Abstract
Objective
This study aimed to improve our understanding of the relationship between bone and cartilage by characterizing the morphological coupling between these mechanosensitive tissues exposed to the same mechanical environment within each knee. Specifically, it reanalyzed a prior dataset to test the hypothesis that the locations of thickest cartilage and densest subchondral bone are correlated in non-osteoarthritic femoral condyles.
Method
Anatomically standardized maps of cartilage thickness (CTh) and subchondral bone mineral density (sBMD) were calculated for 50 non-osteoarthritic distal femurs based on computed tomography arthrography examinations. The locations of thickest CTh and densest sBMD were identified in the load-bearing region of the medial and lateral compartments, and correlation analyses were performed to quantify the associations between these locations, with inclusion of age, gender, femoral bone size and femorotibial angle as cofounding variables. Paired Student's t-tests were also performed to compare CTh and sBMD locations.
Results
Locations of thickest CTh and densest sBMD were positively correlated along the anteroposterior direction in both compartments (r ≥ 0.45, p ≤ 0.001). Furthermore, thickest CTh was more posterior than densest sBMD in the medial (p = 0.014) and lateral (p < 0.001) compartments, and more lateral than densest sBMD in the lateral compartment (p < 0.001). On average, these location differences were of 1.3, 5.3 and 2.1% of the subchondral bone size.
Conclusion
The positive spatial relationship between the locations of thickest CTh and densest sBMD supports the idea of a functional cartilage/subchondral bone unit with morphological coupling conditioned by the individual loading pattern.
Keywords: CT arthrography, Knee joint, Loading, Osteochondral unit
Abbreviations: CT, computerized tomography; OA, osteoarthritis; CTh, cartilage thickness; sBMD, subchondral bone mineral density
1. Introduction
While it is now established that knee osteoarthritis (OA) is a complex disease affecting the whole joint [1,2], there remains a lack of understanding of the interactions between the diverse elements of the disease. Indeed, while numerous knee properties have been described at various stages of the disease [1,2], little is known about their relationships. Characterizing the relationships seems however necessary at this moment because recent disease models have suggested that the relationships could be more meaningful than isolated properties to understand OA pathophysiology [3].
One relationship of particular interest is between cartilage thickness (CTh) and subchondral bone mineral density (sBMD) [4], two hallmarks of the disease. Focusing on these two properties is particularly motivated by the fact that cartilages and subchondral bones are mechanosensitive tissues thought to interact as functional units. There is growing evidence of a biomechanical coupling between these tissues conditioned by gait mechanics [2,5,6]. For example, in non-OA knees the ratio of bone mineral density in the medial and lateral compartments was shown to be positively correlated with loading during gait (knee adduction moment) [7], and a similar positive correlation was described for CTh and the knee adduction moment [6]. Nevertheless, there is a paucity of studies analysing both CTh and sBMD in the same knees [8,9], and therefore few data on the relationship between these two properties.
Interestingly, a recent study measuring CTh and sBMD simultaneously using computed tomography arthrography (CT arthrogram) reported an association between the magnitudes of CTh and sBMD in non-OA femoral condyles [8]. Specifically, thicker CTh was positively correlated with denser sBMD. Although this later study brought new insights to the relationship between CTh and sBMD and thus to the understanding of the osteochondral unit, it only analysed CTh and sBMD magnitudes. Since CTh and sBMD have been shown to vary spatially [[10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20]], our understanding of non-OA femurs could be enhanced by determining if thicker CTh and denser sBMD are also correlated spatially. Establishing such correlations for non-OA knees could be an important step towards characterizing knee homeostasis. It could also contribute to the development of new methods for assessing early changes leading to knee OA, as some relationships between CTh, sBMD and gait mechanics have been reported to differ with the disease [3].
Thus, the purpose of this study was to reanalyse the dataset in Ref. [8], using novel methods, to test the hypothesis that the locations of thickest CTh and densest sBMD are positively correlated along the anteroposterior and mediolateral directions in the medial and lateral load-bearing regions of non-OA femoral condyles. A secondary objective was to compare the locations of thickest CTh and densest sBMD.
