Abstract
Objective
Arthropathy is a major clinical problem in patients with hemochromatosis, the most common genetic disorder of iron overload. The pathological features of hemochromatosis arthropathy (HA) are heterogeneous and its specific nature remains unknown. One important drawback is the lack of proper in vitro models. The aim of the present study was to set up a model to investigate the biological response of cartilage to iron exposure.
Design
Bovine articular cartilage explants were incubated with ferric citrate for up to 9 days. We evaluated chondrocyte viability, iron deposition, and biomarkers of cartilage degradation in the conditioned medium.
Results
Iron accumulated within chondrocytes, which was associated with programmed cell death through chondroptosis. Iron treatment increased the release of sulfated glycosaminoglycans (sGAG), a component of the extracellular matrix, into the medium (p=0.0189). This was dependent on the presence of viable chondrocytes and was associated with increased activity of matrix-degrading metalloproteinases (MMP) (pro/active MMP-9, p=0.0317; pro MMP-2, p=0.0092; active MMP-2, p=0.0288). Co-treatment with the broad MMP/aggrecanase inhibitor prinomastat reduced iron-mediated sGAG release (0.02 μM, p=0.0425; 2 μM, p=0.0014), confirming that iron induces sGAG release via the activation of catabolic enzymes. Notably, iron-treated cartilage continued to release an increased amount of sGAG into the medium for 6 days after termination of the ferric citrate treatment (p=0.0259).
Conclusions
Iron triggers the early stages of cartilage degeneration. Removal of iron exposure does not prevent further damage to the cartilage, thus providing a possible explanation why HA is not prevented after iron depletion by phlebotomy treatment.
Keywords: Iron, Chondrocyte, Hemochromatosis, Arthropathy, Glycosaminoglycan, Metalloproteinase
1. Introduction
HFE related hemochromatosis (HFE-H) is a common genetic disorder of iron overload that is associated with homozigosity for the p.Cys282Tyr variant in the homeostatic iron regulator (HFE) [1]. The most severe manifestations of the disease, due to the accumulation of iron in parenchymal organs such as liver, pancreas and heart, can be prevented by iron depleting phlebotomy treatment [1,2]. In contrast, hemochromatosis arthropathy (HA), which occurs in up to two thirds of HFE-H patients, is not prevented or reverted by phlebotomy treatment and, in some cases, it is even aggravated [3]. Therefore, arthropathy is currently the main cause of morbidity and poor quality of life in HFE-H patients [4], which demands for a better understanding of its etiopathogenesis.
Clinically, HA is a chronic progressive condition that shows a preference for the second and third metacarpophalangeal joint, but large joints of the hip, knee, ankle, shoulder and elbow can also be affected [4]. Symptoms are similar to those reported in idiopathic osteoarthritis (OA), including stiffness, pain on movement and minimal joint swelling. Likewise, the radiologic features include cartilage degeneration, subchondral sclerosis with joint space narrowing, chondrocalcinosis, deposition of calcium pyrophosphate dehydrate crystals, osteoporosis, and presence of osteophytes. Inflammatory reactions are not common and, when present, tend to be mild [4,5]. Histological features are also reminiscent of OA, but neutrophil infiltration is increased in HA and appears to be associated with iron deposition in joints [6].
Iron deposits are found in the synovium and cartilage of some patients [[7], [8], [9]]. However, whether and how iron deposition in different tissues contributes to articular damage remains elusive. Most of the information concerning joint pathology in HA originated from the observation of surgical specimens derived at the time of joint replacement surgery, and is thus representative of advanced disease [4]. There has been a lack of experimental in vitro or ex vivo models that allow the study of early osteoarticular modifications.
The present study aimed at setting up a model to determine whether exposure to excess inorganic iron per se initiates cartilage degeneration. We incubated bovine articular cartilage explants in the presence of ferric citrate and investigated the effects of iron exposure on chondrocyte viability and on specific biomarkers of cartilage degradation. Hydroxyproline and sulfated glycosaminoglycans (sGAG) released from the cartilage to the conditioned medium were quantified and used as a measure of collagen type II and proteoglycan breakdown, respectively. We also measured cartilage oligomeric matrix protein (COMP), an extracellular matrix (ECM) protein that is released into synovial fluid upon cartilage erosion, and the activity of two matrix-degrading metalloproteinases (MMP).
2. Materials and methods
2.1. Reagents
All chemicals and reagents were purchased from Sigma-Aldrich, unless otherwise stated.
2.2. Preparation and culture of bovine articular cartilage explants
Bovine articular cartilage was obtained from the proximal interphalangeal joint of skeletally mature bovines from the local abattoir (Carnes Landeiro, Vila Nova de Famalicão, Portugal), following the directives of the national authority Direção Geral de Alimentação e Veterinária (license number N.12.010.UDER). Joints were aseptically opened and explants were created using a 6 mm diameter biopsy punch (Kruuse) and a scalpel. Explants were weighed and randomly distributed into the wells of 24-well plates. In each well (representing the experimental unit), 3 explants were cultured with 1 mL Dulbecco’s modified Eagle’s medium (DMEM) with high glucose and GlutaMax (Gibco), 5% (v/v) fetal bovine serum (FBS) (Biowest), 1% penicillin-streptomycin and 1% amphotericin B. At the end of 24h, the culture medium was replaced by fresh medium containing 50 μM ferric citrate (Fisher Scientific) or 50 μM sodium citrate (Fisher Scientific), and explants were further incubated for 9 days at 37 °C in a 5% CO2 incubator. The treatments did not alter the pH of culture medium. In some experiments, cartilage explants were treated with ferric citrate in the presence or absence of an iron chelator (desferrioxamine, DFO), an endoplasmic reticulum (ER) stress/chondroptosis inhibitor (tauroursodeoxycholic acid, TUDCA), a pan-caspase inhibitor (Z-VAD-FMK, Santa Cruz Biotechnology), a MMP/aggrecanase inhibitor (prinomastat hydrochloride, AG-3340) or two ferroptosis inhibitors (ferrostatin-1, Fer-1; α-tocopherol). Cartilage was also incubated with ER stress/chondroptosis inducers (tunicamycin or thapsigargin, Santa Cruz Biotechnology) or with ferroptosis inducers (erastin; RSL-3; buthionine-sulfoximine, BSO) for 9 days. Some explants underwent three cycles of freeze/thaw to become metabolically inactive [10,11]. Medium was refreshed every third day and conditioned medium was collected and stored at −80 °C for further analysis. At the end of the culture, explants were collected, weighed once again and fixed for histological analysis.
