Abstract
Objective
It is known that recombinant human gelsolin (rhuGSN) supports wound closure and migration processes in avascular tissue. Since articular cartilage degradation plays an important role in osteoarthritis (OA), we are investigating how rhuGSN affects regeneration processes in human articular cartilage and represents a promising new therapeutic approach for the treatment of OA.
Methods
Primary human chondrocytes (phCs) from articular knee cartilage were cultured with different concentrations of rhuGSN to analyse its direct effect in vitro. In addition, phCs were stimulated with 10 ng/mL IL-1β or TNF-α to simulate osteoarthritis in vitro and treated with different concentrations of rhuGSN to investigate the beneficial effect in disease treatment. Cytokine secretion and gene expression as well as wound assays were performed.
Results
GSN significantly promotes wound closure in phCs after 60 h compared to untreated cells. After 24 h treatment with 30 μg/mL rhuGSN, TGF-β secretion increases significantly in the in vitro osteoarthritis model. Gene expression of MMP1 as well as SPARC is reduced in chondrocytes due to treatment with GSN in the OA model. At the same time, CXCR4 expression increases significantly after 24 h treatment with 3 μg/mL GSN.
Conclusion
In the in vitro model of osteoarthritis, rhuGSN promotes wound closure of chondrocytes by a supported migration as well as expression of reconstructive and down regulated expression of deconstructive genes concentration dependently. Further experiments are needed to fully understand the beneficial effect of gelsolin on human chondrocytes and to verify this promising approach for a pharmacological treatment of osteoarthritis.
Keywords: Gelsolin, Chondrocytes, Osteoarthritis, Migration, Wound closure
1. Introduction
Osteoarthritis is characterised by damage of the cartilage tissue in joints, causing pain and loss of function [1]. In its healthy state the vascularised articular cartilage consists mainly out of collagen II, a structural protein that forms a network for various stabilising proteins. Proteoglycans like aggrecan (ACAN), important for the tissue compression resistance, are embedded in this network [2]. The extracellular matrix (ECM) is mainly formed by chondrocytes in articular cartilage. These cells can react quickly to environmental changes and therefore regulate the composition of the ECM [3].
First signs of pathological changes in articular cartilage are accumulation of chondrocytes and failure of repair processes [4]. Furthermore, the synthesis of tissue-destroying proteinases begins with the activation as well as the secretion of inflammatory cytokines such as interleukin (IL)-6, interleukin-1β and TNF-α. In addition, various collagenases (MMP1, MMP3 and MMP13) and aggrecan-degrading enzymes (ADAMTS-4 and 5) are involved in pathological processes of osteoarthritis [5]. Another important OA-related factor is CXCR4, ligand of SDF1. Overexpression of this axis leads to expression of MMPs in the ECM and leads to their degradation in combination with a loss of aggrecan, which is important for the maintenance of collagen fibrils [[6], [7], [8]]. One cartilage-supporting factor in joints of osteoarthritic state is TGF-β. TGF-β inhibits the maturation and hypertrophy of chondrocytes [9] (Fig. 1). Beside these factors, there are several more involved in pathological processes of osteoarthritis. It is known, that Beclin1, which increases cell viability and decreases apoptosis, is significantly decreased in cartilage tissue of osteoarthritis patients [10]. In addition, HMGB1 (high mobility Group 1 Box 1) and SPARC (acid- and cysteine-rich secreted protein) are closely related to deconstructive processes in cartilage tissue [11,12]. Even though the involvement of these factors in osteoarthritis is known, a complete understanding of the disease is still lacking.
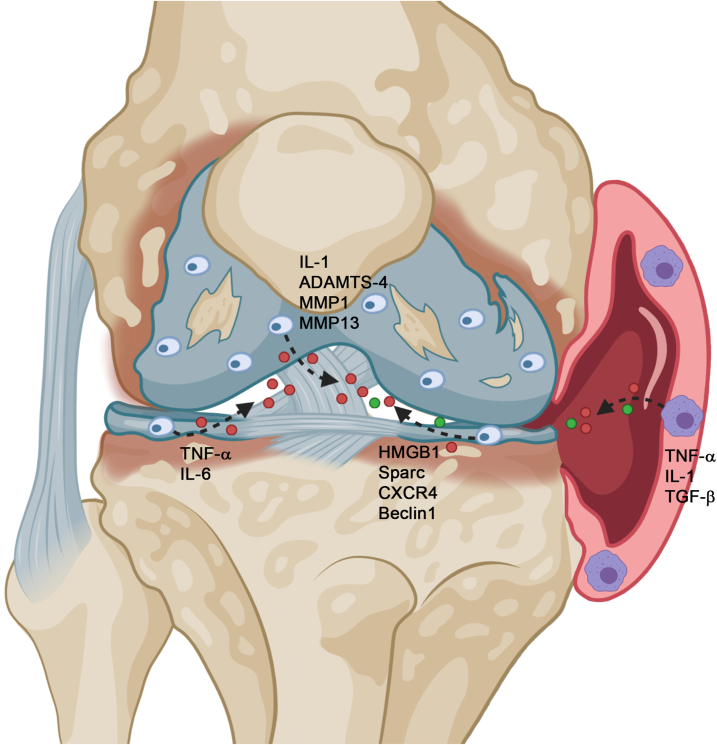
Fig. 1.
Expression of factors in the osteoarthritic articular cartilage. During degeneration of articular cartilage several factors are expressed in the cartilage itself or in the synovial surrounding. Pictured is the knee joint with degenerated cartilage (blue) and its chondrocytes as well the surrounding synovia (right side-red).
One protein considered to be involved in the pathogenesis of osteoarthritis is gelsolin. Gelsolin (GSN) is an important calcium and actin-binding protein closely associated with cell mobility, cell shape and the actin cytoskeleton. Today, three different isoforms of GSN are known, cytoplasmic, plasmatic and the isoform gelsolin 3 [13].
GSN is known to be involved in many processes in humans, such as nerve cell protection, amyloid-beta binding, and modulation of inflammatory responses. Today, GSN is considered to be an indicator of inflammation and its levels are used to predict the severity of diseases or its clinical outcome in patients [14]. In arthritis, a reduction of GSN plasma concentrations and activity in synovial fluid is detected [15]. GSN is a promising biomarker for osteoporosis [16] and is often analysed in age-related studies [5,17]. It was recently shown, that GSN is involved in wound healing processes in the cornea, another non-vascular tissue, where it significantly promotes the re-epithelialisation after wounding [18].
As the supportive effect of GSN on non-vascular tissues was already demonstrated, we investigated the influence of GSN on wound healing and migration processes in human articular cartilage using recombinant human gelsolin (rhuGSN). We further used primary human articular chondrocytes (phCs) to study direct effects of GSN in vitro.