2. Methods
2.1. Population and image acquisition
The dataset reanalyzed in this study consisted of 50 CT arthrograms of non-OA knees from 32 females and 18 males (median age: 58.7 [IQR 6.6] years; biepicondylar femoral diameter: 7.9 [0.9] cm; femorotibial angle: 4.4 [2.6] degrees). The dataset and the acquisition procedure were described previously in details [8]. Briefly, it was composed of consecutive patients aged over 50 years referred to a single imaging center over a 24-month period who had a CT arthrogram and weight-bearing radiographs of their knees the same day. The exclusion criteria were: a Kellgren-Lawrence grade > 1 in any of the medial, lateral or trochlear compartments [21], any imaging sign of previous knee surgery (including knee replacement surgery, ligamentoplasty, cartilage or meniscal repair procedures), post-traumatic or rheumatological disorders (including the presence of intra-articular calcifications), and poor image quality. This pre-existing dataset was of sufficient size to test the hypothesis in this study, as a sample size calculation estimated that a minimum of 47 knees was necessary to test for two-tailed correlation of r ≥ 0.4 with α of 0.05 and power of 0.80. Expected correlations for the sample size calculation were based on a recent systematic review of relationships between CTh, sBMD and gait mechanics [3]. The study was approved by the institutional ethics committee without requirement for informed consent of the participants due to the retrospective study design.
2.2. Image acquisition and processing
CT examinations were performed after intraarticular injection of 10 mL of ionic contrast material [10]. Patients were lying supine, with the knees extended. Acquisitions were made using a 40-detector row CT scanner (Somatom Definition AS; Siemens Healthcare, Forchheim, Germany) with the following parameters: tube voltage of 120 kVp; reference tube current-time product of 350 mAs with the application of a dose modulation protocol (Care Dose 4D; Siemens Healthcare); bone convolution kernel (U70u), voxel size of 0.3 × 0.3 × 0.3 mm.
Two-dimensional (2D) anatomically standardized maps of CTh and sBMD were obtained using previously published methods, summarized as follows and illustrated in Fig. 1. Each CT arthrogram was segmented semi-manually to build three-dimensional (3D) models of distal femoral bone and cartilage [10,22]. CTh and sBMD values were then calculated for each subchondral vertex of the bone model by, respectively, measuring the distance between the bone and cartilage models [22] and averaging the CT intensity of the bone voxels within a distance of 3 mm [10]. The inter-observer reliability of these magnitude measurement methods was shown to be adequate in prior studies [8,23]. Since CTh and sBMD measurements were paired (i.e., performed at the same positions in the CT frame of each knee), the 3D CTh and sBMD maps were automatically registered for each femur. Lastly, a registration technique was applied to standardize the individual 3D CTh and sBMD maps and allow comparing the 50 femurs [22]. This resulted in 2D anatomically standardized maps of CTh and sBMD for each knee.
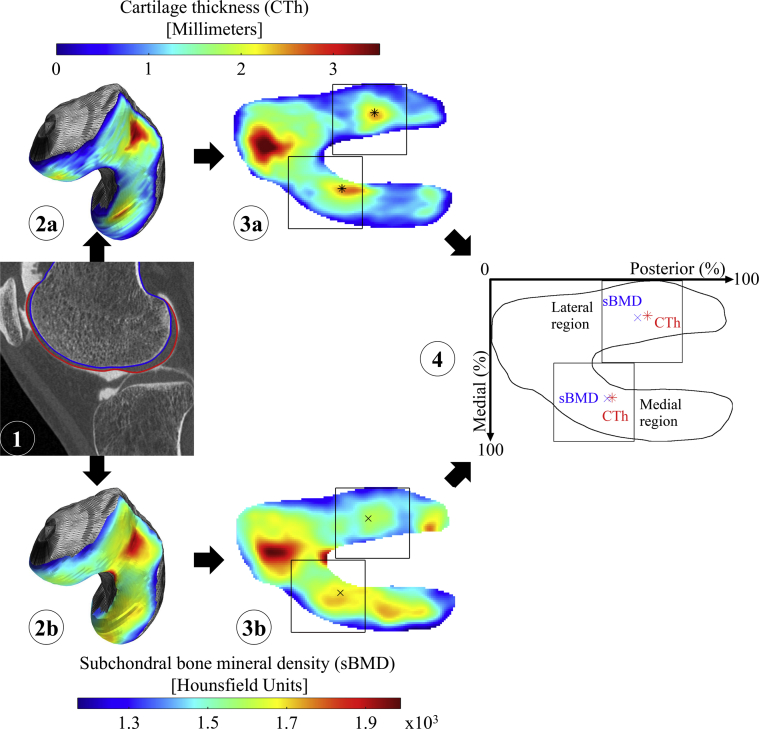
Fig. 1.
Illustration of the method used to measure the location of the thickest CTh and densest sBMD in an individual femur. First, the femoral bone and cartilage are segmented on the CT arthrogram (1), yielding three-dimensional maps of CTh (2a) and sBMD (2b). Each map is then anatomically standardized (3) and the locations of thickest CTh (star in 3a) and densest sBMD (cross in 3b) are identified in the medial (top) and lateral (bottom) load-bearing regions of interest, yielding a pair of locations for each region of interest (4).
