Summary
Objectives
Show the results in pain and functionality, using low-dose radiotherapy in osteoarticular degenerative disorders (OADD). Review of the evidence.
Material and methods
Patients suffering from OADD with no response to other treatments, receive 6Gy in 6 fractions of 1 Gy, each other day, repeating the scheme if necessary. Evaluation of pain based on Visual Analogic Scale, analgesia intake and VonPannewitz score.
Results
Results observed in our series of patients treated with low doses of radiotherapy are similar to those previously published and reinforce the consideration of radiotherapy as an useful option for degenerative musculoskeletal disorders.
Conclusion
Low dose radiotherapy seems to be a good alternative for aged patients suffering from OADD.
Keywords: Low-dose radiotherapy, Benign diseases, Anti-inflammatory effect
1. Introduction
Musculoskeletal disorders represent one of the greatest challenges in medicine. Not only the individuals who suffer them are affected, also national health systems are exposed due to the enormous impact the cause in medical, social and economic terms. Globally, it is estimated that 24% of the general adult population suffers from osteoarthritis, affecting 10% of men and 18% of women above 60 years old in high-income countries. This means pain, stiffness and functional impotence for many activities of daily living. Age, obesity and history of previous joint trauma are the principal risk factors associated with degenerative OA. A WHO report recognized that OA can become the fourth leading cause of disability by 2020 [1]. In Spain, prevalence of OA is 17%, being osteoarthritis of knees and hands the most frequent, affecting 10% and 6% of the population, respectively. We find a higher prevalence in women over men and the economic impact of its treatment reaches 0.5% of GDP, making OA a real sociosanitary problem [2,3].
Degenerative OA can affect any joint, being hands, knees, feet and hips most commonly involved. The pathophysiology of degenerative OA consists of a progressive destruction of the articular cartilage which results in chronic inflammation and other changes that affect the synovial membrane of the periarticular joint, bone and muscle. When osteoarthritis evolves, radiological changes such as loss of joint space, subchondral bone sclerosis and the presence of osteophytes (bone spurs in the margins of the joints) are observed [4]. There is no specific and definitive treatment for degenerative OA. Weight loss, maintaining moderate exercise and physical rehabilitation approaches are some of the conservative measures taken. Analgesics and NSAIDs, Symptomatic slow acting drugs for osteoarthritis (SYSADOAs), corticosteroids, anesthetics and other local injections, have also been proposed for the relief of the symptoms. In the end, a prosthetic replacement of the damaged joint is done. However, none of these options have demonstrated high efficacy, but might have serious side effects (i.e.: gastrointestinal bleeding, kidney disorders, cardiac disorders, etc.) that can even compromise patients' life.
Low-dose radiotherapy has been used for the treatment of different benign conditions, including osteoarticular affections. Nowadays, as we know more about radiotherapy mechanisms of action, and after the publication of guidelines and recommendations about safety and efficacy, there has been a relaunch of low-dose radiation therapy use. Thus, radiotherapy currently represents 10–30% of the daily activity of most departments of Radiation Oncology in Germany, with painful osteoarticular degenerative disorders being the most frequently treated benign pathology [5].
In this paper we present the experience of our centre using low doses of radiotherapy as an alternative treatment for symptomatic degenerative OA.
2. Methods and materials
Adult patients with osteoarticular degenerative diseases refractory to other conventional treatment were offered to enrol our prospective non-randomized study using low-dose radiotherapy delivered to the affected joint. Local Ethics and Clinical Committee approved the study and all the patients signed an informed consent document prior to their inclusion.
2.1. Study endpoints
The primary endpoint was subjective pain relief according to the 10-point Visual Analogic Scale (VAS) (0, no pain; 10, strongest pain), upon the von Pannewitz score (VPS) (painless, markedly improved, slightly improved, or stable). and daily requirements of analgesics (more, equal, less or null) [6].
All patients were evaluated before radiotherapy, immediately after, 8–10 weeks after radiotherapy and at least 3, 6, 9 and 12 months later on.
2.2. Radiation procedures
All patients were treated up to a total dose of 6 Gy delivered in single fractions of 1 Gy every other day, three fractions per week. Radiotherapy was performed maintaining identical quality standards to those used in oncological treatments. A CT-simulation was performed to all patients, using the correct immobilization systems for each location. CTV (Clinical Target Volume) included painful join and surrounding soft tissue; PTV (Planning Target Volume) included CTV and 5 mm isotropic margin. This volumes, nearby organs at risk (guided by ALARA protocol (keep the dose as low as reasonably achievable) and clinical dosimetry were achieved using RayStation® (RaySearch Laboratories AB, Stockholm, Sweden) planning system. Treatments were delivered in an Oncor linear accelerator (Siemens, Erlangen, Germany) using 6 MV photons. Those patients who did not reach a complete response after the treatment, or were subjectively dissatisfied with the relief obtained, received a second course of radiotherapy with identical dose and fractionation 8–12 weeks after first irradiation.
2.3. Statistical analysis
The differences between the parameters studied before and after radiation therapy were calculated for each patient and compared using the Wilcoxon test for non-parametric paired samples [7]. A p value < 0.05 was considered significant for all statistical tests. Statistical analysis was performed using the SPSS 19.0 program for Windows (SPSS Inc., Chicago, IL, USA).
3. Results
Between April 2015 and February 2018, 184 treatments of degenerative osteoarticular disorders were performed in 108 patients in our department. Some patients received treatment in more than one different location and so they have been accounted for differently.
We included 88 (81.5%) women and 20 (18.5%) men with a median age of 64 years (range 30–89 years). The degenerative osteoarticular disorders included in this study were: trochanteric syndrome (20%) gonarthrosis (18%), finger joints osteoarthritis (14%), periarthropathia humeroescapularis (9%), rhizarthrosis (14%), spondyloarthrosis (9%), tibialis tenosynovitis (posterior/anterior) (5%), plantar fasciitis (4%), epicondylopathia humeri (2%) and painful calcinosis (1%). Three percent of treated cases corresponded to disorders of other locations including coxarthrosis and tarsometatarsal joint arthritis.
Characteristics of included patients are detailed in Table 1.
Table 1.