2. Material and methods
2.1. Isolation of phCs and cell cultures
Human articular femoral and tibial cartilage samples were taken from patients with osteoarthritis during knee joint replacement surgeries in the university clinic Erlangen-Nuremberg with institutional review board approval under compliance of the Helsinki Declaration. Articular femoral and tibial cartilage out of intact sectors was cut into small pieces (1 mm2) and transferred to DMEM (Dulbecco's modified eagle's medium) media directly and centrifuged (2000 rpm, 1Min) at room temperature (RT; 22.5°C). The medium was replaced by sterile Pronase E solution (2 mg/mL, Merck, #11459643001) diluted in DMEM and incubated (30Min, 37°C) on a shaker. After centrifugation (1200 rpm) the Pronase solution was replaced by collagenase solution (1 mg/mL, Serva; #17465) in DMEM with 10% FCS (fetal calf serum) and 1% Pen/Strep (penicillin/streptavidin) overnight (37°C) on a shaker. The cell suspension was filtered through a 70 μm cell strainer and centrifuged (5Min, 1200 rpm). Cells were seeded in DMEM (+10% FCS and 1% Pen/Strep) for 3d. For rhuGSN-stimulation cells were seeded and directly stimulated with rhuGSN (pGSN, MyBiosource; #MBS9208646) in DMEM media (10% FCS/1% Pen/Strep) in different concentrations (0, 3, 30, 300 μg/mL) for 3d (72 h). On the second day, fresh media were added, containing rhuGSN respectively. For the osteoarthritis simulation model, IL-1β (Merck; #SRP3083, 10 ng/mL) and TNF-α (Millipore; #01–164, 10 ng/mL) were added alone or in combination for 24 h or 72 h and simultaneously treated with rhuGSN.
2.2. Immunohistochemistry
5 μm thick paraffin slices of healthy (cadavers, Institute of Functional and Clinical Anatomy, FAU) and OA human articular cartilage were dewaxed twice with xylene for 10 min each before rehydration in ethanol. After washing with deionized (DI) water, they were treated with 3% H2O2 (10min). The antigen was obtained by boiling the sections in citrate buffer (pH 6, 10 mM, 10Min). The sections were washed and blocked with 5% goat normal serum (Dako, #20004838, 20Min, RT). Avidin and biotin binding was blocked with a kit (BioLegend; # 927301, SIG-31126) according to the manufacturing protocol. After washing with TBS-T (Tris-buffered saline with Tween 20), the primary antibody (Santa Cruz; #SC-48769, GSN 1:100) was applied overnight (4°C). The sections were washed before the secondary goat anti-rabbit antibody (Dako, #E0432, 1:200) was applied (1 h, RT). An ABC kit (Avidin-Biotin Complex) (Vectastain; PK-6100) was used according to the manufacturer's instructions. The sections were stained with an AEC (3-amino-9-ethylcarbazole) solution (Dako; #K3469) and counterstained with hemalum.
2.3. Protein isolation
The cells were treated with 300 μl Triton buffer containing 0.2% protease and 0.2% phosphatase inhibitors and incubated on ice for 30min. After centrifugation (13000 rpm, 4°C, 5Min) supernatant was decanted and protein concentration was measured by Bradford assay.
2.4. Western blot
OA cartilage phC protein samples were used in a concentration of 25 μg and 5 μl RSB (resuspension buffer) was added, incubated (5Min, 100°C) and separated in a 15% SDS gel. The gel was blotted on a nitrocellulose membrane (GE Healthcare, #10600008). The membrane was blocked in 5% BSA-PBS-T for 1 h (RT) before the primary GSN antibody (Sigma Aldrich; GS-2C4, #G4896, 1:1000) was added overnight (4°C). After washing in PBS-T the secondary antibody (Cell signalling; , #7076S) was added for 2 h (RT). The membrane was washed before an ECL mixture (Millipore; #WBKIS0500) was used for chemiluminescence detection. For GAPDH detection the membrane was treated with stripping buffer (65°C, 45Min), washed in TBS-T and blocked in 5% milk powder-TBS-T before the first GAPDH antibody (Santa Cruz, #Sc-365062, 1:2000) was added overnight (4°C). After washing, the secondary antibody (Cell signalling; #70769, 1:5000) was added (2 h, RT). ECL mixture was used for chemiluminescence.
2.5. RNA isolation and cDNA transcription
Cells were treated with peqGOLD TriFast™ as described before [19].
2.6. PCR
For the polymerase chain reaction (PCR) 2 μl cDNA were used in combination with the Taq DNA Polymerase Kit (Thermo Fisher Scientific, #18038–042). β-actin was used as a loading control.
2.7. Quantitative real time PCR
Analysis of gene expression was performed by quantitative real-time PCR. The LightCyclerR 480 (Roche) in combination with SYBR Green was used. The TAKYON (Eurogentec; #UT-NSMT-B0701) kit was used with the primers as described in Table 1. Results were calculated with the ΔΔCt method.
Table 1.
Primer sequences.
| Primer | Forward | Reverse |
|---|---|---|
| BECN1 | gga tgg tgt ctc tcg cag at | ttg gca ctt tct gtg gac at |
| SPARC | ttc cct gta cac tgg cag ttc | aat gct cca tgg gga tga |
| HMGB1 | tca cag cca ttg cag tac att | agg atc tcc ttt gcc cat gt |
| MMP1 | gct aac ctt tga tgc tat aac tac ga | ttt gtg cgc atg tag aat ctg |
| CXCR4 | ggt ggt cta tgt tgg cgt ct | tgg agt gtg aca gcttgg ag |
| ACAN | cct ccc ctt cac gtg taa aa | gct ccg ctt ctg tag tct gc |
| COL1A1 | ggg att ccc tgg acc taa ag | gga aca cct cgc tct cca |
| COL2A1 | gtg aac ctg gtg tct ctg gtc | ttt cca ggt ttt cca gct tc |
| COL10A | cac ctt ctg cac tgc tcat | ggc agc ata ttc tca gat gga |
2.8. Enzyme linked immunosorbent assay
Commercially available enzyme-linked immunosorbent assays (ELISAs) have been purchased from R&D systems (IL-1β: #DY201-05, IL-6: #DY206-05, TNF-α: #DY210-05, TGF-β. #DY240-05) and performed according to the manufacturing protocols.
2.9. ECIS
The wound closure/migration rate of phC was analysed with an ECIS system (Electrical Cell-Substrate Impedance Sensing; Applied Bioscience). Therefore, a defined amount of phCs was seeded on an electrode and cultivated up to 70% confluence. By applying an electrical voltage, the cells sitting directly on the standardized electrode died and imitated a wound of defined size. Cells were treated with rhuGSN (MyBiosource; #MBS9208646) in different concentrations (0, 3, 30 and 300 μg/mL) for 72 h after wounding.
2.10. Statistics
The results were analysed using One-Way ANOVA tests in combination with Tukey's multiple comparative tests after a D'Agostino-Pearson normality test was performed as well as t-tests. Results are given as mean ± SEM and p-values blow 0.05 are consider significant. Calculation and visualization was performed with GraphPad Prism 6 (GraphPad Prism Software).
