Summary
Background
Rotator cuff injury (RCI) is a leading cause of morbidity in orthopaedics. Advances in regenerative medicine have led to the novel pleiotropic effects of mesenchymal stromal cells (MSCs) as therapeutic agents for RCI.
Objective
Conduct a systematic evaluation of available preclinical studies to quantify the effects of MSCs on RCI.
Methods
A literature search was performed in PubMed, Scopus, Cochrane, CINAHL, and Google Scholar. At least two independent investigators screened animal studies assessing the therapeutic effects of MSCs on: (i) biomechanical testing, imaging, and/or range-of-motion (primary outcome), and (ii) histologic analyses of wound healing, gene/protein expression of regenerative factors, and safety/long-term outcomes (secondary outcome). Meta-analysis data is reported as standardized mean difference (SMD) with 95% confidence interval (CI).
Results
A total of 858 titles and abstracts were screened; 18 studies (n=576) met inclusion criteria. MSC therapy improved ultimate load failure [SMD -0.43 (95% CI -0.65, -0.22), p<0.0001; 15 studies, 28 comparisons], site stiffness [SMD -0.29 (95% CI -0.55, -0.04), p<0.05; 9 studies, 17 comparisons], bone mineral density [SMD -0.77 (95% CI -1.16, -0.38), p<0.0001; 2 studies, 6 comparisons], and stimulated fibrocartilage formation [SMD of -1.37 (95% CI -1.99, -0.74), p<0.0001; 4 studies, 7 comparisons]. Heterogeneity between studies was high and risk of bias was unclear.
Conclusion
Administration of MSCs in preclinical models recapitulating RCI improved aspects of shoulder biomechanics, imaging, and collagen formation. Although these findings are promising, future studies should attempt to limit the risk of bias and focus on optimizing MSCs by standardizing methodologies.
Keywords: Stem cell, Mesenchymal stromal cell, Rotator cuff, Animal, Meta-analysis, Regenerative medicine
1. Introduction
Among musculoskeletal disorders, shoulder pain ranks third only to back and neck pain [1]. Upwards of 50% of individuals aged 50 years and older will succumb to a partial- or full-thickness rotator cuff tear. Despite being a frequently encountered orthopaedic condition, management and outcomes of rotator cuff injuries (RCI) are variable [2,3]. For instance, failure rates of surgical treatment of a RCI range from 11% to 95% (failure defined as re-tears and/or worsening of tear grade) [[2], [3], [4]]. Therefore, novel interventions for RCIs are warranted.
Mesenchymal stem/stromal cells (MSCs) are multipotent cells known to release trophic factors that aid in inflammation, wound healing, and fibrocartilage [5]. Furthermore, advantages to using MSCs for RCI include: ease of obtainment, rapid proliferative capacity, host immune system tolerance, and the capacity for cell-cell interaction and, adaptation to an injured microenvironment. Animal models mimicking RCI pathology have demonstrated therapeutic potential of MSC administration [[6], [7], [8]]. These preclinical MSC findings have now translated into human clinical trials (Supplementary Table 1 depicting ongoing trials per ClinicalTrials.gov). However, there has been no review that has analyzed the quantitative effect of MSCs for RCI.
The aim of our work was threefold: (i) systematically review the current preclinical literature utilizing MSCs for RCI; (ii) quantify the functional of MSCs; and (iii) identify gaps that should be addressed to optimize the regenerative capacity of MSCs.
2. Materials and methods
2.1. Protocol
Our methods adhere to the guidelines established by the Systematic Review Centre for Laboratory Animal Experimentation (SYRCLE) and are described in Supplementary Table 1.
2.2. Literature search
We conducted a literature search on 5 databases, including PubMed, SCOPUS, Cochrane Library, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), and Google Scholar, through March 11, 2019. Our search terms included ‘rotator cuff’ and ‘mesenchymal stem cells’ along with any synonyms for the two main terms. Refer to Supplementary Table 2 for exact terms and synonyms. Screening by title/abstract and subsequent full-text review was conducted independently by two investigators (N.M-G. and D.C.).
2.3. Inclusion and exclusion criteria
Studies were included if they reported the functional effect of MSC administration in animal studies of RCI repair. MSCs were defined per The International Society for Cellular and Gene Therapy [9]. All studies with MSC intervention where included regardless of tissue source and origin, dose, timing, and frequency of administration. Studies were excluded if they did not provide an intervention or have a control/comparison group.
2.4. Primary and secondary endpoints
We defined our primary endpoint as rotator cuff function, reported via biomechanical testing, and/or imaging, and/or range-of-motion. Studies were excluded if the primary outcome criteria were not met. Our secondary outcomes were (i) histologic/microscopic analyses of wound healing, (ii) gene/protein expression, (iii) safety and long-term outcomes.
2.5. Data extraction
Data was collected independently by three investigators (N.M-G, D.C., & C.E.) and compared for consistency. Extracted data included general study design, animal characteristics, details on MSC intervention, and outcome measures. Quantitative data was extracted from manuscript texts, figures, and tables. WebPlotDigitizer (www.automeris.io/WebPlotDigitizer/) was used to obtain data obtained from figures (graphs & plots). Qualitative data was recorded for potential inclusion in narrative findings (Supplemental File 1).
2.6. Risk of bias
SYRCLE's Risk of Bias tool was used to assess methodological quality of animal studies [10].
2.7. Data analysis
Meta-analysis was conducted utilizing a random effects model to generate forest plots if 4 or more comparisons could be made via overlapping outcomes. The estimated efficacy of MSC application on RCI repair was determined using a standardized mean difference (SMD) and a 95% confidence interval (CI). Interpretation for SMD followed Cohen's guidelines where SMD = 0.2 is small, SMD = 0.5 is medium, and SMD = 0.8 is large [11]. If more than 10 studies were included for an outcome, a subgroup analysis was performed to assess for variability in MSC efficacy by: tissue source and origin, dose, timing, and frequency of administration [12]. Heterogeneity between studies was calculated using the I2 metric. Statistical analysis was performed using RevMan Review Manager [13]with a p-value <0.05 set for significance.