Patients and treatment characteristics.
| N | % | |
|---|---|---|
| Median age: | 64 (30–89) | |
| Gender | ||
| Male | 29 | 15,76 |
| Female | 155 | 84,24 |
| Treated location: | ||
| Finger joint osteoarhritis | 26 | 14,13 |
| Bursitis trochanterica | 37 | 20,12 |
| Gonarthrosis | 33 | 17,93 |
| Periarthropathia humeroescapularis | 16 | 8,7 |
| Plantar fasciitis | 8 | 4,35 |
| Rhizarthrosis | 25 | 13,58 |
| Spondyloarthrosis | 16 | 8,7 |
| Epycondilopathia humeri | 4 | 2,17 |
| Painful calcinosis | 3 | 1,63 |
| Tibial Tenosynovitis | 10 | 5,43 |
| Other | 6 | 3,26 |
| Visual Analogic Scale (VAS) of Pain Before Treatment: | ||
| 0–3 | 1 | 0,54 |
| 4–6 | 32 | 17,39 |
| 7–10 | 151 | 82,07 |
| Total dose: | ||
| 6 Gy (1 Gy x 6) | 88 | 48 |
| 12 Gy [(1 Gy x 6) + (1 Gy x 6)] | 96 | 52 |
In 96 cases (52.17%), a second additional course of radiotherapy was given (total accumulated dose 12 Gy) due to persistence of the symptoms in the first evaluation 8 weeks after first treatment.
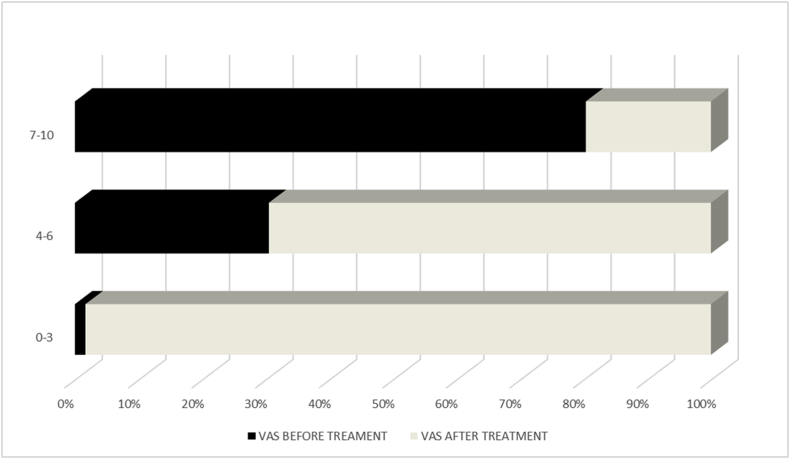
According to the VAS scale, 82.5% of the patients presented with 7–10 score, 17% of patients between 4 and 6 and 0.5% of patients reported pain around 3 or less. . Overall, and with a follow-up of 8 months (range 1–31 months), 91% of patients experienced pain relief. The pain reported according to the VAS scale was 0–3 in 32.6% of the patients, 4–6 in 36.7% and greater or equal to 7 in 20.1% of treated patients. (Table 2). In the comparative analysis, including all the patients, the mean VAS before treatment was 7.43 ± 47 versus mean VAS of 3.3 ± 2.47 after treatment, being this difference statistically significant (p < 0.0001). (Fig. 1). Except EH, all the categories showed significant differences before and after treatment when analysed individually (Table 3).
Table 2.
Response rates and results after radiotherapy (N = 184).
| N | Rate | |
|---|---|---|
| Positive response by location: | ||
| Finger Joint osteoarthritis | 26 | 80,8% |
| Gonarthrosis | 33 | 87,9% |
| Rizarthrosis | 25 | 90,1% |
| Periarthropathia humeroscapularis | 16 | 90,9% |
| Spondyloarthrosis | 16 | 75% |
| Epycondilopathia humeri | 4 | 75% |
| Trochanteric Bursitis | 37 | 97,3% |
| Tibialis Tenosynovitis | 10 | 100% |
| Plantar Fasciitis | 8 | 100% |
| Others | 7 | 87,5 |
| Visual Analogic Scale (VAS) of pain after treatment: | ||
| 0–3 | 60 | 32,61 |
| 4–6 | 73 | 36,67 |
| 7–10 | 37 | 20,11 |
| Von Pannewitz's functional score after treatment: | ||
| Unknown | 38 | 20,66 |
| Stable | 46 | 25 |
| Slightly Improved | 10 | 5,43 |
| Markedly improved | 68 | 36,96 |
| Painless | 22 | 11,96 |
| Analgesic intake after treatment: | ||
| Unknown | 69 | 37,5 |
| No intake | 19 | 26,63 |
| Less | 40 | 21,74 |
| More | 5 | 2,72 |
| Equal | 51 | 27,72 |
Fig. 1.
VAS before and after treatment.
Table 3.
Comparative analysis of mean VAS value before and after LDRT for different locations.
| Mean pre-RT-VAS | Mean post-RT-VAS | ||
|---|---|---|---|
| PHE | 7.75 ± 1.18 | 3.75 ± 2.67 | p = 0.001 |
| EH | 5.5 ± 1.29 | 3.25 ± 3.77 | p = 0.18 |
| FJ | 6.62 ± 1.74 | 3.69 ± 2.25 | p < 0.0001 |
| RA | 7.59 ± 1.6 | 3.48 ± 2.70 | p < 0.0001 |
| BT | 7.75 ± 1.42 | 3.26 ± 2.73 | p < 0.0001 |
| GA | 7.59 ± 1.30 | 3.21 ± 2.21 | p < 0.0001 |
| TT | 8.5 ± 0.70 | 3.10 ± 2.84 | p = 0.007 |
| PF | 7.5 ± 0.75 | 2.75 ± 2.49 | p = 0.011 |
| SA | 7.25 ± 1.23 | 2.81 ± 1.90 | p = 0.001 |
PHE: periarthropathia humeroescapularis; EH: epicondylopathia; FJ: finger joints OA; RA: rhizarthrosis; humeri; BT: bursitis trochanterica; GA: gonarthrosis; TT: tibialis tendinopathy; PF: plantar fasciitis.
Eighty-nine cases with long follow-up (>6 months) have been analysed to assess late response and persistence of response when reached quickly according to the scale proposed by von Pannewitz, and needs of daily analgesia intake. More than two thirds of patients (66.3%) reported improvement of their symptomatology compared to before treatment, being 7.9% of them painfree. Similarly, 46% report that they needed less analgesia intake and 15% did not need to take analgesics of any kind (Table 4).
Table 4.
