Summary
Objective
To examine the feasibility of a 6-week high intensity interval training (HIIT) program in patients with symptomatic knee osteoarthritis (OA). A secondary aim was to evaluate the change in whole-body metabolism.
Design
In a single-arm intervention, 16 adults (mean age 59.9 yrs; BMI: 29.0 ± 4.3 kg/m2; 77% female) with radiographically diagnosed knee OA (Kellgren-Lawrence [KLG]: 2–4) and moderate to severe pain score (≥6) from the Western Ontario and McMasters Universities Index (WOMAC) enrolled in a 6-week, twice weekly, supervised HIIT cycle ergometry intervention. The primary outcome was feasibility; secondary outcomes included change in peak oxygen consumption (VO2peak), WOMAC, and circulating biomarkers of metabolism.
Results
Feasibility was moderate; of the 21 participants screened by phone, 16 were enrolled; 13 completed pre- and post-testing. The average adherence rate (sessions completed/available) was >96%. VO2peak was significantly improved (mean ± SD: 2.6 ± 3.0 mL/min/kg; 95% CI [0.70–4.56]). Significant improvements in WOMAC (mean ± SD: −8.7 ± 12.5; CI: [18.9 to −2.80]), pain [-5.15 to −1.01], and function [-12.9 to −0.98] resulted. There was a significant reduction in concentrations of amino acids: methionine, phenylalanine, and tyrosine (p < 0.02 for all), with trends towards lower concentrations of serine (p = 0.08; [-20.66-1.18]) and greater aspartate/asparagine (p = 0.06; [-1.99-65.73].). Post-training acylcarnitine concentrations were reduced with training.
Conclusions
In this cohort of overweight adults with symptomatic knee OA, 6-weeks of HIIT cycling showed excellent rates of retention and adherence with no adverse events, improved cardiorespiratory fitness and OA symptoms, in concert with metabolic alterations indicative of improved skeletal muscle energetics.
Trial registration number
clinicaltrials.gov: NCT03039452.
Keywords: Exercise therapy, Osteoarthritis, Interval training, Metabolomics, Pain
1. Introduction
Osteoarthritis (OA) is a leading cause of disability in the United States, with symptomatic knee OA present in at least 12–16% of the population [1,2]. Further, OA prevalence is sharply rising due to the obesity epidemic and the aging of the population [3]. Despite the pervasiveness of knee OA, there is no cure, with few non-surgical treatments for mitigating the disease progression. Physical activity is safe, cost-effective and recommended as first-line knee OA treatment [4,5]. Exercise programs have been shown to improve pain and physical function in adults with knee OA [[6], [7], [8], [9]], showing effect sizes comparable to those for simple analgesics and nonsteroidal anti-inflammatory drugs [10]. Unfortunately, few people with knee OA achieve recommended physical activity levels, 150 min/week of moderate intensity [[11], [12], [13], [14]].
One promising means of increasing physical activity in those with knee OA is through high-intensity interval training (HIIT). By interspersing intervals of higher and lower intensities, HIIT allows training at higher intensities than what can be sustained for longer continuous bouts, leading to more rapid gains than seen with traditional aerobic training, and requires much less time (10–20 min) [[15], [16], [17]]. Thus, as compared to traditional existing knee exercise programs, HIIT could potentially minimize one of the key physical activity barriers for people with symptomatic knee OA: lack of time [[18], [19], [20], [21]]. Another important barrier for individuals with knee OA includes pain; HIIT has previously been shown to support greater leg musculature [22], which may increase knee joint support, thereby mitigating pain.
Regardless of time requirements, exercise adherence is problematic for patients with OA; yet, HIIT has promising long-term adherence rates for other clinical populations [23]. HIIT also yields rapid improvements in exercise tolerance and enjoyment [24], which may be beneficial for initiating an exercise program among individuals with high barriers for initiation. Better time efficiency, greater enjoyment, faster acquisition of benefits and flexibility in mode of exercise have made possible the successful implementation of HIIT in those with obesity [25,26], advanced age [7], cardiovascular disease [9], and cancer [8,27]. The effect of HIIT in patients with OA has been evaluated in only two previous studies using an aquatic treadmill and a home-based intervention, respectively; these studies demonstrated improvements in pain and function [6,28]. We are not aware of any supervised HIIT interventions in knee OA similar to ours; that have the potential to be easily implemented in a clinic or at home.
In addition to improvements in pain and function, exercise also produces marked benefits related to metabolism [29]. Although physical activity is one of the few effective OA treatments, and HIIT may overcome barriers and improve uptake, it is unclear how physical activity in general, or HIIT, more specifically, impacts metabolism in the context of knee OA. Systemic metabolic abnormalities are common in OA and likely play a role in its development and progression [30]. One means of evaluating OA metabolic alterations and improvements derived from interventions is through measurement of circulating metabolic intermediates. These measurements, termed “metabolic profiling,” allow identification of metabolite signatures that track specific disease states and are sensitive to exercise adaptations. In persons without knee OA and at-risk for cardiometabolic disease, exercise-induced improvements in cardiorespiratory fitness (VO2peak) and insulin sensitivity are associated with improved skeletal muscle mitochondrial function, reflected, in part, by fewer systemic fatty acids and even chain acylcarnitines [31]. These changes were most pronounced with vigorous intensity training [31]. Increased systemic concentrations of plasma acylcarnitines occurs in conditions of dysfunctional fatty acid oxidation, such as insulin resistance and obesity [32,33]. Systemic acylcarnitine concentrations have been shown to decrease with exercise training and improved cardiorespiratory fitness, suggesting enhanced mitochondrial oxidative capacity [31]. Acutely, targeted metabolomics approaches have shown concentrations of non-esterified fatty acids to be increased for up to 2 h following HIIT, suggesting a shift toward greater lipid metabolism [34]. This has not yet been evaluated in knee osteoarthritis; these results can potentially be used to optimize exercise therapies as strategies to mitigate symptomatic knee OA and cardiometabolic comorbidities. Thus, the purpose of the current project was two-fold; the primary aim was to examine the feasibility and acceptability of a 6-week HIIT program in patients with symptomatic knee OA; the secondary aim was to evaluate the changes in whole-body metabolism induced by 6-weeks of HIIT and the relationship with physiological outcomes, in knee OA.
2. Method
2.1. Participants
Using a targeted medical record review, correspondence was sent to 453 potentially eligible subjects; flyers were also used, as well as contact with previous participants to gauge interest. Fifty-three individuals were called; 48 were interested. Twenty-one of these individuals were screened via phone for eligibility. Sixteen individuals (Mean ± SD; Age: 59.9 ± 8.3 yrs, Height: 169.2 ± 8.6 cm, Weight: 86.0 ± 14.7 kg, BMI: 29.0 ± 4.3 kg/m2; %fat: 39.9 ± 8.1%; 77% female; n = 9 Caucasian; n = 2 African American; n = 1 Asian) with radiographically diagnosed knee OA (Kellgren-Lawrence grade: 2–4) were included and enrolled in the current study. Of the 16 participants, 13 individuals completed both pre and post intervention testing (n = 1 disqualified due to uncontrolled hypertension; n = 2 discontinued for undisclosed personal reasons). Inclusion criteria for participation included body mass index (BMI) between 20 and 40 kg/m2, 40–70 years of age, a normal resting 12-lead ECG with physician clearance, and moderate to severe pain (pain score ≥6) and/or moderate to severe disability (normalized function score ≥ 31) based on the Western Ontario and McMasters Universities Index (WOMAC). Normalized function was used to identify individuals with significant self-reported disability for inclusion; normalized scores were calculated by dividing the recorded score by the maximum possible score. Participants were excluded if they had rheumatoid arthritis, fibromyalgia, gout, diabetes, untreated hypertension or a total knee replacement; and if they had been hospitalized for a cardiovascular event in the previous 6 months, received knee injections in the previous 3 weeks; were currently participating in HIIT, physical therapy for knee OA or greater than 150 min of moderate exercise per week (per self-report). Prior to testing, all participants signed an informed consent approved by the University's Institutional Review Board for the protection of human subjects. This study was registered at clinicaltrials.gov (NCT03039452).