3. Results
3.1. Gelsolin is expressed in human articular cartilage
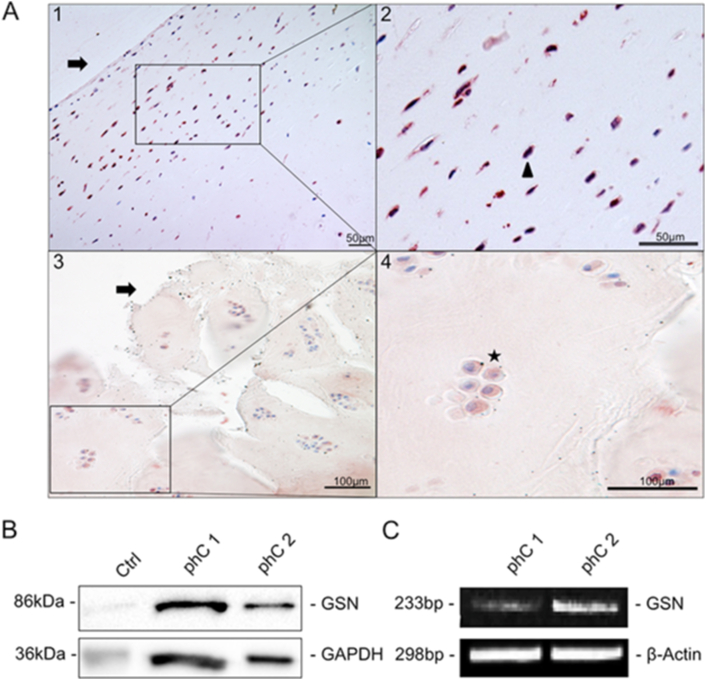
First, we tested whether GSN is present in human articular cartilage tissue and therefore isolated phCs. GSN was localized by immunohistochemical staining in healthy articular cartilage and osteoarthritic tissue. Additionally via Western blot and PCR in cultured chondrocytes using a GSN-specific antibody (Fig. 2). GSN (Fig. 2A1–4, red staining) is detectable in human chondrocytes (Fig. 2A1–2). Low immunohistochemical reactivity was seen in the extracellular matrix surrounding the chondrocytes in osteoarthritic cartilage (Fig. 2A3–4). To analyse whether GSN is detectable in cultured primary cells, we investigated GSN at the protein (86 kDa) and DNA level (233bp) using Western blot (Fig. 2B) and PCR (Fig. 2C). Both methods showed clear evidence of GSN.
Fig. 2.
Localization of gelsolin (GSN) in articular cartilage.(A) Immunohistochemical detection of GSN (red staining) in (A1-2) healthy human articular cartilage, showing single chondrocytes (2, Δ high magnification right picture) under the cartilage surface (→). Both scale bars are showing 50μm. (A 3–4) Osteoarthritic cartilage with gaps (3) and the typical formation of chondrocyte clusters consisting of more than two cells can be seen (4,★ high magnification right picture). The extracellular matrix of the cartilage also has a reddish coloration. Both scale bars are showing 100 μm. (B) Western blot analysis of cultured primary articular cartilage chondrocytes. In both samples shown, a distinct band for GSN appears at 86 kDa. GAPDH (36 kDa) was used as a stress control and human stomach tissue as a positive control. (C) RT-PCR analysis of the two samples already shown in B to investigate GSN at the DNA level. Both samples show a band at 233bp for GSN. β-actin served as a loading control.
3.2. GSN promotes pro-inflammatory cytokine production in primary human articular cartilage chondrocytes
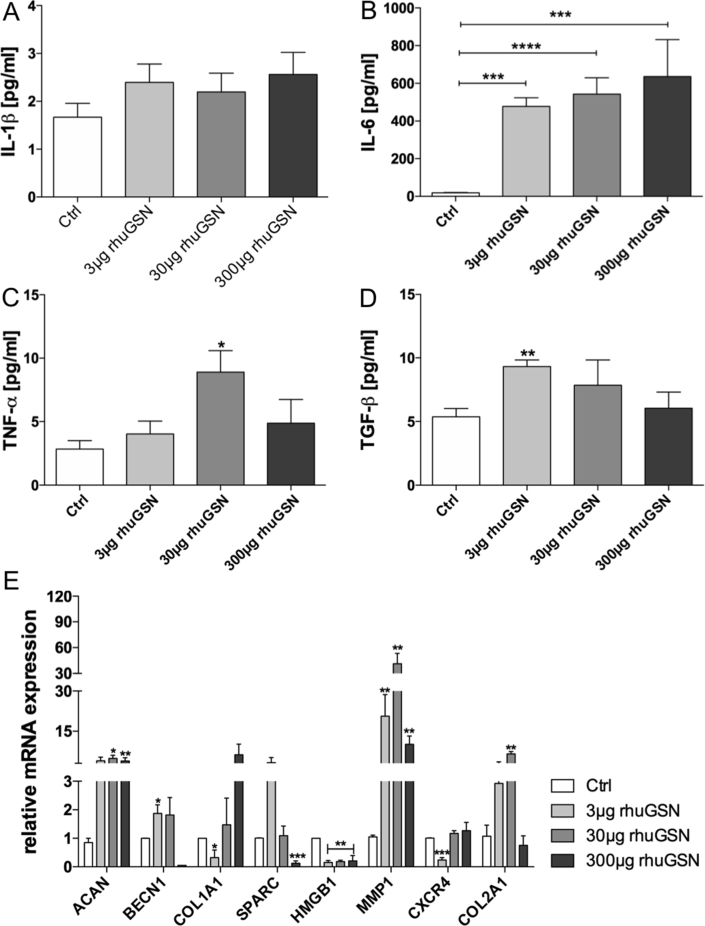
After confirming the presence of GSN in phCs, we were interested in how GSN modulates cytokine production in phCs (No negative influence of rhuGSN on phCs proliferating activity was detected - results not shown). We focused on the pro-inflammatory cytokines Interleukin (IL)-1β, IL-6, TNF-α and the anti-inflammatory TGF-β to test the effect of rhuGSN on phCs (Fig. 3A–D). IL-1β (Fig. 3A) did not showed significant changes, although a modulating effect by rhuGSN is evident. However, we found a significant increase in IL-6 (Fig. 3B) concentration. Without stimulation IL-6 concentration of 18.43 ± 1.85 pg/mL was measured. PhC's stimulated with 3 μg rhuGSN showed a strong increase to 477.5 ± 46.39 pg/mL. Stimulation with 30 μg rhuGSN increased IL-6 production to 542.5 ± 86.87 pg/mL and 300μgrhuGSN stimulated phCs up to 636.0 ± 195.9 pg/mL.
Fig. 3.