Characteristics & results of patientes with longer follow-up (N = 89).
| N (%) | Response rates (%) | |
|---|---|---|
| Median age: | 62 (30–89) | |
| Gender: | ||
| Male | 13 (14.6) | 91,3 |
| Female | 76 (85.4) | 73,7 |
| Treated localization: | ||
| Finger joint osteoarthritis | 16 (18) | 85,7 |
| Bursitis trochanterica | 22 (24.7) | 81,8 |
| Gonarthrosis | 10 (11.2) | 90 |
| Periarthropathia humeroescapularis | 10 (11.2) | 100 |
| Plantar fasciitis | 5 (5.6) | 100 |
| Rhizarthrosis | 11 (12.4) | 88,89 |
| Spondyloarthrosis | 3 (3.4) | 100 |
| Epycondilopathia humeri | 4 (4.5) | 66,67 |
| Painful calcinosis | 2 (2.2) | 100 |
| Tibialis Tenosynovitis | 3 (3.4) | 100 |
| Other | 3 (3.4) | 93,8 |
| Visual Analogic Scale (VAS) of pain before treatment: | ||
| 0–3 | 0 | 0 |
| 4–6 | 16 | 17,98 |
| 7–10 | 73 | 81,94 |
| Visual Analogic Scale (VAS) of pain after treatment: | ||
| 0–3 | 52 | 59,48 |
| 4–6 | 27 | 30,34 |
| 7–10 | 10 | 11,24 |
| Von Pannewitz's functional score after treatment: | ||
| Unknown | 6 | 6,74 |
| Stable | 24 | 26,97 |
| Slightly Improved | 2 | 2,25 |
| Markedly improved | 50 | 56,18 |
| Painless | 7 | 7,87 |
| Analgesic intake after treatment: | ||
| Unknown | 18 | 20,22 |
| No intake | 13 | 14,61 |
| Less | 28 | 31,46 |
| More | 4 | 4,49 |
| Equal | 26 | 29,21 |
Both in the immediate and long-term follow-up, no acute or late side effects have been assessed resulting from treatment.
4. Conclusions
Degenerative osteoarticular diseases represent one of the most prevalent problems in developed countries. They mean high sanitary costs and important social difficulties. The efficacy of available treatments is limited and their potential adverse effects can be occasionally life threatening [4] and so alternative solutions are necessary, especially in the elderly multipathologic and polymedicated population [8].
Anti-inflammatory efficacy of low-dose radiotherapy has been confirmed in several experimental models, both in vitro and in vivo. In contrast to high-dose radiotherapy that induces the production of proinflammatory cytokines in the immune system and the endothelial cells, low doses of radiotherapy (0.5–1.5 Gy) act on the cells that participate in the inflammatory response, producing anti-inflammatory effects, including inhibition of the interactions between leukocytes and endothelial cells, a decrease in the production of adhesion molecules to the endothelium, a decrease of mediators of inflammation and less expression of pro-inflammatory cytokines as well as induction of apoptosis of macrophages and polymorphonuclear. Low-dose irradiation also results in a decrease in levels of NO synthetase (iNOS), L- and E-selectins, reactive oxygen species (ROS), tumor necrosis factor alpha (TNF-α) or the secretion of IL-beta 1 in conjunction to an increase in the production and expression of anti-inflammatory cytokines such as the transforming growth factor of anti-inflammatory cytokine β1 (TGF-β1) and apoptosis mediator such as nuclear factor kappa-beta (NF-κB) [[15], [16], [17], [18]]. All these changes result in a local anti-inflammatory environment that would explain the clinical effects of low-dose radiation therapy [19].
Since 1898 [9,10] low dose radiotherapy is known to be effective for pain relief and functionality recovery of the joints [[11], [12], [13], [14]], however there is wide fear related to its possible side effects, in particular, developing a radio-induced tumor. The evidence supporting the risk of radioinduced tumors comes from historical data based on old planning techniques treatments, obsolete equipments and little controlled dosimetry. Radio-induced tumors require a prolonged latency time before their manifestation, something that must be confronted against the usually advanced age of treated OA patients. It has been estimated that the probability of a fatal cancer for a 25 year old individual is about one magnitude, i.e. nine times, higher than that for a 75 year old person, and the group of OA candidates for treatment are usually the oldest [[20], [21], [22], [23]]. More precisely, the lifetime risk of developing a radioinduced basal cell carcinoma (BCC) has been estimated at approximately 0.006% based on 100 cm2 of skin treated with an average dose of 3Gy, below the risk of spontaneous onset of cancer skin throughout life, estimated at 0.2%. Risk of sarcoma development after radiotherapy is well known, although at a very low frequency (0.05%), and extremely rare with doses below 10 Gy [24].
There are marked regional differences regarding its recommendations (Germany and Eastern Europe, 85% vs. North America and Western Europe, 23%), however, from the end of the 20th century and the beginning of the 21st, increasing knowledge about underlying radiobiology and potential risks of the treatment, has awakened a renewed interest in this old modality. In fact, guidelines and recommendations have been published both from DEGRO in Germany and from the RCR in the United Kingdom that justify and support the use of radiotherapy in osteoarticular diseases [[11], [12], [13], [14],25].
During the last two decades, German groups have published results of surveys about patterns-of-care using radiotherapy for the treatment of different degenerative osteoarticular pathologies. In 2000, Seegenschmiedt et al. analysed the patients affected by different non-tumor pathologies treated in Germany between 1994 and 1996. Eighty-eight per cent of German institutions with radiotherapy facilities treated non-tumor diseases. The authors collected data from a total of 20,082 patients of which 63% (12,600) corresponded to degenerative osteoarticular pathology including periarthropathia humeroscapularis [n = 2,711 (22%)], epicondylopathia humeri [n = 1,555 (12%)], plantar/dorsal heel spur [n = 1,382 (11%)], osteoarthrosis of various joints [n = 2,434 (19%)], and other unspecified disorders [n = 4,518 (36%)]. The mean total dose administered was less than 12 Gy (range 3–12 Gy), in 2/3 fractions per week and daily doses of 0.3–1 Gy [26]. In 2014 they rerun the survey in 116 institutions and the have recently published the results. A total of 36,830 patients were treated for benign diseases: 31,925 (87%) for degenerative osteoarticular diseases including peritendinitis humeroscapularis (8%, n = 2,691, epicondylitis humeri (12%, n = 3,788), plantar/dorsal heel spur (33%, n = 10,510), coxarthrosis (7%, n = 2,230), gonarthrosis (8%, n = 2,623), periarthropathia humeroscapularis (8%, n = 2,691), rhizarthrosis (8%, n = 2,440), polyarthrosis (7%, n = 2297), or other unspecified arthrosis (8%, n = 2,655) [27]. By locations, Micke et al. have published patterns of care data for painful heel spurs from 76 different institutions including a total of 7,947 patients at a dose of 2.5 Gy–8.75 Gy (median 6 Gy–1 Gy per fraction). With a median follow-up 28 months (range 3–335), 59% of patients reported total or partial improvement of pain and motility [28]. Likewise, Mucke et al. collected data from 238 institutions in Germany, of which 188 (79%) used low-dose radiation therapy for the treatment of knee osteoarthritis. The authors analysed data from 4,544 patients treated in 2008 with a median dose of 6 Gy (range 3–12 Gy) by 2 or 3 weekly fractions of 1 Gy of median dose (range 0.25–3 Gy). Thirty per cent of patients received a second series of radiotherapy 6–12 weeks after completion of the first. The authors observed symptomatic response in the form of pain relief in 79.5% of patients. No acute complications or late adverse effects of treatment were observed during follow-up [29]. One of the main limitations of most of these studies are the absence of a control group with standard treatment, although almost all of them included patients refractory to other therapies. Only Canylmaz et al. study, randomized 124 patients with plantar fasciitis to receive radiotherapy (6 Gy in 1 Gy fractions) vs. local corticosteroid and anesthetic injections. With a median follow-up of 12.5 months, pain relief was significantly higher in those receiving radiotherapy over local injections, 68% vs. 28%, p < 0.001 [30].