2.3. Cell viability assay
Chondrocyte viability was determined using the live-dead assay kit (Invitrogen). Briefly, cartilage explants were cut in small fragments, washed with sterile phosphate buffered saline (PBS) and stained with calcein acetoxymethyl (AM)/ethidium homodimer-1. Fluorescence was recorded immediately with a Nikon Eclipse E400 microscope using fluorescein isothiocyanate (FITC)/Texas red filter.
2.4. Culture of SW982 synovial cell line
Human SW982 synovial cells were obtained from the American Type Culture Collection (ATCC HTB93) and cultured with DMEM, 10% (v/v) FBS and 1% penicillin-streptomycin at 37 °C in a 5% CO2 incubator. For the experiments, cells were seeded at 3 × 104 cells/cm2 onto cell culture inserts (polyethylene terephthalate track-etched membrane with 3 μm pore size, Corning). Cells were allowed to attach for 24h, and cell culture inserts were transferred to a 24-well culture plate containing bovine cartilage explants.
2.5. Quantification of sGAG
sGAG released from the cultured cartilage explants into the medium were quantified using the dimehtylene blue (DMB)-binding assay. Briefly, 10 μL conditioned medium was applied to microtiter 96-well plates (Sarstedt). After addition of 200 μL DMB solution (16 mg/L DMB, 2.37 g/L NaCl, 3.04 g/L glycine, at pH=3), absorption was read in a microplate reader (BioTek), with baseline subtraction of the negative peak at OD 590 nm from the reading at OD 530 nm. A dilution series of chondroitin sulfate from shark cartilage was used to generate a standard curve. sGAG release was corrected for explant weight and cumulatively expressed as a fold increase relatively to the amount of sGAG released in the corresponding well during the 24h prior to treatment. Alternatively, we calculated the sGAG release rate for each sample as the slope of the linear regression of cumulative release vs. time, expressed as the amount of sGAG released (μg), per cartilage weight (mg), per day (μg.mg−1.day−1).
2.6. Quantification of hydroxyproline
Levels of hydroxyproline released into the medium were quantified with the hydroxyproline assay kit. Medium samples were concentrated by speedvac (Savant) overnight and subsequently resuspended in ultrapure water. Following addition of 12 M HCl, samples were incubated at 120 °C for 3h. After cooling to room temperature, samples were centrifuged at 13,000×g for 10 min. The supernatant was transferred into a microtiter 96-well plate and evaporated through incubation at 60 °C for 1h. A mixture of chloramine T/oxidation buffer and 4-(dimethylamino)benzaldehyde was added to the samples. Absorbance was read at 560 nm. Hydroxyproline release was corrected for explant weight and cumulatively expressed as a fold increase relatively to the amount released in the corresponding well during the 24h prior to treatment.
2.7. Quantification of COMP
The levels of COMP released into the culture medium were quantified with the bovine COMP enzyme-linked immunosorbent assay (ELISA) kit (MyBioSource), according to the manufacturer’s instructions.
2.8. Gelatine zymography
MMP activity was determined by zymography using 0.1% gelatine as a substrate in 10% sodium dodecyl sulfate (SDS) polyacrylamide gels. Protein concentration in conditioned media was estimated with the protein quantification kit (BioRad). Samples of conditioned media (containing 16 μg of protein) were diluted with sterile PBS and mixed with 3 × 0.25 M Tris at pH=6.8 containing 10% (w/v) SDS, 4% (w/v) sucrose and 2% (w/v) bromophenol blue. The mixture was loaded on the zymography gels and run at 80 V for 3h. Gels were washed twice with 2% (v/v) triton X-100 and incubated in MMP buffer (10 mM CaCl2, 0.02% NaN3, 50 mM Tris-HCl, at pH=7.5) for 16 h at 37 °C and 50 rpm. After staining with 0.1% coomassie blue R-250 (Thermo Scientific) in 40% (v/v) methanol and 10% (v/v) acetic acid, gels were recorded in a calibrated GS800 densitometer (BioRad). Band intensity was determined with Image J software freely available at https://imagej.nih.gov/ij/.
2.9. Histological analyses
Following fixation in 10% (v/v) buffered neutral formalin (Bio-optical), cartilage explants were embedded in paraffin. Following deparaffinization and hydration, 4 μm-thick sections were stained with Perls’ Prussian blue reaction for ferric iron and counterstained with nuclear fast red.
2.10. Terminal deoxynucleotidyltransferase-mediated dUTP nick end labelling (TUNEL) assay
Bovine cartilage explants were fixed in 10% (v/v) buffered neutral formalin. Following incubation with proteinase K (Ambion) at 60 °C for 30 min, 4-μm thick tissue sections were stained with fluorescein in situ cell death detection kit (Roche), according to the manufacturer’s instructions. Cells were counterstained with 4′,6-diamino-2-phenylindole (DAPI). Samples were analysed in a wide-field fluorescence microscope (Zeiss AxioImager Z1, Carl Zeiss). The % of TUNEL-positive cells was determined manually, in a blind manner, using Image J.
2.11. Transmission electron microscopy (TEM)
Cartilage explants were cut in small fragments and fixed by immersion in 2.5% glutaraldehyde and 2% paraformaldehyde in 0.1 M sodium cacodylate buffer (pH=7.4) solution. After post-fixation in 2% osmium tetroxide for 2h, tissues were incubated with 1% uranyl acetate overnight, dehydrated, and embedded in epon. Ultrathin sections (50 nm) stained with uranyl acetate and lead citrate were visualized with a JEM 1400 electron microscope (JEOL) operated at 80 kV. Electron micrographs were captured with an Orius CCD digital camera (Gatan).
2.12. Statistical analyses
Data were analysed using GraphPad Prism 6.07 (GraphPad Software) and the averaged values are presented as mean and standard deviation. All data sets passed the Shapiro-Wilk normality test with 0.05 significance level. To minimize variation between samples from different donors, differences among two group means were compared by paired Student’s t-test, and differences among multiple group means were compared by one-way or two-way repeated measures analysis of variance (ANOVA) with Sidak’s multiple comparisons test, unless stated otherwise. Differences were considered significant when p<0.05.