3. Results
3.1. Study selection
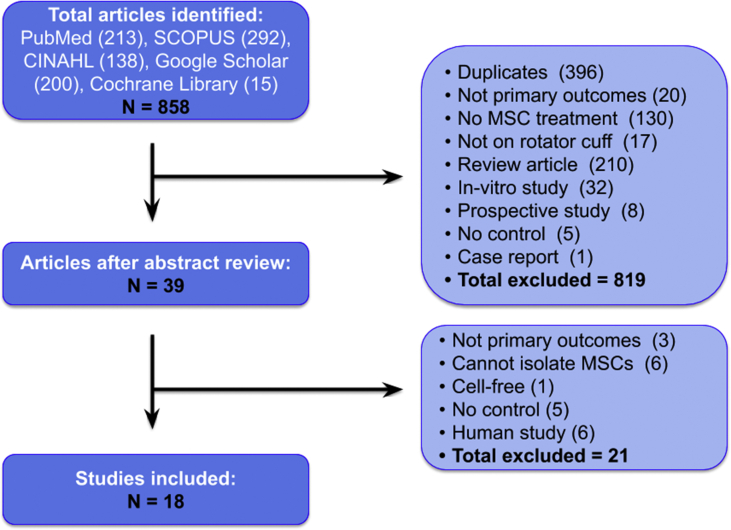
Our literature search identified 858 studies. After excluding duplicate studies, non-interventional studies, studies with no control group, studies with cell-free interventions, in-vitro studies, prospective studies, case reports, and human studies, 18 studies remained for full-text review (Fig. 1). All studies were included in the qualitative synthesis; however, only 14 preclinical studies shared endpoints that could be quantitatively analyzed and included in the meta-analysis.
Fig. 1.
Literature search.
3.2. Study characteristics
The animal studies were published between 2009 and 2019 with nine published in the United States, four in South Korea, three in Spain, one in China, and one in Japan. An overview of the included studies can be found in Table 1.
Table 1.
Overview of study details.
| Author (Year) | Study Design |
Intervention Characteristics |
Outcome Measures |
|||||
|---|---|---|---|---|---|---|---|---|
| Model | RCI model type (acute vs chronic) | Species | Cell type | Total dose | Cell Delivery | Timing of assessment post RCI intervention | Assessment Type | |
| Barco 2014 | Sharp detachment of supraspinatus tendon | Acute | Syngeneic BDIX Rats | Allogenic Adipose-derived stem cells | 2,000,000 | Fibrin sealant | 4, 8 weeks | Ultimate Load Failure Rigidity Ultimate Tensile Strength Energy Absorbed |
| Chen 2015 | Collagenase injection at supraspinatus tendon | Acute | Female Sprague Dawley Rats | Xenogenic Human Adipose-derived stem cells | 3,000,000 | PBS | 3, 7, 14, 21, 28 days | Ultimate Load Failure In-vitro mRNA Expression |
| Degan 2016 | Pressure detachment of supraspinatus tendon | Acute | Athymic Nude Rats | Xenogenic Bone Marrow-derived human MSCs | 1,000,000 | Fibrin sealant | 2, 4 weeks | Ultimate Load Failure Stiffness |
| Gullota 2009 | Sharp detachment of supraspinatus tendon | Acute | Males Lewis Rats | Allogenic Bone Marrow-derived stem cells | 1,000,000 | Fibrin sealant | 2, 4 weeks | Ultimate Load Failure Stiffness Cross-sectional Area Ultimate Tensile Strength Area of Metachromasia Collagen Birefringence MSC Tracking |
| Kaizawa 2019 | Sharp detachment of supraspinatus tendon | Chronic | Male Sprague Dawley Rats | Allogenic “Luciferase-transfected” Adipose-derived stem cells (Luc + ASC) | Not reported | Growth medium | 8 weeks | Ultimate Load Failure Stiffness Micro CT Area of Metachromasia Collagen Birefringence MSC Tracking |
| Kim 2017 | Not reported | Acute | New Zealand White Rabbits | Xenogenic Adipose-derived stem cells | 1,000,000 | Acellular dermal matrix | 8 weeks | Ultimate Load Failure Ultimate Tensile Strength Modified Tendon Maturing Score MSC Tracking |
| Kwon 2018 | Punch biopsy of subscapularis tendon | Chronic | New Zealand White Rabbits | Xenogenic Human Umbilical Cord Blood-derived Mesenchymal Stem Cells (UCB-MSCs) | 1,000,000 | Solution not reported | 4 weeks | Walking Distance Fast Walking time Mean Walking Speed Tear Size |
| Learn 2018 | Sharp detachment of infraspinatus tendon | Acute | New Zealand White Rabbits | Allogenic Bone Marrow from two rabbit femurs | 500,000 | Electrochemically aligned collagen | 3 months | Ultimate Load Failure Stiffness |
| Lipner 2015 | Sharp detachment of supraspinatus tendon | Acute | Sprague Dawley Rats | Allogenic Adipose-derived stem cells | 500,000 | Aligned nanofibrous poly (lactic-co-glycolic acid) scaffold | 14, 28, 56 days | Ultimate Load Failure Stiffnes Ultimate Strain Micro CT Modified Tendon Maturing Score MSC Tracking |
| Liu 2019 | Sharp detachment of infraspinatus tendon | Acute | Mixed Breed Dogs | Autologous Bone Marrow-derived MSCs | 1,000,000 | Tendon-fibrocartilage-bone composite (TFBC) | 6 weeks | Ultimate Load Failure Ultimate Tensile Strength Cross-sectional Area Area of Metachromasia Collagen Birefringence MSC Tracking |
| Oh 2014 | Sharp detachment of subscapularis tendon, the tendon is wrapped in silicone penrose drain | Chronic | New Zealand White Male Rabbits | Allogenic Adipose-derived stem cell | 10,000,000 | Hank's Balanced Salt Solution | 6 weeks | Ultimate Load Failure EMG |
| Omi 2016 | Sharp detachment of supraspinatus tendon | Acute | Adult Female Lewis Rats | Allogenic Bone Marrow stromal cells (BMSC) | 1,000,000 | Composite of multilayer tendon slices (COMTS scaffold) | 6 weeks | Ultimate Load Failure Stiffness MSC Tracking |
| Park 2015 | Punch biopsy of subscapularis tendon | Acute | Male New Zealand White Rabbits | Xenogenic Human Umbilical Vein-derived MSCs | Not reported | Solution not reported | Pre-intervention, 4 weeks | Walking Distance Fast Walking time Mean Walking Speed Tear Size MSC Tracking |
| Rothrauff 2018 | Sharp detachment of supraspinatus and infraspinatus tendons | Acute and Chronic | Adult Male Lewis Rats | Allogenic Adipose-derived stem cells | 1,000,000 | Fibrin sealant or Gelatin methacrylate (GelMA) | 4 weeks | Ultimate Load Failure Stiffness Micro CT |
| Smietana 2017 | Sharp detachment of supraspinatus tendon | Acute and Chronic | Female Fischer Rats | Allogenic Bone Marrow stromal cells from rat femur that was concentrated | Not reported | Tissue engineered tendon | 8 weeks | Ultimate Load Failure Stiffness Area of Metachromasia Collagen Birefringence |
| Tornero 2015 | Sharp detachment of supraspinatus tendon | Chronic | Sprague Dawley Rats | Allogenic Bone marrow-derived stem cells | 1,000,000 | Type 1 collagen membrane | 4, 8 weeks | Ultimate Load Failure Stiffness Max Deformation |
| Valencia 2014 | Sharp detachment of supraspinatus tendon | Acute | Sprague Dawley Rats | Allogenic Adipose-derived MSCs | 2,000,000 | Collagen carrier | 2, 4 weeks | Ultimate Load Failure Stiffness Max Deformation Energy Absorbed Elastic Load MSC Tracking |
| Yokoya 2012 | Sharp detachment of infraspinatus tendon | Acute | Adolescent Japanese White Rabbits | Autologous Bone Marrow-derived MSCs | 5,000,000 | Polyglycolic acid sheed scaffold | 4, 8, 16 weeks | Ultimate Load Failure Ultimate Tensile Strength Cross-sectional Area Young's Modulas Modified Tendon Maturing Score |
The animal studies used rats (n = 11), rabbits (n = 6), and dogs (n = 1). Eleven studies reported gender (40%); of which 7 exclusively used male animals. The age of animals ranged between 6 weeks and 9 months. Twelve studies used an acute RCI model (intervention within 3 days of RCI model creation), 4 studies used a chronic RCI model (intervention within 4–8 weeks after model creation), and 2 studies used a combination. Study characteristics can be found in Table 2.