Table 5 shows the results of studies published since year 2000 using low doses of radiotherapy for the treatment of different OA conditions [[30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59]]. Most studies analyze the response to treatment according to the gain in pain relief measured according to the visual analog scale (VAS) after radiotherapy and during follow-up period. In many cases, pain relief was also measured according to the von Pannewitz four-degree scale (VPS). Globally, response rates, including partial and complete response, in terms of pain relief, varies between 59% −98%. In our experience, 91% of the patients experienced some degree of relief in their symptoms being greater in the cases of plantar fasciitis (100%) and trochanteric syndrome (97.3%) and lower in finger joint OA (80, 8%) and spondyloarthrosis (81%). Recently, Micke et al. have published the results observed in 703 patients treated with radiotherapy at low doses for calcaneodynia, achillodynia, painful gonarthrosis, painful bursitis trochanterica, and painful shoulder syndrome. With a median follow-up of 33 months, there was a better effect of treatment for enthesiopathies in comparison with gonathrosis [59].
Table 5.
Results of studies since year 2000.
| Author & year | Type of Study | Disease | N | Age (mean) | Total dose (dose per fraction) | Second treatment | MFU(months) | Response rates | Adverse effect |
|---|---|---|---|---|---|---|---|---|---|
| Glatzel 2001 [31] | Retrospective | PF | 141 | 55 | 6 Gy (1 Gy) | 15% | 30 | Pain relief: 89% | NR |
| Glatzel 2002 [32] | Retrospective | GA | 114 | 64 | 3–6 Gy (median, 6 Gy) (0.5–1 Gy, median 1 Gy) | 15% | 29 | Pain relief 68% | NR |
| Schneider 2004 [33] | Retrospective | PF | 62 | 54 | 5 Gy (0.25 Gy–1 Gy) | 25% | 40 | Pain relief 90% | NR |
| Schultze 2004 [34] | Retrospective | PHE | 94 | 68 | 6 Gy (0.75 Gy) | 4 | Pain relief 59% | NR | |
| Ruppert 2004 [35] | Retrospective | GA:43%, CA:8%, PHE:23%, RA:26% | 73 | 62 | 6 Gy (0.5 Gy)x.2 serious | 100% | 48 | Pain relief: 63% | NR |
| Miszczyk 2005 [36] | Retrospective | PHE | 30 | 59 | 6 Gy (1 Gy) | 0% | 54 | Pain relief: 74% | NR |
| Niewald 2007 [37] | Retrospective | PHE | 141 | 57 | 4–8 Gy (0.5–7 Gy)& Gy (1 Gy):89% | 0 | 47 | Pain relief: 73% | Acute grade 1 epitelitis: 1 patient No late toxicity |
| Muecke 2007 [38] | Randomized | PF | 502 | 58 | 3 Gy (0,5Gy) x 2 series | 17.5% | 26 | Probability of pain-free at 8y: 61% | NR |
| Heyd 2007 [39] | Randomized | PF | 130 | 59.5 | 3 Gy (0.25 Gy) (50%) 6 Gy (1 Gy) (50%) |
31.5% | 6 | Pain relief: 88% | NR |
| Adamietz 2010 [40] | Retrospective | PHE | 102 | 57 | 3 Gy (0.5 Gy)x 2 series | 100% | 18 | Pain relief: 82% | NR |
| Hajtmanvova 2010 [41] | Retrospective | PF | 323 | 56 | 4 Gy (1 Gy) | 44% | 3 | Pain relief: 75% | NR |
| Niewald 2012 [42] | Randomized | PF | 62 | 56 | 0.6 Gy (0.1 Gy) (53%) 6 Gy (1 Gy) (47%) |
0.6 Gy:64% 6 Gy:17% P < 0.001 |
12 | Pain relief: 83% (6 GY) vs. 47% (0.6 Gy) p = 0.001 | NR |
| Ott 2013 [43] | Randomized | AD | 112 | 51 | 3 Gy (0.5 Gy) (53%) 6 Gy (1 Gy) (47%) |
86% | NE | Pain relief: 100% (1 Gy) vs. 77% (0.5 Gy) p = n.s. | NR |
| Hermann 2013 [44] | Randomized | PF | 250 | 53 | 3 Gy (0.5 Gy) (18%) 6 Gy (1 Gy) (72%) |
0% | 11 | Pain relief: 70% | NR |
| Keller 2013 [45] | Randomized | GA | 1037 | 69% > 60y | Total dose: 0.5–10 Gy (median 4 Gy) Dose/fraction: 0.5–1.5 Gy (median 1 Gy) | 36% ≥ 2 series | NE | Pain relief: 79% | NR |
| Koca 2014 [46] | Retrospective | PF | 62 | 57 | 8 Gy (4 Gy) | 0% | 28 | Pain relief 79% | NR |
| Badakhshi 2014 [47] | Retrospective | PF | 171 | 70 | 3 Gy (0 5 Gy) | 17% | 54 | Pain relief 69% | NR |
| Ott 2014 [48] | Randomized | EH | 216 | 50 | 3Gy (0.5 Gy) (52%) 6 Gy (1 Gy) (48%) | 84% | 35 | Pain relief: 90% (1 Gy) vs. 98% (0.5 Gy) p = n.s. | NR |
| Ott 2014 [49] | Randomized | CD | 457 | 55 | 3 Gy (0.5 Gy) (46%) 6 Gy (1 Gy) (54%) | 80% | 32 | Pain relief: 98% | NR |
| Uysal 2014 [50] | Retrospective | PF | 450 | 52 | 8 Gy (4 Gy) | 2% | 12 | Pain relief: 8% 84% (1 Gy) vs 81% (0.5 Gy)p = n.s. | NR |
| Ott 2014 [51] | Retrospective | PHE | 312 | 62 | 3 Gy (l Gy) (51%) 6 Gy (1 Gy) (49%) |
1% | 35 | Pain relief 8%84% (1 Gy) vs 81% (0.5 Gy)p = n.s. | NR |
| Canyilmaz 2015 [30] | Prospective | PF | 124:64 RT injection CE+AN | 53 | 6 Gy (1 Gy) vs Injection CE+AN | RT: 22% Local injection: 28% | 12.5 | Pain relief: RT:66% Injection:28% p < 0.001 |
NR |
| Niewald 2015 [52] | Retrospective | PF | 117 | 57 | & Gy (l Gyx6) vs 6 Gy (0.5 Gyx12) | 0% | 3 | Pain relief: 66% (AD 100%, no difference between arms |
NR |
| Valduvieco 2016 [53] | Retrospective | BT | 60 | 68 | 10 Gy (l Gy) | 28% (0.3 Gy x 10) | 18.5 | Pain relief: 62% | NR |
| Kaltenborn 2016 [54] | Retrospective | RA | 84 | 62 | 6 Gy (l Gy) | 11% | 3 | Pain relief: 63% | NR |
| Micke 2017 [55] | Prospective | AD (n = 8), CD (n = 51), GA (n = 80), BT (n = 27) | 166 | 77 | 6 Gy (0.5–1 Gy) | 8% | 29 | Pain relief: 66% (AD 100%, CD 89.5%, GA 28%, BT 69%) | NR |