3. Results
To study the impact of iron loading on cartilage integrity, bovine articular cartilage explants were allowed 24h in culture medium to stabilize and subsequently incubated for up to 9 days with medium supplemented with 50 μM ferric citrate or sodium citrate (control). As outlined in Fig. 1, the medium was replaced every 3 days with fresh medium, and stored for quantification of biomarkers of cartilage degradation and MMP activity. At the end of the experiments, cartilage explants were collected to determine chondrocyte viability and iron accumulation. Preliminary experiments showed that, in the current experimental conditions, control chondrocytes remained mostly viable for at least 12 days in culture (Supplementary Fig. 1A). Also, after 9 days in culture, we observed no significant variation in the weight of cartilage explants that received either treatment when compared to untreated cartilage at 24h after collection (Supplementary Fig. 1B).
Fig. 1.
Experimental design overview. Bovine articular cartilage explants were prepared, immediately transferred to culture medium and incubated at 37 °C in a 5% CO2 incubator. At the end of 24h, the culture medium was replaced by fresh medium containing 50 μM ferric citrate or sodium citrate (control), and explants were further incubated for 9 days. Medium was refreshed every third day and conditioned medium was collected for the measurement of biomarkers of cartilage degradation and MMP activity. At the end of the culture, explants were collected for analyses of cell viability and tissue iron accumulation.
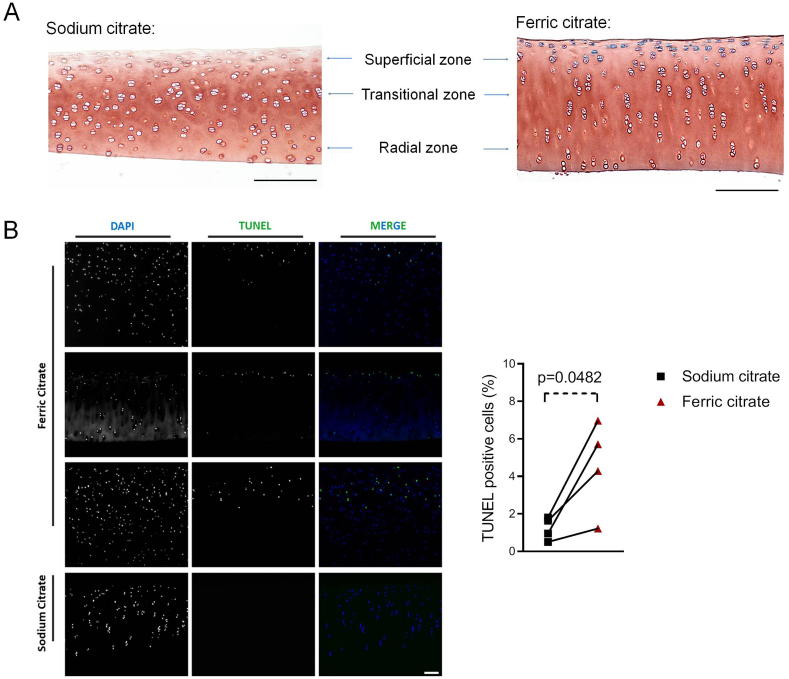
To determine whether ferric citrate was taken up by chondrocytes and whether these cells were able to accumulate iron intracellularly, cartilage sections were stained with Perls’ Prussian blue stain for ferric iron. As expected, control cartilage explants were devoid of iron deposits. In contrast, incubation with ferric citrate for 9 days led to marked intracellular iron loading of the chondrocytes present in the superficial zone of cartilage (Fig. 2A).
Fig. 2.
Iron treatment leads to intracellular iron deposition and increased death of superficial chondrocytes. Cartilage explants were treated for 9 days with 50 μM ferric citrate or sodium citrate (control). A) Cartilage coronal sections were stained with Perls’ Prussian blue stain for ferric iron. Intracellular iron deposits were found exclusively in ferric citrate-treated cartilages, especially in chondrocytes located in the superficial zone. The image is representative of 3 independent experiments. Bar = 200 μm B) Chondrocyte viability as assessed by the TUNEL assay. A significant increase in the percentage of dead (TUNEL-positive) cells was observed in iron-treated cartilage explants incubated with ferric citrate, when compared to control. The graph shows the change within the same experiment, and the magnitude of this change was assessed with the paired Student’s t-test (n=4 independent experiments). Bar=100 μm.
Since excess iron is suggested to cause chondrocyte death in vivo [5], we evaluated the impact of iron accumulation on chondrocyte DNA fragmentation with the TUNEL assay. A significant increase in dead (TUNEL-positive) cells was observed in cartilage explants incubated with ferric citrate for 9 days, when compared to control. TUNEL-positive cells were also found in the cartilage superficial zone, closely matching the distribution pattern of iron-loaded cells (Fig. 2B). This suggests that iron loading induced chondrocytes’ death.
In OA, chondrocytes have been reported to die by classical apoptosis and/or chondroptosis [12], a form of programmed chondrocytic cell death distinct from classical apoptosis that is associated with increased protein synthesis and ER stress [13]. But whether iron-laden chondrocytes die of apoptosis, chondroptosis or an alternative pathway of programmed cell death (e.g. ferroptosis, an iron-dependent form of programmed cell death associated with increased lipid peroxidation [14]) has not yet been determined. We have addressed this question using TEM, which is considered the ‘gold standard’ for detecting chondrocyte death [12]. As depicted in Fig. 3, after 9 days in culture, control chondrocytes retained the ultrastructural characteristics found in cells from freshly collected cartilage, including abundant rough ER and frequent cytoplasmic processes. In contrast, chondrocytes incubated with ferric citrate were characterized by the presence of membrane-bound cytoplasmic bodies containing hemosiderin (siderosomes), chromatin condensation (a common feature of apoptosis and chondroptosis), and a number of morphological changes consistent with death by chondroptosis, namely: expanded and dilated rough ER, autophagic vacuoles, presence of organelles in the extracellular space, and cell disintegration leading to empty lacunae.
Fig. 3.
Iron loading induces morphological alterations in chondrocytes. Transmission electron microscopy images illustrate the ultrastructure of chondrocytes in freshly collected cartilage (A) or in cartilage cultured for 9 days with sodium citrate (control) (B) or ferric citrate (iron loading condition) (C–F). Whilst the morphology of control chondrocytes was indistinct from that of cells from freshly collected cartilage, iron-treated chondrocytes presented siderosomes (white arrows) and a number of morphological changes consistent with cell death by chondroptosis. (D) is a magnification of the area within the square in (C), showing a siderosome and abundant dilated cisternae of rough ER. A chondroptotic cell with convoluted nucleus with condensed chromatin, abundant Golgi and vacuoles (arrow heads), and extrusion of cellular material into the extracellular space (black arrows) is represented in (E). An empty lacuna is depicted in (F), where the remnants of a dead chondrocyte are identified by a dotted line. Samples were contrasted with uranile acetate and lead citrate. Original magnification: 15000 × . Bar=1 μm.
