Summary
Objective
Spherox (CO.DON AG) is an autologous chondrocyte implantation (ACI) product, consisting of spheroids of human autologous matrix-associated chondrocytes. The tendency of primary chondrocytes to dedifferentiate during cultivation and the high biologic variability caused by the autologous nature of the starting material makes it challenging to design a manufacturing process that performs consistently and delivers products that meet their intended function and the high quality criteria for cell-based ATMPs. The current study was submitted during the European authorization procedure, and addresses the requirement to justify the operational ranges of the manufacturing process using clinical data.
Methods
In order to define the operational ranges, statistical correlation analyses were conducted between process parameters and clinical improvement data of 120 patients from Phase II and III treated with ACI (KOOS score, 1 year follow-up).
Results
This approach identified cell culture time as a critical process parameter that negatively correlates with the product's efficacy. Subsequent analyses of the Phase III patients that were treated with chondrocyte spheroids that have been manufactured with shorter monolayer and spheroid cultivation times showed a higher average clinical improvement as well as a higher responder rate compared to the total group. In addition, retrospective analyses demonstrated superiority for the treatment with short-cultivated chondrocyte spheroids over micro-fracture treatment.
Conclusion
These findings underscore the need to use clinical data to optimize the manufacturing process for autologous cell-based therapies. We expect that restricting the cultivation times during manufacturing minimizes the production of suboptimal batches, thus ensuring an efficacious product.
Keywords: ATMP, Manufacturing, Knee, ACI, Cartilage, Clinical trial
1. Introduction
In order to improve cartilage repair treatment, several autologous cartilage implantation (ACI) therapies have been developed that are based on chondrocyte isolation from a cartilage biopsy which are expanded ex vivo and subsequently implanted into the cartilage defect [1,2]. To optimize cell differentiation state in autologous chondrocyte implantation 3-dimensional matrices were developed (M-ACI), using artificial matrices that are seeded with cells [[3], [4], [5]] which is the third generation ACI ([3,6]). Cell-based therapies using chondrocytes to treat cartilage defects seem to result in more durable formed hyaline-like tissue compared to newly formed tissue after the bone marrow stimulating procedure microfracturing [[7], [8], [9]] as shown by histological and clinical data [3,9]. Repair tissue, formed in the treated lesions by MFX was found to be fibrous of nature and may not always be sustainable [10].
Spherox (CO.DON AG, Germany) is a matrix-associated, 3-dimensional ACI for the treatment of knee cartilage defects containing human autologous chondrocytes manufactured in spherical aggregates [11]. The chondrocyte spheroids synthesize cartilage-specific extra-cellular matrix (ECM) proteins like cartilage acidic protein 1 (CRTAC1), glycosaminoglycans (GAGs) and aggrecan (ACAN). The capacity of the spheroids to form new tissue has been studied ex vivo and is related to the level of ACAN expression [12]. The treatment with Spherox (CO.DON AG, Germany) in clinical practice is defined with 10–70 spheroids/cm2 defect for up to 10 cm2 defects and has been approved in July 2017 by the European Commission after a centralized authorisation procedure at the EMA (European Medicines Agency). Spherox’ European Public Assessment report (EPAR) is available at: www.ema.europa.eu.
The clinical relevance for the treatment of cartilage lesions with Spherox (CO.DON AG) has been demonstrated in Phase II and III clinical trials [[13], [14], [15]]. Phase II and Phase III were both conducted as prospective randomised clinical trials to assess the safety and efficacy of the treatment. (of note: Spherox was named chondrosphere® at time of the trials). Phase II study for dose finding demonstrated that the three dose levels had no effect on safety and efficacy of the treatment [[13], [15]]. Phase III study was conducted in comparison to the procedure of micro-fracture (MFX) and showed noninferiority over microfracture (MFX), 2 years after treatment [14].
The manufacturing process of Spherox (CO.DON AG) consists of the isolation of chondrocytes from a patient's cartilage biopsy by enzymatic digestion, followed by expansion of the cells in monolayer that consists of P0 (monolayer cultivation before the first passage), P1 (after the first passage) and P2 (after the second passage). After cell expansion, chondrocytes are transferred into a 3D cultivation process in which chondrocytes aggregate to form spheroids being the active substance of the final product [11,16]. Various factors, such as biopsy quality and cell culture conditions, can influence the quality of the cells and hence the clinical outcome after ACI treatment [17]. In order to develop an effective and consistent manufacturing process to guarantee high cell quality, the operational ranges of the process parameter and acceptance limits of specific quality parameter have been initially defined based on non-clinical studies addressing phenotypic stability and genotypic stability of the chondrocytes during cultivation. These studies revealed and thus defined the maximum population doubling level for chondrocytes allowed during manufacturing before they start to become genetically unstable [18]. However, these studies did not address the impact of possible factors from the manufacturing process on the efficacy of the drug product.
Process-related factors that affect the efficacy of the drug product can be identified using the available data from clinical trials and process parameter from the manufacturing process. The present study aimed to develop a strategy to define and justify the operational ranges of the manufacturing process based on clinical data, as part of a European marketing authorization procedure. A retrospective analysis was conducted in order to further verify if the adjusted ranges could improve the manufacturing process such that a reduction of the non-responder rate of the patient population after ACI treatment with chondrocyte spheroids can be expected.