| Kaltenbon 2017 [56] | Retrospective | BT | 60 | 62 | 3 Gy (0.5 Gy) (39%) 6 Gy (l Gy) |
35% | 18 | Pain relief: 72% | NR |
| Chauhan 2017 [57] | Prospective | PF | 36 | 40 | 6 Gy (l Gy) | 0% | 12 | Pain relief: 88% | NR |
| Kedizierawski 2017 [58] | Retrospective | PF | 47 | 61 | 6 Gy (l Gy) | 8% | 1 to 129 | Pain relief: 100% | NR |
| Micke 2018 [59] | Prospective | GA (n = 139), BT (n = 70)' | 703 | 63 | 6 Gy (0.5 Gy) (85%) 6 Gy (l Gy) (15%) |
7%; | 33 | Pain relief: 73% | NR |
| Current series | Prospective | PHE(n = 16),EH (n = 4), BT (n = 37),SA (n.l6).GA | 184 | 64 | 6 Gy (1Gy) | 52,17% | 8 | Pain relief: 91% | NR |
PF:plantar fasciitis; PHE: periarthropathia humeroescapularis; AD; achillodyina; CD: calcaneocynia; GA: gonarthrosis; CA: coxarthrosis; RA: rhizarthrosis; EH: epicondylopathiahumeri; BT: bursitistrochanterica; FJOA: finger joint OA; TT: tibialis tendinopathy; CE: corticosteroids; AN: anesthetics; MFU_median follow-up.
As in our practice, most authors deliver doses between 0.5 and 1.0 Gy per fraction in 2–3 weekly fractions up to a total doses of 3–6 Gy. Although it is true that laboratory studies [15,17] demonstrated the maximum anti-inflammatory effect of radiotherapy in the environment of 0.3–0.7 Gy per fraction, the observed clinical results in the randomized trials comparing 6 fractions of 1 Gy versus 6 fractions of 0.1–0.5 Gy favors 1 Gy, although the difference is not statistically significant except when compared against the use of 0.1 Gy fractions [48,49,51,52]. Similarly, in vitro experiments demonstrated that the anti-inflammatory effect of low doses of radiotherapy was maximum at 48 h after irradiation and it was lost after 72 h. This explains why it is recommended to administer the dose in separate fractions every 48–72 h [17]. As in many other studies, those patients in our series who did not reach a complete response or who were subjectively dissatisfied with the relief obtained, received a second course of radiotherapy with identical dose and fractionation 8–12 weeks after first irradiation. The median age in our series is 64 years old (range 30–89). As shown in Table 4, the median age in the vast majority of studies exceeds 50 years, and it is exceptional to include patients under the age of 40 years. Even with the renewed interest in the use of low-dose radiation therapy for OA and the availability of advanced planning and treatment techniques, caution should be taken in young patients, especially in the case of children who should only be treated in emergency situations if no other viable therapeutic alternatives are available. [Read 2007] In a recent review of the state of the art and update of the evidence of radiotherapy in benign osteoarticular processes, Seegenschmiedt et al. conclude that irradiation at low doses in patients resistant to other standard treatments should be performed with a total dose of 3–6 Gy and a fractionation 0.5–1 Gy two/three times a week and otherwise restricted to patients older than 40 years [5].
Delayed onset of analgesic effects low dose radiotherapy has been previously established by different authors and results in a significantly improved long-term efficacy in comparison to the results immediately after radiotherapy [45,55,59]. It has been suggested that this delayed response to radiotherapy would be related to the previously mentioned radiobiological mechanisms that justify its efficacy. In our series, in those patients with more than 6 months of follow-up after treatment, the percentage reporting absence or only mild pain (VAS 0–3) increased from 33% at the end of radiotherapy to the 59.5%, and the percentage of patients with severe pain (VAS 7–10) was reduced from 20% to 11%. In addition, the percentage of patients who reported having improved with treatment according to VPS increased from 53% to 66% with more than 6 months of follow-up with respect to the moment immediately following the end of treatment.
Neither in our series nor in any other paper published since 2000 have shown late complications attributable to treatment, and only Niewald et al. reported the appearance of grade 1 epithelitis in one patient treated of PHE. However, it is advisable to maintain a long follow-up of patients to rule out the appearance of late complications.
All in all, the use of low doses of radiotherapy achieves significant pain relief in patients with different degenerative OA disorders. In addition to the improvement of pain, patients report subjective functional improvement and require less daily analgesia. The relief achieved at the end of the treatment was maintained or even improved during follow-up. All in all, it appears to improve quality of life with no acute or long-term side effects.
5. Contributions
-
•
Conception and design: Ángel Montero, Beatriz Álvarez.
-
•
Analysis and interpretation of the data: Ángel Montero.
-
•
Critical revision of the article for important intellectual content: Carmen Rubio.