Table 2.
Summary of study characteristics.
| ANIMAL CHARACTERISTICS | N (%) |
|---|---|
| Animal Type | |
| Rat | 11 (61.1) |
| Rabbit | 6 (33.3) |
| Canine | 1 (5.6) |
| Rat | |
| Sprague Dawley Rat | 5 (27.8) |
| Lewis Rat | 3 (16.7) |
| Athymic nude Rat | 1 (5.6) |
| Fisher Rat | 1 (5.6) |
| Syngeneic BDIX Rat | 1 (5.6) |
| Rabbit | |
| New Zealand white Rabbit | 5 (27.8) |
| Japanese white Rabbit | 1 (5.6) |
| Canine | |
| Mixed-breed dogs | 1 (5.6) |
| Age | |
| <3 Months | 1 (5.6) |
| ≥3 months and <6 months | 5 (27.8) |
| ≥8 months | 3 (16.7) |
| Not reported | 9 (50) |
| Sex | |
| Male | 7 (38.9) |
| Female | 4 (22.2) |
| Not reported |
7 (38.9) |
|
STEM CELL CHARACTERISTICS |
N (%) |
| Source | |
| Adipose | 8 (44.4) |
| Bone Marrow | 8 (44.4) |
| Umbilical Cord | 2 (11.1) |
| Origin | |
| Allogenic | 11 (61.1) |
| Autologous | 2 (11.1) |
| Xenogenic | 5 (27.8) |
| Dose | |
| ≤1 Million cells | 10 (55.6) |
| >1 Million cells | 5 (27.8) |
| Not reported |
3 (16.7) |
| EXPERIMENTAL CHARACTERISTICS |
N (%) |
| RCI Model | |
| Acute Model (intervention given ≤ 3 days after model creation) | 12 (66.7) |
| Chronic Model (intervention given 4–8 weeks after model creation) | 4 (22.2) |
| Both | 2 (11.1) |
| Delivery Method | |
| Direct Application | 4 (22.2) |
| Scaffold | 14 (77.8) |
| Measurement Modality | |
| Biomechanical - Ultimate Load Failure | 15 (83.3) |
| Biomechanical - Stiffness | 9 (50) |
| Biomechanical - Tendon Cross-sectional Area | 3 (16.7) |
| Imaging - MicroCT | 3 (16.7) |
| Histologic - Collagen Birefringence | 4 (22.2) |
| Histologic - Total Area of Metachromasia | 4 (22.2) |
| Safety | |
| Presence of infection | 2 (11.1) |
| Complication due to surgical procedure | 1 (5.6) |
| No complications noted | 6 (33.3) |
| Complications were not addressed | 9 (50) |
Amongs studies, MSC origin was variable with most studies using allogenic sources (n = 11, 61%). Adipose tissue and bone marrow were the most commonly used sources. For each study, MSC intervention was applied once, and most investigators delivered MSCs in conjunction with a scaffold (i.e. fibrin sealant, collagen carrier, textile product etc.). Total MSC dose ranged from 500,000 to 10, 000, 000 cells.
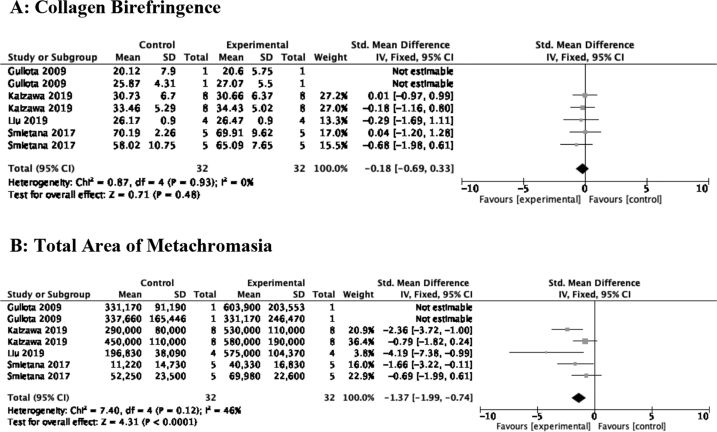
The biomechanical variables most commonly assessed between studies were ultimate load failure (n = 16) and stiffness (n = 9). In terms of secondary outcomes, collagen organization was assessed by collagen birefringence (n = 4), while new fibrocartilage formation was examined through the total area of metachromasia stained (n = 4).
3.3. MSC characteristics
A summary of MSC characteristics for the included animal studies can be found in Supplementary Table 3. Eighty-three percent of studies self-isolated MSCs (n = 15); however, many studies did not report the minimum criteria necessary to define their cells as MSCs (n = 3; 16.7%). The criteria most commonly reported was plastic adherence (n = 6), followed by positive flow cytometry markers (n = 8). MSC differentiation capacity was reported in 4 studies. The tissue source of MSCs was evenly split between adipose and bone marrow-derived, where 61% of studies performed an allogeneic transplantation of cells.