2. Method
In total 120 patients of the Phase II and III clinical trials were used for statistical correlation analyses between clinical outcome and the operational ranges of the process parameter. The primary endpoint to measure for clinical efficacy used in Phase II and III was the ‘Knee injury and Osteoarthritis Outcome Scores’ (KOOS) change from baseline. Phase II and III clinical trials are registered at www.clinicaltrials.gov with ID: NCT01225575 and ID: NCT01222559, respectively, and were approved by the local ethics committees and the federal authority as stated in the original publications [13,15]. All patients signed a written consent. Data from 72 patients from Phase II [15], and from Phase III, 48 patients with ACI and 49 patients with micro-fracture (MFX) treatment [14] were assessed in the present study.
The overall delta KOOS score (including all subscales) before arthroscopy (= base line) and after 1 year after implantation were used [19]. The clinical outcome was defined as positive at a difference of at least 8 points overall change from baseline, and is based on the minimal important change (MIC) of 8–10 as described in the user's guide for KOOS questionnaires (www.koos.nu). Since the current study was part of a European marketing authorization procedure 1 year follow-up data were used that were available at the time.
The operational ranges of monolayer cultivation times between the different cell culture passages (P0, P1 and P2), total monolayer cultivation time, spheroid cultivation time and the total cultivation time (monolayer and spheroids together) were statistically assessed against clinical outcome for every batch produced for the clinical trials.
Correlation analyses were performed using Spearman's correlation coefficient for non- Gaussian distributed variables, with a P < 0.05 considered significant. For statistical analysis of differences between cell cultivation times between responder and non-responder patient groups the Mann-Whitney's U test was used since these variables do not show a Gaussian distribution (two-tailed, with P-value<0.05 considered significant). Superiority/non-inferiority analysis of the ACI treatment compared to MFX treatment was performed by an unpaired t-test with Welch's correction with a 95% confidence interval. All statistical analyses were performed using GraphPad Prism v6 (GraphPad Software, USA).
3. Results
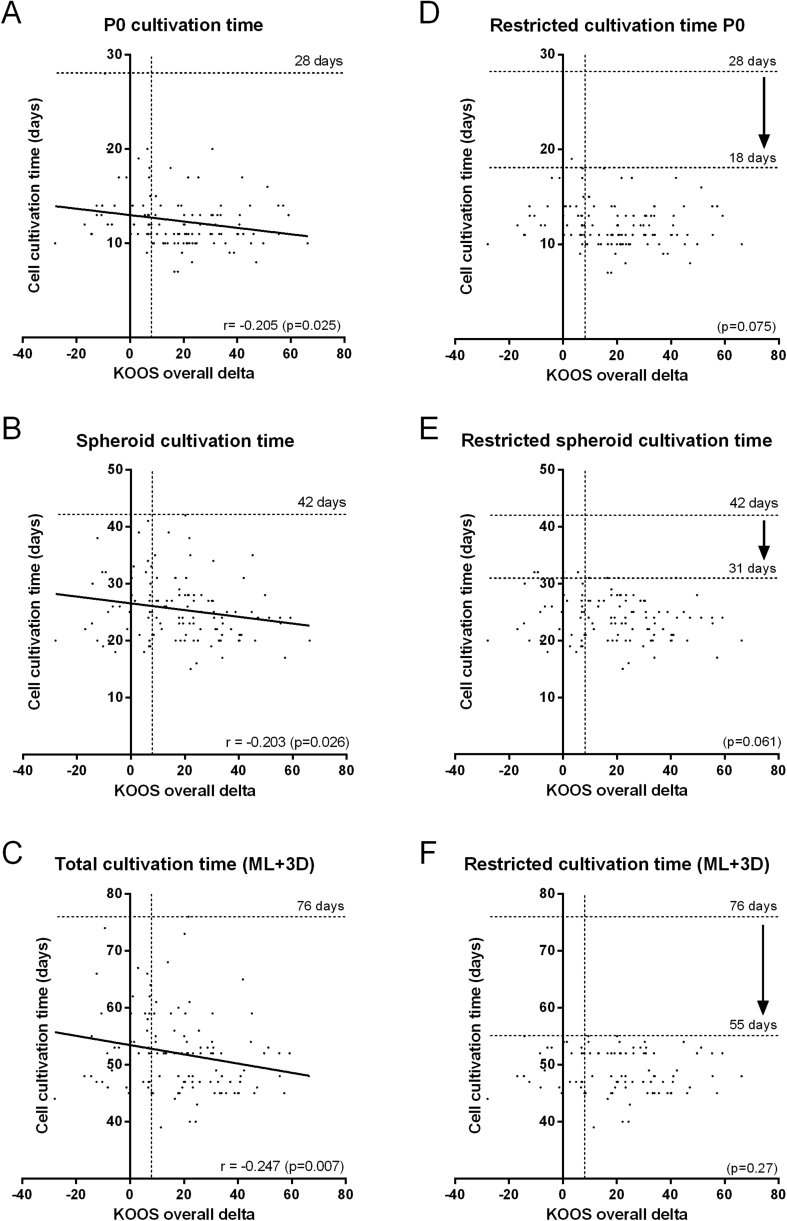
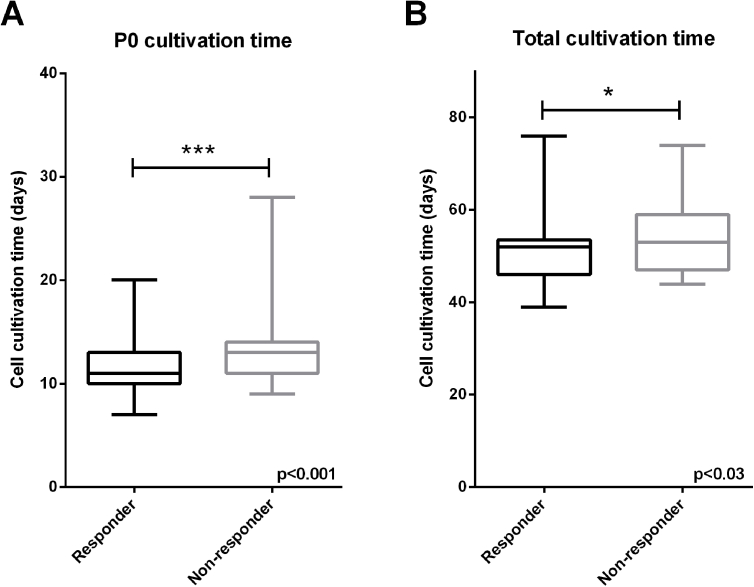
In Fig. 1 the clinical improvement after 1 year after implantation (KOOS overall change from baseline) is plotted against the monolayer (ML) cultivation time before the first passage (P0), against spheroid culture (3D) and against the total cell cultivation time (ML and 3D) for every transplant used to treat Phase II and III patients. The negative correlations that were found for P0 (Spearman, P = 0.025) (Fig. 1A), the spheroid cultivation time (P = 0.026) (Fig. 1C) and the total cultivation time of chondrocytes in ML and 3D together (P = 0.007) (Fig. 1E) are first indication that the operational ranges could have an impact on the product's efficacy. This is supported by the observation that the non-responder group (KOOS <8) had received spheroid batches that have been manufactured with significant longer cultivation times in P0 compared to the responder group (Supplementary Fig. S1A). Also the total cell culture duration of ML and 3D, appeared to be longer on average in the non-responder group (Supplementary Fig. S1B). This underscores the need to newly define and restrict the operational ranges for cell culture time based on clinical data to avoid a negative impact on the product's efficacy.
Fig. 1.