Chondrocytes are responsible for maintaining cartilage homeostasis. Consequently, we have also investigated the impact of iron on cartilage integrity. To address this, we measured the release of specific constituents of the cartilage ECM into the culture medium, namely hydroxyproline, COMP and sGAG. Hydroxyproline and sGAG were measured every 3 days and results were expressed as the cumulative fold change compared to day 0. COMP levels were quantified at the end of the 9-day incubation period. Iron treatment did not significantly change hydroxyproline (p=0.3488) (Fig. 4A) or COMP levels (p=0.4657) (Supplementary Fig. 2). On the other hand, iron significantly increased the release of sGAG into the medium compared to control (p=0.0189). The effect of iron was significant from day 6 in culture (Fig. 4B). Confirming a dependence on redox-active iron, the rate of sGAG release (between days 0 and 9) from cartilage explants treated with ferric citrate was significantly decreased by the specific iron chelator DFO (Fig. 4C).
Fig. 4.
Iron differently affects the release of cartilage ECM constituents into the conditioned medium. Cartilage explants were treated for 9 days with 50 μM ferric citrate or sodium citrate (control). Hydroxyproline and sGAG were measured every 3 days and results were expressed as the cumulative fold change compared to day 0 (A–B) or as the release rate between days 0–9 (C). A) Hydroxyproline release into the culture medium was not significantly altered by iron treatment. Statistical significance was assessed by two-way repeated measures ANOVA (n=4 independent experiments). B) Iron treatment significantly increased the release of sGAG into the medium compared to control from day 6. Statistical significance was assessed by two-way repeated measures ANOVA (p=0.0189) with Sidak’s multiple comparisons test (respective p-values depicted) (n=5 independent experiments). C) Ferric citrate-induced sGAG release was abrogated upon co-incubation with the iron chelator desferrioxamine (DFO, 100 μM). Statistical significance was assessed by one-way repeated measures ANOVA with Sidak’s multiple comparisons test (n=5 independent experiments).
There is increasing evidence associating cartilage degradation with chondrocyte death in OA [15]. However, it is unclear whether chondrocyte death is a cause of cartilage degeneration [12]. To investigate if the death of chondrocytes could trigger sGAG release in our experimental system, we have rendered cartilage explants metabolically inactive by repetitive freeze-thawing. In this condition, sGAG release was decreased when compared with the active cartilage. Importantly, no change in sGAG release was observed upon exposure of metabolically inactive explants to iron (Fig. 5A). This supports the hypothesis that the iron-dependent degradation observed in active explants depends on the presence of viable chondrocytes, and is neither the result of a direct chemical interaction between redox-active iron and the ECM, nor a direct consequence of chondrocyte death.
Fig. 5.
Iron-mediated s-GAG release requires a metabolically active cartilage and metalloproteinase activity. A) Cartilage explants were rendered metabolically inactive (by repetitive freeze-thawing) prior to incubation with 50 μM ferric citrate or sodium citrate (control) for 9 days. sGAG release was decreased when compared with the active cartilage, and iron increased sGAG release exclusively in metabolically active explants. Statistical significance was assessed by one-way repeated measures ANOVA with Sidak’s multiple comparisons test (n=3 independent experiments). B) Cartilage explants were incubated with 50 μM ferric citrate (FC) or sodium citrate (SC, control) for 9 days. MMP-2 and MMP-9 activity in the 9-day conditioned medium was measured by gelatine zymography (top panel). Iron significantly increased the gelatinolytic activity levels of both MMPs (bottom panels). The graphs show the change within the same experiment, and the magnitude of this change was assessed with the paired Student’s t-test (n=3 independent experiments). C) Cartilage explants were incubated with ferric citrate (FC) (50 μM) in the presence or absence of the metalloproteinase inhibitor prinomastat (AG-3340) (0, 0.02 or 2 μM) for 9 days. Prinomastat significantly reduced sGAG release. Statistical significance was assessed by one-way repeated measures ANOVA with Sidak’s multiple comparisons test (n=5 independent experiments).
The putative contribution of cell death was further investigated by measuring sGAG release into the culture medium upon treatment of cartilage with known apoptotis/chondroptosis stimuli with an ER stress component, namely tunicamycin, a specific inhibitor of N-glycosylation in ER [16], and thapsigargin, which depletes ER calcium storage [17]. Both ER stress inducers were previously demonstrated to be effective in cartilage [[18], [19], [20]], but treatment of cartilage explants for 9 days failed to increase sGAG release (Suppl. Figure 3). Likewise, we incubated cartilage with different inducers of ferroptosis. Erastin and buthionine-sulfoximine (BSO) induce ferroptosis in a variety of cell types by inactivating glutathione peroxidase enzymes indirectly through glutathione depletion (via inhibition of the cystine glutamate antiporter or of γ-glutamyl cysteine synthetase, respectively), whereas RSL-3 binds to and directly inhibits glutathione peroxidase 4 [21]. Ferroptosis inducers also failed to increase sGAG release (Suppl. Figure 3).
Conversely, we have incubated cartilage explants with ferric citrate in the presence or absence of ER stress/chondroptosis, apoptosis or ferroptosis inhibitors and evaluated sGAG release. The rate of sGAG release from cartilage explants treated with ferric citrate remained unaltered in the presence of the ER stress inhibitor TUDCA [22,23], the pan-caspase inhibitor Z-VAD-FMK [24,25] or the lipophilic antioxidant inhibitors of ferroptosis, Fer-1 [26] and α-tocopherol [27,28] (Suppl. Figure 4). It is thus unlikely that iron-induced sGAG release could be explained by the death of chondrocytes.
Instead, we hypothesized that iron loading could promote sGAG release by altering the catabolic profile of chondrocytes. To investigate this hypothesis, we measured the activity of two MMPs that are secreted into the extracellular space (MMP-2 and MMP-9) by gelatine zymography. In agreement with our hypothesis, we found significantly higher activity levels of both MMPs in the culture medium of cartilage explants incubated with ferric citrate (Fig. 5B). Our data thus suggest that iron-induced sGAG release is associated with increased metalloproteinase activity. To confirm this, we incubated cartilage explants with ferric citrate in the presence of increasing concentrations of the broad spectrum MMP/aggrecanase inhibitor prinomastat (AG-3340). Notably, iron-mediated sGAG released was significantly reduced in a concentration-dependent manner (Fig. 5C).
