Abstract
Objectives
To investigate the role of osteoarthritis (OA) in the incidence of musculoskeletal, metabolic, cardiovascular, digestive, neuro-psychological, kidney and other comorbidities/adverse events after (i) incident non-steroidal anti-inflammatory drug (NSAID) initiation and (ii) NSAID discontinuation.
Methods
We used register data for the population of Skåne, Sweden. For (i), we analysed the association between starting NSAIDs and the risk of incident outcome events in the 6 years following NSAID dispensation among people with prevalent OA vs no OA. For (ii) we studied the effect of discontinuing NSAIDs among people with and without OA up to 120 days. We used flexible parametric models to estimate adjusted differences in cumulative incidence with NSAIDs as time-varying exposure in the presence of non-proportional hazards.
Results
For (i) we included between 243,832 and 409,749 persons. In the whole cohort, over the 6 years of follow-up, NSAID initiation was associated with a 3% (metabolic) to 16% (musculoskeletal) higher cumulative incidence of outcomes compared to non-users. The difference between those initiating NSAIDs vs non-users was similar in those with and without OA for most outcomes, but in those with OA the increase was lower for neuro-psychological (95%CI: 3.7%–4.6% vs 7.1%–7.9%) and musculoskeletal comorbidities (12%–14.5% vs 16.2%–17.2%).
In (ii), we found no interaction between OA and NSAID discontinuation. NSAID discontinuation was associated with decreased risks for most of the outcomes, from −1.3% for musculoskeletal to −0.4% for cardiovascular comorbidities.
Conclusions
OA appears to have little influence on the increased risk of comorbidities observed after NSAID initiation or decrease after discontinuation.
Keywords: Osteoarthritis, Knee, Hip, NSAID, Comorbidity
1. Introduction
Osteoarthritis (OA) is the most common joint disease worldwide [1]. Pain is a cardinal symptom and, in the absence of disease-modifying treatments, pain is the main target of current management strategies [2]. Historically, OA has been considered a joint-specific “wear and tear” disease, however, decades of research has revealed that it is a complex disorder with multiple genetic and environmental risk factors [3], which may also increase the risk of other chronic conditions [4,5]. In accordance, a recent systematic review reported a pooled comorbidity prevalence of 67% in people with OA; about 20% higher than age and sex-matched controls without OA [6].
Oral non-steroidal anti-inflammatory drugs (NSAIDs) are the most commonly used pharmaceutical for pain control and are at least conditionally recommended by most clinical practice guidelines as second-line treatments when exercise, weight-loss and self-management do not provide satisfactory pain reductions [2,7]. However, NSAIDs are often preferred to other treatment strategies and are together with opioids the most frequently used treatment for people with OA in the US, ahead of physical therapy [8]. Considering the current “opioid crisis” and the effort to reduce opioids prescriptions, NSAID use is likely to further increase in the management of long-lasting pain [9].
NSAIDs have shown short term effectiveness in pain reduction in OA [10], however, their long-term use may be a leading cause of drug-related morbidity [11]. This can potentially be accentuated in people with OA who often are elderly and/or at higher risk of developing comorbidities, e.g. due to low-grade inflammation and/or physical inactivity [5]. NSAIDs and OA may even hypothetically interact and result in more than additive risk. A recent report has indicated that the use of NSAIDs mediates up to 40% of the association between OA and cardiovascular (CV) disease [12]. Given the chronic nature of OA symptoms and the resulting need for long-term therapeutic solutions, assessing the risks of developing comorbidities in people with an incident NSAID dispensation is important [10].
Thus, our aim was to use data from everyday clinical settings to investigate whether people with OA experience a higher risk of comorbidities and adverse events (AEs) after NSAIDs dispensation and discontinuation when compared to controls without OA.
2. Methods
2.1. Data source
We used register data for the entire population of Skåne, the southernmost region in Sweden with 1.4 million inhabitants (13% of the total Swedish population). The Skane Healthcare Register (SHR) holds details for primary, secondary and in-patient care given in the region. For each visit to a physician, the date, personal identification number, details on the clinic or primary care unit, and diagnostic codes according to ICD-10 system are registered. The Prescribed Drug Register contains information on all drugs prescribed and dispensed at one of the pharmacies in the country with data available from July 2005. The study was approved by the Regional Ethical Review Board in Gothenburg (1059–16).
2.2. Inclusion criteria
We identified individuals aged 40 years or older on Dec 31st, 2009 and with residence in the Skåne region between January 1st, 1998 and Dec 31st, 2009 with at least one healthcare visit during this time.
2.3. Exclusion criteria
All people with any prescription of NSAIDs within two years before the start of the study (January 1, 2010) were excluded.
2.4. Exposure
For the current study, we analysed the use of the following NSAIDs: Ketoprofen (M01AE03), Tenoxicam (M01AC02), Ketorolac (M01AB15), Diclofenac (M01AB05), Ibuprofen (M01AE01), Naproxen (M01AE02), Dexibuprofen (M01AE14), Nabumetone (M01AX01), indomethacin (M01AB01), piroxicam (M01AC01), Naproxen (M01AE52), Celecoxib (M01AH01), Rofecoxib (M01AH02), Valdecoxib (M01AH03), Parecoxib (M01AH04), Etoricoxib (M01AH05), Lumiracoxib (M01AH06), Polmacoxib (M01AH07), Meloxicam (M01AC06). We performed two sub-studies to evaluate both initiation and discontinuation of NSAID use. For substudy one we used the incident use of NSAIDs (any type) as exposure. Substudy two was done only in persons receiving NSAID and the exposure of interest was stopping the NSAIDs, i.e. to evaluate recent and past drug use vs current use. To establish the duration of NSAID use we used the number of recommended daily-dose included in one dispensation.
