for improvement in OA symptoms [8], we encouraged participants to aim to lose at least 10% or more of their body weight. The 10% weight loss target was selected due to a dose-response relationship between weight loss and symptom improvement in knee OA [36].
Prior to their first dietitian consultation, participants were posted a variety of Optifast® meal replacement products (Nestlé Health Science, Rhodes, Australia) sufficient for two meals per day for one month. Participants received additional Optifast® meal replacement products of their choice as required over the subsequent five months. Optifast® meal replacement products are formulated to provide an adequate supply of micronutrients (vitamins, minerals, and metals), and come as bars, shakes, desserts or soups in a variety of flavours. Additional educational resources to support the participant's weight loss were also provided via post (online supplementary Appendix 1).
The first dietitian consultation was approximately 45 min and took place two to three days following the first physiotherapy consultation. Follow-up consultations were approximately 30 min. Consultations were recommended to occur in weeks 1, 3, 6, 10, between weeks 14–17, and between weeks 19–23, but timing was negotiated between each participant and their dietitian. During the first consultation, appropriate weight loss goals, including a target weight, and a management plan were developed. Participants were encouraged to commence the ketogenic VLCD, which involved replacing two meals, generally breakfast and lunch, with meal replacements. One low-carbohydrate meal (generally dinner) included a source of protein (e.g. white or red meat, fish or seafood, eggs, or tofu) and non-starchy vegetables/salad. A small amount (i.e. 1 tablespoon) of oil/fat was also recommended for this meal to reduce the risk of gallstone formation. In total, the diet contained approximately 800 kilocalories (3280 kJ) per day. The participant could use a modified version of the diet (e.g. one meal replacement only) if deemed necessary (e.g. if they found the meal replacements unpalatable or were having difficulty adhering).
Participants were asked to weigh themselves at home weekly and record their progress in their logbook. During subsequent consultations, dietitians discussed progress and used motivational interviewing principles and techniques to assist motivation [37], self-efficacy, and in overcoming obstacles to completing the agreed self-management plan. Dietitians guided participants through the resource booklets provided, which included activities to help them adhere to their self-management plan such as planning for unforeseen events (e.g. eating out) and choosing a support person.
Once the target weight was achieved, participants were able to choose whether to transition to a weight maintenance phase or continue with the VLCD and aim for further weight loss. Transitioning off the VLCD involved reintroduction of foods containing carbohydrates and consuming only one meal replacement per day. This transition phase lasted at least 2 weeks, after which participants commenced a healthy eating diet consistent with the principles of the Commonwealth Scientific and Industrial Research Organisation Total Wellbeing diet [38] (high protein, low glycaemic index carbohydrate, low fat). In the weight maintenance phase, participants were encouraged to monitor their weight regularly (e.g. once per week). Participants who regained 2 kg or more were advised to restart the ketogenic VLCD for 1–2 weeks. Dietitians used online consultation notes to record details of agreed weight management plans and barriers/motivators that were discussed, and which resources/activities participants were asked to read/complete.
2.7. Physiotherapists
Three private practice physiotherapists in Melbourne delivered the exercise intervention. The physiotherapists had an average of 16 (range 7–29) years of clinical experience since qualification and 11 (range 4–24) years of post-graduate clinical musculoskeletal experience. Only physiotherapists who had treated at least five individuals with hip OA in the past 12 months were eligible to participate. All physiotherapists attended a 4-h training session and were provided with a treatment manual describing the intervention. Telephone meetings were conducted with physiotherapists to address study issues as needed.
2.8. Dietitians
Five dietitians in private practices across Melbourne, Australia delivered the diet intervention. The dietitians had an average of 9 (range 4–19) years of clinical experience since qualification. Only dietitians who had at least two years of clinical experience and some experience assisting people with weight loss were eligible to participate. Dietitians already delivering a comparable weight management plan to people with knee OA in another clinical trial [23] were recruited and provided with a treatment manual describing the intervention for the current study, study protocol and procedures. As part of the clinical trial evaluating the weight management plan in knee OA [23], dietitians completed training in i) best-practice OA management (half day workshop led by researcher); ii) motivational interviewing skills (2-day training course delivered by Health and Wellbeing Training Consultants who specialised in training clinicians in motivational training), and; iii) VLCD (1-h webinar delivered by research team). Further details on training have been previously published [23]. Telephone meetings were conducted with dietitians where necessary to address study issues.
2.9. Descriptive data (baseline)
Age, sex, duration of hip OA symptoms, problems in other joints, comorbidities [39], and OA pain medication were obtained using a questionnaire. Body weight and height were also self-reported and used to determine BMI.
2.10. Adherence and adverse events
The number of dietitian and physiotherapist consultations attended were documented by the therapist. Participants rated their adherence to the i) weight loss management plan, and; ii) exercise program using an 11-point NRS (not at all = 0 to completely as instructed = 10) by responding to “Over the past 6 months, how closely did you follow your weight management program?” and “Over the past 6 months, overall how closely did you follow the strengthening exercise program provided to you by the physiotherapist?” Participants were instructed to report any adverse event to the study coordinator as soon as possible for documentation in an adverse events treatment log. Adverse events were also documented by the physiotherapist when they were made aware of them and collected via self-report in the 6-month questionnaires.
2.11. Acceptability of treatment (6-month follow-up)
Participants rated their perceived usefulness of the dietitian consultations, weight management educational resources, and VLCD on an11-point NRS ranging from “not at all useful” to “extremely useful”. Participants were also asked about how easy it was for them to use the videoconferencing software using an 11-point NRS scale ranging from “not at all easy” to “extremely easy”. Overall satisfaction with treatment was scored on a 7-point Likert scale (“Overall, how satisfied are you with the program you received for your hip pain?”) ranging from “extremely unsatisfied” to “extremely satisfied”.
2.12. Pain (baseline, 3 months and 6 months follow-up)
Overall average hip pain intensity over the past week and during walking was rated via a NRS with terminal descriptors ‘no pain’ (score 0) and ‘worst pain possible’ (score 10). The NRS pain outcome has demonstrated reliability in OA [40,41]. A minimum clinically important change was defined as a change in pain of 1.8 units [42].