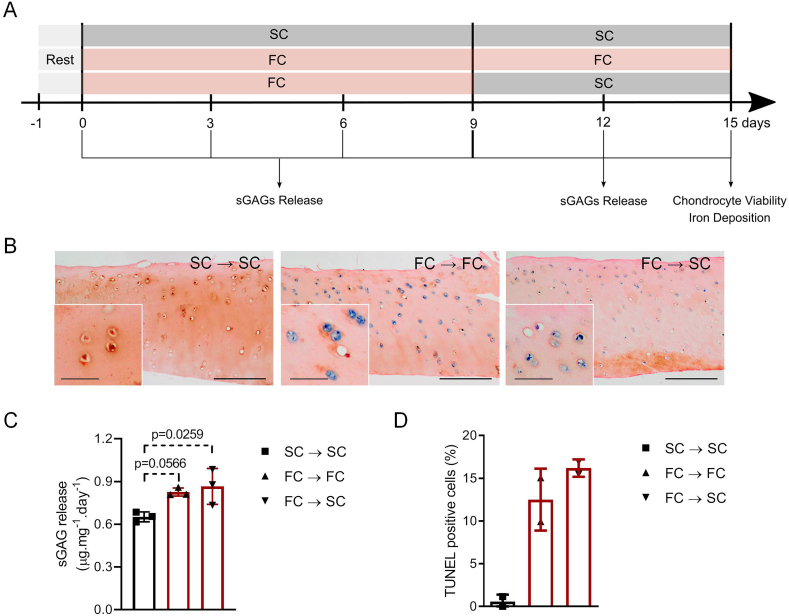
We also evaluated the reversibility of the effect of iron on cartilage degradation. After 9 days of incubation with ferric citrate, cartilage explants were exposed to sodium citrate for an additional 6-day period (Fig. 6A). Iron loading and sGAG release were assessed in comparison with cartilage that was further incubated with ferric citrate and with control cartilage (sodium citrate). As depicted in Fig. 6B, sustained incubation with ferric citrate led to greater iron deposition within chondrocytes, which has extended to the transitional and radial zones of cartilage. The withdrawal of iron excess from the medium at day 9 of culture has apparently prevented the load of iron in chondrocytes at some extent. Nevertheless, chondrocytes remained iron-loaded, especially at the superficial zone of cartilage (Fig. 6B). Despite the iron removal, the rate of sGAG release into the medium between days 9 and 15 of culture remained significantly elevated when compared to control (Fig. 6C). We noted a trend for sustained iron exposure to increase the percentage of dead (TUNEL-positive) chondrocytes, when compared to what we had observed after just 9 days (Fig. 2B), which could not be prevented by iron withdrawal (Fig. 6D). Overall, these results suggest that chondrocyte iron loading leads to an irreversible onset of cartilage degeneration.
Fig. 6.
Increased sGAG release persists after iron withdrawal from the culture medium. A) Cartilage explants were incubated with 50 μM ferric citrate or sodium citrate (SC, control) for 9 days, after which the iron-treated explants have either received 50 μM ferric citrate (FC, sustained iron loading condition) or sodium citrate (iron withdrawal condition) for an additional 6-day period. B) Cartilage coronal sections were stained with Perls’ Prussian blue stain for ferric iron. Sustained incubation with ferric citrate (FC→FC) led to iron deposition within chondrocytes throughout the whole cartilage, including the transitional and radial zones; chondrocytes submitted to iron withdrawal condition (FC→SC) remained iron loaded, especially in the superficial zone. Bar=200 μm (insets denoting iron deposits in superficial chondrocytes, bar=50 μm). C) Despite the removal of iron excess from the medium at day 9 in culture, the cartilage samples cultured under iron withdrawal conditions displayed increased sGAG release between days 9–15, when compared to control. Statistical significance was assessed by one-way ANOVA with Dunnett’s multiple comparisons test (n=3 independent experiments). D) Chondrocyte death was assessed with the TUNEL assay. Sustained iron exposure for 15 days increased the percentage of dead (TUNEL-positive) chondrocytes when compared to control, which did not appear to be prevented by iron withdrawal from day 9 (n=2 independent experiments).
Finally, we hypothesized that iron-induced cartilage degradation may be further modulated in the presence of other cellular components of the joint. To explore this hypothesis, we have treated bovine articular cartilage explants with ferric citrate (or sodium citrate as control) for 9 days in the presence or absence of SW982 synovial cells previously seeded on cell culture inserts. Medium was refreshed every third day and conditioned medium was collected for the measurement of sGAG (Supplementary Fig. 5A). Interestingly, iron-induced sGAG release was exacerbated in the co-culture (Supplementary Fig. 5B). Furthermore, when cartilage explants were rendered metabolically inactive (by repetitive freeze-thawing) prior to incubation in the presence of SW982 cells, we observed no further increase in sGAG release into the medium (Supplementary Fig. 5C). This suggests that iron-induced cartilage degradation is augmented as a result of a crosstalk between chondrocytes and synovial cells, while confirming that the release of sGAG from articular cartilage is mediated by metabolically active chondrocytes.
4. Discussion
To study iron-induced cartilage degeneration, we incubated bovine articular cartilage explants with ferric citrate, which is predicted to be the dominant non-transferrin-bound iron (NTBI) species in plasma [29], increased in hemochromatosis patients [30]. We used ferric citrate at 50 μM, a concentration that is expected to saturate the transferrin present in culture medium containing 5% FBS [31]. Hence, we expect that iron was presented to cells in both transferrin-bound and non-transferrin-bound states.
We provide histological and ultrastructural evidence that the treatment of cartilage with ferric citrate causes iron accumulation within chondrocytes, especially at the cartilage surface. The presence of intracellular iron deposits in chondrocytes has been previously reported in the articular cartilage from the affected joints of haemophilic patients [32] and in the cartilage of some hemochromatosis patients [7,8]. However, whether and how iron contributes to cartilage degradation has remained elusive.
In the present work, we showed that iron-treated chondrocytes present expansion and dilatation of ER, which is suggestive of increased protein synthesis and ER stress [33]. In OA, chondrocyte ER stress arises as a consequence of increased protein synthesis of both ECM molecules and matrix-degrading proteases [33]. Chronic ER stress, in turn, is known to induce programmed cell death of chondrocytes [34]. We showed here that iron loading causes programmed cell death (chondroptosis) of superficial chondrocytes.