3. Outcomes
A large set of >50 conditions were selected from a previous systematic review and previous studies from Sweden and England studying the association between OA and incident comorbidities [[4], [5], [6]]. The conditions were grouped into musculoskeletal (MSK), metabolical, neuro-psychological, CV, kidney, digestive and other AEs/comorbidities (supplementary file 1A). The outcome of interest was a new diagnosis (ICD-10 code) of any of the outcome events grouped as above. In the analysis of the incidence of each comorbidity, individuals with a diagnostic code of that specific condition between January 1, 1998 and December 31, 2009 were excluded from the analysis for that outcome event but were included in the others. Among the analysed conditions, we included several common NSAID AEs that we analysed separately from the other conditions in a sensitivity analysis expecting an increased risk of development of these conditions after incident NSAIDs dispensation as well as a reduction after NSAID cessation (supplementary file 1B).
3.1. Definition of OA
We identified persons with an OA diagnosis in peripheral joints (spine excluded), i.e. of the hip, knee, ankle/foot, wrist/hand, generalized or unspecific as recorded by a physician at a healthcare visit, i.e., at least one M15 to M19 ICD-10 code, between January 1st, 1998 and Dec 31st, 2019. All persons without OA diagnosed during this time were considered as not having OA.
3.2. Study design
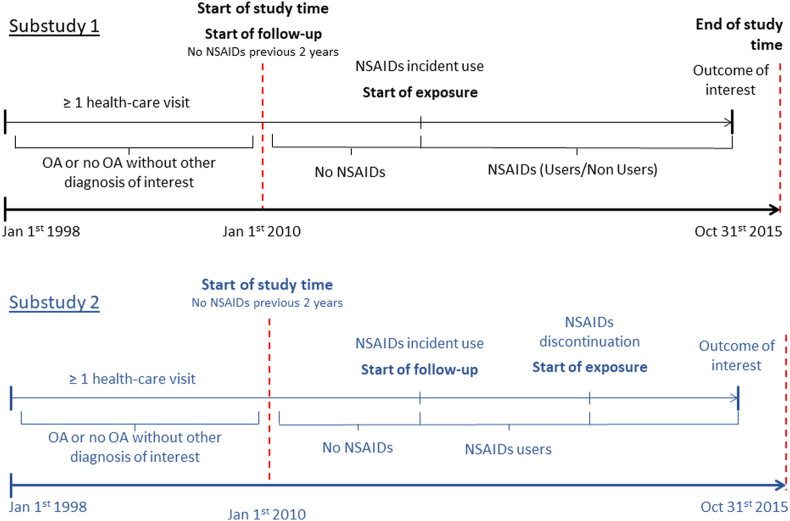
Substudy 1): In this substudy, we used January 2010 as the start of follow-up for all persons (Fig. 1). We considered a person as an incident NSAID user from the first date of NSAID dispensation. We followed all persons until the end of the study (Oct 31st, 2015), death or the development of the outcome event. Time from start of follow-up to the first NSAID dispensation date was treated as unexposed time (non-user) and so the exposure was time-varying.
Fig. 1.
Study design. OA: Osteoarthritis; NSAIDs: non-steroidal anti-inflammatory drugs.
Substudy 2): we performed this substudy in all persons receiving at least one NSAID dispensation, with or without OA. The start of the exposure was the end of the NSAID dispensation. To account for long-term use, all drug dispensations with a break shorter than 90 days were considered as a single drug episode. Thus, the exposure (stopping NSAID use) started at the end of the first drug episode, i.e. at least 90 days after the first dispensation. The participants were followed for the following 120 days, death or the development of one of the outcomes. We chose 120 days based on the observed NSAID use patterns, so that there are both exposure and unexposed during the whole follow-up.
3.3. Confounders
Sex, age, marital status, if born in Sweden, residential area, income, education in years, comorbidities and healthcare use were considered confounders as they can potentially influence both exposure (incident NSAID use) and outcome (incident comorbidity). For included individuals, individual-level information on income, education, marital status, and country of birth, as reported in the year 2009, were retrieved from the LISA register held by Statistics Sweden. We categorized education according to its length: <10 years, 10–12 years, 13–14 years and ≥15 years. Marital status (married/registered partner or other) and country of birth (Sweden or outside Sweden) were binary, while income was divided into 6 categories by 10th, 25th, 50th, 75th and 90th percentile. The residential area was extracted from the Swedish Population Register and was included as municipality (Lund, Malmö, Helsingborg [the three largest municipalities in the region] or other). Information on comorbidities between January 1, 1998 and December 31, 2009 was retrieved from the SHR and the presence of each comorbidity of interest was included as dummy variable. Healthcare use was operationalised as: a) if having an inpatient stay, visits to a physician in specialist care (0,1–2, 3+), visits to a physician in primary care (0,1–2, 3+) or visits to other (not physicians) healthcare professionals (0, 1–2, 3+). Information on all covariates was collected up to the beginning of follow-up (January 1, 2010) and was not updated after to avoid adjusting for intermediates.
3.4. Analysis
In both substudy one and two we used flexible parametric models fitted on hazard scale using restricted cubic splines to estimate differences in cumulative incidence as well as the hazard with NSAIDs as time-varying exposure in the presence of non-proportional hazards [13]. Separate analyses were performed for each group of conditions. As the primary outcome measure, we provided the standardized difference in the cumulative incidence of the outcome event at 6 years follow-up for substudy one and 120 days for substudy two. We also provided hazard per 100,000 persons. The model included OA status (yes or no), NSAID use (yes or no, as a time-varying variable), their interaction, and was adjusted for age, sex, education, presence of comorbidities (including all the outcome events), location of OA, and healthcare use (only for substudy one). We allowed for non-proportional hazards for OA, NSAID use and their interaction. We used three degrees of freedom for baseline hazard function and two for each time-dependent effect. We chose the time-on-drug as the time scale and used robust standard errors in the estimation [14]. This was done separately for each group of outcome events.