2.13. Physical function (baseline, 3 months and 6 months follow-up)
The physical function subscale of the Western Ontario and McMaster Universities (WOMAC) Osteoarthritis Index was used to assess physical function. The subscale contains 17 items, each answered on a Likert scale (no dysfunction = 0 and extreme dysfunction = 4) and ranges from 0 to 68, with higher scores indicating poorer function. The WOMAC is a self-report disease-specific instrument that has established validity, reliability and responsiveness in several OA studies [43,44]. A minimum clinically important change was defined as a change in physical function of 6 units [45].
2.14. Overall change (6-month follow-up)
Participants rated their overall global change in a) pain related to study hip, and; b) physical function related to study hip. The terminal descriptors on the 7-point Likert scales were ‘much worse’ to ‘much better’ [46]. Participants reporting that they were “moderately better” or “much better” were classified as “improved”.
2.15. Body weight and composition (baseline, 3 months [self-reported only] and 6 months follow-up)
Self-reported body weight was recorded at baseline, 3 months and 6 months. Participants were asked to use the same set of scales at the same time of day across the time points. Total body mass, fat mass, lean mass and visceral adipose tissue were measured at baseline and 6 months follow-up using dual-energy x-ray absorptiometry (GE Lunar DXA narrow-angle dual energy x-ray densitometer) according to the manufacturer recommendations for positioning the participant, scan protocols and scan analysis. Participants were asked to fast for 2 h before each DEXA scan, and to avoid engaging in exercise for 2 h before the scan. Excellent test re-test reliability has been reported with GE Lunar DXA for fat mass, lean mass and android mass (intra-class correlations 0.96–0.99) [47], albeit with a protocol not directly comparable our current study. Body weight was expressed in kg and as a percentage of change in weight from baseline, while fat mass and lean mass were expressed as percentage of body mass.
2.16. Sample size
A formal sample size calculation was not performed, due to this being a pilot study [48,49]. We aimed to include 20 participants as we considered this an adequate number to assess the feasibility of the intervention. However, we chose to cease recruitment after 18 participants to ensure we would have pilot data to inform an upcoming grant application.
2.17. Statistical analysis
As this was a pilot study, no hypothesis testing was performed [22]. Data for complete cases (i.e. participants who had data at baseline and follow-up) were used to calculate change scores (follow-up minus baseline) with 95% confidence intervals for continuous outcomes. Categorical data were expressed as number participants (percentage). The number (percentage) of participants who lost 5% and 10% body weight from baseline, as well as the number of participants (percentage) who reached the minimal clinically importance change in each of self-reported pain and physical function were calculated. The percentage of participants are expressed relative to the number of participants with available data per outcome. Stata version 16.0 (Statacorp, College Station, TX, USA) was used for descriptive statistics.
3. Results
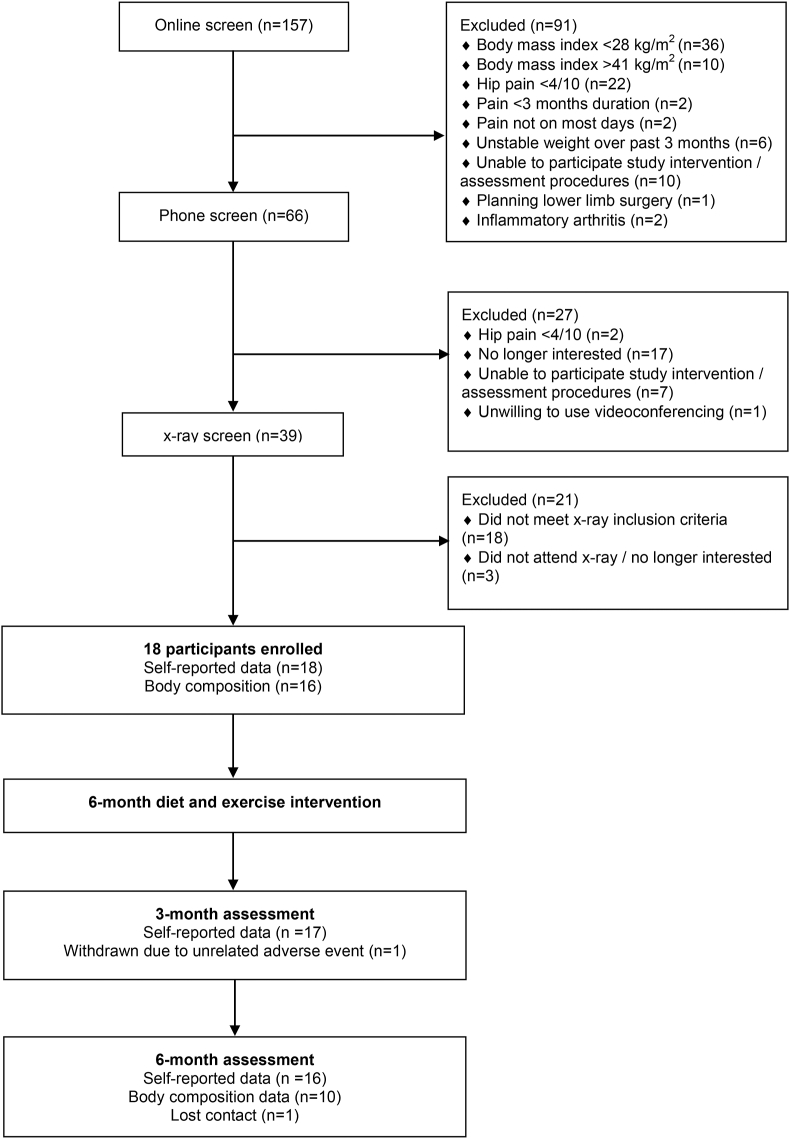
Of the 157 individuals who completed the initial online screening, 66 (42%) completed phone screening, 39 (25%) completed x-ray screening and 18 (11%) participants were enrolled into the study (Fig. 1). Two participants withdrew from the study (one due to an unrelated adverse event and one lost to 6-month follow-up). Sixteen participants underwent a DXA scan at baseline and 10 participants underwent scan at 6 months. Two participants did not undergo DXA scans at baseline due to no available radiographer. At follow-up, four participants were unable to attend their DXA scan due to COVID-19 related closure of the DXA facility, and two participants had withdrawn from the study. Participant characteristics are described in Table 2.