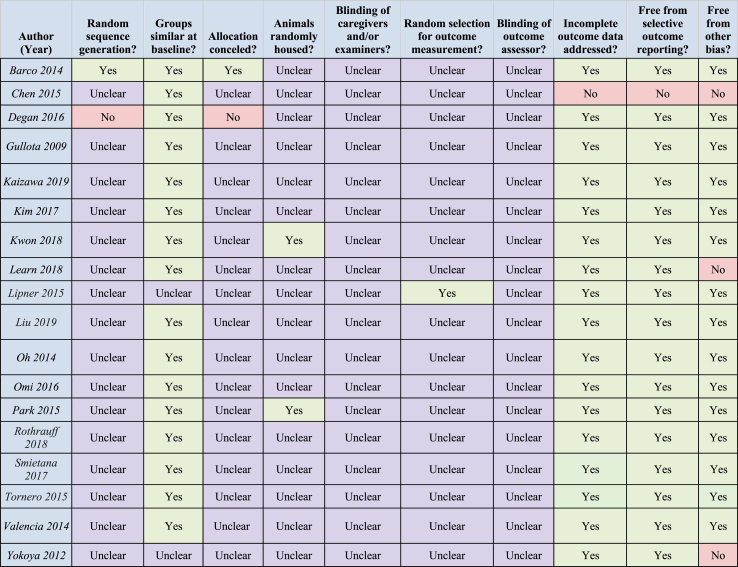
3.4. Risk of bias
The risk of bias was assessed for all 18 of the included animal studies using the SYRCLE Risk of Bias Tool (summarized in Table 3). Based on the information supplied in each published manuscript, none of the included experiments met criteria for low bias risk across all domains. All studies failed to disclose details regarding blinding of animal caregivers and investigators. Only one study endorsed the use of a random sequence generator. Two (11%) studies met the criteria of low risk of bias with regards to housing by caging animals individually. A high risk of bias was warranted for 3 (16%) studies that declared receipt of funding from possible influencers.
Table 3.
SYRCLE risk of bias assessment for included studies. Yes-green (minimal risk of bias); No-red (risk of bias); Unclear-purple (intermediate risk of bias).
3.5. Meta-analysis for primary outcomes
3.5.1. Biomechanics
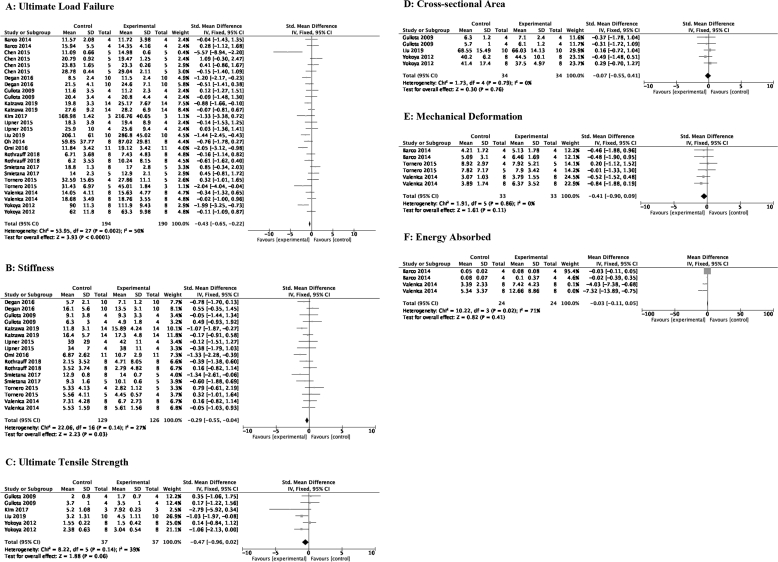
Biomechanical properties of RCI repair were assessed by: (i) ultimate load failure (N), (ii) stiffness (N/mm), (iii) ultimate tensile strength (MPA), (iv) tendon cross-sectional area (mm2), (v) deformation (mm), and (vi) absorbed energy (j). Of the biomechanics measures, ultimate load failure and stiffness demonstrated benefit after MSC administration. Data is depicted in Fig. 2A–F.
-
(i)
Ultimate load failure: MSC therapy after RCI increased ultimate load failure [SMD of −0.43 (95% CI -0.65, −0.22) p < 0.0001; 15 studies, 28 comparisons]. Heterogeneity between the ultimate load failure studies was substantial [I2 = 50%, p < 0.0001].
-
(ii)
Stiffness: RC repair site stiffness improved with MSC delivery [SMD of −0.29 (95% CI -0.50, −0.04) 9 studies, 17 comparisons].
-
(iii)
Ultimate tensile strength: No statistical significance was observed with regards to tensile strength after MSC application [SMD of −0.47 (95% CI -0.96, 0.02) 4 studies, 6 comparisons].
-
(iv)
Cross-sectional area: RCI tendon cross-sectional area was also not significant between the MSC intervention groups and the control groups [SMD of −0.07 (95% CI -0.55, 0.41) 3 studies, 5 comparisons].
-
(v)
Mechanical Deformation: No statistical significance was observed with regards to mechanical deformation after MSCs were given to animals with RCI [SMD of −0.41 (95% CI -0.90, 0.09) p = 0.11].
-
(vi)
Energy Absorbed: No statistical significance was seen after MSC intervention [SMD of −0.03 (95% CI -0.11, 0.5) p = 0.41; 2 studies, 4 comparisons].
Fig. 2.
A–F: Effects of MSC therapy on RCI biomechanics. A: Ultimate load failure; B: Stiffness; C: Ultimate tensile strength; D: Cross-sectional area; E: Mechanical deformation; F: Energy absorbed.
3.5.2. Imaging
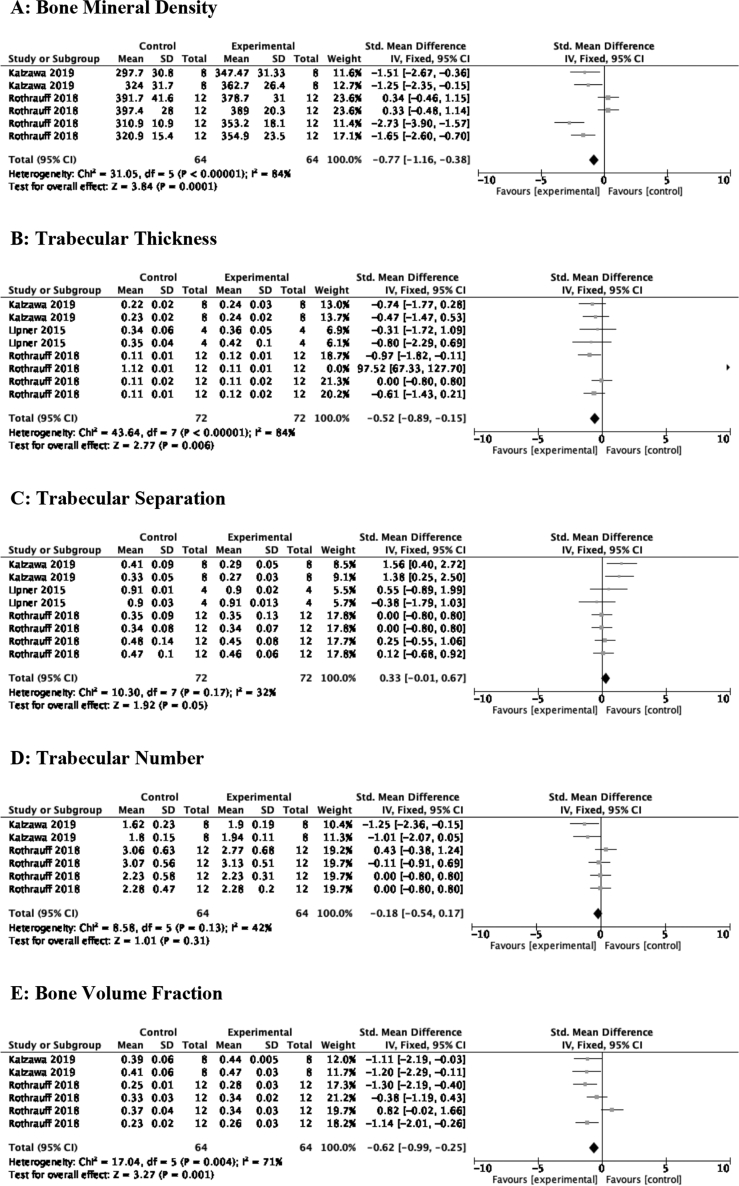
Bone morphology of the RCI intervention site was assessed by MicroCT using the following measures: (i) bone mineral density (BMD), (ii) trabecular thickness (T.Th), (iii) trabecular separation (T.Sp), (iv) trabecular number (T.N), and (v) bone volume fraction (BVF). MSC therapy improved three of the five imaging measures (refer to Fig. 3A–E).