Cytokines and gene expression modulated in phC's because of rhuGSN stimulation. Primary human chondrocytes (phCs) were stimulated with different concentrations of rhuGSN for three days (72 h) during cultivation. The supernatant was decanted and used for the ELISA and cells were harvested and used for qPCR. (A) IL-1β production of phCs stimulated. RhuGSN has no significant influence on the production of IL-1β. (B) IL-6 cytokine production in stimulated phCs. Stimulation with 3 μg/mL and increasing concentrations significantly increases the production of IL-6. (C) TNF-α production increases due to rhuGSN stimulation significantly in with 30 μg rhuGSN stimulated phCs and decreases at high concentrations of rhuGSN. (D) TGF-β production shows significant increase due to 3 μg rhuGSN stimulation in phCs and decreases in higher concentrations. (E) Quantitative real-time PCR analysis of cartilage ECM-related genes after treatment with different concentrations of rhuGSN. The treatment of phCs with rhuGSN significantly modulates the expression of cartilage-related genes in vitro. Anova test, n = 4, ∗ <0.5, ∗∗ <0.1 ∗∗∗ <0.05, ∗∗∗∗ <0.001.
TNF-α showed a significant increase from 2.83 ± 0.66 pg/mL in the control group up to 8.90 ± 1.69 pg/mL when the phCs were stimulated with 30 μg rhuGSN. A higher concentration did not lead to a further increase in TNF-α secretion. For TGF-β an opposite course was found. Stimulation of phCs with 3 μg rhuGSN resulted in an increase from 5.36 ± 0.65 g/mL up to 9.317 ± 0.51 pg/mL.
3.3. Cartilage ECM related genes are modulated by rhuGSN
We further wanted to test how cartilage-related genes in phCs are influenced by rhuGSN. Therefore, we stimulated phCs as described above for three days and performed quantitative real-time PCR to analyse genes that are important components of the ECM of human cartilage (Fig. 3E). The expression of aggrecan (ACAN) increased significantly by 3–4.8-fold when cells were treated with 30 μg or 300 μg of rhuGSN. Beclin1 showed a significant increase when cells were treated with 3 μg rhuGSN up to 1.8-fold. Within the 300 μg rhuGSN group Beclin was almost undetectable and decreased dramatically. The expression of COL1A1 decreases significantly to 0.32-fold when cells were treated with 3 μg rhuGSN, but increased up to 6.4-fold with higher concentrations in the 300 μg rhuGSN group. A different course is seen in SPARC, where expression increased at low rhuGSN concentrations, but significantly decreased to 0.12-fold at high rhuGSN concentrations (300 μg). The expression of HMGB1 showed a significant decrease when cells were treated with any concentration of rhuGSN. CXCR4 expression decreased when treated with 3 μg rhuGSN but was not effected at higher concentrations. Outstanding was the increase of MMP1 after 30 μg rhuGSN stimulation, with cells expressing 60 times more MMP1 compared to the control group. COL2A1 could be significantly increased due to 30 μg rhuGSN stimulation. The expression of ADAMTS-4 and Sox 9 was not changed (results not shown) and COL10A1 did not show clear results.
3.4. RhuGSN supports wound healing in primary human chondrocytes
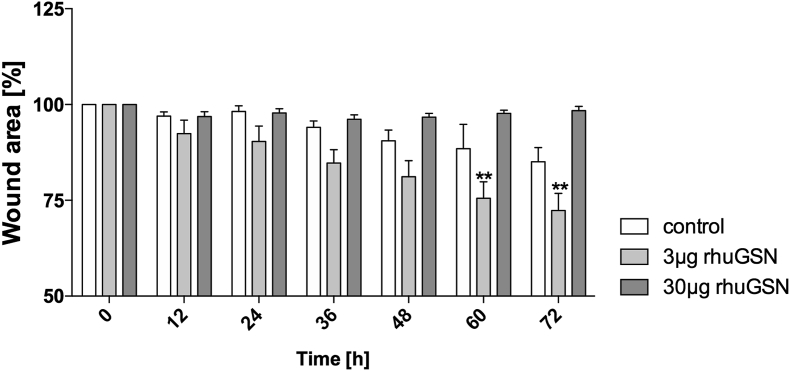
As we know that rhuGSN has supportive wound closing and migrative effects in the cornea, we were interested in the wound healing effects in phCs. Using electrical cell-substrate impedance measurement (ECIS) we tested whether stimulation of phCs with rhuGSN has an influence on the migration of phCs into the wound (Fig. 4). For this purpose, we divided the cells into three groups (control, 3 μg rhuGSN and 30 μg rhuGSN) and analysed them after wounding for 72 h. 300 μg rhuGSN was not further used, because previous results showed that 3 and 30 μg rhuGSN already give significant results. Already 24 h after wounding a first supportive wound closure effect of 3 μg rhuGSN was visible, which continued at further times. After 60 h a significant reduction of the wound area down to 75.5 ± 4.3% in the 3 μg rhuGSN group compared to the control (88.5 ± 6.3%) was observed. This significant effect persisted until 72 h between the control group (85.05 ± 3.7%) and the group treated with 3 μg rhuGSN (72.3 ± 4.4%). The group treated with 30 μg rhuGSN revealed no differences.
Fig. 4.
GSN supports wound closure in phC's. phCs were cultivated in ECIS wells until confluence and electrically wounded. After wounding cells were treated with 0 (control), 3 μg or 30 μg rhuGSN. Shown are the percentages of the wound area after the wound in a time-dependent manner (h). 3 μg rhuGSN support wound closure directly (12 h), but over time this supporting function becomes clearer (72 h). N = 3, unpaired t-test, ∗<0.03; ∗∗<0.0021.
3.5. IL-1β/TNF-α stimulation followed by rhuGSN treatment showed modulated cytokine and gene expression in phCs
IL-1β and TNF-α stimulation mimics osteoarthritis in phCs in vitro. Therefore, human chondrocytes were isolated from articular cartilage and stimulated with 10 ng/mL of IL-1β, TNF-α or a combination of these for 24 h or 72 h. Additionally, cells were treated with different concentrations of rhuGSN during cultivation (0, 3, 30 μg/mL) to investigate wound-healing effects under osteoarthritic conditions (Fig. 5).
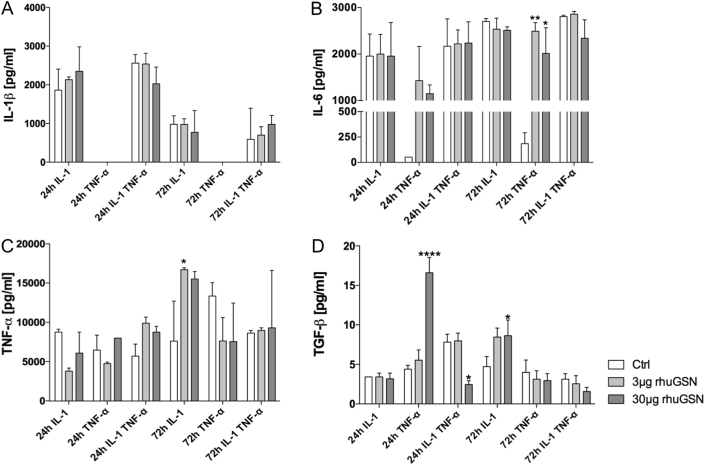
Fig. 5.