2.3. Locations of thickest CTh and densest sBMD
For each femur, the locations of thickest CTh and densest sBMD were identified in medial and lateral load-bearing regions of interest common to both properties using a weighted average method (Fig. 1) [24]. The regions of interest were defined as squares with sides' length equal to half the mediolateral size of the standardized maps. Mediolaterally, they were positioned in each half of the map to avoid any overlap. Their anteroposterior positions were determined independently for the medial and lateral compartments using an automatized iterative method [11]. Each iteration of this method consisted in determining the locations of thickest CTh and densest sBMD of the 50 femurs in the current regions of interest and then in updating the position of the regions of interest by centering them on the average location of the 50 thickest CTh and densest sBMD locations. Iterations were repeated until the convergence of both regions of interest to a constant position. The regions used in this study to locate the thickest CTh and densest sBMD in 2D anatomically standardized maps agreed with regions described in literature [11]. In addition, five knees randomly selected were processed twice by different observers (segmentation and location of thickest CTh and densest sBMD) to assess the inter-observer reliability. This evaluation reported excellent reliability, with an intraclass correlation coefficient (ICC) of 0.99. The locations of thickest CTh and densest sBMD were expressed in percent of the anteroposterior and mediolateral sizes of the standardized maps.
2.4. Statistical analysis
First, four separate Pearson correlations were used to assess the relationships between the locations of thickest CTh and densest sBMD along the anteroposterior and mediolateral directions in the medial and lateral compartments. Second, since gender, age, biepicondylar diameter and femorotibial angle have been shown to be associated with CTh and sBMD [25,26], partial correlations were calculated to describe the relationship between the locations of thickest CTh and densest sBMD while controlling for these four confounding variables [22]. Additionally, paired Student's t-tests were performed to compare the locations of thickest CTh and densest sBMD along both directions in both compartments. Parametric statistics was used after confirmation of the normal distribution of the data using Kolmogorov-Smirnov tests. All statistical calculations were done with R version 3.4.2 (R Core Team, 2017), and an α-level of 5% was considered statistically significant for all tests.
3. Results
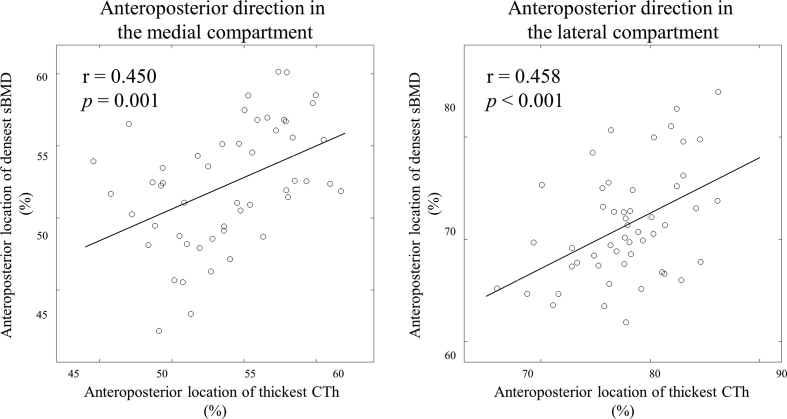
The Pearson correlations indicated that, along the anteroposterior direction, the locations of thickest CTh was statistically significantly positively correlated to the location of densest sBMD in both the medial (r = 0.45, 95% confidence interval (CI) [0.20, 0.65], p = 0.001) and the lateral (r = 0.46, 95% CI [0.21, 0.65], p < 0.001) compartments (Table 1, Fig. 2). No relationship along the mediolateral direction achieved statistical significance (p ≥ 0.124). The partial correlation analyses resulted in the same statistically significant relationships between the locations of thickest CTh and densest sBMD (Table 1).
Table 1.
Relationships between the locations of thickest cartilage thickness (CTh) and densest subchondral bone mineral density (sBMD) in the medial and lateral femoral compartments.
| Pearson correlation |
Partial correlationa |
|||
|---|---|---|---|---|
| R [95% CI] | P–value | R [95% CI] | P–value | |
| Medial compartment | ||||
| Anteroposterior direction | 0.45 [0.20, 0.65] | 0.001 | 0.46 [0.19, 0.66] | 0.001 |
| Mediolateral direction | 0.22 [-0.06, 0.47] | 0.124 | 0.22 [-0.07, 0.48] | 0.133 |
| Lateral compartment | ||||
| Anteroposterior direction | 0.46 [0.21, 0.65] | <0.001 | 0.38 [0.10, 0.61] | 0.009 |
| Mediolateral direction | 0.07 [-0.21, 0.34] | 0.636 | 0.09 [-0.21, 0.37] | 0.553 |
P-values in bold indicate statistically significant relationships between thickest CTh and densest sBMD locations (p < 0.05). Statistically significant relationships are illustrated in Fig. 2.