4. Results
4.1. Substudy one, incident use of NSAID
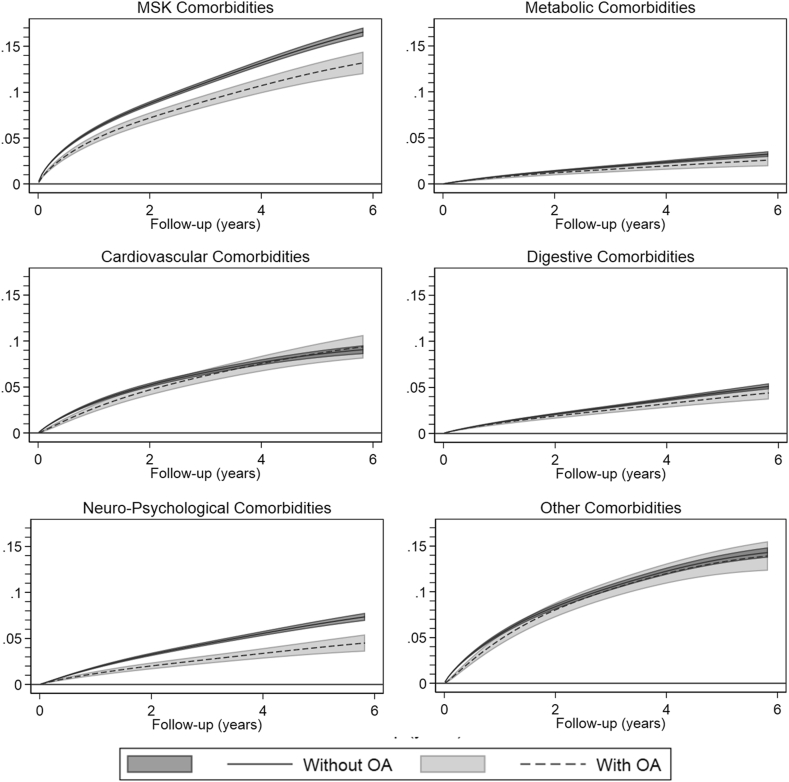
We included between 243,832 and 409,749 persons in the analyses, with mean age of 60 years, 50% women and 8% with OA in at least one peripheral joint (Table 1). Over the 6-year study period, the difference in cumulative incidence of comorbidities between people who took NSAIDs and people who did not varied between 3.2% (95% CIs 3.1%; 3.3%) for metabolic comorbidities to 16.3% (95% CIs 16.2%; 16.4%) for MSK comorbidities signifying a higher incidence of all the analysed comorbidities in people using NSAIDs. (Fig. 2; supplementary file 2). For most comorbidities, we found no essential interaction between OA status and NSAID use, suggesting that the risks associated with NSAIDs are not exacerbated in persons with OA. However, the association between NSAID use and neuro-psychological and MSK disorders was different among persons with and without OA. In those with OA, NSAID use was associated with a 4.7% (95% CIs: 3.7%; 5.6%) cumulative incidence of mental disorders at 6 years of follow-up, while in those without OA the corresponding estimate was 7.5% (95% CIs: 7.1%; 7.9%). Similarly, in those with OA NSAID use was associated with a 13.3% (95% CIs: 12.0%; 14.5%) cumulative incidence of MSK disorders at 6 years of follow-up, while in those without OA the corresponding estimate was 16.7% (95% CIs: 16.2%; 17.2%). The hazard (instantaneous risk per 100,000 persons) of the comorbidities increased sharply after initiation of the treatment with NSAIDs for all the studied outcome events and diminished slowly over time, while it was slowly increasing in persons not initiating an NSAID treatment (Supplementary file 3). The sensitivity analysis showed a similarly increased risk of developing well-known AEs after NSAID initiation regardless of the presence of OA.
Table 1.
Demographics stratified by outcome for substudy one.
| Musculoskeletal | Metabolic | Cardiovascular | Digestive | Neuro-Psychological | Kidney | Other | Adverse Events | |
|---|---|---|---|---|---|---|---|---|
| Subjects, n | 372,251 | 428,277 | 329,416 | 431,351 | 395,347 | 478,800 | 278,685 | 334,842 |
| Age in years, mean (SD) | 59.2 (13.1) | 59.1 (13.2) | 55.8 (11.6) | 59.6 (13.3) | 59.8 (13.2) | 59.9 (13.3) | 55.7 (11.2) | 56.5 (12.0) |
| Sex, n (%) | ||||||||
| Women | 184,477 (49.6%) | 216,409 (50.5%) | 169,701 (51.5%) | 219,389 (50.9%) | 195,460 (49.4%) | 246,408 (51.5%) | 134,891 (48.4%) | 170,671 (51.0%) |
| Men | 187,774 (50.4%) | 211,868 (49.5%) | 159,715 (48.5%) | 211,962 (49.1%) | 199,887 (50.6%) | 232,392 (48.5%) | 143,794 (51.6%) | 164,171 (49.0%) |
| OA, n (%) | 31,328 (8.4%) | 41,295 (9.6%) | 23,421 (7.1%) | 42,017 (9.7%) | 39,588 (10.0%) | 49,852 (10.4%) | 19,119 (6.9%) | 24,439 (7.3%) |
| OA + NSAID, n (%) | 8657 (2.3%) | 12,862 (3.0%) | 7324 (2.2%) | 12,869 (3.0%) | 11,918 (3.0%) | 15,922 (3.3%) | 5464 (2.0%) | 7203 (2.2%) |
| Education, n (%) | ||||||||
| 9 years or less | 98,949 (26.6%) | 113,120 (26.4%) | 73,316 (22.3%) | 117,240 (27.2%) | 107,580 (27.2%) | 132,795 (27.7%) | 64,701 (23.2%) | 76,469 (22.8%) |
| 10 to 12 years | 161,187 (43.3%) | 188,622 (44.0%) | 150,037 (45.5%) | 188,595 (43.7%) | 171,644 (43.4%) | 209,315 (43.7%) | 127,312 (45.7%) | 150,987 (45.1%) |