Clinical outcome correlate with cell cultivation times. From 120 spheroid batches used for ACI in Phase II and III clinical trials, the operational ranges were assessed against clinical outcome after 1 year after implantation. Long cultivation times in monolayer before the first passage (P0) (A), in 3D (spheroids) (B) and total in ML and 3D (C) correlated with low KOOS overall delta scores. Eliminating spheroid batches from the data set that were produced with long cultivation time resulted in loss of correlation in P0 (D), 3D (E) and total cultivation time (F). This enabled to set provisional limits (arrow) for maximum cultivation times such that cultivation time does not have a negative effect on the drug product's efficacy. Maximum cultivation time for P0 was set at 18 days (D), for 3D at 31 days (E) and for the total cultivation time in ML and 3D at 55 days (F). r = Spearman correlation efficient with a P-value <0.05 is considered significant. KOOS= Knee injury and Osteoarthritis Outcome Score.
To determine a provisional maximum time for chondrocyte cultivation, the batches with the longest cultivation time were subsequently deleted from the data set until the negative correlation disappeared (Spearman's correlation coefficient). For the ML cultivation time before the first passage, P0, a maximum cultivation time without a negative effect on clinical outcome was defined at 18 days (Fig. 1D). Following the same strategy, a provisional maximum allowed time for spheroid cultivation was set at 31 days (Fig. 1E).
Since the correlation coefficients between the clinical outcome and P0, 3D and total cultivation time were low (−0.2), the batches that were produced with high cultivation times were further analysed for the occurrence of non-responders (KOOS score <8). This revealed a remarkable high non-responder rate of 80% in the group of patients treated with spheroid batches cultivated in ML P0 between 19 and 28 days, and 61.5% for batches cultivated between 16 and 28 days (Supplementary Table S1). Batches cultivated up until 16 days displayed a non-responder rate of 28%, which is significantly lower compared to the 31.7% of non-responders in the complete clinical study group.
Furthermore, patients treated with spheroids cultivated in 3D between 32 and 42 days showed a relatively high non-responder rate of 59% (n = 17) (Supplementary Table S2) underscoring the requirement to also limit 3D cultivation time. A possible restriction up until 28 days was considered to further reduce the non-responder rate. Indeed, between 29 and 42 days of cultivation, a non-responder rate of 50% indicates that further limitation of the cultivation time in 3D could improve the responder rate. In line with this, the non-responder rate was lower when cultivation was restricted until maximal 28 days; 26.6% (Supplementary Table S2). Considering the non-responder rate together with the feasibility of cultivation times during manufacturing, the provisional maximum cultivation times for P0 and 3D were defined at, but not further restricted than 16 days and 28 days, respectively.
As expected from the separate assessments of ML and 3D cultivation times, the total cultivation time also correlated negatively with clinical outcome (Fig. 1C). In order to identify additive effects of ML and 3D during the manufacturing process, first the individual effects were excluded by eliminating batches that were cultivated over 16 days in P0 and over 28 days in 3D from the data set. In the group of spheroid batches that were cultivated within max 55 days, no correlation could be found between cultivation time and clinical outcome, thus demonstrating that no additive effects of P0 and 3D together were present when batches were cultivated within the newly defined cultivation times (Fig. 1F). The group of batches that have been cultivated longer than 55 days showed a non-responder rate of 52%, whereas the group grown within 55 days showed a rate of 26.1% (Supplementary Table S3). Therefore, the total cultivation time was limited to 55 days and not further restricted.