Partial correlation between CTh and sBMD locations while controlling for age, gender, biepicondylar femoral diameter and femorotibial angle confounding variables.
Fig. 2.
Scatter plots of the location of densest sBMD with respect to the location of thickest CTh along the anteroposterior direction in the medial (left) and lateral (right) compartments.
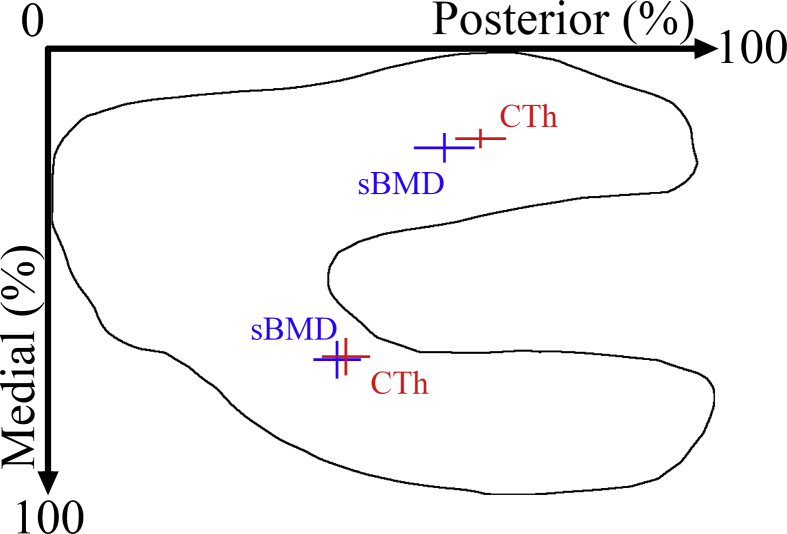
The location of thickest CTh was more posterior than the location of densest sBMD in both the medial (43.6 ± 3.5 vs. 44.9 ± 3.6% of the anteroposterior standardized map size; p = 0.014) and lateral (59.5 ± 4.5 vs. 64.8 ± 3.8%; p < 0.001) compartments (Table 2, Fig. 3). In addition, the location of the densest sBMD along the mediolateral direction was more medial than the location of thickest CTh in the lateral compartment (23.0 ± 3.3 vs. 20.9 ± 2.2% of the mediolateral standardized map size; p < 0.001). To help the interpretation, for an average knee with an anteroposterior and mediolateral subchondral bone size of 96 mm and 64 mm [22], respectively, these differences would correspond on average to 1.2 mm along the anteroposterior direction in the medial compartment, to 5.1 mm along the anteroposterior direction in the lateral compartment, and to 1.3 mm along the mediolateral direction in the lateral compartment.
Table 2.
Locations and magnitudes of thickest cartilage thickness (CTh) and densest subchondral bone mineral density (sBMD) in the medial and lateral femoral compartments.
| Thickest CTh | Densest sBMD | P-values | |
|---|---|---|---|
| Medial compartment | |||
| Anteroposterior location | 44.9 ± 3.6% | 43.6 ± 3.5% | 0.014 |
| Mediolateral location | 69.3 ± 4.2% | 70.0 ± 4.2% | 0.357 |
| Magnitude | 2.1 ± 0.4 mm | 1697.5 ± 70.1 HU | n/a |
| Lateral compartment | |||
| Anteroposterior location | 64.8 ± 3.8% | 59.5 ± 4.5% | <0.001 |
| Mediolateral location | 20.9 ± 2.2% | 23.0 ± 3.3% | <0.001 |
| Magnitude | 2.1 ± 0.5 mm | 1577.3 ± 71.8 HU | n/a |
Location and magnitude data are presented as mean ± standard deviation of the 50 knees. Location data are in percent of the anteroposterior or mediolateral sizes of the standardized maps (see Fig. 3), whereas magnitude data are in mm for CTh and in Hounsfield units (HU) for sBMD.
P-values in bold indicate statistically significant differences between CTh and sBMD locations (p < 0.05).
Fig. 3.
Average (± standard deviation) location of thickest CTh (red) and densest sBMD (blue) for the 50 knees.
4. Discussion
In this paper, we showed that the locations of thickest CTh and densest sBMD are positively correlated along the anteroposterior direction of non-OA femoral condyles, suggesting that spatial morphological variations are coupled between subchondral bones and cartilages consistently with the main motion of the joint. These results are consistent with the current understanding of the knee function. Indeed, cartilage growth and bone densification are both stimulated by mechanical loading [24,27]; load experienced by both tissues should occur in neighboring locations within each femur [2,5]; ambulatory mechanics differ among individuals, leading to variations in the areas of the joint exposed to larger loads [28,29].
