Abstract
Introduction
The wait times crisis for hip and knee total joint replacement surgery has been a significant health care issue in Alberta and across Canada. Significant resource and financial efforts have been put forward to reduce wait times for surgery as a means of treating patients with osteoarthritis (OA), but the gains achieved were not sustained.
Objective
To effectively address wait time issues, an alternative perspective on this problem is presented – that the wait times are an immediate problem for those needing surgery, but are also a symptom of the bigger issue of an inability of health care systems in Canada to address the needs of individuals with early OA with first-line treatment protocols.
Discussion
In considering this more comprehensive understanding of the overall OA management problem, encapsulated by the concept of an “osteoarthritis funnel”, we outline potential approaches for a solution on a systemic level that integrates services delivery, health care resource allocation and conceptualization of OA in research activities. It also emphasizes the need for a more effective and relevant program of research to address this complex problem that requires unique solutions.
Conclusions
New approaches and understanding are needed to address integrated implementation of effective first-line treatments for newly diagnosed osteoarthritis to prevent the expanding demand for joint replacement surgery. While the focus here is on the Canadian perspective, the need to develop and implement better first-line treatments for those with early OA and those at risk for development of OA is not unique to Canada.
Keywords: Osteoarthritis, Hip and knee osteoarthritis, Arthroplasty, Surgery, First-line treatment, Self-management, Research needs, Wait times, Osteoarthritis prevention, Mitigating risk
1. Background
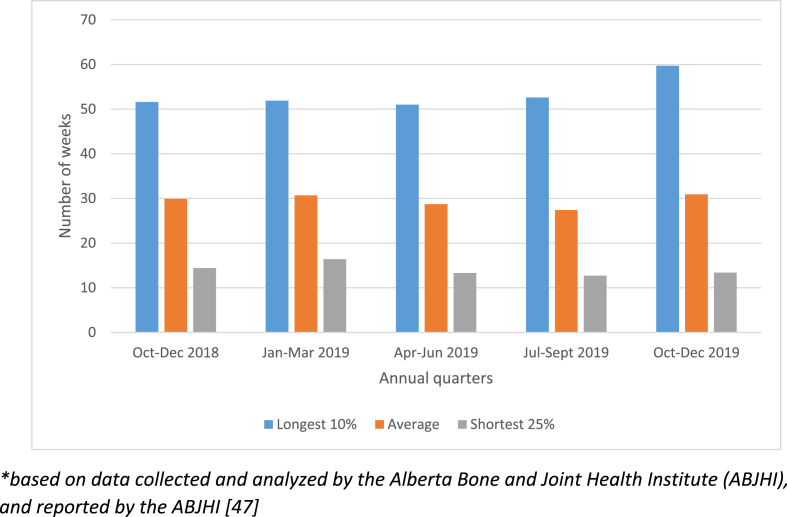
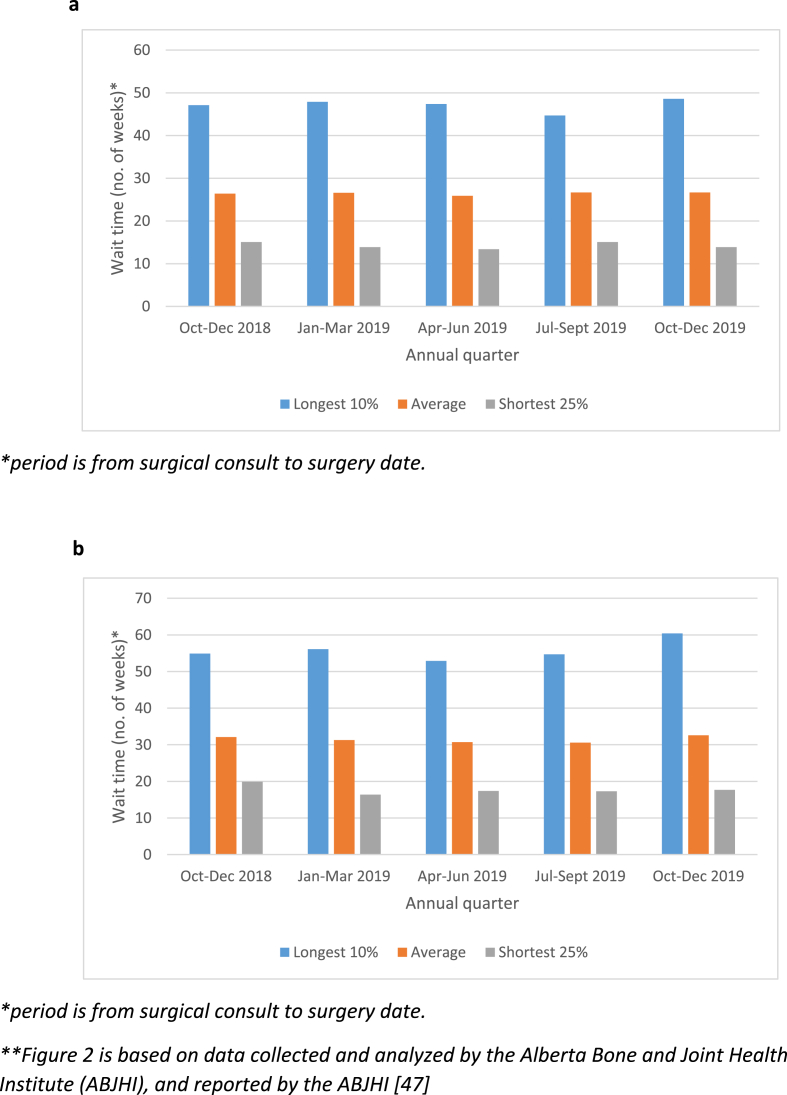
In the early to mid-2000s, Alberta, like other Canadian and international jurisdictions, was facing a wait-times crisis for hip and knee total joint replacement surgery. Almost all of these surgeries were directed at joints that had been damaged by the chronic and degenerative disease called osteoarthritis (an umbrella term for degenerative loss of function from various causes). In 2009, the government of Alberta allocated an additional $40 million to decrease the wait times for hip and knee replacement surgeries and set an aggressive benchmark of 14 weeks for wait times from surgical consult to surgery. The initiative – a 5-year plan to reach this benchmark - was launched in 2010. It was primarily based on increasing surgical volumes to offset the number of patients in the surgical queue. Wait times began to decrease from a high of 47 weeks–40 weeks in the 2012/2013 fiscal year. Since 2013, wait times remained relatively stable at around 41 weeks in an environment of “zero surgical volume growth”; however, since 2018, wait times have again lengthened for both orthopedic consultation (Fig. 1) and joint replacement surgery (Fig. 2). These increases in wait times have occurred against a backdrop of 10848 hip and knee total joint replacement surgeries in 2018, and 10,574 in 2019. The problem of growing wait times is also compounded by the COVID pandemic. In Alberta, it is estimated that the wait lists for surgery have increased from ∼9836 in February, 2020 to ∼14,112 by June, 2020 (Alberta Bone & Joint Health Institute, internal reports).