2.2. Experimental design
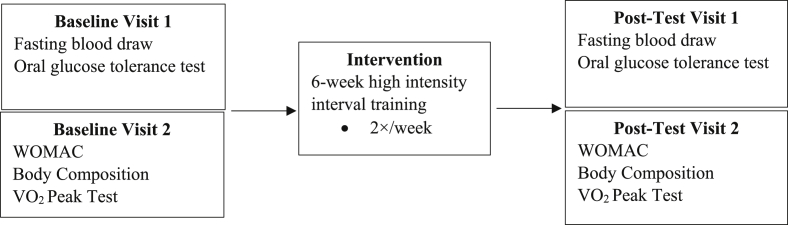
The present study evaluated blood biomarkers, perceived pain, stiffness and function, body composition, and cardiorespiratory fitness at baseline and following a 6-week HIIT intervention. Participants completed two testing sessions at baseline and two post-interventions (Fig. 1). For the first baseline and post-test sessions, participants were assessed at the Clinical and Translation Research Center (CTRC) following abstention from food (8 h), caffeine (12 h), and strenuous exercise, alcohol, and tobacco (24 h each). For the second baseline and post-intervention visits, participants were assessed for body composition and peak oxygen consumption following a 4-h fast, and abstention from strenuous exercise, alcohol, and tobacco for 24 h. Upon arrival, height was measured using a portable stadiometer (Perspective Enterprises, Portage, MI, USA) and weight was measured using a mechanical scale (Detecto, Webb City, MO, USA).
Fig. 1.
Experimental overview of study design.
2.3. Blood biomarkers
Before and after the 6-week intervention (16–24 h after the last exercise bout), fasting EDTA plasma was collected from each participant and stored at −80 degrees Celsius. Circulating metabolites were measured using a targeted mass spectrometry-based platform, as previously described [35,36]. Fasting concentrations of 15 amino acids, 45 acylcarnitines, glucose, total ketone, non-esterified fatty acids (NEFA), lactate, and insulin were determined. Because skeletal muscle insulin sensitivity is best reflected in a post-prandial rather than fasting state, a 2-h oral glucose tolerance test (OGTT) was performed with blood sampled at baseline, 30 min, 60 min, and 120 min; areas under the curve for glucose and insulin were calculated. Glucose, insulin, and metabolic intermediate determinations were performed at the Duke Molecular Physiology Institute Metabolomics Core Facility. Coefficients of variations for all analyses were less than 4.5%.
2.4. Western Ontario and McMaster osteoarthritis index
Participants completed the WOMAC; a 24 item questionnaire that has been shown to be valid and reliable in individuals with knee OA [37]. Participants were asked to complete the pain (5 items), stiffness (2 items), and function (17 items) subscales, which were rated on a 5-point Likert scale from no symptoms to extreme symptoms.
2.5. Body composition
Body composition variables including fat mass (FM), lean mass (LM), body fat percentage (%fat), regional (trunk, legs and arms) FM and LM, visceral fat (VAT), and bone mineral content (BMC) were obtained from a whole body DXA scan (GE Lunar iDXA, GE Medical Systems Ultrasound & Primary Care Diagnostics, Madison, WI). Prior to each scan, birthdate, height, weight, and ethnicity were entered into the software. Participants were asked to remove all metal (i.e., jewelry, glasses, clothing with zippers, ect.), thick clothing and heavy plastic to reduce interference. Each participant was positioned supine in the center of the scanning table and instructed to breathe normally and minimize movement for the duration of the 7–13 min scan. The same technician performed and analyzed all scans by manually adjusting the regions of interest. The Lunar iDXA, test-retest reliability from our laboratory for FM, LM and %fat have demonstrated intraclass correlation coefficients (ICC) of 0.998, 0.998, 0.995, respectively, and standard error of the measurements (SEM) of 0.462 kg, 0.806 kg, 0.807%, respectively.
2.6. Cardiorespiratory fitness
Cardiorespiratory Fitness was measured by peak oxygen consumption (VO2peak) recorded during a graded exercise test on an electronically braked cycle ergometer (Corival Lode, Gronigen, The Netherlands). Participants completed a standardized 2-min warm up at 20 watts (W) followed by an increase of 1 W every 3 s until volitional fatigue or failure to maintain the power output (cadence dropped below 50 rpm). Participants were instructed to maintain a pedal cadence between 70 and 80 rpm. Respiratory gases were monitored breath-by-breath with open-circuit spirometry using a calibrated metabolic cart (True One 2400®, Parvo-Medics, Inc., Provo, UT). The three highest measured VO2 values were averaged to obtain VO2peak. Heart rate (HR) was monitored throughout the duration of the test via a polar HR monitor (Polar FT1, Polar USA, Port Washington, NT, USA). Peak power output (PPO), time to exhaustion, maximum heart rate were recorded. Ventilatory threshold (VT) was determined from Parvomedics standard software by automatically regressing ventilation (VE) against VO2 as previously described [38]. Two linear regression lines were fit to the lower and upper portions of the VE vs. VO2 curve, before and after the break points, respectively. The intersection of these two lines was defined as VT; power output (W), oxygen consumption (l/min), and HR were automatically captured at the VT delineation. Test-retest reliability from our laboratory for the VO2peak protocol in the present study has demonstrated ICC = 0.98 and SEM = 1.74 mL/kg/min.
2.7. Intervention
All training was performed on an electronically braked cycle ergometer under one-on-one supervision with trained research staff. Participants trained twice a week for six weeks for a total of 12 training sessions. Training sessions were performed a minimum of 24 h apart, preferentially scheduling visits on non-consecutive days each week. Training intensity was set at 90% of the PPO obtained during the graded exercise test. Participants completed a self-selected warm-up, followed by ten repetitions of 1-min bouts at 90% PPO with 1-min rest periods between each work interval. Participants were also instructed to keep outside activity consistent with what they were doing prior to study enrollment.
2.7.1. Statistical analysis
Due to the nature of this pilot trial, the data were descriptively summarized with mean and standard deviations, along with 95% confidence intervals with a modified intention to treat analysis, calculated using t-distribution and paired t-test to evaluate the change in physiologic and metabolic outcomes over the 6-week intervention. Data were adjusted for multiple comparisons using a Bonferroni correction. For metabolic intermediates, percent changes were computed as [(Post-intervention – Pre-intervention)/Pre-intervention]∗100%. Paired t-tests were used to evaluate significance responses to the intervention. Spearman correlations were used to determine associations between changes in metabolic factors and changes in clinical and physiologic responses. All data were analyzed using R software version 3.5.1 (The R Foundation). The criterion for statistical significance was set a priori at α = 0.05.
3. Results
3.1. Feasibility outcomes
Two participants withdrew from the HIIT intervention; one completed baseline cardiorespiratory fitness testing but declined to return for the OGTT test due to an aversion to blood draws; one completed 6 training sessions but stopped at 4 weeks due to personal reasons. An additional participant withdrawn at baseline by the study team upon discovery of very high blood pressure. Recruitment and enrollment was more difficult than anticipated. Initial reservations from participants was often high; for those that enrolled, perceived barriers did not appear to be as large as they anticipated. Common concerns were related to their ability to walk to the lab (~500 feet from the parking lot), mount/dismount the bike, obtain required intensity, pain, time. For the 13 that remained enrolled, training compliance was extremely high with a 96% average compliance rate, calculated from total number of sessions completed/total available for each subject. For the 13 compliant participants, some barriers were apparent; namely the walk to the lab was taxing; the bike seat had to be lowered to the shortest height for mount/dismount and then adjusted during exercise. No adverse events related to the HIIT program were reported.
3.2. Training responses
Fitness: The training intervention improved cardiorespiratory fitness, measured as absolute VO2peak (L/min) (95%CI [-0.04-0.39]), relative VO2peak (mL/min/kg) [0.70–4.56], peak watts, and watts at VT [W; 95%CI [1.00–32.20](Table 1); however, there were little to no body composition changes, except for a slight reduction in left lower extremity lean mass (kg) [0.06–0.69]; which was not beyond the error of the device (i.e. SEM).
Table 1.