-
(i)
BMD: There was significant improvement in bone mineral density after MSC therapy [SMD of −0.77 (95% CI -1.16, −0.38) 2 studies, 6 comparisons].
-
(ii)
T.Th: MSC administration enhanced trabecular thickness [SMD of −0.52 (95% CI -0.89, −0.15) 3 studies, 8 comparisons].
-
(iii)
T.Sp: No difference was appreciated in trabecular separation after MSC transplantation [SMD of 0.33 (95% CI -0.01, 0.67) 3 studies, 8 comparisons].
-
(iv)
T.N: MSCs had no impact on bone trabecular number [SMD of −0.18 (95% CI -0.54, 0.17) 2 studies, 6 comparisons].
-
(v)
BVF: A −0.62 SMD [95% CI -0.99, −0.25] difference was seen after MSC treatment to animals with RCI.
Fig. 3.
A–E: Effects of MSC therapy on bone morphology. A: Bone mineral density; B: Trabecular thickness; C: Trabecular separation; D: Trabecular number; E: Bone volume fraction.
3.6. Meta-analysis for secondary outcomes
3.6.1. Histological
(i) Collagen birefringence and (ii) total area of metachromasia were surrogates of collagen organization and neofibrocartilage formation, respectively. MSCs stimulated fibrocartilage formation [SMD of −1.37 (95% CI -1.99, −0.74) 4 studies, 7 comparisons], but did not confer a benefit in collagen organization [SMD of −0.18 (95% CI -0.69, 0.33) p = 0.48]. Outcomes addressing gene/protein expression in the reviewed articles was not substantial and thus was not included in this text. Data is depicted in Fig. 4A–B.
Fig. 4.
A–B: Effect of MSC therapy on semiquantitative histologic outcomes. A: Collagen birefringence; B: Total area of metachromasia.
3.7. Subgroup analysis
3.7.1. Ultimate load failure
Supplementary Figs. 1A–G depict subgrouping of the treatment effect. Also refer to Supplementary Table 4 for condensed overview.
-
⁃
Tissue source: Bone marrow derived stem cells yielded a moderately significant SMD, while no significance was observed with the use of adipose-derived cells versus control [SMD of −0.66 (95% CI -0.99, −0.32) vs. −0.27 (95% CI -0.55, 0.01)].
-
⁃
Autologous vs. Allogeneic/Xenogeneic: A larger effect size was appreciated with the use of autologous MSCs [SMD -1.05 (95% CI -1.66, −0.44) p < 0.0001].
-
⁃
Dose: With respect to the varying MSC doses utilized in the reviewed studies, it was observed that ultimate load failure improved most when a dose of less than or equal 1 million cells was used [SMD of −0.77 (95% CI -1.03, −0.38) p < 0.0001; 8 studies, 13 comparisons].
-
⁃
Early vs. Late MSC administration: Minimal difference was noted between early (≤3 days) and late (>4 weeks) delivery of MSCs [SMDs of −0.45 vs. −0.39, respectively].
-
⁃
Timing of biomechanical assessment: Evaluation 4 weeks after RCI injury demonstrated better outcomes and was the only significant period of assessment observed [SMD of −0.67 (95% CI -0.99, −0.35)].
-
⁃
Animal model: A canine model of RCI had the highest effect size [SMD -1.44 (95% CI -2.45, −0.43)], although this is limited to an analysis of one study with 20 animals.
-
⁃
Scaffold vs. Scaffold-less: Scaffold delivery of MSCs improved outcomes with a moderate SMD of −0.43 [95% CI -0.65, −0.22]. Studies delivering MSCs without a scaffold were found to have no improvement in outcomes [95% CI -0.90, 0.04; p = 0.07].