| 13 to 14 years | 46,370 (12.5%) | 52,873 (12.3%) | 44,478 (13.5%) | 52,303 (12.1%) | 48,341 (12.2%) | 57,175 (11.9%) | 36,654 (13.2%) | 44,619 (13.3%) |
| 15 years or more | 62,984 (16.9%) | 70,648 (16.5%) | 59,696 (18.1%) | 70,025 (16.2%) | 64,943 (16.4%) | 75,976 (15.9%) | 48,339 (17.3%) | 60,725 (18.1%) |
| Married, n (%) | 270,133 (72.6%) | 312,326 (72.9%) | 242,393 (73.6%) | 312,998 (72.6%) | 291,484 (73.7%) | 347,995 (72.7%) | 205,336 (73.7%) | 246,142 (73.5%) |
| Outcome, n (%) | 42,600 (11.4%) | 21,371 (5.0%) | 53,566 (16.3%) | 21,493 (5.0%) | 40,738 (10.3%) | 8317 (1.7%) | 59,895 (21.5%) | 58,447 (17.5%) |
| Comorbidities, n (%) | ||||||||
| MSK | – | 81,181 (19.0%) | 54,070 (16.4%) | 79,646 (18.5%) | 70,975 (18.0%) | 94,942 (19.8%) | 42,561 (15.3%) | 54,568 (16.3%) |
| Metabolic | 35,975 (9.7%) | – | 16,980 (5.2%) | 43,811 (10.2%) | 39,532 (10.0%) | 50,693 (10.6%) | 19,828 (7.1%) | 18,137 (5.4%) |
| Cardiovascular | 99,784 (26.8%) | 109,607 (25.6%) | – | 121,775 (28.2%) | 110,691 (28.0%) | 141,134 (29.5%) | 54,124 (19.4%) | 20,019 (6.0%) |
| Digestive | 31,308 (8.4%) | 40,671 (9.5%) | 25,772 (7.8%) | – | 34,769 (8.8%) | 48,449 (10.1%) | 18,404 (6.6%) | 10,512 (3.1%) |
| Neuro-Psychological | 55,467 (14.9%) | 69,633 (16.3%) | 48,259 (14.6%) | 67,687 (15.7%) | – | 81,356 (17.0%) | 38,298 (13.7%) | 47,750 (14.3%) |
| Kidney | 3682 (1.0%) | 3992 (0.9%) | 1268 (0.4%) | 4699 (1.1%) | 4501 (1.1%) | – | 1535 (0.6%) | – |
| Other | 135,120 (36.3%) | 160,562 (37.5%) | 101,523 (30.8%) | 162,088 (37.6%) | 148,568 (37.6%) | 189,301 (39.5%) | – | 105,007 (31.4%) |
n: number; SD: Standard Deviation; OA: Osteoarthritis; MSK: Musculoskeletal
Fig. 2.
Difference in cumulative incidence between persons initiating non-steroidal anti-inflammatory drugs (NSAIDs) and non-users, stratified by the presence of OA. Positive values signify an increase in the incidence among people initiating NSAIDs. OA: Osteoarthritis; MSK: Musculoskeletal.
4.2. Substudy two, stopping NSAID use
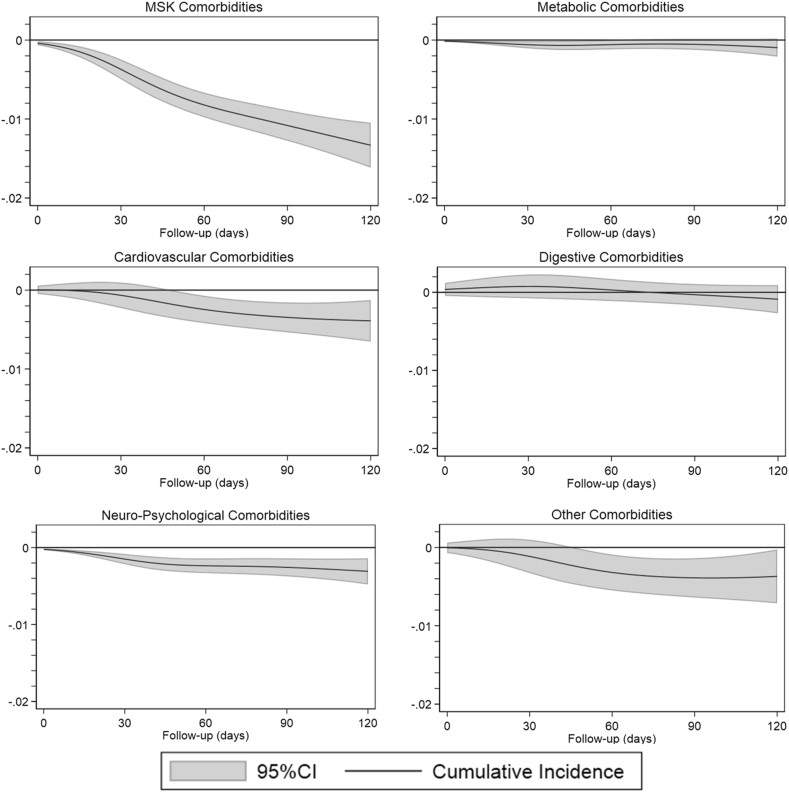
We included between 151,178 and 239,003 persons in the analysis, with mean age between 54 and 59 years, 50% women and 5% with OA in at least one peripheral joint (Table 2). During the 120 days of study period, the difference in cumulative incidence between people who stopped using NSAIDs and people who continued varied between −1.3% (95% CIs: 1.6%; −1.0%) for MSK comorbidities to −0.1% (95% CIs: 0.2%; 0.0%) for metabolic comorbidities signifying a reduction in the incidence of comorbidities for people who stopped using NSAIDs (Fig. 3, supplementary file 4). Overall, we found no interaction between OA status and stopping NSAIDs, suggesting that stopping NSAIDs is equally beneficial for persons with and without OA (Supplementary file 5). Stopping NSAIDs was associated with lower hazards of developing our outcome events when compared to people using NSAIDs (Supplementary file 6). However, only for MSK comorbidities, the hazard of people stopping NSAIDs was lower for the whole study period when compared to the hazard experienced by people using NSAIDs. The sensitivity analysis showed a decrease in the risk of developing well-known AEs after NSAID discontinuation regardless of the presence of OA.
Table 2.