Physiological Variables (Mean ± SD) and absolute change scores for subjects completing pre-and post testing (77% female).
| Pre N | PRE | Post N | POST | Change (N = 13) | |
|---|---|---|---|---|---|
| Weight (kg) | 13 | 83.17 ± 14.29 | 13 | 83.13 ± 14.36 | −0.04 ± 1.61 |
| Fat Mass (kg) | 13 | 32.36 ± 9.46 | 13 | 32.07 ± 9.29 | −0.29 ± 1.13 |
| Lean Mass (kg) | 13 | 47.41 ± 10.75 | 13 | 48.00 ± 10.76 | 0.59 ± 1.29 |
| Percent Body Fat (%) | 13 | 39.09 ± 8.75 | 13 | 38.62 ± 8.58 | 0.47 ± 1.21 |
| BMC (kg) | 13 | 2.68 ± 0.68 | 13 | 2.69 ± 0.69 | −0.004 ± 0.03 |
| Legs Lean/Muscle Mass (kg) | 13 | 16.76 ± 3.53 | 13 | 17.14 ± 3.64 | −0.30 ± 0.49 |
| Right leg Lean/Muscle Mass (kg) | 13 | 8.43 ± 1.83 | 13 | 8.54 ± 1.74 | −0.10 ± 0.30 |
| Left leg Lean/Muscle Mass (kg) | 13 | 8.32 ± 1.73 | 13 | 8.60 ± 1.90∗ | −0.25 ± 0.35 |
| Legs Fat Mass (kg) | 13 | 11.21 ± 3.64 | 13 | 11.11 ± 3.67 | 0.09 ± 0.49 |
| Trunk Fat (kg) | 13 | 17.53 ± 5.22 | 13 | 16.51 ± 4.87 | 0.20 ± 0.63 |
| Visceral Mass (kg) | 13 | 1.16 ± 0.59 | 13 | 1.18 ± 0.54 | −0.02 ± 0.10 |
| Visceral Fat Volume (in3) | 13 | 75.25 ± 38.12 | 13 | 76.38 ± 35.04 | −1.13 ± 6.38 |
| VO2peak (l·min−1) | 13 | 1.62 ± 0.47 | 12 | 1.90 ± 0.51∗ | 0.22 ± 0.23 |
| VO2peak (ml·kg−1·min−1) | 13 | 19.49 ± 5.99 | 12 | 22.68 ± 5.41∗ | 2.63 ± 3.04 |
| VO2 TTE (sec) | 13 | 493.69 ± 120.28 | 13 | 513.17 ± 114.58 | 36.25 ± 26.49 |
| Peak Power (W) | 13 | 143.38 ± 38.52 | 13 | 160.42 ± 42.41∗ | 10.43 ± 10.72 |
| VT (l·min−1) | 13 | 1.11 ± 0.26 | 13 | 1.23 ± 0.32 | 0.10 ± 0.20 |
| VT (W) | 13 | 84.85 ± 20.13 | 13 | 100.25 ± 26.61∗ | 11.21 ± 20.38 |
| VT HR (bpm) | 13 | 113.38 ± 9.06 | 13 | 120.83 ± 13.23 | 6.07 ± 9.03 |
| WOMAC Total | 13 | 36.15 ± 8.60 | 13 | 25.46 ± 16.09∗ | −8.69 ± 12.46 |
| WOMAC Pain | 13 | 7.85 ± 2.08 | 13 | 4.77 ± 3.72∗ | −2.50 ± 3.31 |
| WOMAC Stiffness | 13 | 3.46 ± 1.45 | 13 | 2.77 ± 1.83 | −0.60 ± 1.59 |
| WOMAC Function | 13 | 24.85 ± 6.76 | 13 | 17.92 ± 11.51∗ | −6.00 ± 9.42 |
∗Bold = significantly different from PRE (p < 0.05).
BMC = bone mineral content; VO2peak = peak oxygen consumption; TTE = time to exhaustion; VT = ventilatory threshold; HR = heart rate; WOMAC = Western Ontario and McMaster Universities Osteoarthritis Index.
OA Symptoms: The intervention improved OA symptoms with reductions in total WOMAC (95%CI [-18.9 to −2.80]), pain [-5.15 to −1.01], and adverse function [-12.9 to −0.98] scores (p < 0.05 for all) (Table 1).
Systemic Measures: Intervention responses for metabolic function and metabolic intermediates are shown in Table 2. Measures of glucose handling (AUCs and Matsuda) were largely unchanged while fasting glucose was greater (p < 0.02) with a trend towards greater fasting insulin (p < 0.08). After 6 weeks of HIIT, persons with knee OA had significantly lower concentrations of the following amino acids: methionine, phenylalanine, and tyrosine (p < 0.02 for all), with trends towards lower concentrations of serine (p = 0.08; [-20.66-1.18]) and greater aspartate/asparagine (p = 0.06; [-1.99-65.73].). There were trends towards significantly lower post-training fasting concentrations of non-esterified free fatty acids (p = 0.08; [-0.021-0.014]) and ketones (p = 0.10; [-80.15-8.57]). Overall, most post-training acylcarnitine concentrations were lower, with significant reductions in linoleyl carnitine (C18:2, p = 0.004; [-0.019 to −0.005]), 3-hydroxyoctadecanoly or hexadecanedioyl carnitine (C18–OH/C16, p = 0.04; [-0.005 to −0.000]), and 3-hydroxydodecanoyl or sebacoyl carnitine (C12:2-OH/C10:2DC, p = 0.04; [-0.007 to −0.000]).
Table 2.
Metabolic assessments and fasting metabolic intermediate concentrations (Mean ± SD).
| N | PRE | POST | p-value | |
|---|---|---|---|---|
| Glucose (mg/dL) | 13 | 99.8 ± 5.0 | 102.2 ± 5.7 | 0.02 |
| Insulin (pg/mL) | 13 | 235.8 ± 113.6 | 290.3 ± 172.0 | 0.08 |
| AUC Glucose (mg/dL ∗ 120 min) | 13 | 15483.46 ± 2977.02 | 15506.54 ± 3047.80 | 0.98 |
| AUC Insulin (pg/mL ∗ 120 min) | 13 | 281022.4 ± 148791.9 | 286286.1 ± 174255.7 | 0.79 |
| Matsuda Index | 13 | 0.17 ± 0.11 | 0.16 ± 0.11 | 0.51 |
| Ketones (μM) | 13 | 85.05 ± 63.75 | 49.26 ± 23.69 | 0.10 |
| NEFA (mM) | 13 | 0.49 ± 0.16 | 0.39 ± 0.16 | 0.08 |
| Lactate (mM) | 13 | 1.11 ± 0.67 | 1.18 ± 0.50 | 0.65 |
| AMINO ACID INTERMEDIATES (μM units) | ||||
| Glycine | 13 | 318.14 ± 68.97 | 300.83 ± 52.68 | 0.22 |
| L-Alanine | 13 | 379.27 ± 61.92 | 401.00 ± 68.26 | 0.28 |
| L-Serine | 13 | 101.97 ± 17.89 | 92.24 ± 13.73 | 0.08 |
| L-Proline | 13 | 170.65 ± 65.06 | 163.31 ± 40.35 | 0.58 |
| L-Valine | 13 | 230.68 ± 55.83 | 227.85 ± 36.73 | 0.83 |
| l-Leucine, L-Isoleucine | 13 | 150.23 ± 43.19 | 138.81 ± 21.15 | 0.19 |
| L-Methionine | 13 | 26.01 ± 4.16 | 23.90 ± 3.52∗ | 0.02 |
| L-Histidine | 13 | 76.32 ± 8.37 | 73.39 ± 6.82 | 0.14 |
| L-Phenylalanine | 13 | 57.55 ± 7.17 | 52.55 ± 6.50∗ | 0.00 |
| L-Tyrosine | 13 | 64.44 ± 8.15 | 57.50 ± 8.89∗ | 0.00 |
| l-Aspartic acid, L-Asparagine | 13 | 308.55 ± 74.31 | 340.42 ± 42.29 | 0.06 |
| l-Glutamic acid, L-Glutamate | 13 | 82.48 ± 22.40 | 82.47 ± 16.44 | 0.99 |
| L-Ornithine | 13 | 61.10 ± 12.52 | 55.78 ± 9.88 | 0.09 |