RhuGSN modulates cytokine secretion in vitro osteoarthritis cell model. Primary human chondrocytes were stimulated with 10 ng/mL IL-1β, TNF-α or a combination of these for 24 h or 72 h. During stimulation cells were divided into three groups and treated with 0, 3 or 30 μg/mL rhuGSN. The supernatants were decanted and used for ELISA analysis. (A) The secretion of IL-1β is slightly modulated but not significantly altered due to rhuGSN. (B) IL-6 secretion is significantly increased in cells stimulated with TNF-α for 72 h and treated with rhuGSN. (C) TNF-α secretion is significantly modulated by treatment with 3 μg rhuGSN after 72 h in IL-1β stimulated cells. (D) TGF-β secretion is significantly modulated by 30 μg rhuGSN treatment in phCs stimulated with TNF-α alone or in combination with IL-β for 24 h and in the 72 h group stimulated with IL-1β. N = 3, one-way Anova test, ∗ <0.5, ∗∗ <0.1 ∗∗∗ <0.05, ∗∗∗∗ <0.001.
We focused on the four cytokines analysed before (IL-1β, IL-6, TNF-α and TGF-β). IL-1β (Fig. 5A) showed no significant modulation after stimulation with different concentrations of rhuGSN after 24 h or 72 h. IL-6 (Fig. 5B) secretion was significantly increased when cells were stimulated with TNF-α for 72 h (ctrl = 185.08 ± 107.64 pg/mL) and additionally treated with 3 μg (2490.40 ± 187.14 pg/mL) or 30 μg (2014.20 ± 551.05 pg/mL) rhuGSN. TNF-α production (Fig. 5C) was significantly increased when cells were stimulated with IL-1β for 72 h (8290.21 ± 2371.23 pg/mL) and additionally treated with 3 μg rhuGSN (16703.79 ± 235.81 pg/mL). Other combinations didn't reveal any changes. TGF-β (Fig. 5D) significantly increased when phCs were stimulated with TNF-α (4.39 ± 0.49 pg/mL) for 24 h and treated with 30 μg rhuGSN (16.60 ± 1.95 pg/mL) in contrast to the control. A combined stimulation of IL-1β and TNF-α without rhuGSN (24 h) revealed an increase in TGF-β expression (7.81 ± 1.02 pg/mL), while the additional stimulation with 30 μg rhuGSN resulted in the opposite effect and a significant decrease to 2.44 ± 0.49 pg/mL.
Further, we investigated gene expression in cells stimulated with IL-1β (Fig. 6A), TNF-α (Fig. 6B) or a combination of these (Fig. 6C). A stimulation of the phCs with IL-1β alone and a stimulation with different concentrations of rhuGSN led to a massive modulation of the expressed genes, as described below. Beclin1 (BECN1) expression decreased significantly when cells were stimulated with 30 μg rhuGSN after 24 h (0.06-fold) and 72 h (0.08-fold). However, this reduction was already visible when the cells were treated with 3 μg rhuGSN for 72 h (0.38-fold). A similar expression pattern could be seen in SPARC, whose expression was significantly reduced after 72 h when the cells were treated with either 3 or 30 μg rhuGSN. This significantly reduced expression was also observed in HMGB1, except for the 24 h 30 μg rhuGSN group, MMP1 and for COL2A1 in the 30 μg rhuGSN 24 h group. IL-1β and TNF-α showed an opposite result, as they were decreased in the low concentrations of rhuGSN but significantly upregulated in high rhuGSN concentrations. COL10A was not detectable in the 3 μg rhuGSN groups but decreased significantly when treated with 30 μg rhuGSN.
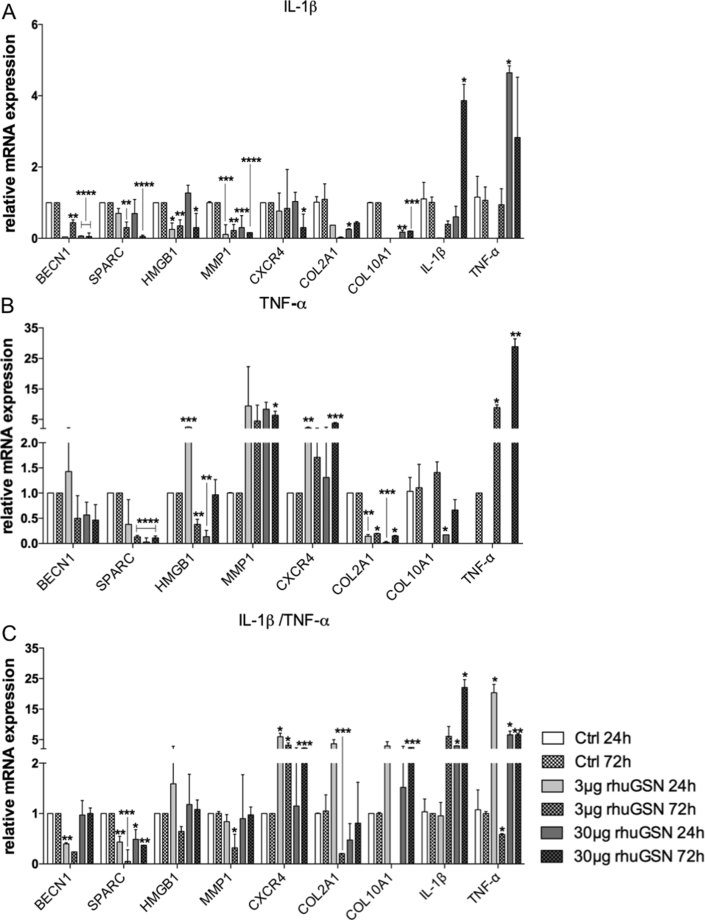
Fig. 6.
RhuGSN influences the gene expression of phCs in an in vitro osteoarthritis model. Primary human chondrocytes were stimulated with 10 ng/mL IL-1β, TNF-α or a combination of these for 24 h or 72 h. During stimulation cells were divided into three groups and treated with 0, 3 or 30 μg/mL rhuGSN. Cells were harvested and used for real-time quantitative PCR analysis of cartilage-related genes. (A) Cells stimulated during treatment with rhuGSN in different concentrations for 24 h and 72 h with IL-1β rhuGSN significantly reduces the expression of most genes. (B) Cells stimulated with TNF-α during treatment with rhuGSN in different concentrations for 24 h and 72 h. Treatment with rhuGSN shows a decreasing effect in SPARC, Col2A1 and an increasing effect in MMP1 and CXCR4 as well as in TNF-α (C) Cells stimulated with IL-1β in combination with TNF-α during treatment with rhuGSN at different concentrations for 24 h and 72 h. Gene expression of Beclin1, SPARC, COL2A1 and MMP1 is largely down regulated, while CXCR4, IL-1β and TNF- α are significantly upregulated. Missing of bars indicates no detection in these groups. N = 3, one-way Anova test, ∗ <0.5, ∗∗ <0.1 ∗∗∗ <0.05, ∗∗∗∗ <0.001.