Fig. 1.
Wait times for surgeon consultation for hip and knee joint replacement surgery in Alberta [47].
Fig. 2.
a. Wait times for hip joint replacement surgery in Alberta. b. Wait times for surgeon consultation for knee joint replacement surgery in Alberta [47].
So we are back to where we started a decade ago and the projections in Alberta for total joint replacement surgery demand are troubling [1]. It would appear that we have gone full circle, but with the problem further exacerbated by the pandemic, and yet the focus remains on the wait times for total joint replacement surgery, suggesting that timely access to surgery continues to be the core problem that needs to be tackled. We would like to propose an alternate perspective – while wait times are a real problem for those in the queue for surgery, which has been further complicated by the COVID pandemic, wait times are also, and importantly, a symptom of a much larger and complex problem referred by some as the OA epidemic. The OA epidemic is defined by increasing numbers of individuals diagnosed with OA (the majority of whom are not surgical candidates) and more OA diagnoses in increasingly younger people. The OA epidemic results in a systemic problem defined by several important facets that we will discuss here: lack of attention and resource allocation to early-OA interventions and OA prevention strategies in Canada, perception by patients that total joint replacement is the solution to OA, and, current thinking and understanding about OA as a disease state, and, hence, how it continues to be researched.
Once initiated by one of several possible threats to the integrity of the joint (discussed in more detail below), the progression of OA is as a degenerative disease affecting joints, resulting in the physical breakdown of the joint structure. This loss of mobility and related symptoms such as pain can result in secondary consequences of the disease, including sedentary behavior and development of obesity, and impact on other physiologic systems such as the cardiovascular and respiratory systems, and cognition loss [2,3]. Thus, the predictions for increased incidence of OA will likely have a number of primary and secondary implications for both the health of patients, and impact on the Canadian health care system (and others) as it relates to both acute and chronic care. While the discussion has thus far had a Canadian perspective, likely the issues around wait lists is broader in many countries with a similar health care system, but not in others that differ.
The prevalence of OA in Alberta is similar to Canada – approximately 10–12% of Albertans have been diagnosed with OA, with higher rates evident in females and older adults. After menopause, the incidence of OA is higher in women than corresponding aged males [2,4,5]. A troublesome projection is that the prevalence of OA is expected to rise up to 25% in the next generation [6,7].
It is evident that OA is a significant health care issue negatively impacting many and presenting a significant challenge to the health care system. However, the current Canadian approach to addressing OA, and specifically wait times, is almost singularly focused on an elective surgery at the end stage of the disease. It is important to consider that this reflects services for approximately 5% of the individuals living with OA in Alberta.
We determined that addressing the OA problem health systems are grappling with, a more comprehensive, if not balanced, perspective is needed. The OA Funnel, depicted in Fig. 3, is a way of understanding the full breadth of the OA epidemic and potentially informative in how to address it across the continuum of care, and not predominantly at the surgical end-stage. The end of the OA Funnel (the funnel) is where most of the current attention is focused: the end stage of a disease where the only viable treatment option is surgery. Given real world resource constraints, access to surgery is limited. And with the prevalence of OA increasing, this end stage becomes a “bottleneck”. In front of the “bottle neck” for joint replacement in Canada is a large “funnel” holding a huge number of people with a diagnosis of Hip and/or Knee OA. Furthermore, this group is diverse – including men, women, various age groups and various stages of OA. While many of these individuals may or may not be waiting for surgery, they should all be engaged in some form of conservative (non-surgical) or first-line treatment management for their OA, particularly those at the early or moderate stages of the disease. Research has demonstrated that consistent first-line or conservative management of early and moderate OA could alter the disease trajectory to modify the dynamics of navigating the “funnel” leading to joint replacement surgery.
Fig. 3.
The osteoarthritis funnel.
Ahead of even this ever expanding “funnel” of patients with early and moderate OA, as well as those with advanced OA and in need of a joint replacement, are the likely large youth and adult populations at risk for developing OA, but are individuals who have not yet expressed symptoms or dysfunction. For this population, prevention strategies are critical, and they may have a real impact on lowering their risk of developing OA and disrupting their contribution to the chronically refilling funnel and bottleneck. Although there is potential for real impact here to minimize the development of OA, there is little attention or committed resources.
And here in lies part of the real problem we are faced with – it is not only the needs of current end-stage patients, which are actually a small proportion of the overall OA population, but rather the lack of intense attention and resource allocation to the large numbers sitting in the “funnel” and the potential for that “funnel” to continue expanding without effective surgical, conservative and preventative interventions, further exacerbating the bottleneck and its repeated development. Certainly, in other countries such as the Scandinavian countries and others [[8], [9], [10], [11]], first-line treatments for OA management are having an impact and some efforts for first-line treatment management are being made in Canada [12]. However, a significant shift in attention and resource allocation is required to move further upstream in the disease course, which will have an impact on wait times into the future.