In order to quantitate a possible difference of the drug product's efficacy if batches have been manufactured using long or shorter cultivation times, these limits were applied retrospectively to the Phase III group of produced batches as described below.
From this point on, only patients that were included in Phase III were further analysed, since this study group enables a comparison to the micro-fracture (MFX) treatment group. In Phase III, 25% of the total patient group did not show a relevant clinical improvement (KOOS>8) to the ACI treatment after 1 year after implantation. The over-representation of batches that were manufactured out of the newly restricted cultivation times in the non-responder groups (66.7%) compared to the responder groups (22.2%), demonstrates again the need to adjust the cultivation times.
In order to test if restriction of cell cultivation times is effective such that improvement of clinical efficacy can be expected, the possible improvement for Phase III clinical trial batches was quantified retrospectively by comparison with the comparator MFX. To do so, patients were divided into two groups. This division is based on the elimination of ACI batches from the Phase III data set as described before. The subgroups consist of spheroid batches that were either produced within the newly defined operational ranges for the process parameter P0, 3D and ML+3D, Subgroup 1, or out of these ranges, in at least one of the cultivation phases, Subgroup 2. The differences in cultivation times between the two subgroups are depicted in Fig. 2.
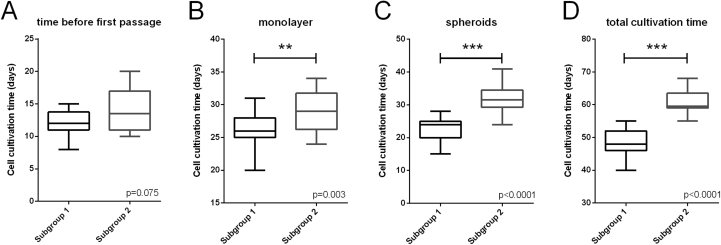
Fig. 2.
Chondrocyte cultivation times in Subgroup 1 and Subgroup 2. Phase III clinical trial batches were divided into two subgroups; subgroup 1 that have been cultivated within the newly defined maximum cultivation times, and subgroup 2 that have been cultivated outside these limits. Spheroid batches were cultivated significantly longer in subgroup 2 considering ML total cultivation time, P = 0.003 (B), 3D, P < 0.001 (C) and total cultivation time in ML and 3D, P < 0.001 (D). In P0 (A), cultivation time was not significantly different between the two subgroups (P = 0.075) because this subgroup contains batches with long 3D cultivation time (>28 days) that have been cultivated within the ORs in P0 (<16 days). Means between groups were analysed using the Mann-Witney t-test (two-sided) with P- value < 0.05 considered significant.
The cultivation times in monolayer and 3D and the total cultivation time (ML and 3D) were significantly longer in the batches of Subgroup 2 (Fig. 2B–D). For P0 however, there was no statistical significant difference between the two subgroups (Fig. 2A). This is due to the fact that some batches with extended 3D cultivation times, and therefore assigned to Subgroup 2, still had P0 cultivation time within the newly defined range.
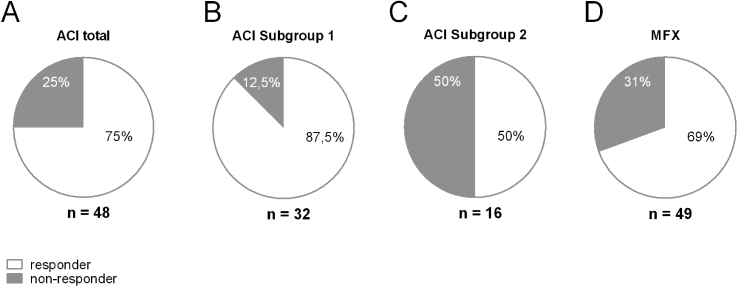
Subgroup 1, was compared with the total group of Phase III patients to find differences in clinical outcome. This subgroup (n = 32) contains a higher amount of responder of 87.5% (Fig. 3B), compared to 75% in the total Phase III group (Fig. 3A). In contrast, subgroup 2 contained only 50% of the patients with a positive clinical outcome after 1 year after implantation (Fig. 3C).
Fig. 3.
Spheroid batches with short cultivation times show improved responder rate. From Phase III clinical trial, the patients that underwent ACI (n = 49) were divided into two subgroups: Subgroup 1 consisting of patients treated with spheroid batches manufactured with restricted cultivation times (n = 32), and Subgroup 2 that were treated with spheroid batches with longer cultivation times (n = 16). A: From the total Phase III group of patients, 75% of the spheroid batches belong to responder patients (KOOS>8). B: In subgroup 1, 87,5% of the patients showed a relevant clinical improvement (KOOS>8), whereas in subgroup 2 (C) only 50% of the patients. D: In the Phase III comparator group (MFX) 69% of the patients showed a clinical improvement after 1 year (KOOS>8).