-
•
Final approval of the article: Carmen Rubio, Paloma García de la Peña
-
•
Provision of study materials or patients: Francisco Aramburu, Enrique Calvo, Silvia Rodríguez, Rosa Alonso, Jeannette Valero, Ovidio Hernando, Mariola García-Aranda, Mercedes López, Raquel Ciérvide.
-
•
Statistical expertise: Ángel Montero.
-
•
Administrative, technical, or logistic support: Miguel Ángel de la Casa.
-
•
Collection and assembly of data: Beatriz Álvarez.
Ángel Montero (angel.monteroluis@gmail.com) and Beatriz Álvarez (bea.alvarez.rguez@gmail.com) take responsibility for the integrity of the work as a whole, from inception to finished article.
Declaration of Competing Interest
None.
Footnotes
No financial support.
References
- 1.Woolf A.D., Pfleger B. Burden of major musculoskeletal conditions. Bull. World Health Organ. 2003;81(9):646–656. [PMC free article] [PubMed] [Google Scholar]
- 2.Carmona-Terés V., Lumillo-Gutiérrez I., Jodar-Fernández L., Rodriguez-Blanco T., Moix-Queraltó J., Pujol-Ribera E., Berenguera A. Effectiveness and cost-effectiveness of a health coaching intervention to improve the lifestyle of patients with knee osteoarthritis: cluster randomized clinical trial. BMC Muscoskelet. Disord. 2015;16:38. doi: 10.1186/s12891-015-0501-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Loza E., Lopez-Gomez J.M., Abasolo L., Maese J., Carmona L., Batlle-Gualda E. Artrocad Study Group. Economic burden of knee and hip osteoarthritis in Spain. Arthritis Rheum. 2009;61(2):158–165. doi: 10.1002/art.24214. 15. [DOI] [PubMed] [Google Scholar]
- 4.Wieland H.A., Michaelis M., Kirschbaum B.J., Rudolphi K.A. Osteoarthritis - an untreatable disease? Nat. Rev. Drug Discov. 2005;4(4):331–344. doi: 10.1038/nrd1693. Review. Erratum in: Nat Rev Drug Discov. 2005 Jul;4(7):543. [DOI] [PubMed] [Google Scholar]
- 5.Seegenschmiedt M.H., Micke O., Muecke R., the German Cooperative Group on Radiotherapy for Non-malignant Diseases (GCG-BD) Radiotherapy for non-malignant disorders: state of the art and update of the evidence-based practice guidelines. Br. J. Radiol. 2015;88(1051) doi: 10.1259/bjr.20150080. 20150080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Von Pannewitz G. Roentgen therapy for deforming arthritis. Ergebnisse der medizinischen Strahlenforschung. 1933:61–126. [Google Scholar]
- 7.Wilcoxon F. Individual comparisons by ranking methods. Int. Biom. Soc. 1945;1:80–83. [Google Scholar]
- 8.Micke O., Seegenschmiedt M.H., Adamietz I.A., Kundt G., Fakhrian K., Schaefer U., Muecke R. Low-dose radiation therapy for benign painful skeletal disorders: the typical treatment for the elderly patient? Int. J. Radiat. Oncol. Biol. Phys. 2017;98(4):958–963. doi: 10.1016/j.ijrobp.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 9.Sokoloff N. Röntgenstrahlen gegen Gelenkrheumatismus. Fortschr Röntgenstr. 1898;1:209–213. [Google Scholar]
- 10.Stenbek O. On the treatment of rheumatism of chronic arthritis with X-rays. Läk Förh. 1898;1:117. [Google Scholar]
- 11.Reichl B., Block A., Schäfer U., Bert C., Mueller R., Jung H., Rödel F. DEGRO practical guidelines for radiotherapy of non-malignant disorders: Part I: physical principles, radiobiological mechanisms, and radiogenic risk. Strahlenther. Onkol.: Organ der Deutschen Rontgengesellschaft. 2015;191(9):701–709. doi: 10.1007/s00066-015-0865-8. [DOI] [PubMed] [Google Scholar]
- 12.Ott O.J., Niewald M., Weitmann H.D., Jacob I., Adamietz I.A., Schaefer U., German Cooperative Group on Radiotherapy for Benign Diseases (GCG-BD DEGRO guidelines for the radiotherapy of non-malignant disorders. Strahlenther. Onkol. 2015;191(1):1–6. doi: 10.1007/s00066-014-0757-3. [DOI] [PubMed] [Google Scholar]
- 13.Seegenschmiedt M.H., Micke O., Niewald M., Mücke R., Eich H.T., Kriz J., Heyd R. DEGRO guidelines for the radiotherapy of non-malignant disorders. Strahlenther. Onkol. 2015;191:541–548. doi: 10.1007/s00066-015-0818-2. [DOI] [PubMed] [Google Scholar]
- 14.Reinartz G., Eich H.T., Pohl F., German Cooperative Group on Radiotherapy for Benign Diseases (GCG-BD) DEGRO practical guidelines for the radiotherapy of non-malignant disorders–Part IV. Strahlenther. Onkol. 2015;191(4):295–302. doi: 10.1007/s00066-014-0789-8. [DOI] [PubMed] [Google Scholar]
- 15.Rödel F., Keilholz L., Herrmann M., Sauer R., Hildebrandt G. Radiobiological mechanisms in inflammatory diseases of low-dose radiation therapy. Int. J. Radiat. Biol. 2007;83(6):357–366. doi: 10.1080/09553000701317358. [DOI] [PubMed] [Google Scholar]
- 16.Rödel F., Frey B., Manda K., Hildebrandt G., Hehlgans S., Keilholz L., Rödel C. Immunomodulatory properties and molecular effects in inflammatory diseases of low-dose x-irradiation. Front. Oncol. 2012;2:120. doi: 10.3389/fonc.2012.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arenas M., Sabater S., Hernández V., Rovirosa A., Lara P.C., Biete A., Panés J. Anti-inflammatory effects of low-dose radiotherapy. Strahlenther. Onkol. 2012;188(11):975–981. doi: 10.1007/s00066-012-0170-8. [DOI] [PubMed] [Google Scholar]
- 18.Lödermann B., Wunderlich R., Frey S., Schorn C., Stangl S., Rödel F., Frey B. Low dose ionising radiation leads to a NF-κB dependent decreased secretion of active IL-1β by activated macrophages with a discontinuous dose-dependency. Int. J. Radiat. Biol. 2012;88(10):727–734. doi: 10.3109/09553002.2012.689464. [DOI] [PubMed] [Google Scholar]