2. Perceptions on joint replacement surgery
Another facet defining the actual problem we are faced with is the perception of and expectations related to joint replacement surgery. Restoration of hips and knees with an artificial joint that replaces a joint damaged beyond repair is one of the most successful orthopedic procedures ever developed, and likely the closest intervention we currently have to “curing” OA – effectively cutting the dysfunctional parts of the joint out of the body and replacing them with a prosthesis. Most people (∼80% or more) who have had their joints replaced go on to live functional pain-free lives, with a return to a good quality of life [13], but a subset still have significant pain [reviewed in 14]. Although effective, it needs to be situated correctly – it is an effective solution for the end-stage of a progressive disease, but it should not be the only “goal” for patients or clinicians. Many years can pass before surgery is a viable option. And these years can be filled with pain, dysfunction and progressive decline in quality of life [15]. Further, research on patient experience suggests that patients would rather avoid surgery if they are able to effectively manage the OA-related symptoms [16]. However, it is challenging to broaden the perspective on OA care within a healthcare system and culture oriented towards crisis management, rather than a longer term approach that involves focused attention and commitment to managing non-curable conditions (i.e. a chronic disease) at earlier stages – where impact can be made by slowing the progression of a disease or preventing its contribution to disability and loss of quality of life. And, delaying or preventing surgery.
3. First-line options for the OA patient prior to surgery
For the patient with recently diagnosed OA of the hip or knee, most of them will be treated to alleviate the pain associated with the condition. However, as the condition progresses, there are a number of both surgical (Table 1) and first-line or conservative (Table 2) treatment options available to them prior to total joint replacement. The advantages and limitations of these surgical options (Table 1) have been recently reviewed [[17], [18], [19]] and some patients derive longer term benefit from these interventions than others. Recently, Shimomura et al. [20] have reported on an experimental tissue engineering approach to use stem cells to repair cartilage defects which often progress to OA. Thus, some of the newer surgical interventions using “regenerative” cell therapies may offer some hope in the future for repair of damaged tissues in early OA with a return to joint integrity and function.
Table 1.
Surgical approaches for treatment of OA.
| Intervention type | Description |
|---|---|
| Total Joint Replacement | Joint components (e.g. menisci, bone, diseased cartilage) replaced (fully or partially) with an artificial prosthesis [46] |
| Chondrocyte Transplantation | Repair of damaged or defective articular cartilage through implantation of autologous cartilage cells/chondrocytes [48,49] |
| High Tibial Osteotomy/Distal Femoral Osteotomy | Realignment of the knee joint by dividing and internally fixing either the tibia or the femur to alter load across the joint [50] |
| Microfracture | Holes are bored into the subchondral bone of OA lesions to release bone marrow cells which repopulate a clot in the lesions, leading to fibrocartilage formation which then covers the cartilage defect/exposed bone [51]. |
| Transplantation of Osteochondral Plugs (autologous/allogeneic) | Bone-cartilage plugs are taken from either non-weight bearing areas (autologous) or allogeneic tissue, and then press fit into defects in the damaged cartilage [52] |
| Stem Cell Implantation | Mesenchymal stem cells (MSCs) derived from bone marrow, fat, synovium, or other tissues are expanded in vitro and implanted into cartilage as either free cells [53,54,56]; MSC incorporated into a scaffold of synthetic or organic molecules and implanted into OA lesions [55,56]; or the MSC in an endogenously produced matrix (TEC) are implanted into OA lesions [20] |
Table 2.
Non-surgical first-line approaches for management and treatment of OA and associated symptoms – from evidence-based to emerging.
| Intervention type | Description |
|---|---|
| Education | Education interventions, in the form of group classes, online support, and counselling sessions, involves the provision of information to patients to ensure an understanding about OA, disease progression, and appropriate interventions and self-management options. Education is recognized as a standard of care and core component of any treatment plan for OA [57]. |
| Exercise | Physical activity or prescribed exercise or supervised exercise programs that effectively manage symptoms of OA and play a role in prevention of OA. For OA specifically, types of exercises recommended include neuromuscular training programs (e.g. Good Living with Arthritis-Denmark (GLA:D) [58,59], aquatic exercises, strength exercises, and land-based exercise [57]. |
| Weight management | Excess weight, and obesity in particular, are identified as a risk factor for development of OA and exacerbation of symptoms for those with an OA diagnosis. Morbid obesity has also been identified as a risk factor for complications during arthroplasty [60,61]. As such, weight loss and weight management programs that introduce various types of nutritional composition are available and recommended [57]. Many are under the supervision of a family physician or registered dietician; however, patients also opt to undertake diets independently. |
| Prebiotics (oral) | Many individuals with obesity have a metabolic syndrome, a syndrome which can influence the symptoms of OA. Part of this syndrome is mediated by change to the gut microbiome. However, treatment with a prebiotic such as inulin/poly-fructose can correct some of these alterations [62], and modify the metabolic syndrome and influence OA symptoms and disease progression [63]. |
| Physical therapy | Physical therapy (or physiotherapy) is a regulated health care profession across Canada. Physical therapist are physical movement specialists, qualified to deliver interventions that aim to rehabilitate and maximize the functioning of joints and muscles [64]. |
| Occupational therapy | Occupational Therapy is a regulated health care profession across Canada. Occupational Therapists provided a range of interventions and services to support and enable the functioning of individuals. They address physical limitations as well as psychosocial factors to enable individuals to carry out their daily activities including self-care, occupational roles and functions, educational pursuits, leisure activities and more. |
| Complementary and Integrative therapies | This group of therapies includes acupuncture, traditional Chinese medicine, naturopathic pharmacotherapy (e.g. vitamins, chondroitin, glucosamine) [65], variably regulated across Canada. The research evidence is limited to support the efficacy or effectiveness of these interventions; however, they are used by individuals living with OA to manage a range of symptoms, including pain and swelling related to osteoarthritis [66]. |
| Anti-inflammatory drugs | Drugs that have active ingredients that reduce inflammation or swelling. Commonly used anti-inflammatories are non-steroidal anti-inflammatory drugs (NSAIDs) [57]. |
| Steroids (corticosteroids, glucocorticoids) | Chemically similar to cortisol, steroids produce an anti-inflammatory and immunosuppressive response. However, frequent use via direct injection into a knee can have adverse effects, so caution is recommended [57]. |