| L-Citrulline | 13 | 35.15 ± 5.16 | 35.26 ± 6.80 | 0.94 |
| L-Arginine | 13 | 94.17 ± 18.22 | 93.27 ± 16.45 | 0.88 |
| ACYLCARNITINE INTERMEDIATES (μM units) | ||||
| Acetyl carnitine | 13 | 7.40 ± 1.62 | 6.36 ± 1.85 | 0.14 |
| Propionyl carnitine | 13 | 0.34 ± 0.08 | 0.34 ± 0.10 | 0.81 |
| Tiglyl carnitine | 13 | 0.18 ± 0.05 | 0.18 ± 0.03 | 0.51 |
| Isovaleryl carnitine, 3-methylbutyryl carnitine or 2-Methylbutyryl carnitine | 13 | 0.14 ± 0.07 | 0.12 ± 0.02 | 0.30 |
| 3-Hydroxy-isovaleryl carnitine or Malonyl carnitine | 13 | 0.04 ± 0.02 | 0.12 ± 0.27 | 0.35 |
| Glutaryl carnitine | 13 | 0.06 ± 0.02 | 0.05 ± 0.02 | 0.33 |
| Pimeloyl carnitine, heptanedioyl carnitine | 13 | 0.01 ± 0.02 | 0.02 ± 0.02 | 0.77 |
| Butyryl carnitine or Isobutyryl carnitine | 13 | 0.20 ± 0.06 | 0.18 ± 0.05 | 0.17 |
| 3-Hydroxy-butyryl carnitine, b-hydroxy butyryl carnitine | 13 | 0.12 ± 0.27 | 0.11 ± 0.28 | 0.26 |
| Hexanoyl carnitine | 13 | 0.17 ± 0.25 | 0.15 ± 0.29 | 0.52 |
| Methylmalonyl carnitine or Succinyl carnitine | 13 | 0.03 ± 0.01 | 0.04 ± 0.01 | 0.24 |
| Octenoyl carnitine | 13 | 0.31 ± 0.15 | 0.34 ± 0.19 | 0.50 |
| Octanoyl carnitine | 13 | 0.21 ± 0.14 | 0.17 ± 0.11 | 0.29 |
| 3-Hydroxy-cis-5-octenoyl carnitine or Hexenedioyl carnitine | 13 | 0.05 ± 0.03 | 0.04 ± 0.02 | 0.22 |
| Adipoyl carnitine | 13 | 0.06 ± 0.02 | 0.06 ± 0.02 | 0.89 |
| Decatrienoyl carnitine | 13 | 0.09 ± 0.04 | 0.09 ± 0.05 | 0.98 |
| Decadienoyl carnitine | 13 | 0.04 ± 0.02 | 0.04 ± 0.02 | 0.29 |
| Decenoyl carnitine | 13 | 0.22 ± 0.11 | 0.19 ± 0.10 | 0.28 |
| Decanoyl carnitine | 13 | 0.340 ± 0.24 | 0.31 ± 0.24 | 0.65 |
| Octenedioyl carnitine | 13 | 0.03 ± 0.01 | 0.03 ± 0.02 | 0.49 |
| 3-Hydroxy-decanoyl carnitine or Suberoyl carnitine | 13 | 0.06 ± 0.05 | 0.05 ± 0.02 | 0.31 |
| Dodecenoyl carnitine | 13 | 0.12 ± 0.05 | 0.11 ± 0.05 | 0.27 |
| Lauroyl carnitine | 13 | 0.10 ± 0.05 | 0.08 ± 0.056 | 0.20 |
| 3-Hydroxy-dodecanoyl carnitine or Sebacoyl carnitine | 13 | 0.01 ± 0.004 | 0.01 ± 0.01∗ | 0.04 |
| Tetradecadienoyl carnitine | 13 | 0.05 ± 0.02 | 0.04 ± 0.03 | 0.77 |
| Tetradecenoyl carnitine | 13 | 0.08 ± 0.03 | 0.07 ± 0.03 | 0.39 |
| Myristoyl carnitine | 13 | 0.04 ± 0.01 | 0.03 ± 0.01 | 0.17 |
| 3-Hydroxy-tetradecenoyl carnitine | 13 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.75 |
| 3-Hydroxy-tetradecanoyl carnitine or Dodecanedioyl carnitine | 13 | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.20 |
| Hexadecadienoyl carnitine | 13 | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.34 |
| Palmitoleoyl carnitine | 13 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.90 |
| Palmitoyl carnitine | 13 | 0.09 ± 0.02 | 0.08 ± 0.02 | 0.16 |
| 3-Hydroxy-palmitoleoyl carnitine or cis-5-Tetradecenedioyl carnitine | 13 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.40 |
| 3-Hydroxy-hexadecanoyl carnitine or Tetradecanedioyl carnitine | 13 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.95 |
| Linoleyl carnitine | 13 | 0.05 ± 0.01 | 0.04 ± 0.01∗ | 0.00 |
| Oleyl carnitine | 13 | 0.09 ± 0.02 | 0.08 ± 0.02 | 0.17 |
| Stearoyl carnitine | 13 | 0.04 ± 0.01 | 0.04 ± 0.01 | 0.62 |
| 3-Hydroxy-linoleyl carnitine | 13 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.40 |
| 3-Hydroxy-octadecenoyl carnitine | 13 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.86 |
| 3-Hydroxy-octadecanoyl carnitine or Hexadecanedioyl carnitine, thapsoyl carnitine | 13 | 0.01 ± 0.00 | 0.01 ± 0.00∗ | 0.04 |
| Arachidonoyl carnitine | 13 | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.32 |
| Arachidoyl carnitine, eicosanoyl carnitine | 13 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.10 |
| Octadecenedioyl carnitine | 13 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.85 |
| 3-Hydroxy-eicosanoyl carnitine or Octadecanedioyl carnitine | 13 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.33 |
| Behenoyl carnitine, docosanoyl carnitine | 13 | 0.01 ± 0.00 | 0.01 ± 0.00 | 0.68 |
C-reactive protein (CRP); Area under the curve (AUC); Oral Glucose Tolerance Test (OGTT); Non-esterified fatty acids (NEFA). ∗Bold indicates significant difference from PRE (p < 0.05).
3.3. Relationships between OA symptoms and other training responses
To evaluate connections between intervention-derived changes in physiology, body composition, and improved OA symptoms, we evaluated associations between changes in OA symptoms with physiologic and body composition responses (Table 3). For measures of cardiorespiratory fitness, improvements in ventilatory threshold (L/min) were associated with improvements in pain (r = −0.70; p < 0.02); similarly, there were trends for relationships between improvements in ventilatory thresholds and total WOMAC scores (p < 0.06). For body composition responses, change in lean mass were associated with improved stiffness (r = −0.78, p < 0.003); there were trends for associations between reductions in fat and trunk fat mass with reduced stiffness (r = 0.55–0.56; p < 0.06 for both).
Table 3.
Relationships for changes in OA symptoms with changes in body composition, physiologic, and metabolic responses to exercise training.