The positive relationships in non-OA knees between CTh and sBMD along the anterior-posterior direction are in line with previous reports showing that the spatial variations in thickest CTh location were associated with variations in sagittal plane kinematics during walking [24,30]. Thus, the current results taken together with prior gait studies provide additional support for the idea of a local coupling between articular cartilage and subchondral bone related to each tissue adapting to gait mechanics. In particular, this observation suggests that mechanical signals can transcend scales from whole-body mechanics to a response detectable at the scale of the tissue. The coupling observed in the present study reveals a clinical importance when considered together with prior descriptions of spatial shifts in femorotibial loading during walking with pathological conditions associated to knee OA, such as rupture of the anterior cruciate ligament [24]. In fact, changes in loading pattern without adaptation of cartilage and/or bone to the new loading condition can lead to a degenerative pathway [6].
The results in this study bring additional support to OA pathophysiology models such as the “Integrated Joint System (IJS) [3]”, which postulate that knee health depends on homeostatic relationships where tissue properties are mutually adapted. Although only a few studies so far focused on the relationships between knee properties, some relationships have been shown to differ with knee OA [3]. Specifically, a previous work reported a positive relationship between CTh and sBMD magnitudes in non-OA femurs and a negative relationship in OA femurs [8]. The transition from positive to negative relationships with OA could provide a basis for early disease detection as it might reflect metabolic changes that occur early in the disease [31]. For example, the greater metabolic activity of bone relative to cartilage would suggest different morphological adaptations to loading changes in cartilage compared to bone as OA develops. Thus, the detection sensitivity could be enhanced by using a metric based on the coupling of CTh and sBMD rather than by analysing these properties in isolation. Altogether, these observations warrant further research on the relationship between CTh and sBMD as well as the extension to other properties. The interest of extending to other properties in the future is particularly well supported by a recent study reporting positive correlations between thicker CTh and thicker subchondral bone plate in normal sheep knees and changes in the relationships between these properties after partial meniscectomy [15].
This study presents some limitations, including its retrospective design that limited the confounding variables that could be included in the analyses. Further studies should consider other confounding variables such as participants' size and body mass index. Similarly, taking into account the inter-individual differences in local knee morphology could help explain the absence of correlation along the mediolateral direction [32,33]. Another limitation is the absence of mechanical loading data. Although the locations of thickest CTh and densest sBMD agree with prior works reporting the areas of the femur in contact with the tibia during daily activities [16,29,34,35] and although the location of thickest CTh has been related to the knee flexion angle during walking [24,30], future studies should consider knee dynamics and loading patterns in addition to bone and cartilage properties for a global understanding of the osteochondral unit. In addition, the methodology is only applicable to non-OA knees, as measuring the location of thickest CTh is irrelevant in knees with substantial cartilage loss. A previous work on CTh and sBMD magnitudes has shown different relationships in non-OA and severe OA knees [8]. It is therefore of particular interest to develop new methods allowing the characterization of the spatial relationship between CTh and sBMD in both OA and non-OA knees. Lastly, BMD quantified by clinical CT is a measure of apparent density. As such, it includes calcified cartilage, whose thickness varies topographically, and non-mineralized tissues in the attenuation calculation [4]. In particular, BMD should not be mistaken for true tissue mineral density (TMD) which requires a segmentation of the bone to distinguish calcified bone tissue from surrounding tissue [36].
In conclusion, this study showed a positive in vivo relationship between locations of thickest CTh and densest sBMD. These results support the idea of a functional unit with morphological coupling between articular cartilage and subchondral bone, and more generally support OA pathophysiology models based on relationships between joint properties. Future research characterizing the spatial relationship in intermediate and severe OA stages could further improve our understanding of knee OA.
Contributions
Hugo Babel made substantial contributions to: analysis and interpretation of the data; drafting of the article; critical revision of the article for important intellectual content; final approval of the article.
Patrick Omoumi made substantial contributions to: conception and design; acquisition of data; analysis and interpretation of data; drafting of the article; critical revision of the article for important intellectual content; final approval of the article; obtaining of funding. PO made equal contributions to this work than BMJ and JF.
Thomas P. Andriacchi made substantial contributions to: analysis and interpretation of data; drafting of the article; critical revision of the article for important intellectual content; final approval of the article.
Brigitte M. Jolles made substantial contributions to: analysis and interpretation of data; critical revision of the article for important intellectual content; final approval of the article; obtaining of funding. BMJ made equal contributions to this work than PO and JF.
Julien Favre made substantial contributions to: conception and design; analysis and interpretation of data; drafting of the article; critical revision of the article for important intellectual content; final approval of the article; obtaining of funding.