| Pain medications | This class of drugs blocks signals that produce pain. Examples of pain medications include acetaminophen, duloxetine, NSAIDs, aspirin [57]. |
| Hyaluronic Acid (injections; supplements) | A treatment that introduces cross-linked hyaluronic acid into the synovial fluid of a joint such as a knee. Such HA is sold as a device to improve lubrication, but efficacy remains somewhat variable and the evidence is not strong. HA (endogenous) occurs naturally and functions as a joint lubricant. However, with OA progression, its lubricating function is negatively impacted [67,68]. |
| Platelet-Rich Plasma (PRP) injections | Platelets in blood contain growth factors that may function to assist in tissue repair and cell regeneration. Injection of the Platelet-rich Plasma (PRP) fraction from blood into a joint has been reported to improve symptoms of OA such as pain, but results are variable and the evidence is not strong [21]. |
With regard to first-line treatment options, or non-surgical approaches for patients with hip and knee OA, a number of these do require intra-articular injections but others are more focused on exercise and neuromuscular training (Table 2). Some of these approaches are backed by evidence and have been endorsed by organizations [21], but others such as using free autologous stem cells derived from fat or bone marrow, or other sources are only allowed in research trials as Health Canada has placed a moratorium on other uses outside of research [22]. Interestingly, only a subset of patients respond well to a number of the interventions listed in Table 2. For interventions such as glucocorticoids, Platelet-Rich Plasma (PRP), Hyaluronic Acid, and even exercise, there are responders and non-responders [discussed in 21], and evidence for their effectiveness remains lacking or remains controversial. With exercise, it has been reported that some people respond to aerobic exercise, but others respond to resistive exercises [discussed in 23]. The basis for such variability is not yet understood, but its existence means that for many patients they do not have a variety of effective first line treatment options to improve their quality of life with OA prior to total joint replacement. Furthermore, many of the first-line treatment options are often viewed as individual programs, and not as an integrated platform of options optimized for the individual patient. In addition, many patients rely mainly on over-the-counter pain medications until they no longer are effective before seeking out other options when the OA has progressed instead of embracing other options early when they may be more effective in slowing disease progression.
In addition to the variable response to first-line treatment options for OA, many of the surgical treatments are also not available to many patients, in part due to risks associated with factors such as co-morbidities (i.e. poorly controlled diabetes, excessive obesity, cardiovascular complications), and they are deemed not appropriate for a specific intervention based on established criteria, or other factors. Not only do such interventions use many resources for a variable outcome in some patients, some such as the microfracture technique yield a temporary solution even when “successful” due to the fact that the procedure yields fibrocartilage which is less durable than the natural hyaline cartilage and only lasts 3–5 years at best [[24], [25], [26]].
Thus, while exercise protocols do offer relief for many patients with OA (8–12), patients with early OA need new protocols to address their disease to either slow/stop progression, or in the best case scenario, reverse the joint damage to restore function and alleviate the protracted disease course leading to the need for total joint replacement surgery. Furthermore, it is not just new options that are needed, but also integrations of options to develop individualized “platforms” for care early after diagnosis.
4. The “complexity” of osteoarthritis
The other very real issue impacting the effective treatment of OA, and one that may account for some of the variation discussed above, is our understanding of the complexity of the disease. For many years, it was viewed as a disease of articular cartilage degeneration. While still a prevalent concept in some quarters, it is clear that, consistent with the name “osteoarthritis”, OA is an inflammatory disease [27,28]. While OA likely has its origins in the biomechanics of the joint (27), such dysregulation of the mechanical integrity of a joint such as a knee can lead to an inflammatory environment as the OA progresses. Furthermore, OA involves the whole joint as an adaptive and integrative unit [[30], [31], [32]]. Therefore, it is not just a disease of articular cartilage, but also involves other tissues of the joint such as the menisci, ligaments, synovium, fat pads, and the joint capsule, as well as muscles [33,34]. This concept leads to the idea that the joint is an organ system whose optimal workings are derived from the integrated functioning of all of the component parts [[30], [31], [32]], and loss of this integrity leads to OA development and subsequent degeneration of the joint.
Usually, the first indication that the knee or hip “unit” has been compromised is the presentation of pain. Pain, often persistent pain, is the usual symptom that brings a patient to the health professional for advice and diagnosis. Unfortunately, there is often a “disconnect” between the pain and evidence for structural alterations or damage [35]. Because of such patient-to-patient variation, and lack of other tools to definitively diagnosis OA (e.g. biomarkers), often the patients are left to address the symptoms such as pain, and not the disease itself.
5. Is OA one disease or many?
For many years, OA was presented in the literature as a single disease, and many patients were treated as if it was a single disease. More recent thinking has led to the idea that the term “Osteoarthritis” should actually be considered an umbrella term, and likely there are multiple factors leading to end stage joint destruction and loss of function. Thus, multiple mechanisms can contribute to the end stage of loss of cartilage and joint integrity. Such subsets of OA likely include post-traumatic OA (PTOA) following an overt joint injury [36]; “metabolic OA” associated with the metabolic syndrome of obesity [27,28] (although there may also be a biomechanical element due to the increased stress on joints of the lower extremities due to weight); with the remainder loosely defined as idiopathic OA (which is a default term meaning the basis is unknown at the present time) [3].
While not currently defined as a separate form of OA, we would also hypothesize that OA arising in post-menopausal females should perhaps be considered a mechanistically unique form of the disease due to the loss of tissue regulation following decline in hormonal involvement. Before the age of 50, the incidence of knee and hip OA is ∼1:1 (F/M), while after age 50 (and the onset of menopause), the incidence is at a minimum >2:1(F/M) [37], and this is not apparently due to a number of reproductive factors [38]. However, it could also relate to bone shape which differs between males and females [[39], [40]] and changes over time more in females [38]. While commonly categorized with other idiopathic cases of OA at present, the post-menopausal cases may represent a unique form of OA based on how it arises in this subset of females. However, since only a subset of post-menopausal females develop OA, variables other than just loss of hormones must also be at play in these individuals.