Iron loading has also resulted in matrix degradation, observed as loss of sGAG. Loss of sGAG-containing aggrecans is a key pathophysiological event in joint diseases such as OA and rheumatoid arthritis (RA). It compromises both the functional and structural integrity of the cartilage matrix, leading to irreversible cartilage erosion [35]. As such, the presence of sGAG in the synovial fluid is a useful indicator for cartilage degeneration [36].
While the levels of sGAG released in our control cultures (0.5–0.6 μg mg−1.day−1) are similar to those reported in studies using bovine cartilage explants [[37], [38], [39]], the increase in sGAG release induced by iron exposure is of a much lower magnitude than that induced by IL-1b and/or TNF [39,40]. This is consistent with the chronic, late-onset nature of HA, when compared with typical inflammatory conditions such as RA.
There is evidence of an association between cartilage degradation and chondrocyte death in OA. Nonetheless, it remains to be established whether chondrocyte death is a cause or a result of cartilage degeneration [12]. We showed that, while iron-induced chondrocyte death is likely to contribute to the progression of joint disease in HA, it cannot explain the release of ECM components. This is supported by the fact that iron-induced sGAG release required a metabolically viable cartilage and was not inhibited by a variety of apoptosis, chondroptosis and ferroptosis inhibitors.
Instead, we showed that iron-induced loss of sGAG from articular cartilage is due to the activation of matrix-degrading proteinases. In RA and OA cartilage, chondrocytes have an increased capacity to synthesize and secrete proteins that cleave membrane collagens and proteoglycans, including MMPs and aggrecanases [41]. We showed that iron loading promotes the production of matrix degrading metalloproteases, such as MMP-2 and MMP-9. While there is ample evidence that some MMPs (namely MMP-3 and MMP-13) are able to degrade aggrecans in vitro, it is now recognized that the main proteinases that are responsible for in situ aggrecanolysis are aggrecanases [35]. We showed that prinomastat (AG-3340), a hydroxamate inhibitor with high specificity for MMP-2, MMP-9 and two MMPs known to be capable of degrading the interglobular domain of the aggrecan core protein, MMP-3 and MMP-13, prevented iron-induced sGAG release in a concentration-dependent manner. Notably, while far more potent against MMPs, prinomastat is also known to inhibit aggrecanase at concentrations around 10−6 M [42].
In summary, we showed that iron triggers the early stages of cartilage degeneration, which is associated with the activation of catabolic metabolism in chondrocytes leading to ECM degradation and programmed chondrocytic cell death. Moreover, our data suggest that the damage is irreversible and therefore removal of iron from the extracellular medium (mimicking the iron withdrawal achieved through therapeutic phlebotomy) is unlikely to prevent further damage to the cartilage. This may partly explain the progressive and irreversible nature of the joint disease in HFE-H patients even after venesection regimens.
Our study represents a first attempt to generate an ex vivo model to study HA. We acknowledge that it has limitations. The use of young adult bovine cartilage in a highly controlled experimental setting does not mimic the full spectrum of physical insults to which human cartilage is exposed to over several decades of life. The amount of iron and how it is presented to cells (i.e. transferrin-bound and/or NTBI) in synovial fluid of hemochromatosis patients is unknown, which raises doubts on the physiological relevance of the iron concentration employed herein. Also, the oxygen levels present in standard cell culture conditions may increase the redox-cycling of iron, which could be avoided by culturing cartilage under hypoxic environment. Finally, we employed a simplified experimental model to study the impact of iron loading on cartilage stability, focusing on the role of chondrocytes. However, iron is known to accumulate also in the synovial cells of hemochromatosis patients [43]. Our preliminary co-culture experiments showed that iron-induced, chondrocyte-mediated cartilage degradation is further augmented as a result of a crosstalk with synovial cells. Future experiments should aim at characterizing the mechanisms governing this crosstalk. Likewise, the current experimental model could be modified to allow co-culture of cartilage explants with other cellular players that are also likely to contribute to cartilage degeneration, such as inflammatory cells (e.g. neutrophils), to better mimic the in vivo situation.
Author’s credits
Anaísa V Ferreira: Methodology, Investigation, Formal analysis, Writing – review & editing Tiago L Duarte: Conceptualization, Investigation, Formal analysis, Writing – original draft Sandra Marques: Investigation, Formal analysis Paula Costa: Investigation Sara C. Neves: Conceptualization, Methodology, Writing – review & editing. Tiago dos Santos: Methodology, Resources. Pedro L. Granja: Conceptualization, Methodology, Writing – review & editing. Graça Porto: Conceptualization, Methodology, Funding acquisition, Writing – review & editing.
Declaration of competing interest
The authors declare no conflicts of interest.
Acknowledgements
The authors acknowledge support of the i3S Histology and Electron Microscopy Service (HEMS), member of the PPBI-Portuguese Platform of Bioimaging (PPBI–POCI-01-0145-FEDER-022122). We are grateful to HEMS′ staff Rui Fernandes and Ana Rita Malheiro for technical assistance in TEM, and Rossana Correia for technical assistance in histology.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ocarto.2021.100145.
Contributor Information
A.V. Ferreira, Email: anaisa.valido@gmail.com.
T.L. Duarte, Email: tduarte@ibmc.up.pt.
S. Marques, Email: marques.sandra075@gmail.com.
P. Costa, Email: paulacostafp@hotmail.com.
S.C. Neves, Email: saracneves@gmail.com.
T. dos Santos, Email: Tiago.f.Santos@ineb.up.pt.
P.L. Granja, Email: pgranja@i3s.up.pt.