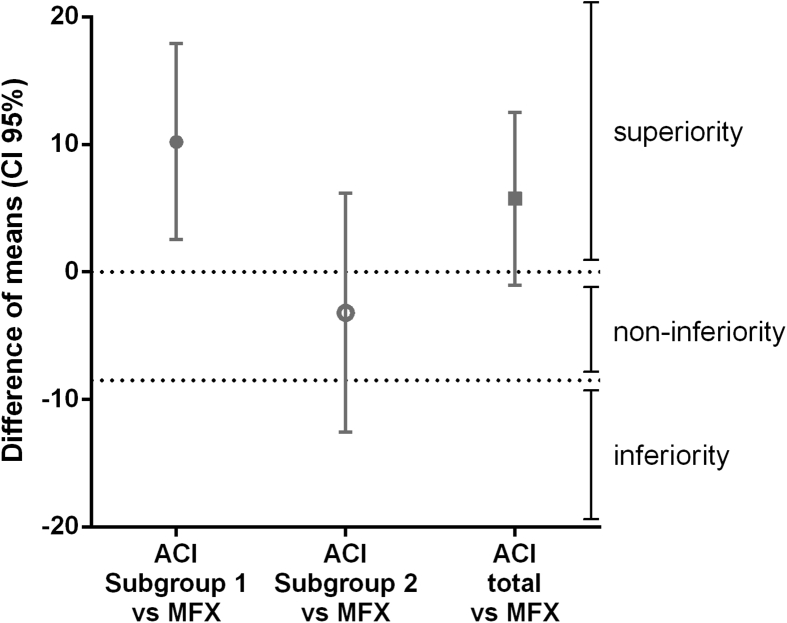
The difference between the KOOS (overall delta) means of the chondrocyte spheroid-based ACI compared to MFX was in favour of the ACI treatment with 5.75 points after 1 year after implantation (unpaired t-test with Welch's correction; Supplementary Table S4). With a confidence interval of 95% (CI 95%), a lower bound of −1.03 thus crossing the non-inferiority limit as defined here at 0 points of the ACI treatment compared to MFX (Fig. 4). In order to address a possible improvement of KOOS-based clinical outcome of the subgroup that has been manufactured within the newly defined operational ranges, the difference of the means between the ACI Subgroup 1 and MFX KOOS scores was calculated. This Subgroup of spheroid batches represents the current commercial manufacturing process of Spherox and a mean difference of 10.21 compared to MFX (CI 95%) and a lower bound of 2.53 (Supplementary Table S4) remained above the non-inferiority limit as defined here at 0 points thus demonstrating superiority over MFX (Fig. 4). The retrospective elimination of a specific group of batches with long cultivation times from the data set results in a higher average KOOS scores in this group.
Fig. 4.
Superiority/non-inferiority analysis of Subgroup 1 and 2. The clinical outcome from patients included in Phase III, 1 year after spheroid-based ACI shows non-inferiority compared to microfracture (MFX). The two subgroups derived from Phase III clinical trial that contains spheroid batches either cultivated within the newly defined ORs, Subgroup 1, or outside the newly defined ORs, Subgroup 2. The means of KOOS overall delta scores of the total group of patients, ACI total (n = 48), Subgroup 1 (n = 32) and Subgroup 2 (n = 16) were compared with the mean KOOS overall delta scores of the MFX treated patients (n = 49), 1 year after treatment. The retrospective selection of spheroid batches that have been manufactured within the more restricted cultivation times results in the selection of patients with a clinical outcome that show superiority over MFX treatment. Difference of means with 95% Confidence Interval was calculated using unpaired t-test with Welch's correction.
4. Discussion
If hyaline cartilage lesions are left untreated they may progress into degeneration of the cartilage tissue which will lead to significant pain and reduced function of the joint and may progress into osteoarthritis (OA) [20]. Patients with cartilage defects up till 10 cm2 benefit from treatment with chondrocyte spheroid-based ACI as demonstrated by Phase II and III clinical trials [[13], [14], [15]]. In the present study that was part of the dossier for EU marketing authorization, clinical efficacy data revealed that the operational ranges used in manufacturing of Spherox (CO.DON AG) needed to be restricted.
At the start of the manufacturing process of chondrocyte spheroids a minimum yield of chondrocytes isolated from a cartilage biopsy is required. This cell number needs to be sufficient from the start to obtain enough cells during monolayer cultivation for the proper dose of chondrocyte spheroids (10–70 spheroids/cm2 defect) within a maximum allowed cell expansion (population doubling level). Since it is an autologous cell-based product, for every batch, the cell yield after digestion of the biopsy is variable. Also the amount of chondrocytes required to produce enough spheroids is variable per batch because of the different defect sizes per patient (data not shown). These two factors are the main causes for variable cultivation times in P0 and during ML cultivation in general. To rule out that the defect size could be responsible for worse clinical outcome rather than the cell cultivation time itself, a correlation analysis was performed, demonstrating no statistical dependency (Spearman's r = −0.1151; p = 0.2108). Moreover, cultivation time in P0 and 3D are both independent of the defect size. Also the number of cells applied per patient did not correlate with clinical outcome (Spearman's r = −0.04857; p = 0.5999).