Demographics stratified by outcome for substudy two.
| MSK | Metabolic | Cardiovascular | Digestive | Neuro-Psychological | Kidney | Other | Adverse Events | |
|---|---|---|---|---|---|---|---|---|
| Subjects, n | 156,375 | 208,418 | 153,543 | 206,401 | 184,865 | 239,003 | 120,013 | 151,178 |
| Age in years, mean (SD) | 57.6 (11.8) | 57.7 (12.1) | 54.5 (10.6) | 58.1 (12.2) | 58.6 (12.1) | 58.4 (12.2) | 54.3 (10.0) | 54.8 (10.8) |
| Sex, n (%) | ||||||||
| Women, n (%) | 78,625 (50.3%) | 106,679 (51.2%) | 81,070 (52.8%) | 106,623 (51.7%) | 91,394 (49.4%) | 125,215 (52.4%) | 58,626 (48.8%) | 78,850 (52.2%) |
| Men, n (%) | 77,750 (49.7%) | 101,739 (48.8%) | 72,473 (47.2%) | 99,778 (48.3%) | 93,471 (50.6%) | 113,788 (47.6%) | 61,387 (51.2%) | 72,328 (47.8%) |
| OA, n (%) | 15,464 (9.9%) | 23,303 (11.2%) | 13,170 (8.6%) | 23,270 (11.3%) | 21,566 (11.7%) | 28,886 (12.1%) | 9805 (8.2%) | 12,943 (8.6%) |
| OA + NSAID, n (%) | 6807 (4.4%) | 10,441 (5.0%) | 5846 (3.8%) | 10,401 (5.0%) | 9648 (5.2%) | 12,964 (5.4%) | 4341 (3.6%) | 5740 (3.8%) |
| Total daily doses, mean (SD) | 43.8 (93.2) | 45.0 (97.0) | 44.8 (95.4) | 45.3 (96.9) | 44.9 (96.6) | 45.3 (97.6) | 44.6 (93.5) | 44.8 (95.5) |
| Education, n (%) | ||||||||
| 9 years or less | 38,278 (24.5%) | 51,984 (24.9%) | 32,200 (21.0%) | 52,723 (25.5%) | 47,562 (25.7%) | 62,852 (26.3%) | 26,095 (21.7%) | 31,654 (20.9%) |
| 10 to 12 years | 70,728 (45.2%) | 95,915 (46.0%) | 72,952 (47.5%) | 94,238 (45.7%) | 83,927 (45.4%) | 109,129 (45.7%) | 57,803 (48.2%) | 71,261 (47.1%) |
| 13 to 14 years | 20,125 (12.9%) | 26,149 (12.5%) | 20,913 (13.6%) | 25,635 (12.4%) | 22,966 (12.4%) | 29,038 (12.1%) | 15,812 (13.2%) | 20,713 (13.7%) |
| 15 years or more | 26,550 (17.0%) | 33,474 (16.1%) | 26,953 (17.6%) | 32,809 (15.9%) | 29,579 (16.0%) | 36,818 (15.4%) | 19,902 (16.6%) | 27,023 (17.9%) |
| Married, n (%) | 119,242 (76.3%) | 158,967 (76.3%) | 117,317 (76.4%) | 156,911 (76.0%) | 142,767 (77.2%) | 181,864 (76.1%) | 91,938 (76.6%) | 115,544 (76.4%) |
| Outcome, n (%) | 4116 (2.6%) | 1076 (0.5%) | 2860 (1.9%) | 1302 (0.6%) | 1914 (1.0%) | 415 (0.2%) | 3289 (2.7%) | 3019 (2.0%) |
| Comorbidities, n (%) | ||||||||
| MSK | – | 69,184 (33.2%) | 45,789 (29.8%) | 66,681 (32.3%) | 57,892 (31.3%) | 82,018 (34.3%) | 34,275 (28.6%) | 44,239 (29.3%) |
| Metabolic | 18,715 (12.0%) | – | 10,569 (6.9%) | 26,163 (12.7%) | 23,018 (12.5%) | 32,264 (13.5%) | 11,045 (9.2%) | 10,422 (6.9%) |
| Cardiovascular | 48,883 (31.3%) | 64,390 (30.9%) | – | 69,489 (33.7%) | 61,905 (33.5%) | 84,730 (35.5%) | 28,904 (24.1%) | 9836 (6.5%) |
| Digestive | 18,181 (11.6%) | 28,079 (13.5%) | 17,574 (11.4%) | – | 22,582 (12.2%) | 34,519 (14.4%) | 11,333 (9.4%) | 6597 (4.4%) |
| Neuro-Psychological | 30,557 (19.5%) | 46,124 (22.1%) | 31,180 (20.3%) | 43,775 (21.2%) | – | 55,541 (23.2%) | 22,410 (18.7%) | 29,537 (19.5%) |
| Kidney | 1559 (1.0%) | 2098 (1.0%) | 718 (0.5%) | 2450 (1.2%) | 2266 (1.2%) | – | 759 (0.6%) | – |
| Other | 70,474 (45.1%) | 98,100 (47.1%) | 61,970 (40.4%) | 96,360 (46.7%) | 86,256 (46.7%) | 117,995 (49.4%) | – | 60,350 (39.9%) |
n: number; SD: Standard Deviation; OA: Osteoarthritis; MSK: Musculoskeletal.
Fig. 3.
Difference in cumulative incidence between people using non-steroidal anti-inflammatory drugs (NSAIDs) and people stopping NSAIDs. Negative values signify a decrease in the incidence among people stopping NSAIDs. OA: Osteoarthritis; MSK: Musculoskeletal.
5. Discussion
Our findings of this population-based cohort study using register data indicated that people who initiate NSAIDs are at higher risk of developing a large set of comorbidities and AEs and that stopping the therapy may reduce the risk. Overall, OA in peripheral joints did not seem to essentially modify this association. However, there may be two exceptions. The risk of developing MSK and neuro-psychological comorbidities after NSAID initiation was somewhat lower in people with OA.
Certain CV and digestive system comorbidities such as myocardial infarction and gastrointestinal bleed are well established AEs associated with NSAID use [15,16]. Our results confirm these findings but also suggest that people initiating NSAIDs are exposed to a higher risk of developing numerous comorbidities not normally associated with NSAID use (e.g. metabolic and MSK disorders).