Stimulation of cells only with TNF-α (Fig. 6B) showed the same decreasing trend in Beclin1 and SPARC as in IL-1β stimulation. HMGB1 expression was significantly up- or down regulated when cells were stimulated with 3 and 30 μg rhuGSN, respectively, as a function of time. MMP1 expression increased due to the combination of TNF-α and rhuGSN at any concentration and CXCR4. On the other hand, COL2A1 decreased significantly in all concentrations of rhuGSN in combination with TNF-α, to a minimum of 0.02-fold (30 μg rhuGSN 24 h). IL-1β was not detectable in the phCs treated with TNF-α, but TNF-α itself showed a significant increase in increasing rhuGSN concentrations up to a 28-fold expression increase compared to the control cells just treated with TNF-α without rhuGSN. COL10A was not detectable in the 3 μg rhuGSN 24 h group but showed an increase after 72 h in this group. In cells treated with 30 μg rhuGSN the expression of COL10A decreased significantly after 24 h but not after 72 h.
A combination of IL-1β and TNF-α stimulation (Fig. 6C) revealed a supported regenerative trend in the regulation of gene expression of cartilage ECM-related genes.
Beclin1 was down regulated when phCs were treated with 3 μg rhuGSN (0.4 fold), but showed a comparable expression to the control when cells were stimulated with 30 μg rhuGSN. SPARC was down regulated due to treatment with rhuGSN independent of time and concentration. After 24 h stimulation with rhuGSN, the expression of HMGB1 concentrations initially increased (24 h), but decreased after 72 h. MMP1 expression decreased significantly when cells were treated with 3 μg rhuGSN for 72 h. A higher dosage of rhuGSN led to no changes of MMP1, which indicated a dose-dependent function of rhuGSN. CXCR4 expression was increased in all cells treated with a combination of IL-1β/TNF-α and rhuGSN. Treatment of stimulated phCs with 3μgrhuGSN led to a significant decrease of COL2A1 after 72 h (0.2-fold) after an initial increase. COL10A was not detectable in the 3 μg rhuGSN 72 h group but was elevated in all others. IL-1β expression was significantly upregulated when cells were treated with 30 μg rhuGSN for 24 (2.9-fold) or 72 h (22-fold). The expression of TNF-α was initially regulated after 24 h, but decreased at 72 h treatment in both rhuGSN concentrations compared to the 24 h time point. The decrease in expression in the 72 h 3 μg rhuGSN even decreased (0.5-fold) compared to the control cells. ADAMTS-4 and MMP13 are other factors we included in our gene expression experiments, but without clear results.
4. Discussion
In this study, we present a new approach for a therapeutic methodology to support the own repair processes of cartilage by treating phCs with rhuGSN. With our experiments we could show that in phCs, similar to other non-vascular tissue cells, like human corneal cells (HCE) [18] and skin, GSN was found to be involved in wound closure processes [20]. This indicates that GSN might is a major factor in overall wound-healing and migration processes.
To analyse the effect of rhuGSN on phCs, we studied cytokine secretion and gene expression in phCs stimulated with rhuGSN alone compared to phCs in an in vitro model of osteoarthritis. One of the most significant upregulation can be seen in the TGF-β secretion after 24 h treated with 30 μg rhuGSN phC (Fig. 5D). After 72 h this positive upregulation is no longer visible, indicating that rhuGSN promotes the secretion of TGF-β in a limited time after treatment. TGF-β is an important factor in the development of osteoarthritis, as its inhibition is believed to participate in the onset of this disease. As an inhibitor of hypertrophy and maturation in chondrocytes [9], the upregulation due to rhuGSN treatment in vitro indicates a positive effect for the treatment of osteoarthritis.
Further, phCs treated with rhuGSN (Fig. 3A–D) showed a significant up-regulation for IL-6 (Fig. 3B). This effect can also be seen in mouse fibroblasts, where a stimulation with GSN leads to an increase of IL-6 secretion [21]. It was already shown that IL-6 secretion is a mediator of wound-healing processes in human corneal cells (HCE) by stimulating cell proliferation and migration [22]. Our hypothesis is, that GSN might play a role, not only in wound healing of corneal, but also in healing processes of all non-vascularised tissues. Further stimulation of phCs with rhuGSN shows a significant increase in aggrecan expression as well as a decreased expression of degrading genes such as CXCR4 and SPARC or harmful genes such as collagen 1 [23] (Fig. 3E), indicating a beneficial effect of rhuGSN in cartilage. Lower concentrations indicate more promising effects. For example, TNF-α, which stimulates the (abnormal) proliferation of fibroblasts like synoviocytes - a sign of arthritis [24] - is significantly increased in 30 μg rhuGSN treatments (Fig. 3C).
Moreover we tested the ability of GSN to promote wound healing in chondrocytes. We could show that stimulation with 3 μg rhuGSN significantly supports wound closure in vitro (Fig. 4B). In comparison, in mouse fibroblast a concentration up to 12.5 μg of GSN [22] and in HCE cells up to 300 μg of rhuGSN is needed to get positive wound healing effects [18]. This indicates that GSN needs to be evaluated tissue specific to determine which concentrations show the most beneficial effect. Further, it must be considered that chondrocytes themselves have a low turnover rate [25] and nearly no proliferative activity as chondrocytes are just proliferative active during interstitial growth. Because of this, we think that the supportive wound closure effect of rhuGSN is associated with a higher migration activity of the stimulated cells rather than with proliferation. This thesis is supported by findings of Kubler and Watt, which state that in the edge of epidermal wounds actin-related proteins, like GSN, are reduced [26]. Therefore a stimulation of these cells with rhuGSN helps to support migration into the wound area.
Additionally we examined how genes are involved in the supportive wound healing effect of rhuGSN in chondrocytes. A clearer difference between the “healthy” (without cytokine stimulation) phCs culture and the osteoarthritis model in vitro becomes apparent when focusing on gene expression (Figs. 3E and 6C). Treatment with rhuGSN of healthy cell cultures showed supportive effects on cartilage-degenerative genes such as MMP1 and (Fig. 3E). MMP1, induced by IL-1β and TNF-α, is highly associated with arthritis as it breaks down collagen (especially type II) in the ECM of cartilage and is found in high amounts on the surface of arthritic joints [27]. However, there has been a significant decrease in HMGB1, which is associated with OA in humans [28].