| WOMAC Total | WOMAC Pain | WOMAC Stiffness | WOMAC Function | |
|---|---|---|---|---|
| Visceral Fat (kg) | 0.43 | 0.49 | 0.34 | 0.42 |
| Trunk Fat (kg) | 0.31 | 0.33 | 0.55 | 0.31 |
| Leg Fat (kg) | 0.37 | 0.22 | 0.37 | 0.28 |
| Total Fat (kg) | 0.30 | 0.20 | 0.56 | 0.29 |
| Weight (kg) | −0.07 | 0.09 | −0.08 | −0.12 |
| Lean Mass (kg) | −0.29 | 0.01 | −0.77∗ | −0.34 |
| Leg Lean Muscle (kg) | 0.03 | 0.25 | −0.41 | −0.14 |
| Arm Lean Muscle (kg) | 0.02 | 0.22 | −0.32 | −0.05 |
| Absolute Peak VO2 (l·min-1) | 0.06 | 0.02 | −0.27 | 0.06 |
| Relative Peak VO2 (ml·kg·min-1) | 0.03 | 0.01 | −0.27 | 0.06 |
| Power (W)at Peak VO2 | −0.23 | 0.06 | −0.25 | −0.28 |
| Maximum Heart Rate | −0.50 | −0.25 | −0.52 | −0.50 |
| Ventilatory Threshold (l·min-1) | −0.58 | −0.70∗ | −0.29 | −0.45 |
| Power (W) at Ventilatory Threshold | −0.28 | −0.14 | −0.18 | −0.14 |
| Heart Rate at Ventilatory Threshold | −0.54 | −0.38 | −0.29 | −0.54 |
| Glucose AUC | 0.09 | 0.08 | 0.22 | −0.07 |
| Insulin AUC | 0.55∗ | 0.58∗ | 0.36 | 0.54∗ |
| Matsuda Index | −0.38 | −0.43 | −0.25 | −0.37 |
| Total ketones | −0.01 | 0.25 | −0.32 | −0.21 |
| NEFAs | 0.14 | 0.27 | 0.20 | −0.02 |
| Lactate | 0.19 | 0.33 | 0.05 | 0.28 |
| AMINO ACID INTERMEDIATES | ||||
| Glycine | −0.22 | −0.36 | 0.27 | −0.27 |
| Alanine | 0.16 | 0.03 | 0.40 | 0.36 |
| Serine | −0.18 | −0.20 | −0.20 | −0.10 |
| Proline | −0.03 | −0.19 | 0.021 | 0.20 |
| Valine | −0.03 | 0.21 | −0.24 | −0.10 |
| Leucine/Isoleucine | 0.20 | 0.47 | 0.05 | 0.08 |
| Methionine | −0.11 | 0.00 | 0.18 | −0.19 |
| Histidine | −0.09 | 0.01 | −0.20 | −0.12 |
| Phenylalanine | −0.29 | −0.17 | −0.12 | −0.40 |
| Tyrosine | −0.26 | −0.16 | −0.07 | −0.23 |
| Aspartic acid/Asparagine | −0.08 | −0.04 | −0.35 | −0.16 |
| Glutamic acid/Glutamate | 0.08 | 0.23 | −0.07 | −0.06 |
| Ornithine | −0.53 | −0.54 | 0.00 | −0.49 |
| Citrulline | −0.57∗ | −0.59∗ | −0.46 | −0.56∗ |
| Arginine | −0.40 | −0.43 | 0.00 | −0.30 |
| ACYLCARNITINE INTERMEDIATES | ||||
| Acetyl carnitine (C2) | −0.24 | −0.20 | −0.34 | −0.302 |
| Propionyl carnitine (C3) | −0.40 | −0.18 | −0.63∗ | −0.32 |
| Tiglyl carnitine (C5:1) | 0.46 | 0.32 | 0.51 | 0.58∗ |
| Isovaleryl carnitine, 3-methylbutyryl carnitine or 2-Methylbutyryl carnitine (C5) | −0.22 | 0.10 | −0.36 | −0.34 |
| 3-Hydroxy-isovaleryl carnitine or Malonyl carnitine (C5OH) | −0.38 | −0.55 | −0.23 | −0.17 |
| Glutaryl carnitine (C5DC) | −0.24 | −0.05 | −0.01 | −0.32 |
| Butyryl carnitine or Isobutyryl carnitine (C4/C4i) | −0.48 | −0.24 | −0.64∗ | −0.59∗ |
| 3-Hydroxy-butyryl carnitine, b-hydroxy butyryl carnitine (C4OH) | 0.34 | 0.24 | 0.60∗ | 0.36 |
| Hexanoyl carnitine (C6) | −0.51 | −0.37 | −0.24 | −0.51 |
| Methylmalonyl carnitine or Succinyl carnitine (C4DC) | 0.12 | 0.12 | 0.31 | 0.09 |
| Octenoyl carnitine (C8:1) | −0.19 | 0.11 | −0.17 | −0.37 |
| Octanoyl carnitine (C8) | −0.31 | −0.28 | 0.12 | −0.38 |
| 3-Hydroxy-cis-5-octenoyl carnitine or Hexenedioyl carnitine (C8:1OH) | 0.24 | 0.22 | −0.01 | 0.23 |
| Adipoyl carnitine (C6DC) | 0.07 | −0.09 | 0.29 | 0.05 |
| Decatrienoyl carnitine (C10:3) | −0.11 | −0.08 | 0.24 | −0.18 |
| Decadienoyl carnitine (C10:2) | −0.60∗ | −0.48 | −0.47 | −0.48 |
| Decenoyl carnitine (C10:1) | 0.06 | −0.05 | −0.15 | −0.04 |
| Decanoyl carnitine (C10) | −0.37 | −0.39 | −0.15 | −0.39 |
| Octenedioyl carnitine (C8:1DC) | −0.28 | −0.11 | −0.19 | −0.26 |
| 3-Hydroxy-decanoyl carnitine or Suberoyl carnitine (C10OH/C8DC) | −0.08 | −0.12 | 0.12 | −0.11 |
| Dodecenoyl carnitine (C12:1) | −0.12 | −0.13 | 0.24 | −0.20 |
| Lauroyl carnitine (C12) | −0.09 | −0.24 | −0.22 | −0.10 |
| 3-Hydroxy-dodecanoyl carnitine or Sebacoyl carnitine (C12OH) | −0.42 | −0.45 | −0.14 | −0.31 |
| Tetradecadienoyl carnitine (C14:2) | 0.25 | 0.41 | 0.37 | 0.10 |
| Tetradecenoyl carnitine (C14:1) | 0.00 | 0.00 | −0.08 | −0.13 |
| Myristoyl carnitine (C14) | 0.42 | 0.45 | 0.21 | 0.40 |
| 3-Hydroxy-tetradecenoyl carnitine (C14:1OH) | 0.13 | −0.04 | −0.09 | 0.12 |
| 3-Hydroxy-tetradecanoyl carnitine or Dodecanedioyl carnitine (C14OH/C12DC) | −0.22 | 0.07 | −0.59 | −0.22 |
| Hexadecadienoyl carnitine (C16:2) | −0.38 | −0.11 | −0.75∗ | −0.38 |
| Palmitoleoyl carnitine (C16:1) | 0.13 | 0.16 | −0.08 | 0.13 |
| Palmitoyl carnitine (C16) | 0.48 | 0.36 | 0.36 | 0.45 |
| 3-Hydroxy-palmitoleoyl carnitine or cis-5-Tetradecenedioyl carnitine (C16:1OH/C14:1DC) | −0.06 | 0.23 | −0.02 | −0.13 |
| 3-Hydroxy-hexadecanoyl carnitine or Tetradecanedioyl carnitine (C16OH/C14DC) | −0.13 | −0.30 | −0.64∗ | −0.11 |
| Linoleyl carnitine (C18:2) | 0.07 | −0.27 | 0.19 | 0.12 |
| Oleyl carnitine (C18:1) | −0.07 | −0.00 | −0.32 | −0.14 |
| Stearoyl carnitine (C18) | 0.36 | 0.54 | 0.09 | 0.32 |
| 3-Hydroxy-linoleyl carnitine (C18:2OH) | −0.01 | −0.18 | 0.58∗ | 0.04 |
| 3-Hydroxy-octadecenoyl carnitine (C18:1OH/C16:1DC) | −0.35 | −0.04 | −0.65 | −0.48 |
| 3-Hydroxy-octadecanoyl carnitine or Hexadecanedioyl carnitine, thapsoyl carnitine (C18OH/C16DC) | −0.01 | 0.04 | 0.09 | −0.15 |
| Arachidonoyl carnitine (C20:4) | 0.18 | 0.20 | 0.25 | 0.07 |
| Arachidoyl carnitine, eicosanoyl carnitine (C20) | −0.51 | −0.48 | 0.03 | −0.49 |
| Octadecenedioyl carnitine (C18:1DC) | 0.19 | 0.039 | 0.41 | 0.17 |
| 3-Hydroxy-eicosanoyl carnitine or Octadecanedioyl carnitine (C20OH/C18DC) | −0.20 | −0.17 | 0.38 | −0.32 |
| Behenoylcarnitine (C22) | 0.30 | 0.31 | 0.19 | 0.13 |
∗Bold indicates significant difference from PRE (p < 0.05).
To evaluate connections between intervention-mediated alterations in metabolism and OA symptoms, we evaluated associations between OA symptoms and change in inflammatory markers, metabolic function, and metabolic intermediates. WOMAC improvements in total, pain, and function scores were associated with improvements (reductions) in insulin area under the curve (AUC) from OGTT tests; also, these three WOMAC score improvements were associated with increases in citrulline (p < 0.05 for all). Additionally, improvements in function were related to increases in circulating IL-6 and C4 acylcarnitine and reductions in C5:1 acylcarnitine concentrations (p < 0.05 for all). Improvements in stiffness were associated with increases in C3, C4, C14OH/C12DC, C16:2, C16OH/C14DC, C18:1OH/C16:1DC acylcarnitines and reductions in C4OH and C18:2OH acylcarnitines (p < 0.05 for all).