NSAIDs are a large class of analgesic commonly used in the management of painful conditions such as OA [17]. The presence of painful conditions is known to be associated with several comorbidities such as depression, pain in other body sites, diabetes, obesity and CV disorders [4,[18], [19], [20]]. People requiring analgesic management may thus be exposed to a higher risk of developing comorbidities which does not depend on the pharmacological effect of the drug but on the underlying condition for which the drug is prescribed. These results underline the importance of monitoring patients for which pharmacological management with analgesics is needed. The National Institute for Health and Care Excellence (NICE) already recommends annual follow-ups for people with OA, however, this is not a formal requirement and it is likely not implemented in primary care [21].
In recent years, several studies have shown that OA is strongly associated with CV diseases [[22], [23], [24]]. A previous study has indicated that a large part of the risk of developing comorbidities experienced by people with OA can be explained by the frequent use of NSAIDs to manage OA pain. Our study confirmed and extended these findings showing that in the absence of a recorded CV diagnosis and previous NSAID use, initiating NSAIDs can lead to similar risks of developing CV diseases in both people with and without OA [12,24]. Findings from a randomised trial studying the safety of Colexib, naproxen and ibuprofen in people with OA or rheumatoid arthritis appear to further support our findings showing a similar cumulative incidence of CV events 30 months after NSAID initiation [25]. However, due to differences in inclusion criteria, NSAIDs classification and outcomes definition, comparison between our and previous studies remain speculative. Moreover, we believe it is important to consider that the exclusion of people with pre-existing CV conditions in our study may somewhat underestimate the rate of CV events that may be observed in clinical practice where, despite discouraged by clinical practice guidelines, people with high CV risk are often prescribed NSAIDs [11].
As expected, NSAID initiation was also associated with a higher cumulative incidence of MSK disorders. In fact, the presence of painful disorders for which NSAIDs are prescribed is a known risk factor for the development of other painful MSK conditions [[26], [27], [28]]. Thus, the high rate of MSK comorbidities likely reflects a bias by indication rather than the effect of treatment with NSAIDs. However, this increase was smaller in persons with OA compared to those without. This result cannot be solely explained by the presence of a bias-by-indication which should be equally present in both people with and without OA. It may be hypothesised that the lower rate of incident MSK comorbidities among people with OA is due to the cases having one condition (OA) already diagnosed which can result in a lower number of MSK diagnoses following NSAIDs prescription.
The incidence of neuro-psychological comorbidities showed a similar pattern to the one showed by cardiovascular comorbidities with a lower increase in the incidence of comorbidities after NSAID initiation in persons with OA. Considering that several disorders included in the neuro-psychological group such as depression and sleep disorders are commonly found in people with OA [29,30], we believe that the depletion of susceptible subjects (e.g. those with prevalent neuro-psychological disorders) may explain the lower rates in people with OA.
Stopping NSAIDs is expected to reduce the risk of developing AEs, CV and digestive comorbidities. We evaluated this during the first 120 days after NSAIDs termination and observed reductions in cumulative incidence of most comorbidities, however with rather wide confidence intervals due to the low number of events during the short follow-up time. Substudy two also suggested that stopping NSAIDs was associated with a general reduction in the risk of developing comorbidities. This reduction may again be linked to the risk associated with the need for prolonged analgesic treatment (120 days) rather than to the NSAIDs pharmacological effect. Moreover, it appeared that OA has little or no effect on the risk of developing comorbidities after NSAID discontinuation which confirms the results from substudy one.
This study has several important limitations that need to be considered when interpreting the results. This is an observational study that relies on observational but still prospectively ascertained register data from everyday healthcare in Sweden. We cannot be sure that the persons who received the NSAIDs actually took all (if any) of the daily doses dispensed. On the same point, certain NSAIDs in small packages and low doses (i.e. ibuprofen, diclofenac [up to June 2020] and naproxen) can be sold over the counter (OTC) in which case they would not be recorded in the prescribed drug register. However, in Sweden, analgesic drugs are eligible for reimbursement if prescribed for the relief of chronic pain. Hence, most OTC analgesics are used for non-chronic conditions or as supplements to prescribed analgesics which should limit the impact on our results.
The use of a regional register to ascertain diagnosis implies that certain conditions may be missed if the persons did not seek care for that specific problem or if they received the diagnosis in another region or country. However, a study assessing the quality of the SHR has shown that nearly all the residents in the region (97%) had consulted some type of healthcare in the period 2012–2016 suggesting that the problem of out-of-the-region diagnosis may be limited [31]. Moreover, starting from 2004, over 80% of the visits to a physician have at least one diagnostic code registered [31]. Finally, up-to-date prevalence figures of common diseases from SHR are in line with what could be expected suggesting that also the problem of lack of consultation for many conditions may be limited [[32], [33], [34], [35], [36]]. For what concerns OA, the positive predictive value of a knee OA diagnosis in SHR has previously been reported to be high at 88% [37]. We analysed a large set of comorbidities which we grouped to facilitate analysis and interpretation. Thus, it is not possible to draw direct conclusions regarding the association of NSAID use or termination with a single condition while the low number of events did not allow us to produce estimates for kidney comorbidities. Moreover, different comorbidity groupings may yield different results. Similarly, we grouped a large number of NSAIDs belonging to different COX-inhibition classes. Therefore, no conclusions can be drawn on the role of OA in the association of a specific COX-inhibition class and the comorbidities. Finally, we could not control for obesity and smoking which are important risk factors for many of the analysed comorbidities. A previous study analysing the risk of CV events linked to NSAID use in people with and without OA showed that excluding BMI from the analysis had little influence on the results [12]. It is possible that controlling for a large number of comorbidities, most of which associated with obesity, may minimise the risk of bias linked to the lack of BMI in the analysis.
6. Conclusion
The presence of OA appeared to have little influence on the increased risk of comorbidities observed after NSAID initiation or on its reduction observed after NSAID termination. People in need of analgesic management, especially if prolonged, are generally at higher risk of developing comorbidities. Thus, close monitoring and follow-ups may be warranted with any NSAID prescription intended for long term use.
Author contributions
Conception and design: AD, AT, WZ, AK, SB-Z, JR, DP-A, ME. Collection and assembly of data: AT and ME. Analysis and interpretation of data: AD, AT and ME. Drafting manuscript: AD, AT. Revising manuscript and approving the final version of manuscript: AD, AT, WZ, AK, SB-Z, JR, DP-A, ME. Final approval of the version to be published: AD, AT, WZ, AK, SB-Z, JR, DP-A, ME. All authors take responsibility for the integrity of the data analysis.