G. Porto, Email: gporto@ibmc.up.pt.
Role of the funding source
This work was funded by Project Norte-01-0145-FEDER-000012, supported by Norte Portugal Regional Operational Programme (NORTE 2020), under the PORTUGAL 2020 Partnership Agreement, through the European Regional Development Fund (FEDER). The funding source had no involvement in the study design, collection, analysis and interpretation of data; in the writing of the manuscript; or in the decision to submit the manuscript for publication.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Porto G., Brissot P., Swinkels D.W., Zoller H., Kamarainen O., Patton S., et al. EMQN best practice guidelines for the molecular genetic diagnosis of hereditary hemochromatosis (HH) Eur. J. Hum. Genet. 2016;24:479–495. doi: 10.1038/ejhg.2015.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prabhu A., Cargill T., Robert N., Ryan J.D. Systematic review of the clinical outcomes of iron reduction in Hereditary Hemochromatosis. Hepatology. 2020;72:1469–1482. doi: 10.1002/hep.31405. [DOI] [PubMed] [Google Scholar]
- 3.Husar-Memmer E., Stadlmayr A., Datz C., Zwerina J. HFE-related hemochromatosis: an update for the rheumatologist. Curr. Rheumatol. Rep. 2014;16:393. doi: 10.1007/s11926-013-0393-4. [DOI] [PubMed] [Google Scholar]
- 4.Carroll G.J., Breidahl W.H., Olynyk J.K. Characteristics of the arthropathy described in hereditary hemochromatosis. Arthritis Care Res. 2012;64:9–14. doi: 10.1002/acr.20501. [DOI] [PubMed] [Google Scholar]
- 5.van Vulpen L.F.D., Roosendaal G., van Asbeck B.S., Mastbergen S.C., Lafeber F.P.J.G., Schutgens R.E.G. The detrimental effects of iron on the joint: a comparison between haemochromatosis and haemophilia. J. Clin. Pathol. 2015;68:592–600. doi: 10.1136/jclinpath-2015-202967. [DOI] [PubMed] [Google Scholar]
- 6.Heiland G.R., Aigner E., Dallos T., Sahinbegovic E., Krenn V., Thaler C., et al. Synovial immunopathology in haemochromatosis arthropathy. Ann. Rheum. Dis. 2010;69:1214–1219. doi: 10.1136/ard.2009.120204. [DOI] [PubMed] [Google Scholar]
- 7.Schumacher H.R. Articular cartilage in the degenerative arthropathy of hemochromatosis. Arthritis Rheum. 1982;25:1460–1468. doi: 10.1002/art.1780251212. [DOI] [PubMed] [Google Scholar]
- 8.Axford J.S., Bomford A., Revell P., Watt I., Williams R., Hamilton E.B. Hip arthropathy in genetic hemochromatosis. Radiographic and histologic features. Arthritis Rheum. 1991;34:357–361. doi: 10.1002/art.1780340314. [DOI] [PubMed] [Google Scholar]
- 9.Montgomery K.D., Williams J.R., Sculco T.P., DiCarlo E. Clinical and pathologic findings in hemochromatosis hip arthropathy. Clin. Orthop. Relat. Res. 1998;347:179–187. https://journals.lww.com/clinorthop/Abstract/1998/02000/Clinical_and_Pathologic_Findings_in.20.aspx [PubMed] [Google Scholar]
- 10.Steenvoorden M.M.C., Bank R.A., Ronday H.K., Toes R.E.M., Huizinga T.W.J., DeGroot J. Fibroblast-like synoviocyte-chondrocyte interaction in cartilage degradation. Clin. Exp. Rheumatol. 2007;25:239–245. [PubMed] [Google Scholar]
- 11.Sumer E.U., Sondergaard B.C., Rousseau J.C., Delmas P.D., Fosang A.J., Karsdal M.A., Christiansen C., Qvist P. MMP and non-MMP-mediated release of aggrecan and its fragments from articular cartilage: a comparative study of three different aggrecan and glycosaminoglycan assays. Osteoarthritis Cartilage. 2007;15:212–221. doi: 10.1016/j.joca.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 12.Zamli Z., Sharif M. Chondrocyte apoptosis: a cause or consequence of osteoarthritis? Int. J. Rheum. Dis. 2011;14:159–166. doi: 10.1111/j.1756-185X.2011.01618.x. [DOI] [PubMed] [Google Scholar]
- 13.Roach H.I., Aigner T., Kouri J.B. Chondroptosis: a variant of apoptotic cell death in chondrocytes? Apoptosis. 2004;9:265–277. doi: 10.1023/B:APPT.0000025803.17498.26. [DOI] [PubMed] [Google Scholar]
- 14.Hassannia B., Van Coillie S., Vanden Berghe T. Ferroptosis: biological rust of lipid membranes. Antioxidants Redox Signal. 2020 doi: 10.1089/ars.2020.8175. [DOI] [PubMed] [Google Scholar]
- 15.Paterson S.I., Eltawil N.M., Simpson A.H.R.W., Amin A.K., Hall A.C. Drying of open animal joints in vivo subsequently causes cartilage degeneration. Bone Joint Res. 2016;5:137–144. doi: 10.1302/2046-3758.54.2000594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martina J.A., Daniotti J.L., Maccioni H.J. Influence of N-glycosylation and N-glycan trimming on the activity and intracellular traffic of GD3 synthase. J. Biol. Chem. 1998;273:3725–3731. doi: 10.1074/jbc.273.6.3725. [DOI] [PubMed] [Google Scholar]
- 17.Sagara Y., Inesi G. Inhibition of the sarcoplasmic reticulum Ca2+ transport ATPase by thapsigargin at subnanomolar concentrations. J. Biol. Chem. 1991;266:13503–13506. doi: 10.1016/S0021-9258(18)92726-2. [DOI] [PubMed] [Google Scholar]
- 18.Yokose S., Tajima Y. In vivo effects of tunicamycin on chondrocytes of rat mandibular condyles as revealed by lectin cytochemistry. Cell Tissue Res. 1992;269:235–239. doi: 10.1007/BF00319614. [DOI] [PubMed] [Google Scholar]
- 19.Pritchard S., Guilak F. Effects of interleukin-1 on calcium signaling and the increase of filamentous actin in isolated and in situ articular chondrocytes. Arthritis Rheum. 2006;54:2164–2174. doi: 10.1002/art.21941. [DOI] [PubMed] [Google Scholar]
- 20.Mouw J.K., Imler S.M., Levenston M.E. Ion-channel regulation of chondrocyte matrix synthesis in 3D culture under static and dynamic compression. Biomech. Model. Mechanobiol. 2007;6:33–41. doi: 10.1007/s10237-006-0034-1. [DOI] [PubMed] [Google Scholar]
- 21.Yang W.S., SriRamaratnam R., Welsch M.-E., Shimada K., Skouta R., Viswanathan V.S., et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317–331. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu C., Cao Y., Yang X., Shan P., Liu H. Tauroursodeoxycholic acid suppresses endoplasmic reticulum stress in the chondrocytes of patients with osteoarthritis. Int. J. Mol. Med. 2015;36:1081–1087. doi: 10.3892/ijmm.2015.2295. [DOI] [PubMed] [Google Scholar]