4. Discussion
In this cohort of older, overweight/obese adults with radiographic and symptomatic knee OA, six weeks of twice-weekly high-intensity interval cycle training showed excellent rates of retention and compliance with no noted adverse events. Recruitment and enrollment was a bit challenging; this appeared to be due to perceived barriers from potential participants, such as the ability to complete ‘high intensity’ exercise, mounting/dismounting the cycle ergometer, and completing the cycle motion due to pain and stiffness. Once enrolled, these did not reveal them as actual barriers to completing the exercise program. Additionally, with 12 sessions of supervised exercise training, persons with knee OA improved cardiorespiratory fitness and OA symptoms in concert with metabolic alterations indicative of improved skeletal muscle energetics.
With few treatment options for knee OA, it is remarkable that OA symptoms improved after six weeks of twice weekly interval-based exercise training. To date, there are minimal data evaluating the effects of HIIT in OA. Only two previous studies have evaluated HIIT in patients with OA [6,28]. One protocol was completed on an aquatic treadmill, potentially inhibiting implementation into most clinics and exercise settings [6]. The other protocol evaluated a home-based cycling protocol with a similar work to rest ratio as used in the present study [28]. Both studies demonstrated improvements in WOMAC scores for pain. Together, it appears as if HIIT conducted via cycling may be a feasible and effective approach to reduce symptoms of knee OA. There will likely be initial perceived barriers for beginning this type of exercise; of which are highly likely to be overcome and improved with participation in HIIT cycling.
After six weeks of HIIT, overall fasting glucose was greater while there were reductions in several acylcarnitines (linoleyl carnitine (C18:2), 3-hydroxyoctadecanoly or hexadecanedioyl carnitine (C18OH/C16DC), 3-hydroxydodecanoyl or sebacoyl carnitine (C12:2OH/C10:2DC) and amino acids (methionine, phenylalanine, and tyrosine). These findings suggest HIIT aerobic training on a cycle ergometer increased fatty acid and amino acid utilization resulting in reduced systemic accumulation. Acylcarnitines are intermediates of fatty acid oxidation, and accumulation in the systemic circulation occurs in the setting of dysfunctional fatty acid oxidation [39]. For example, accumulation of certain acylcarnitines species (e.g. medium chain acylcarnitines) signifies genetic deficiencies in fatty acid catabolic enzymes (e.g. medium-chain acyl-CoA dehydrogenase deficiency). Additionally, plasma acylcarnitines accumulate in a number of chronic metabolic insults including in persons with greater fat mass [40], poorer skeletal muscle insulin sensitivity and pancreatic insulin response [41], poorer physical performance [42], heart failure [43], pulmonary hypertension [44], or increased risk for cardiovascular events [45]. Systemic acylcarnitine reductions occur with exercise training, in concert with increases in skeletal muscle oxidative machinery and cardiorespiratory fitness [31,46]. Thus, reduced acylcarnitine concentrations after six weeks of HIIT suggest a beneficial metabolic response including improved skeletal muscle fatty acid oxidation and mitochondrial function.
Reductions in systemic concentrations of the amino acids, methionine, phenylalanine, and tyrosine could reflect either increased oxidation of these amino acids or increased protein synthesis. In the setting of minimal weight loss and full availability of other substrates, amino acid catabolism appears an unlikely contributor. Alternatively, it is well-recognized that exercise, as well as protein ingestion, stimulate protein synthesis [[47], [48], [49]]. In the context of this HIIT intervention, reduced fasting amino acid concentrations likely represent an exercise training-mediated induction of biosynthesis for muscle structural and metabolic machinery [[47], [48], [49]]. Additionally, phenylalanine and tyrosine are catecholamine precursors associated with exercise capacity, such that training-induced reductions may represent an upregulation of catecholamine synthesis that promotes exercise capacity. Nonetheless, reductions in these amino acids after six weeks of HIIT reinforces an overall beneficial metabolic adaptation to exercise training. Results from the present study may be unique to cycling exercise, due to the large demand on lower body leg musculature.
Metabolic adaptations to exercise training are likely to play a role in training program-induced improvements in OA symptoms. As above, overall improvements were noted in pain, function, and total OA symptoms. Interestingly, improvements in pain were associated with improvements in ventilatory threshold, the point—indicated by increased ventilatory rate, CO2 accumulation and lactate production—representing the capacity for oxygen consumption to produce ATP for the work of peripheral muscle during exercise. Thus, ventilatory threshold provides a measure of skeletal muscle aerobic capacity; associated improvements with pain imply that underlying skeletal muscle fitness may contribute to improvements in OA pain. This is further supported by the improvements in aerobic capacity seen for all participants.
Improvements in pain, as well as function and total WOMAC, were also associated with improved glucose-stimulated insulin kinetics, as measured by insulin AUCs during an oral glucose tolerance test. As skeletal muscle is the main organ responsible for insulin-stimulated glucose uptake, this association further supports the link between exercise training, skeletal muscle metabolic health, and OA pain and function improvements. Similarly, OA pain and function improvements were related to greater concentrations of citrulline, a precursor for arginine, nitric oxide, and creatine. Citrulline promotes exercise tolerance, especially at greater intensities, perhaps through serving as a ready substrate for synthesis of nitric oxide and creatine phosphate [50]. The nitric oxide -mediated vasodilatory activity can increase speed of blood delivery to skeletal muscle; creatine phosphate is a critical energy source for short, intense exercise bouts [50].
Intriguingly, improvements in OA pain and function appear distinct from training-mediated improvements in OA stiffness. OA stiffness improvements were related to improved body composition, defined as a reduction in visceral fat volume and trend toward a decrease in fat mass. Additionally, improvements in OA stiffness were associated with increases in a number of short-, medium- and long-chain acylcarnitine concentrations (C3, C4, C14OH/C12DC, C16:2, C16OH/C14DC, C18:1OH/C16:1DC acylcarnitines and reductions in C4OH and C18:2OH). Circulating acylcarnitines may reflect a surplus of energy substrates and incomplete muscle oxidation of fatty acids. However, in the context of an intervention imposing muscle energy demands, increased acylcarnitines may reflect a reduction in liver fatty acid catabolism—associated with reduced trunk adiposity—as a means of shifting these substrates towards skeletal muscle. How reductions in visceral adiposity or shifts from liver to muscle fat oxidation would lead to OA stiffness remains unclear.
Limitations within the study must be addressed. This single-arm pilot study provides an initial evaluation of the effect of supervised HIIT with cycle ergometry in knee OA. The lack of a control group is a limitation. A randomized-controlled trial in a larger sample is needed, particularly given these promising early results. The majority of the present sample (77%) were female. Knee OA is more prevalent in women [51], but with potential differences in cardiovascular and metabolic adaptations, a sample evaluating the influence of sex on these outcomes is warranted. Additionally, peripheral metabolites cannot directly describe skeletal muscle metabolic alterations; results from the present study demonstrating some positive peripheral metabolic changes, support the needs for skeletal muscle analytes to be evaluated in the future.
In sum, six weeks of HIIT in symptomatic knee OA was associated with improvements in fitness, visceral fat, and OA symptoms. Peripheral whole-body metabolic adaptations and associated outcomes suggest improved skeletal muscle function, and specifically improved mitochondrial function. Metabolic and physiologic mechanisms driving OA symptom improvements appear specific to pain and function, as compared to stiffness, an intriguing finding meriting additional investigation. Overall, our findings of connections between improved OA symptoms and exercise training suggest mediation by skeletal muscle metabolism.
Author contributions
AESR, KDA, YMG, KMH conceived the idea;
AESR, MNMB, YMG, KMH planned the experiments.
AESR, MNMB, KCA, KRH, JLH, MJM, ORI, CS, VK, WK, KMH performed the experiments.
AESR, MNMB, KRH, KMH compiled and completed the results and analyses.
AESR, MNMB, KMH took the lead in writing the manuscript.
All authors (AESR, MNMB, KCA, KRH, KDA, JLH, MJM, ORI, CS, VBK, WEK, YMG, KMH) provided critical feedback that helped shape the research, analysis, and manuscript.
Role of the funding source
This study was funded by the National Institutes of Health Resource to Enhance Clinical Trials (REACT) Pilot and Feasibility Award under Grant 1P2CHD086851. The sponsor had no role in the study design, analysis, or interpretation.
Declaration of Competing Interest
None to disclose.
Acknowledgements
We would like to thank the participants for their commitment to the exercise training.
Contributor Information
Abbie E. Smith-Ryan, Email: abbsmith@email.unc.edu.
Malia N.M. Blue, Email: mnm3303@live.unc.edu.
Kara C. Anderson, Email: ka2zwg@virginia.edu.
Katie R. Hirsch, Email: ktrose23@live.unc.edu.