Funding source
This work was funded by the "Swiss National Science Foundation, Switzerland (SNSF Grant #CRSII5_177155)" and the “Lausanne Orthopedic Research Foundation, Switzerland”. The funding sources had no involvement in the study design, collection, analysis and interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication.
Declaration of Competing Interest
None.
Acknowledgements
None.
Contributor Information
Hugo Babel, Email: hugo.babel@chuv.ch.
Patrick Omoumi, Email: patrick.omoumi@chuv.ch.
Thomas P. Andriacchi, Email: tandriac@stanford.edu.
Brigitte M. Jolles, Email: brigitte.jolles-haeberli@chuv.ch.
Julien Favre, Email: julien.favre@chuv.ch.
References
- 1.Wieland H.A., Michaelis M., Kirschbaum B.J., Rudolphi K.A. Osteoarthritis—an untreatable disease? Nat. Rev. Drug Discov. 2005;4(4):331–345. doi: 10.1038/nrd1693. [DOI] [PubMed] [Google Scholar]
- 2.Goldring S.R., Goldring M.B. Changes in the osteochondral unit during osteoarthritis: structure, function and cartilage-bone crosstalk. Nat. Rev. Rheumatol. 2016;12(11):632–644. doi: 10.1038/nrrheum.2016.148. Epub 2016/10/21 PubMed PMID: 27652499. [DOI] [PubMed] [Google Scholar]
- 3.Edd S.N., Omoumi P., Andriacchi T.P., Jolles B.M., Favre J. Modeling knee osteoarthritis pathophysiology using an integrated joint system (IJS): a systematic review of relationships among cartilage thickness, gait mechanics, and subchondral bone mineral density. Osteoarthritis Cartilage. 2018;26(11):1425–1437. doi: 10.1016/j.joca.2018.06.017. Epub 2018/07/30 PubMed PMID: 30056214. [DOI] [PubMed] [Google Scholar]
- 4.Bousson V., Lowitz T., Laouisset L., Engelke K., Laredo J.D. CT imaging for the investigation of subchondral bone in knee osteoarthritis. Osteoporos. Int. 2012;23(Suppl 8):S861–S865. doi: 10.1007/s00198-012-2169-5. Epub 2012/11/28 PubMed PMID: 23179574. [DOI] [PubMed] [Google Scholar]
- 5.Imhof H., Sulzbacher I., Grampp S., Czerny C., Youssefzadeh S., Kainberger F. Subchondral bone and cartilage disease: a rediscovered functional unit. Invest. Radiol. 2000;35(10):581–588. doi: 10.1097/00004424-200010000-00004. [DOI] [PubMed] [Google Scholar]
- 6.Andriacchi T.P., Koo S., Scanlan S.F. Gait mechanics influence healthy cartilage morphology and osteoarthritis of the knee. J. Bone Jt. Surg. Am. Vol. 2009;91(Suppl 1):95–101. doi: 10.2106/JBJS.H.01408. Epub 2009/02/21 PubMed PMID: 19182033; PubMed Central PMCID: PMCPMC2663350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hurwitz D.E., Sumner D.R., Andriacchi T.P., Sugar D.A. Dynamic knee loads during gait predict proximal tibial bone distribution. J. Biomech. 1998;31(5):423–430. doi: 10.1016/s0021-9290(98)00028-1. [DOI] [PubMed] [Google Scholar]
- 8.Omoumi P., Babel H., Jolles B.M., Favre J. Relationships between cartilage thickness and subchondral bone mineral density in non-osteoarthritic and severely osteoarthritic knees: in vivo concomitant 3D analysis using CT arthrography. Osteoarthritis Cartilage. 2019;27(4):621–629. doi: 10.1016/j.joca.2018.12.014. PubMed PMID: WOS:000461674500010. [DOI] [PubMed] [Google Scholar]
- 9.Cao Y., Stannus O.P., Aitken D., Cicuttini F., Antony B., Jones G., et al. Cross-sectional and longitudinal associations between systemic, subchondral bone mineral density and knee cartilage thickness in older adults with or without radiographic osteoarthritis. Ann. Rheum. Dis. 2014;73(11):2003–2009. doi: 10.1136/annrheumdis-2013-203691. Epub 2013/08/02 PubMed PMID: 23904471. [DOI] [PubMed] [Google Scholar]
- 10.Omoumi P., Babel H., Jolles B.M., Favre J. Quantitative regional and sub-regional analysis of femoral and tibial subchondral bone mineral density (sBMD) using computed tomography (CT): comparison of non-osteoarthritic (OA) and severe OA knees. Osteoarthritis Cartilage. 2017;25(11):1850–1857. doi: 10.1016/j.joca.2017.07.014. Epub 2017/07/27. PubMed PMID: 28743608. [DOI] [PubMed] [Google Scholar]