3.8. Publication bias
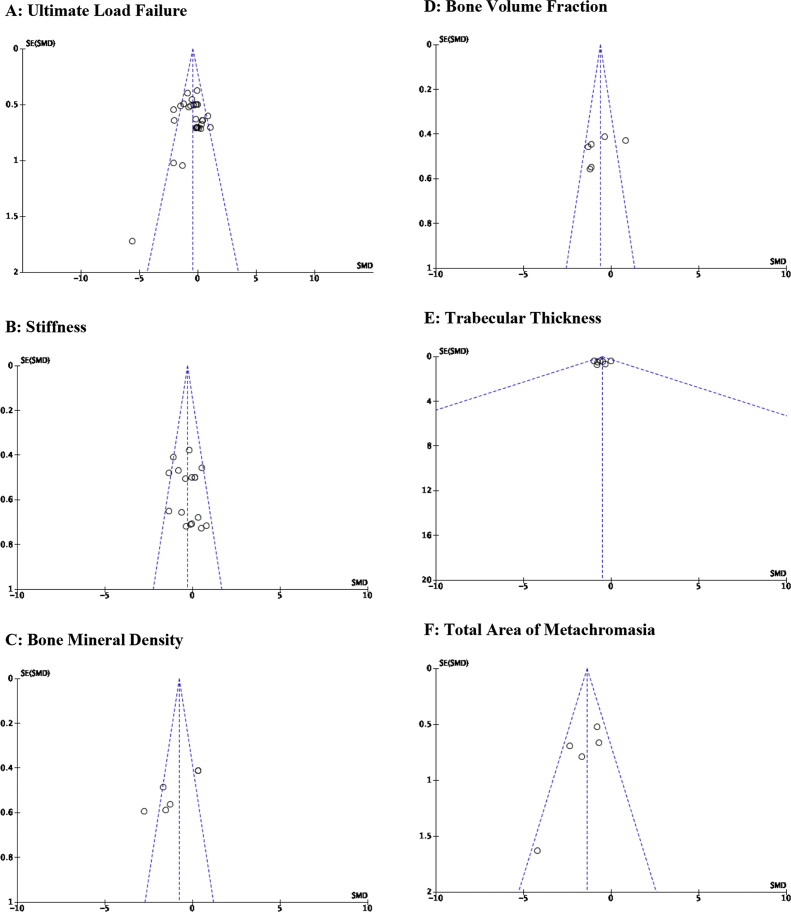
Considering the heterogeneity observed across all 18 studies, funnel plot assessment was conducted to determine the relationship between study quality and observed effect size (Fig. 5A–F). Interpretation of these plots was limited to the studies that were included in the meta-analysis. Qualitatively, the funnel plot for the primary outcome, ultimate load failure (Fig. 5A), indicates relatively symmetrical outcomes with only 3 points lying outside the boundaries where 95% of SMDs would be predicted to fall based on SEM. Stiffness depicted relatively similar findings as ultimate load failure (Fig. 5B). Of the 3 significant bone morphology comparisons included in the meta-analysis, BMD and BVF had relatively close boundaries representing 95% of SMDs (Fig. 5C–D). Trabecular thickness, however, had a very-wide 95% confidence interval (Fig. 5E). With regards to the new-fibrocartilage as represented by total area of metachromasia, the funnel plot generated signifies symmetrical outcomes with no studies falling outside the 95% confidence interval (Fig. 5F).
Fig. 5.
A–F: Publication bias. A: Ultimate load failure; B: Stiffness; C: Bone mineral density; D: Bone volume fraction; E: Trabecular thickness; F: Total area of metachromasia.
Narrative Findings can be found in Supplemental File 1.
4. Discussion
Affecting greater than 50% of people 60 years and older, rotator cuff tears are a common cause of shoulder pain and dysfunction [28,29]. As a result, surgical repair of the rotator cuff is one of the most common orthopaedic interventions for shoulder pain, and methods to improve functional outcomes are continuing to evolve. With the ability to differentiate into mesenchymal tissues such as tendon, muscle, and bone, MSCs provide an opportunity to bridge the advances in regenerative medicine and help reduce surgical failure rates for RCIs [30].
This review suggests that MSC application to RCI improves outcomes in animal models by: (1) increasing ultimate load failure [SMD of −0.42], (2) enhancing resistance to mechanical deformation (stiffness), (3) improving local bone quality, and (4) recruiting new fibrocartilage formation at the repair site [SMD of −1.37]. As such, the findings in this study offer hope in potentiating RCI healing by improving the quality of tissue surrounding the area under repair.
Several preclinical studies reviewing tendon- and ligament-potentiated healing via MSCs have found improvement in ultimate load failure and are consistent with the findings of this study. Using a rabbit model, Soon et al. discovered that ultimate load failure of reconstructed anterior cruciate ligaments increased by 56% at 8 weeks post-repair in groups subjected to cell-based therapy [31]. Similarly, Adams et al. found that surgical repair with MSC adjuvant significantly improved achilles tendon ultimate load failure in rat models [32]. In contrast, Okamota et al. found that isolated MSCs only improved ultimate load failure at 7 and 14 days, but not at 28 days post-repair [33]. Furthermore, Okamota et al. found that ultimate load failure improved significantly more at 7, 14, and 28 days post-intervention with the use of bone marrow concentrate compared to isolated MSCs. Therefore, the importance of growth factors, as found in bone marrow concentrate, is still unclear [2].
Neofibrocartilage formation, as measured by total area metachromasia, was significantly improved with MSC intervention and the most robust finding of this review. In a review from Lee et al. examining MSC application to large cartilage defects in a porcine model, morphologic and histologic evidence indicated that cell-based therapy resulted in improved cartilage regeneration at 6 and 12 weeks post-therapy [34]. In congruence, Zhang et al. and Guo et al. also found significant improvement in cartilage formation after MSC therapy in rabbits models [35,36]. The clinical application of MSC induced cartilage regeneration is still, however, unclear. Recently, multiple clinical trials have reviewed functional and imaging-based outcomes related to cell-based therapy and have yielded conflicting results [[37], [38], [39], [40]]. These conflicting outcomes between preclinical and clinical studies highlight gaps in translation research.
Our subgroup analysis of ultimate load failure suggests improved weight bearing with: (1) autogenic origin, (2) bone marrow source, (3) RCI assessment 4 week post-MSC intervention, (4) the use of rabbit or canine models, (5) an MSC dose of less than or equal to 1,000,000 cells, (6) early administration of MSCs (≤3 days) after RCI model creation, and (7) utilization of a scaffold for cell delivery. The notion that early therapy improves outcomes is not a novel finding of this study nor is it restricted to the musculoskeletal application of MSCs. In a study reviewing the application of cell-based therapy to myocardial infarctions, Wu et al. suggested the importance of early MSC therapy, as it translated to greater infarction reduction [41]. The clinical implications of early MSC application to rotator cuff tears is not necessarily practical, as many surgical repairs are not performed without some considerable delay after injury [7,21]. Though some improvement was observed in both acute and chronic RCI models in our study, cell-based therapy in rotator pathology is likely not a ‘one-size-fits-all’ application. Later in the review, Wu et al. goes on to suggest that although early therapy is crucial, so is the use of scaffolds for cellular delivery [41]. In this, it is suggested that even if cells were to be delivered early in the disease course, without a scaffold to retain cells, MSCs would simply leak out of the myocardium or wash away as a result of blood flow.
Publication bias is a significant concern, as it may lead to conclusions that overestimate the true effect of therapy. This study utilized trim and fill statistical adjustments and identified a notable reduction in treatment effect. However, the treatment effect remained statistically and clinically significant. Among the 18 included studies, many met criteria for low risk of bias or were unclear across multiple domains. It was not clear whether animals were housed randomly or if the examiners were blinded, which introduces the potential for a type 1 statistical error. This ambiguity makes it challenging to accurately replicate and validate the findings of these animal studies. In addition, we noted a high degree of heterogeneity, with values ranging between 27 and 71% (I2) across the primary and secondary outcomes. However, this is expected among preclinical studies given differences in animal species, study design, and functional outcomes. Specifically, for this study, there were noticeable differences in origin and source of MSCs, timing of delivery, timing of assessment, and the use of a cellular scaffolds. Therefore, it is important to standardize these parameters for future preclinical trials.