Given the heterogeneity of humans, and the variety of factors that can potentially contribute to risk for development of OA and its progression, there may be a need for more personalized first-line treatment management of a patient's disease [41]. Thus, if first line treatments move beyond current modalities, new treatments focused on individual initiators of disease may be warranted.
6. Why has progress in development and implementation of interventions for early OA been so slow to yield success?
There are multiple reasons why progress to enhance the development of early effective interventions has not progressed as rapidly as one might have hoped. Some of these are disease related as discussed above, while others may be research related. Humans are very heterogeneous at multiple levels. Such factors include race, sex, age, genetics, epigenetics, nutrition, anatomy, and other variables. At a species level, such heterogeneity likely serves to enhance survival of the species in response to a variety of threats, but may also complicate development of population-wide interventions for conditions such as OA. Thus, OA may have multiple mechanistic initiators and pathways to progression, with a final common endpoint of loss of cartilage and joint dysfunction. Focusing on the common endpoint means that for the most part, the upstream heterogeneity is not being adequately addressed.
Furthermore, the pharma approach fundamentally has a biological perspective of disease processes. In contrast, OA is a disease of a joint, an integrated organ system that is designed to function in a biomechanically loaded dynamic environment, with specific tissue elements evolved to perform specific tasks which yield a functioning system and element cross-talk, but one that may be at risk for organ failure if adaptation fails when one element is compromised. Thus, appreciation of the biomechanical-biological interface is critical when considering interventions. This is of particular relevance as OA has been suggested to be a disease of biomechanics/mechanics [29].
It is also likely that the preclinical research effort is partially to blame for the slow progress in developing effective interventions to stop and reverse OA progression. First of all, only ∼12% of human OA is likely related to PTOA. However, in part due to a lack of very many “spontaneous” OA models (other than a few preclinical models such as in guinea pigs and some genetic models), most OA research at this level is focused on PTOA. Therefore, progression/translation of findings from such models to heterogeneous patient populations involving individuals with multiple OA subtypes may not be destined for success.
Furthermore, much of the preclinical OA research has been and continues to be performed with inbred rodent models (mostly inbred mice and mostly PTOA models although some chemical and enzymatic models do exist) [[42], [43], [44]]. Therefore, findings with PTOA models may not translate to disease arising from other initiating factors. A limitation of working with such small preclinical models is that it is difficult and challenging to investigate both the biological and biomechanical consequences of a joint injury in sufficient detail. Both biology and biomechanics are critical to the functioning of a joint such as a knee. Larger animal models, such as sheep models [45] can overcome some of these limitations, as both biomechanics and biology parameters can be assessed in detail. Furthermore, one of the important lessons learned from such models is that each sheep must be viewed as an individual research subject due to heterogeneity, just like humans.
Thus, one might ask the question “why do we continue to support research that has a low potential to translate to human patients?” The answer to that question is complex, but likely resides in part, due to the fact that much of it is considered “good science”, readily manipulated using sophisticated methodology, and more focused on experiments that can be done and viewed as “good science”. In addition, there is a primary focus on biology. Thus, much of the current basic research approach likely needs to be revisited and new more translational paradigms implemented.
7. Conclusions
From the above discussion, wait times for hip and knee OA patients in Canada are both the problem and via their cyclic nature, a symptom of a larger problem associated with a lack of attention and resourcing along the entire continuum of OA care, and the lack of progress on understanding the disease origins and progression pathways for subtypes of hip and knee OA. Unless we apply a multi-pronged plan with both short- and long-term orientation that better integrates health care operations and advances in research, the current wait time crisis in Canada will likely continue to be repeated. In the long run, the patient numbers waiting to get through the joint replacement bottleneck will outpace the resources of the Canadian health care system and we will continue on our trajectory of escalating crisis management with increasingly limited access to needed and effective care. While the focus of this article has been on the Canadian perspective, it should be noted that wait times, independent of country or health care system, need new and improved approaches for first-line treatment or even preventative measures to enhance the quality of life for both patients with early OA and those at risk for development of OA, and to effectively manage the continuous wait times crises.
Author contributions
DAH conceived of the manuscript and wrote the first drafts. AKR provided input on the conception and drafting of the manuscript. JW and JR provided input into the final manuscript. All agree with its submission to Osteoarthritis & Cartilage OPEN.
Declaration of competing interest
The authors declare they have no conflicts of interest to disclose.
Acknowledgements
The authors thank Drs. Cyril B. Frank (deceased), Nigel G. Shrive, and many colleagues at the Canadian Arthritis Network for interesting discussions on this topic over the past two decades. In addition, discussions with members of the Alberta Innovates Health Solutions OA Team, the BJH SCN Leadership Team, the BJH SCN OA Conservative Working Group, as well as those with our partners at the Alberta Bone and Joint Health Institute (ABJHI)and the McCaig Institute for Bone & Joint Health are gratefully acknowledged. The ABJHI has a critical function in orthopedic data management and analytics in Alberta. Financial support for the preparation of this manuscript was provided by the AHS Strategic Clinical Networks Program.
Contributor Information
David A. Hart, Email: hartd@ucalgary.ca.
Jason Werle, Email: Jason.Werle@albertahealthservices.ca.
Jill Robert, Email: Jill.Robert@albertahealthservices.ca.
Ania Kania-Richmond, Email: Anna.kania-richmond@ahs.ca.