Kelli D. Allen, Email: kdallen@email.unc.edu.
Janet L. Huebner, Email: janet.heubner@duke.edu.
Michael J. Muehlbauer, Email: Michael.muehlbauer@duke.edu.
Olga R. Ilkayeva, Email: olga.ilkayeva@duke.edu.
Virginia Byers Kraus, Email: kraus004@duke.edu.
William E. Kraus, Email: william.kraus@duke.edu.
Yvonne M. Golightly, Email: golight@email.unc.edu.
Kim M. Huffman, Email: kim.huffman@duke.edu.
References
- 1.Dillon C.F., Rasch E.K., Gu Q., Hirsch R. Prevalence of knee osteoarthritis in the United States: arthritis data from the third national health and nutrition examination survey 1991-94. J. Rheumatol. 2006;33:2271–2279. [PubMed] [Google Scholar]
- 2.Jordan J.M., Helmick C.G., Renner J.B., Luta G., Dragomir A.D., Woodard J., et al. Prevalence of knee symptoms and radiographic and symptomatic knee osteoarthritis in African Americans and caucasians: the Johnston County osteoarthritis project. J. Rheumatol. 2007;34:172–180. [PubMed] [Google Scholar]
- 3.Lawrence R.C., Felson D.T., Helmick C.G., Arnold L.M., Choi H., Deyo R.A., et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aamot I.L., Karlsen T., Dalen H., Stoylen A. Long-term exercise adherence after high-intensity interval training in cardiac rehabilitation: a randomized study. Physiother. Res. Int. 2016;21:54–64. doi: 10.1002/pri.1619. [DOI] [PubMed] [Google Scholar]
- 5.Gibala M.J. High-intensity interval training: a time-efficient strategy for health promotion? Curr. Sports Med. Rep. 2007;6:211–213. [PubMed] [Google Scholar]
- 6.Bressel E., Wing J.E., Miller A.I., Dolny D.G. High-intensity interval training on an aquatic treadmill in adults with osteoarthritis: effect on pain, balance, function, and mobility. J. Strength Condit Res. 2014;28:2088–2096. doi: 10.1519/JSC.0000000000000258. [DOI] [PubMed] [Google Scholar]
- 7.Knowles A.M., Herbert P., Easton C., Sculthorpe N., Grace F.M. Impact of low-volume, high-intensity interval training on maximal aerobic capacity, health-related quality of life and motivation to exercise in ageing men. Age. 2015;37:25. doi: 10.1007/s11357-015-9763-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Persoon S., Kersten M.J., Chinapaw M.J., Buffart L.M., Burghout H., Schep G., et al. Design of the EXercise Intervention after Stem cell Transplantation (EXIST) study: a randomized controlled trial to evaluate the effectiveness and cost-effectiveness of an individualized high intensity physical exercise program on fitness and fatigue in patients with multiple myeloma or (non-) Hodgkin's lymphoma treated with high dose chemotherapy and autologous stem cell transplantation. BMC Canc. 2010;10:671. doi: 10.1186/1471-2407-10-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wisloff U., Stoylen A., Loennechen J.P., Bruvold M., Rognmo O., Haram P.M., et al. Superior cardiovascular effect of aerobic interval training versus moderate continuous training in heart failure patients: a randomized study. Circulation. 2007;115:3086–3094. doi: 10.1161/CIRCULATIONAHA.106.675041. [DOI] [PubMed] [Google Scholar]
- 10.Fransen M., McConnell S. Exercise for osteoarthritis of the knee. Cochrane Database Syst. Rev. 2008 doi: 10.1002/14651858.CD004376.pub2. [DOI] [PubMed] [Google Scholar]
- 11.Aglamiş B., Toraman N.F., Yaman H. Change of quality of life due to exercise training in knee osteoarthritis: SF-36 and WOMAC. J. Back Musculoskelet. Rehabil. 2009;22:43–48. doi: 10.3233/BMR-2009-0219. PMID: 20023363. [DOI] [PubMed] [Google Scholar]
- 12.Tucker J.M., Welk G.J., Beyler N.K. Physical activity in U.S. Adults: compliance with the physical activity guidelines for Americans. Am. J. Prev. Med. 2011;40:454–461. doi: 10.1016/j.amepre.2010.12.016. PMID: 21406280. [DOI] [PubMed] [Google Scholar]
- 13.Farr J.N., Going S.B., Lohman T.G., Rankin L., Kasle S., Cornett M., et al. Physical activity levels in patients with early knee osteoarthritis measured by accelerometry. Arthritis Rheum. 2008;59:1229–1236. doi: 10.1002/art.24007. PMCID: PMC2595140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wallis J.A., Webster K.E., Levinger P., Taylor N.F. What proportion of people with hip and knee osteoarthritis meet physical activity guidelines? A systematic review and meta-analysis. Osteoarthritis Cartilage. 2013;21:1648–1659. doi: 10.1016/j.joca.2013.08.003. PMID: 23948979. [DOI] [PubMed] [Google Scholar]
- 15.Freyssin C., Verkindt C., Prieur F., Benaich P., Maunier S., Blanc P. Cardiac rehabilitation in chronic heart failure: effect of an 8-week, high-intensity interval training versus continuous training. Arch. Phys. Med. Rehabil. 2012;93:1359–1364. doi: 10.1016/j.apmr.2012.03.007. Epub 2012 Mar 1321. [DOI] [PubMed] [Google Scholar]
- 16.Masuki S., Morikawa M., Nose H. Interval walking training can increase physical fitness in middle-aged and older people. Exerc. Sport Sci. Rev. 2017;45:154–162. doi: 10.1249/JES.0000000000000113. doi: 110.1249/JES.0000000000000113. [DOI] [PubMed] [Google Scholar]
- 17.Milanovic Z., Sporis G., Weston M. Effectiveness of high-intensity interval training (HIT) and continuous endurance training for VO2max improvements: a systematic review and meta-analysis of controlled trials. Sports Med. 2015;45:1469–1481. doi: 10.1007/s40279-015-0365-0. [DOI] [PubMed] [Google Scholar]
- 18.Fransen M., McConnell S. Exercise for osteoarthritis of the knee. Cochrane Database Syst. Rev. 2008;8 doi: 10.1002/14651858.CD004376.pub2. PMID: 18843657. [DOI] [PubMed] [Google Scholar]
- 19.Fransen M., McConnell S. Land-based exercise for osteoarthritis of the knee: a metaanalysis of randomized controlled trials. J. Rheumatol. 2009;36:1109–1117. doi: 10.3899/jrheum.090058. PMID: 19447940. [DOI] [PubMed] [Google Scholar]
- 20.Fransen M., McConnell S., Harmer A.R., Van der Esch M., Simic M., Bennell K.L. Exercise for osteoarthritis of the knee. Cochrane Database Syst. Rev. 2015;1 doi: 10.1002/14651858.CD004376.pub3. PMID: 25569281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Golightly Y.M., Allen K.D., Caine D.J. A comprehensive review of the effectiveness of different exercise programs for patients with osteoarthritis. Physician Sportsmed. 2012;40:52–65. doi: 10.3810/psm.2012.11.1988. PMCID: PMC4077018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blue M.N.M., Smith-Ryan A.E., Trexler E.T., Hirsch K.R. The effects of high intensity interval training on muscle size and quality in overweight and obese adults. J. Sci. Med. Sport. 2018;21:207–212. doi: 10.1016/j.jsams.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aamot I.L., Karlsen T., Dalen H., Stoylen A. Long-term exercise adherence after high-intensity interval training in cardiac rehabilitation: a randomized study. Physiother. Res. Int. 2016;21:54–64. doi: 10.1002/pri.1619. [DOI] [PubMed] [Google Scholar]
- 24.Smith-Ryan A.E. Enjoyment of high-intensity interval training in an overweight/obese cohort: a short report. Clin. Physiol. Funct. Imag. 2015 doi: 10.1111/cpf.12262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith-Ryan A.E., Melvin M.N., Wingfield H.L. High-intensity interval training: modulating interval duration in overweight/obese men. Physician Sportsmed. 2015;43:107–113. doi: 10.1080/00913847.2015.1037231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith-Ryan A.E., Trexler E.T., Wingfield H.L., Blue M.N. Effects of high intensity interval training on cardiometabolic risk factors in overweight/obese women. J. Sports Sci. 2016;34:2038–2046. doi: 10.1080/02640414.2016.1149609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wood W.A., Phillips B., Smith-Ryan A.E., Wilson D., Deal A.M., Bailey C., et al. Personalized home-based interval exercise training may improve cardiorespiratory fitness in cancer patients preparing to undergo hematopoietic cell transplantation. Bone Marrow Transplant. 2016;15:967–972. doi: 10.1038/bmt.2016.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Keogh J.W., Grigg J., Vertullo C.J. Is high-intensity interval cycling feasible and more beneficial than continuous cycling for knee osteoarthritic patients? Results of a randomised control feasibility trial. PeerJ. 2018;6:e4738. doi: 10.7717/peerj.4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sparks L.M. Exercise training response heterogeneity: physiological and molecular insights. Diabetologia. 2017;60:2329–2336. doi: 10.1007/s00125-017-4461-6. [DOI] [PubMed] [Google Scholar]