- 23.Arai Y., Choi B., Kim B.J., Rim W., Park S., Park H., et al. Tauroursodeoxycholic acid (TUDCA) counters osteoarthritis by regulating intracellular cholesterol levels and membrane fluidity of degenerated chondrocytes. Biomater. Sci. 2019;7:3178–3189. doi: 10.1039/C9BM00426B. [DOI] [PubMed] [Google Scholar]
- 24.Dang A.C., Warren A.P., Kim H.T. Beneficial effects of intra-articular caspase inhibition therapy following osteochondral injury. Osteoarthritis Cartilage. 2006;14:526–532. doi: 10.1016/j.joca.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 25.D’Lima D., Hermida J., Hashimoto S., Colwell C., Lotz M. Caspase inhibitors reduce severity of cartilage lesions in experimental osteoarthritis. Arthritis Rheum. 2006;54:1814–1821. doi: 10.1002/art.21874. [DOI] [PubMed] [Google Scholar]
- 26.Yao X., Sun K., Yu S., Luo J., Guo J., Lin J., et al. Chondrocyte ferroptosis contribute to the progression of osteoarthritis. J. Orthop. Translat. 2020;27:33–43. doi: 10.1016/j.jot.2020.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shimada K., Skouta R., Kaplan A., Yang W.S., Hayano M., Dixon S.J., et al. Global survey of cell death mechanisms reveals metabolic regulation of ferroptosis. Nat. Chem. Biol. 2016;12:497–503. doi: 10.1038/nchembio.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chin K.Y., Ima-Nirwana S. The role of vitamin E in preventing and treating osteoarthritis - a review of the current evidence. Front. Pharmacol. 2018;9:946. doi: 10.3389/fphar.2018.00946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.May P.M., Linder P.W., Williams D.R. Computer-simulation of metal-ion equilibria in biofluids - models for low-molecular-weight complex distribution of calcium(ii), magnesium(ii), manganese(ii), iron(iii), copper(ii), zinc(ii), and lead(ii) ions in human-blood plasma. J. Chem. Soc-Dalton Transactions. 1977;6:588–595. doi: 10.1039/DT9770000588. [DOI] [Google Scholar]
- 30.Grootveld M., Bell J.D., Halliwell B., Aruoma O.I., Bomford A., Sadler P.J. Non-transferrin-bound iron in plasma or serum from patients with idiopathic hemochromatosis. Characterization by high performance liquid chromatography and nuclear magnetic resonance spectroscopy. J. Biol. Chem. 1989;264:4417–4422. doi: 10.1016/S0021-9258(18)83758-9. [DOI] [PubMed] [Google Scholar]
- 31.Young S.P., Garner C. Delivery of iron to human cells by bovine transferrin. Implications for the growth of human cells in vitro. Biochem. J. 1990;265:587–591. doi: 10.1042/bj2650587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rippey J.J., Hill R.R., Lurie A., Sweet M.B., Thonar E.J., Handelsman J.E. Articular cartilage degradation and the pathology of haemophilic arthropathy. S. Afr. Med. J. 1978;54:345–351. [PubMed] [Google Scholar]
- 33.Rellmann Y., Dreier R. Different forms of ER stress in chondrocytes result in short stature disorders and degenerative cartilage diseases: new insights by cartilage-specific ERp57 knockout mice. Oxid. Med. Cell Longev. 2018;17:8421394. doi: 10.1155/2018/8421394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim C., Kim B. Anti-cancer natural products and their bioactive compounds inducing ER stress-mediated apoptosis: a review. Nutrients. 2018;10:1021. doi: 10.3390/nu10081021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caterson B., Flannery C.R., Hughes C.E., Little C.B. Mechanisms involved in cartilage proteoglycan catabolism. Matrix Biol. 2000;19:333–344. doi: 10.1016/S0945-053X(00)00078-0. [DOI] [PubMed] [Google Scholar]
- 36.Kulkarni P., Deshpande S., Koppikar S., Patil S., Ingale D., Harsulkar A. Glycosaminoglycan measured from synovial fluid serves as a useful indicator for progression of Osteoarthritis and complements Kellgren-Lawrence Score. BBA Clin. 2016;6:1–4. doi: 10.1016/j.bbacli.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nishimuta J.F., Bendernagel M.F., Levenston M.E. Co-culture with infrapatellar fat pad differentially stimulates proteoglycan synthesis and accumulation in cartilage and meniscus tissues, Connect. Tissue Res. 2017;58:447–455. doi: 10.1080/03008207.2016.1245728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Caramés B., Taniguchi N., Seino D., Blanco F.J., D’Lima D., Lotz M. Mechanical injury suppresses autophagy regulators and pharmacologic activation of autophagy results in chondroprotection. Arthritis Rheum. 2012;64:1182–1192. doi: 10.1002/art.33444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stevens A.L., Wheeler C.A., Tannenbaum S.R., Grodzinsky A.J. Nitric oxide enhances aggrecan degradation by aggrecanase in response to TNF-alpha but not IL-1beta treatment at a post-transcriptional level in bovine cartilage explants. Osteoarthritis Cartilage. 2008;16:489–497. doi: 10.1016/j.joca.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Matta C., Fellows C.R., Quasnichka H., Williams A., Jeremiasse B., Allaway D., et al. Clusterin secretion is attenuated by pro-inflammatory cytokines in culture models of cartilage degradation. bioRxiv. 2020 doi: 10.1101/2020.05.05.078105. [DOI] [PubMed] [Google Scholar]
- 41.Okada Y. In: tenth ed. Firestein G.S., Budd R.C., Gabriel S.E., McInnes I.B., O’Dell J.R., editors. ume 1. Elsevier Inc.; 2017. Chapter 8 - proteinases and matrix degradation; pp. 106–125. (Kelley and Firestein’s Textbook of Rheumatology). [DOI] [Google Scholar]
- 42.Sugimoto K., Takahashi M., Yamamoto Y., Shimada K., Tanzawa K. Identification of aggrecanase activity in medium of cartilage culture. J. Biochem. 1999;126:449–455. doi: 10.1093/oxfordjournals.jbchem.a022471. [DOI] [PubMed] [Google Scholar]
- 43.Schumacher H.R., Jr. Ultrastructural characteristics of the synovial membrane in idiopathic haemochromatosis. Ann. Rheum. Dis. 1972;31:465–473. doi: 10.1136/ard.31.6.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.