Statistical analyses revealed a correlation between the operational ranges and clinical outcome and it became clear that cultivation times defined for chondrocytes in monolayer and 3D needed to be more restricted to prevent a negative impact on the product's efficacy. Based on these finding, the commercial manufacturing process has been adjusted to enforce a significant improvement of the drug product's quality and it is expected to improve the product's clinical efficacy and a reduction of non-responder patients. Adjusting the operational ranges will however not completely prevent the occurrence of non-responder patients. Also process-independent factors can impair the efficacy of the ACI treatment with chondrocyte spheroids. In the group of batches, that have been cultivated within the newly restricted cultivation times, that is, with cultivation times that do not correlate with efficacy of the ACI product, still 12.5% of the patients did not show a clinical improvement after treatment. This means that not responding to the ACI treatment is in 12.5% of the patients may not be caused by long cultivation times in ML and 3D but is most likely caused by process-independent factors as discussed in publications regarding other ACI products [17,21]. These factors were not subject of the present study.
The correlation that was found between long cultivation times of chondrocytes and a low KOOS overall change from baseline (KOOS overall delta) after 1 year after implantation suggests a negative impact of the manufacturing process on the efficacy of the ACI product. Indeed, taking out the batches with long cultivation times from the data set resulted in a loss of correlation between cultivation time and KOOS overall change from baseline. In line with the correlation analysis, spheroid batches with long cultivation times result in a four times higher occurrence of non-responder patients (8 out of 16: 50%), 1 year after implantation compared to 12.5% (4 out of 32) non-responder in the group of batches that have been cultivated within the new limits. The higher occurrence of non-responders in the group with longer cultivation times suggests that impaired clinical improvement may be at least partly caused by a suboptimal manufacturing process. From these non-responders the majority (7/8) has been cultivated too long in 3D and only 2 out of 8 in P0. (Of note: one batch out of 8 has been cultivated too long in both P0 and 3D). This means that the highest occurrence among the non-responders is an extended cultivation time in 3D. Extended cultivation times in 3D are not the consequence of cell growth characteristics but are controlled by the coordinated appointments made with the clinic and the patient. Further analyses of the non-responder patients as a group confirms the previous findings and show that batches with long cultivation times are overrepresented in this group compared to the responder group. The most striking difference was again found for 3D cultivation time. 58.3% of the non-responder patient batches have been treated with batches cultivated too long in 3D. This occurrence is much higher compared to the 17.1% found in the responder group. This overrepresentation suggests that long cultivation in 3D forms a high risk to affect the efficacy of the ACI product in the Phase III clinical trial. This impact is cell growth independent, since chondrocytes stop to proliferate after forming cell aggregates in 3D culture [22] and can be avoided by planning the implantation date with the clinic and the patient accordingly. Therefore, a maximum cultivation time of 28 days has been implemented immediately in the commercial process.
A possible cause for loss of efficacy of the spheroid ACI product after prolonged, extensive cultivation could be found in loss of cartilage specific gene expression. From previous studies it is known that during the expansion Phase of chondrocytes in monolayer, the cells tend to de-differentiate and loose the expression of chondrocyte specific proteins like collagen type II and aggrecan [[23], [24], [25], [26], [27]]. Loss of expression of key components during chondrocyte differentiation [28] coincides with increased expression of genes that mark the loss of a chondrocyte phenotype into a more fibroblast phenotype [29]. This phenomenon could therefore negatively affect the efficacy of the drug product [23,24]. Using an in vivo model it was demonstrated that dedifferentiated chondrocytes, caused by extended cultivation times in monolayer, were impaired in cartilage repair and coincides with decreased expression of cartilage specific ECM proteins with increasing passage number [30]. In line with these findings, mRNA levels for collagen type I and II were found to be expressed highly time-dependent, and the increased ratio of type I/type II over time demonstrate loss of the chondrocyte phenotype during ML cultivation [31]. The maintenance of gene expression levels of ECM proteins, when chondrocytes are cultured in micromass suggests that cultivation in 3D, might protect/prevent -at least partly-chondrocytes to lose their phenotype during the first 3 weeks of cultivation, compared to cultivation in ML [32]. These published studies indicate that the loss of efficacy of chondrocyte spheroids by long cultivation times in 3D may be mediated by the loss of expression of chondrocyte specific genes. Although it cannot be excluded that long 3D cultivation times sometimes coincided with impaired efficacy because of inherited impaired cell quality.
Similar to the treatment of cartilage defects with ACI treatment methods like MACI [3], also the treatment with Spherox results in a higher overall KOOS scores as well as a better responder rate of the treated patients compared to MFX, already after one year. Moreover, retrospective assessment of the group of batches produced with restricted cultivation time showed superiority over MFX treatment. It needs to be taken into account though, that Subgroup 2 of Phase III (n = 16) is very small for a statistical relevant power.
Altogether, the overall response-rate in the Phase III trial after treatment with spheroid-based ACI at 1 year follow-up was 75%, whereas the subgroup of patients treated with chondrocyte spheroids manufactured according to the more stringent cultivation times shows a responder rate of 87%.
This underscores the fact that manufacturing of autologous cell-based therapies such as ACI requires the justification of the operational ranges based on clinical data. This enabled to restrict the operational ranges such that the manufacturing process does not affect the ACI product's efficacy. Since dedifferentiation of chondrocytes during the expansion Phase in ML may cause degeneration and loss of chondrocyte phenotype, the efficacy of this cell-based therapy may directly depend on cell cultivation time which may need to be improved. Thus, after implementing the maximum cultivation times, it is expected that the efficacy of the final product will improve and the occurrence of non-responders will be reduced.