Role of the funding sources
This work was supported by funds from the Foundation for Research in Rheumatology (FOREUM), The Swedish Research Council, Greta and Johan Kock Foundation, The Swedish Rheumatology Association, and Governmental Funding of Clinical Research within National Health Service (ALF). The funders did not have any role in the study other than to provide funding.
Declaration of competing interest
AD, AK, JR have no conflict of interest to disclose. AT has received personal fees from Osteoarthritis and Cartilage outside the submitted work (<10,000$). WZ has received personal fees from Eli Lily (<10,000$) and Regeneron (<10,000$) outside the submitted work. SB-Z has received grants from The Netherlands Organisation for Health Research and Development, CZ, European Union, FOREUM, Dutch Arthritis Association, personal fees from Pfizer (<10,000$), personal fees from OARSI (<10,000$); all outside the submitted work. DP-A has received, outside the submitted work, non-financial support from UCB Biopharma, grants from Les Laboratoires Servier, support from Janssen on behalf of IMI-funded EHDEN and EMIF and Synapse Management Partners consortiums for training programmes organised by DP-A ‘s department. ME has received personal fees from Pfizer outside the submitted work (<10,000$).
Acknowledgements
We would like to thank the registers used in this study for providing the data.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ocarto.2022.100253.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Global regional. And national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1859–1922. doi: 10.1016/S0140-6736(18)32335-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bannuru R.R., Osani M.C., Vaysbrot E.E., Arden N.K., Bennell K., Bierma-Zeinstra S.M.A., et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage. 2019;27(11):1578–1589. doi: 10.1016/j.joca.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 3.Litwic A., Edwards M.H., Dennison E.M., Cooper C. Epidemiology and burden of osteoarthritis. Br. Med. Bull. 2013;105:185–199. doi: 10.1093/bmb/lds038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swain S., Coupland C., Mallen C., Kuo C.F., Sarmanova A., Bierma-Zeinstra S.M.A., et al. Temporal relationship between osteoarthritis and comorbidities: a combined case control and cohort study in the UK primary care setting. Rheumatology. 2021 doi: 10.1093/rheumatology/keab067. Oxford) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dell'Isola A., Pihl K., Turkiewicz A., Hughes V., Zhang W., Bierma-Zeinstra S., et al. Risk of comorbidities following physician-diagnosed knee or hip osteoarthritis: a register-based cohort study. Arthritis Care Res. 2021 doi: 10.1002/acr.24717. n/a(n/a) [DOI] [PubMed] [Google Scholar]
- 6.Swain S., Sarmanova A., Coupland C., Doherty M., Zhang W. Comorbidities in osteoarthritis: a systematic review and meta-analysis of observational studies. Arthritis Care Res. 2020;72(7):991–1000. doi: 10.1002/acr.24008. [DOI] [PubMed] [Google Scholar]
- 7.Katz J.N., Arant K.R., Loeser R.F. Diagnosis and treatment of hip and knee osteoarthritis: a review. JAMA. 2021;325(6):568–578. doi: 10.1001/jama.2020.22171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gore M., Tai K.S., Sadosky A., Leslie D., Stacey B.R. Use and costs of prescription medications and alternative treatments in patients with osteoarthritis and chronic low back pain in community-based settings. Pain Pract. 2012;12(7):550–560. doi: 10.1111/j.1533-2500.2012.00532.x. [DOI] [PubMed] [Google Scholar]
- 9.Thorlund J.B., Turkiewicz A., Prieto-Alhambra D., Englund M. Opioid use in knee or hip osteoarthritis: a region-wide population-based cohort study. Osteoarthritis Cartilage. 2019;27(6):871–877. doi: 10.1016/j.joca.2019.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Osani M.C., Vaysbrot E.E., Zhou M., McAlindon T.E., Bannuru R.R. Duration of symptom relief and early trajectory of adverse events for oral nonsteroidal antiinflammatory drugs in knee osteoarthritis: a systematic review and meta-analysis. Arthritis Care Res. 2020;72(5):641–651. doi: 10.1002/acr.23884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wehling M. Non-steroidal anti-inflammatory drug use in chronic pain conditions with special emphasis on the elderly and patients with relevant comorbidities: management and mitigation of risks and adverse effects. Eur. J. Clin. Pharmacol. 2014;70(10):1159–1172. doi: 10.1007/s00228-014-1734-6. [DOI] [PubMed] [Google Scholar]
- 12.Atiquzzaman M., Karim M.E., Kopec J., Wong H., Anis A.H. Role of nonsteroidal antiinflammatory drugs in the association between osteoarthritis and cardiovascular diseases: a longitudinal study. Arthritis Rheumatol. 2019;71(11):1835–1843. doi: 10.1002/art.41027. [DOI] [PubMed] [Google Scholar]
- 13.Lambert P.C., Royston P. Further development of flexible parametric models for survival analysis. STATA J. 2009;9(2):265–290. [Google Scholar]
- 14.Westreich D., Cole S.R., Tien P.C., Chmiel J.S., Kingsley L., Funk M.J., et al. Time scale and adjusted survival curves for marginal structural cox models. Am. J. Epidemiol. 2010;171(6):691–700. doi: 10.1093/aje/kwp418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bally M., Dendukuri N., Rich B., Nadeau L., Helin-Salmivaara A., Garbe E., et al. Risk of acute myocardial infarction with NSAIDs in real world use: bayesian meta-analysis of individual patient data. BMJ. 2017;357:j1909. doi: 10.1136/bmj.j1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coxib, traditional N.T.C., Bhala N., Emberson J., Merhi A., Abramson S., et al. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet. 2013;382(9894):769–779. doi: 10.1016/S0140-6736(13)60900-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.da Costa B.R., Reichenbach S., Keller N., Nartey L., Wandel S., Juni P., et al. Effectiveness of non-steroidal anti-inflammatory drugs for the treatment of pain in knee and hip osteoarthritis: a network meta-analysis. Lancet. 2017;390(10090):e21–e33. doi: 10.1016/S0140-6736(17)31744-0. [DOI] [PubMed] [Google Scholar]