When phCs were stimulated with IL-1β in combination with TNF-α and subsequently treated with rhuGSN (Fig. 6C) the expression of cartilage genes associated with cartilage degeneration, like MMP1 and SPARC are reduced in phCs due to treatment with rhuGSN. On the other hand, regenerative or protective articular cartilage genes such as Beclin1 and COL2A1 are suppressed and some degenerative genes such as CXCR4 and COL10 are increased, indicating a negative effect of rhuGSN on phCs. An increased expression of CXCR4 leads to the release of more catabolic genes such as MMPs and is therefore a promotive factor of OA [6,7,29]. These results give a first impression of the effect GSN may have on chondrocyte gene expression but further experiments are needed to evaluate how rhuGSN affects gene expression in osteoarthritic cartilage and how a supportive effect occurs in “healthy” cell cultures and during migration processes.
Because of limited excess to phCs during this studies further experiments are needed to fully examine how wound healing in chondrocytes is supported by rhuGSN. In addition, different degrees of OA severity should be included in further experiments as well as in vivo experiments with osteoarthritic animals, also to test different application methods for rhuGSN, like intra synovial applications. Further additional analyses of proteins such as Snorc (small cartilage-rich novel), a specific trans membrane proteoglycan involved in endochondral ossification [30], or other factors such as fibronectin [31] should be included in further experiments to understand how accurate the positive effect of rhuGSN is in chondrocytes. New experiments with 3D models [32] and tissue samples for the cultivation of chondrocytes [33] could provide more detailed insight into how rhuGSN affects chondrocytes and the surrounding extracellular matrix. Other promising therapeutic approaches, such as cell-based surfaces [34], autologous chondrocyte implantation [35] or stem cell therapies [36] must also be included in further experiments to see if rhuGSN shows comparable or better results. A combination of these methods with rhuGSN could also be very interesting to see if an additional administration of rhuGSN would support the regeneration of articular cartilage tissue.
Here we demonstrate a new approach for treating chondrogenic defects in diseases such as osteoarthritis by stimulating phCs with rhuGSN in vitro. GSN significantly supports wound closure of phCs in cell culture-based experiments. Additionally degenerative factors (MMP, SPARC) are down regulated by rhuGSN in vitro and cytokines are significantly modulated. More experiments are necessary to fully understand the effect of GSN in non-vascular tissues such as articular cartilage as well as to find the perfect concentration for treatments of the cartilage. Therefore, we conclude that rhuGSN is a promising new approach to find a suitable therapy for degenerative cartilage diseases like osteoarthritis by supporting healthy articular cartilage areas in its regenerative processes.
5. Contributions
JF planed and performed the experiments and analysed as well as visualised the results. JW helped analysing results and gave revision of the article for important intellectual content. VS designed Fig. 1 in collaboration with JF. KG and MT provided samples of human articular cartilage for isolation of cells (KG) and for histology (MT). FP contributes by drafting and the final approval of the article as well as obtaining the funding.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgement
The authors would like to thank all patients who willingly donated their joint cartilage for research purposes after it has been removed during a necessary knee replacement operation. The authors would also like to thank Maike Hemmerlein, Anke Fischer-Gößwein, Melanie Pflügner and Hong Nguyen for their professional technical assistance.
References
- 1.Sacitharan P.K. Ageing and osteoarthritis. Subcell. Biochem. 2019;91:123–159. doi: 10.1007/978-981-13-3681-2_6. [DOI] [PubMed] [Google Scholar]
- 2.Glyn-Jones S., Palmer A.J., Agricola R., Price A.J., Vincent T.L., Weinans H., et al. Osteoarthritis. Lancet. 2015;386:376–387. doi: 10.1016/S0140-6736(14)60802-3. [DOI] [PubMed] [Google Scholar]
- 3.Buckwalter J.A., Mankin H.J., Grodzinsky A.J. Articular cartilage and osteoarthritis. Instr. Course Lect. 2005;54:465–480. [PubMed] [Google Scholar]
- 4.Bijlsma J.W., Berenbaum F., Lafeber F.P. Osteoarthritis: an update with relevance for clinical practice. Lancet. 2011;377:2115–2126. doi: 10.1016/S0140-6736(11)60243-2. [DOI] [PubMed] [Google Scholar]
- 5.Fodor I., Urban P., Kemenes G., Koene J.M., Pirger Z. Aging and disease-relevant gene products in the neuronal transcriptome of the great pond snail (Lymnaea stagnalis): a potential model of aging, age-related memory loss, and neurodegenerative diseases. Invertebr. Neurosci. 2020;20:9. doi: 10.1007/s10158-020-00242-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jia D., Li Y., Han R., Wang K., Cai G., He C., et al. miR146a5p expression is upregulated by the CXCR4 antagonist TN14003 and attenuates SDF1induced cartilage degradation. Mol. Med. Rep. 2019;19:4388–4400. doi: 10.3892/mmr.2019.10076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zheng X., Zhao F.C., Pang Y., Li D.Y., Yao S.C., Sun S.S., et al. Downregulation of miR-221-3p contributes to IL-1 beta-induced cartilage degradation by directly targeting the SDF1/CXCR4 signaling pathway. J. Mol. Med. (Berl.) 2017;95:615–627. doi: 10.1007/s00109-017-1516-6. [DOI] [PubMed] [Google Scholar]
- 8.Martinez-Lopez J.I. ECG of the month. The plot thickens. Second-degree AV block and ventricular escape rhythm. J. La. State Med. Soc. 1989;141:7–10. [PubMed] [Google Scholar]
- 9.Blaney Davidson E.N., Vitters E.L., van der Kraan P.M., van den Berg W.B. Expression of transforming growth factor-beta (TGFbeta) and the TGFbeta signalling molecule SMAD-2P in spontaneous and instability-induced osteoarthritis: role in cartilage degradation, chondrogenesis and osteophyte formation. Ann. Rheum. Dis. 2006;65:1414–1421. doi: 10.1136/ard.2005.045971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song B., Song H., Wang W., Wang H., Peng H., Cui J., et al. Beclin 1 overexpression inhibits chondrocyte apoptosis and downregulates extracellular matrix metabolism in osteoarthritis. Mol. Med. Rep. 2017;16:3958–3964. doi: 10.3892/mmr.2017.7064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakamura S., Kamihagi K., Satakeda H., Katayama M., Pan H., Okamoto H., et al. Enhancement of SPARC (osteonectin) synthesis in arthritic cartilage. Increased levels in synovial fluids from patients with rheumatoid arthritis and regulation by growth factors and cytokines in chondrocyte cultures. Arthritis Rheum. 1996;39:539–551. doi: 10.1002/art.1780390402. [DOI] [PubMed] [Google Scholar]