Fig. 1.
Flow of participants through the study.
Table 2.
Participant characteristics (means ± SD, unless otherwise stated).
| n = 18 | |
|---|---|
| Age, years | 64.8 ± 6.5 |
| Sex, female (%) | 16 (89%) |
| Height, m | 1.65 (0.07) |
| Weight, kg | 90.08 (14.26) |
| Body mass index, kg/m2 | 33.1 (4.0) |
| Bilateral symptoms, n(%) | 5 (28%) |
| Duration of symptoms, n(%) | |
| <1 year | 2 (11%) |
| 1–2 years | 9 (50%) |
| 2–5 years | 7 (39%) |
| 5–10 years | 0 (0%) |
| >5 years | 0 (0%) |
| Problem in other joints, number of participants (%) | 18 (100%) |
| Hand | 8 (44%) |
| Neck | 4 (22%) |
| Back | 10 (56%) |
| Knee | 11 (61%) |
| Foot/ankle | 6 (75%) |
| Shoulder | 6 (33%) |
| Current pain medication use, n(%)a | |
| Non-steroidal anti-inflammatory tablets | 6 (33%) |
| Cyclooxygenase-2 inhibitors | 0 (0%) |
| Analgesia, paracetamol | 13 (72%) |
| Topical anti-inflammatories | 7 (39%) |
| Glucosamine or chondroitin | 2 (11%) |
| Topical liniments | 3 (17%) |
| Oral corticosteroids | 0 (0%) |
| Oral opioids | 1 (6%) |
| Comorbidities, n(%) | |
| Heart condition | 2 (11%) |
| High blood pressure | 10 (56%) |
| Lung condition | 0 (0%) |
| Diabetes | 2 (11%) |
| Ulcer | 0 (0%) |
| Kidney condition | 0 (0%) |
| Liver condition | 0 (0%) |
| Anemia | 1 (6%) |
| Cancer | 1 (6%) |
| Depression | 3 (17%) |
| Hypothyroidism | 1 (6%) |
| Vertigo | 1 (6%) |
| Sleep apnoea | 1 (6%) |
| History of fractures | 1 (6%) |
Defined as at least once per week over the past month.
3.1. Adherence and adverse events
All participants attended all 6 dietitian sessions and 16 participants attended at least 5 physiotherapy sessions. Participants reported very good adherence to the weight management plan (mean (SD), [observed range] 7.6 (2.6), [0 to 10]) and the prescribed strengthening exercises (8.3 (1.5), [5 to 10]). Twenty-one adverse events (see online supplementary Appendix 4) were reported by 16 (89%) participants. Eleven were considered unrelated to the intervention and 10 were related to the exercise component of the intervention.
3.2. Perceived usefulness and satisfaction
Eleven (69%) participants were “extremely satisfied” (i.e. selected 10 out of 10 on the NRS) with the usefulness of the weight management education resources, mean (SD), [observed range]: 9.3 (1.4), [[5], [6], [7], [8], [9], [10]]. Fifteen (94%) participants found the dietitian consultations “extremely useful” (i.e. selected 10 out of 10 on the NRS), mean (SD) [observed range]: 9.8 (1), [[6], [7], [8], [9], [10]]. Nine (56%) participants found the VLCD diet “extremely useful” (i.e. selected 10 out of 10 on the NRS) with a mean (SD) [observed range]: 8.9 (1.5), [[5], [6], [7], [8], [9], [10]]. Ten (63%) participants found the videoconferencing software “extremely easy to use” (i.e. selected 10 out of 10 on the NRS), with a mean (SD), [observed range] 9.4 (1.4), [[7], [8], [9], [10]]. Fourteen (88%) participants were “extremely satisfied” (i.e. selected 10 out of 10 on the NRS) with the overall treatment, mean (SD) [observed range]: 9.9 (0.3), [9,10].
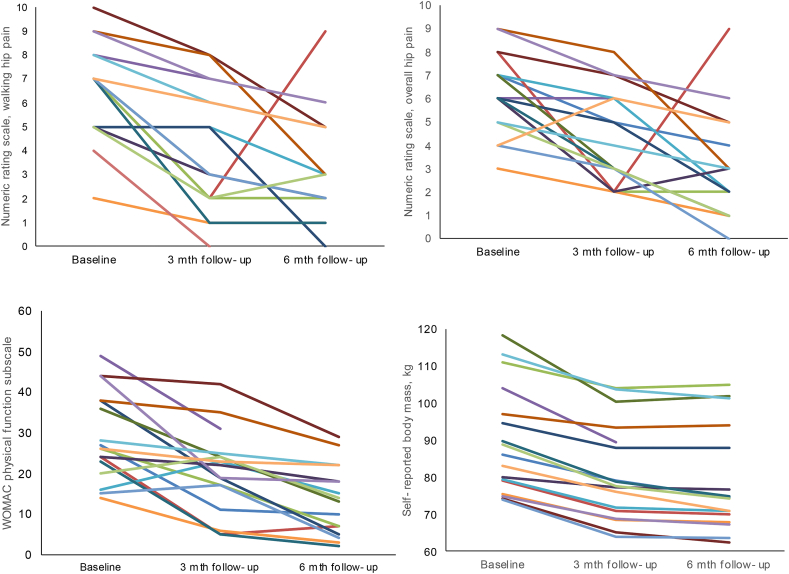
3.3. Symptoms
Pain and physical function data are presented in Table 3. Individual participant trajectories for overall hip pain intensity, hip pain intensity during walking and physical function are illustrated in Fig. 2. All (100%) participants reported their pain on the global rating scale to be at least “slightly better”, with 12 (75%) participants considered to have “improved”. The majority (94%) of participants reported at least “slightly better” for physical function on the global rating scale, with 13 (81%) participants considered to have “improved”. See online supplementary Appendix 5 for details.