- 19.Park S.H., Lee J.E. Radiotherapy, a new treatment option for non-malignant disorders: radiobiological mechanisms, clinical applications, and radiation risk. J. Rheum. Dis. 2017;24(2):74–84. [Google Scholar]
- 20.Grant E.J., Brenner A., Sugiyama H., Sakata R., Sadakane A., Utada M., Preston D.L. Solid cancer incidence among the Life Span Study of atomic bomb survivors: 1958–2009. Radiat. Res. 2017;187(5):513–537. doi: 10.1667/RR14492.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jansen J.T.M., Broerse J.J., Zoetelief J., Klein C., Seegenschmiedt H.M. Estimation of the carcinogenic risk of radiotherapy of benign diseases from shoulder to heel. Radiother. Oncol. 2005;76(3):270–277. doi: 10.1016/j.radonc.2005.06.034. [DOI] [PubMed] [Google Scholar]
- 22.Trott K.R., Kamprad F. Estimation of cancer risks from radiotherapy of benign diseases. Strahlenther. Onkol. 2006;182(8):431–436. doi: 10.1007/s00066-006-1542-8. [DOI] [PubMed] [Google Scholar]
- 23.Mazonakis M., Damilakis J. Cancer risk after radiotherapy for benign diseases. Phys. Med.: Eur. J. Med. Plants. 2017;42:285–291. doi: 10.1016/j.ejmp.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 24.McKeown S.R., Hatfield P., Prestwich R.J., Shaffer R.E., Taylor R.E. Radiotherapy for benign disease; assessing the risk of radiation-induced cancer following exposure to intermediate dose radiation. Br. J. Radiol. 2015;88(1056):20150405. doi: 10.1259/bjr.20150405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.A Review of the Use of Radiotherapy in the UK for the Treatment of Benign Clinical Conditions and Benign Tumors. The Royal College of Radiologists; London: 2015. [Google Scholar]
- 26.Seegenschmiedt M.H., Katalinic A., Makoski H.B., Haase W., Gademann G., Hassenstein E. Radiation therapy for benign diseases: patterns of care study in Germany. Int. J. Radiat. Oncol. Biol. Phys. 2000;47(1):195–202. doi: 10.1016/s0360-3016(99)00537-4. [DOI] [PubMed] [Google Scholar]
- 27.Kriz J., Seegenschmiedt M.H., Bartels A., Micke O., Muecke R., Schaefer U., Eich H.T. Updated strategies in the treatment of benign diseases–a patterns of care study of the German cooperative group on benign diseases. Adv. Radiat. Oncol. 2018;3(3):240–244. doi: 10.1016/j.adro.2018.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Micke O., Seegenschmiedt M.H. Radiotherapy in painful heel spurs (plantar fasciitis)—results of a national patterns of care study. Int. J. Radiat. Oncol. Biol. Phys. 2004;58(3):828–843. doi: 10.1016/S0360-3016(03)01620-1. [DOI] [PubMed] [Google Scholar]
- 29.Mücke R., Seegenschmiedt M.H., Heyd R., Schäfer U., Prott F.J., Glatzel M., Micke O. Radiotherapy in painful gonarthrosis. Results of a national patterns-of-care study. Strahlenther. Onkol.: Organ der Deutschen Rontgengesellschaft. 2010;186(1):7–17. doi: 10.1007/s00066-009-1995-7. [DOI] [PubMed] [Google Scholar]
- 30.Canyilmaz E., Canyilmaz F., Aynaci O., Colak F., Serdar L., Uslu G.H., Yoney A. Prospective randomized comparison of the effectiveness of radiation therapy and local steroid injection for the treatment of plantar fasciitis. Int. J. Radiat. Oncol. Biol. Phys. 2015;92(3):659–666. doi: 10.1016/j.ijrobp.2015.02.009. [DOI] [PubMed] [Google Scholar]
- 31.Glatzel M., Bäsecke S., Krauß A., Fröhlich D. Radiotherapy of the painful plantar heel spur. Benig News. 2001;2:18–19. [Google Scholar]
- 32.Glatzel M., Frohlich D., Kraub A., Basecke S. Results of radiotherapy for. gonarthrosis. Benign News. 2002;3:9–11. 43. [Google Scholar]
- 33.Schneider O., Stückle C.A., Bosch E., Gott C., Adamietz I.A. Effectiveness and prognostic factors of radiotherapy for painful plantar heel spurs. Strahlenther. Onkol. 2004;180(8):502–509. doi: 10.1007/s00066-004-1204-7. [DOI] [PubMed] [Google Scholar]
- 34.Schultze J., Schlichting G., Galalae R., Koltze H., Kimmig B. Results of radiation therapy in periarthritis humeroscapularis. Rontgenpraxis; Zeitschrift fur radiologische Technik. 2004;55(4):160–164. [PubMed] [Google Scholar]
- 35.Ruppert R., Seegenschmiedt M.H., Sauer R. Radiotherapy of osteoarthritis. Indication, technique and clinical results. Der Orthopäde. 2004;33(1):56–62. doi: 10.1007/s00132-003-0568-1. [DOI] [PubMed] [Google Scholar]
- 36.Miszczyk L., Woźniak G., Walichiewicz P., Spindel J. The effectiveness of radiotherapy for painful humeroscapular periarthritis (PHS). Nowotwory. J. Concol. 2005;55(1) 49-49. [Google Scholar]
- 37.Niewald M., Fleckenstein J., Naumann S., Ruebe C. Long-term results of radiotherapy for periarthritis of the shoulder: a retrospective evaluation. Radiat. Oncol. 2007;2(1):34. doi: 10.1186/1748-717X-2-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muecke R., Micke O., Reichl B., Heyder R., Prott F.J., Seegenschmiedt M.H., Kundt G. Demographic, clinical and treatment related predictors for event-free probability following low-dose radiotherapy for painful heel spurs–a retrospective multicenter study of 502 patients. Acta Oncol. 2007;46(2):239–246. doi: 10.1080/02841860600731935. [DOI] [PubMed] [Google Scholar]
- 39.Heyd R., Tselis N., Ackermann H., Röddiger S.J., Zamboglou N. Radiation therapy for painful heel spurs. Strahlenther. Onkol. 2007;183(1):3–9. doi: 10.1007/s00066-007-1589-1. [DOI] [PubMed] [Google Scholar]
- 40.Adamietz B., Schulz-Wendtland R., Alibek S., Uder M., Sauer R., Ott O., Keilholz L. Calcifying tendonitis of the shoulder joint. Strahlenther. Onkol. 2010;186(1):18–23. doi: 10.1007/s00066-009-2025-5. [DOI] [PubMed] [Google Scholar]