References
- 1.Alberta bone and joint health Institute (ABJHI-1), the osteoarthritis crisis in Alberta: access, quality, and long-term planning. https://www.albertaboneandjoint.com/wp-content/uploads/2019/10/ABJHI_Osteoarthritis_Crisis_in_Alberta_2019.pdf Retrieved from:
- 2.Vina E.R., Kwoh C.K. Epidemiology of osteoarthritis: a literature update. Curr. Opin. Rheumatol. 2018;30:160–167. doi: 10.1097/BOR.0000000000000479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mobasheri A., van Spil W.E., Budd E., Uzieliene I., Bernotiene E., Bay-Jensen A., et al. Molecular taxonomy of osteoarthritis for patient stratification, disease management, and drug development: biochemical markers associated with emerging clinical phenotypes and molecular endotypes. Curr. Opin. Rheumatol. 2019;31:80–89. doi: 10.1097/BOR.0000000000000567. [DOI] [PubMed] [Google Scholar]
- 4.Nevitt M.C., Felson D.T. Sex hormones and the risk for osteoarthritis in women: epidemiological evidence. Ann. Rheum. Dis. 1996;55:673–676. doi: 10.1136/ard.55.9.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hussain S.M., Cicuttini F.M., Alyousef B., Wang Y. Female hormonal factors and osteoarthritis of the knee, hip and hand. A narrative review. Climacteric. 2018;21:132–139. doi: 10.1080/13697137.2017.1421926. [DOI] [PubMed] [Google Scholar]
- 6.Marshall D.A., Vanderby S., Barnabe C., MacDonald K., Maxwell C., Mosher D., et al. Estimating the burden of osteoarthritist to plan for the future. Arthritis Care Res. 2015;67:1379–1386. doi: 10.1002/acr.22612. [DOI] [PubMed] [Google Scholar]
- 7.Marshall D.A., Liu X., Shahid R., Bertazzon S., Seidel J.E., Patel A.B., et al. Geographic variation in osteoarthritis prevalence in Alberta: a spatial analysis approach. Appl. Geogr. 2019;103:112–121. doi: 10.1016/j.apgeog.2019.01.004. [DOI] [Google Scholar]
- 8.Better management of patients with OsteoArthritis. http://boa.registercentrum.se/in-english/better-management-of-patients-with-osteoarthritis-boa/p/By_o8GxVg
- 9.ActiveOA-Active living with osteoarthritis. http://aktivmedartrose.no/english
- 10.Treat hip and knee pain from home (Joint Academy) http://www.jointacademy.com/us/en/
- 11.Allen K.D., Woolson S., Hoenig H.M., Bongiomi D., Byrd J., Caves K., et al. Stepped exercise program for patients with knee osteoarthritis: a randomized controlled trial. Ann. Intern. Med. 2020 doi: 10.7326/M20-4447. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis A.M., Wong K.D., Robarts S., Skou S., McGlasson R., Roos E. Cross-cultural adaptation and implementation of Good Life with osteoarthritis in Denmark (GLA:D); group education and exercise for hip and knee osteoarthritis is feasible in Canada. Osteoarthritis Cartilage. 2018;26:112–219. doi: 10.1016/j.joca.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 13.Hawker G.A., Conner-Spady B.L., Bohm E., Dunbar M.J., Jones C.A., Ravi B., et al. The relationship between patient-reported readiness for total knee arthroplasty and likelihood of a good outcome at one year. Arthritis Care Res. 2021 doi: 10.1002/acr.24562. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 14.Wluka A.E., Yan M.K., Lim K.Y., Hussain S.M., Cicuttini F.M. Does preoperative neuropathic-like pain and central sensitisation affect post-operative outcomes of knee replacement for osteoarthritis? A systematic review and meta analysis. Osteoarthrtitis Cartilage. 2020;28:1403–1411. doi: 10.1016/j.joca.2020.07.010. [DOI] [PubMed] [Google Scholar]
- 15.Bryk C., Lewis T.R., Penman C., Miller J., Teare S. University of Calgary; Calgary, AB: 2013. The Experience of Waiting for Help with Osteoarthritis (Unpublished Internship Report)https://dspace.ucalgary.ca/handle/1880/109968 Retrieved from: [Google Scholar]
- 16.Miller J.L., Teare S.R., Marlett N., Shklarov S., Marshall D.A. Support for living a meaningful life with osteoarthritis: a patient-to-patient research study. Patient. 2016;9:457–464. doi: 10.1007/s40271-016-0169-9. [DOI] [PubMed] [Google Scholar]
- 17.Chimutengwende-Gordon M., Donaldson J., Bentley G. Current solutions for treatment of chronic articular cartilage defects in the knee. EFFORT Open Rev. 2020;5:156–163. doi: 10.1302/2058-5241.5.190031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Du D., Peichun H., Zhenzhong Z., Zhang C. Current surgical options and innovations for repairing cartilage defects in the femoral head. J. Orthop. Translat. 2019;21:122–128. doi: 10.1016/j.jot.2019.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Simon T.M., Jackson D.W. Articular Cartilage: injury pathways and treatment options. Sports Med. Arthrosc. Rev. 2018;26:31–39. doi: 10.1097/00132585-200609000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Shimomura K., Yasui Y., Koizumi K., Chijimatsu R., Hart D.A., Yonetani Y., et al. First-in-human pilot study of implantation of a scaffold-free tissue-engineered construct generated from autologous synovial mesenchymal stem cells for repair of knee chondral lesions. Am. J. Sports Med. 2018;46:2384–2393. doi: 10.1177/0363546518781825. [DOI] [PubMed] [Google Scholar]
- 21.Kydd A.S.R., Hart D.A. Efficacy and safety of platelet-rich plasma injections for osteoarthritis. Curr. Treat. Opt. Rheumatol. 2020;6:87–98. [Google Scholar]