Table 3.
Exploratory outcomes.
| Baseline Mean (SD) |
3 months | 6-months Mean (SD) |
Mean (95%CI) 3-mth change 3-mth minus baseline |
Number (%) of participants who met or exceeded MCID at 3-months | Mean (95%CI) 6-mth change 6-mth minus baseline |
Number (%) of participants who met or exceeded MCID at 6-months | |
|---|---|---|---|---|---|---|---|
| Self-reported outcomes | n = 18 | n = 17 | n = 16 | ||||
| Self-reported body weight, kg | 90.3 (14.1) | 81.0 (13.2) | 79.0 (14.3) | −8.8 (−10.7, −6.9) | – | −9.9 (−12.0, −7.8) | – |
| aOverall hip pain, (0–10) | 6.2 (1.7) | 4.4 (2.0) | 3.0 (2.3) | −1.9 (−2.8, −0.9) | 8 (47%) | −3.3 (−4.3, −2.2) | 14 (88%) |
| aHip pain during walking, (0–10) | 6.5 (2.0) | 4.2 (2.4) | 3.2 (2.3) | −2.4 (−3.3, −1.5) | 11 (65%) | −3.4 (−4.5, −2.4) | 14 (88%) |
| WOMAC physical function, (0–68) | 28.3 (10.8) | 20.5 (10.2) | 13.5 (8.6) | −8.5 (−13.2, −3.6) | 9 (53%) | −14.2 (−18.9, −9.5) | 14 (88%) |
| Body composition | n = 16 | n = 10 | |||||
| Total body mass, kg | 89.8 (13.4) | – | 81.4 (15.5) | – | – | −8.4 (−11.7, −5.1) | – |
| Lean mass, kg | 46.5 (9.4) | – | 44.7 (9.5) | – | – | −1.4 (−2.3, −0.6) | – |
| Fat mass, kg | 40.8 (6.6) | – | 34.0 (9.3) | – | – | −7.0 (−9.7, −4.3) | – |
| bLean mass, % | 51.6 (4.5) | – | 55.1 (5.9) | – | – | 3.9 (2.0, 5.7) | – |
| bFat mass, % | 45.6 (4.7) | – | 41.6 (6.2) | – | – | −4.2 (−6.4, −2.3) | – |
| Visceral adipose, kg | 1.61 (0.80) | 1.12 (0.61) | – | – | −0.39 (−0.74, −0.04) | – |
NRS: numeric rating scale (higher score indicates worse pain); WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index (higher score indicates worse function).
Expressed as a percentage of total body mass; SD: standard deviation; CI: confidence interval; MCID: minimal clinical important difference.
Fig. 2.
Individual participant trajectories from baseline to 3- and 6-month follow-up for i) hip pain intensity during walking; ii) overall hip pain intensity; iii) physical function subscale of the Western Ontario and McMaster Universities Osteoarthritis Index, and; iv) self-reported body mass. Higher scores on indicate worse pain and worse physical function.
3.4. Body weight and body composition
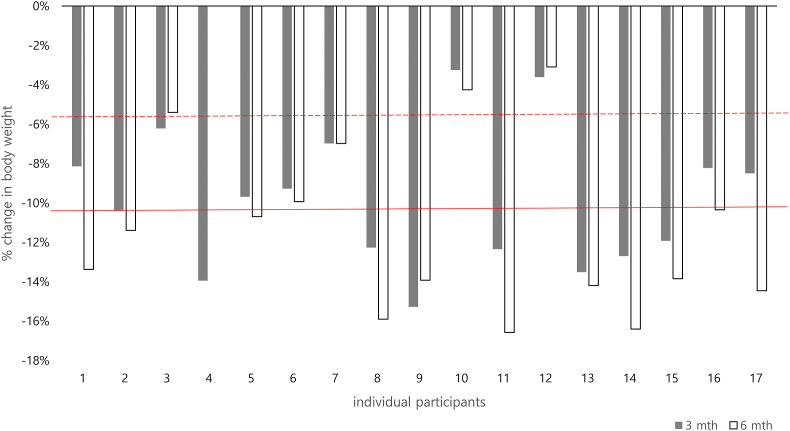
Body weight and body composition data are presented in Table 3. Average self-reported body weight reduced by 10% [95%CI -12% to −8%] at 3 months, and by 11% [-14% to −9%] at 6 months. At 3 months, 15 (88%) participants had lost at least 5% of body weight from baseline, and 8 (50%) participants had lost at least 10% body weight. At 6 months, 14 (82%) participants had lost at least 5% of body weight from baseline, and 11 (75%) participants had lost at least 10% body weight. Individual participant changes in body weight from baseline over time is presented in Figs. 2 and 3. Participants (n = 10) who underwent DXA assessment at 6 months lost 10% [95%CI -13% to −6%] of baseline body weight. On average, fat mass reduced by 18% [95%CI -25% to −11%], lean mass reduced by 3% [95%CI -5% to −1%] and visceral adipose tissue reduced by 25% [95% CI -40% to −11%] from baseline.
Fig. 3.
Individual participant change in self-reported body weight from baseline to 3- and 6-month follow-up. Dashed and solid red horizontal lines indicate 5% and 10% body weight loss from baseline, respectively. Data is missing from one participant at 6 months and from one participant at 3-months and 6-months.
4. Discussion
Our pilot data support the feasibility of a the 6-month online exercise and weight management intervention in hip OA and inform development of a future trial. The majority of participants reported good adherence and perceived the weight management component to be “extremely useful” and the overall intervention as “extremely satisfactory”. Of the 16 out of 18 participants who completed the study at 6 months, 75% of participants lost 10% or more of baseline body weight (which was the goal of the weight loss intervention) and 88% met or exceeded the minimal clinically important change threshold in overall hip pain, hip pain during walking and physical function.