Author contributions
G.R. and C.E. conceived the presented concept and performed the analyses including assessment of data. G.R. and C.E. wrote the manuscript with consultation of C.K. C.K., Ph.N., S.F. and W.W. reviewed the manuscript and gave substantial input for improvements. S.F., Ph.N and W.Z. were the clinical study investigators and thus responsible for the conduction of the studies at their respective study site in Germany. W.W. was the coordinating investigator of Phase III clinical trials in Poland.
Role of the funding source
CO.DON AG was sponsor of the Phase II and Phase III clinical trials and supported the submission of the manuscript for publication.
Declaration of Competing Interest
The authors C.E, C.K. and G.R. are employees of CO.DON AG and are involved in the patent portfolio of CO.DON AG. S.F. is a consultant for Arthrex and Bauerfeind. W.Z. has been paid honorary by CO.DON AG for consultant activities. Ph.N. has been paid by CO.DON AG for scientific consultation and expert testimony.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ocarto.2020.100033.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
Figs1.
References
- 1.Brittberg M., Lindahl A., Nilsson A., Ohlsson C., Isaksson O., Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N. Engl. J. Med. 1994;331:889–895. doi: 10.1056/NEJM199410063311401. [DOI] [PubMed] [Google Scholar]
- 2.Brittberg M., Winalski C.S. Evaluation of cartilage injuries and repair. J Bone Joint Surg Am. 2003;85–A(Suppl 2):58–69. doi: 10.2106/00004623-200300002-00008. [DOI] [PubMed] [Google Scholar]
- 3.Saris D., Price A., Widuchowski W., Bertrand-Marchand M., Caron J., Drogset J.O., et al. Matrix-applied characterized autologous cultured chondrocytes versus microfracture: two-year follow-up of a prospective randomized trial. Am. J. Sports Med. 2014;42:1384–1394. doi: 10.1177/0363546514528093. [DOI] [PubMed] [Google Scholar]
- 4.Kon E., Roffi A., Filardo G., Tesei G., Marcacci M. Scaffold-based cartilage treatments: with or without cells? A systematic review of preclinical and clinical evidence. Arthroscopy. 2015;31:767–775. doi: 10.1016/j.arthro.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 5.Kreuz P.C., Muller S., Freymann U., Erggelet C., Niemeyer P., Kaps C., et al. Repair of focal cartilage defects with scaffold-assisted autologous chondrocyte grafts: clinical and biomechanical results 48 months after transplantation. Am. J. Sports Med. 2011;39:1697–1705. doi: 10.1177/0363546511403279. [DOI] [PubMed] [Google Scholar]
- 6.Brittberg M. Cell carriers as the next generation of cell therapy for cartilage repair: a review of the matrix-induced autologous chondrocyte implantation procedure. Am. J. Sports Med. 2010;38:1259–1271. doi: 10.1177/0363546509346395. [DOI] [PubMed] [Google Scholar]
- 7.Steadman J.R., Rodkey W.G., Rodrigo J.J. Microfracture: surgical technique and rehabilitation to treat chondral defects. Clin. Orthop. Relat. Res. 2001:S362–S369. doi: 10.1097/00003086-200110001-00033. [DOI] [PubMed] [Google Scholar]
- 8.Miller B.S., Joseph T.A., Barry E.M., Rich V.J., Sterett W.I. Patient satisfaction after medial opening high tibial osteotomy and microfracture. J. Knee Surg. 2007;20:129–133. doi: 10.1055/s-0030-1248031. [DOI] [PubMed] [Google Scholar]
- 9.Peterson L., Brittberg M., Kiviranta I., Akerlund E.L., Lindahl A. Autologous chondrocyte transplantation. Biomechanics and long-term durability. Am. J. Sports Med. 2002;30:2–12. doi: 10.1177/03635465020300011601. [DOI] [PubMed] [Google Scholar]
- 10.Saris D.B., Vanlauwe J., Victor J., Haspl M., Bohnsack M., Fortems Y., et al. Characterized chondrocyte implantation results in better structural repair when treating symptomatic cartilage defects of the knee in a randomized controlled trial versus microfracture. Am. J. Sports Med. 2008;36:235–246. doi: 10.1177/0363546507311095. [DOI] [PubMed] [Google Scholar]
- 11.Anderer U., Libera J. In vitro engineering of human autogenous cartilage. J. Bone Miner. Res. 2002;17:1420–1429. doi: 10.1359/jbmr.2002.17.8.1420. [DOI] [PubMed] [Google Scholar]
- 12.Bartz C., Meixner M., Giesemann P., Roel G., Bulwin G.C., Smink J.J. An ex vivo human cartilage repair model to evaluate the potency of a cartilage cell transplant. J. Transl. Med. 2016;14:317. doi: 10.1186/s12967-016-1065-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Niemeyer P., Laute V., John T., Becher C., Diehl P., Kolombe T., et al. The effect of cell dose on the early magnetic resonance morphological outcomes of autologous cell implantation for articular cartilage defects in the knee: a randomized clinical trial. Am. J. Sports Med. 2016 doi: 10.1177/0363546516646092. [DOI] [PubMed] [Google Scholar]