- 30.Sellam J., Berenbaum F. Is osteoarthritis a metabolic disease? Joint Bone Spine. 2013;80:568–573. doi: 10.1016/j.jbspin.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 31.Huffman K.M., Koves T.R., Hubal M.J., Abouassi H., Beri N., Bateman L.A., et al. Metabolite signatures of exercise training in human skeletal muscle relate to mitochondrial remodelling and cardiometabolic fitness. Diabetologia. 2014;57:2282–2295. doi: 10.1007/s00125-014-3343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huffman K.M., Shah S.H., Stevens R.D., Bain J.R., Muehlbauer M., Slentz C.A., et al. Relationships between circulating metabolic intermediates and insulin action in overweight to obese, inactive men and women. Diabetes Care. 2009;32:1678–1683. doi: 10.2337/dc08-2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Redman L.M., Huffman K.M., Landerman L.R., Pieper C.F., Bain J.R., Muehlbauer M.J., et al. Effect of caloric restriction with and without exercise on metabolic intermediates in nonobese men and women. J. Clin. Endocrinol. Metab. 2011;96:E312–E321. doi: 10.1210/jc.2010-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peake J.M., Tan S.J., Markworth J.F., Broadbent J.A., Skinner T.L., Cameron-Smith D. Metabolic and hormonal responses to isoenergetic high-intensity interval exercise and continuous moderate-intensity exercise. Am. J. Physiol. Endocrinol. Metab. 2014;307:E539–E552. doi: 10.1152/ajpendo.00276.2014. [DOI] [PubMed] [Google Scholar]
- 35.Lowe W.L., Jr., Bain J.R., Nodzenski M., Reisetter A.C., Muehlbauer M.J., Stevens R.D., et al. Maternal BMI and glycemia impact the fetal metabolome. Diabetes Care. 2017;40:902–910. doi: 10.2337/dc16-2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shah S.H., Bain J.R., Muehlbauer M.J., Stevens R.D., Crosslin D.R., Haynes C., et al. Association of a peripheral blood metabolic profile with coronary artery disease and risk of subsequent cardiovascular events. Circ Cardiovasc Genet. 2010;3:207–214. doi: 10.1161/CIRCGENETICS.109.852814. [DOI] [PubMed] [Google Scholar]
- 37.McConnell S., Kolopack P., Davis A.M. The Western Ontario and McMaster Universities osteoarthritis index (WOMAC): a review of its utility and measurement properties. Arthritis Rheum. 2001;45:453–461. doi: 10.1002/1529-0131(200110)45:5<453::aid-art365>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 38.Orr G.W., Green H.J., Hughson R.L., Bennett G.W. A computer linear regression model to determine ventilatory anaerobic threshold. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1982;52:1349–1352. doi: 10.1152/jappl.1982.52.5.1349. [DOI] [PubMed] [Google Scholar]
- 39.Baker P.R., 2nd, Boyle K.E., Koves T.R., Ilkayeva O.R., Muoio D.M., Houmard J.A., et al. Metabolomic analysis reveals altered skeletal muscle amino acid and fatty acid handling in obese humans. Obesity. 2015;23:981–988. doi: 10.1002/oby.21046. Epub 22015 Apr 21010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Redman L.M., Huffman K.M., Landerman L.R., Pieper C.F., Bain J.R., Muehlbauer M.J., et al. Effect of caloric restriction with and without exercise on metabolic intermediates in nonobese men and women. J. Clin. Endocrinol. Metab. 2011;96:E312–E321. doi: 10.1210/jc.2010-1971. Epub 2010 Dec 1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huffman K.M., Shah S.H., Stevens R.D., Bain J.R., Muehlbauer M., Slentz C.A., et al. Relationships between circulating metabolic intermediates and insulin action in overweight to obese, inactive men and women. Diabetes Care. 2009;32:1678–1683. doi: 10.2337/dc08-2075. Epub 2009 Jun 1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lum H., Sloane R., Huffman K.M., Kraus V.B., Thompson D.K., Kraus W.E., et al. Plasma acylcarnitines are associated with physical performance in elderly men. J. Gerontol. A Biol. Sci. Med. Sci. 2011;66:548–553. doi: 10.1093/gerona/glr006. Epub 2011 Mar 1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ahmad T., Kelly J.P., McGarrah R.W., Hellkamp A.S., Fiuzat M., Testani J.M., et al. Prognostic implications of long-chain acylcarnitines in heart failure and reversibility with mechanical circulatory support. J. Am. Coll. Cardiol. 2016;67:291–299. doi: 10.1016/j.jacc.2015.10.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Luo N., Craig D., Ilkayeva O., Muehlbauer M., Kraus W.E., Newgard C.B., et al. Plasma acylcarnitines are associated with pulmonary hypertension. Pulm. Circ. 2017;7:211–218. doi: 10.1086/690554. eCollection 692017 Mar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shah S.H., Sun J.L., Stevens R.D., Bain J.R., Muehlbauer M.J., Pieper K.S., et al. Baseline metabolomic profiles predict cardiovascular events in patients at risk for coronary artery disease. Am. Heart J. 2012;163:844–850. doi: 10.1016/j.ahj.2012.02.005. e841. [DOI] [PubMed] [Google Scholar]
- 46.Gong Y., Selzer F., Deshpande B., Losina E. Trends in procedure type, patient characteristics, and outcomes among persons with knee osteoarthritis undergoing bariatric surgery, 2005-2014. Osteoarthritis Cartilage. 2018;26:1487–1494. doi: 10.1016/j.joca.2018.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Churchward-Venne T.A., Pinckaers P.J.M., Smeets J.S.J., Peeters W.M., Zorenc A.H., Schierbeek H., et al. Myofibrillar and mitochondrial protein synthesis rates do not differ in young men following the ingestion of carbohydrate with milk protein, whey, or micellar casein after concurrent resistance- and endurance-type exercise. J. Nutr. 2019;149:198–209. doi: 10.1093/jn/nxy244. doi: 110.1093/jn/nxy1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim I.Y., Schutzler S., Schrader A., Spencer H.J., Azhar G., Ferrando A.A., et al. The anabolic response to a meal containing different amounts of protein is not limited by the maximal stimulation of protein synthesis in healthy young adults. Am. J. Physiol. Endocrinol. Metab. 2016;310:E73–E80. doi: 10.1152/ajpendo.00365.02015. Epub 02015 Nov 00363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trommelen J., Holwerda A.M., Kouw I.W., Langer H., Halson S.L., Rollo I., et al. Resistance exercise augments postprandial overnight muscle protein synthesis rates. Med. Sci. Sports Exerc. 2016;48:2517–2525. doi: 10.1249/MSS.0000000000001045. doi: 2510.1249/MSS.0000000000001045. [DOI] [PubMed] [Google Scholar]
- 50.Bailey S.J., Blackwell J.R., Lord T., Vanhatalo A., Winyard P.G., Jones A.M. Vol. 119. 2015. pp. 385–395. (L-Citrulline Supplementation Improves O2 Uptake Kinetics and High-Intensity Exercise Performance in Humans. 1985)). Epub 02015 May 00128. [DOI] [PubMed] [Google Scholar]
- 51.Deshpande B.R., Katz J.N., Solomon D.H., Yelin E.H., Hunter D.J., Messier S.P., et al. Number of persons with symptomatic knee osteoarthritis in the US: impact of race and ethnicity, age, sex, and obesity. Arthritis Care Res. 2016;68:1743–1750. doi: 10.1002/acr.22897. [DOI] [PMC free article] [PubMed] [Google Scholar]