The feasibility of a weight management and exercise intervention is evidenced by recruitment and participation rates. Eighteen participants were enrolled over a 6-week period, extrapolating to a recruitment rate of 12 participants per month. Approximately 11% of those who completed the online, phone screening and x-ray screening were enrolled. A greater number of females (89%) compared to males were enrolled. This is unsurprising as hip OA is more common in females than males [50]. Future clinical trials should consider stratifying by sex to balance the sexes across trial arms [51].
The main reason people failed to be enrolled into the study was failure to fulfil the BMI inclusion criterion (between 28 kg/m2 and 41 kg/m2). The lower limit (28 kg/m2) was set in accordance with recommendations [52] that VLCDs are not indicated for weight loss in people with BMI ≤27 kg/m2. The upper BMI limit was set because people with class 3 obesity (BMI ≥40 kg/m2) may be more likely to have complications of obesity that require medical monitoring during an intensive weight loss intervention, and to require earlier consideration of adjunctive treatments that would exclude them from participation, or require them to discontinue the study (e.g. obesity medications or bariatric surgery). Clinical trialists may wish to consider adjusting the range of BMI to enhance the recruitment rate in future studies. Overall, retention of participants who completed all self-reported outcomes at 6 months was excellent (89%).
Participants adhered to the majority of consultations with both the dietitian and physiotherapist and self-reported relatively high adherence to the weight management plan and exercises prescribed. Overall, these adherence data indirectly indicate the intervention components were acceptable to our participants, supporting use of our exercise and weight management intervention. We consider our intervention safe for our population of interest. Although the majority of our participants reported an adverse event, only 10 of the adverse events reported were related to our intervention, and these were generally minor and consistent with exercise rather than weight management. Although other exercise-related studies in hip OA [[53], [54], [55], [56]] appear to generally report fewer adverse events from fewer participants, the number of adverse events in the present study is consistent with those observed in our previous exercise trials in hip OA [26,27]. It is difficult to compare adverse events across studies, as adverse events depend on various factors such as number of participants, duration of intervention and definition used by investigators. Nevertheless, a review of the exercise program will be undertaken to determine if any of the exercises require modification for a future trial.
All participants reported being almost “extremely satisfied” (9/10) with the overall intervention, warranting further evaluation in a future clinical trial. We also assessed perceived usefulness of the intervention, particularly the weight management components and telehealth-delivery mode to understand if any changes would be required prior to subsequent evaluation in a future clinical trial. Most participants were “extremely satisfied” with usefulness of the weight management education resources (69%) and dietitian consultations (94%). Just over 50% of participants rated the VLCD as “extremely useful”, with all but one participant rating at least 7/10. The majority (63%) of participants rated the videoconferencing software as “extremely easy to use” with all participants rating the ease of use at least 7/10. Overall, our findings support the use of videoconferencing as an acceptable mode to deliver dietitian consultations, supporting previous reports that videoconference dietetic consultations are feasible and well accepted [57].
Our preliminary data support the use of an exercise and weight management intervention involving a ketogenic VLCD to reduce body mass. Based on 6-month data from DXA scans, we observed a 17% [95% CI -25%, −11%] reduction in fat mass from baseline. Of note is the 25% [95% CI -40%, −11%] reduction in visceral adipose tissue, a predictor of all-cause mortality [58]. Future research using DXA scans should consider factors such as hydration status and time of day as these factors may influence accuracy of body composition data. Although participants were encouraged to lose at least 10% of their body weight, clinical recommendations for hip and knee OA suggest that at least 5% of weight loss is associated with changes in clinical outcomes [8] based on knee OA literature. By 3 months, 88% of our participants had already achieved at least 5% weight loss. The use of self-reported body weight may be considered as a limitation and in a large-scale clinical trial it would be preferable to access body weight with a blinded assessor using standardised scales.
In relation to symptom improvement, by 3 months–50% or more participants had reached or exceeded the minimal clinically important change for pain and physical function. By 6 months, most participants (88%) reached or exceeded the clinically meaningful threshold for each of the pain-related and physical function outcomes. Data regarding global change for pain and physical function also support potentially meaningful improvements at 6 months for the majority of participants. No inferences should be made using our data and we caution any extrapolation beyond the exercise and weight management programs used in the current study given the lack of a control group. These data may be used to inform sample size calculations for future clinical trials that aim to investigate whether weight loss and exercise has symptomatic benefits in people with hip OA.
In conclusion, this pilot study provides feasibility and safety data for a remotely delivered exercise and weight management intervention for individuals with hip OA and overweight or obesity that can help inform the conduct of a randomised controlled trial. The weight loss component of the intervention is scalable, given that the meal replacement products are widely available in stores throughout Australia, as is access to dietitians who can deliver the program. The scalability of the intervention is enhanced through delivery of the program via videoconferencing, and well received among patients with obesity and knee OA [18].
Acknowledgments
We sincerely thank the participants who volunteered for this study and the clinicians involved in delivering the weight management (Jodie Prendergast, Marisa Nastasi, Erin Dwyer, Lyndal Collins and Katherine Roberts-Thomson) and exercise program (Andrew Dalwood, Jon Dodd and Paul Kemel).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ocarto.2021.100174.
Authors’ contributions
MH, RSH, KB conceived the idea of the study. KB and MH obtained funding for the study. RSH, PS and KB designed the original intervention for knee OA upon which this intervention was adapted, including participant educational booklets/resources. MH, LS, PS and KLB designed the study protocol and exercise program; LS and MH trained the physiotherapists; LS and GK recruited participants and LS coordinated the study; LS and MH were responsible for data analysis; MH and GK drafted the manuscript and all authors read and approved the final version for submission.
Role of the funding source
The work was supported by the Australia & New Zealand Musculoskeletal Clinical Trials Network and National Health and Medical Research Council (NHMRC) program grant (#109132). MH is supported by a NHMRC Investigator Grant Emerging Leader 1 (#1172928). KLB is supported by a NHMRC Investigator Grant Leadership 2 (#1174431). RSH is supported by a NHMRC Senior Research Fellowship (#1154217). PS is supported by a NHMRC Investigator Grant. Funders had no role in design and conduct of the study; collection, management, data analysis and interpretation; and preparation, review or approval of the manuscript or the decision to submit for publication.