- 12.Aulin C., Lassacher T., Palmblad K., Erlandsson Harris H. Early stage blockade of the alarmin HMGB1 reduces cartilage destruction in experimental OA. Osteoarthritis Cartilage. 2020;28:698–707. doi: 10.1016/j.joca.2020.01.003. [DOI] [PubMed] [Google Scholar]
- 13.Feldt J., Schicht M., Garreis F., Welss J., Schneider U.W., Paulsen F. Structure, regulation and related diseases of the actin-binding protein gelsolin. Expet Rev. Mol. Med. 2019;20:e7. doi: 10.1017/erm.2018.7. [DOI] [PubMed] [Google Scholar]
- 14.Piktel E., Levental I., Durnas B., Janmey P.A., Bucki R. Plasma gelsolin: indicator of inflammation and its potential as a diagnostic tool and therapeutic target. Int. J. Mol. Sci. 2018;19 doi: 10.3390/ijms19092516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osborn T.M., Verdrengh M., Stossel T.P., Tarkowski A., Bokarewa M. Decreased levels of the gelsolin plasma isoform in patients with rheumatoid arthritis. Arthritis Res. Ther. 2008;10:R117. doi: 10.1186/ar2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Porcelli T., Pezzaioli L., Delbarba A., Maffezzoni F., Cappelli C., Ferlin A. Protein markers in osteoporosis. Protein Pept. Lett. 2020 doi: 10.2174/1871530320666200425204634. [DOI] [PubMed] [Google Scholar]
- 17.Ji L., Chauhan A., Muthaiyah B., Wegiel J., Chauhan V. Gelsolin levels are increased in the brain as a function of age during normal development in children that are further increased in Down syndrome. Alzheimer Dis. Assoc. Disord. 2009;23:319–322. doi: 10.1097/WAD.0b013e31819d494e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wittmann J., Dieckow J., Schroder H., Hampel U., Garreis F., Jacobi C., et al. Plasma gelsolin promotes re-epithelialization. Sci. Rep. 2018;8:13140. doi: 10.1038/s41598-018-31441-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schroder A., Abrar D.B., Hampel U., Schicht M., Paulsen F., Garreis F. In vitro effects of sex hormones in human meibomian gland epithelial cells. Exp. Eye Res. 2016;151:190–202. doi: 10.1016/j.exer.2016.08.009. [DOI] [PubMed] [Google Scholar]
- 20.Cowin A.J., Hatzirodos N., Teusner J.T., Belford D.A. Differential effect of wounding on actin and its associated proteins, paxillin and gelsolin, in fetal skin explants. J. Invest. Dermatol. 2003;120:1118–1129. doi: 10.1046/j.1523-1747.2003.12231.x. [DOI] [PubMed] [Google Scholar]
- 21.Vaid B., Chopra B.S., Raut S., Sagar A., Badmalia M.D., Ashish, et al. Antioxidant and wound healing property of gelsolin in 3T3-L1 cells. Oxid Med Cell Longev. 2020;2020:4045365. doi: 10.1155/2020/4045365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arranz-Valsero I., Soriano-Romani L., Garcia-Posadas L., Lopez-Garcia A., Diebold Y. IL-6 as a corneal wound healing mediator in an in vitro scratch assay. Exp. Eye Res. 2014;125:183–192. doi: 10.1016/j.exer.2014.06.012. [DOI] [PubMed] [Google Scholar]
- 23.Steinberg J., Ritchie G.R.S., Roumeliotis T.I., Jayasuriya R.L., Clark M.J., Brooks R.A., et al. Integrative epigenomics, transcriptomics and proteomics of patient chondrocytes reveal genes and pathways involved in osteoarthritis. Sci. Rep. 2017;7:8935. doi: 10.1038/s41598-017-09335-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang K.S., Wang J.F., Zhang S.L., Li Z., Pei Z., Guan Z.P. Effects of tumor necrosis factor Alpha on the expression of programmed cell death factor 5 in arthritis. Orthop. Surg. 2019;11:698–704. doi: 10.1111/os.12497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Freman M.A.R. 1979. Pitman Medical. Adult Articular Cartilage; pp. 145–214. [Google Scholar]
- 26.Kubler M.D., Watt F.M. Changes in the distribution of actin-associated proteins during epidermal wound healing. J. Invest. Dermatol. 1993;100:785–789. doi: 10.1111/1523-1747.ep12476492. [DOI] [PubMed] [Google Scholar]
- 27.Burrage P.S., Mix K.S., Brinckerhoff C.E. Matrix metalloproteinases: role in arthritis. Front. Biosci. 2006;11:529–543. doi: 10.2741/1817. [DOI] [PubMed] [Google Scholar]
- 28.Amin A.R., Islam A.B. Genomic analysis and differential expression of HMG and S100A family in human arthritis: upregulated expression of chemokines, IL-8 and nitric oxide by HMGB1. DNA Cell Biol. 2014;33:550–565. doi: 10.1089/dna.2013.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang W., Ha C., Lin T., Wang D., Wang Y., Gong M. Celastrol attenuates pain and cartilage damage via SDF-1/CXCR4 signalling pathway in osteoarthritis rats. J. Pharm. Pharmacol. 2018;70:81–88. doi: 10.1111/jphp.12835. [DOI] [PubMed] [Google Scholar]
- 30.Heinonen J., Zhang F.P., Surmann-Schmitt C., Honkala S., Stock M., Poutanen M., et al. Defects in chondrocyte maturation and secondary ossification in mouse knee joint epiphyses due to Snorc deficiency. Osteoarthritis Cartilage. 2017;25:1132–1142. doi: 10.1016/j.joca.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 31.Kalkreuth R.H., Kruger J.P., Lau S., Niemeyer P., Endres M., Kreuz P.C., et al. Fibronectin stimulates migration and proliferation, but not chondrogenic differentiation of human subchondral progenitor cells. Regen. Med. 2014;9:759–773. doi: 10.2217/rme.14.40. [DOI] [PubMed] [Google Scholar]
- 32.Khurshid M., Mulet-Sierra A., Adesida A., Sen A. Osteoarthritic human chondrocytes proliferate in 3D co-culture with mesenchymal stem cells in suspension bioreactors. J Tissue Eng Regen Med. 2018;12:e1418–e1432. doi: 10.1002/term.2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Youngstrom D.W., Cakstina I., Jakobsons E. Cartilage-derived extracellular matrix extract promotes chondrocytic phenotype in three-dimensional tissue culture. Artif Cells Nanomed Biotechnol. 2016;44:1040–1047. doi: 10.3109/21691401.2015.1014091. [DOI] [PubMed] [Google Scholar]
- 34.Caldwell K.L., Wang J. Cell-based articular cartilage repair: the link between development and regeneration. Osteoarthritis Cartilage. 2015;23:351–362. doi: 10.1016/j.joca.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hinckel B.B., Gomoll A.H. Patellofemoral cartilage restoration: indications, techniques, and outcomes of autologous chondrocytes implantation, matrix-induced chondrocyte implantation, and particulated juvenile allograft cartilage. J. Knee Surg. 2018;31:212–226. doi: 10.1055/s-0037-1607294. [DOI] [PubMed] [Google Scholar]
- 36.Kubosch E.J., Lang G., Furst D., Kubosch D., Izadpanah K., Rolauffs B., et al. The potential for synovium-derived stem cells in cartilage repair. Curr. Stem Cell Res. Ther. 2018;13:174–184. doi: 10.2174/1574888X12666171002111026. [DOI] [PubMed] [Google Scholar]