- 22.Murray I.R., Chahla J., Frank R.M., Piuzzi N.S. Rogue stem cells clinics. Bone Joint Lett. J. 2020;102-B:148–152. doi: 10.1302/0301-620X.102B2.BJJ-2019-1104.R1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hart D.A., Zernicke R.F. Optimal human functioning requires exercise across the lifespan: mobility in a 1g environment is intrinsic to the integrity of multiple biological systems. Front. Physiol. 2020;11:156. doi: 10.3389/fphys.2020.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Erggelet C., Vavken P. Microfracture for the treatment of cartilage defects in the knee joint- a golden standard? J. Clin. Orthop. Trauma. 2016;7:145–152. doi: 10.1016/j.jcot.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Haien Z., Jiachang W., Qiang L., Yufeng M., Zhenwei J. Osteochondral autologous transplantation compared to microfracture for treating osteochondral defect: an updated meta-analysis of randomized controlled trials. J. Knee Surg. 2018;31:341–347. doi: 10.1055/s-0037-1603798. [DOI] [PubMed] [Google Scholar]
- 26.York P.J., Wydra F.B., Belton M.E., Vidal A.F. Joint preservation techniques in orthopaedic surgery. Sports Health. 2017;9:545–554. doi: 10.1177/1941738117712203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Berenbaum F., Walker C. Osteoarthritis and inflammation: a serious disease with overlapping phenotypic patterns. Postgrad. Med. 2020;132:377–384. doi: 10.1080/00325481.2020.1730669. [DOI] [PubMed] [Google Scholar]
- 28.Courties A., Berenbaum F., Sellam J. The phenotypic approach to osteoarthritis: a look at metabolic syndrome-associated osteoarthritis. Joint Bone Spine. 2019;86:725–730. doi: 10.1016/j.jbspin.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 29.Felson D. Osteoarthritis is a disease of mechanics. Ostoearthritis Cartilage. 2013;21:10–15. doi: 10.1016/j.joca.2012.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Radin E.L., Burr D.B., Caterson B., Fyhrie D., Brown T.D., Boyd RD R.D. Mechanical determinants of osteoarthritis. Semin. Arthritis Rheum. 1991;21:12–21. doi: 10.1016/0049-0172(91)90036-y. [DOI] [PubMed] [Google Scholar]
- 31.Frank C.B., Shrive N.G., Boorman R.S., Lo I.K.Y., Hart D.A. New perspectives on bioengineering of joint tissues: joint adaptation creates a moving target for engineering replacement tissues. Ann. Biomed. Eng. 2004;32:458–465. doi: 10.1023/b:abme.0000017548.85451.b7. [DOI] [PubMed] [Google Scholar]
- 32.Loeser R.F., Goldring S.R., Scanzello C.R., Goldring M.B. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64:1697–1707. doi: 10.1002/art.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Godziuk K., Prado C.M., Woodhouse L.J., Forhan M. The impact of sarcopenic obesity on knee and hip osteoarthritis: a scoping review. BMC Musculoskelet Disord. 2018;19:271. doi: 10.1186/s12891-018-2175-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Herzog W., Longino D., Clarke A. The role of muscles in joint adaptation and degeneration. Langenbeck's Arch. Surg. 2003;388:305–315. doi: 10.1007/s00423-003-0402-6. [DOI] [PubMed] [Google Scholar]
- 35.Pan F., Tian J., Munugoda I.P., Graves S., Lorimer M., Cicuttini F., et al. Do knee pain phenotypes have different risks for total knee replacement? J. Clin. Med. 2020;9:632. doi: 10.3390/jcm9030632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lohmander L.S., Englund P.M., Dahl L.L., Roos E.M. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am. J. Sports Med. 2017;35:1760–1769. doi: 10.1177/0363546507307396. [DOI] [PubMed] [Google Scholar]
- 37.Sirkanth V.K., Fryer J.L., Zhai G., Winzenberg T.M., Hosmer D., Jones G. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthritis Cartilage. 2015;13:769–781. doi: 10.1016/j.joca.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 38.Wang A., Zawadzki N., Hedlin H., LeBlanc E., Budrys N., Van Horn L., et al. Reproductive history and osteoarthritis in the women's health initiative. Scand. J. Rheumatol. 2020;6:10. doi: 10.1080/03009742.2020.1751271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wise B.L., Niu J., Zhang Y., Pang J., Lynch J.A., Lane N.E. Bone shape mediates the relationship between sex and incident knee osteoarthritis. BMC Muscoskel. Disord. 2018;19:331. doi: 10.1186/s12891-018-2251-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li K., Cavaignac E., Xu W., Cheng Q., Telmon N., Huang W. Morphometric evaluation of the knee in Chinese population reveals sexual dimorphism and age-related differences. Int. Orthop. 2018;42:2349–2356. doi: 10.1007/s00264-018-3826-x. [DOI] [PubMed] [Google Scholar]
- 41.Karsdal M.A., Christensen C., Ladel C., Henriksen K., Bay-Jensen A.C. Osteoarthritis—a case for personalized health care? Osteoarthritis Cartilage. 2014;22:7–16. doi: 10.1016/j.joca.2013.10.018. [DOI] [PubMed] [Google Scholar]
- 42.Malfait A.M., Little C.B. On the predictive utility of animal models of osteoarthritis. Arthritis Res. Ther. 2015;17:225. doi: 10.1186/s13075-015-0747-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blaker C.L., Clarke E.C., Little C.B. Using mouse models to investigate the parthophysiology, treatment, and prevention of post-traumatic osteoarthritis. J. Orthop. Res. 2017;35:424–439. doi: 10.1002/jor.23343. [DOI] [PubMed] [Google Scholar]
- 44.Van der Kraan P.M. Factors that influence outcome on experimental osteoarthritis. Osteoarthritis Cartilage. 2017;25:369–375. doi: 10.1016/j.joca.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 45.Barton K.I., Shekarforoush M., Heard B.J., Sevick J.L., Vakil P., Atarod M., et al. Use of pre-clinically induced models to understand biomechanical and biological consequences of PTOA development. J. Orthop. Res. 2017;35:454–465. doi: 10.1002/jor.23322. [DOI] [PubMed] [Google Scholar]
- 46.Gooch K., Marshall D.A., D Faris P., Khong H., Wasylak T., Pearce T., et al. Comparative effectiveness of alternative clinical pathways for primary hip and knee joint replacement patients: a pragmatic randomized, controlled trial. Osteoarthritis Cartilage. 2020;20:1086–1094. doi: 10.1016/j.joca.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 47.Alberta bone and joint health Institute, wait times for hip and knee arthroplasty. https://www.albertaboneandjoint.com/for-patients/wait-times/ Retrieved from:
- 48.Mistry H., Connock M., Pink J., Shyangdan D., Clar C., Royle P., et al. Autologous chondrocyte implantation in the knee: systematic review and economic evaluation. Health Technol. Assess. 2017;21:1–294. doi: 10.3310/hta21060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vasiliadis S., Wasiak J. Autologous chondrocyte implantation for full thickness articular cartilage defects of the knee. Cochrane Database Syst. Rev. 2010;10 doi: 10.1002/14651858.CD003323.pub3. CD003323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Coventry M.B., Ilstrup D.M., Wallrichs S.L. Proximal tibial osteotomy. A critical long-term study of eighty-seven cases. J. Bone Joint Surg. 1993;75:196–201. doi: 10.2106/00004623-199302000-00006. [DOI] [PubMed] [Google Scholar]
- 51.Miller B.S., Steadman J.R., Briggs K.K., Rodrigo J.J., Rodkey W.G. Patient satisfaction and outcome after microfracture of the degenerative knee. J. Knee Surg. 2014;17:13–17. doi: 10.1055/s-0030-1247141. [DOI] [PubMed] [Google Scholar]
- 52.Gracitelli G.C., Moraes V.Y., Franciozi C.E.S., Luzo M.V., Belloti J.C. Surgical interventions (microfracture, drilling, mosaicplasty, and allograft transplantation) for treating isolated cartilage defects of the knee in adults. Cochrane Database Syst. Rev. 2016;9 doi: 10.1002/14651858.CD010675.pub2. CD010675. Published online 2016 Sep. 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De Bari C., Roelofs A.J. Stem cell-based therapeutic strategies for cartilage defects and osteoarthritis. Curr. Opin. Pharmacol. 2018;40:74–80. doi: 10.1016/j.coph.2018.03.009. doi.org/10.1016/j.coph.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 54.Debnath U.K. Mesenchymal stem cell therapy in chondral defects of knee: current concept review. Indian J. Orthop. 2020;54:1–9. doi: 10.1007/s43465-020-00198-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yamagata K., Nakayamada S., Tanaka Y. Use of mesenchymal stem cells seeded on the scaffold in articular cartilage repair. Inflamm. Regen. 2018;38:4. doi: 10.1186/s41232-018-0061-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Le H., Xu W., Zhuang X., Chang F., Wang Y., Ding J. Mesenchymal stem cells for cartilage regeneration. J. Tissue Eng. 2020;26:11. doi: 10.1177/2041731420943839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.R Bannuru R., C Osani M., E Vaysbrot E., K Arden N., Bennell K., A Bierma-Zeinstr S.M., et al. OARSI guidelines for the non-surgical management of knee, hip and polyarticular osteoarthritis. Osteoarthritis Cartilage. 2019;27:1578–1589. doi: 10.1016/j.joca.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 58.Skou T.S., Roos E.M. Good Life with osteoArthritis in Denmark (GLA:D™): evidence-based education and supervised neuromuscular exercise delivered by certified physiotherapists nationwide. BMC Muscoskel. Disord. 2017;18 doi: 10.1186/s12891-017-1439-y. Article number 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jones C., Ellis K., Beaupre L.A., Robert J., Slomp M., D.A. Hart A., et al. GLA:D experience – evaluation of implementing a group-based osteoarthritis exercise program in Alberta, Canada. Osteoarthritis and Cartilage – abstract only. April 1, 2020;28(supplement 1) S449. [Google Scholar]
- 60.Godziuk K., Kania-Richmond A., Hart D.A. Obesity: implications for patients with obesity. a knowledge synthesis of review articles (2010-2017) on treatment of hip and knee osteoarthritis in patients with obesity. https://www.albertahealthservices.ca/assets/about/scn/ahs-scn-bjh-osteoarthritis-and-obesity-white-paper-final.pdf Retrieved from: A knowledge synthesis of review articles (2010-2017) on treatment of hip and knee osteoarthritis in patients with obesity. Accessed on Sept 4 2020. Retrieved from:
- 61.Koonce R.C., Bravman J.T. Obesity and osteoarthritis: more than just wear and tear. J. Am. Acad. Orthop. Surg. 2013;21:161–169. doi: 10.1093/rheumatology/keu464. [DOI] [PubMed] [Google Scholar]
- 62.Klancic T., Reimer R.A. Gut microbiota and obesity: impact of antibiotics and prebiotics and potential for musculoskeletal health. J. Sport Health Sci. 2020;9:110–118. doi: 10.1016/j.jshs.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fortuna R., Sharkey K.A., Hart D.A., Reimer R.A. Proceed. Ann. Meeting Orthop. Res. Soc. Poster 2266. 2020. A 3-month prebiotic diet intervention can improve knee joint function in adults with obesity suffering from knee osteoarthritis.https://www.org.ors/2020annualmeeting/ [Google Scholar]
- 64.Physiotherapy Alberta College and Association About physiotherapy. https://www.physiotherapyalberta.ca/public_and_patients/about_physiotherapy Accessed on Sept 4 2020. Retrieved from:
- 65.Onishi K., Utturkar A., Chang E., Panush R., Hata J., Perret-Karimi D. Osteoarthritis: a critical review. Crit. Rev. Phys. Rehabil. Med. 2012;24:251–264. doi: 10.1615/CritRevPhysRehabilMed.2013007630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mayo clinic. “Acupuncture”. www.mayoclinic.org/tests-procedures/acupuncture/about/pac-20392763 Retrieved from:
- 67.Bowman S., Awad M.E., Hamrick M.W., Hunter M., Fulzele S. Recent advances in hyaluronic acide based therapy for osteoarthritis. Clin. Transl. Med. 2018;7:6. doi: 10.1186/s40169-017-0180-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hermans J., A Bierma-Zeinstra S.M., Bos P.K., Niesten D.D., Verhaar J.A.N., Reijman M. The effectiveness of high molecular weight hyaluronic acid for knee osteoarthritis in patients in the working age: a randomised controlled trial. BMC Muscoskel. Disord. 2019;20 doi: 10.1186/s12891-019-2546-8. Article number 196. [DOI] [PMC free article] [PubMed] [Google Scholar]