Declaration of competing interest
The authors have no conflicts of interest to declare.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Kim C., Linsenmeyer K.D., Vlad S.C., Guermazi A., Clancy M.M., Niu J., et al. Prevalence of radiographic and symptomatic hip osteoarthritis in an urban United States community: the Framingham osteoarthritis study. Arthritis Rheumatology. 2014;66:3013–3017. doi: 10.1002/art.38795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reyes C., Leyland K.M., Peat G., Cooper C., Arden N.K., Prieto-Alhambra D. Association between overweight and obesity and risk of clinically diagnosed knee, hip, and hand osteoarthritis: a population-based cohort study. Arthritis Rheumatology. 2016;68:1869–1875. doi: 10.1002/art.39707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ackerman I.N., Bohensky M.A., de Steiger R., Brand C.A., Eskelinen A., Fenstad A.M., et al. Lifetime risk of primary total hip replacement surgery for osteoarthritis from 2003 to 2013: a multinational analysis using national registry data. Arthritis Care Res. 2017;69:1659–1667. doi: 10.1002/acr.23197. [DOI] [PubMed] [Google Scholar]
- 4.Royal Australian College of General Practitioners . second ed. RACGP; East Melbourne: 2018. Guideline for the Management of Knee and Hip Osteoarthritis. [Google Scholar]
- 5.Bannuru R.R., Osani M.C., Vaysbrot E.E., Arden N.K., Bennell K., Bierma-Zeinstra S.M.A., et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 2019;27:1578–1589. doi: 10.1016/j.joca.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 6.Fernandes L., Hagen K.B., Bijlsma J.W.J., Andreassen O., Christensen P., Conaghan P.G., et al. EULAR recommendations for the non-pharmacological core management of hip and knee osteoarthritis. Ann. Rheum. Dis. 2013;72:1125–1135. doi: 10.1136/annrheumdis-2012-202745. [DOI] [PubMed] [Google Scholar]
- 7.National Clinical Guideline Centre. Osteoarthritis. Care and Management in Adults. NICE clinical guideline CG177. London; National Institute for Health and Clinical Excellence.
- 8.Kolasinski S.L., Neogi T., Hochberg M.C., Oatis C., Guyatt G., Block J., et al. American College of Rheumatology/Arthritis Foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Rheumatology. 2019;72:220–233. doi: 10.1002/art.41142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moseng T., Dagfinrud H., Smedslund G., Østerås N. The importance of dose in land-based supervised exercise for people with hip osteoarthritis. A systematic review and meta-analysis. Osteoarthritis Cartilage. 2017;25:1563–1576. doi: 10.1016/j.joca.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 10.Fransen M., McConnell S., Hernandez-Molina G., Reichenbach S. Cochrane Database Syst Rev; 2014. Exercise for Osteoarthritis of the Hip; p. CD007912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moseng T., Dagfinrud H., Smedslund G., Østerås N. Corrigendum to 'The importance of dose in land-based supervised exercise for people with hip osteoarthritis. A systematic review and meta-analysis. Osteoarthritis Cartilage. 2018;26:707–709. doi: 10.1016/j.joca.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 12.Svege I., Nordsletten L., Fernandes L., Risberg M.A. Exercise therapy may postpone total hip replacement surgery in patients with hip osteoarthritis: a long-term follow-up of a randomised trial. Ann. Rheum. Dis. 2015;74:164–169. doi: 10.1136/annrheumdis-2013-203628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paans N., van den Akker-Scheek I., Dilling R.G., Bos M., van der Meer K., Bulstra S.K., et al. Effect of exercise and weight loss in people who have hip osteoarthritis and are overweight or obese: a prospective cohort study. Phys. Ther. 2013;93:137–146. doi: 10.2522/ptj.20110418. [DOI] [PubMed] [Google Scholar]
- 14.Bennell K.L., Nelligan R., Dobson F., Rini C., Keefe F., Kasza J., et al. Effectiveness of an internet-delivered exercise and pain-coping skills training intervention for persons with chronic knee pain: a randomized trial. Ann. Intern. Med. 2017;166:453–462. doi: 10.7326/M16-1714. [DOI] [PubMed] [Google Scholar]
- 15.Kelly J.T., Reidlinger D.P., Hoffmann T.C., Campbell K.L. Telehealth methods to deliver dietary interventions in adults with chronic disease: a systematic review and meta-analysis. Am. J. Clin. Nutr. 2016;104:1693–1702. doi: 10.3945/ajcn.116.136333. [DOI] [PubMed] [Google Scholar]
- 16.Lawford B.J., Bennell K.L., Hinman R.S. Consumer perceptions of and willingness to use remotely delivered service models for exercise management of knee and hip osteoarthritis: a cross-sectional survey. Arthritis Care Res. 2017;69:667–676. doi: 10.1002/acr.23122. [DOI] [PubMed] [Google Scholar]
- 17.Lawford B.J., Delany C., Bennell K.L., Hinman R.S. I was really sceptical...But it worked really well": a qualitative study of patient perceptions of telephone-delivered exercise therapy by physiotherapists for people with knee osteoarthritis. Osteoarthritis Cartilage. 2018;26:741–750. doi: 10.1016/j.joca.2018.02.909. [DOI] [PubMed] [Google Scholar]
- 18.Lawford B.J., Bennell K.L., Jones S.E., Keating C., Brown C., Hinman R.S. “It's the single best thing I’ve done in the last 10 years: a qualitiative study exploring patienti and dietitian experience with, and perception of, multi-component dietary weight loss program for knee osteoarthritis. Osteoarthritis Cartilage. 2021;29:507–517. doi: 10.1016/j.joca.2021.01.001. [DOI] [PubMed] [Google Scholar]