- 11.Favre J., Scanlan S.F., Erhart-Hledik J.C., Blazek K., Andriacchi T.P. Patterns of femoral cartilage thickness are different in asymptomatic and osteoarthritic knees and can be used to detect disease-related differences between samples. J. Biomech. Eng. 2013;135(10):101002–101010. doi: 10.1115/1.4024629. Epub 2013/06/01 PubMed PMID: 23722563; PubMed Central PMCID: PMCPMC3792405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Terukina M., Fujioka H., Yoshiya S., Kurosaka M., Makino T., Matsui N., et al. Analysis of the thickness and curvature of articular cartilage of the femoral condyle. Arthrosc. J. Arthrosc. Relat. Surg. 2003;19(9):969–973. doi: 10.1016/j.arthro.2003.09.006. [DOI] [PubMed] [Google Scholar]
- 13.Cohen Z.A., Mccarthy D.M., Kwak S.D., Legrand P., Fogarasi F., Ciaccio E.J., et al. Knee cartilage topography, thickness, and contact areas from MRI: in-vitro calibration and in-vivo measurements. Osteoarthritis Cartilage. 1999;7(1):95–109. doi: 10.1053/joca.1998.0165. [DOI] [PubMed] [Google Scholar]
- 14.Cohen Z.A., Mow V.C., Henry J.H., Levine W.N., Ateshian G.A. Templates of the cartilage layers of the patellofemoral joint and their use in the assessment of osteoarthritic cartilage damage. Osteoarthritis Cartilage. 2003;11(8):569–579. doi: 10.1016/s1063-4584(03)00091-8. [DOI] [PubMed] [Google Scholar]
- 15.Oláh T., Reinhard J., Gao L., Haberkamp S., Goebel L.K., Cucchiarini M., et al. Topographic modeling of early human osteoarthritis in sheep. Sci. Transl. Med. 2019;11(508) doi: 10.1126/scitranslmed.aax6775. [DOI] [PubMed] [Google Scholar]
- 16.Li G., Park S.E., DeFrate L.E., Schutzer M.E., Ji L., Gill T.J., et al. The cartilage thickness distribution in the tibiofemoral joint and its correlation with cartilage-to-cartilage contact. Clin. Biomech. 2005;20(7):736–744. doi: 10.1016/j.clinbiomech.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Armstrong S.J., Read R.A., Price R. Topographical variation within the articular cartilage and subchondral bone of the normal ovine knee joint: a histological approach. Osteoarthritis Cartilage. 1995;3(1):25–33. doi: 10.1016/s1063-4584(05)80035-4. [DOI] [PubMed] [Google Scholar]
- 18.Johnston J.D., Masri B.A., Wilson D.R. Computed tomography topographic mapping of subchondral density (CT-TOMASD) in osteoarthritic and normal knees: methodological development and preliminary findings. Osteoarthritis Cartilage. 2009;17(10):1319–1326. doi: 10.1016/j.joca.2009.04.013. Epub 2009/05/12 PubMed PMID: 19427927. [DOI] [PubMed] [Google Scholar]
- 19.Bennell K.L., Creaby M.W., Wrigley T.V., Hunter D.J. Tibial subchondral trabecular volumetric bone density in medial knee joint osteoarthritis using peripheral quantitative computed tomography technology. Arthritis Rheum. 2008;58(9):2776–2785. doi: 10.1002/art.23795. Epub 2008/09/02 PubMed PMID: 18759296. [DOI] [PubMed] [Google Scholar]
- 20.Phillips A., Burton N.J., Warren-Smith C.M.R., Kulendra E.R., Parsons K.J. Topographic bone density of the radius and ulna in Greyhounds and Labrador Retrievers with and without medial coronoid process disease. Vet. Surg. 2015;44(2):180–190. doi: 10.1111/j.1532-950X.2014.12294.x. [DOI] [PubMed] [Google Scholar]
- 21.Felson D.T., McAlindon T.E., Anderson J.J., Weissman B.W., Aliabadi P., Evans S., et al. Defining radiographic osteoarthritis for the whole knee. Osteoarthritis Cartilage. 1997;5(4):241–250. doi: 10.1016/s1063-4584(97)80020-9. [DOI] [PubMed] [Google Scholar]
- 22.Favre J., Erhart-Hledik J.C., Blazek K., Fasel B., Gold G.E., Andriacchi T.P. Anatomically standardized maps reveal distinct patterns of cartilage thickness with increasing severity of medial compartment knee osteoarthritis. J. Orthop. Res. 2017;35(11):2442–2451. doi: 10.1002/jor.23548. Epub 2017/02/25 PubMed PMID: 28233332. [DOI] [PubMed] [Google Scholar]
- 23.Koo S., Gold G.E., Andriacchi T.P. Considerations in measuring cartilage thickness using MRI: factors influencing reproducibility and accuracy. Osteoarthritis Cartilage. 2005;13(9):782–789. doi: 10.1016/j.joca.2005.04.013. Epub 2005/06/18 PubMed PMID: 15961328. [DOI] [PubMed] [Google Scholar]