- 41.Hajtmanová E., Kinclová I., Kostková L., Hajtman A., Péc M. Low-dose radiotherapy in the treatment of plantar fasciitis. Klin. Onkol. 2010;23(2):104–110. [PubMed] [Google Scholar]
- 42.Niewald M., Seegenschmiedt M.H., Micke O., Graeber S., Muecke R., Schaefer V., Ruebe C. Randomized, multicenter trial on the effect of radiation therapy on plantar fasciitis (painful heel spur) comparing a standard dose with a very low dose: mature results after 12 months' follow-up. Int. J. Radiat. Oncol. Biol. Phys. 2012;84(4):e455–e462. doi: 10.1016/j.ijrobp.2012.06.022. [DOI] [PubMed] [Google Scholar]
- 43.Ott O.J., Jeremias C., Gaipl U.S., Frey B., Schmidt M., Fietkau R. Radiotherapy for achillodynia. Strahlenther. Onkol. 2013;189(2):142–146. doi: 10.1007/s00066-012-0240-y. [DOI] [PubMed] [Google Scholar]
- 44.Hermann R.M., Meyer A., Becker A., Schneider M., Reible M., Carl U.M., Nitsche M. Effect of field size and length of plantar spur on treatment outcome in radiation therapy of plantar fasciitis: the bigger the better? Int. J. Radiat. Oncol. Biol. Phys. 2013;87(5):1122–1128. doi: 10.1016/j.ijrobp.2013.08.042. [DOI] [PubMed] [Google Scholar]
- 45.Keller S., Müller K., Kortmann R.D., Wolf U., Hildebrandt G., Liebmann A., Baaske D. Efficacy of low-dose radiotherapy in painful gonarthritis: experiences from a retrospective East German bicenter study. Radiat. Oncol. 2013;8(1):29. doi: 10.1186/1748-717X-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koca T., Aydın A., Sezen D., Başaran H., Karaca S. Painful plantar heel spur treatment with Co-60 teletherapy: factors influencing treatment outcome. SpringerPlus. 2014;3(1):21. doi: 10.1186/2193-1801-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Badakhshi H., Buadch V. Low dose radiotherapy for plantar fasciitis. Treatment outcome of 171 patients. Foot. 2014;24(4):172–175. doi: 10.1016/j.foot.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 48.Ott O.J., Hertel S., Gaipl U.S., Frey B., Schmidt M., Fietkau R. The Erlangen Dose Optimization trial for low-dose radiotherapy of benign painful elbow syndrome. Strahlenther. Onkol. 2014;190(3):293. doi: 10.1007/s00066-013-0504-1. [DOI] [PubMed] [Google Scholar]
- 49.Ott O.J., Jeremias C., Gaipl U.S., Frey B., Schmidt M., Fietkau R. Radiotherapy for benign calcaneodynia. Strahlenther. Onkol. 2014;190(7):671–675. doi: 10.1007/s00066-014-0618-0. [DOI] [PubMed] [Google Scholar]
- 50.Uysal B., Beyzadeoglu M., Sager O., Demıral S., Gamsız H., Dıncoglan F., Dırıcan B. Role of radiotherapy in the management of heel spur. Eur. J. Orthop. Surg. Traumatol. 2015;25(2):387–389. doi: 10.1007/s00590-014-1482-4. [DOI] [PubMed] [Google Scholar]
- 51.Ott O.J., Hertel S., Gaipl U.S., Frey B., Schmidt M., Fietkau R. The Erlangen dose optimization trial for radiotherapy of benign painful shoulder syndrome. Strahlenther. Onkol. 2014;190(4):394. doi: 10.1007/s00066-013-0520-1. [DOI] [PubMed] [Google Scholar]
- 52.Niewald M., Holtmann H., Prokein B., Hautmann M.G., Rösler H.P., Graeber S., Fleckenstein J. Randomized multicenter follow-up trial on the effect of radiotherapy on painful heel spur (plantar fasciitis) comparing two fractionation schedules with uniform total dose: first results after three months' follow-up. Radiat. Oncol. 2015;10(1):174. doi: 10.1186/s13014-015-0471-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Valduvieco I., Biete A., Moreno L.A., Gallart X., Rovirosa A., Saez J., Peris P. Is anti-inflammatory radiotherapy an effective treatment in trochanteritis? Br. J. Radiol. 2016;90(1069):20160520. doi: 10.1259/bjr.20160520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kaltenborn A., Bulling E., Nitsche M., Carl U.M., Hermann R.M. The field size matters: low dose external beam radiotherapy for thumb carpometacarpal osteoarthritisRelevanz der Feldgröße in der Reizbestrahlung bei Rhizarthrose. Strahlenther. Onkol. 2016;192(8):582–588. doi: 10.1007/s00066-016-0995-7. [DOI] [PubMed] [Google Scholar]
- 55.Micke O., Seegenschmiedt M.H., Adamietz I.A., Kundt G., Fakhrian K., Schaefer U., Muecke R. Low-dose radiation therapy for benign painful skeletal disorders: the typical treatment for the elderly patient? Int. J. Radiat. Oncol. Biol. Phys. 2017;98(4):958–963. doi: 10.1016/j.ijrobp.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 56.Kaltenborn A., Carl U.M., Hinsche T., Nitsche M., Hermann R.M. Low-dose external beam radiotherapy for greater trochanteric pain syndrome. Strahlenther. Onkol. 2017;193(4):260–268. doi: 10.1007/s00066-016-1071-z. [DOI] [PubMed] [Google Scholar]
- 57.Chauhan A., Verm Y., Sangwan S., Kumar S., Paramjit D.K. Efficacy of external beam radiotherapy for the management of refractory plantar fasciitis: a prospective study. Int. J. Curr. Res. 2017;9:51230–51236. [Google Scholar]
- 58.Kedzierawski Stando P., Paweł R.M. Retrospective evaluation of the effectiveness of radiotherapy in patients with plantar fascitis (heel spurs) Rep. Pract. Oncol. Radiother. 2017;22:209–211. doi: 10.1016/j.rpor.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Micke O., Ugrak E., Bartmann S., Adamietz I.A., Schaefer U., Bueker R., Muecke R. Radiotherapy for calcaneodynia, achillodynia, painful gonarthrosis, bursitis trochanterica, and painful shoulder syndrome-Early and late results of a prospective clinical quality assessment. Radiat. Oncol. 2018;13(1):71. doi: 10.1186/s13014-018-1025-y. [DOI] [PMC free article] [PubMed] [Google Scholar]