- 19.Lawford B.J., Bennell K.L., Kasza J. R.S. Physical therapists' perceptions of telephone- and internet video-mediated service models for exercise management of people with osteoarthritis. Arthritis Care Res. 2018;70:398–408. doi: 10.1002/acr.23260. [DOI] [PubMed] [Google Scholar]
- 20.Australian Physiotherapy Association . 2020. Telehealth Guidelines Response to COVID-19. Melbourne. [Google Scholar]
- 21.Kelly J.T., Allman-Farinelli M., Chen J., Partridge S.R., Collins C., Rollo M., et al. Dietitians Australia position statement on telehealth. Nutr. Diet. 2020;77:406–415. doi: 10.1111/1747-0080.12619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eldridge S.M., Chan C.L., Campbell M.J., Bond C.M., Hopewell S., Thabane L., et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ. 2016;355:i5239. doi: 10.1136/bmj.i5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bennell K.L., Keating C., Lawford B.J., Kimp A.J., Egerton T., Brown C., et al. Better Knee, Better Me™: effectiveness of two scalable health care interventions supporting self-management for knee osteoarthritis - protocol for a randomized controlled trial. BMC Muscoskel. Disord. 2020;21 doi: 10.1186/s12891-020-3166-z. 160-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michie S., van Stralen M.M., West R. The behaviour change wheel: a new method for characterising and designing behaviour change interventions. Implement. Sci. 2011;6:42. doi: 10.1186/1748-5908-6-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bennell K.L., Campbell P.K., Egerton T., Metcalf B., Kasza J., Forbes A., et al. Telephone coaching to enhance a home-based physical activity program for knee osteoarthritis: a randomized clinical trial. Arthritis Care Res. 2017;69:84–94. doi: 10.1002/acr.22915. [DOI] [PubMed] [Google Scholar]
- 26.Bennell K.L., Egerton T., Martin J., Abbott J.H., Metcalf B., McManus F., et al. Effect of physical therapy on pain and function in patients with hip osteoarthritis: a randomized clinical trial. J. Am. Med. Assoc. 2014:3111987–3111997. doi: 10.1001/jama.2014.4591. [DOI] [PubMed] [Google Scholar]
- 27.Bennell K.L., Nelligan R.K., Rini C., Keefe F.J., Kasza J., French S., et al. Effects of internet-based pain coping skills training before home exercise for individuals with hip osteoarthritis (HOPE trial): a randomised controlled trial. Pain. 2018;159:1833–1842. doi: 10.1097/j.pain.0000000000001281. [DOI] [PubMed] [Google Scholar]
- 28.Hinman R.S., Campbell P.K., Lawford B.J., Briggs A.M., Gale J., Bills C., et al. Does telephone-delivered exercise advice and support by physiotherapists improve pain and/or function in people with knee osteoarthritis? Telecare randomised controlled trial. Br. J. Sports Med. 2020;54:790–797. doi: 10.1136/bjsports-2019-101183. [DOI] [PubMed] [Google Scholar]
- 29.Nicolson P.J.A., Hinman R.S., French S.D., Lonsdale C., Bennell K.L. Improving adherence to exercise: do people with knee osteoarthritis and physical therapists agree on the behavioral approaches likely to succeed? Arthritis Care Res. 2018;70:388–397. doi: 10.1002/acr.23297. [DOI] [PubMed] [Google Scholar]
- 30.Michie S., Ashford S., Sniehotta F.F., Dombrowski S.U., Bishop A., French D.P. A refined taxonomy of behaviour change techniques to help people change their physical activity and healthy eating behaviours: the CALO-RE taxonomy. Psychol. Health. 2011;26:1479–1498. doi: 10.1080/08870446.2010.540664. [DOI] [PubMed] [Google Scholar]
- 31.Hoffmann T.C., Glasziou P.P., Boutron I., Milne R., Perera R., Moher D., et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ. 2014;348:g1687. doi: 10.1136/bmj.g1687. [DOI] [PubMed] [Google Scholar]
- 32.Borg G. Human Kinetics; Champaign, IL, US: 1998. Borg's Perceived Exertion and Pain Scales. [Google Scholar]
- 33.Sumithran P., Prendergast L.A., Delbridge E., Purcell K., Shulkes A., Kriketos A., et al. Ketosis and appetite-mediating nutrients and hormones after weight loss. Eur. J. Clin. Nutr. 2013;67:759–764. doi: 10.1038/ejcn.2013.90. [DOI] [PubMed] [Google Scholar]
- 34.Purcell K., Sumithran P., Prendergast L.A., Bouniu C.J., Delbridge E., Proietto J. The effect of rate of weight loss on long-term weight management: a randomised controlled trial. Lancet Diabetes Endocrinol. 2014;2:954–962. doi: 10.1016/S2213-8587(14)70200-1. [DOI] [PubMed] [Google Scholar]
- 35.Nordmann A.J., Nordmann A., Briel M., Keller U., Yancy W.S., Jr., Brehm B.J., et al. Effects of low-carbohydrate vs low-fat diets on weight loss and cardiovascular risk factors: a meta-analysis of randomized controlled trials. Arch. Intern. Med. 2006;166:285–293. doi: 10.1001/archinte.166.3.285. [DOI] [PubMed] [Google Scholar]
- 36.Messier S.P., Resnik A.E., Beavers D.P., Mihalko S.L., Miller G.D., Nicklas B.J., et al. Intentional weight loss in overweight and obese patients with knee osteoarthritis: is more better? Arthritis Care Res. 2018;70:1569–1575. doi: 10.1002/acr.23608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O'Halloran P.D., Blackstock F., Shields N., Holland A., Iles R., Kingsley M., et al. Motivational interviewing to increase physical activity in people with chronic health conditions: a systematic review and meta-analysis. Clin. Rehabil. 2014;28:1159–1171. doi: 10.1177/0269215514536210. [DOI] [PubMed] [Google Scholar]
- 38.Noakes M., Clifton P. Penguin; VIC. Australia: 2005. The CSIRO Total Wellbeing Diet. Camberwell. [Google Scholar]
- 39.Sangha O., Stucki G., Liang M.H., Fossel A.H., Katz J.N. The Self-Administered Comorbidity Questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum. 2003;49:156–163. doi: 10.1002/art.10993. [DOI] [PubMed] [Google Scholar]