- 24.Scanlan S.F., Favre J., Andriacchi T.P. The relationship between peak knee extension at heel-strike of walking and the location of thickest femoral cartilage in ACL reconstructed and healthy contralateral knees. J. Biomech. 2013;46(5):849–854. doi: 10.1016/j.jbiomech.2012.12.026. Epub 2013/02/05 PubMed PMID: 23375789. [DOI] [PubMed] [Google Scholar]
- 25.Frobell R.B., Nevitt M.C., Hudelmaier M., Wirth W., Wyman B.T., Benichou O., et al. Femorotibial subchondral bone area and regional cartilage thickness: a cross-sectional description in healthy reference cases and various radiographic stages of osteoarthritis in 1,003 knees from the Osteoarthritis Initiative. Arthritis Care Res (Hoboken). 2010;62(11):1612–1623. doi: 10.1002/acr.20262. Epub 2010/05/25 PubMed PMID: 20496431. [DOI] [PubMed] [Google Scholar]
- 26.Baum T., Sauerschnig M., Penzel J., Jungmann P.M., Waldt S., Rummeny E.J., et al. Early changes of trabecular bone structure in asymptomatic subjects with knee malalignment. 2014;38(1):137–141. doi: 10.1097/RCT.0b013e3182a90f08. [DOI] [PubMed] [Google Scholar]
- 27.Turner C. Three rules for bone adaptation to mechanical stimuli. Bone. 1998;23(5):399–407. doi: 10.1016/s8756-3282(98)00118-5. [DOI] [PubMed] [Google Scholar]
- 28.Chehab E., Andriacchi T., JJJob Favre. Speed, age, sex, and body mass index provide a rigorous basis for comparing the kinematic and kinetic profiles of the lower extremity during walking. 2017;58:11–20. doi: 10.1016/j.jbiomech.2017.04.014. [DOI] [PubMed] [Google Scholar]
- 29.Akpinar B., Thorhauer E., Tashman S., Irrgang J.J., Fu F.H., WJJOjosm Anderst. Tibiofemoral cartilage contact differences between level walking and downhill running. 2019;7(4) doi: 10.1177/2325967119836164. 2325967119836164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koo S., Rylander J.H., Andriacchi T.P. Knee joint kinematics during walking influences the spatial cartilage thickness distribution in the knee. J. Biomech. 2011;44(7):1405–1409. doi: 10.1016/j.jbiomech.2010.11.020. Epub 2011/03/05 PubMed PMID: 21371712; PubMed Central PMCID: PMCPMC3078989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lohmander L.S., Ionescu M., Jugessur H., Poole A.R. Changes in joint cartilage aggrecan after knee injury and in osteoarthritis. Arthritis Rheum.: Off. J. Am. coll. Rheumatol. 1999;42(3):534–544. doi: 10.1002/1529-0131(199904)42:3<534::AID-ANR19>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 32.Koo S., Andriacchi T.P. A comparison of the influence of global functional loads vs. local contact anatomy on articular cartilage thickness at the knee. J. Biomech. 2007;40(13):2961–2966. doi: 10.1016/j.jbiomech.2007.02.005. Epub 2007/04/10 PubMed PMID: 17418219; PubMed Central PMCID: PMCPMC2358971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clouthier A.L., Smith C.R., Vignos M.F., Thelen D.G., Deluzio K.J., MJJMe Rainbow, et al. The effect of articular geometry features identified using statistical shape modelling on knee biomechanics. 2019;66:47–55. doi: 10.1016/j.medengphy.2019.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li J.-S., Hosseini A., Cancre L., Ryan N., Rubash H.E., Li G.J.G., et al. Kinematic characteristics of the tibiofemoral joint during a step-up activity. 2013;38(4):712–716. doi: 10.1016/j.gaitpost.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yin P., Li J.-S., Kernkamp W.A., Tsai T.-Y., Baek S.-H., Hosseini A., et al. Analysis of in-vivo articular cartilage contact surface of the knee during a step-up motion. 2017;49:101–106. doi: 10.1016/j.clinbiomech.2017.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bouxsein M.L., Boyd S.K., Christiansen B.A., Guldberg R.E., Jepsen K.J., RJJob Müller, et al. Guidelines for assessment of bone microstructure in rodents using micro–computed tomography. 2010;25(7):1468–1486. doi: 10.1002/jbmr.141. [DOI] [PubMed] [Google Scholar]