- 14.Niemeyer P., Laute V., Zinser W., Becher C., Kolombe T., Fay J., et al. A prospective, randomized, open-label, multicenter, phase III noninferiority trial to compare the clinical efficacy of matrix-associated autologous chondrocyte implantation with spheroid technology versus arthroscopic microfracture for cartilage defects of the knee. Orthop. J. Sports Med. 2019;7 doi: 10.1177/2325967119854442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Becher C., Laute V., Fickert S., Zinser W., Niemeyer P., John T., et al. Safety of three different product doses in autologous chondrocyte implantation: results of a prospective, randomised, controlled trial. J. Orthop. Surg. Res. 2017;12:71. doi: 10.1186/s13018-017-0570-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schubert T., Anders S., Neumann E., Scholmerich J., Hofstadter F., Grifka J., et al. Long-term effects of chondrospheres on cartilage lesions in an autologous chondrocyte implantation model as investigated in the SCID mouse model. Int. J. Mol. Med. 2009;23:455–460. doi: 10.3892/ijmm_00000151. [DOI] [PubMed] [Google Scholar]
- 17.Niemeyer P., Pestka J.M., Salzmann G.M., Sudkamp N.P., Schmal H. Influence of cell quality on clinical outcome after autologous chondrocyte implantation. Am. J. Sports Med. 2012;40:556–561. doi: 10.1177/0363546511428879. [DOI] [PubMed] [Google Scholar]
- 18.Wallenborn M., Petters O., Rudolf D., Hantmann H., Richter M., Ahnert P., et al. Comprehensive high-resolution genomic profiling and cytogenetics of human chondrocyte cultures by GTG-banding, locus-specific FISH, SKY and SNP array. Eur. Cell. Mater. 2018;35:225–241. doi: 10.22203/eCM.v035a16. [DOI] [PubMed] [Google Scholar]
- 19.Roos E.M., Lohmander L.S. The Knee injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health Qual. Life Outcome. 2003;1:64. doi: 10.1186/1477-7525-1-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldring M.B., Marcu K.B. Cartilage homeostasis in health and rheumatic diseases. Arthritis Res. Ther. 2009;11:224. doi: 10.1186/ar2592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gursoy S., Akkaya M., Simsek M.E., Gursoy M., Dogan M., Bozkurt M. Factors influencing the results in matrix-associated autologous chondrocyte implantation: a 2 - 5 Year follow-up study. J. Clin. Med. Res. 2019 doi: 10.14740/jocmr3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caron M.M., Emans P.J., Coolsen M.M., Voss L., Surtel D.A., Cremers A., et al. Redifferentiation of dedifferentiated human articular chondrocytes: comparison of 2D and 3D cultures. Osteoarthritis Cartilage. 2012;20:1170–1178. doi: 10.1016/j.joca.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 23.Chen S., Fu P., Cong R., Wu H., Pei M. Strategies to minimize hypertrophy in cartilage engineering and regeneration. Genes Dis. 2015;2:76–95. doi: 10.1016/j.gendis.2014.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen J.L., Duan L., Zhu W., Xiong J., Wang D. Extracellular matrix production in vitro in cartilage tissue engineering. J. Transl. Med. 2014;12:88. doi: 10.1186/1479-5876-12-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dell'Accio F., De Bari C., Luyten F.P. Molecular markers predictive of the capacity of expanded human articular chondrocytes to form stable cartilage in vivo. Arthritis Rheum. 2001;44:1608–1619. doi: 10.1002/1529-0131(200107)44:7<1608::AID-ART284>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 26.Jakob M., Demarteau O., Schafer D., Hintermann B., Dick W., Heberer M., et al. Specific growth factors during the expansion and redifferentiation of adult human articular chondrocytes enhance chondrogenesis and cartilaginous tissue formation in vitro. J. Cell. Biochem. 2001;81:368–377. doi: 10.1002/1097-4644(20010501)81:2%3C368::aid-jcb1051%3E3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 27.Schulze-Tanzil G., de Souza P., Villegas Castrejon H., John T., Merker H.J., Scheid A., et al. Redifferentiation of dedifferentiated human chondrocytes in high-density cultures. Cell Tissue Res. 2002;308:371–379. doi: 10.1007/s00441-002-0562-7. [DOI] [PubMed] [Google Scholar]
- 28.Mendler M., Eich-Bender S.G., Vaughan L., Winterhalter K.H., Bruckner P. Cartilage contains mixed fibrils of collagen types II, IX, and XI. J. Cell Biol. 1989;108:191–197. doi: 10.1083/jcb.108.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Benya P.D., Shaffer J.D. Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels. Cell. 1982;30:215–224. doi: 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- 30.Lin L., Zhou C., Wei X., Hou Y., Zhao L., Fu X., et al. Articular cartilage repair using dedifferentiated articular chondrocytes and bone morphogenetic protein 4 in a rabbit model of articular cartilage defects. Arthritis Rheum. 2008;58:1067–1075. doi: 10.1002/art.23380. [DOI] [PubMed] [Google Scholar]
- 31.Marlovits S., Hombauer M., Truppe M., Vecsei V., Schlegel W. Changes in the ratio of type-I and type-II collagen expression during monolayer culture of human chondrocytes. J. Bone Joint Surg. Br. 2004;86:286–295. doi: 10.1302/0301-620x.86b2.14918. [DOI] [PubMed] [Google Scholar]
- 32.Dehne T., Schenk R., Perka C., Morawietz L., Pruss A., Sittinger M., et al. Gene expression profiling of primary human articular chondrocytes in high-density micromasses reveals patterns of recovery, maintenance, re- and dedifferentiation. Gene. 2010;462:8–17. doi: 10.1016/j.gene.2010.04.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.