- 40.Farrar J.T., Young J.P., Jr., LaMoreaux L., Werth J.L., Poole R.M. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94:149–158. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 41.Bellamy N. Osteoarthritis clinical trials: candidate variables and clinimetric properties. J. Rheumatol. 1997;24:768–778. [PubMed] [Google Scholar]
- 42.Bellamy N., Carette S., Ford P.M., Kean W.F., le Riche N.G., Lussier A., et al. Osteoarthritis antirheumatic drug trials. III. Setting the delta for clinical trials--results of a consensus development (Delphi) exercise. J. Rheumatol. 1992;19:451–457. [PubMed] [Google Scholar]
- 43.Bellamy N., Buchanan W.W., Goldsmith C.H., Campbell J., Stitt L.W. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J. Rheumatol. 1988;15:1833–1840. [PubMed] [Google Scholar]
- 44.McConnell S., Kolopack P., Davis A.M. The western Ontario and McMaster Universities osteoarthritis index (WOMAC): a review of its utility and measurement properties. Arthritis Care Res. 2001;45:453–461. doi: 10.1002/1529-0131(200110)45:5<453::aid-art365>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 45.Tubach F., Ravaud P., Martin-Mola E., Awada H., Bellamy N., Bombardier C., et al. Minimum clinically important improvement and patient acceptable symptom state in pain and function in rheumatoid arthritis, ankylosing spondylitis, chronic back pain, hand osteoarthritis, and hip and knee osteoarthritis: results from a prospective multinational study. Arthritis Care Res. 2012;64:1699–1707. doi: 10.1002/acr.21747. [DOI] [PubMed] [Google Scholar]
- 46.ten Klooster P.M., Drossaers-Bakker K.W., Taal E., van de Laar M.A. Patient-perceived satisfactory improvement (PPSI): interpreting meaningful change in pain from the patient's perspective. Pain. 2006;121:151–157. doi: 10.1016/j.pain.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 47.Carvar T.E., Christou N.V., Anderson R.E. In vivo precision of the GE iDXA for the assessment of total body composition and fat distribution in severly obese patients. Obesity. 2013;21:1367–1369. doi: 10.1002/oby.20323. [DOI] [PubMed] [Google Scholar]
- 48.Arain M., Campbell M.J., Cooper C.L., et al. What is a pilot or feasibility study? A review of current practice and editorial policy. BMC Med. Res. Methodol. 2010;10:67. doi: 10.1186/1471-2288-10-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Billingham S.A., Whitehead AL A.L., Julious S.A. An audit of sample sizes for pilot and feasibility trials being undertaken in the United Kingdom registered in the United Kingdom Clinical Research Network database. BMC Med. Res. Methodol. 2013;13:104. doi: 10.1186/1471-2288-13-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Y., Jordan J.M. Epidemiology of osteoarthritis. Clin. Geriatr. Med. 2010;26:355–369. doi: 10.1016/j.cger.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Beavers K.M., Neiberg R.H., Kritchevsky S.B., Nicklas B.J., Kitzman D.W., Messier S.P., et al. Association of sex or race with the effect of weight loss on physical function: a secondary analysis of 8 randomized clinical trials. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.14631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.National Health. Medical Research Council . 2013. Clinical Practice Guidelines for the Management of Overweight and Obesity in Adults, Adolescents and Children in Australia. [Google Scholar]
- 53.Fernandes L., Storheim K., Sandvik L., Nordsletten L., Risberg M.A. Efficacy of patient education and supervised exercise vs patient education alone in patients with hip osteoarthritis: a single blind randomized clinical trial. Osteoarthritis Cartilage. 2010;18:1237–1243. doi: 10.1016/j.joca.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 54.Hermann A., Holsgaard-Larsen A., Zerahn B., Mejdahl S., Overgaard S. Preoperative progressive explosive-type resistance training is feasible and effective in patients with hip osteoarthritis scheduled for total hip arthroplasty--a randomized controlled trial. Osteoarthritis Cartilage. 2016;24:91–98. doi: 10.1016/j.joca.2015.07.030. [DOI] [PubMed] [Google Scholar]
- 55.Hoeksma H.L., Dekker J., Ronday H.K., Heering A., Van Der Lubbe N., Vel C., et al. Comparison of manual therapy and exercise therapy in osteoarthritis of the hip: a randomized clinical trial. Arthritis Care Res. 2004;51:722–729. doi: 10.1002/art.20685. [DOI] [PubMed] [Google Scholar]
- 56.Krauß I., Steinhilber B., Haupt G., Miller R., Martus P., Janßen P. Exercise therapy in hip osteoarthritis--a randomized controlled trial. Dtsch Arztebl Int. 2014;111:592–599. doi: 10.3238/arztebl.2014.0592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Raven M., Bywood P. Primary Health Care Research & Information Service; Adelaide: 2013. Allied Health Video Consultation Services. PHCRIS Policy Issue Review. [Google Scholar]
- 58.Kuk J.L., Katzmarzyk P.T., Nichaman M.Z., Church T.S., Blair S.N., Ross R. Visceral fat is an independent predictor of all-cause mortality in men. Obesity. 2006;14:336–341. doi: 10.1038/oby.2006.43. [DOI] [PubMed] [Google Scholar]
- 59.Altman R., Alarcón G., Appelrouth D., Bloch D., Borenstein D., Brandt K., et al. The American College of Rheumatology criteria for the classification and reporting of osteoarthritis of the hip. Arthritis Rheum. 1991;34:505–514. doi: 10.1002/art.1780340502. [DOI] [PubMed] [Google Scholar]
- 60.Norton K., Norton L. Exercise and Sports Science Australia; Australia: 2012. Pre-existing Screening: Guide to the Australian Adult Pre-exercise Screening System. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.