4.1. Clinical relevance
Arthroscopic surgery is the most common management option for treating chronic shoulder pain; however, these can be technically challenging and are subject to long term complications such as stiffness, nerve injury, implant failure and adhesive capsulitis. Therefore, mesenchymal stem cell-based therapy has emerged as a promising option for the treatment of RCI. As of November 3rd, 2019, clinicaltrials.gov indicates that there are 3 ongoing clinical trials and 6 completed trials evaluating the role of MSCs as a treatment for RCI [[42], [43], [44], [45], [46], [47]]. All clinical studies included MSCs derived from bone marrow or adipose-derived tissues. Of the 6 published trials, half of them used MSCs as an adjunct to surgery while the other half utilized ultrasound-guided injections without surgery. Of the completed trials, some of the early findings of these MSCs have shown:
-
1.
Significantly decreased number of ruptures at 10-year follow-ups
-
2.
Improved functional outcomes and tendon integrity as measured by various functional scoring system and follow-up MRI analysis
-
3.
Lower volumes of articular- and bursal-side defects on arthroscopic examination
-
4.
Very few treatment-related adverse events
Despite these promising findings, it is important to recognize that the majority of these early phase clinical trials enrolled a small subset of patients. Although magnetic resonance imaging is an effective way of assessing healing of rotator cuff tendons, the sutures and metallic debris from arthroscopic instrumentation can introduce artifacts and can impair the quality of imaging. Finally, these studies were not blinded and were non-randomized, and therefore the results for clinical and functional assessments could be biased by the Hawthorne effect.
4.2. Limitations
There are several limitations to our systematic review and meta-analysis. First, we had a limited number of studies and experiments, which may influence the results of the combined effect. Second, most of the studies included in this review used MSCs as an adjuvant to surgery; however the specific surgical techniques varied across studies, with some using MSC-laden scaffolds and others using MSC in solution (i.e. PBS or culture media). Therefore, functional outcomes and imaging findings may be affected by the type of surgery performed. Typically, rotator cuff injury is secondary to degeneration, impingement or overload, however, most studies reviewed performed controlled incisions to simulate rotator cuff injury. Although all studies met the inclusion criteria of assessing functional outcomes, some studies could not be included in meta-analysis since they did not report quantifiable data or failed to use common metrics of functional outcomes. Although MSCs are generally deemed to be safe, there are some studies that suggest that MSCs are associated with rapid proliferation of cells and malignant transformation. Though complications were addressed in 50% of included studies, no studies performed long-term safety analysis of the MSCs on the animals.
5. Conclusion
This systematic review and meta-analysis demonstrates that MSCs may provide benefit in animal models of RCI. The field of regenerative medicine is exciting, rapidly advancing, and has now resulted in multiple early-phase human RCI clinical trials. However, with the heterogeneity of current studies and lack of methodologic standardization, further preclinical work is necessary. Given that preclinical studies are the basis for clinical studies, due diligence is warranted and recommended to optimize the potential clinical effects of MSCs.
Author contributions
N.M-G.: Conceived and designed the review, collected data, performed analysis & interpretation, created tables & figures, and wrote the manuscript.
D.C.: Collected data, created tables & figures, and contributed to writing of the manuscript.
C.E.: Collected data.
K.C.: Contributed to writing of the manuscript and performed critical review.
A.M.: Contributed to statistical analysis & interpretation, as well as made significant contribution to writing of the manuscript and conducting critical review.
Funding
Supported by NIH NCATS, KL2 TR001118, UT Health San Antonio School of Medicine pilot grant, and the Parker B. Francis Foundation (provided to senior author).
Declaration of Competing Interest
The authors declare no conflict of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ocarto.2020.100047.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Nixon A., Watts A., Schnabel L. Cell- and gene-based approaches to tendon regeneration. J. Shoulder Elbow Surg. 2012;21(2):278–294. doi: 10.1016/j.jse.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 2.Beitzel K., Solovyova O., Cote M., et al. The future role of mesenchymal stem cells in the management of shoulder disorders. Arthrosc. J. Arthrosc. Relat. Surg. 2013;29(10):1702–1711. doi: 10.1016/j.arthro.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 3.Galatz L., Ball C., Teefey S., Middleton W., Yamaguchi K. The outcome and repair integrity of completely arthroscopically repaired large and massive rotator cuff tears. J. Bone Joint Surg. 2004;86(2):219–224. doi: 10.2106/00004623-200402000-00002outcomes. [DOI] [PubMed] [Google Scholar]
- 4.Lafosse L., Brzoska R., Toussaint B., Gobezie R. The outcome and structural integrity of arthroscopic rotator cuff repair with use of the double-row suture anchor technique. J. Bone Jt. Surg. Am. Vol. 2008;90:275–286. doi: 10.2106/jbjs.h.00388. [DOI] [PubMed] [Google Scholar]
- 5.Caplan A.I., Dennis J.E. Mesenchymal stem cells as trophic mediators. J. Cell. Biochem. 2006;1084:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 6.Omi R., Gingery A., Steinmann S., Amadio P., An K., Zhao C. Rotator cuff repair augmentation in a rat model that combines a multilayer xenograft tendon scaffold with bone marrow stromal cells. J. Shoulder Elbow Surg. 2016;25(3):469–477. doi: 10.1016/j.jse.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaizawa Y., Franklin A., Leyden J., et al. Augmentation of chronic rotator cuff healing using adipose-derived stem cell-seeded human tendon-derived hydrogel. J. Orthop. Res. 2019;37(4):877–886. doi: 10.1002/jor.24250. [DOI] [PubMed] [Google Scholar]
- 8.Yokoya S., Mochizuki Y., Natsu K., Omae H., Nagata Y., Ochi M. Rotator cuff regeneration using a bioabsorbable material with bone marrow–derived mesenchymal stem cells in a rabbit model. Am. J. Sports Med. 2012;40(6):1259–1268. doi: 10.1177/0363546512442343. [DOI] [PubMed] [Google Scholar]