- 18.Grimby-Ekman A., Gerdle B., Bjork J., Larsson B. Comorbidities, intensity, frequency and duration of pain, daily functioning and health care seeking in local, regional, and widespread pain - a descriptive population-based survey (SwePain) BMC Muscoskel. Disord. 2015;16(1):165. doi: 10.1186/s12891-015-0631-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bair M.J., Robinson R.L., Katon W., Kroenke K. Depression and pain comorbidity: a literature review. Arch. Intern. Med. 2003;163(20):2433–2445. doi: 10.1001/archinte.163.20.2433. [DOI] [PubMed] [Google Scholar]
- 20.Duruoz M.T., Turan Y., Gurgan A., Deveci H. Evaluation of metabolic syndrome in patients with chronic low back pain. Rheumatol. Int. 2012;32(3):663–667. doi: 10.1007/s00296-010-1693-x. [DOI] [PubMed] [Google Scholar]
- 21.UK N.C.G.C. 2014. Osteoarthritis: Care and Management in Adults. [PubMed] [Google Scholar]
- 22.Watt F.E., Wise E.M. Osteoarthritis and associated comorbidities: new answers and more questions. Rheumatology. 2021 doi: 10.1093/rheumatology/keab405. Oxford) [DOI] [PubMed] [Google Scholar]
- 23.Rahman M.M., Kopec J.A., Anis A.H., Cibere J., Goldsmith C.H. Risk of cardiovascular disease in patients with osteoarthritis: a prospective longitudinal study. Arthritis Care Res. 2013;65(12):1951–1958. doi: 10.1002/acr.22092. [DOI] [PubMed] [Google Scholar]
- 24.Wang H., Bai J., He B., Hu X., Liu D. Osteoarthritis and the risk of cardiovascular disease: a meta-analysis of observational studies. Sci. Rep. 2016;6(1):39672. doi: 10.1038/srep39672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nissen S.E., Yeomans N.D., Solomon D.H., Luscher T.F., Libby P., Husni M.E., et al. Cardiovascular safety of Celecoxib, naproxen, or ibuprofen for arthritis. N. Engl. J. Med. 2016;375(26):2519–2529. doi: 10.1056/NEJMoa1611593. [DOI] [PubMed] [Google Scholar]
- 26.Croft P., Dunn K.M., Von Korff M. Chronic pain syndromes: you can't have one without another. Pain. 2007;131(3):237–238. doi: 10.1016/j.pain.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 27.Smith B.H., Elliott A.M., Hannaford P.C., Chambers W.A., Smith W.C. Factors related to the onset and persistence of chronic back pain in the community: results from a general population follow-up study. Spine. 2004;29(9):1032–1040. doi: 10.1097/00007632-200405010-00016. [DOI] [PubMed] [Google Scholar]
- 28.de Cassia Pereira Fernandes R., Pataro S.M.S., de Carvalho R.B., Burdorf A. The concurrence of musculoskeletal pain and associated work-related factors: a cross sectional study. BMC Publ. Health. 2016;16(1):628. doi: 10.1186/s12889-016-3306-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dai Z., Neogi T., Brown C., Nevitt M., Lewis C.E., Torner J., et al. Sleep quality is related to worsening knee pain in those with widespread pain: the multicenter osteoarthritis study. J. Rheumatol. 2020;47(7):1019–1025. doi: 10.3899/jrheum.181365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leonard B.E. Pain, depression and inflammation: are interconnected causative factors involved? Mod. Trend. Pharmacopsychiatr. 2015;30:22–35. doi: 10.1159/000435930. [DOI] [PubMed] [Google Scholar]
- 31.Löfvendahl S., Schelin M.E.C., Jöud A. The value of the Skåne Health-care Register: prospectively collected individual-level data for population-based studies. Scand. J. Publ. Health. 2020;48(1):56–63. doi: 10.1177/1403494819868042. [DOI] [PubMed] [Google Scholar]
- 32.Andréasson K., Saxne T., Bergknut C., Hesselstrand R., Englund M. Prevalence and incidence of systemic sclerosis in southern Sweden: population-based data with case ascertainment using the 1980 ARA criteria and the proposed ACR-EULAR classification criteria. Ann. Rheum. Dis. 2014;73(10):1788–1792. doi: 10.1136/annrheumdis-2013-203618. [DOI] [PubMed] [Google Scholar]
- 33.Haglund E., Bremander A.B., Petersson I.F., Strömbeck B., Bergman S., Jacobsson L.T., et al. Prevalence of spondyloarthritis and its subtypes in southern Sweden. Ann. Rheum. Dis. 2011;70(6):943–948. doi: 10.1136/ard.2010.141598. [DOI] [PubMed] [Google Scholar]
- 34.Jöud A., Petersson I.F., Englund M. Low back pain: epidemiology of consultations. Arthritis Care Res. 2012;64(7):1084–1088. doi: 10.1002/acr.21642. [DOI] [PubMed] [Google Scholar]
- 35.Jöud A., Petersson I.F., Jordan K.P., Löfvendahl S., Grahn B., Englund M. Socioeconomic status and the risk for being diagnosed with spondyloarthritis and chronic pain: a nested case-control study. Rheumatol. Int. 2014;34(9):1291–1298. doi: 10.1007/s00296-014-3039-6. [DOI] [PubMed] [Google Scholar]
- 36.Löfvendahl S., Theander E., Svensson Å., Carlsson K.S., Englund M., Petersson I.F. Validity of diagnostic codes and prevalence of physician-diagnosed psoriasis and psoriatic arthritis in southern Sweden--a population-based register study. PLoS One. 2014;9(5):e98024. doi: 10.1371/journal.pone.0098024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Turkiewicz A., Petersson I.F., Bjork J., Hawker G., Dahlberg L.E., Lohmander L.S., et al. Current and future impact of osteoarthritis on health care: a population-based study with projections to year 2032. Osteoarthritis Cartilage. 2014;22(11):1826–1832. doi: 10.1016/j.joca.2014.07.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.