- 9.Dominici M., Le Blanc K., Mueller I., et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8(4):315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 10.Hooijmans C., Rovers M., de Vries R., Leenaars M., Ritskes-Hoitinga M., Langendam M. SYRCLE's risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014;14(1) doi: 10.1186/1471-2288-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faraone S. Interpreting estimates of treatment effects. Pharm. Therapeut. 2008;33(12):700–703. [PMC free article] [PubMed] [Google Scholar]
- 12.Higgins J.P.T., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., Welch V.A. Cochrane. 2019. Cochrane handbook for systematic reviews of interventions.www.training.cochrane.org/handbook version 6.0. [Google Scholar]
- 13.Review Manager (RevMan) [Computer Program] The Nordic Cochrane Centre. The Cochrane Collaboration; Copenhagen: 2014. Version 5.3. [Google Scholar]
- 21.Rothrauff B., Smith C., Ferrer G., et al. The effect of adipose-derived stem cells on enthesis healing after repair of acute and chronic massive rotator cuff tears in rats. J. Shoulder Elbow Surg. 2019;28(4):654–664. doi: 10.1016/j.jse.2018.08.044. [DOI] [PubMed] [Google Scholar]
- 28.Milgrom C., Schaffler M., Gilbert S., van Holsbeeck M. Rotator-cuff changes in asymptomatic adults. The effect of age, hand dominance and gender. J. Bone Joint Surg. 1995;77-B(2):296–298. doi: 10.1302/0301-620x.77b2.7706351. [DOI] [PubMed] [Google Scholar]
- 29.Sher J., Uribe J., Posada A., Murphy B., Zlatkin M. Abnormal findings on magnetic resonance images of asymptomatic shoulders. J. Bone Joint Surg. 1995;77(1):10–15. doi: 10.2106/00004623-199501000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Castro-Malaspina H., Gay R., Resnick G., et al. Characterization of human bone marrow fibroblast colony-forming cells (CFU-F) and their progeny. Blood. 1980;56(2):289–301. doi: 10.1182/blood.v56.2.289.289. [DOI] [PubMed] [Google Scholar]
- 31.Soon M., Hassan A., Hui J., Goh J., Lee E. An analysis of soft tissue allograft anterior cruciate ligament reconstruction in a rabbit model. Am. J. Sports Med. 2007;35(6):962–971. doi: 10.1177/0363546507300057. [DOI] [PubMed] [Google Scholar]
- 32.Adams S., Thorpe M., Parks B., Aghazarian G., Allen E., Schon L. Stem cell-bearing suture improves achilles tendon healing in a rat model. Foot Ankle Int. 2014;35(3):293–299. doi: 10.1177/1071100713519078. [DOI] [PubMed] [Google Scholar]
- 33.Okamoto N., Kushida T., Oe K., Umeda M., Ikehara S., Iida H. Treating achilles tendon rupture in rats with bone-marrow-cell transplantation therapy. J. Bone Jt. Surg. Am. Vol. 2010;92(17):2776–2784. doi: 10.2106/jbjs.i.01325. [DOI] [PubMed] [Google Scholar]
- 34.Lee K., Hui J., Song I., Ardany L., Lee E. Injectable mesenchymal stem cell therapy for large cartilage defects-a porcine model. Stem Cell. 2007;25(11):2964–2971. doi: 10.1634/stemcells.2006-0311. [DOI] [PubMed] [Google Scholar]
- 35.Guo X., Zheng Q., Yang S., et al. Repair of full-thickness articular cartilage defects by cultured mesenchymal stem cells transfected with the transforming growth factor β1gene. Biomed. Mater. 2006;1(4):206–215. doi: 10.1088/1748-6041/1/4/006. [DOI] [PubMed] [Google Scholar]
- 36.Zhang H., Li L., Leng P., Wang Y., Lv C. Uninduced adipose-derived stem cells repair the defect of full-thickness hyaline cartilage. Chin. J. Traumatol. 2019;12(2):92–97. [PubMed] [Google Scholar]
- 37.Cui G.H., Wang Y.Y., Li C.J., Shi C.H., Wang W.S. Efficacy of mesenchymal stem cells in treating patients with osteoarthritis of the knee: a meta-analysis. Exp. and Therapeut. Med. 2016;12(5):3390–3400. doi: 10.3892/etm.2016.3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim S.H., Ha C.W., Park Y.B., Nam E., Lee J.E., Lee H.J. Intra-articular injection of mesenchymal stem cells for clinical outcomes and cartilage repair in osteoarthritis of the knee: a meta-analysis of randomized controlled trials. Arch. Orthop. Trauma Surg. 2019;139(7):971–980. doi: 10.1007/s00402-019-03140-8. [DOI] [PubMed] [Google Scholar]
- 39.Shin Y.-S., Yoon J.-R., Kim H.-S., Lee S.-H. Intra-articular injection of bone marrow-derived mesenchymal stem cells leading to better clinical outcomes without difference in MRI outcomes from baseline in patients with knee osteoarthritis. Knee Surg.Relat. Res. 2018;30(3):206–214. doi: 10.5792/ksrr.17.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu S., Liu H., Xie Y., Sang L., Liu J., Chen B. Effect of mesenchymal stromal cells for articular cartilage degeneration treatment: a meta-analysis. Cytotherapy. 2015;17(10):1342–1352. doi: 10.1016/j.jcyt.2015.05.005. [DOI] [PubMed] [Google Scholar]
- 41.Wu R., Hu X., Wang J. Concise review: optimized strategies for stem cell-based therapy in myocardial repair: clinical translatability and potential limitation. Stem Cell. 2018;36(4):482–500. doi: 10.1002/stem.2778. [DOI] [PubMed] [Google Scholar]
- 42.Centeno C., Bashir J., Freeman M., Al-Sayegh H., Goodyear S. A prospective multi-site registry study of a specific protocol of autologous bone marrow concentrate for the treatment of shoulder rotator cuff tears and osteoarthritis. J. Pain Res. 2015:269. doi: 10.2147/jpr.s80872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gomes J., da Silva R., Silla L., Abreu M., Pellanda R. Conventional rotator cuff repair complemented by the aid of mononuclear autologous stem cells. Knee Surg. Sports Traumatol. Arthrosc. 2011;20(2):373–377. doi: 10.1007/s00167-011-1607-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hernigou P., Flouzat Lachaniette C., Delambre J., et al. Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: a case-controlled study. Int. Orthop. 2014;38(9):1811–1818. doi: 10.1007/s00264-014-2391-1. [DOI] [PubMed] [Google Scholar]
- 45.Jo C., Chai J., Jeong E., et al. Intratendinous injection of autologous adipose tissue-derived mesenchymal stem cells for the treatment of rotator cuff disease: a first-in-human trial. Stem Cell. 2018;36(9):1441–1450. doi: 10.1002/stem.2855. [DOI] [PubMed] [Google Scholar]
- 46.Kim S., Song D., Park J., Park S., Kim S. Effect of bone marrow aspirate concentrate-platelet-rich plasma on tendon-derived stem cells and rotator cuff tendon tear. Cell Transplant. 2017;26(5):867–878. doi: 10.3727/096368917x694705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim Y., Sung C., Chung S., Kwak S., Koh Y. Does an injection of adipose-derived mesenchymal stem cells loaded in fibrin glue influence rotator cuff repair outcomes? a clinical and magnetic resonance imaging study. Am. J. Sports Med. 2017;45(9):2010–2018. doi: 10.1177/0363546517702863. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.